Attention-Deficit Hyperactivity Disorder (ADHD): A Comprehensive Overview of the Mechanistic Insights from Human Studies to Animal Models
Abstract
1. Introduction
2. Diagnosing ADHD in Children and Adults: Similarities and Differences
3. Brain Structural Changes Associated with ADHD
4. Neurotransmitters and ADHD
4.1. Dopaminergic Signaling in the Brain
4.2. Noradrenergic Signaling in the Brain
4.3. Serotonergic Signaling in the Brain
4.4. NMDAR and Dopamine Interactions
5. Genetic Contributions to ADHD
6. ADHD Medications
7. Modes of Action of Various ADHD Medications
7.1. Methylphenidate Based Medications
7.2. Amphetamine Based Medications
7.3. Non-Stimulant Medications
8. Pharmacological Treatment for ADHD in Humans and Animal Models: Discrepancies and Possible Explanations
8.1. ADHD Medications Generally Improve Cognition in Humans
8.2. Amphetamine Effects on Cognition
8.3. Methylphenidate Effects on Cognition
8.4. Atomoxetine Effects on Cognition
9. Implications of In Utero Exposure to ADHD Medications for Long-Term Cognition
9.1. Prescription of ADHD Medications During Pregnancy
9.2. Continued Evidence Suggests That ADHD Medications Are Safe During Pregnancy
9.3. In Utero Exposure to AMPH: Time-Dependent Changes in Metabolism and Behavior in Animal Studies
9.4. In Utero Exposure to Methylphenidate: Alterations in DA Pathways and Behavior in Animal Studies
10. Transcranial Stimulation for ADHD Treatment
11. A Paradigm Shift in ADHD Research: Integrating Synaptic and Systems-Level Neuroscience
11.1. Reframing ADHD Treatment: From Disorder to Divergence
11.2. Improving ADHD Diagnosis and Interpretation of Human Data
11.3. Further Longitudinal Studies Are Required to Assess the Impact of In Utero Exposure to ADHD Medications on Cognition
11.4. Considerations for Animal Models and In Utero Exposure Studies
12. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADHD | Attention-deficit/hyperactivity disorder |
| ASD | Autism spectrum disorder |
| DSM-5 | Diagnostic and Statistical Manual of Mental Disorders 5 |
| ADHD-I | Primarily inattentive ADHD |
| ADHD-H | Primarily hyperactive ADHD |
| ADHD-C | Combined ADHD |
| PFC | Prefrontal cortex |
| TDIs | Typically developing individuals |
| NAc | Nucleus accumbens |
| CC | Corpus callosum |
| BG | Basal ganglia |
| DA | Dopamine |
| NE | Norepinephrine |
| AMPH | Amphetamine |
| MPH | Methylphenidate |
| ATX | Atomoxetine |
| 5-HT | Serotonin |
| CNS | Central nervous system |
| VTA | Ventral tegmental area |
| SN | Substantia nigra |
| DS | Dorsal striatum |
| GPCRs | G-protein-coupled-receptors |
| cAMP | Cyclic adenosine monophosphate |
| PKA | Protein kinase A |
| CREB | Cyclic adenosine monophosphate |
| SNc | Substantia nigra pars compacta |
| IRK | Inward rectifying potassium channels |
| PET | Positron emission tomography |
| DAT | Dopamine transporter |
| NET | Norepinephrine transporter |
| DOPA | Dopamine β-hydroxylase |
| LC | Locus coeruleus |
| DRn | Dorsal raphe nucleus |
| SERT | Serotonin transporter |
| NMDARs | N-methyl-D-aspartate receptors |
| AMPARs | α-amino-3-hydroxy-5-methyl-4-isoxazole propionic receptors |
| EPSCs | Excitatory postsynaptic potentials |
| VMAT2 | Vesicular monoamine transporter 2 |
| MAO | Monoamine oxidase |
| VWM | Visual working memory |
| SWM | Spatial working memory |
| LTP | Long-term potentiation |
| BDNF | Brain-derived neurotrophic factor |
| rTMS | Repetitive transcranial magnetic stimulation |
| tDCS | Transcranial direct current stimulation |
| dlPFC | Dorsolateral prefrontal cortex |
| VNS | Vagus nerve stimulation |
| eTNS | External trigeminal nerve stimulation |
References
- Astle, D.E.; Bassett, D.S.; Viding, E. Understanding divergence: Placing developmental neuroscience in its dynamic context. Neurosci. Biobehav. Rev. 2024, 157, 105539. [Google Scholar] [CrossRef]
- Friedman, L.A.; Rapoport, J.L. Brain development in ADHD. Curr. Opin. Neurobiol. 2015, 30, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Zha, M.; Yang, Q.; Zhang, Y.; Li, X.; Rudan, I. The prevalence of adult attention-deficit hyperactivity disorder: A global systematic review and meta-analysis. J. Glob. Health 2021, 11, 04009. [Google Scholar] [CrossRef]
- Willcutt, E.G. The Prevalence of DSM-IV Attention-Deficit/Hyperactivity Disorder: A Meta-Analytic Review. Neurotherapeutics 2012, 9, 490–499. [Google Scholar] [CrossRef]
- Young, S.; Adamo, N.; Ásgeirsdóttir, B.B.; Branney, P.; Beckett, M.; Colley, W.; Cubbin, S.; Deeley, Q.; Farrag, E.; Gudjonsson, G.; et al. Females with ADHD: An expert consensus statement taking a lifespan approach providing guidance for the identification and treatment of attention-deficit/hyperactivity disorder in girls and women. BMC Psychiatry 2020, 20, 404. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; DSM-5-TR; American Psychiatric Association Publishing: Washington, DC, USA, 2022; ISBN 978-0-89042-575-6. [Google Scholar]
- Posner, J.; Polanczyk, G.V.; Sonuga-Barke, E. Attention-deficit hyperactivity disorder. Lancet 2020, 395, 450–462. [Google Scholar] [CrossRef]
- Eiland, L.S.; Gildon, B.L. Diagnosis and Treatment of ADHD in the Pediatric Population. J. Pediatr. Pharmacol. Ther. 2024, 29, 107–118. [Google Scholar] [CrossRef]
- Jangmo, A.; Stålhandske, A.; Chang, Z.; Chen, Q.; Almqvist, C.; Feldman, I.; Bulik, C.M.; Lichtenstein, P.; D’Onofrio, B.; Kuja-Halkola, R.; et al. Attention-Deficit/Hyperactivity Disorder, School Performance, and Effect of Medication. J. Am. Acad. Child Adolesc. Psychiatry 2019, 58, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Weyandt, L.L.; Iwaszuk, W.; Fulton, K.; Ollerton, M.; Beatty, N.; Fouts, H.; Schepman, S.; Greenlaw, C. The Internal Restlessness Scale: Performance of College Students With and Without ADHD. J. Learn. Disabil. 2003, 36, 382–389. [Google Scholar] [CrossRef]
- Martel, M.M.; von Eye, A.; Nigg, J. Developmental differences in structure of attention-deficit/hyperactivity disorder (ADHD) between childhood and adulthood. Int. J. Behav. Dev. 2012, 36, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Williams, O.C.; Prasad, S.; McCrary, A.; Jordan, E.; Sachdeva, V.; Deva, S.; Kumar, H.; Mehta, J.; Neupane, P.; Gupta, A. Adult attention deficit hyperactivity disorder: A comprehensive review. Ann. Med. Surg. 2023, 85, 1802–1810. [Google Scholar] [CrossRef] [PubMed]
- Bálint, S.; Czobor, P.; Komlósi, S.; Mészáros, Á.; Simon, V.; Bitter, I. Attention deficit hyperactivity disorder (ADHD): Gender- and age-related differences in neurocognition. Psychol. Med. 2009, 39, 1337–1345. [Google Scholar] [CrossRef]
- Henning, C.; Summerfeldt, L.J.; Parker, J.D.A. ADHD and Academic Success in University Students: The Important Role of Impaired Attention. J. Atten. Disord. 2022, 26, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Fuermaier, A.B.M.; Tucha, L.; Butzbach, M.; Weisbrod, M.; Aschenbrenner, S.; Tucha, O. ADHD at the workplace: ADHD symptoms, diagnostic status, and work-related functioning. J. Neural Transm. 2021, 128, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, A.; Fleischmann, R.H. Advantages of an ADHD Diagnosis in Adulthood: Evidence from Online Narratives. Qual. Health Res. 2012, 22, 1486–1496. [Google Scholar] [CrossRef]
- Young, S.; Bramham, J.; Gray, K.; Rose, E. The Experience of Receiving a Diagnosis and Treatment of ADHD in Adulthood: A Qualitative Study of Clinically Referred Patients Using Interpretative Phenomenological Analysis. J. Atten. Disord. 2008, 11, 493–503. [Google Scholar] [CrossRef]
- Girard-Joyal, O.; Gauthier, B. Creativity in the Predominantly Inattentive and Combined Presentations of ADHD in Adults. J. Atten. Disord. 2022, 26, 1187–1198. [Google Scholar] [CrossRef]
- Stolte, M.; Trindade-Pons, V.; Vlaming, P.; Jakobi, B.; Franke, B.; Kroesbergen, E.H.; Baas, M.; Hoogman, M. Characterizing Creative Thinking and Creative Achievements in Relation to Symptoms of Attention-Deficit/Hyperactivity Disorder and Autism Spectrum Disorder. Front. Psychiatry 2022, 13, 909202. [Google Scholar] [CrossRef]
- Ten, W.; Tseng, C.-C.; Chiang, Y.-S.; Wu, C.-L.; Chen, H.-C. Creativity in children with ADHD: Effects of medication and comparisons with normal peers. Psychiatry Res. 2020, 284, 112680. [Google Scholar] [CrossRef]
- González-Carpio Hernández, G.; Serrano Selva, J.P. Medication and creativity in Attention Deficit Hyperactivity Disorder (ADHD). Psicothema 2016, 28, 20–25. [Google Scholar] [CrossRef]
- Zentall, S.S. Production deficiencies in elicited language but not in the spontaneous verbalizations of hyperactive children. J. Abnorm. Child Psychol. 1988, 16, 657–673. [Google Scholar] [CrossRef]
- White, H.A.; Shah, P. Creative style and achievement in adults with attention-deficit/hyperactivity disorder. Personal. Individ. Differ. 2011, 50, 673–677. [Google Scholar] [CrossRef]
- Brod, M.; Pohlman, B.; Lasser, R.; Hodgkins, P. Comparison of the burden of illness for adults with ADHD across seven countries: A qualitative study. Health Qual. Life Outcomes 2012, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Oram, R.; Rogers, M. Inattentive Behavior and Homework Performance in Elementary School: The Mediating Effects of Academic Enablers. Contemp. Sch. Psychol. 2022, 26, 448–457. [Google Scholar] [CrossRef]
- Committee on Quality Improvement, Subcommittee on Attention-Deficit/Hyperactivity Disorder. Clinical Practice Guideline: Diagnosis and Evaluation of the Child with Attention-Deficit/Hyperactivity Disorder. Pediatrics 2000, 105, 1158–1170. [Google Scholar] [CrossRef] [PubMed]
- Asherson, P. Clinical assessment and treatment of attention deficit hyperactivity disorder in adults. Expert Rev. Neurother. 2005, 5, 525–539. [Google Scholar] [CrossRef]
- Palmini, A. Attention-deficit/hyperactivity disorder (ADHD) in adults: A multilayered approach to a serious disorder of inattention to the future. Arq. Neuropsiquiatr. 2024, 82, 001–012. [Google Scholar] [CrossRef]
- Posner, K.; Melvin, G.A.; Murray, D.W.; Gugga, S.S.; Fisher, P.; Skrobala, A.; Cunningham, C.; Vitiello, B.; Abikoff, H.B.; Ghuman, J.K.; et al. Clinical Presentation of Attention-Deficit/Hyperactivity Disorder in Preschool Children: The Preschoolers with Attention-Deficit/Hyperactivity Treatment Study (PATS). J. Child Adolesc. Psychopharmacol. 2007, 17, 547–562. [Google Scholar] [CrossRef]
- Haskell, B.; Carter-Orbke, M.; Vick, R.; Madden, B.; Smith, L. Diagnosis and Treatment of Adults with Attention-Deficit/Hyperactivity Disorder (ADHD). J. Nurse Pract. 2024, 20, 105217. [Google Scholar] [CrossRef]
- Wender, P.H.; Wolf, L.E.; Wasserstein, J. Adults with ADHD: An Overview. Ann. N. Y. Acad. Sci. 2001, 931, 1–16. [Google Scholar] [CrossRef]
- Lahey, B.B.; Pelham, W.E.; Loney, J.; Lee, S.S.; Willcutt, E. Instability of the DSM-IV Subtypes of ADHD from preschool through elementary school. Arch. Gen. Psychiatry 2005, 62, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Schrevel, S.J.C.; Dedding, C.; van Aken, J.A.; Broerse, J.E.W. “Do I need to become someone else?” A qualitative exploratory study into the experiences and needs of adults with ADHD. Health Expect. 2016, 19, 39–48. [Google Scholar] [CrossRef]
- Schachar, R.J.; Dupuis, A.; Arnold, P.D.; Anagnostou, E.; Kelley, E.; Georgiades, S.; Nicolson, R.; Townes, P.; Burton, C.L.; Crosbie, J. Autism Spectrum Disorder and Attention-Deficit/Hyperactivity Disorder: Shared or Unique Neurocognitive Profiles? Res. Child Adolesc. Psychopathol. 2023, 51, 17–31. [Google Scholar] [CrossRef]
- Crisci, G.; Cardillo, R.; Mammarella, I.C. Social Functioning in Children and Adolescents with ADHD and Autism Spectrum Disorder: A Cross-Disorder Comparison. J. Clin. Child Adolesc. Psychol. 2024, 53, 489–502. [Google Scholar] [CrossRef]
- De Giacomo, A.; Craig, F.; Medicamento, S.; Gradia, F.; Sardella, D.; Costabile, A.; Matera, E.; Turi, M. Identifying Autistic-Like Symptoms in Children with ADHD: A Comparative Study Using ADOS-2. Neuropsychiatr. Dis. Treat. 2024, 20, 1367–1376. [Google Scholar] [CrossRef]
- Canals, J.; Morales-Hidalgo, P.; Voltas, N.; Hernández-Martínez, C. Prevalence of comorbidity of autism and ADHD and associated characteristics in school population: EPINED study. Autism Res. 2024, 17, 1276–1286. [Google Scholar] [CrossRef]
- Pedersen, S.L.; Kennedy, T.M.; Joseph, H.M.; Riston, S.J.; Kipp, H.L.; Molina, B.S.G. Real-World Changes in Adolescents’ ADHD Symptoms within the Day and across School and Non-school Days. J. Abnorm. Child Psychol. 2020, 48, 1543–1553. [Google Scholar] [CrossRef]
- Duh-Leong, C.; Fuller, A.; Brown, N.M. Associations Between Family and Community Protective Factors and Attention-Deficit/Hyperactivity Disorder Outcomes Among US Children. J. Dev. Behav. Pediatr. 2020, 41, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sibley, M.H.; Swanson, J.M.; Arnold, L.E.; Hechtman, L.T.; Owens, E.B.; Stehli, A.; Abikoff, H.; Hinshaw, S.P.; Molina, B.S.G.; Mitchell, J.T.; et al. Defining ADHD symptom persistence in adulthood: Optimizing sensitivity and specificity. J. Child Psychol. Psychiatry 2017, 58, 655–662. [Google Scholar] [CrossRef]
- Faraone, S.V.; Biederman, J.; Mick, E. The age-dependent decline of attention deficit hyperactivity disorder: A meta-analysis of follow-up studies. Psychol. Med. 2006, 36, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Lowe, C.T.; Bath, A.C.; Callahan, B.L.; Climie, E.A. Positive Childhood Experiences and the Indirect Relationship with Improved Emotion Regulation in Adults with ADHD Through Social Support. J. Atten. Disord. 2024, 28, 1615–1626. [Google Scholar] [CrossRef]
- Brown, N.M.; Brown, S.N.; Briggs, R.D.; Germán, M.; Belamarich, P.F.; Oyeku, S.O. Associations Between Adverse Childhood Experiences and ADHD Diagnosis and Severity. Acad. Pediatr. 2017, 17, 349–355. [Google Scholar] [CrossRef]
- Roy, A.; Hechtman, L.; Arnold, L.E.; Sibley, M.H.; Molina, B.S.G.; Swanson, J.M.; Howard, A.L. MTA Cooperative Group Childhood Factors Affecting Persistence and Desistence of Attention-Deficit/Hyperactivity Disorder Symptoms in Adulthood: Results from the MTA. J. Am. Acad. Child Adolesc. Psychiatry 2016, 55, 937–944.e4. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Xiao, Y.; Yu, C.; Pei, Y.; Cao, F. Association of positive childhood experiences with flourishing among children with ADHD: A population-based study in the United States. Prev. Med. 2024, 179, 107824. [Google Scholar] [CrossRef]
- Mastoras, S.M.; Saklofske, D.H.; Schwean, V.L.; Climie, E.A. Social Support in Children with ADHD: An Exploration of Resilience. J. Atten. Disord. 2018, 22, 712–723. [Google Scholar] [CrossRef] [PubMed]
- Atta, M.H.R.; Amin, S.M.; Hamad, N.I.M.; Othman, A.A.; Sayed, Y.M.; Sanad, H.S.; El-Sayed, A.A.I. The Role of Perceived Social Support in the Association Between Stress and Creativity Self-Efficacy Among Adolescents with Attention Deficit Hyperactivity Disorder. J. Psychiatr. Ment. Health Nurs. 2025, 32, 897–909. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, D.; Basurto, K.; Guilfoyle, J.; VanLandingham, H.B.; Gonzalez, C.; Ovsiew, G.P.; Rodriguez, V.J.; Resch, Z.J.; Ulrich, D.M.; Soble, J.R. The Effect of Adverse Childhood Experiences on ADHD Symptom Reporting, Psychological Symptoms, and Cognitive Performance Among Adult Neuropsychological Referrals. J. Atten. Disord. 2024, 28, 43–50. [Google Scholar] [CrossRef]
- Bedford, S.A.; Lai, M.-C.; Lombardo, M.V.; Chakrabarti, B.; Ruigrok, A.; Suckling, J.; Anagnostou, E.; Lerch, J.P.; Taylor, M.; Nicolson, R.; et al. Brain-Charting Autism and Attention-Deficit/Hyperactivity Disorder Reveals Distinct and Overlapping Neurobiology. Biol. Psychiatry 2025, 97, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.; Eckstrand, K.; Sharp, W.; Blumenthal, J.; Lerch, J.P.; Greenstein, D.; Clasen, L.; Evans, A.; Giedd, J.; Rapoport, J.L. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc. Natl. Acad. Sci. USA 2007, 104, 19649–19654. [Google Scholar] [CrossRef]
- Shaw, P.; Lerch, J.; Greenstein, D.; Sharp, W.; Clasen, L.; Evans, A.; Giedd, J.; Castellanos, F.X.; Rapoport, J. Longitudinal Mapping of Cortical Thickness and Clinical Outcome in Children and Adolescents with Attention-Deficit/Hyperactivity Disorder. Arch. Gen. Psychiatry 2006, 63, 540–549. [Google Scholar] [CrossRef]
- Emond, V.; Joyal, C.; Poissant, H. Neuroanatomie structurelle et fonctionnelle du trouble déficitaire d’attention avec ou sans hyperactivité (TDAH). L’Encéphale 2009, 35, 107–114. [Google Scholar] [CrossRef]
- Costa Dias, T.G.; Wilson, V.B.; Bathula, D.R.; Iyer, S.P.; Mills, K.L.; Thurlow, B.L.; Stevens, C.A.; Musser, E.D.; Carpenter, S.D.; Grayson, D.S.; et al. Reward circuit connectivity relates to delay discounting in children with attention-deficit/hyperactivity disorder. Eur. Neuropsychopharmacol. 2013, 23, 33–45. [Google Scholar] [CrossRef]
- Smith, S.M.; Fox, P.T.; Miller, K.L.; Glahn, D.C.; Fox, P.M.; Mackay, C.E.; Filippini, N.; Watkins, K.E.; Toro, R.; Laird, A.R.; et al. Correspondence of the brain’s functional architecture during activation and rest. Proc. Natl. Acad. Sci. USA 2009, 106, 13040–13045. [Google Scholar] [CrossRef] [PubMed]
- Zaher, A.; Leonards, J.; Reif, A.; Grimm, O. Functional connectivity of the nucleus accumbens predicts clinical course in treated and non-responder adult ADHD. MedRxiv, 2024; preprint. [Google Scholar] [CrossRef]
- Kortz, M.W.; Lillehei, K.O. Insular Cortex. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK570606/ (accessed on 30 May 2025).
- Yasumura, A.; Omori, M.; Fukuda, A.; Takahashi, J.; Yasumura, Y.; Nakagawa, E.; Koike, T.; Yamashita, Y.; Miyajima, T.; Koeda, T.; et al. Age-related differences in frontal lobe function in children with ADHD. Brain Dev. 2019, 41, 577–586. [Google Scholar] [CrossRef]
- Sheridan, M.A.; Hinshaw, S.; D’Esposito, M. Efficiency of the Prefrontal Cortex During Working Memory in Attention-Deficit/Hyperactivity Disorder. J. Am. Acad. Child Adolesc. Psychiatry 2007, 46, 1357–1366. [Google Scholar] [CrossRef]
- Isaac, V.; Lopez, V.; Escobar, M.J. Can attention-deficit/hyperactivity disorder be considered a form of cerebellar dysfunction? Front. Neurosci. 2025, 19, 1453025. [Google Scholar] [CrossRef]
- Wyciszkiewicz, A.; Pawlak, M.A.; Krawiec, K. Cerebellar Volume in Children with Attention-Deficit Hyperactivity Disorder (ADHD): Replication Study. J. Child Neurol. 2017, 32, 215–221. [Google Scholar] [CrossRef]
- Mackie, S.; Shaw, P.; Lenroot, R.; Pierson, R.; Greenstein, D.K.; Nugent, T.F.; Sharp, W.S.; Giedd, J.N.; Rapoport, J.L. Cerebellar Development and Clinical Outcome in Attention Deficit Hyperactivity Disorder. Am. J. Psychiatry 2007, 164, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.E.; Yeo, R.A.; Campbell, R.A.; Hart, B.; Vigil, J.; Brooks, W. Magnetic resonance imaging correlates of attention-deficit/hyperactivity disorder in children. Neuropsychology 2003, 17, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Sun, L.; Gong, G.; Lv, Y.; Cao, X.; Shuai, L.; Zhu, C.; Zang, Y.; Wang, Y. The macrostructural and microstructural abnormalities of corpus callosum in children with attention deficit/hyperactivity disorder: A combined morphometric and diffusion tensor MRI study. Brain Res. 2010, 1310, 172–180. [Google Scholar] [CrossRef]
- Goldstein, A.; Covington, B.P.; Mahabadi, N.; Mesfin, F.B. Neuroanatomy, Corpus Callosum. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK448209/ (accessed on 30 May 2025).
- Dramsdahl, M.; Westerhausen, R.; Haavik, J.; Hugdahl, K.; Plessen, K.J. Adults with attention-deficit/hyperactivity disorder—A diffusion-tensor imaging study of the corpus callosum. Psychiatry Res. Neuroimaging 2012, 201, 168–173. [Google Scholar] [CrossRef]
- Luders, E.; Kurth, F.; Das, D.; Oyarce, D.E.; Shaw, M.E.; Sachdev, P.; Easteal, S.; Anstey, K.J.; Cherbuin, N. Associations between corpus callosum size and ADHD symptoms in older adults: The PATH through life study. Psychiatry Res. Neuroimaging 2016, 256, 8–14. [Google Scholar] [CrossRef]
- Qiu, A.; Crocetti, D.; Adler, M.; Mahone, E.M.; Denckla, M.B.; Miller, M.I.; Mostofsky, S.H. Basal ganglia volume and shape in children with attention deficit hyperactivity disorder. Am. J. Psychiatry 2009, 166, 74–82. [Google Scholar] [CrossRef]
- Shaw, P.; De Rossi, P.; Watson, B.; Wharton, A.; Greenstein, D.; Raznahan, A.; Sharp, W.; Lerch, J.P.; Chakravarty, M.M. Mapping the Development of the Basal Ganglia in Children with Attention-Deficit/Hyperactivity Disorder. J. Am. Acad. Child Adolesc. Psychiatry 2014, 53, 780–789.e11. [Google Scholar] [CrossRef]
- Pasini, A.; D’agati, E. Pathophysiology of NSS in ADHD. World J. Biol. Psychiatry 2009, 10, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Haber, S.N. Neuroanatomy of Reward: A View from the Ventral Striatum. In Neurobiology of Sensation and Reward; Gottfried, J.A., Ed.; Frontiers in Neuroscience; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011; ISBN 978-1-4200-6726-2. Available online: http://www.ncbi.nlm.nih.gov/books/NBK92777/ (accessed on 30 May 2025).
- Greven, C.U.; Bralten, J.; Mennes, M.; O’Dwyer, L.; Van Hulzen, K.J.E.; Rommelse, N.; Schweren, L.J.S.; Hoekstra, P.J.; Hartman, C.A.; Heslenfeld, D.; et al. Developmentally Stable Whole-Brain Volume Reductions and Developmentally Sensitive Caudate and Putamen Volume Alterations in Those with Attention-Deficit/Hyperactivity Disorder and Their Unaffected Siblings. JAMA Psychiatry 2015, 72, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Mooney, M.A.; Bhatt, P.; Hermosillo, R.J.M.; Ryabinin, P.; Nikolas, M.; Faraone, S.V.; Fair, D.A.; Wilmot, B.; Nigg, J.T. Smaller total brain volume but not subcortical structure volume related to common genetic risk for ADHD. Psychol. Med. 2021, 51, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, F.X. Developmental Trajectories of Brain Volume Abnormalities in Children and Adolescents with Attention-Deficit/Hyperactivity Disorder. JAMA 2002, 288, 1740. [Google Scholar] [CrossRef]
- MacDonald, H.J.; Kleppe, R.; Szigetvari, P.D.; Haavik, J. The dopamine hypothesis for ADHD: An evaluation of evidence accumulated from human studies and animal models. Front. Psychiatry 2024, 15, 1492126. [Google Scholar] [CrossRef]
- Cortese, S.; Adamo, N.; Del Giovane, C.; Mohr-Jensen, C.; Hayes, A.J.; Carucci, S.; Atkinson, L.Z.; Tessari, L.; Banaschewski, T.; Coghill, D.; et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: A systematic review and network meta-analysis. Lancet Psychiatry 2018, 5, 727–738. [Google Scholar] [CrossRef]
- McCabe, S.E.; Knight, J.R.; Teter, C.J.; Wechsler, H. Non-medical use of prescription stimulants among US college students: Prevalence and correlates from a national survey. Addiction 2005, 100, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, F.T.; Kim, J.M.; Mandl, K.D. Premarket safety and efficacy studies for ADHD medications in children. PLoS ONE 2014, 9, e102249. [Google Scholar] [CrossRef][Green Version]
- Besag, F.M.C. ADHD treatment and pregnancy. Drug Saf. 2014, 37, 397–408. [Google Scholar] [CrossRef]
- Jiang, Y.; Zou, D.; Li, Y.; Gu, S.; Dong, J.; Ma, X.; Xu, S.; Wang, F.; Huang, J.H. Monoamine Neurotransmitters Control Basic Emotions and Affect Major Depressive Disorders. Pharmaceuticals 2022, 15, 1203. [Google Scholar] [CrossRef]
- Nikolaus, S.; Mamlins, E.; Giesel, F.L.; Schmitt, D.; Müller, H.-W. Monoaminergic hypo- or hyperfunction in adolescent and adult attention-deficit hyperactivity disorder? Rev. Neurosci. 2022, 33, 347–364. [Google Scholar] [CrossRef]
- Bromberg-Martin, E.S.; Matsumoto, M.; Hikosaka, O. Dopamine in motivational control: Rewarding, aversive, and alerting. Neuron 2010, 68, 815–834. [Google Scholar] [CrossRef] [PubMed]
- Berke, J.D. What does dopamine mean? Nat. Neurosci. 2018, 21, 787–793. [Google Scholar] [CrossRef]
- Ott, T.; Nieder, A. Dopamine and Cognitive Control in Prefrontal Cortex. Trends Cogn. Sci. 2019, 23, 213–234. [Google Scholar] [CrossRef]
- Elliott, B.L.; D’Ardenne, K.; Mukherjee, P.; Schweitzer, J.B.; McClure, S.M. Limbic and Executive Meso- and Nigrostriatal Tracts Predict Impulsivity Differences in Attention-Deficit/Hyperactivity Disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2022, 7, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.X.; Huang, E.J. Dopaminergic Neurons and Brain Reward Pathways. Am. J. Pathol. 2016, 186, 478–488. [Google Scholar] [CrossRef]
- Qi-Lytle, X.; Sayers, S.; Wagner, E.J. Current Review of the Function and Regulation of Tuberoinfundibular Dopamine Neurons. Int. J. Mol. Sci. 2023, 25, 110. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.O.; Battagello, D.S.; Cardoso, A.R.; Hauser, D.N.; Bittencourt, J.C.; Correa, R.G. Dopamine: Functions, Signaling, and Association with Neurological Diseases. Cell. Mol. Neurobiol. 2019, 39, 31–59. [Google Scholar] [CrossRef]
- Gonzalez-Lopez, E.; Vrana, K.E. Dopamine beta-hydroxylase and its genetic variants in human health and disease. J. Neurochem. 2020, 152, 157–181. [Google Scholar] [CrossRef] [PubMed]
- Bari, B.A.; Chokshi, V.; Schmidt, K. Locus coeruleus-norepinephrine: Basic functions and insights into Parkinson’s disease. Neural Regen. Res. 2020, 15, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cordeiro Matos, S.; Jego, S.; Adamantidis, A.; Séguéla, P. Norepinephrine Drives Persistent Activity in Prefrontal Cortex via Synergistic α1 and α2 Adrenoceptors. PLoS ONE 2013, 8, e66122. [Google Scholar] [CrossRef]
- Zaborszky, L. Sleep-wake mechanisms and basal forebrain circuitry. Front. Biosci. 2003, 8, d1146–d1169. [Google Scholar] [CrossRef]
- España, R.A.; Berridge, C.W. Organization of noradrenergic efferents to arousal-related basal forebrain structures. J. Comp. Neurol. 2006, 496, 668–683. [Google Scholar] [CrossRef]
- Galvez, R.; Mesches, M.H.; McGaugh, J.L. Norepinephrine release in the amygdala in response to footshock stimulation. Neurobiol. Learn. Mem. 1996, 66, 253–257. [Google Scholar] [CrossRef]
- Moreno-Castilla, P.; Pérez-Ortega, R.; Violante-Soria, V.; Balderas, I.; Bermúdez-Rattoni, F. Hippocampal release of dopamine and norepinephrine encodes novel contextual information. Hippocampus 2017, 27, 547–557. [Google Scholar] [CrossRef]
- Stanley, A.T.; Post, M.R.; Lacefield, C.; Sulzer, D.; Miniaci, M.C. Norepinephrine release in the cerebellum contributes to aversive learning. Nat. Commun. 2023, 14, 4852. [Google Scholar] [CrossRef] [PubMed]
- Hoxha, E.; Tempia, F.; Lippiello, P.; Miniaci, M.C. Modulation, Plasticity and Pathophysiology of the Parallel Fiber-Purkinje Cell Synapse. Front. Synaptic Neurosci. 2016, 8, 35. [Google Scholar] [CrossRef]
- Inoshita, T.; Hirano, T. Norepinephrine Facilitates Induction of Long-term Depression through β-Adrenergic Receptor at Parallel Fiber-to-Purkinje Cell Synapses in the Flocculus. Neuroscience 2021, 462, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Margules, D.L.; Lewis, M.J.; Dragovich, J.A.; Margules, A.S. Hypothalamic Norepinephrine: Circadian Rhythms and the Control of Feeding Behavior. Science 1972, 178, 640–643. [Google Scholar] [CrossRef]
- Greco, A.M.; Gambardella, P.; Sticchi, R.; D’Aponte, D.; De Franciscis, P. Circadian rhythms of hypothalamic norepinephrine and of some circulating substances in individually housed adult rats. Physiol. Behav. 1992, 52, 1167–1172. [Google Scholar] [CrossRef]
- Rodenkirch, C.; Liu, Y.; Schriver, B.J.; Wang, Q. Locus coeruleus activation enhances thalamic feature selectivity via norepinephrine regulation of intrathalamic circuit dynamics. Nat. Neurosci. 2019, 22, 120–133. [Google Scholar] [CrossRef]
- Af Bjerkén, S.; Stenmark Persson, R.; Barkander, A.; Karalija, N.; Pelegrina-Hidalgo, N.; Gerhardt, G.A.; Virel, A.; Strömberg, I. Noradrenaline is crucial for the substantia nigra dopaminergic cell maintenance. Neurochem. Int. 2019, 131, 104551. [Google Scholar] [CrossRef]
- Gu, B.-M.; Kim, J.G.; Hossain, A.; Cron, G.O.; Lee, J.H. Substantia nigra modulates breathing rate via locus coeruleus. iScience 2025, 28, 112423. [Google Scholar] [CrossRef]
- Sara, S.J. The locus coeruleus and noradrenergic modulation of cognition. Nat. Rev. Neurosci. 2009, 10, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Puig, M.V.; Gulledge, A.T. Serotonin and Prefrontal Cortex Function: Neurons, Networks, and Circuits. Mol. Neurobiol. 2011, 44, 449–464. [Google Scholar] [CrossRef]
- Sargin, D.; Jeoung, H.-S.; Goodfellow, N.M.; Lambe, E.K. Serotonin Regulation of the Prefrontal Cortex: Cognitive Relevance and the Impact of Developmental Perturbation. ACS Chem. Neurosci. 2019, 10, 3078–3093. [Google Scholar] [CrossRef] [PubMed]
- Zangen, A.; Nakash, R.; Overstreet, D.; Yadid, G. Association between depressive behavior and absence of serotonin-dopamine interaction in the nucleus accumbens. Psychopharmacology 2001, 155, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Hon, O.J.; DiBerto, J.F.; Mazzone, C.M.; Sugam, J.; Bloodgood, D.W.; Hardaway, J.A.; Husain, M.; Kendra, A.; McCall, N.M.; Lopez, A.J.; et al. Serotonin modulates an inhibitory input to the central amygdala from the ventral periaqueductal gray. Neuropsychopharmacology 2022, 47, 2194–2204. [Google Scholar] [CrossRef] [PubMed]
- Balasubramani, P.P.; Chakravarthy, V.S.; Ravindran, B.; Moustafa, A.A. A network model of basal ganglia for understanding the roles of dopamine and serotonin in reward-punishment-risk based decision making. Front. Comput. Neurosci. 2015, 9, 76. [Google Scholar] [CrossRef]
- Portas, C.M.; Bjorvatn, B.; Ursin, R. Serotonin and the sleep/wake cycle: Special emphasis on microdialysis studies. Prog. Neurobiol. 2000, 60, 13–35. [Google Scholar] [CrossRef]
- Lin, M.T.; Tsay, H.J.; Su, W.H.; Chueh, F.Y. Changes in extracellular serotonin in rat hypothalamus affect thermoregulatory function. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1998, 274, R1260–R1267. [Google Scholar] [CrossRef]
- Kawashima, T. The role of the serotonergic system in motor control. Neurosci. Res. 2018, 129, 32–39. [Google Scholar] [CrossRef]
- Yoshida, K.; Drew, M.R.; Mimura, M.; Tanaka, K.F. Serotonin-mediated inhibition of ventral hippocampus is required for sustained goal-directed behavior. Nat. Neurosci. 2019, 22, 770–777. [Google Scholar] [CrossRef]
- Buhot, M.C.; Martin, S.; Segu, L. Role of serotonin in memory impairment. Ann. Med. 2000, 32, 210–221. [Google Scholar] [CrossRef]
- Schwarting, R.K.W.; Thiel, C.M.; Müller, C.P.; Huston, J.P. Relationship between anxiety and serotonin in the ventral striatum. NeuroReport 1998, 9, 1025–1029. [Google Scholar] [CrossRef]
- Ford, C.P. The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience 2014, 282, 13–22. [Google Scholar] [CrossRef]
- Beaulieu, J.-M.; Gainetdinov, R.R. The Physiology, Signaling, and Pharmacology of Dopamine Receptors. Pharmacol. Rev. 2011, 63, 182–217. [Google Scholar] [CrossRef]
- Forssberg, H.; Fernell, E.; Waters, S.; Waters, N.; Tedroff, J. Altered pattern of brain dopamine synthesis in male adolescents with attention deficit hyperactivity disorder. Behav. Brain Funct. 2006, 2, 40. [Google Scholar] [CrossRef]
- Cheon, K.-A.; Ryu, Y.H.; Kim, Y.-K.; Namkoong, K.; Kim, C.-H.; Lee, J.D. Dopamine transporter density in the basal ganglia assessed with [123I]IPT SPET in children with attention deficit hyperactivity disorder. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 306–311. [Google Scholar] [CrossRef]
- Dougherty, D.D.; Bonab, A.A.; Spencer, T.J.; Rauch, S.L.; Madras, B.K.; Fischman, A.J. Dopamine transporter density in patients with attention deficit hyperactivity disorder. Lancet 1999, 354, 2132–2133. [Google Scholar] [CrossRef]
- Ludolph, A.G.; Kassubek, J.; Schmeck, K.; Glaser, C.; Wunderlich, A.; Buck, A.K.; Reske, S.N.; Fegert, J.M.; Mottaghy, F.M. Dopaminergic dysfunction in attention deficit hyperactivity disorder (ADHD), differences between pharmacologically treated and never treated young adults: A 3,4-dihdroxy-6-[18F]fluorophenyl-l-alanine PET study. Neuroimage 2008, 41, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M.; Zametkin, A.J.; Matochik, J.A.; Pascualvaca, D.; Jons, P.H.; Cohen, R.M. High midbrain [18F]DOPA accumulation in children with attention deficit hyperactivity disorder. Am. J. Psychiatry 1999, 156, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Jucaite, A.; Fernell, E.; Halldin, C.; Forssberg, H.; Farde, L. Reduced midbrain dopamine transporter binding in male adolescents with attention-deficit/hyperactivity disorder: Association between striatal dopamine markers and motor hyperactivity. Biol. Psychiatry 2005, 57, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Wang, G.-J.; Kollins, S.H.; Wigal, T.L.; Newcorn, J.H.; Telang, F.; Fowler, J.S.; Zhu, W.; Logan, J.; Ma, Y.; et al. Evaluating Dopamine Reward Pathway in ADHD: Clinical Implications. JAMA 2009, 302, 1084–1091. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.-J.; Newcorn, J.H.; Kollins, S.H.; Wigal, T.L.; Telang, F.; Fowler, J.S.; Goldstein, R.Z.; Klein, N.; Logan, J.; et al. Motivation deficit in ADHD is associated with dysfunction of the dopamine reward pathway. Mol. Psychiatry 2011, 16, 1147–1154. [Google Scholar] [CrossRef]
- Itagaki, S.; Ohnishi, T.; Toda, W.; Sato, A.; Matsumoto, J.; Ito, H.; Ishii, S.; Yamakuni, R.; Miura, I.; Yabe, H. Reduced dopamine transporter availability in drug-naive adult attention-deficit/hyperactivity disorder. Psychiatry Clin. Neurosci. Rep. 2024, 3, e177. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.A.; Conlon, M.M.; Stutt, H.R.; Wendt, L.; Ten Eyck, P.; Narayanan, N.S. Quantifying the inverted U: A meta-analysis of prefrontal dopamine, D1 receptors, and working memory. Behav. Neurosci. 2022, 136, 207–218. [Google Scholar] [CrossRef]
- Wagatsuma, A.; Okuyama, T.; Sun, C.; Smith, L.M.; Abe, K.; Tonegawa, S. Locus coeruleus input to hippocampal CA3 drives single-trial learning of a novel context. Proc. Natl. Acad. Sci. USA 2018, 115, E310–E316. [Google Scholar] [CrossRef]
- Roozendaal, B.; Hermans, E.J. Norepinephrine effects on the encoding and consolidation of emotional memory: Improving synergy between animal and human studies. Curr. Opin. Behav. Sci. 2017, 14, 115–122. [Google Scholar] [CrossRef]
- Del Campo, N.; Chamberlain, S.R.; Sahakian, B.J.; Robbins, T.W. The Roles of Dopamine and Noradrenaline in the Pathophysiology and Treatment of Attention-Deficit/Hyperactivity Disorder. Biol. Psychiatry 2011, 69, e145–e157. [Google Scholar] [CrossRef]
- Hannestad, J.; Gallezot, J.-D.; Planeta-Wilson, B.; Lin, S.-F.; Williams, W.A.; Van Dyck, C.H.; Malison, R.T.; Carson, R.E.; Ding, Y.-S. Clinically Relevant Doses of Methylphenidate Significantly Occupy Norepinephrine Transporters in Humans In Vivo. Biol. Psychiatry 2010, 68, 854–860. [Google Scholar] [CrossRef]
- Vanicek, T.; Spies, M.; Rami-Mark, C.; Savli, M.; Höflich, A.; Kranz, G.S.; Hahn, A.; Kutzelnigg, A.; Traub-Weidinger, T.; Mitterhauser, M.; et al. The norepinephrine transporter in attention-deficit/hyperactivity disorder investigated with positron emission tomography. JAMA Psychiatry 2014, 71, 1340–1349. [Google Scholar] [CrossRef] [PubMed]
- Ulke, C.; Rullmann, M.; Huang, J.; Luthardt, J.; Becker, G.-A.; Patt, M.; Meyer, P.M.; Tiepolt, S.; Hesse, S.; Sabri, O.; et al. Adult attention-deficit/hyperactivity disorder is associated with reduced norepinephrine transporter availability in right attention networks: A (S,S)-O-[11C]methylreboxetine positron emission tomography study. Transl. Psychiatry 2019, 9, 301. [Google Scholar] [CrossRef] [PubMed]
- Sigurdardottir, H.L.; Kranz, G.S.; Rami-Mark, C.; James, G.M.; Vanicek, T.; Gryglewski, G.; Berroterán-Infante, N.; Kautzky, A.; Hienert, M.; Traub-Weidinger, T.; et al. Association of norepinephrine transporter methylation with in vivo NET expression and hyperactivity-impulsivity symptoms in ADHD measured with PET. Mol. Psychiatry 2021, 26, 1009–1018. [Google Scholar] [CrossRef]
- Sigurdardottir, H.L.; Kranz, G.S.; Rami-Mark, C.; James, G.M.; Vanicek, T.; Gryglewski, G.; Kautzky, A.; Hienert, M.; Traub-Weidinger, T.; Mitterhauser, M.; et al. Effects of norepinephrine transporter gene variants on NET binding in ADHD and healthy controls investigated by PET. Hum. Brain Mapp. 2016, 37, 884–895. [Google Scholar] [CrossRef]
- Shang, C.-Y.; Lin, H.-Y.; Gau, S.S.-F. The norepinephrine transporter gene modulates intrinsic brain activity, visual memory, and visual attention in children with attention-deficit/hyperactivity disorder. Mol. Psychiatry 2021, 26, 4026–4035. [Google Scholar] [CrossRef]
- Hohmann, S.; Hohm, E.; Treutlein, J.; Blomeyer, D.; Jennen-Steinmetz, C.; Schmidt, M.H.; Esser, G.; Banaschewski, T.; Brandeis, D.; Laucht, M. Association of norepinephrine transporter (NET, SLC6A2) genotype with ADHD-related phenotypes: Findings of a longitudinal study from birth to adolescence. Psychiatry Res. 2015, 226, 425–433. [Google Scholar] [CrossRef]
- Barkley, R.A.; Smith, K.M.; Fischer, M.; Navia, B. An examination of the behavioral and neuropsychological correlates of three ADHD candidate gene polymorphisms (DRD4 7+, DBH TaqI A2, and DAT1 40 bp VNTR) in hyperactive and normal children followed to adulthood. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2006, 141B, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.M.; Daly, M.; Fischer, M.; Yiannoutsos, C.T.; Bauer, L.; Barkley, R.; Navia, B.A. Association of the dopamine beta hydroxylase gene with attention deficit hyperactivity disorder: Genetic analysis of the Milwaukee longitudinal study. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2003, 119B, 77–85. [Google Scholar] [CrossRef]
- Kieling, C.; Genro, J.P.; Hutz, M.H.; Rohde, L.A. The −1021 C/T DBH polymorphism is associated with neuropsychological performance among children and adolescents with ADHD. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2008, 147B, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Bellgrove, M.A.; Mattingley, J.B.; Hawi, Z.; Mullins, C.; Kirley, A.; Gill, M.; Robertson, I.H. Impaired Temporal Resolution of Visual Attention and Dopamine Beta Hydroxylase Genotype in Attention-Deficit/Hyperactivity Disorder. Biol. Psychiatry 2006, 60, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Watabe-Uchida, M.; Zhu, L.; Ogawa, S.K.; Vamanrao, A.; Uchida, N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron 2012, 74, 858–873. [Google Scholar] [CrossRef]
- Pollak Dorocic, I.; Fürth, D.; Xuan, Y.; Johansson, Y.; Pozzi, L.; Silberberg, G.; Carlén, M.; Meletis, K. A Whole-Brain Atlas of Inputs to Serotonergic Neurons of the Dorsal and Median Raphe Nuclei. Neuron 2014, 83, 663–678. [Google Scholar] [CrossRef]
- Grace, A.A.; Floresco, S.B.; Goto, Y.; Lodge, D.J. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007, 30, 220–227. [Google Scholar] [CrossRef]
- Predescu, E.; Vaidean, T.; Rapciuc, A.-M.; Sipos, R. Metabolomic Markers in Attention-Deficit/Hyperactivity Disorder (ADHD) among Children and Adolescents—A Systematic Review. Int. J. Mol. Sci. 2024, 25, 4385. [Google Scholar] [CrossRef]
- Kenna, G.A.; Roder-Hanna, N.; Leggio, L.; Zywiak, W.H.; Clifford, J.; Edwards, S.; Kenna, J.A.; Shoaff, J.; Swift, R.M. Association of the 5-HTT gene-linked promoter region (5-HTTLPR) polymorphism with psychiatric disorders: Review of psychopathology and pharmacotherapy. Pharmgenom. Pers. Med. 2012, 5, 19–35. [Google Scholar] [CrossRef]
- Vanicek, T.; Kutzelnigg, A.; Philippe, C.; Sigurdardottir, H.L.; James, G.M.; Hahn, A.; Kranz, G.S.; Höflich, A.; Kautzky, A.; Traub-Weidinger, T.; et al. Altered interregional molecular associations of the serotonin transporter in attention deficit/hyperactivity disorder assessed with PET. Hum. Brain Mapp. 2017, 38, 792–802. [Google Scholar] [CrossRef]
- Karlsson, L.; Tuominen, L.; Huotarinen, A.; Leppämäki, S.; Sihvola, E.; Helin, S.; Sipilä, M.; Tani, P.; Hirvonen, J.; Hietala, J.; et al. Serotonin transporter in attention-deficit hyperactivity disorder—Preliminary results from a positron emission tomography study. Psychiatry Res. Neuroimaging 2013, 212, 164–165. [Google Scholar] [CrossRef]
- Jocoy, E.; Cepeda, C.; Levine, M.; André, V. NMDA and Dopamine: Diverse Mechanisms Applied to Interacting Receptor Systems. In Biology of the NMDA Receptor; VanDongen, A., Ed.; Frontiers in Neuroscience; CRC Press: Boca Raton, FL, USA, 2008; Volume 20085482, pp. 41–57. ISBN 978-1-4200-4414-0. Available online: https://www.ncbi.nlm.nih.gov/books/NBK5280/ (accessed on 17 April 2025).
- Li, F.; Tsien, J.Z. Memory and the NMDA receptors. N. Engl. J. Med. 2009, 361, 302–303. [Google Scholar] [CrossRef]
- Varela, J.A.; Hirsch, S.J.; Chapman, D.; Leverich, L.S.; Greene, R.W. D1/D5 Modulation of Synaptic NMDA Receptor Currents. J. Neurosci. 2009, 29, 3109–3119. [Google Scholar] [CrossRef]
- Faraone, S.V.; Larsson, H. Genetics of attention deficit hyperactivity disorder. Mol. Psychiatry 2019, 24, 562–575. [Google Scholar] [CrossRef]
- Grimm, O.; Kranz, T.M.; Reif, A. Genetics of ADHD: What Should the Clinician Know? Curr. Psychiatry Rep. 2020, 22, 18. [Google Scholar] [CrossRef]
- Uchida, M.; DiSalvo, M.; Walsh, D.; Biederman, J. The Heritability of ADHD in Children of ADHD Parents: A Post-Hoc Analysis of Longitudinal Data. J. Atten. Disord. 2023, 27, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Brikell, I.; Burton, C.; Mota, N.R.; Martin, J. Insights into attention-deficit/hyperactivity disorder from recent genetic studies. Psychol. Med. 2021, 51, 2274–2286. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.K.; Bhat, A.A.; Hashem, S.; Nisar, S.; Kamal, M.; Syed, N.; Temanni, M.-R.; Gupta, R.K.; Kamran, S.; Azeem, M.W.; et al. Genetic variations influence brain changes in patients with attention-deficit hyperactivity disorder. Transl. Psychiatry 2021, 11, 349. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-H.; Hahn, M.K.; Joung, Y.; Anderson, S.L.; Steele, A.H.; Mazei-Robinson, M.S.; Gizer, I.; Teicher, M.H.; Cohen, B.M.; Robertson, D.; et al. A polymorphism in the norepinephrine transporter gene alters promoter activity and is associated with attention-deficit hyperactivity disorder. Proc. Natl. Acad. Sci. USA 2006, 103, 19164–19169. [Google Scholar] [CrossRef]
- Liu, L.; Cheng, J.; Li, H.; Yang, L.; Qian, Q.; Wang, Y. The possible involvement of genetic variants of NET1 in the etiology of attention-deficit/hyperactivity disorder comorbid with oppositional defiant disorder. J. Child Psychol. Psychiatry 2015, 56, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.B.; Biederman, J.; Valera, E.M.; Doyle, A.E.; Bush, G.; Spencer, T.; Monuteaux, M.C.; Mick, E.; Whitfield-Gabrieli, S.; Makris, N.; et al. Effect of dopamine transporter gene (SLC6A3) variation on dorsal anterior cingulate function in attention-deficit/hyperactivity disorder. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2010, 153B, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Kuc, K.; Bielecki, M.; Racicka-Pawlukiewicz, E.; Czerwinski, M.B.; Cybulska-Klosowicz, A. The SLC6A3 gene polymorphism is related to the development of attentional functions but not to ADHD. Sci. Rep. 2020, 10, 6176. [Google Scholar] [CrossRef] [PubMed]
- Reith, M.E.A.; Kortagere, S.; Wiers, C.E.; Sun, H.; Kurian, M.A.; Galli, A.; Volkow, N.D.; Lin, Z. The dopamine transporter gene SLC6A3: Multidisease risks. Mol. Psychiatry 2022, 27, 1031–1046. [Google Scholar] [CrossRef]
- Park, S.; Lee, J.-M.; Kim, J.-W.; Cho, D.-Y.; Yun, H.J.; Han, D.H.; Cheong, J.H.; Kim, B.-N. Associations between serotonin transporter gene (SLC6A4) methylation and clinical characteristics and cortical thickness in children with ADHD. Psychol. Med. 2015, 45, 3009–3017. [Google Scholar] [CrossRef]
- Provenzi, L.; Giorda, R.; Beri, S.; Montirosso, R. SLC6A4 methylation as an epigenetic marker of life adversity exposures in humans: A systematic review of literature. Neurosci. Biobehav. Rev. 2016, 71, 7–20. [Google Scholar] [CrossRef]
- Kwon, H.J.; Jin, H.J.; Lim, M.H. Association Between Monoamine Oxidase Gene Polymorphisms and Attention Deficit Hyperactivity Disorder in Korean Children. Genet. Test. Mol. Biomark. 2014, 18, 505–509. [Google Scholar] [CrossRef]
- Luca, P.; Laurin, N.; Misener, V.L.; Wigg, K.G.; Anderson, B.; Cate-Carter, T.; Tannock, R.; Humphries, T.; Lovett, M.W.; Barr, C.L. Association of the dopamine receptor D1 gene, DRD1, with inattention symptoms in families selected for reading problems. Mol. Psychiatry 2007, 12, 776–785. [Google Scholar] [CrossRef]
- RibaséS, M.; Ramos-Quiroga, J.A.; HerváS, A.; Sánchez-Mora, C.; Bosch, R.; Bielsa, A.; Gastaminza, X.; Lesch, K.-P.; Reif, A.; Renner, T.J.; et al. Candidate system analysis in ADHD: Evaluation of nine genes involved in dopaminergic neurotransmission identifies association with DRD1. World J. Biol. Psychiatry 2012, 13, 281–292. [Google Scholar] [CrossRef]
- Kebir, O.; Joober, R. Neuropsychological endophenotypes in attention-deficit/hyperactivity disorder: A review of genetic association studies. Eur. Arch. Psychiatry Clin. Neurosci. 2011, 261, 583–594. [Google Scholar] [CrossRef]
- Cervantes-Henriquez, M.L.; Acosta-López, J.E.; Ahmad, M.; Sánchez-Rojas, M.; Jiménez-Figueroa, G.; Pineda-Alhucema, W.; Martinez-Banfi, M.L.; Noguera-Machacón, L.M.; Mejía-Segura, E.; De La Hoz, M.; et al. ADGRL3, FGF1 and DRD4: Linkage and Association with Working Memory and Perceptual Organization Candidate Endophenotypes in ADHD. Brain Sci. 2021, 11, 854. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Qian, A.; Tao, J.; Zhou, R.; Fu, C.; Yang, C.; Lin, Q.; Zhou, J.; Li, J.; Huang, X.; et al. Different effects of the DRD4 genotype on intrinsic brain network connectivity strength in drug-naïve children with ADHD and healthy controls. Brain Imaging Behav. 2022, 16, 464–475. [Google Scholar] [CrossRef] [PubMed]
- Tovo-Rodrigues, L.; Rohde, L.A.; Menezes, A.M.B.; Polanczyk, G.V.; Kieling, C.; Genro, J.P.; Anselmi, L.; Hutz, M.H. DRD4 Rare Variants in Attention-Deficit/Hyperactivity Disorder (ADHD): Further Evidence from a Birth Cohort Study. PLoS ONE 2013, 8, e85164. [Google Scholar] [CrossRef]
- Langley, K.; Fowler, T.A.; Grady, D.L.; Moyzis, R.K.; Holmans, P.A.; Van Den Bree, M.B.M.; Owen, M.J.; O’Donovan, M.C.; Thapar, A. Molecular genetic contribution to the developmental course of attention-deficit hyperactivity disorder. Eur. Child Adolesc. Psychiatry 2009, 18, 26–32. [Google Scholar] [CrossRef]
- Wu, J.; Xiao, H.; Sun, H.; Zou, L.; Zhu, L.-Q. Role of Dopamine Receptors in ADHD: A Systematic Meta-Analysis. Mol. Neurobiol. 2012, 45, 605–620. [Google Scholar] [CrossRef]
- Kang, J.; Choi, B.-S.; Kim, B. The Role of Gene–Gene Interaction Between ADRA2A and SLC6A2 Polymorphisms in Attention System and Treatment Outcomes for Children with ADHD. Children 2025, 12, 704. [Google Scholar] [CrossRef]
- Schmitz, M.; Denardin, D.; Silva, T.L.; Pianca, T.; Roman, T.; Hutz, M.H.; Faraone, S.V.; Rohde, L.A. Association Between Alpha-2a-adrenergic Receptor Gene and ADHD Inattentive Type. Biol. Psychiatry 2006, 60, 1028–1033. [Google Scholar] [CrossRef]
- Faraone, S.V.; Perlis, R.H.; Doyle, A.E.; Smoller, J.W.; Goralnick, J.J.; Holmgren, M.A.; Sklar, P. Molecular genetics of attention-deficit/hyperactivity disorder. Biol. Psychiatry 2005, 57, 1313–1323. [Google Scholar] [CrossRef] [PubMed]
- Kessi, M.; Duan, H.; Xiong, J.; Chen, B.; He, F.; Yang, L.; Ma, Y.; Bamgbade, O.A.; Peng, J.; Yin, F. Attention-deficit/hyperactive disorder updates. Front. Mol. Neurosci. 2022, 15, 925049. [Google Scholar] [CrossRef]
- Levitan, R.D.; Masellis, M.; Basile, V.S.; Lam, R.W.; Jain, U.; Kaplan, A.S.; Kennedy, S.H.; Siegel, G.; Walker, M.L.; Vaccarino, F.J.; et al. Polymorphism of the serotonin-2A receptor gene (HTR2A) associated with childhood attention deficit hyperactivity disorder (ADHD) in adult women with seasonal affective disorder. J. Affect. Disord. 2002, 71, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Quist, J.F.; Barr, C.L.; Schachar, R.; Roberts, W.; Malone, M.; Tannock, R.; Basile, V.S.; Beitchman, J.; Kennedy, J.L. The serotonin 5-HT1B receptor gene and attention deficit hyperactivity disorder. Mol. Psychiatry 2003, 8, 98–102. [Google Scholar] [CrossRef]
- Rivero, O.; Selten, M.M.; Sich, S.; Popp, S.; Bacmeister, L.; Amendola, E.; Negwer, M.; Schubert, D.; Proft, F.; Kiser, D.; et al. Cadherin-13, a risk gene for ADHD and comorbid disorders, impacts GABAergic function in hippocampus and cognition. Transl. Psychiatry 2015, 5, e655. [Google Scholar] [CrossRef]
- Salatino-Oliveira, A.; Genro, J.P.; Polanczyk, G.; Zeni, C.; Schmitz, M.; Kieling, C.; Anselmi, L.; Menezes, A.M.B.; Barros, F.C.; Polina, E.R.; et al. Cadherin-13 gene is associated with hyperactive/impulsive symptoms in attention/deficit hyperactivity disorder. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2015, 168, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, G.C.; Ehlis, A.-C.; Weber, H.; Vitale, M.R.; Zöller, J.E.M.; Ku, H.-P.; Schiele, M.A.; Kürbitz, L.I.; Romanos, M.; Pauli, P.; et al. A Common CDH13 Variant Is Associated with Low Agreeableness and Neural Responses to Working Memory Tasks in ADHD. Genes 2021, 12, 1356. [Google Scholar] [CrossRef] [PubMed]
- Lesch, K.-P.; Timmesfeld, N.; Renner, T.J.; Halperin, R.; Röser, C.; Nguyen, T.T.; Craig, D.W.; Romanos, J.; Heine, M.; Meyer, J.; et al. Molecular genetics of adult ADHD: Converging evidence from genome-wide association and extended pedigree linkage studies. J. Neural Transm. 2008, 115, 1573–1585. [Google Scholar] [CrossRef]
- Olfson, E.; Farhat, L.C.; Liu, W.; Vitulano, L.A.; Zai, G.; Lima, M.O.; Parent, J.; Polanczyk, G.V.; Cappi, C.; Kennedy, J.L.; et al. Rare de novo damaging DNA variants are enriched in attention-deficit/hyperactivity disorder and implicate risk genes. Nat. Commun. 2024, 15, 5870. [Google Scholar] [CrossRef]
- Terracciano, A.; Esko, T.; Sutin, A.R.; De Moor, M.H.M.; Meirelles, O.; Zhu, G.; Tanaka, T.; Giegling, I.; Nutile, T.; Realo, A.; et al. Meta-analysis of genome-wide association studies identifies common variants in CTNNA2 associated with excitement-seeking. Transl. Psychiatry 2011, 1, e49. [Google Scholar] [CrossRef]
- Breiderhoff, T.; Christiansen, G.B.; Pallesen, L.T.; Vaegter, C.; Nykjaer, A.; Holm, M.M.; Glerup, S.; Willnow, T.E. Sortilin-related receptor SORCS3 is a postsynaptic modulator of synaptic depression and fear extinction. PLoS ONE 2013, 8, e75006. [Google Scholar] [CrossRef]
- Demontis, D.; Walters, G.B.; Athanasiadis, G.; Walters, R.; Therrien, K.; Nielsen, T.T.; Farajzadeh, L.; Voloudakis, G.; Bendl, J.; Zeng, B.; et al. Genome-wide analyses of ADHD identify 27 risk loci, refine the genetic architecture and implicate several cognitive domains. Nat. Genet. 2023, 55, 198–208. [Google Scholar] [CrossRef]
- Subkhangulova, A.; Malik, A.R.; Hermey, G.; Popp, O.; Dittmar, G.; Rathjen, T.; Poy, M.N.; Stumpf, A.; Beed, P.S.; Schmitz, D.; et al. SORCS1 and SORCS3 control energy balance and orexigenic peptide production. EMBO Rep. 2018, 19, e44810. [Google Scholar] [CrossRef]
- Balogh, L.; Pulay, A.J.; Réthelyi, J.M. Genetics in the ADHD Clinic: How Can Genetic Testing Support the Current Clinical Practice? Front. Psychol. 2022, 13, 751041. [Google Scholar] [CrossRef]
- Boyle, E.A.; Li, Y.I.; Pritchard, J.K. An Expanded View of Complex Traits: From Polygenic to Omnigenic. Cell 2017, 169, 1177–1186. [Google Scholar] [CrossRef]
- He, Q.; Keding, T.J.; Zhang, Q.; Miao, J.; Russell, J.D.; Herringa, R.J.; Lu, Q.; Travers, B.G.; Li, J.J. Neurogenetic mechanisms of risk for ADHD: Examining associations of polygenic scores and brain volumes in a population cohort. J. Neurodev. Disord. 2023, 15, 30. [Google Scholar] [CrossRef]
- Vidal, O.M.; Vélez, J.I.; Arcos-Burgos, M. ADGRL3 genomic variation implicated in neurogenesis and ADHD links functional effects to the incretin polypeptide GIP. Sci. Rep. 2022, 12, 15922. [Google Scholar] [CrossRef]
- Bruxel, E.M.; Moreira-Maia, C.R.; Akutagava-Martins, G.C.; Quinn, T.P.; Klein, M.; Franke, B.; Ribasés, M.; Rovira, P.; Sánchez-Mora, C.; Kappel, D.B.; et al. Meta-analysis and systematic review of ADGRL3 (LPHN3) polymorphisms in ADHD susceptibility. Mol. Psychiatry 2021, 26, 2277–2285. [Google Scholar] [CrossRef]
- McNeill, R.V.; Palladino, V.S.; Brunkhorst-Kanaan, N.; Grimm, O.; Reif, A.; Kittel-Schneider, S. Expression of the adult ADHD-associated gene ADGRL3 is dysregulated by risk variants and environmental risk factors. World J. Biol. Psychiatry 2021, 22, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Abraham, E.; Scott, M.A.; Blair, C. Catechol-O-methyltransferase Val158Met Genotype and Early-Life Family Adversity Interactively Affect Attention-Deficit Hyperactivity Symptoms Across Childhood. Front. Genet. 2020, 11, 724. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Yuan, F.; Shen, X.; Xiong, G.; Wu, J. Role of COMT in ADHD: A Systematic Meta-Analysis. Mol. Neurobiol. 2014, 49, 251–261. [Google Scholar] [CrossRef]
- Fageera, W.; Chaumette, B.; Fortier, M.-È.; Grizenko, N.; Labbe, A.; Sengupta, S.M.; Joober, R. Association between COMT methylation and response to treatment in children with ADHD. J. Psychiatr. Res. 2021, 135, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Gizer, I.R.; Ficks, C.; Waldman, I.D. Candidate gene studies of ADHD: A meta-analytic review. Hum. Genet. 2009, 126, 51–90. [Google Scholar] [CrossRef]
- Schote, A.B.; Bonenberger, M.; Pálmason, H.; Seitz, C.; Meyer, J.; Freitag, C.M. Glucocorticoid receptor variants in childhood attention-deficit/hyperactivity disorder and comorbid psychiatric disorders. Psychiatry Res. 2016, 246, 275–283. [Google Scholar] [CrossRef]
- Plieger, T.; Felten, A.; Splittgerber, H.; Duke, É.; Reuter, M. The role of genetic variation in the glucocorticoid receptor (NR3C1) and mineralocorticoid receptor (NR3C2) in the association between cortisol response and cognition under acute stress. Psychoneuroendocrinology 2018, 87, 173–180. [Google Scholar] [CrossRef]
- Liu, L.; Feng, X.; Li, H.; Cheng Li, S.; Qian, Q.; Wang, Y. Deep learning model reveals potential risk genes for ADHD, especially Ephrin receptor gene EPHA5. Brief. Bioinform. 2021, 22, bbab207. [Google Scholar] [CrossRef]
- Cooper, M.A.; Crockett, D.P.; Nowakowski, R.S.; Gale, N.W.; Zhou, R. Distribution of EphA5 receptor protein in the developing and adult mouse nervous system. J. Comp. Neurol. 2009, 514, 310–328. [Google Scholar] [CrossRef] [PubMed]
- Akaneya, Y.; Sohya, K.; Kitamura, A.; Kimura, F.; Washburn, C.; Zhou, R.; Ninan, I.; Tsumoto, T.; Ziff, E.B. Ephrin-A5 and EphA5 Interaction Induces Synaptogenesis during Early Hippocampal Development. PLoS ONE 2010, 5, e12486. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-C.; Kuo, H.-C.; Huang, L.-H.; Chou, W.-J.; Lee, S.-Y.; Chan, W.-C.; Wang, L.-J. DNA Methylation in LIME1 and SPTBN2 Genes Is Associated with Attention Deficit in Children. Children 2021, 8, 92. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence (NICE). Attention Deficit Hyperactivity Disorder: Diagnosis and Management; National Institute for Health and Care Excellence: Guidelines; National Institute for Health and Care Excellence (NICE): London, UK, 2019; ISBN 978-1-4731-2830-9. Available online: http://www.ncbi.nlm.nih.gov/books/NBK493361/ (accessed on 23 June 2025).
- Jerome, D.; Jerome, L. Approach to diagnosis and management of childhood attention deficit hyperactivity disorder. Can. Fam. Physician 2020, 66, 732–736. [Google Scholar] [PubMed]
- Wolraich, M.L.; Hagan, J.F.; Allan, C.; Chan, E.; Davison, D.; Earls, M.; Evans, S.W.; Flinn, S.K.; Froehlich, T.; Frost, J.; et al. Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of Attention-Deficit/Hyperactivity Disorder in Children and Adolescents. Pediatrics 2019, 144, e20192528. [Google Scholar] [CrossRef]
- Quintero, J.; Gutiérrez-Casares, J.R.; Álamo, C. Molecular Characterisation of the Mechanism of Action of Stimulant Drugs Lisdexamfetamine and Methylphenidate on ADHD Neurobiology: A Review. Neurol. Ther. 2022, 11, 1489–1517. [Google Scholar] [CrossRef]
- Calipari, E.S.; Jones, S.R. Sensitized nucleus accumbens dopamine terminal responses to methylphenidate and dopamine transporter releasers after intermittent-access self-administration. Neuropharmacology 2014, 82, 1–10. [Google Scholar] [CrossRef]
- Grund, T.; Lehmann, K.; Bock, N.; Rothenberger, A.; Teuchert-Noodt, G. Influence of methylphenidate on brain development—An update of recent animal experiments. Behav. Brain Funct. 2006, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Gatley, S.J.; Pan, D.; Chen, R.; Chaturvedi, G.; Ding, Y.-S. Affinities of methylphenidate derivatives for dopamine, norepinephrine and serotonin transporters. Life Sci. 1996, 58, PL231–PL239. [Google Scholar] [CrossRef] [PubMed]
- Stevens, T.; Sangkuhl, K.; Brown, J.T.; Altman, R.B.; Klein, T.E. PharmGKB summary: Methylphenidate pathway, pharmacokinetics/pharmacodynamics. Pharmacogenet. Genom. 2019, 29, 136–154. [Google Scholar] [CrossRef] [PubMed]
- Volz, T.J.; Farnsworth, S.J.; King, J.L.; Riddle, E.L.; Hanson, G.R.; Fleckenstein, A.E. Methylphenidate Administration Alters Vesicular Monoamine Transporter-2 Function in Cytoplasmic and Membrane-Associated Vesicles. J. Pharmacol. Exp. Ther. 2007, 323, 738–745. [Google Scholar] [CrossRef]
- Volz, T.J.; Farnsworth, S.J.; Rowley, S.D.; Hanson, G.R.; Fleckenstein, A.E. Methylphenidate-induced increases in vesicular dopamine sequestration and dopamine release in the striatum: The role of muscarinic and dopamine D2 receptors. J. Pharmacol. Exp. Ther. 2008, 327, 161–167. [Google Scholar] [CrossRef]
- Mantle, T.J.; Tipton, K.F.; Garrett, N.J. Inhibition of monoamine oxidase by amphetamine and related compounds. Biochem. Pharmacol. 1976, 25, 2073–2077. [Google Scholar] [CrossRef]
- Miller, H.H.; Shore, P.A.; Clarke, D.E. In vivo monoamine oxidase inhibition by d-amphetamine. Biochem. Pharmacol. 1980, 29, 1347–1354. [Google Scholar] [CrossRef]
- Sitte, H.H.; Freissmuth, M. Amphetamines, new psychoactive drugs and the monoamine transporter cycle. Trends Pharmacol. Sci. 2015, 36, 41–50. [Google Scholar] [CrossRef]
- Martin, D.; Le, J.K. Amphetamine. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK556103/ (accessed on 12 January 2025).
- Jones, S.; Kauer, J.A. Amphetamine depresses excitatory synaptic transmission via serotonin receptors in the ventral tegmental area. J. Neurosci. 1999, 19, 9780–9787. [Google Scholar] [CrossRef]
- Groom, M.J.; Cortese, S. Current Pharmacological Treatments for ADHD. In New Discoveries in the Behavioral Neuroscience of Attention-Deficit Hyperactivity Disorder; Stanford, S.C., Sciberras, E., Eds.; Current Topics in Behavioral Neurosciences; Springer International Publishing: Cham, Switzerland, 2022; Volume 57, pp. 19–50. ISBN 978-3-031-11801-2. Available online: https://link.springer.com/10.1007/7854_2022_330 (accessed on 20 May 2025).
- Amiri, D.; Eriksson, T. Long-Term Safety and Efficacy of Stimulant vs. Non-Stimulant Medications in ADHD Treatment: A Comparative Meta-Analysis Over Two Years. J. Psychiatry Cogn. Behav. 2023, 7, 166. [Google Scholar] [CrossRef]
- Carboni, E.; Silvagni, A.; Vacca, C.; Di Chiara, G. Cumulative effect of norepinephrine and dopamine carrier blockade on extracellular dopamine increase in the nucleus accumbens shell, bed nucleus of stria terminalis and prefrontal cortex. J. Neurochem. 2006, 96, 473–481. [Google Scholar] [CrossRef]
- Fedder, D.; Patel, H.; Saadabadi, A. Atomoxetine. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK493234/ (accessed on 20 May 2025).
- Swanson, C.J.; Perry, K.W.; Koch-Krueger, S.; Katner, J.; Svensson, K.A.; Bymaster, F.P. Effect of the attention deficit/hyperactivity disorder drug atomoxetine on extracellular concentrations of norepinephrine and dopamine in several brain regions of the rat. Neuropharmacology 2006, 50, 755–760. [Google Scholar] [CrossRef]
- Bymaster, F. Atomoxetine Increases Extracellular Levels of Norepinephrine and Dopamine in Prefrontal Cortex of Rat A Potential Mechanism for Efficacy in Attention Deficit/Hyperactivity Disorder. Neuropsychopharmacology 2002, 27, 699–711. [Google Scholar] [CrossRef]
- Mathew, B.M.; Pellegrini, M.V. Viloxazine. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK576423/ (accessed on 20 May 2025).
- Yu, C.; Garcia-Olivares, J.; Candler, S.; Schwabe, S.; Maletic, V. New Insights into the Mechanism of Action of Viloxazine: Serotonin and Norepinephrine Modulating Properties. J. Exp. Pharmacol. 2020, 12, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Neuchat, E.E.; Bocklud, B.E.; Kingsley, K.; Barham, W.T.; Luther, P.M.; Ahmadzadeh, S.; Shekoohi, S.; Cornett, E.M.; Kaye, A.D. The Role of Alpha-2 Agonists for Attention Deficit Hyperactivity Disorder in Children: A Review. Neurol. Int. 2023, 15, 697–707. [Google Scholar] [CrossRef]
- Bello, N.T. Clinical utility of guanfacine extended release in the treatment of ADHD in children and adolescents. Patient Prefer. Adherence 2015, 9, 877–885. [Google Scholar] [CrossRef]
- Alamo, C.; López-Muñoz, F.; Sánchez-García, J. Mechanism of action of guanfacine: A postsynaptic differential approach to the treatment of attention deficit hyperactivity disorder (adhd). Actas Esp. Psiquiatr. 2016, 44, 107–112. [Google Scholar]
- Russell, V.A.; Sagvolden, T.; Johansen, E.B. Animal models of attention-deficit hyperactivity disorder. Behav. Brain Funct. 2005, 1, 9. [Google Scholar] [CrossRef] [PubMed]
- Gainetdinov, R.R. Strengths and limitations of genetic models of ADHD. ADHD Atten. Def. Hyp Disord. 2010, 2, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Yadav, D.; Song, M. An updated review on animal models to study attention-deficit hyperactivity disorder. Transl. Psychiatry 2024, 14, 187. [Google Scholar] [CrossRef]
- Schaare, H.L.; Blöchl, M.; Kumral, D.; Uhlig, M.; Lemcke, L.; Valk, S.L.; Villringer, A. Associations between mental health, blood pressure and the development of hypertension. Nat. Commun. 2023, 14, 1953. [Google Scholar] [CrossRef] [PubMed]
- Gąsecki, D.; Kwarciany, M.; Nyka, W.; Narkiewicz, K. Hypertension, brain damage and cognitive decline. Curr. Hypertens. Rep. 2013, 15, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Fuemmeler, B.F.; Østbye, T.; Yang, C.; McClernon, F.J.; Kollins, S.H. Association between attention-deficit/hyperactivity disorder symptoms and obesity and hypertension in early adulthood: A population-based study. Int. J. Obes. 2011, 35, 852–862. [Google Scholar] [CrossRef]
- Sora, I.; Wichems, C.; Takahashi, N.; Li, X.F.; Zeng, Z.; Revay, R.; Lesch, K.P.; Murphy, D.L.; Uhl, G.R. Cocaine reward models: Conditioned place preference can be established in dopamine- and in serotonin-transporter knockout mice. Proc. Natl. Acad. Sci. USA 1998, 95, 7699–7704. [Google Scholar] [CrossRef]
- Gainetdinov, R.R.; Jones, S.R.; Caron, M.G. Functional hyperdopaminergia in dopamine transporter knock-out mice. Biol. Psychiatry 1999, 46, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.R.; Gainetdinov, R.R.; Jaber, M.; Giros, B.; Wightman, R.M.; Caron, M.G. Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc. Natl. Acad. Sci. USA 1998, 95, 4029–4034. [Google Scholar] [CrossRef]
- Li, Q.; Lu, G.; Antonio, G.E.; Mak, Y.T.; Rudd, J.A.; Fan, M.; Yew, D.T. The usefulness of the spontaneously hypertensive rat to model attention-deficit/hyperactivity disorder (ADHD) may be explained by the differential expression of dopamine-related genes in the brain. Neurochem. Int. 2007, 50, 848–857. [Google Scholar] [CrossRef]
- Rahi, V.; Kumar, P. Animal models of attention-deficit hyperactivity disorder (ADHD). Int. J. Dev. Neurosci. 2021, 81, 107–124. [Google Scholar] [CrossRef]
- Drolet, G.; Proulx, K.; Pearson, D.; Rochford, J.; Deschepper, C.F. Comparisons of behavioral and neurochemical characteristics between WKY, WKHA, and Wistar rat strains. Neuropsychopharmacology 2002, 27, 400–409. [Google Scholar] [CrossRef]
- Raber, J.; Mehta, P.P.; Kreifeldt, M.; Parsons, L.H.; Weiss, F.; Bloom, F.E.; Wilson, M.C. Coloboma hyperactive mutant mice exhibit regional and transmitter-specific deficits in neurotransmission. J. Neurochem. 1997, 68, 176–186. [Google Scholar] [CrossRef]
- Hess, E.J.; Jinnah, H.A.; Kozak, C.A.; Wilson, M.C. Spontaneous locomotor hyperactivity in a mouse mutant with a deletion including the Snap gene on chromosome 2. J. Neurosci. 1992, 12, 2865–2874. [Google Scholar] [CrossRef] [PubMed]
- Bruno, K.J.; Freet, C.S.; Twining, R.C.; Egami, K.; Grigson, P.S.; Hess, E.J. Abnormal latent inhibition and impulsivity in coloboma mice, a model of ADHD. Neurobiol. Dis. 2007, 25, 206–216. [Google Scholar] [CrossRef]
- Jones, M.D.; Williams, M.E.; Hess, E.J. Abnormal presynaptic catecholamine regulation in a hyperactive SNAP-25-deficient mouse mutant. Pharmacol. Biochem. Behav. 2001, 68, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Hess, E.J.; Collins, K.A.; Wilson, M.C. Mouse Model of Hyperkinesis Implicates SNAP-25 in Behavioral Regulation. J. Neurosci. 1996, 16, 3104–3111. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.C. Coloboma mouse mutant as an animal model of hyperkinesis and attention deficit hyperactivity disorder. Neurosci. Biobehav. Rev. 2000, 24, 51–57. [Google Scholar] [CrossRef]
- Abeliovich, A.; Schmitz, Y.; Fariñas, I.; Choi-Lundberg, D.; Ho, W.H.; Castillo, P.E.; Shinsky, N.; Verdugo, J.M.; Armanini, M.; Ryan, A.; et al. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 2000, 25, 239–252. [Google Scholar] [CrossRef]
- Bouchatta, O.; Manouze, H.; Bouali-benazzouz, R.; Kerekes, N.; Ba-M’hamed, S.; Fossat, P.; Landry, M.; Bennis, M. Neonatal 6-OHDA lesion model in mouse induces Attention-Deficit/Hyperactivity Disorder (ADHD)-like behaviour. Sci. Rep. 2018, 8, 15349. [Google Scholar] [CrossRef]
- Bouchatta, O.; Manouze, H.; Ba-M’Hamed, S.; Landry, M.; Bennis, M. Neonatal 6-OHDA Lesion Model in Mouse Induces Cognitive Dysfunctions of Attention-Deficit/Hyperactivity Disorder (ADHD) During Young Age. Front. Behav. Neurosci. 2020, 14, 27. [Google Scholar] [CrossRef]
- Ghasemi, A.; Jeddi, S.; Kashfi, K. The laboratory rat: Age and body weight matter. EXCLI J. 2021, 20, Doc1431. [Google Scholar] [CrossRef]
- Maldonado, K.A.; Alsayouri, K. Physiology, Brain. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK551718/ (accessed on 21 May 2025).
- Lin, J.H. Species similarities and differences in pharmacokinetics. Drug Metab. Dispos. 1995, 23, 1008–1021. [Google Scholar] [CrossRef]
- Rubio Morell, B.; Hernández Expósito, S. Differential long-term medication impact on executive function and delay aversion in ADHD. Appl. Neuropsychol. Child 2019, 8, 140–157. [Google Scholar] [CrossRef] [PubMed]
- MacQueen, D.A.; Minassian, A.; Kenton, J.A.; Geyer, M.A.; Perry, W.; Brigman, J.L.; Young, J.W. Amphetamine improves mouse and human attention in the 5-choice continuous performance test. Neuropharmacology 2018, 138, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Martin, I.L.; Baker, G.B.; Mitchell, P.R. The effect of viloxazine hydrochloride on the transport of noradrenaline, dopamine, 5-hydroxytryptamine and γ-amino-butyric acid in rat brain tissue. Neuropharmacology 1978, 17, 421–423. [Google Scholar] [CrossRef]
- Blackburn, T.P.; Foster, G.A.; Greenwood, D.T.; Howe, R. Effects of viloxazine, its optical isomers and its major metabolites on biogenic amine uptake mechanisms in vitro and in vivo. Eur. J. Pharmacol. 1978, 52, 367–374. [Google Scholar] [CrossRef]
- Lobato-Camacho, F.J.; López, J.C.; Vargas, J.P. Enhancing spatial memory and pattern separation: Long-term effects of stimulant treatment in individuals with ADHD. Behav. Brain Res. 2024, 475, 115211. [Google Scholar] [CrossRef]
- Fuermaier, A.B.M.; Tucha, L.; Koerts, J.; Weisbrod, M.; Lange, K.W.; Aschenbrenner, S.; Tucha, O. Effects of methylphenidate on memory functions of adults with ADHD. Appl. Neuropsychol. Adult 2017, 24, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Arnold, L.E.; Hodgkins, P.; Kahle, J.; Madhoo, M.; Kewley, G. Long-Term Outcomes of ADHD: Academic Achievement and Performance. J. Atten. Disord. 2020, 24, 73–85. [Google Scholar] [CrossRef]
- Whitehurst, L.N.; Mednick, S.C. Psychostimulants may block long-term memory formation via degraded sleep in healthy adults. Neurobiol. Learn. Mem. 2021, 178, 107342. [Google Scholar] [CrossRef] [PubMed]
- Ballard, M.E.; Gallo, D.A.; de Wit, H. Amphetamine increases errors during episodic memory retrieval. J. Clin. Psychopharmacol. 2014, 34, 85–92. [Google Scholar] [CrossRef]
- Spencer, T.J.; Brown, A.; Seidman, L.J.; Valera, E.M.; Makris, N.; Lomedico, A.; Faraone, S.V.; Biederman, J. Effect of psychostimulants on brain structure and function in ADHD: A qualitative literature review of magnetic resonance imaging-based neuroimaging studies. J. Clin. Psychiatry 2013, 74, 902–917. [Google Scholar] [CrossRef]
- Wu, F.; Zhang, W.; Ji, W.; Zhang, Y.; Jiang, F.; Li, G.; Hu, Y.; Wei, X.; Wang, H.; Wang, S.-Y.; et al. Stimulant medications in children with ADHD normalize the structure of brain regions associated with attention and reward. Neuropsychopharmacology 2024, 49, 1330–1340. [Google Scholar] [CrossRef]
- Walhovd, K.B.; Amlien, I.; Schrantee, A.; Rohani, D.A.; Groote, I.; Bjørnerud, A.; Fjell, A.M.; Reneman, L. Methylphenidate Effects on Cortical Thickness in Children and Adults with Attention-Deficit/Hyperactivity Disorder: A Randomized Clinical Trial. AJNR Am. J. Neuroradiol. 2020, 41, 758–765. [Google Scholar] [CrossRef]
- Fotopoulos, N.H.; Devenyi, G.A.; Guay, S.; Sengupta, S.M.; Chakravarty, M.M.; Grizenko, N.; Karama, S.; Joober, R. Cumulative exposure to ADHD medication is inversely related to hippocampus subregional volume in children. Neuroimage Clin. 2021, 31, 102695. [Google Scholar] [CrossRef]
- Kassim, F.M. Systematic reviews of the acute effects of amphetamine on working memory and other cognitive performances in healthy individuals, with a focus on the potential influence of personality traits. Hum. Psychopharmacol. 2023, 38, e2856. [Google Scholar] [CrossRef] [PubMed]
- de Wit, H.; Enggasser, J.L.; Richards, J.B. Acute administration of d-amphetamine decreases impulsivity in healthy volunteers. Neuropsychopharmacology 2002, 27, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, J.S.; Patrick, K.S. The Clinical Pharmacokinetics of Amphetamines Utilized in the Treatment of Attention-Deficit/Hyperactivity Disorder. J. Child Adolesc. Psychopharmacol. 2017, 27, 678–689. [Google Scholar] [CrossRef]
- Barch, D.M.; Carter, C.S. Amphetamine improves cognitive function in medicated individuals with schizophrenia and in healthy volunteers. Schizophr. Res. 2005, 77, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Mintzer, M.Z.; Griffiths, R.R. Triazolam-amphetamine interaction: Dissociation of effects on memory versus arousal. J. Psychopharmacol. 2003, 17, 17–29. [Google Scholar] [CrossRef]
- Ward, A.S.; Kelly, T.H.; Foltin, R.W.; Fischman, M.W. Effects of d-amphetamine on task performance and social behavior of humans in a residential laboratory. Exp. Clin. Psychopharmacol. 1997, 5, 130–136. [Google Scholar] [CrossRef]
- Willson, M.C.; Wilman, A.H.; Bell, E.C.; Asghar, S.J.; Silverstone, P.H. Dextroamphetamine causes a change in regional brain activity in vivo during cognitive tasks: A functional magnetic resonance imaging study of blood oxygen level-dependent response. Biol. Psychiatry 2004, 56, 284–291. [Google Scholar] [CrossRef]
- Fleming, K.; Bigelow, L.B.; Weinberger, D.R.; Goldberg, T.E. Neuropsychological effects of amphetamine may correlate with personality characteristics. Psychopharmacol. Bull. 1995, 31, 357–362. [Google Scholar] [PubMed]
- Makris, A.P.; Rush, C.R.; Frederich, R.C.; Taylor, A.C.; Kelly, T.H. Behavioral and subjective effects of D-amphetamine and modafinil in healthy adults. Exp. Clin. Psychopharmacol. 2007, 15, 123–133. [Google Scholar] [CrossRef]
- Wardle, M.C.; Yang, A.; De Wit, H. Effect of d-amphetamine on post-error slowing in healthy volunteers. Psychopharmacology 2012, 220, 109–115. [Google Scholar] [CrossRef]
- Lewandowski, L.; Hendricks, K.; Gordon, M. Test-Taking Performance of High School Students with ADHD. J. Atten. Disord. 2015, 19, 27–34. [Google Scholar] [CrossRef]
- Dan, O.; Raz, S. The Relationships Among ADHD, Self-Esteem, and Test Anxiety in Young Adults. J. Atten. Disord. 2015, 19, 231–239. [Google Scholar] [CrossRef]
- Cools, R.; D’Esposito, M. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol. Psychiatry 2011, 69, e113–e125. [Google Scholar] [CrossRef] [PubMed]
- Prasad, V.; Brogan, E.; Mulvaney, C.; Grainge, M.; Stanton, W.; Sayal, K. How effective are drug treatments for children with ADHD at improving on-task behaviour and academic achievement in the school classroom? A systematic review and meta-analysis. Eur. Child Adolesc. Psychiatry 2013, 22, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Ilieva, I.P.; Farah, M.J. Attention, Motivation, and Study Habits in Users of Unprescribed ADHD Medication. J. Atten. Disord. 2019, 23, 149–162. [Google Scholar] [CrossRef]
- Choe, E. Amphetamine Increases Phosphorylation of Extracellular Signal-regulated Kinase and Transcription Factors in the Rat Striatum via Group I Metabotropic Glutamate Receptors. Neuropsychopharmacology 2002, 27, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.-M.; Xue, B.; Jin, D.-Z.; Wang, J.Q. Dynamic increases in AMPA receptor phosphorylation in the rat hippocampus in response to amphetamine. J. Neurochem. 2015, 133, 795–805. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-García, L.E.; Tendilla-Beltrán, H.; Vázquez-Roque, R.A.; Jurado-Tapia, E.E.; Díaz, A.; Aguilar-Alonso, P.; Brambila, E.; Monjaraz, E.; De La Cruz, F.; Rodríguez-Moreno, A.; et al. Amphetamine sensitization alters hippocampal neuronal morphology and memory and learning behaviors. Mol. Psychiatry 2021, 26, 4784–4794. [Google Scholar] [CrossRef]
- Chidambaram, S.B.; Rathipriya, A.G.; Bolla, S.R.; Bhat, A.; Ray, B.; Mahalakshmi, A.M.; Manivasagam, T.; Thenmozhi, A.J.; Essa, M.M.; Guillemin, G.J.; et al. Dendritic spines: Revisiting the physiological role. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 92, 161–193. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H.; Kantor, L.; Wang, K.K.W.; Gnegy, M.E. Repeated, intermittent treatment with amphetamine induces neurite outgrowth in rat pheochromocytoma cells (PC12 cells). Brain Res. 2002, 951, 43–52. [Google Scholar] [CrossRef]
- Angelucci, F.; Gruber, S.H.M.; El Khoury, A.; Tonali, P.A.; Mathé, A.A. Chronic amphetamine treatment reduces NGF and BDNF in the rat brain. Eur. Neuropsychopharmacol. 2007, 17, 756–762. [Google Scholar] [CrossRef]
- Li, M.-H.; Underhill, S.M.; Reed, C.; Phillips, T.J.; Amara, S.G.; Ingram, S.L. Amphetamine and Methamphetamine Increase NMDAR-GluN2B Synaptic Currents in Midbrain Dopamine Neurons. Neuropsychopharmacology 2017, 42, 1539–1547. [Google Scholar] [CrossRef]
- Lu, W.; Monteggia, L.M.; Wolf, M.E. Withdrawal from repeated amphetamine administration reduces NMDAR1 expression in the rat substantia nigra, nucleus accumbens and medial prefrontal cortex. Eur. J. Neurosci. 1999, 11, 3167–3177. [Google Scholar] [CrossRef]
- Lu, W.; Chen, H.; Xue, C.-J.; Wolf, M.E. Repeated amphetamine administration alters the expression of mRNA for AMPA receptor subunits in rat nucleus accumbens and prefrontal cortex. Synapse 1997, 26, 269–280. [Google Scholar] [CrossRef]
- Mao, L.-M.; Wang, W.; Chu, X.-P.; Zhang, G.-C.; Liu, X.-Y.; Yang, Y.-J.; Haines, M.; Papasian, C.J.; Fibuch, E.E.; Buch, S.; et al. Stability of surface NMDA receptors controls synaptic and behavioral adaptations to amphetamine. Nat. Neurosci. 2009, 12, 602–610. [Google Scholar] [CrossRef]
- Lohr, W.D.; Wanta, J.W.; Baker, M.; Grudnikoff, E.; Morgan, W.; Chhabra, D.; Lee, T. Intentional Discontinuation of Psychostimulants Used to Treat ADHD in Youth: A Review and Analysis. Front. Psychiatry 2021, 12, 642798. [Google Scholar] [CrossRef] [PubMed]
- Beutler, L.R.; Wanat, M.J.; Quintana, A.; Sanz, E.; Bamford, N.S.; Zweifel, L.S.; Palmiter, R.D. Balanced NMDA receptor activity in dopamine D1 receptor (D1R)- and D2R-expressing medium spiny neurons is required for amphetamine sensitization. Proc. Natl. Acad. Sci. USA 2011, 108, 4206–4211. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R.; Sahakian, B.J.; Matthews, K.; Bannerjea, A.; Rimmer, J.; Robbins, T.W. Effects of methylphenidate on spatial working memory and planning in healthy young adults. Psychopharmacology 1997, 131, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Agay, N.; Yechiam, E.; Carmel, Z.; Levkovitz, Y. Methylphenidate enhances cognitive performance in adults with poor baseline capacities regardless of attention-deficit/hyperactivity disorder diagnosis. J. Clin. Psychopharmacol. 2014, 34, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Hammerness, P.; Fried, R.; Petty, C.; Meller, B.; Biederman, J. Assessment of cognitive domains during treatment with OROS methylphenidate in adolescents with ADHD. Child Neuropsychol. 2014, 20, 319–327. [Google Scholar] [CrossRef]
- Linssen, A.M.W.; Vuurman, E.F.P.M.; Sambeth, A.; Riedel, W.J. Methylphenidate produces selective enhancement of declarative memory consolidation in healthy volunteers. Psychopharmacology 2012, 221, 611–619. [Google Scholar] [CrossRef]
- Tucha, O.; Mecklinger, L.; Laufkötter, R.; Klein, H.E.; Walitza, S.; Lange, K.W. Methylphenidate-induced improvements of various measures of attention in adults with Attention Deficit Hyperactivity Disorder. J. Neural Transm. 2006, 113, 1575–1592. [Google Scholar] [CrossRef]
- Maul, J.; Advokat, C. Stimulant medications for attention-deficit/hyperactivity disorder (ADHD) improve memory of emotional stimuli in ADHD-diagnosed college students. Pharmacol. Biochem. Behav. 2013, 105, 58–62. [Google Scholar] [CrossRef]
- Andersen, S.L.; Arvanitogiannis, A.; Pliakas, A.M.; LeBlanc, C.; Carlezon, W.A. Altered responsiveness to cocaine in rats exposed to methylphenidate during development. Nat. Neurosci. 2002, 5, 13–14. [Google Scholar] [CrossRef]
- Crawford, C.A.; McDougall, S.A.; Meier, T.L.; Collins, R.L.; Watson, J.B. Repeated methylphenidate treatment induces behavioral sensitization and decreases protein kinase A and dopamine-stimulated adenylyl cyclase activity in the dorsal striatum. Psychopharmacology 1998, 136, 34–43. [Google Scholar] [CrossRef]
- Beverley, J.A.; Piekarski, C.; Van Waes, V.; Steiner, H. Potentiated gene regulation by methylphenidate plus fluoxetine treatment: Long-term gene blunting (Zif268, Homer1a) and behavioral correlates. Basal Ganglia 2014, 4, 109–116. [Google Scholar] [CrossRef]
- Motaghinejad, M.; Motevalian, M.; Falak, R.; Heidari, M.; Sharzad, M.; Kalantari, E. Neuroprotective effects of various doses of topiramate against methylphenidate-induced oxidative stress and inflammation in isolated rat amygdala: The possible role of CREB/BDNF signaling pathway. J. Neural Transm. 2016, 123, 1463–1477. [Google Scholar] [CrossRef] [PubMed]
- Di Miceli, M.; Omoloye, A.; Gronier, B. Characterisation of methylphenidate-induced excitation in midbrain dopamine neurons, an electrophysiological study in the rat brain. Prog. Neuropsychopharmacol. Biol. Psychiatry 2022, 112, 110406. [Google Scholar] [CrossRef]
- Zhang, C.-L.; Feng, Z.-J.; Liu, Y.; Ji, X.-H.; Peng, J.-Y.; Zhang, X.-H.; Zhen, X.-C.; Li, B.-M. Methylphenidate enhances NMDA-receptor response in medial prefrontal cortex via sigma-1 receptor: A novel mechanism for methylphenidate action. PLoS ONE 2012, 7, e51910. [Google Scholar] [CrossRef]
- Zhou, B.-Y.; Li, Z.-X.; Li, Y.-W.; Li, J.-N.; Liu, W.-T.; Liu, X.-Y.; Hu, Z.-B.; Zhao, L.; Chen, J.-Y.; Hu, L.; et al. Central Med23 deficiency leads to malformation of dentate gyrus and ADHD-like behaviors in mice. Neuropsychopharmacology 2025, 50, 1224–1236. [Google Scholar] [CrossRef]
- Urban, K.R.; Li, Y.-C.; Gao, W.-J. Treatment with a clinically-relevant dose of methylphenidate alters NMDA receptor composition and synaptic plasticity in the juvenile rat prefrontal cortex. Neurobiol. Learn. Mem. 2013, 101, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Jalloh, K.; Roeder, N.; Hamilton, J.; Delis, F.; Hadjiargyrou, M.; Komatsu, D.; Thanos, P.K. Chronic oral methylphenidate treatment in adolescent rats promotes dose-dependent effects on NMDA receptor binding. Life Sci. 2021, 264, 118708. [Google Scholar] [CrossRef]
- Van Der Marel, K.; Bouet, V.; Meerhoff, G.F.; Freret, T.; Boulouard, M.; Dauphin, F.; Klomp, A.; Lucassen, P.J.; Homberg, J.R.; Dijkhuizen, R.M.; et al. Effects of long-term methylphenidate treatment in adolescent and adult rats on hippocampal shape, functional connectivity and adult neurogenesis. Neuroscience 2015, 309, 243–258. [Google Scholar] [CrossRef]
- Shang, C.-Y.; Gau, S.S.-F. Improving Visual Memory, Attention, and School Function with Atomoxetine in Boys with Attention-Deficit/Hyperactivity Disorder. J. Child Adolesc. Psychopharmacol. 2012, 22, 353–363. [Google Scholar] [CrossRef]
- Kratochvil, C.J.; Newcorn, J.H.; Arnold, L.E.; Duesenberg, D.; Emslie, G.J.; Quintana, H.; Sarkis, E.H.; Wagner, K.D.; Gao, H.; Michelson, D. Atomoxetine Alone or Combined with Fluoxetine for Treating ADHD with Comorbid Depressive or Anxiety Symptoms. J. Am. Acad. Child Adolesc. Psychiatry 2005, 44, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Geller, D.; Donnelly, C.; Lopez, F.; Rubin, R.; Newcorn, J.; Sutton, V.; Bakken, R.; Paczkowski, M.; Kelsey, D.; Sumner, C. Atomoxetine Treatment for Pediatric Patients with Attention-Deficit/Hyperactivity Disorder with Comorbid Anxiety Disorder. J. Am. Acad. Child Adolesc. Psychiatry 2007, 46, 1119–1127. [Google Scholar] [CrossRef]
- Griffiths, K.R.; Leikauf, J.E.; Tsang, T.W.; Clarke, S.; Hermens, D.F.; Efron, D.; Williams, L.M.; Kohn, M.R. Response inhibition and emotional cognition improved by atomoxetine in children and adolescents with ADHD: The ACTION randomized controlled trial. J. Psychiatr. Res. 2018, 102, 57–64. [Google Scholar] [CrossRef]
- Epperson, C.N.; Pittman, B.; Czarkowski, K.A.; Bradley, J.; Quinlan, D.M.; Brown, T.E. Impact of atomoxetine on subjective attention and memory difficulties in perimenopausal and postmenopausal women. Menopause 2011, 18, 542–548. [Google Scholar] [CrossRef]
- de Jong, C.G.W.; Van De Voorde, S.; Roeyers, H.; Raymaekers, R.; Allen, A.J.; Knijff, S.; Verhelst, H.; Temmink, A.H.; Smit, L.M.E.; Rodriques-Pereira, R.; et al. Differential effects of atomoxetine on executive functioning and lexical decision in attention-deficit/hyperactivity disorder and reading disorder. J. Child Adolesc. Psychopharmacol. 2009, 19, 699–707. [Google Scholar] [CrossRef]
- Marquand, A.F.; De Simoni, S.; O’Daly, O.G.; Williams, S.C.; Mourão-Miranda, J.; Mehta, M.A. Pattern Classification of Working Memory Networks Reveals Differential Effects of Methylphenidate, Atomoxetine, and Placebo in Healthy Volunteers. Neuropsychopharmacol. 2011, 36, 1237–1247. [Google Scholar] [CrossRef]
- Fumagalli, F.; Cattaneo, A.; Caffino, L.; Ibba, M.; Racagni, G.; Carboni, E.; Gennarelli, M.; Riva, M.A. Sub-chronic exposure to atomoxetine up-regulates BDNF expression and signalling in the brain of adolescent spontaneously hypertensive rats: Comparison with methylphenidate. Pharmacol. Res. 2010, 62, 523–529. [Google Scholar] [CrossRef]
- Tzavara, E.T.; Bymaster, F.P.; Overshiner, C.D.; Davis, R.J.; Perry, K.W.; Wolff, M.; McKinzie, D.L.; Witkin, J.M.; Nomikos, G.G. Procholinergic and memory enhancing properties of the selective norepinephrine uptake inhibitor atomoxetine. Mol. Psychiatry 2006, 11, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Piña, R.; Rozas, C.; Contreras, D.; Hardy, P.; Ugarte, G.; Zeise, M.L.; Rojas, P.; Morales, B. Atomoxetine Reestablishes Long Term Potentiation in a Mouse Model of Attention Deficit/Hyperactivity Disorder. Neuroscience 2020, 439, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Callahan, P.M.; Plagenhoef, M.R.; Blake, D.T.; Terry, A.V. Atomoxetine improves memory and other components of executive function in young-adult rats and aged rhesus monkeys. Neuropharmacology 2019, 155, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yang, J.; Lei, G.; Wang, G.; Wang, Y.; Sun, R. Atomoxetine Increases Histamine Release and Improves Learning Deficits in an Animal Model of Attention-Deficit Hyperactivity Disorder: The Spontaneously Hypertensive Rat. Basic Clin. Pharmacol. Toxicol. 2008, 102, 527–532. [Google Scholar] [CrossRef]
- Nomura, H.; Shimizume, R.; Ikegaya, Y. Histamine: A Key Neuromodulator of Memory Consolidation and Retrieval. In The Functional Roles of Histamine Receptors; Yanai, K., Passani, M.B., Eds.; Current Topics in Behavioral Neurosciences; Springer International Publishing: Cham, Switzerland, 2021; Volume 59, pp. 329–353. ISBN 978-3-031-16996-0. Available online: https://link.springer.com/10.1007/7854_2021_253 (accessed on 1 May 2025). [CrossRef]
- Ludolph, A.; Udvardi, P.; Föhr, K.; Henes, C.; Liebau, S.; Dreyhaupt, J.; Boeckers, T.M.; Ludolph, A.G. Atomoxetine affects transcription/translation of the NMDA receptor and the norepinephrine transporter in the rat brain—An in vivo study. Drug Des. Dev. Ther. 2013, 2013, 1433–1446. [Google Scholar] [CrossRef]
- Di Miceli, M.; Gronier, B. Psychostimulants and atomoxetine alter the electrophysiological activity of prefrontal cortex neurons, interaction with catecholamine and glutamate NMDA receptors. Psychopharmacology 2015, 232, 2191–2205. [Google Scholar] [CrossRef]
- Joya, X.; Pujadas, M.; Falcón, M.; Civit, E.; Garcia-Algar, O.; Vall, O.; Pichini, S.; Luna, A.; De La Torre, R. Gas chromatography–mass spectrometry assay for the simultaneous quantification of drugs of abuse in human placenta at 12th week of gestation. Forensic Sci. Int. 2010, 196, 38–42. [Google Scholar] [CrossRef]
- Li, L.; Sujan, A.C.; Butwicka, A.; Chang, Z.; Cortese, S.; Quinn, P.; Viktorin, A.; Öberg, A.S.; D’Onofrio, B.M.; Larsson, H. Associations of Prescribed ADHD Medication in Pregnancy with Pregnancy-Related and Offspring Outcomes: A Systematic Review. CNS Drugs 2020, 34, 731–747. [Google Scholar] [CrossRef]
- Ornoy, A. Pharmacological Treatment of Attention Deficit Hyperactivity Disorder During Pregnancy and Lactation. Pharm. Res. 2018, 35, 46. [Google Scholar] [CrossRef]
- Ross, E.J.; Graham, D.L.; Money, K.M.; Stanwood, G.D. Developmental consequences of fetal exposure to drugs: What we know and what we still must learn. Neuropsychopharmacology 2015, 40, 61–87. [Google Scholar] [CrossRef]
- Sauer, J.-M.; Ring, B.J.; Witcher, J.W. Clinical Pharmacokinetics of Atomoxetine. Clin. Pharmacokinet. 2005, 44, 571–590. [Google Scholar] [CrossRef] [PubMed]
- McAllister-Williams, R.H.; Baldwin, D.S.; Cantwell, R.; Easter, A.; Gilvarry, E.; Glover, V.; Green, L.; Gregoire, A.; Howard, L.M.; Jones, I.; et al. British Association for Psychopharmacology consensus guidance on the use of psychotropic medication preconception, in pregnancy and postpartum 2017. J. Psychopharmacol. 2017, 31, 519–552. [Google Scholar] [CrossRef]
- Baker, A.S.; Wales, R.; Noe, O.; Gaccione, P.; Freeman, M.P.; Cohen, L.S. The Course of ADHD during Pregnancy. J. Atten. Disord. 2022, 26, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Scoten, O.; Tabi, K.; Paquette, V.; Carrion, P.; Ryan, D.; Radonjic, N.V.; Whitham, E.A.; Hippman, C. Attention-deficit/hyperactivity disorder in pregnancy and the postpartum period. Am. J. Obstet. Gynecol. 2024, 231, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Louik, C.; Kerr, S.; Kelley, K.E.; Mitchell, A.A. Increasing use of ADHD medications in pregnancy. Pharmacoepidemiol. Drug Saf. 2015, 24, 218–220. [Google Scholar] [CrossRef]
- Anderson, K.N.; Ailes, E.C.; Danielson, M.; Lind, J.N.; Farr, S.L.; Broussard, C.S.; Tinker, S.C. Attention-Deficit/Hyperactivity Disorder Medication Prescription Claims Among Privately Insured Women Aged 15–44 Years—United States, 2003–2015. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 66–70. [Google Scholar] [CrossRef]
- Bang Madsen, K.; Robakis, T.K.; Liu, X.; Momen, N.; Larsson, H.; Dreier, J.W.; Kildegaard, H.; Groth, J.B.; Newcorn, J.H.; Hove Thomsen, P.; et al. In utero exposure to ADHD medication and long-term offspring outcomes. Mol. Psychiatry 2023, 28, 1739–1746. [Google Scholar] [CrossRef]
- Cohen, J.M.; Hernández-Díaz, S.; Bateman, B.T.; Park, Y.; Desai, R.J.; Gray, K.J.; Patorno, E.; Mogun, H.; Huybrechts, K.F. Placental Complications Associated with Psychostimulant Use in Pregnancy. Obstet. Gynecol. 2017, 130, 1192–1201. [Google Scholar] [CrossRef] [PubMed]
- Bang Madsen, K.; Larsson, H.; Skoglund, C.; Liu, X.; Munk-Olsen, T.; Bergink, V.; Newcorn, J.H.; Cortese, S.; Lichtenstein, P.; Kuja-Halkola, R.; et al. In utero exposure to methylphenidate, amphetamines and atomoxetine and offspring neurodevelopmental disorders—A population-based cohort study and meta-analysis. Mol. Psychiatry 2025, 30, 3885–3894. [Google Scholar] [CrossRef] [PubMed]
- Huybrechts, K.F.; Bröms, G.; Christensen, L.B.; Einarsdóttir, K.; Engeland, A.; Furu, K.; Gissler, M.; Hernandez-Diaz, S.; Karlsson, P.; Karlstad, Ø.; et al. Association Between Methylphenidate and Amphetamine Use in Pregnancy and Risk of Congenital Malformations: A Cohort Study From the International Pregnancy Safety Study Consortium. JAMA Psychiatry 2018, 75, 167–175. [Google Scholar] [CrossRef]
- Ladhani, N.N.N.; Shah, P.S.; Murphy, K.E. Prenatal amphetamine exposure and birth outcomes: A systematic review and metaanalysis. Am. J. Obstet. Gynecol. 2011, 205, 219.e1–219.e7. [Google Scholar] [CrossRef]
- Eriksson, M.; Jonsson, B.; Steneroth, G.; Zetterström, R. Amphetamine abuse during pregnancy: Environmental factors and outcome after 14–15 years. Scand. J. Public Health 2000, 28, 154–157. [Google Scholar] [CrossRef]
- Chen, V.S.; Morrison, J.P.; Southwell, M.F.; Foley, J.F.; Bolon, B.; Elmore, S.A. Histology Atlas of the Developing Prenatal and Postnatal Mouse Central Nervous System, with Emphasis on Prenatal Days E7.5 to E18.5. Toxicol. Pathol. 2017, 45, 705–744. [Google Scholar] [CrossRef] [PubMed]
- Flores, G.; De Jesús Gómez-Villalobos, M.; Rodríguez-Sosa, L. Prenatal Amphetamine Exposure Effects on Dopaminergic Receptors and Transporter in Postnatal Rats. Neurochem. Res. 2011, 36, 1740–1749. [Google Scholar] [CrossRef]
- Gong, S.; Fayette, N.; Heinsbroek, J.A.; Ford, C.P. Cocaine shifts dopamine D2 receptor sensitivity to gate conditioned behaviors. Neuron 2021, 109, 3421–3435.e5. [Google Scholar] [CrossRef]
- Volkow, N.D.; Chang, L.; Wang, G.-J.; Fowler, J.S.; Ding, Y.-S.; Sedler, M.; Logan, J.; Franceschi, D.; Gatley, J.; Hitzemann, R.; et al. Low Level of Brain Dopamine D2 Receptors in Methamphetamine Abusers: Association with Metabolism in the Orbitofrontal Cortex. Am. J. Psychiatry 2001, 158, 2015–2021. [Google Scholar] [CrossRef]
- Bigl, V.; Dalitz, E.; Kunert, E.; Biesold, D.; Leonard, B.E. The effect of d-amphetamine and amitriptyline administered to pregnant rats on the locomotor activity and neurotransmitters of the offspring. Psychopharmacology 1982, 77, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Nasello, A.G.; Ramirez, O.A. Brain catecholamines metabolism in offspring of amphetamine treated rats. Pharmacol. Biochem. Behav. 1978, 9, 17–20. [Google Scholar] [CrossRef]
- Tavares, M.A.; Silva, M.C.; Silva-Araújo, A.; Xavier, M.R.; Ali, S.F. Effects of prenatal exposure to amphetamine in the medial prefrontal cortex of the rat. Int. J. Dev. Neurosci. 1996, 14, 585–596. [Google Scholar] [CrossRef]
- Bell, R.W.; Drucker, R.R.; Woodruff, A.B. The effects of prenatal injections of adrenalin chloride and d- amphetamine sulfate on subsequent emotionality and ulcer-proneness of offspring. Psychon. Sci. 1965, 2, 269–270. [Google Scholar] [CrossRef]
- Nasello, A.G.; Ramirez, O.A. Open-field and Lashley III maze behaviour of the offspring of amphetamine-treated rats. Psychopharmacology 1978, 58, 171–173. [Google Scholar] [CrossRef]
- Nasello, A.G.; Astrada, C.A.; Ramirez, O.A. Effects on the acquisition of conditioned avoidance responses and seizure threshold in the offspring of amphetamine treated gravid rats. Psychopharmacologia 1974, 40, 25–31. [Google Scholar] [CrossRef]
- Ke, T.; Poquette, K.E.; Amro Gazze, S.L.; Carvelli, L. Amphetamine Exposure during Embryogenesis Alters Expression and Function of Tyrosine Hydroxylase and the Vesicular Monoamine Transporter in Adult C. elegans. Int. J. Mol. Sci. 2024, 25, 4219. [Google Scholar] [CrossRef] [PubMed]
- Korchynska, S.; Krassnitzer, M.; Malenczyk, K.; Prasad, R.B.; Tretiakov, E.O.; Rehman, S.; Cinquina, V.; Gernedl, V.; Farlik, M.; Petersen, J.; et al. Life-long impairment of glucose homeostasis upon prenatal exposure to psychostimulants. EMBO J. 2020, 39, e100882. [Google Scholar] [CrossRef]
- Plessinger, M.A. PRENATAL EXPOSURE TO AMPHETAMINES. Obstet. Gynecol. Clin. N. Am. 1998, 25, 119–138. [Google Scholar] [CrossRef] [PubMed]
- Lepelletier, F.-X.; Tauber, C.; Nicolas, C.; Solinas, M.; Castelnau, P.; Belzung, C.; Emond, P.; Cortese, S.; Faraone, S.V.; Chalon, S.; et al. Prenatal Exposure to Methylphenidate Affects the Dopamine System and the Reactivity to Natural Reward in Adulthood in Rats. Int. J. Neuropsychopharmacol. 2015, 18, pyu044. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, S.A.; Oltean, C.; Pass, H.; Phillips, B.; Staton, K.; Robertson, C.L.; Shanks, R.A. Prenatal exposure to psychostimulants increases impulsivity, compulsivity, and motivation for rewards in adult mice. Physiol. Behav. 2013, 119, 43–51. [Google Scholar] [CrossRef]
- Aoki, S.; Kaizaki-Mitsumoto, A.; Hattori, N.; Numazawa, S. Fetal methylphenidate exposure induced ADHD-like phenotypes and decreased Drd2 and Slc6a3 expression levels in mouse offspring. Toxicol. Lett. 2021, 344, 1–10. [Google Scholar] [CrossRef]
- McFadyen-Leussis, M.P.; Lewis, S.P.; Bond, T.L.Y.; Carrey, N.; Brown, R.E. Prenatal exposure to methylphenidate hydrochloride decreases anxiety and increases exploration in mice. Pharmacol. Biochem. Behav. 2004, 77, 491–500. [Google Scholar] [CrossRef]
- Levin, E.D.; Sledge, D.; Roach, S.; Petro, A.; Donerly, S.; Linney, E. Persistent behavioral impairment caused by embryonic methylphenidate exposure in zebrafish. Neurotoxicol. Teratol. 2011, 33, 668–673. [Google Scholar] [CrossRef]
- Priori, A.; Hallett, M.; Rothwell, J.C. Repetitive transcranial magnetic stimulation or transcranial direct current stimulation? Brain Stimul. 2009, 2, 241–245. [Google Scholar] [CrossRef]
- Westwood, S.J.; Radua, J.; Rubia, K. Noninvasive brain stimulation in children and adults with attention-deficit/hyperactivity disorder: A systematic review and meta-analysis. J. Psychiatry Neurosci. 2021, 46, E14–E33. [Google Scholar] [CrossRef]
- Dayan, E.; Censor, N.; Buch, E.R.; Sandrini, M.; Cohen, L.G. Noninvasive brain stimulation: From physiology to network dynamics and back. Nat. Neurosci. 2013, 16, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wei, Z.-Y.; Zhao, N.; Zhuang, Q.; Zhang, H.; Fang, H.-L.; Zang, Y.-F.; Feng, Z.-J. Transcranial magnetic stimulation in attention-deficit/hyperactivity disorder: A systematic review and meta-analysis of cortical excitability and therapeutic efficacy. Front. Psychiatry 2025, 16, 1544816. [Google Scholar] [CrossRef] [PubMed]
- Miao, S.; Han, J.; Gu, Y.; Wang, X.; Song, W.; Li, D.; Liu, Z.; Yang, J.; Li, X. Reduced Prefrontal Cortex Activation in Children with Attention-Deficit/Hyperactivity Disorder during Go/No-Go Task: A Functional Near-Infrared Spectroscopy Study. Front. Neurosci. 2017, 11, 367. [Google Scholar] [CrossRef]
- Li, Y.; Ma, S.; Zhang, X.; Gao, L. ASD and ADHD: Divergent activating patterns of prefrontal cortex in executive function tasks? J. Psychiatr. Res. 2024, 172, 187–196. [Google Scholar] [CrossRef]
- Cao, P.; Xing, J.; Cao, Y.; Cheng, Q.; Sun, X.; Kang, Q.; Dai, L.; Zhou, X.; Song, Z. Clinical effects of repetitive transcranial magnetic stimulation combined with atomoxetine in the treatment of attention-deficit hyperactivity disorder. Neuropsychiatr. Dis. Treat. 2018, 14, 3231–3240. [Google Scholar] [CrossRef] [PubMed]
- Nagy, N.A.S.; Amin, G.R.; Khalil, S.A.; Mahmoud, D.A.M.; Elkholy, H.; Shohdy, M. The therapeutic role of repetitive transcranial magnetic stimulation in children with attention deficit/hyperactivity disorder in Egypt a randomized sham controlled clinical trial. Middle East. Curr. Psychiatry 2022, 29, 55. [Google Scholar] [CrossRef]
- Cao, P.; Wang, L.; Cheng, Q.; Sun, X.; Kang, Q.; Dai, L.; Zhou, X.; Song, Z. Changes in serum miRNA-let-7 level in children with attention deficit hyperactivity disorder treated by repetitive transcranial magnetic stimulation or atomoxetine: An exploratory trial. Psychiatry Res. 2019, 274, 189–194. [Google Scholar] [CrossRef]
- Wang, J.; Zou, Z.; Huang, H.; Zhang, Y.; He, X.; Su, H.; Wang, W.; Chen, Y.; Liu, Y. Effects of repetitive transcranial magnetic stimulation on prefrontal cortical activation in children with attention deficit hyperactivity disorder: A functional near-infrared spectroscopy study. Front. Neurol. 2024, 15, 1503975. [Google Scholar] [CrossRef] [PubMed]
- Mendes Dos Santos, R.; Nunes, M.; Peres De Souza, L.; Nayara De Araújo Val, S.; Machado Santos, Á.; Cristina Vieira Da Costa, A.; Emanuelle Sousa Lima, L.; Souza, H.; Teixeira, S. Hypothesis on the potential of repetitive transcranial magnetic stimulation to modulate neurochemical pathways and circadian rhythm in ADHD. Med. Hypotheses 2024, 189, 111411. [Google Scholar] [CrossRef]
- Allenby, C.; Falcone, M.; Bernardo, L.; Wileyto, E.P.; Rostain, A.; Ramsay, J.R.; Lerman, C.; Loughead, J. Transcranial direct current brain stimulation decreases impulsivity in ADHD. Brain Stimul. 2018, 11, 974–981. [Google Scholar] [CrossRef]
- Soff, C.; Sotnikova, A.; Christiansen, H.; Becker, K.; Siniatchkin, M. Transcranial direct current stimulation improves clinical symptoms in adolescents with attention deficit hyperactivity disorder. J. Neural Transm. 2017, 124, 133–144. [Google Scholar] [CrossRef]
- Salehinejad, M.A.; Nejati, V.; Mosayebi-Samani, M.; Mohammadi, A.; Wischnewski, M.; Kuo, M.-F.; Avenanti, A.; Vicario, C.M.; Nitsche, M.A. Transcranial Direct Current Stimulation in ADHD: A Systematic Review of Efficacy, Safety, and Protocol-induced Electrical Field Modeling Results. Neurosci. Bull. 2020, 36, 1191–1212. [Google Scholar] [CrossRef]
- Cachoeira, C.T.; Leffa, D.T.; Mittelstadt, S.D.; Mendes, L.S.T.; Brunoni, A.R.; Pinto, J.V.; Blazius, V.; Machado, V.; Bau, C.H.D.; Rohde, L.A.; et al. Positive effects of transcranial direct current stimulation in adult patients with attention-deficit/hyperactivity disorder—A pilot randomized controlled study. Psychiatry Res. 2017, 247, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, B.; Reis, J.; Martinowich, K.; Schambra, H.M.; Ji, Y.; Cohen, L.G.; Lu, B. Direct Current Stimulation Promotes BDNF-Dependent Synaptic Plasticity: Potential Implications for Motor Learning. Neuron 2010, 66, 198–204. [Google Scholar] [CrossRef]
- Wong, H.C.; Zaman, R. Neurostimulation in Treating ADHD. Psychiatr. Danub. 2019, 31, 265–275. [Google Scholar] [PubMed]
- Zhi, J.; Zhang, S.; Huang, M.; Qin, H.; Xu, H.; Chang, Q.; Wang, Y. Transcutaneous auricular vagus nerve stimulation as a potential therapy for attention deficit hyperactivity disorder: Modulation of the noradrenergic pathway in the prefrontal lobe. Front. Neurosci. 2024, 18, 1494272. [Google Scholar] [CrossRef] [PubMed]
- Aniwattanapong, D.; List, J.J.; Ramakrishnan, N.; Bhatti, G.S.; Jorge, R. Effect of Vagus Nerve Stimulation on Attention and Working Memory in Neuropsychiatric Disorders: A Systematic Review. Neuromodul. Technol. Neural Interface 2022, 25, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Ben-Menachem, E. Vagus Nerve Stimulation, Side Effects, and Long-Term Safety. J. Clin. Neurophysiol. 2001, 18, 415–418. [Google Scholar] [CrossRef]
- De Cicco, V.; Tramonti Fantozzi, M.P.; Cataldo, E.; Barresi, M.; Bruschini, L.; Faraguna, U.; Manzoni, D. Trigeminal, Visceral and Vestibular Inputs May Improve Cognitive Functions by Acting through the Locus Coeruleus and the Ascending Reticular Activating System: A New Hypothesis. Front. Neuroanat. 2017, 11, 130. [Google Scholar] [CrossRef] [PubMed]
- Cook, I.A.; Schrader, L.M.; Degiorgio, C.M.; Miller, P.R.; Maremont, E.R.; Leuchter, A.F. Trigeminal nerve stimulation in major depressive disorder: Acute outcomes in an open pilot study. Epilepsy Behav. 2013, 28, 221–226. [Google Scholar] [CrossRef]
- McGough, J.J.; Sturm, A.; Cowen, J.; Tung, K.; Salgari, G.C.; Leuchter, A.F.; Cook, I.A.; Sugar, C.A.; Loo, S.K. Double-Blind, Sham-Controlled, Pilot Study of Trigeminal Nerve Stimulation for Attention-Deficit/Hyperactivity Disorder. J. Am. Acad. Child Adolesc. Psychiatry 2019, 58, 403–411.e3. [Google Scholar] [CrossRef]
- Rubia, K.; Johansson, L.; Carter, B.; Stringer, D.; Santosh, P.; Mehta, M.A.; Conti, A.A.; Bozhilova, N.; Eraydin, I.E.; Cortese, S. The efficacy of real versus sham external Trigeminal Nerve Stimulation (eTNS) in youth with Attention-Deficit/Hyperactivity Disorder (ADHD) over 4 weeks: A protocol for a multi-centre, double-blind, randomized, parallel-group, phase IIb study (ATTENS). BMC Psychiatry 2024, 24, 326. [Google Scholar] [CrossRef]
- McGough, J.J.; Loo, S.K.; Sturm, A.; Cowen, J.; Leuchter, A.F.; Cook, I.A. An eight-week, open-trial, pilot feasibility study of trigeminal nerve stimulation in youth with attention-deficit/hyperactivity disorder. Brain Stimul. 2015, 8, 299–304. [Google Scholar] [CrossRef]
- Frolli, A.; Cerciello, F.; Esposito, C.; Ricci, M.C.; Laccone, R.P.; Bisogni, F. Universal Design for Learning for Children with ADHD. Children 2023, 10, 1350. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.L. David H. Rose, Anne Meyer, Teaching Every Student in the Digital Age: Universal Design for Learning: Association for Supervision and Curriculum Development, 1703 N. Beauregard St., Alexandria, VA 22311-1714, Product no. 101042, $22.95 ASCD members, $26.95 nonmembers, ISBN-0-87120-599-8. Educ. Technol. Res. Dev. 2007, 55, 521–525. [Google Scholar] [CrossRef]
- McDonnell-Dowling, K.; Kelly, J.P. Sources of variation in the design of preclinical studies assessing the effects of amphetamine-type stimulants in pregnancy and lactation. Behav. Brain Res. 2015, 279, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef]
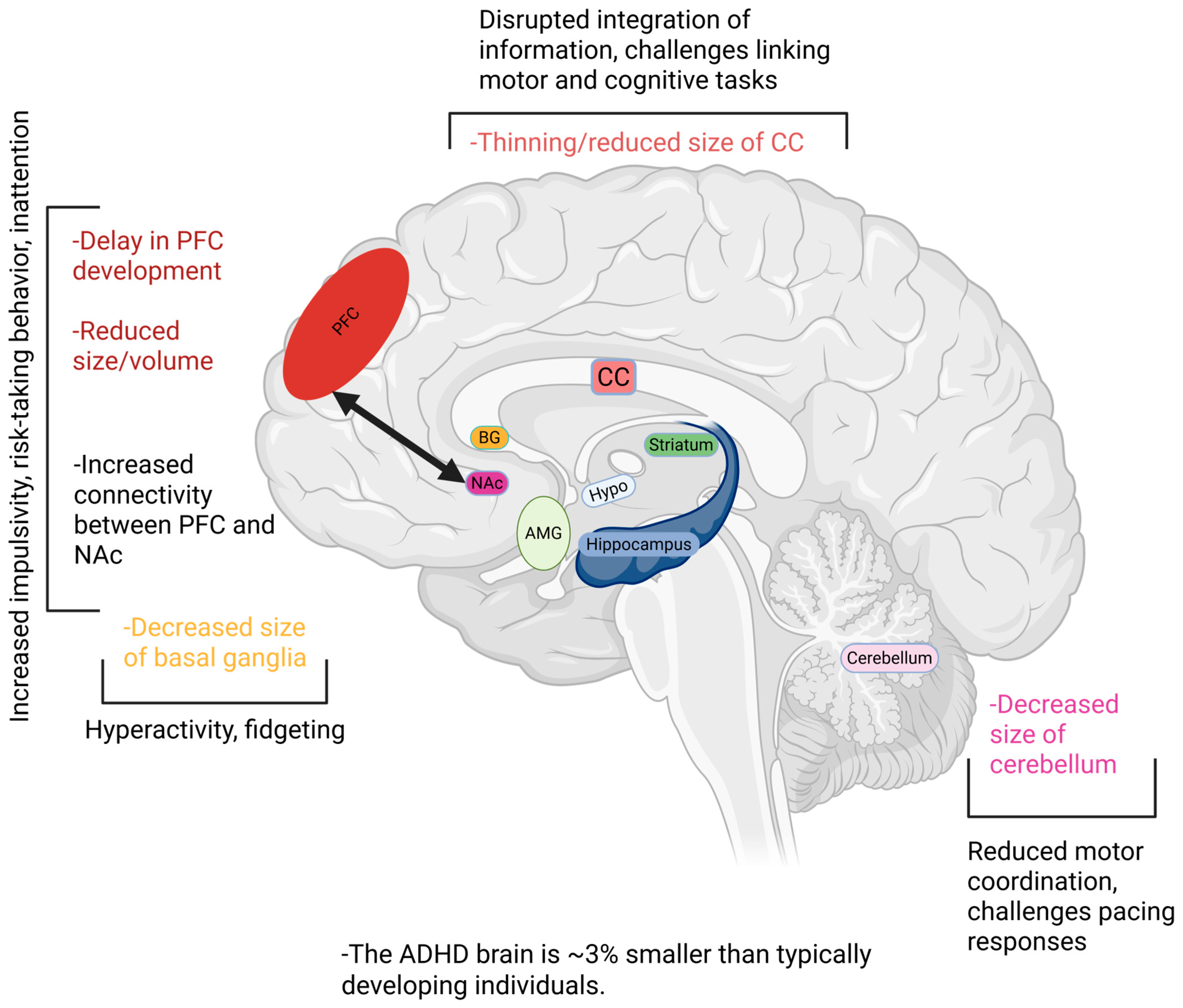


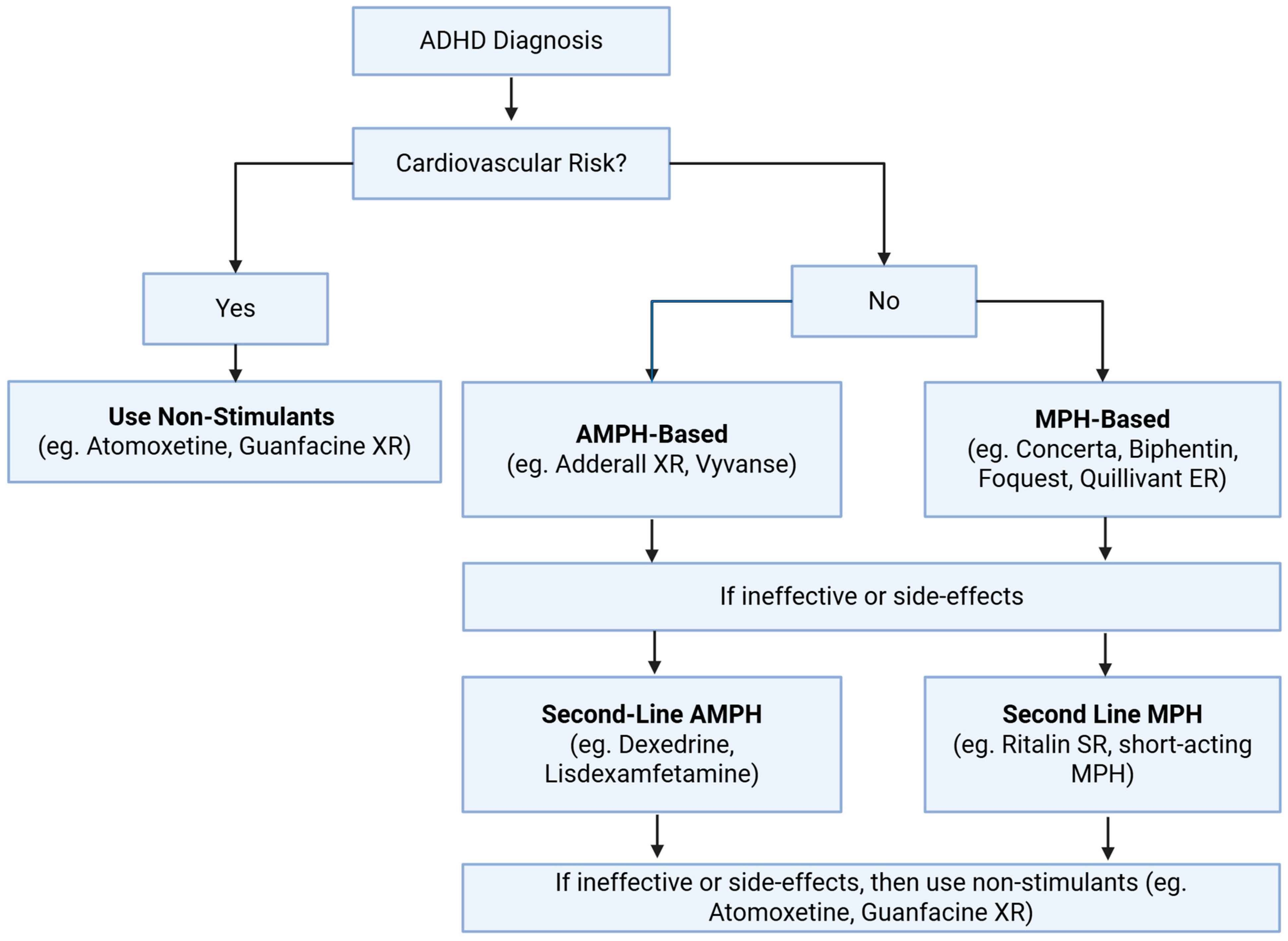
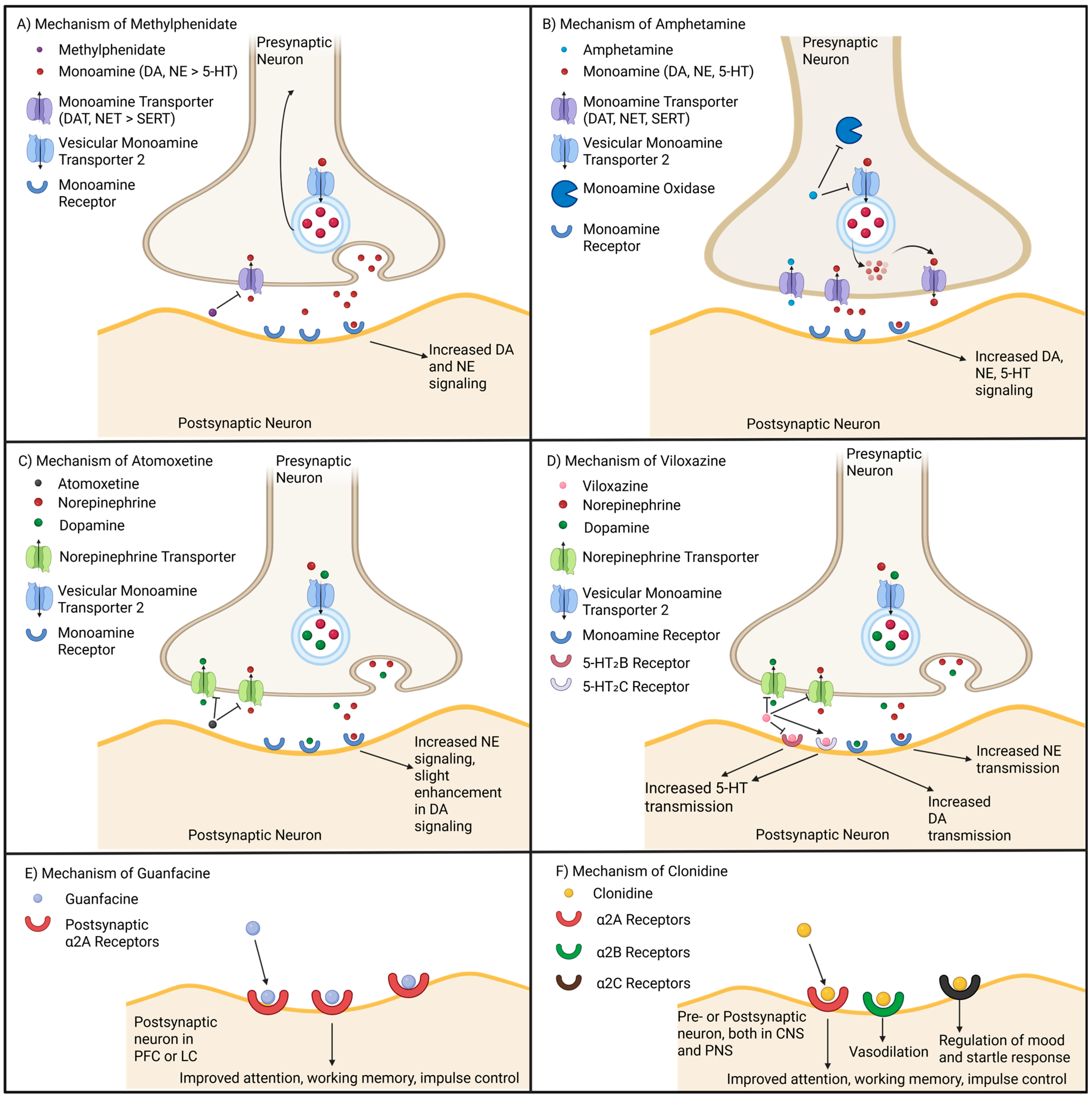

| Subtype | Diagnostic Criteria | Inattention Symptoms | Hyperactivity/Impulsivity Symptoms | Example Presentations |
|---|---|---|---|---|
| Primarily Inattentive (ADHD-I) | ≥6 inattention symptoms (≥5 for adults); fewer than 6 hyperactivity/impulsivity symptoms (<5 for adults) [6] |
| Not meeting threshold [6] | Examples for Children:
|
| Primarily Hyperactive (ADHD-H) | ≥6 hyperactivity/impulsivity symptoms (≥5 for adults); fewer than 6 inattention symptoms (<5 for adults) [6] | Not meeting threshold [6] |
| Examples for Children:
|
| Combined (ADHD-C) | ≥6 inattention AND ≥ 6 hyperactivity/impulsivity symptoms (≥5 each for adults) [6] | Same 9 symptoms as ADHD-I [6] | Same 9 symptoms as ADHD-H [6] | Mixed profile of inattentive + hyperactive symptoms; may also include sensory processing difficulties, sleep difficulties [6]. |
| Candidate Gene | Basal Function | Gene Variation/Single Nucleotide Polymorphism | Clinical Phenotype | References |
|---|---|---|---|---|
| SLC6A2/NET | Encodes for the NE transporter which regulates NE homeostasis. | rs36011 | Deficits in visual memory and attention | [134,156] |
| rs1566652 | ||||
| rs11568324 | Implicated in ADHD heritability and etiology | [157] | ||
| SLC6A3/DAT | Human DA transporter gene; regulates DA reuptake and neurotransmission dynamics | 10-repeat (10R) allele in 3′ UTR | Homozygosity implicated with worse response inhibition and sustained attention | [158,159,160] |
| SLC6A4/SERT | 5-HT transporter gene associated with socio-emotional development and stress susceptibility. | Promoter methylation | Associated with more hyperactive-impulsive symptoms, and reduced cortical thickness in occipito-temporal regions | [161,162] |
| DBH | The protein encoded by this gene converts DA to NE. | TaqI polymorphism (T) | Poorer sustained and visual attention | [138,139,140] |
| ADGRL3 (LPHN3) | Encodes for protein ADGRL3 and signals for cell-cell adhesion, signal transduction, and contributes to CNS development. | rs35106420 | Variants associated with increased susceptibility to developing ADHD | [190,191,192] |
| rs61747658 | ||||
| rs734644 | ||||
| DRD4 | Encodes for highly expressed DA receptor 4, regulates the dopaminergic pathway, and is involved in perception and response to sensory stimuli and executive function. | Exon III 48 base pair variable number tandem repeats | Effects on functional brain connectivity, deficiencies in perceptual organization, and attention symptoms | [167,168,169] |
| rs916457 | ||||
| DRD1 | Encodes DA receptor 1 and plays a role in attention. | rs265977 | Linked to hyperactivity and impulsivity symptoms | [164,165] |
| COMT | Enzyme product of gene catabolizes extra-neuronal DA and is linked to cognition, attention, and social behavior. | rs4680 | Linked to inattentive and hyperactive symptoms, as well as decreased grey matter volume | [193,194,195] |
| ADRA2A | Encodes for the alpha-2A-adrenergic receptor which is linked to working memory and focused attention through noradrenergic projections to the PFC. | rs553668 | Gene polymorphisms have been linked to severity of inattentive symptoms, and impacts on auditory/visual attention | [172,173] |
| MspI site in the promoter region (C→G) SNP | ||||
| MAOA | The protein product of the gene is an enzyme that affects metabolism of dopamine to noradrenaline. | rs5906883 | Gene polymorphisms potentially alter catecholamine regulation and are correlated with increased susceptibility to ADHD development and symptoms | [163] |
| rs3027407 | ||||
| CDH13 | Encodes for an atypical cadherin that drives neurodevelopmental processes and may have multiple functions related to signaling, neurite growth, and synapse development. | rs2199430 | Linked with excitatory/inhibitory imbalance, hyperactive-impulsive symptoms, deficits in learning and memory, and lower agreeableness | [178,179,180] |
| rs11150556 | ||||
| CTNNA2 | Encodes an alpha-catenin protein involved in neuronal migration, synaptic plasticity, and is critical for synaptic contact. | rs7600563 | Variants are associated with impulsivity, excitement seeking | [181,182,183] |
| rs13395022 | ||||
| DRD2 | Encodes for D2 receptors. | rs2075654 | Greater rate of commission errors. | [166] |
| rs1079596 | ||||
| DRD5 | Encodes for D5 receptors which stimulates adenyl cyclase activity. | Dinucleotide repeat of 148-bp allele (risk factor) is located 18.5 kb from 5′ end. | 148-bp allele is a risk factor for ADHD, and also significantly predicts baseline persistent ADHD diagnosis. 136-bp allele is a protective factor. | [170,171] |
| 136-bp allele was a protective factor. | ||||
| SNAP-25 | Encodes for the SNAP-25 protein, which is involved in the SNARE complex to facilitate synaptic vesicle fusion and neurotransmitter release. | rs3746544 located in the 3′ UTR | Association with ADHD. | [196] |
| PPP1R16A | Encodes a myosin phosphatase targeting subunit, which guides catalytic protein phosphatase 1 subunit to its intended targets to facilitate control of the actin cytoskeleton and cell-cell junctions. | Chromosome 1 | Reduced expression in ADHD cases, linked to educational attainment | [185] |
| B4GALT2 | Encodes for an enzyme involved in the synthesis of glycoconjugates and saccharide structures. | Chromosome 8 | Reduced expression in ADHD cases, linked to educational attainment. | [185] |
| SORCS3 | Encodes for the SORC3 protein which is an intracellular trafficking receptor for tropomyosin-related kinase B. Highly expressed in the CA1 region of the hippocampus and localized to the postsynaptic region. Regulates postsynaptic depression and influences the extinguishing of fear-related memories. | Deleterious variants | Linked to increased risk of ADHD. | [184,185,186] |
| NR3C1 | Encodes for the glucocorticoid receptor, which regulates processes including stress response, metabolism, immune regulation, mood, and cognition. | Haplotype TthIIII-NR3C1-I-ER22/23EK | Potentially linked to increased risk of ADHD. | [197,198] |
| EPHA5 | Encodes for the EphA5 receptor which plays a role in synaptic plasticity. Interactions between ephrin-A5 and the EphA5 receptor induces NMDAR-PSD95 complexes, spine maturation, and neuronal activity during early hippocampal development. | rs4860671 | Reduced performance in working memory and processing speed. | [199,200,201] |
| CDH13 | Encodes for an atypical cadherin that drives neurodevelopmental processes, and may have multiple functions related to signaling, neurite growth, and synapse development. | rs8055161 | Linked with excitatory/inhibitory imbalance, hyperacuity/impulsivity symptoms, deficits in learning and memory, and lower agreeableness | [178,179,180] |
| rs6565113 | ||||
| rs11150556 | ||||
| rs2199430 | ||||
| LIME1 | Encodes a transmembrane adaptor protein and plays role in inflammatory signaling pathway. | Increased methylation at: | Increased attention deficits as measured by Conner’s Continuous Performance Test | [202] |
| cg00446123 | ||||
| cg20513976 | ||||
| SPTBN2 | Regulates glutamate signaling pathway and is associated with neurodevelopment. | Reduced methylation at cg02506324 | Increased attention deficits as measured by Conner’s Continuous Performance Test | [202] |
| HTR1B | Encodes for the 5-HT 1B receptor (HTR1B). | rs6296 | Controls the release of DA, 5-HT, and acetylcholine in the brain. Knockout mice demonstrate behavioral disinhibition, hyperactivity, and increased aggressiveness. | [174,175,176,177] |
| rs6297 | ||||
| rs11568817 | ||||
| rs130058 | ||||
| rs6298 | ||||
| rs130060 |
| ADHD Animal Model | Manipulation | ADHD Relevant Features | Behavioral Phenotypes | Biological Alterations | Advantages | Disadvantages | References |
|---|---|---|---|---|---|---|---|
| DAT mutant mice | DAT KnockOut/Down | Hyperactivity | Excessive locomotion. | Fivefold-higher extracellular DA concentrations, 300-fold reduced DA reuptake. | Achieves strong hyperactivity phenotype. Useful for investigating molecular mechanisms linking DA signaling and hyperactivity. | Knockout of DAT prevents AMPH and MPH from exerting one of their core mechanisms to improve ADHD symptoms. Reduced DA is not a universal hallmark of human ADHD. Complete knockout of DAT may lead to developmental compensations that limit translational value. | [230,231,235,236,237] |
| Spontaneously Hypertensive Rat Model | Crossing male Wistar rats with spontaneous hypertension with females with moderately high blood pressure. Mate hypertensive rats again. | Attention deficit, hyperactivity, impulsiveness. Psychostimulants improve core ADHD-like symptoms in model. | More sensitive to delays in reinforcement similar to ADHD children. | Increased D1/D5 receptor density in neostriatum and NAc for young males. Reduced D4 gene and protein expression in PFC. Increased NE production and release in PFC. | Behavioral symptoms resemble core ADHD symptoms. Responds to stimulants like AMPH and MPH. | Confounding influences of hypertension on ADHD symptoms may reduce translatability of findings to human ADHD. Most studies use Wistar-Kyoto rats as controls, which have increased depression behaviors. | [231,238,239,240] |
| Coloboma Mouse | Generated through an irradiated deletion of 2cM region on chromosome 2. | Hyperactivity, impulsivity, inattention Are somewhat responsive to AMPH treatment, but not MPH. | Reduced patience in delayed reinforcement task. Increased locomotor activity. | Reduced DA release from dorsal striatum. Increased TH mRNA alongside increased α2A-receptor mRNA in LC. DA levels are increased in ventral striatum and cerebral cortex. | SNAP-25 variants are linked to ADHD in humans. Do show ADHD-like symptoms that are somewhat treated by stimulants. | Lethality in homozygotes, only heterozygotes. Generation of strain involves deletion of other genes beyond SNAP25, leading to confounding causes of the phenotype. | [231,241,242,243,244,245,246] |
| α-synuclein-lacking mice | Knocking out α-synuclein. | Hyperactive | Memory deficits | Elevated DA | Impacts DA function, allowing for investigating how DA impacts ADHD symptoms. | Does not strongly mimic inattention associated with ADHD. Reduced effect of D-AMPH as compared to WT mice. | [239,247] |
| Neonatal 6-hydroxydopamne Lesion Rat | Neonatal rats are injected with 6-hydroxydopamine which targets developing dopaminergic neurons to yield DA depletion in striatal/cortical targets. | Hyperactivity, attention-deficit, and impulsiveness. MPH reduced hyperactivity symptoms. | Adolescent mice exhibit anxiety, and antisocial behavior. | Reduced TH in the striatum, SN, and VTA. | Targets the DA system which is implicated in ADHD. Lesions in the neonatal model produce a neurodevelopmental origin of ADHD rather than adult-onset pathology. Allows for relatively selective DA manipulation. | More invasive as compared to SHR or genetic models. Exposure to DA neurotoxin during early development may result in compensatory changes. | [248,249] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yacoub, M.W.; Smith, S.R.; Abbas, B.; Iqbal, F.; Jazieh, C.M.O.; Al Shaer, N.S.H.; Luk, C.C.-F.; Syed, N.I. Attention-Deficit Hyperactivity Disorder (ADHD): A Comprehensive Overview of the Mechanistic Insights from Human Studies to Animal Models. Cells 2025, 14, 1367. https://doi.org/10.3390/cells14171367
Yacoub MW, Smith SR, Abbas B, Iqbal F, Jazieh CMO, Al Shaer NSH, Luk CC-F, Syed NI. Attention-Deficit Hyperactivity Disorder (ADHD): A Comprehensive Overview of the Mechanistic Insights from Human Studies to Animal Models. Cells. 2025; 14(17):1367. https://doi.org/10.3390/cells14171367
Chicago/Turabian StyleYacoub, Matthew William, Sophia Rose Smith, Badra Abbas, Fahad Iqbal, Cham Maher Othman Jazieh, Nada Saed Homod Al Shaer, Collin Chill-Fone Luk, and Naweed Imam Syed. 2025. "Attention-Deficit Hyperactivity Disorder (ADHD): A Comprehensive Overview of the Mechanistic Insights from Human Studies to Animal Models" Cells 14, no. 17: 1367. https://doi.org/10.3390/cells14171367
APA StyleYacoub, M. W., Smith, S. R., Abbas, B., Iqbal, F., Jazieh, C. M. O., Al Shaer, N. S. H., Luk, C. C.-F., & Syed, N. I. (2025). Attention-Deficit Hyperactivity Disorder (ADHD): A Comprehensive Overview of the Mechanistic Insights from Human Studies to Animal Models. Cells, 14(17), 1367. https://doi.org/10.3390/cells14171367








