Rosmarinic Acid as Bioactive Compound: Molecular and Physiological Aspects of Biosynthesis with Future Perspectives
Abstract
1. Introduction
2. Rosmarinic Acid Biosynthetic Pathways in Plants
2.1. L-Phenylalanine
2.2. L-Tyrosine
2.3. Rosmarinic Acid Synthase
3. Molecular Mechanisms of Rosmarinic Acid Accumulation
Effect of Elicitor on Rosmarinic Acid Accumulation
4. Synthetic Biology and Rosmarinic Acid Synthesis
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Scarpati, M.L.; Oriente, G. Isolamento e costituzione dell’acido rosmarinico (dal Rosmarinus off.). Ric. Sci. 1958, 28, 2329–2333. [Google Scholar]
- Janicsák, G.; Máthé, I.; Miklóssy-Vári, V.; Blunden, G. Comparative studies of the rosmarinic and caffeic acid contents of Lamiaceae species. Biochem. Syst. Ecol. 1999, 27, 733–738. [Google Scholar] [CrossRef]
- Petersen, M.; Simmonds, M.S.J. Molecules of interest—Rosmarinic acid. Phytochemistry 2003, 62, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M. Rosmarinic acid: New aspects. Phytochem. Rev. 2013, 12, 207–227. [Google Scholar] [CrossRef]
- Kim, G.D.; Park, Y.S.; Jin, Y.H.; Park, C.S. Production and applications of rosmarinic acid and structurally related compounds. Appl. Microbiol. Biotechnol. 2015, 99, 2083–2092. [Google Scholar] [CrossRef]
- Agata, I.; Kusakabe, H.; Hatano, T.; Nishibe, S.; Okuda, T. Melitric acids A and B, new trimeric caffeic acid derivatives from Melissa officinalis. Chem. Pharm. Bull. 1993, 41, 1608–1611. [Google Scholar] [CrossRef]
- Lu, Y.R.; Foo, L.Y. Rosmarinic acid derivatives from Salvia officinalis. Phytochemistry 1999, 51, 91–94. [Google Scholar] [CrossRef]
- Satake, T.; Kamiya, K.; Saiki, Y.; Hama, T.; Fujimoto, U.; Kitanaka, S.; Kimura, Y.; Uzawa, J.; Endang, H.; Umar, M. Studies on the constituents of fruits of Helicteres isora L. Chem. Pharm. Bull. 1999, 47, 1444–1447. [Google Scholar] [CrossRef]
- Habtemariam, S. Molecular pharmacology of rosmarinic and salvianolic acids: Potential seeds for alzheimer’s and vascular dementia drugs. Int. J. Mol. Sci. 2018, 19, 458. [Google Scholar] [CrossRef]
- Bulgakov, V.P.; Inyushkina, Y.V.; Fedoreyev, S.A. Rosmarinic acid and its derivatives: Biotechnology and applications. Crit. Rev. Biotechnol. 2012, 32, 203–217. [Google Scholar] [CrossRef]
- Khojasteh, A.; Mirjalili, M.H.; Alcalde, M.A.; Cusido, R.M.; Eibl, R.; Palazon, J. Powerful plant antioxidants: A new biosustainable approach to the production of rosmarinic acid. Antioxidants 2020, 9, 1273. [Google Scholar] [CrossRef] [PubMed]
- Harindranath, H.; Susil, A.; Sekar, M.; Kumar, B.P. Unlocking the potential of rosmarinic acid: A review on extraction, isolation, quantification, pharmacokinetics and pharmacology. Phytomed. Plus 2024, 5, 100726. [Google Scholar] [CrossRef]
- Jakovljević, D.; Topuzović, M.; Stanković, M. Nutrient limitation as a tool for the induction of secondary metabolites with antioxidant activity in basil cultivars. Ind. Crops. Prod. 2019, 138, 111462. [Google Scholar] [CrossRef]
- Arikan, B.; Ozfidan-Konakci, C.; Alp, F.N.; Zengin, G.; Yildiztugay, E. Rosmarinic acid and hesperidin regulate gas exchange, chlorophyll fluorescence, antioxidant system and the fatty acid biosynthesis-related gene expression in Arabidopsis thaliana under heat stress. Phytochemistry 2022, 198, 113157. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, V.; Alooparampil, S.; Pandya, R.V.; Tank, J.G. Physiological function of phenolic compounds in plant defense. In Phenolic Compounds: Chemistry, Synthesis, Diversity, Non-Conventional Industrial, Pharmaceutical and Therapeutic Applications; Badria, F.A., Ed.; IntechOpen: London, UK, 2022; p. 185. [Google Scholar]
- Zhou, P.P.; Yue, C.L.; Zhang, Y.C.; Li, Y.; Da, X.Y.; Zhou, X.Q.; Ye, L.D. Alleviation of the byproducts formation enables highly efficient biosynthesis of rosmarinic acid in Saccharomyces cerevisiae. J. Agric. Food Chem. 2022, 70, 5077–5087. [Google Scholar] [CrossRef]
- Ku, Y.S.; Cheng, S.S.; Luk, C.Y.; Leung, H.S.; Chan, T.Y.; Lam, H.M. Deciphering metabolite signalling between plant roots and soil pathogens to design resistance. BMC Plant Biol. 2025, 25, 308. [Google Scholar] [CrossRef]
- Bais, H.P.; Walker, T.S.; Schweizer, H.P.; Vivanco, J.A. Root specific elicitation and antimicrobial activity of rosmarinic acid in hairy root cultures of Ocimum basilicum. Plant Physiol. Biochem. 2002, 40, 983–995. [Google Scholar] [CrossRef]
- Lanoue, A.; Burlat, V.; Henkes, G.J.; Koch, I.; Schurr, U.; Röse, U.S.R. De novo biosynthesis of defense root exudates in response to Fusarium attack in barley. New Phytol. 2010, 185, 577–588. [Google Scholar] [CrossRef]
- Park, S.U.; Uddin, M.R.; Xu, H.; Kim, Y.K.; Lee, S.Y. Biotechnological applications for rosmarinic acid production in plant. Afr. J. Biotechnol. 2008, 7, 4959–4965. [Google Scholar]
- Chen, Y.; Jiang, X.; Yang, Q.; Luo, S.; Xie, X.; Peng, C. Bioactivity of rosmarinic acid and its application in food industry: A review. Food Ferment. Ind. 2023, 49, 318–325. [Google Scholar]
- Trócsányi, E.; György, Z.; Zámboriné-Németh, V. New insights into rosmarinic acid biosynthesis based on molecular studies. Curr. Plant Biol. 2020, 23, 100162. [Google Scholar] [CrossRef]
- Babaei, M.; Zamfir, G.M.B.; Chen, X.; Christensen, H.B.; Kristensen, M.; Nielsen, J.; Borodina, I. Metabolic engineering of Saccharomyces cerevisiae for rosmarinic acid production. ACS Synth. Biol. 2020, 9, 1978–1988. [Google Scholar] [CrossRef]
- Petersen, M.; Abdullah, Y.; Benner, J.; Eberle, D.; Gehlen, K.; Hücherig, S.; Janiak, V.; Kim, K.H.; Sander, M.; Weitzel, C.; et al. Evolution of rosmarinic acid biosynthesis. Phytochemistry 2009, 70, 1663–1679. [Google Scholar] [CrossRef]
- Ellis, B.E.; Towers, G.H.N. Biogenesis of rosmarinic acid in Mentha. Biochem. J. 1970, 118, 291–297. [Google Scholar] [CrossRef]
- Tariq, H.; Asif, S.; Andleeb, A.; Hano, C.; Abbasi, B.H. Flavonoid production: Current trends in plant metabolic engineering and de novo microbial production. Metabolites 2023, 13, 124. [Google Scholar] [CrossRef]
- Zhang, X.B.; Liu, C.J. Multifaceted regulations of gateway enzyme phenylalanine ammonialyase in the biosynthesis of phenylpropanoids. Mol. Plant 2015, 8, 17–27. [Google Scholar] [CrossRef]
- Jakovljević, D. Exploring molecular diversity of plants for enhancement of natural products. In Biosynthesis of Natural Products in Plants: Bioengineering in Post-Genomics Era; Kumar, N., Ed.; Springer: Singapore, 2024; pp. 315–327. [Google Scholar]
- Petersen, M. Cytochrome P450-dependent hydroxylation in the biosynthesis of rosmarinic acid in Coleus. Phytochemistry 1997, 45, 1165–1172. [Google Scholar] [CrossRef]
- Maeda, H.; Dudareva, N. The shikimate pathway and aromatic amino acid biosynthesis in plants. Annu. Rev. Plant Biol. 2012, 63, 73–105. [Google Scholar] [CrossRef]
- Xu, J.J.; Fang, X.; Li, C.Y.; Yang, L.; Chen, X.Y. General and specialized tyrosine metabolism pathways in plants. Abiotech 2020, 1, 97–105. [Google Scholar] [CrossRef]
- Mizukami, H.; Ellis, B.E. Rosmarinic acid formation and differential expression of tyrosine aminotransferase isoforms in Anchusa officinalis cell suspension cultures. Plant Cell Rep. 1991, 10, 321–324. [Google Scholar] [CrossRef]
- Huang, B.B.; Yi, B.; Duan, Y.B.; Sun, L.N.; Yu, X.J.; Guo, J.; Chen, W.S. Characterization and expression profiling of tyrosine aminotransferase gene from Salvia miltiorrhiza (Dan-shen) in rosmarinic acid biosynthesis pathway. Mol. Biol. Rep. 2008, 35, 601–612. [Google Scholar] [CrossRef]
- Lu, X.L.; Hao, L.; Wang, F.; Huang, C.; Wu, S.W. Molecular cloning and overexpression of the tyrosine aminotransferase (TAT) gene leads to increased rosmarinic acid yield in Perilla frutescens. PCTOC 2013, 115, 69–83. [Google Scholar] [CrossRef]
- Kim, Y.B.; Uddin, M.R.; Kim, Y.; Park, C.G.; Park, S.U. Molecular cloning and characterization of tyrosine aminotransferase and hydroxyphenylpyruvate reductase, and rosmarinic acid accumulation in Scutellaria baicalensis. Nat. Prod. Commun. 2014, 9, 1311–1314. [Google Scholar] [CrossRef]
- Ru, M.; Wang, K.R.; Bai, Z.Q.; Peng, L.; He, S.X.; Wang, Y.; Liang, Z.S. A tyrosine aminotransferase involved in rosmarinic acid biosynthesis in Prunella vulgaris L. Sci. Rep. 2017, 7, 4892. [Google Scholar] [CrossRef]
- Busch, T.; Petersen, M. Identification and biochemical characterisation of tyrosine aminotransferase from Anthoceros agrestis unveils the conceivable entry point into rosmarinic acid biosynthesis in hornworts. Planta 2021, 253, 98. [Google Scholar] [CrossRef]
- Mehta, P.K.; Hale, T.I.; Christen, P. Aminotransferases: Demonstration of homology and division into evolutionary subgroups. Eur. J. Biochem. 1993, 214, 549–561. [Google Scholar] [CrossRef]
- Frey, P.A.; Hegeman, A.D. Enzymatic Reaction Mechanisms; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Petersen, M.; Hausler, E.; Karwatzki, B.; Meinhard, J. Proposed biosynthetic pathway for rosmarinic acid in cell cultures of Coleus blumei Benth. Planta 1993, 189, 10–14. [Google Scholar] [CrossRef]
- Matsuno, M.; Nagatsu, A.; Ogihara, Y.; Ellis, B.E.; Mizukami, H. CYP98A6 from Lithospermum erythrorhizon encodes 4-coumaroyl-4′-hydroxyphenyllactic acid 3-hydroxylase involved in rosmarinic acid biosynthesis. FEBS Lett. 2002, 514, 219–224. [Google Scholar] [CrossRef]
- Xiao, Y.; Gao, S.H.; Di, P.; Chen, J.F.; Chen, W.S.; Zhang, L. Lithospermic acid B is more responsive to silver ions (Ag+) than rosmarinic acid in Salvia miltiorrhiza hairy root cultures. Biosci. Rep. 2010, 30, 33–40. [Google Scholar] [CrossRef]
- Xu, Y.P.; Geng, L.J.; Zhao, S.J. Biosynthesis of bioactive ingredients of Salvia miltiorrhiza and advanced biotechnologies for their production. Biotechnol. Biotechnol. Equip. 2018, 32, 1367–1377. [Google Scholar] [CrossRef]
- Berger, A.; Meinhard, J.; Petersen, M. Rosmarinic acid synthase is a new member of the superfamily of BAHD acyltransferases. Planta 2006, 224, 1503–1510. [Google Scholar] [CrossRef]
- Weitzel, C.; Petersen, M. Cloning and characterisation of rosmarinic acid synthase from Melissa officinalis L. Phytochemistry 2011, 72, 572–578. [Google Scholar] [CrossRef]
- Landmann, C.; Hücherig, S.; Fink, B.; Hoffmann, T.; Dittlein, D.; Coiner, H.A.; Schwab, W. Substrate promiscuity of a rosmarinic acid synthase from lavender (Lavandula angustifolia L.). Planta 2011, 234, 305–320. [Google Scholar] [CrossRef]
- Levsh, O.; Pluskar, T.; Carballo, V.; Mitchell, A.J.; Weng, J.K. Independent evolution of rosmarinic acid biosynthesis in two sister families under the Lamiids clade of flowering plants. J. Biol. Chem. 2019, 294, 15193–15205. [Google Scholar] [CrossRef]
- Eberle, D.; Ullmann, P.; Werck-Reichhart, D.; Petersen, M. cDNA cloning and functional characterisation of CYP98A14 and NADPH:cytochrome P450 reductase from Coleus blumei involved in rosmarinic acid biosynthesis. Plant Mol. Biol. 2009, 69, 239–253. [Google Scholar] [CrossRef]
- Zhou, J.L.; Zou, X.F.; Deng, Z.X.; Duan, L. Analysing a group of homologous BAHD enzymes provides insights into the evolutionary transition of rosmarinic acid synthases from hydroxycinnamoyl-CoA: Shikimate/quinate hydroxycinnamoyl transferases. Plants 2024, 13, 512. [Google Scholar] [CrossRef]
- Caretto, S.; Linsalata, V.; Colella, G.; Mita, G.; Lattanzio, V. Carbon fluxes between primary metabolism and phenolic pathway in plant tissues under stress. Int. J. Mol. Sci. 2015, 16, 26378–26394. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Luo, W.F.; Tanaka, G.; Konishi, Y.; Matsuura, H.; Takahashi, K. Wounding stress induces phenylalanine ammonia lyases, leading to the accumulation of phenylpropanoids in the model liverwort Marchantia polymorpha. Phytochemistry 2018, 155, 30–36. [Google Scholar] [CrossRef]
- Moure, A.; Cruz, J.M.; Franco, D.; Domínguez, J.M.; Sineiro, J.; Domínguez, H.; Núñez, M.J.; Parajó, J.C. Natural antioxidants from residual sources. Food Chem. 2001, 72, 145–171. [Google Scholar] [CrossRef]
- Salami, M.; Rahimmalek, M.; Ehtemam, M.H.; Szumny, A.; Fabian, S.; Matkowski, A. Essential oil composition, antimicrobial activity and anatomical characteristics of Foeniculum vulgare Mill. fruits from dif-ferent regions of Iran. J. Essent. Oil-Bear. Plants 2016, 19, 1614–1626. [Google Scholar] [CrossRef]
- Zali, A.G.; Ehsanzadeh, P.; Szumny, A.; Matkowski, A. Genotype-specific response of Foeniculum vulgare grain yield and essential oil composition to proline treatment under different irrigation conditions. Ind. Crops. Prod. 2018, 124, 177–185. [Google Scholar] [CrossRef]
- Kiferle, C.; Maggini, R.; Pardossi, A. Influence of nitrogen nutrition on growth and accumulation of rosmarinic acid in sweet basil (Ocimum basilicum L.) grown in hydroponic culture. Aust. J. Crop Sci. 2013, 7, 321–327. [Google Scholar]
- Li, M.X.; Xu, J.S.; Wang, X.X.; Fu, H.; Zhao, M.L.; Wang, H.; Shi, L.X. Photosynthetic characteristics and metabolic analyses of two soybean genotypes revealed adaptive strategies to low-nitrogen stress. J. Plant Physiol. 2018, 229, 132–141. [Google Scholar] [CrossRef]
- Gholamnia, A.; Arani, A.M.; Sodaeizadeh, H.; Esfahani, S.T.; Ghasemi, S. Expression profiling of rosmarinic acid biosynthetic genes and some physiological responses from Mentha piperita L. under salinity and heat stress. Physiol. Mol. Biol. Plants 2022, 28, 545–557. [Google Scholar] [CrossRef]
- Farhadi, N.; Moghaddam, M. Application of recent advanced technologies for the improvement of medicinal and aromatic plants. In Biosynthesis of Bioactive Compounds in Medicinal and Aromatic Plants: Manipulation by Conventional and Biotechnological Approaches; Jafari, S.M., Ed.; Springer: Cham, Switzerland, 2023; pp. 235–255. [Google Scholar]
- Kleinwächter, M.; Selmar, D. New insights explain that drought stress enhances the quality of spice and medicinal plants: Potential applications. Agron. Sustain. Dev. 2015, 35, 121–131. [Google Scholar] [CrossRef]
- Yahyazadeh, M.; Meinen, R.; Hänsch, R.; Abouzeid, S.; Selmar, D. Impact of drought and salt stress on the biosynthesis of alkaloids in Chelidonium majus L. Phytochemistry 2018, 152, 204–212. [Google Scholar] [CrossRef]
- Shakya, P.; Marslin, G.; Siram, K.; Beerhues, L.; Franklin, G. Elicitation as a tool to improve the profiles of high-value secondary metabolites and pharmacological properties of Hypericum perforatum. J. Pharm. Pharmacol. 2019, 71, 70–82. [Google Scholar] [CrossRef]
- Ramirez-Estrada, K.; Vidal-Limon, H.; Hidalgo, D.; Moyano, E.; Golenioswki, M.; Cusidó, R.M.; Palazon, J. Elicitation, an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories. Molecules 2016, 21, 182. [Google Scholar] [CrossRef]
- Farmer, E.E.; Alméras, E.; Krishnamurthy, V. Jasmonates and related oxylipins in plant responses to pathogenesis and herbivory. Curr. Opin. Plant Biol. 2003, 6, 372–378. [Google Scholar] [CrossRef]
- Baraik, B.; Kumari, T.; Lal, S. Role of induced mutation and stresses in the production of bioactive compounds in plants. In Biosynthesis of Bioactive Compounds in Medicinal and Aromatic Plants: Manipulation by Conventional and Biotechnological Approaches; Jafari, S.M., Ed.; Springer: Cham, Switzerland, 2023; pp. 151–179. [Google Scholar]
- Garcia-Brugger, A.; Lamotte, O.; Vandelle, E.; Bourque, S.; Lecourieux, D.; Poinssot, B.; Wendehenne, D.; Pugin, A. Early signaling events induced by elicitors of plant defenses. Mol. Plant-Microbe Interact. 2006, 19, 711–724. [Google Scholar] [CrossRef]
- Delaunois, B.; Farace, G.; Jeandet, P.; Clément, C.; Baillieul, F.; Dorey, S.; Cordelier, S. Elicitors as alternative strategy to pesticides in grapevine? Current knowledge on their mode of action from controlled conditions to vineyard. Environ. Sci. Pollut. Res. Int. 2014, 21, 4837–4846. [Google Scholar] [CrossRef]
- Pesaraklu, A.; Radjabian, T.; Salami, S.A. Methyl jasmonate and Ag+ as effective elicitors for enhancement of phenolic acids contents in Salvia officinalis and Salvia verticillata, as two traditional medicinal plants. S. Afr. J. Bot. 2021, 141, 105–115. [Google Scholar] [CrossRef]
- Kianersi, F.; Azarm, D.A.; Pour-Aboughadareh, A.; Poczai, P. Change in secondary metabolites and expression pattern of key rosmarinic acid related genes in iranian lemon balm (Melissa officinalis L.) ecotypes using methyl jasmonate treatments. Molecules 2022, 27, 1715. [Google Scholar] [CrossRef]
- Ru, M.; Li, Y.H.; Guo, M.; Chen, L.Y.; Tan, Y.; Peng, L.; Liang, Z.S. Increase in rosmarinic acid accumulation and transcriptional responses of synthetic genes in hairy root cultures of Prunella vulgaris induced by methyl jasmonate. PCTOC 2022, 149, 371–379. [Google Scholar] [CrossRef]
- Kianersi, F.; Azarm, D.A.; Fatemi, F.; Jamshidi, B.; Pour-Aboughadareh, A.; Janda, T. The influence of methyl jasmonate on expression patterns of rosmarinic acid biosynthesis genes, and phenolic compounds in different species of Salvia subg. Perovskia Kar L. Genes 2023, 14, 871. [Google Scholar] [CrossRef]
- Khojasteh, A.; Mirjalili, M.H.; Palazon, J.; Eibl, R.; Cusido, R.M. Methyl jasmonate enhanced production of rosmarinic acid in cell cultures of Satureja khuzistanica in a bioreactor. Eng. Life Sci. 2016, 16, 740–749. [Google Scholar] [CrossRef]
- Vergara-Martínez, V.M.; Estrada-Soto, S.E.; Valencia-Díaz, S.; Garcia-Sosa, K.; Peña-Rodríguez, L.M.; Arellano-García, J.D.; Perea-Arango, I. Methyl jasmonate enhances ursolic, oleanolic and rosmarinic acid production and sucrose induced biomass accumulation, in hairy roots of Lepechinia caulescens. PeerJ 2021, 9, e11279. [Google Scholar] [CrossRef]
- Kim, Y.B.; Kim, J.K.; Uddin, M.R.; Xu, H.; Park, W.T.; Tuan, P.A.; Li, X.; Chung, E.; Lee, J.H.; Park, S.U. Metabolomics analysis and biosynthesis of rosmarinic acid in Agastache rugosa Kuntze treated with methyl jasmonate. PLoS ONE 2013, 8, e64199. [Google Scholar] [CrossRef]
- Krzyzanowska, J.; Czubacka, A.; Pecio, L.; Przybys, M.; Doroszewska, T.; Stochmal, A.; Oleszek, W. The effects of jasmonic acid and methyl jasmonate on rosmarinic acid production in Mentha x piperita cell suspension cultures. PCTOC 2012, 108, 73–81. [Google Scholar] [CrossRef]
- Zhou, W.; Shi, M.; Deng, C.P.; Lu, S.J.; Huang, F.F.; Wang, Y.; Kai, G.Y. The methyl jasmonate-responsive transcription factor SmMYB1 promotes phenolic acid biosynthesis in Salvia miltiorrhiza. Hortic. Res. 2021, 8, 10. [Google Scholar] [CrossRef]
- Jafari, S.H.; Arani, A.M.; Esfahani, S.T. The combined effects of Rhizobacteria and methyl jasmonate on rosmarinic acid production and gene expression profile in Origanum vulgare L. under salinity conditions. J. Plant Growth Regul. 2023, 42, 1472–1487. [Google Scholar] [CrossRef]
- Khoshsokhan, F.; Babalar, M.; Salami, S.A.; Sheikhakbari-Mehr, R.; Mirjalili, M.H. An efficient protocol for production of rosmarinic acid in Salvia nemorosa L. In Vitro Cell. Dev. Biol. Plan 2023, 59, 298–314. [Google Scholar] [CrossRef]
- Li, X.H.; Guo, H.B.; Qi, Y.X.; Liu, H.L.; Zhang, X.R.; Ma, P.D.; Liang, Z.S.; Dong, J.N. Salicylic acid-induced cytosolic acidification increases the accumulation of phenolic acids in Salvia miltiorrhiza cells. PCTOC 2016, 126, 333–341. [Google Scholar] [CrossRef]
- Pérez-Tortosa, V.; López-Orenes, A.; Martínez-Pérez, A.; Ferrer, M.A.; Calderón, A.A. Antioxidant activity and rosmarinic acid changes in salicylic acid-treated Thymus membranaceus shoots. Food Chem. 2012, 130, 362–369. [Google Scholar] [CrossRef]
- Ejtahed, R.S.; Radjabian, T.; Tafreshi, S.A.H. Expression analysis of phenylalanine ammonia lyase gene and rosmarinic acid production in Salvia officinalis and Salvia virgata shoots under salicylic acid elicitation. Appl. Biochem. Biotechnol. 2015, 176, 1846–1858. [Google Scholar] [CrossRef]
- Hao, W.F.; Guo, H.B.; Zhang, J.Y.; Hu, G.G.; Yao, Y.Q.; Dong, J.E. Hydrogen peroxide is involved in salicylic acid-elicited rosmarinic acid production in Salvia miltiorrhiza cell cultures. Sci. World J. 2014, 1, 843764. [Google Scholar]
- Khalil, N.; Fekry, M.; Bishr, M.; El-Zalabani, S.; Salama, O. Foliar spraying of salicylic acid induced accumulation of phenolics, increased radical scavenging activity and modified the composition of the essential oil of water stressed Thymus vulgaris L. Plant Physiol. Biochem. 2018, 123, 65–74. [Google Scholar] [CrossRef]
- Stasinska-Jakubas, M.; Hawrylak-Nowak, B.; Dresler, S.; Wójciak, M.; Rubinowska, K. Application of chitosan lactate, selenite, and salicylic acid as an approach to induce biological responses and enhance secondary metabolism in Melissa officinalis L. Ind. Crops. Prod. 2023, 205, 117571. [Google Scholar] [CrossRef]
- Park, W.T.; Arasu, M.V.; Al-Dhabi, N.A.; Yeo, S.K.; Jeon, J.; Park, J.S.; Lee, S.Y.; Park, S.U. Yeast extract and silver nitrate induce the expression of phenylpropanoid biosynthetic genes and induce the accumulation of rosmarinic acid in Agastache rugosa cell culture. Molecules 2016, 21, 426. [Google Scholar] [CrossRef]
- Nasiri-Bezenjani, M.A.; Riahi-Madvar, A.; Baghizadeh, A.; Ahmadi, A.R. Rosmarinic acid production and expression of tyrosine aminotransferase gene in Melissa officinalis seedlings in response to yeast extract. J. Agric. Sci. Technol. 2014, 16, 921–930. [Google Scholar]
- Xiao, Y.; Zhang, L.; Gao, S.H.; Saechao, S.K.; Di, P.; Chen, J.F.; Chen, W.S. The c4h, tat, hppr and hppd genes prompted engineering of rosmarinic acid biosynthetic pathway in Salvia miltiorrhiza hairy root cultures. PLoS ONE 2011, 6, e29713. [Google Scholar] [CrossRef]
- Gonçalves, S.; Mansinhos, I.; Rodríguez-Solana, R.; Pérez-Santín, E.; Coelho, N.; Romano, A. Elicitation improves rosmarinic acid content and antioxidant activity in Thymus lotocephalus shoot cultures. Ind. Crops. Prod. 2019, 137, 214–220. [Google Scholar] [CrossRef]
- Guo, H.B.; Zhu, N.; Deyholos, M.K.; Liu, J.; Zhang, X.R.; Dong, J.E. Calcium mobilization in salicylic acid-induced Salvia miltiorrhiza cell cultures and its effect on the accumulation of rosmarinic acid. Appl. Biochem. Biotechnol. 2015, 175, 2689–2702. [Google Scholar] [CrossRef]
- Ho, C.C.; Kumaran, A.; Hwang, L.S. Bio-assay guided isolation and identification of anti-Alzheimer active compounds from the root of Angelica sinensis. Food Chem. 2009, 114, 246–252. [Google Scholar] [CrossRef]
- Eudes, A.; Mouille, M.; Robinson, D.S.; Benites, V.T.; Wang, G.; Roux, L.; Tsai, Y.L.; Baidoo, E.E.K.; Chiu, T.Y.; Heazlewood, J.L.; et al. Exploiting members of the BAHD acyltransferase family to synthesize multiple hydroxycinnamate and benzoate conjugates in yeast. Microb. Cell Fact. 2016, 15, 198. [Google Scholar] [CrossRef]
- Rainha, J.; Gomes, D.; Rodrigues, L.R.; Rodrigues, J.L. Synthetic biology approaches to engineer Saccharomyces cerevisiae towards the industrial production of valuable poly-phenolic compounds. Life 2020, 10, 56. [Google Scholar] [CrossRef]
- Braga, A.; Rocha, I.; Faria, N. Microbial hosts as a promising platform for polyphenol production. In Natural Bio-Active Compounds: Volume 1: Production and Applications; Akhtar, M.S., Swamy, M.K., Sinniah, U.R., Eds.; Springer: Singapore, 2019; pp. 71–103. [Google Scholar]
- Hanson, P.K. Saccharomyces cerevisiae: A unicellular model genetic organism of enduring importance. Curr. Protoc. Essent. Lab. Tech. 2018, 16, e21. [Google Scholar] [CrossRef]
- Weitzel, C.; Petersen, M. Enzymes of phenylpropanoid metabolism in the important medicinal plant Melissa officinalis L. Planta 2010, 232, 731–742. [Google Scholar] [CrossRef]
- Prabhu, P.R.; Hudson, A.O. Identification and partial characterization of an L-tyrosine aminotransferase (TAT) from Arabidopsis thaliana. Biochem. Res. Int. 2010, 2010, 549572. [Google Scholar] [CrossRef]
- Yan, Y.; Jia, P.; Bai, Y.J.; Fan, T.P.; Zheng, X.H.; Cai, Y.J. Production of rosmarinic acid with ATP and CoA double regenerating system. Enzyme. Microb. Technol. 2019, 131, 109392. [Google Scholar] [CrossRef]
- Bloch, S.E.; Schmidt-Dannert, C. Construction of a chimeric biosynthetic pathway for the de novo biosynthesis of rosmarinic acid in Escherichia coli. Chembiochem 2014, 15, 2393–2401. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.W.; Li, J.J.; Zhu, C.A.; Jing, B.Y.; Shi, K.; Yu, J.Q.; Hu, Z.J. Exogenous rosmarinic acid application enhances thermotolerance in tomatoes. Plants 2022, 11, 1172. [Google Scholar] [CrossRef] [PubMed]

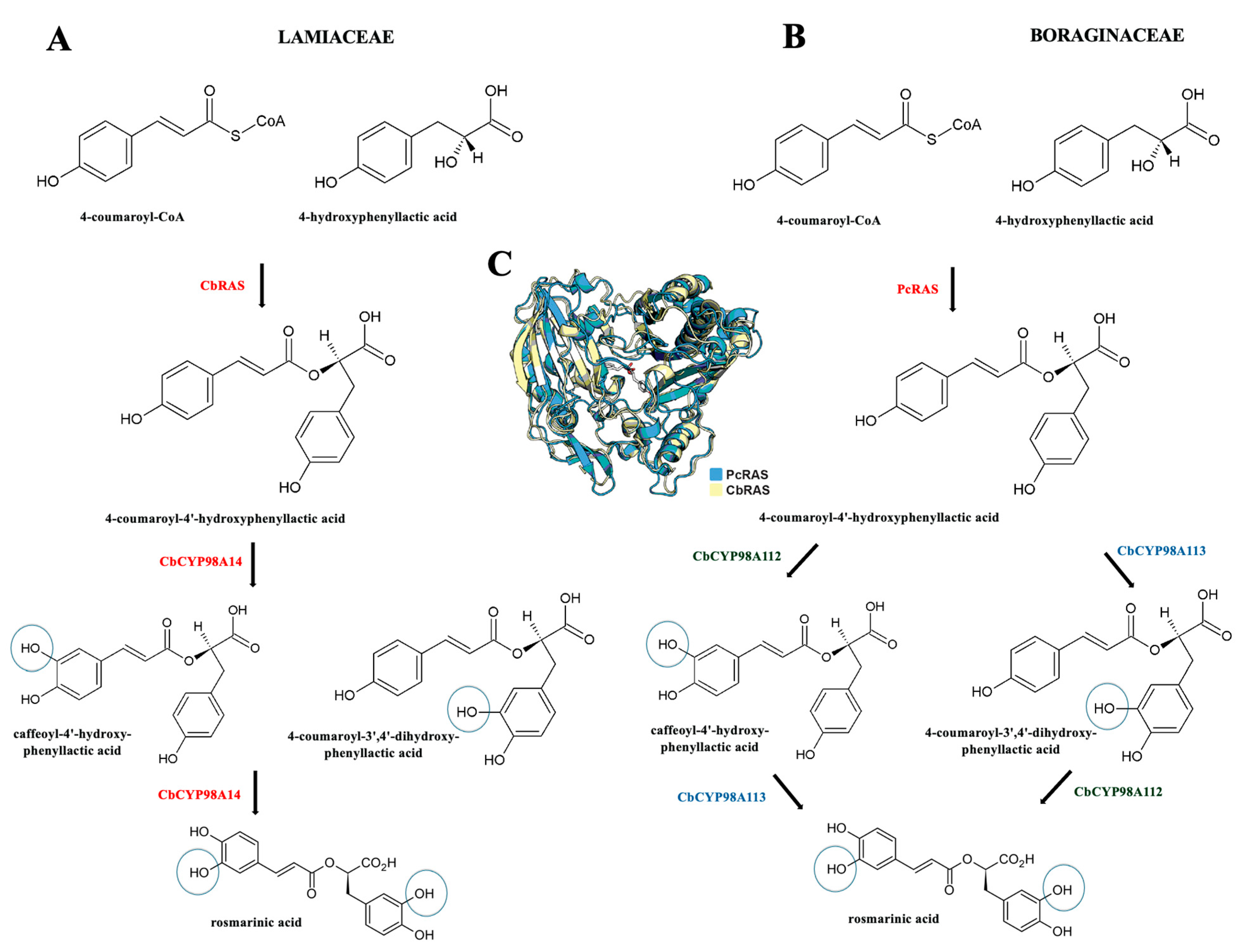
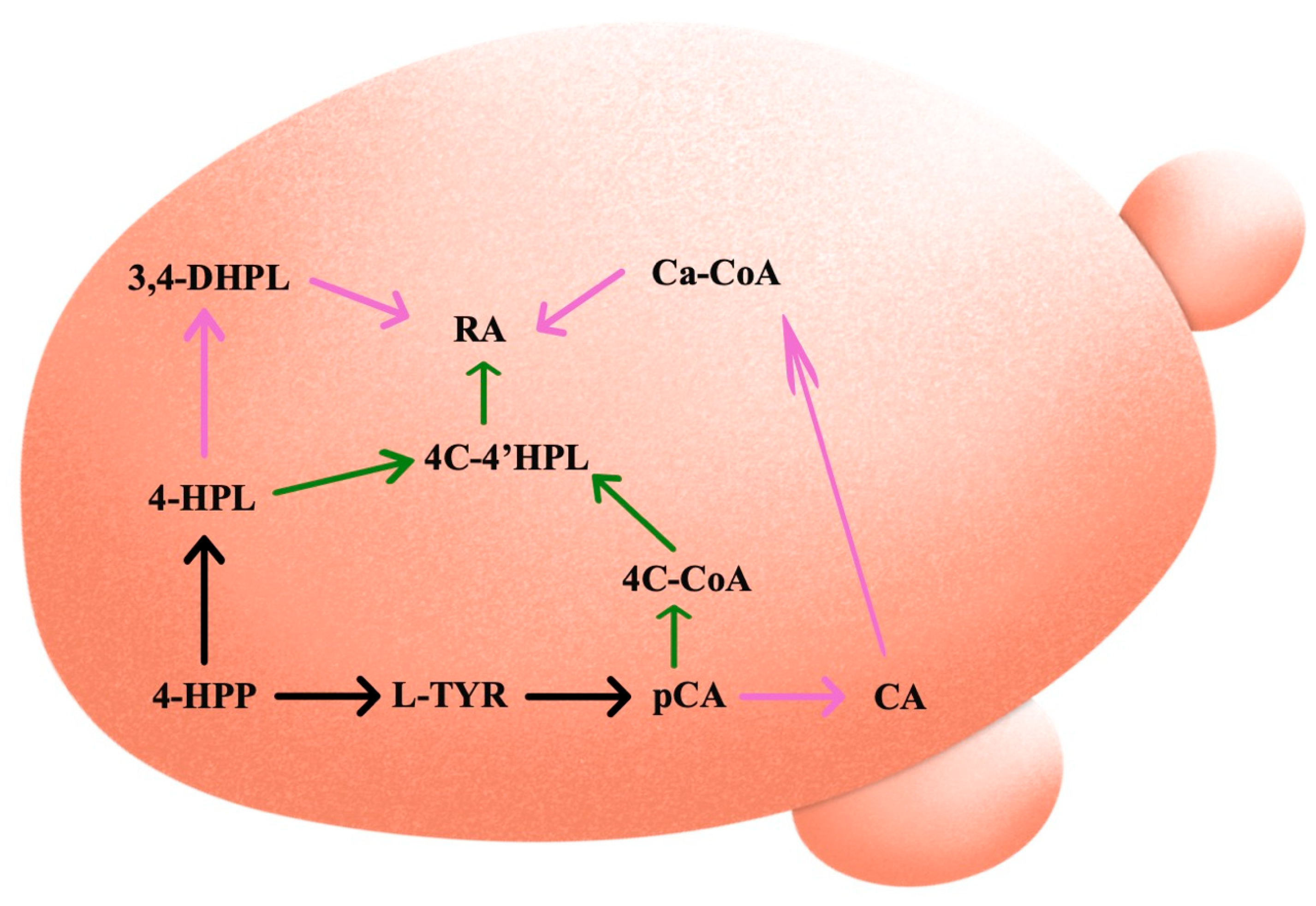
| Plant Species | Elicitor Used | Culture Conditions | Experimental Outcomes | References |
|---|---|---|---|---|
| Salvia officinalis Salvia verticillata | 50 μM MeJA and 15 μM AgNO3 | Foliar application of elicitors | MeJA and Ag+ influenced the expression of the key genes PAL, TAT, HPPR, RAS, and CYP98A14 in both phenylpropanoid and tyrosine pathways | [67] |
| Melissa officinalis | 150 μM MeJA | Aerial plant parts at vegetative development stage were sprayed | RA accumulation was associated with the transcript level of MoPAL, Mo4CL, and MoRAS | [68] |
| Prunella vulgaris | 100 μM MeJA | Hairy root culture | RA accumulation linked with transcript expression of PvPAL, PvHPPR, PVC4H, PvCL1, PvCL2, and PvCYP98A101 | [69] |
| Salvia yangii Salvia abrotanoides | 150 μM MeJA | Aerial plant parts at vegetative development stage were sprayed | 1.66- and 1.54-fold increase in RA content due to the increased number of RAS, 4CL, and PAL transcripts | [70] |
| Satureja khuzistanica | 100 μM MeJA | Cell suspension culture | Elicitor tripled RA production (3.9 g L−1) | [71] |
| Lepechinia caulescens | 300 μM MeJA | Hairy root culture | The highest concentration of RA was 24 h after elicitor treatment | [72] |
| Agastache rugosa | 50 μM MeJA | Cell cultures | Increased transcript levels of ArPAL, Ar4CL, and ArC4H | [73] |
| Mentha × piperita | 100 μM MeJA and 200 μM jasmonic acid | Cell suspension culture | 1.5 times higher RA concentration (117.95 mg g−1 DW and 110.12 mg g−1 DW) than in elicitor-free culture | [74] |
| Salvia miltiorrhiza | 50 μM MeJA | transgenic lines with hairy roots | SmMYB1 overexpression increased RA accumulation | [75] |
| Origanum vulgare | 0.1 mM MeJA, and Azotobacter chroococcum, Azospirillium brasilense, Pseudomonas fluorescens consortium | MeJA solutions were sprayed on aerial parts of the plants | The expression of RAS and C4H genes increased 3.37 and 6.6 times, respectively | [76] |
| Salvia nemorosa | 0.5 μM SA | Callus culture | 8-fold increase in RA content compared to the control | [77] |
| Salvia miltiorrhiza | 0.16 mM SA | Cell cultures | SA up-regulated the expression of TAT, PAL, and RAS and enhanced the RA accumulation | [78] |
| Thymus membranaceus | 10 μM SA | In vitro shoot culture | Increased RA and phenolic levels | [79] |
| Salvia officinalis Salvia virgata | 250 and 500 μM SA | In vitro shoot culture | Up-regulation of SoPAL and SvPAL caused by elicitor treatments | [80] |
| Salvia miltiorrhiza | SA and H2O2 | Cell cultures | Synergistic effects of applied elicitors positively influenced RA accumulation and PAL activity | [81] |
| Thymus vulgaris | 3 mM SA | Drought-induced stress | Increased RA accumulation | [82] |
| Melissa officinalis | 100 mg L−1 SA | Foliar application of elicitor | The application of SA in combination with chitosan lactate had the strongest impact on RA accumulation | [83] |
| Agastache rugosa | 500 mg L−1 YE and 30 mg L−1 silver nitrate | Cell suspension culture | 4.98 mg L−1 of RA after YE elicitation with several times higher transcript levels of HPPR | [84] |
| Melisa officinalis | 0.1% YE | Elicitor applied on 30-days old seedlings | The highest TAT gene expression in relation to RA accumulation | [85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakovljević, D.; Warchoł, M.; Skrzypek, E. Rosmarinic Acid as Bioactive Compound: Molecular and Physiological Aspects of Biosynthesis with Future Perspectives. Cells 2025, 14, 850. https://doi.org/10.3390/cells14110850
Jakovljević D, Warchoł M, Skrzypek E. Rosmarinic Acid as Bioactive Compound: Molecular and Physiological Aspects of Biosynthesis with Future Perspectives. Cells. 2025; 14(11):850. https://doi.org/10.3390/cells14110850
Chicago/Turabian StyleJakovljević, Dragana, Marzena Warchoł, and Edyta Skrzypek. 2025. "Rosmarinic Acid as Bioactive Compound: Molecular and Physiological Aspects of Biosynthesis with Future Perspectives" Cells 14, no. 11: 850. https://doi.org/10.3390/cells14110850
APA StyleJakovljević, D., Warchoł, M., & Skrzypek, E. (2025). Rosmarinic Acid as Bioactive Compound: Molecular and Physiological Aspects of Biosynthesis with Future Perspectives. Cells, 14(11), 850. https://doi.org/10.3390/cells14110850








