Heterochannels Kv(1.1-1.2)2 and Their Interactions with Pore Blockers
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents, Recombinant Peptides, and Proteins
2.2. Expression Plasmids
2.3. Cells and Experiments with Cells
2.4. Confocal Microscopy
2.5. Quantitative Analysis of Interactions Between Heterochannels and Ligands
2.6. Electrophysiology Measurements
3. Results
3.1. Design of Concatemers K-Kv1.1-1.2 and K-Kv1.2-1.1
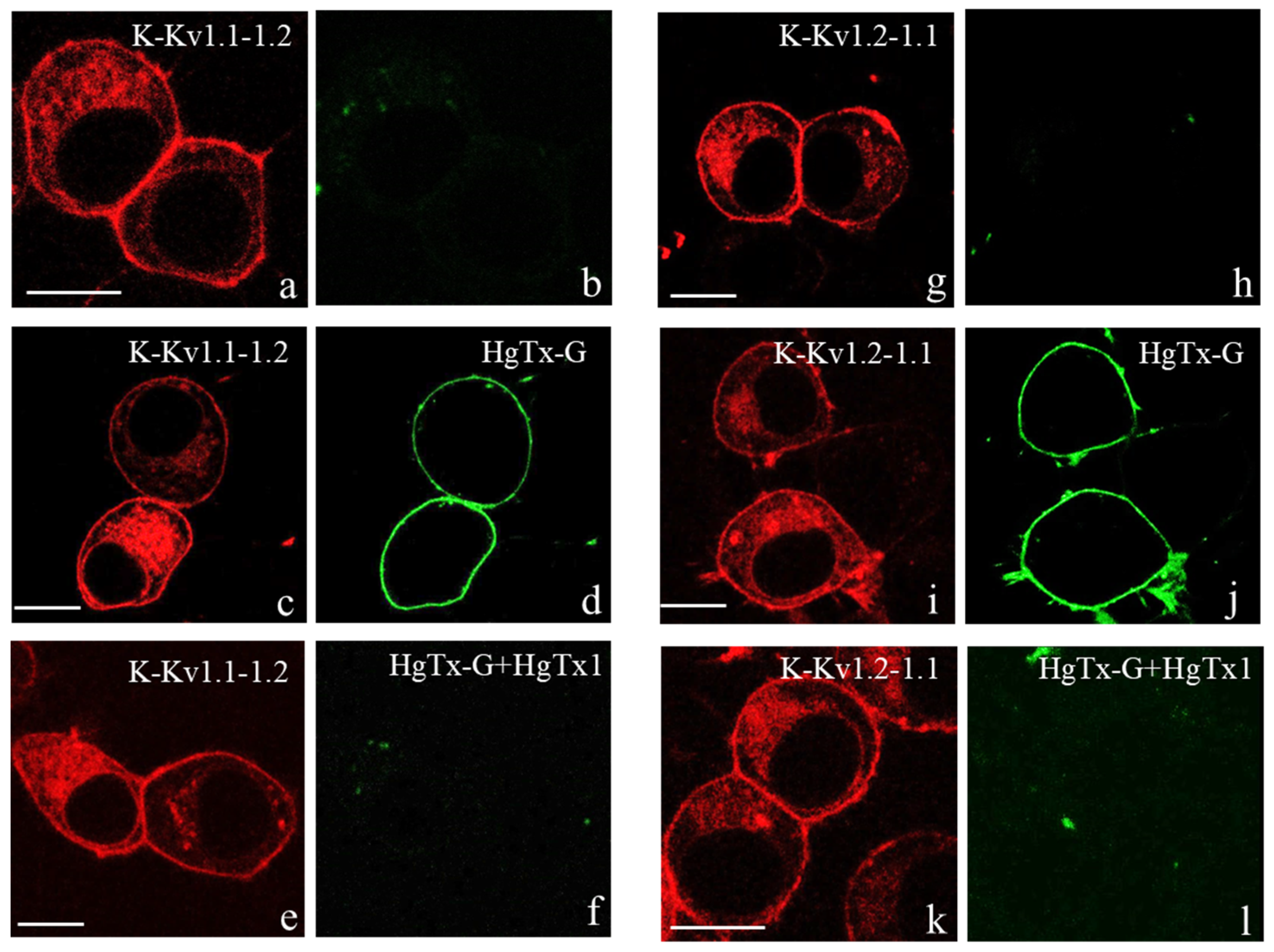
3.2. Expression and Distribution of Concatemers K-Kv1.1-1.2 and K-Kv1.2-1.1 in Neuro-2a Cells
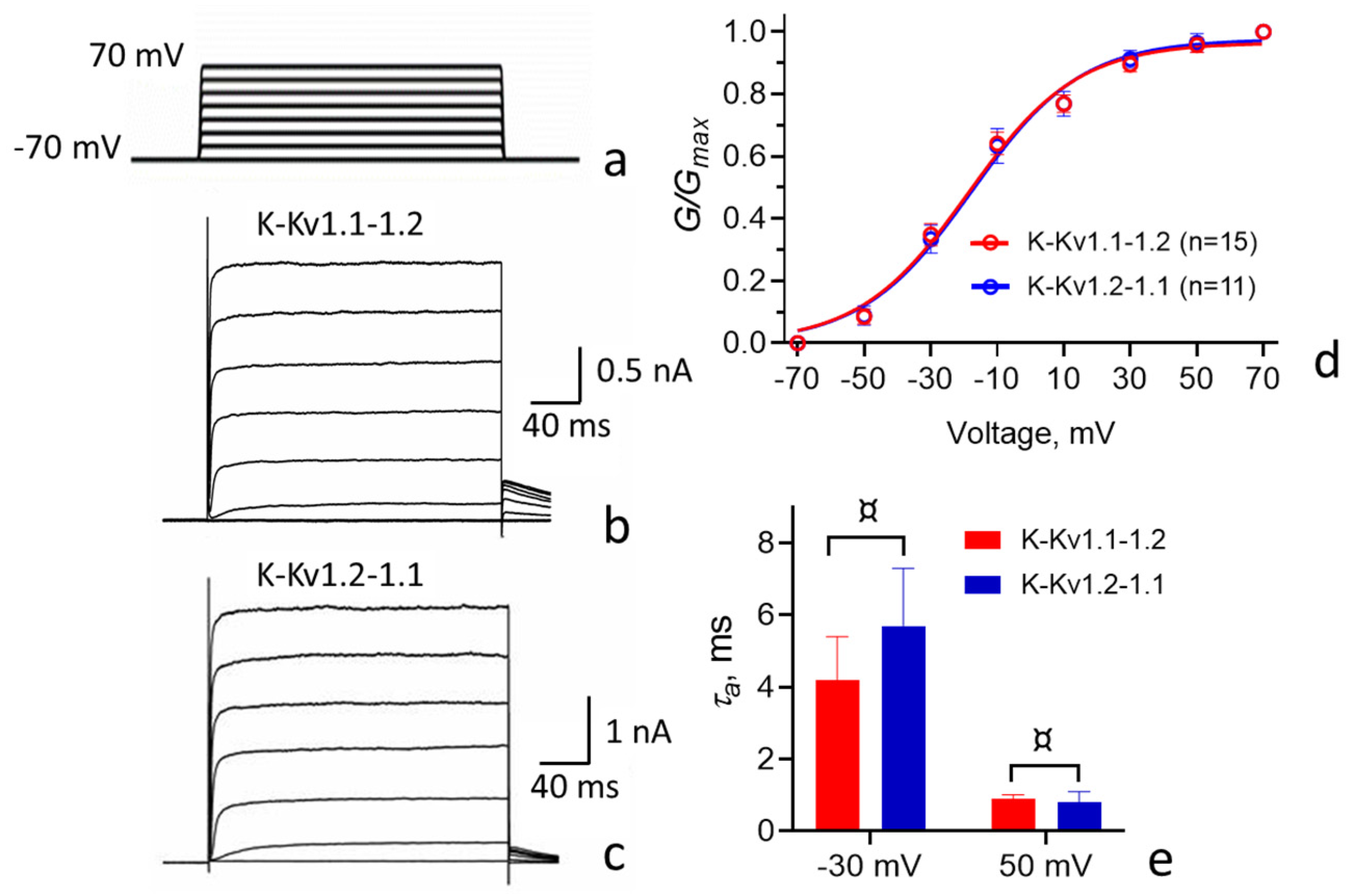
3.3. Properties of Heterochannels Formed by Concatemers K-Kv1.1-1.2 and K-Kv1.2-1.1
3.4. Interactions of HgTx-G and Some Peptide Blockers with Heterochannels
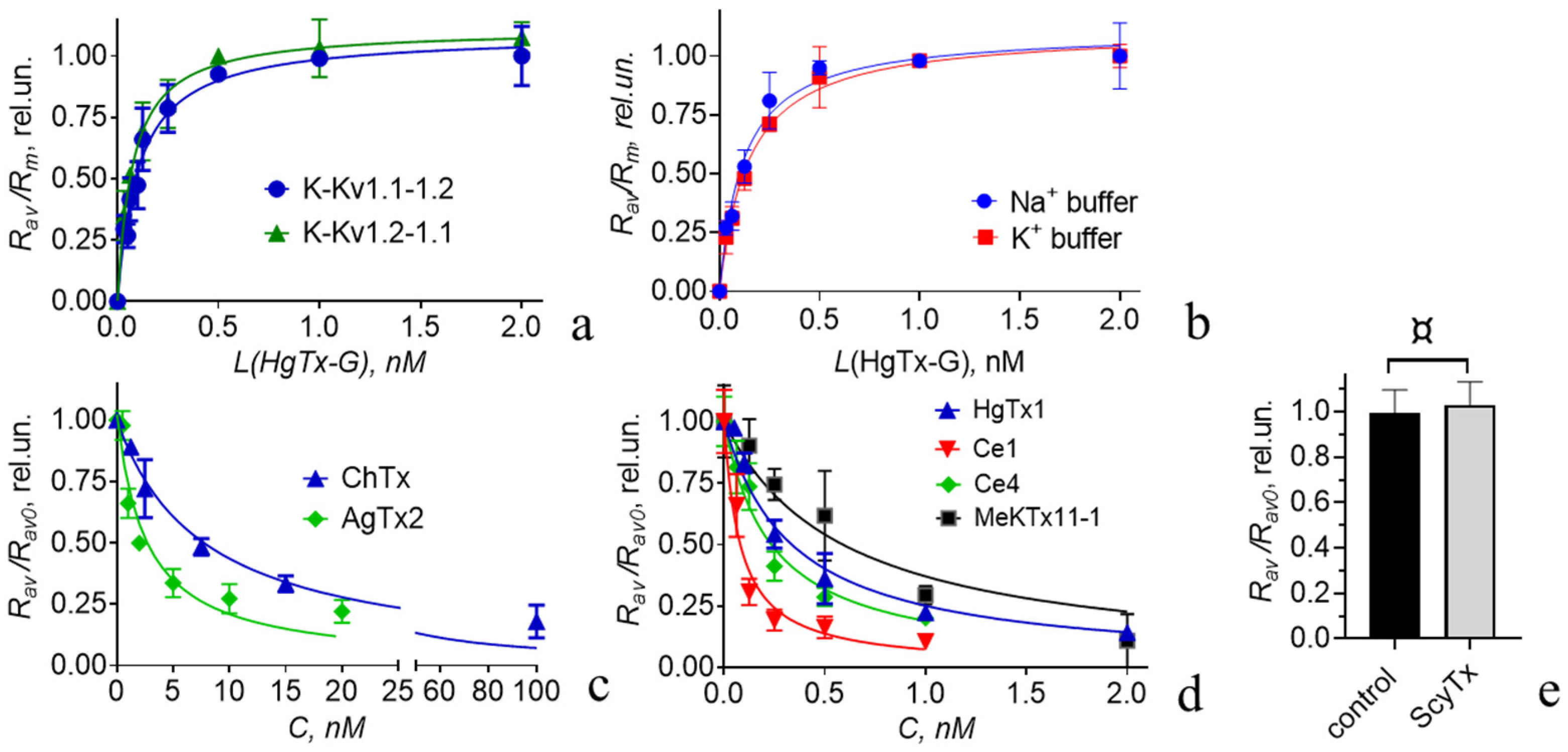
3.4.1. Interactions of HgTx-G with Heterochannels
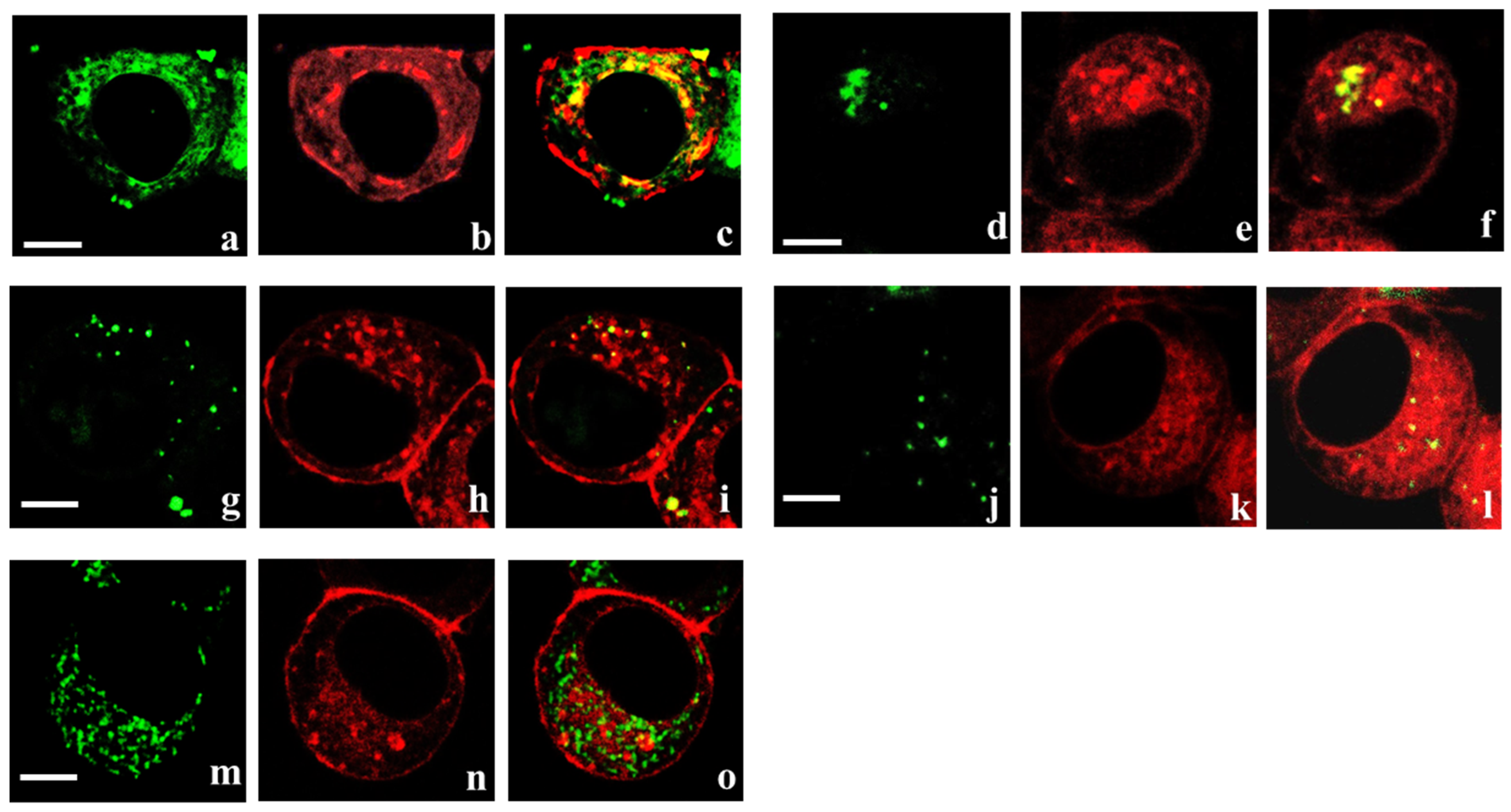
3.4.2. Interactions of Some Recombinant Peptide Blockers with Heterochannels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AgTx2 | agitoxin 2 |
| ChTx | charybdotoxin |
| CNS | central nervous system |
| DTX | dendrotoxin |
| HgTx1 | hongotoxin 1 |
| HgTx-G | hongotoxin 1 fused with fluorescent protein GFP |
| ScyTx | Scyllatoxin |
| K-Kv1.1 | Kv1.1 α-subunit fused with fluorescent protein mKate2 |
| K-Kv1.1-1.2 | concatenated dimer Kv1.1-Kv1.2 fused with mKate2 |
| K-Kv1.2 | Kv1.2 α-subunit fused with mKate2 |
| K-Kv1.2-1.1 | concatenated dimer Kv1.2-Kv1.1 fused with mKate2 |
| Kv(1.1-1.2)2 | heterochannels formed by concatemers Kv1.1-Kv1.2 fused with mKate2 |
References
- Gonzalez, C.; Baez-Nieto, D.; Valencia, I.; Oyarzun, I.; Rojas, P.; Naranjo, D.; Latorre, R. K+ Channels: Function-Structural Overview. Compr. Physiol. 2012, 2, 2087–2149. [Google Scholar] [CrossRef] [PubMed]
- Pinatel, D.; Faivre-Sarrailh, C. Assembly and Function of the Juxtaparanodal Kv1 Complex in Health and Disease. Life 2020, 11, 8. [Google Scholar] [CrossRef]
- Kuzmenkov, A.I.; Grishin, E.V.; Vassilevski, A.A. Diversity of Potassium Channel Ligands: Focus on Scorpion Toxins. Biochemistry 2015, 80, 1764–1799. [Google Scholar] [CrossRef]
- Strang, C.; Cushman, S.J.; DeRubeis, D.; Peterson, D.; Pfaffinger, P.J. A Central Role for the T1 Domain in Voltage-Gated Potassium Channel Formation and Function. J. Biol. Chem. 2001, 276, 28493–28502. [Google Scholar] [CrossRef]
- Kurata, H.T.; Fedida, D. A Structural Interpretation of Voltage-Gated Potassium Channel Inactivation. Prog. Biophys. Mol. Biol. 2006, 92, 185–208. [Google Scholar] [CrossRef]
- Li, D.; Takimoto, K.; Levitan, E.S. Surface Expression of Kv1 Channels Is Governed by a C-Terminal Motif. J. Biol. Chem. 2000, 275, 11597–11602. [Google Scholar] [CrossRef]
- Trimmer, J.S.; Rhodes, K.J. Localization of Voltage-Gated Ion Channels in Mammalian Brain. Annu. Rev. Physiol. 2004, 66, 477–519. [Google Scholar] [CrossRef] [PubMed]
- Tiffany, A.M.; Manganas, L.N.; Kim, E.; Hsueh, Y.P.; Sheng, M.; Trimmer, J.S. PSD-95 and SAP97 Exhibit Distinct Mechanisms for Regulating K+ Channel Surface Expression and Clustering. J. Cell Biol. 2000, 148, 147–157. [Google Scholar] [CrossRef]
- Poliak, S.; Gollan, L.; Martinez, R.; Custer, A.; Einheber, S.; Salzer, J.L.; Trimmer, J.S.; Shrager, P.; Peles, E. Caspr2, a New Member of the Neurexin Superfamily, Is Localized at the Juxtaparanodes of Myelinated Axons and Associates with K+ Channels. Neuron 1999, 24, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- Schulte, U.; Thumfart, J.O.; Klöcker, N.; Sailer, C.A.; Bildl, W.; Biniossek, M.; Dehn, D.; Deller, T.; Eble, S.; Abbass, K.; et al. The Epilepsy-Linked Lgi1 Protein Assembles into Presynaptic Kv1 Channels and Inhibits Inactivation by Kvβ1. Neuron 2006, 49, 697–706. [Google Scholar] [CrossRef]
- Isacoff, E.Y.; Jan, Y.N.; Jan, L.Y. Evidence for the Formation of Heteromultimeric Potassium Channels in Xenopus Oocytes. Nature 1990, 345, 530–534. [Google Scholar] [CrossRef]
- Shamotienko, O.; Akhtar, S.; Sidera, C.; Meunier, F.A.; Ink, B.; Weir, M.; Dolly, J.O. Recreation of Neuronal Kv1 Channel Oligomers by Expression in Mammalian Cells Using Semliki Forest Virus. Biochemistry 1999, 38, 16766–16776. [Google Scholar] [CrossRef] [PubMed]
- Scott, V.E.S.; Muniz, Z.M.; Sewing, S.; Lichtinghagen, R.; Parcej, D.N.; Pongs, O.; Dolly, J.O. Antibodies Specific for Distinct Kv Subunits Unveil a HeteroOligomeric Basis for Subtypes of α-Dendrotoxin-Sensitive K+ Channels in Bovine Brain. Biochemistry 1994, 33, 1617–1623. [Google Scholar] [CrossRef] [PubMed]
- Koschak, A.; Bugianesi, R.M.; Mitterdorfer, J.; Kaczorowski, G.J.; Garcia, M.L.; Knaus, H.G. Subunit Composition of Brain Voltage-Gated Potassium Channels Determined by Hongotoxin-1, a Novel Peptide Derived from Centruroides Limbatus Venom. J. Biol. Chem. 1998, 273, 2639–2644. [Google Scholar] [CrossRef]
- Bagchi, B.; Al-Sabi, A.; Kaza, S.; Scholz, D.; O’Leary, V.B.; Dolly, J.O.; Ovsepian, S.V. Disruption of Myelin Leads to Ectopic Expression of K(V)1.1 Channels with Abnormal Conductivity of Optic Nerve Axons in a Cuprizone-Induced Model of Demyelination. PLoS ONE 2014, 9, e87736. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Kunkel, D.D.; Martin, T.M.; Schwartzkroin, P.A.; Tempel, B.L. Heteromultimeric K+ Channels in Terminal and Juxtaparanodal Regions of Neurons. Nature 1993, 365, 75–79. [Google Scholar] [CrossRef]
- Shamotienko, O.G.; Parcej, D.N.; Dolly, J.O. Subunit Combinations Defined for K+ Channel Kv1 Subtypes in Synaptic Membranes from Bovine Brain. Biochemistry 1997, 36, 8195–8201. [Google Scholar] [CrossRef]
- Wang, F.C.; Parcej, D.N.; Dolly, J.O. Alpha Subunit Compositions of Kv1.1-Containing K+ Channel Subtypes Fractionated from Rat Brain Using Dendrotoxins. Eur. J. Biochem. 1999, 263, 230–237. [Google Scholar] [CrossRef]
- Meneses, D.; Vega, A.V.; Torres-Cruz, F.M.; Barral, J. KV1 and KV3 Potassium Channels Identified at Presynaptic Terminals of the Corticostriatal Synapses in Rat. Neural Plast. 2016, 2016, 8782518. [Google Scholar] [CrossRef]
- Sharma, K.; Kang, K.W.; Seo, Y.W.; Glowatzki, E.; Yi, E. Low-Voltage Activating K+ Channels in Cochlear Afferent Nerve Fiber Dendrites. Exp. Neurobiol. 2022, 31, 243–259. [Google Scholar] [CrossRef]
- Norton, R.S.; Chandy, K.G. Venom-Derived Peptide Inhibitors of Voltage-Gated Potassium Channels. Neuropharmacology 2017, 127, 124–138. [Google Scholar] [CrossRef] [PubMed]
- Koch, R.O.; Wanner, S.G.; Koschak, A.; Hanner, M.; Schwarzer, C.; Kaczorowski, G.J.; Slaughter, R.S.; Garcia, M.L.; Knaus, H.G. Complex Subunit Assembly of Neuronal Voltage-Gated K+ Channels. Basis for High-Affinity Toxin Interactions and Pharmacology. J. Biol. Chem. 1997, 272, 27577–27581. [Google Scholar] [CrossRef]
- Dodson, P.D.; Barker, M.C.; Forsythe, I.D. Two Heteromeric Kv1 Potassium Channels Differentially Regulate Action Potential Firing. J. Neurosci. 2002, 22, 6953–6961. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, S.; Shamotienko, O.; Papakosta, M.; Ali, F.; Oliver Dolly, J. Characteristics of Brain Kv1 Channels Tailored to Mimic Native Counterparts by Tandem Linkage of Alpha Subunits: Implications for K+ Channelopathies. J. Biol. Chem. 2002, 277, 16376–16382. [Google Scholar] [CrossRef]
- Sokolov, M.V.; Shamotienko, O.; Dhochartaigh, S.N.; Sack, J.T.; Dolly, J.O. Concatemers of Brain Kv1 Channel α Subunits That Give Similar K+ Currents Yield Pharmacologically Distinguishable Heteromers. Neuropharmacology 2007, 53, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Al-Sabi, A.; Shamotienko, O.; Ni Dhochartaigh, S.; Muniyappa, N.; Le Berre, M.; Shaban, H.; Wang, J.; Sack, J.T.; Oliver Dolly, J. Arrangement of Kv1 Alpha Subunits Dictates Sensitivity to Tetraethylammonium. J. Gen. Physiol. 2010, 136, 273–282. [Google Scholar] [CrossRef]
- Al-Sabi, A.; Kaza, S.K.; Oliver Dolly, J.; Wang, J. Pharmacological Characteristics of Kv1.1- and Kv1.2-Containing Channels Are Influenced by the Stoichiometry and Positioning of Their α Subunits. Biochem. J. 2013, 454, 101–108. [Google Scholar] [CrossRef]
- Kryukova, E.V.; Efremenko, A.V.; Kazakov, O.V.; Feofanov, A.V.; Nekrasova, O.V. The Properties of a Fluorescent Kv1.2 Channel Assembled from Concatemers of Alpha-Subunits. Biophysics 2025, 70, 86–92. [Google Scholar] [CrossRef]
- Efremenko, A.V.; Kryukova, E.V.; Kazakov, O.V.; Kirpichnikov, M.P.; Nekrasova, O.V.; Feofanov, A.V. Properties of Potassium Channel Kv1.1 on the Basis of Fluorescent Dimer of Alpha-Subunits MKate2-Kv1.1-Kv1.1 in Neuro-2a Cells. Russ. J. Bioorg. Chem. 2025, 51. in press. [Google Scholar]
- Zhu, J.; Watanabe, I.; Gomez, B.; Thornhill, W.B. Heteromeric Kv1 Potassium Channel Expression. Amino Acid Determinants Involved in Processing and Trafficking to the Cell Surface. J. Biol. Chem. 2003, 278, 25558–25567. [Google Scholar] [CrossRef]
- Wang, W.; Kim, H.J.; Lv, P.; Tempel, B.; Yamoah, E.N. Association of the Kv1 Family of K+ Channels and Their Functional Blueprint in the Properties of Auditory Neurons as Revealed by Genetic and Functional Analyses. J. Neurophysiol. 2013, 110, 1751–1764. [Google Scholar] [CrossRef][Green Version]
- Cordeiro, S.; Finol-Urdaneta, R.K.; Köpfer, D.; Markushina, A.; Song, J.; French, R.J.; Kopec, W.; De Groot, B.L.; Giacobassi, M.J.; Leavitt, L.S.; et al. Conotoxin ΚM-RIIIJ, a Tool Targeting Asymmetric Heteromeric K v 1 Channels. Proc. Natl. Acad. Sci. USA 2019, 116, 1059–1064. [Google Scholar] [CrossRef]
- Orlov, N.A.; Ignatova, A.A.; Kryukova, E.V.; Yakimov, S.A.; Kirpichnikov, M.P.; Nekrasova, O.V.; Feofanov, A.V. Combining MKate2-Kv1.3 Channel and Atto488-Hongotoxin for the Studies of Peptide Pore Blockers on Living Eukaryotic Cells. Toxins 2022, 14, 858. [Google Scholar] [CrossRef]
- Orlov, N.A.; Kryukova, E.V.; Efremenko, A.V.; Yakimov, S.A.; Toporova, V.A.; Kirpichnikov, M.P.; Nekrasova, O.V.; Feofanov, A.V. Interactions of the Kv1.1 Channel with Peptide Pore Blockers: A Fluorescent Analysis on Mammalian Cells. Membranes 2023, 13, 645. [Google Scholar] [CrossRef] [PubMed]
- Ignatova, A.A.; Kryukova, E.V.; Novoseletsky, V.N.; Kazakov, O.V.; Orlov, N.A.; Korabeynikova, V.N.; Larina, M.V.; Fradkov, A.F.; Yakimov, S.A.; Kirpichnikov, M.P.; et al. New High-Affinity Peptide Ligands for Kv1.2 Channel: Selective Blockers and Fluorescent Probes. Cells 2024, 13, 2096. [Google Scholar] [CrossRef] [PubMed]
- Nekrasova, O.; Kudryashova, K.; Fradkov, A.; Yakimov, S.; Savelieva, M.; Kirpichnikov, M.; Feofanov, A. Straightforward Approach to Produce Recombinant Scorpion Toxins—Pore Blockers of Potassium Channels. J. Biotechnol. 2017, 241, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Orlov, N.A.; Yakimov, S.A.; Nekrasova, O.V.; Feofanov, A.V. Recombinant Peptides Ce1 and Ce4 from the Venom of Scorpion Centruroides Elegans and Their Interactions with Hybrid Channels KcsA-Kv1.x (x = 1, 3, 6). Mosc. Univ. Biol. Sci. Bull. 2022, 77, 119–125. [Google Scholar] [CrossRef]
- Kuzmenkov, A.I.; Nekrasova, O.V.; Peigneur, S.; Tabakmakher, V.M.; Gigolaev, A.M.; Fradkov, A.F.; Kudryashova, K.S.; Chugunov, A.O.; Efremov, R.G.; Tytgat, J.; et al. K V 1.2 Channel-Specific Blocker from Mesobuthus Eupeus Scorpion Venom: Structural Basis of Selectivity. Neuropharmacology 2018, 143, 228–238. [Google Scholar] [CrossRef]
- Solé, L.; Sastre, D.; Colomer-Molera, M.; Vallejo-Gracia, A.; Roig, S.R.; Pérez-Verdaguer, M.; Lillo, P.; Tamkun, M.M.; Felipe, A. Functional Consequences of the Variable Stoichiometry of the Kv1.3-KCNE4 Complex. Cells 2020, 9, 1128. [Google Scholar] [CrossRef]
- Zhu, J.; Gomez, B.; Watanabe, I.; Thornhill, W.B. Amino Acids in the Pore Region of Kv1 Potassium Channels Dictate Cell-Surface Protein Levels: A Possible Trafficking Code in the Kv1 Subfamily. Biochem. J. 2005, 388, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Racapé, J.; Lecoq, A.; Romi-Lebrun, R.; Liu, J.; Kohler, M.; Garcia, M.L.; Ménez, A.; Gasparini, S. Characterization of a Novel Radiolabeled Peptide Selective for a Subpopulation of Voltage-Gated Potassium Channels in Mammalian Brain. J. Biol. Chem. 2002, 277, 3886–3893. [Google Scholar] [CrossRef] [PubMed]
- Efremenko, A.V.; Nekrasova, O.V.; Feofanov, A.V. Formation of Heterotetrameric Potassium Channels Kv1.1-Kv1.2 in Neuro-2A Cells: Analysis by the Förster Resonance Energy Transfer Technique. Biophysics 2025, 70, 69–75. [Google Scholar] [CrossRef]
- Ranjan, R.; Logette, E.; Marani, M.; Herzog, M.; Tâche, V.; Scantamburlo, E.; Buchillier, V.; Markram, H. A Kinetic Map of the Homomeric Voltage-Gated Potassium Channel (Kv) Family. Front. Cell Neurosci. 2019, 13, 358. [Google Scholar] [CrossRef]
- Shakkottai, V.G.; Regaya, I.; Wulff, H.; Fajloun, Z.; Tomita, H.; Fathallah, M.; Cahalan, M.D.; Gargus, J.J.; Sabatier, J.M.; Chandy, K.G. Design and Characterization of a Highly Selective Peptide Inhibitor of the Small Conductance Calcium-Activated K+ Channel, SkCa2. J. Biol. Chem. 2001, 276, 43145–43151. [Google Scholar] [CrossRef]
- Garcia, M.L.; Garcia-Calvo, M.; Hidalgo, P.; Lee, A.; MacKinnon, R. Purification and Characterization of Three Inhibitors of Voltage-Dependent K+ Channels from Leiurus Quinquestriatus Var. Hebraeus Venom. Biochemistry 1994, 33, 6834–6839. [Google Scholar] [CrossRef]
- Kuzmenkov, A.I.; Nekrasova, O.V.; Kudryashova, K.S.; Peigneur, S.; Tytgat, J.; Stepanov, A.V.; Kirpichnikov, M.P.; Grishin, E.V.; Feofanov, A.V.; Vassilevski, A.A. Fluorescent Protein-Scorpion Toxin Chimera Is a Convenient Molecular Tool for Studies of Potassium Channels. Sci. Rep. 2016, 6, 33314. [Google Scholar] [CrossRef] [PubMed]
- Anangi, R.; Koshy, S.; Huq, R.; Beeton, C.; Chuang, W.J.; King, G.F. Recombinant Expression of Margatoxin and Agitoxin-2 in Pichia Pastoris: An Efficient Method for Production of KV1.3 Channel Blockers. PLoS ONE 2012, 7, e52965. [Google Scholar] [CrossRef] [PubMed]
- Mourre, C.; Chernova, M.N.; Martin-Eauclaire, M.F.; Bessone, R.; Jacquet, G.; Gola, M.; Alper, S.L.; Crest, M. Distribution in Rat Brain of Binding Sites of Kaliotoxin, a Blocker of Kv1.1 and Kv1.3 Alpha-Subunits. J. Pharmacol. Exp. Ther. 1999, 291, 943–952. [Google Scholar] [CrossRef]
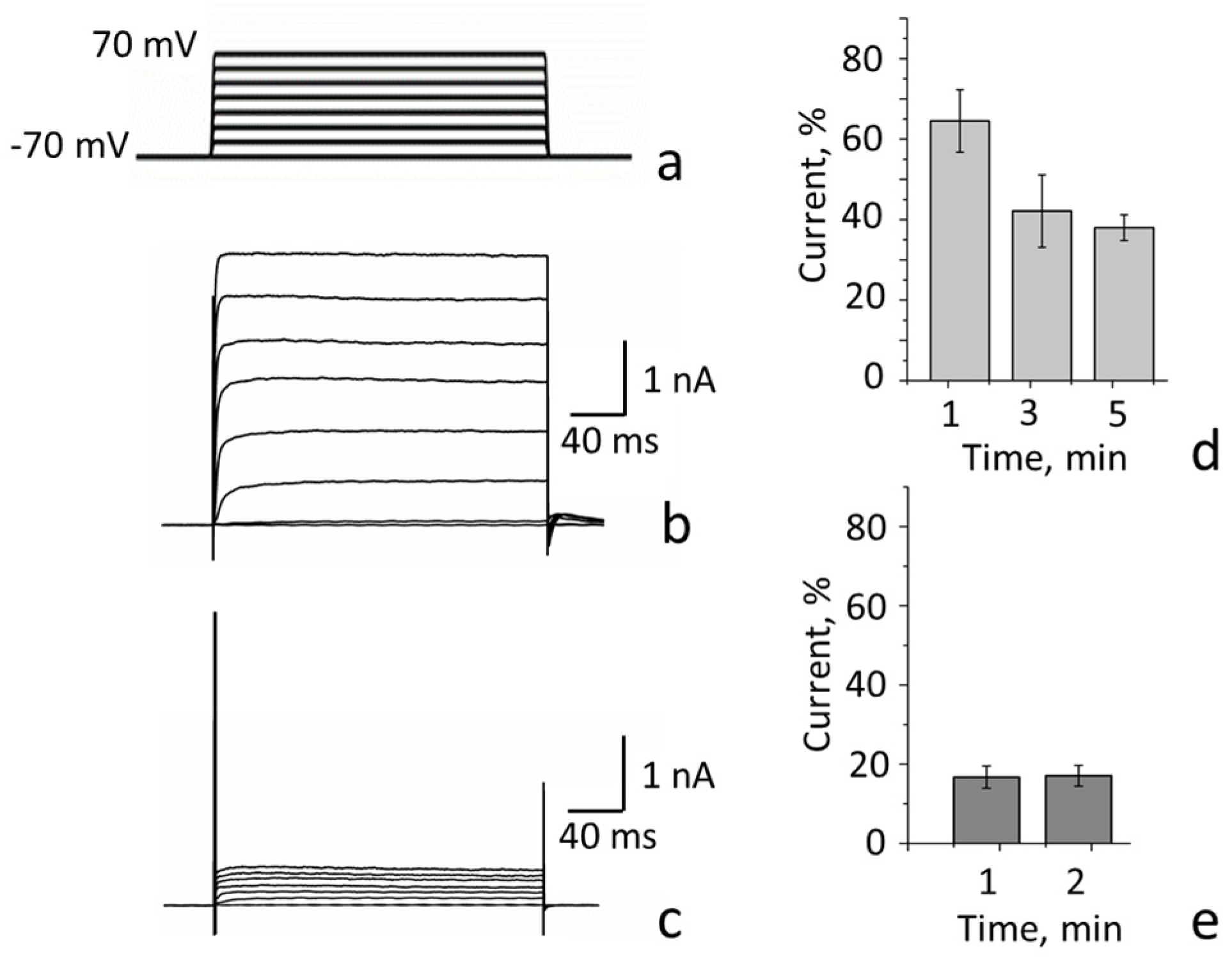
| Channel | Kv(1.1-1.2)2 (n = 14) # | Kv(1.1-1.2)2 (n = 11) | Kv1.1 * (n = 12) | Kv1.2 ** (n = 16) |
|---|---|---|---|---|
| Subunit | K-Kv1.1-1.2 | K-Kv1.2-1.1 | K-Kv1.1 | K-Kv1.2 |
| τa(−30 mV &), ms | 4.2 ± 1.2 | 5.7 ± 1.6 ¤ | 4.0 ± 0.5 ¤ | 11 ± 2 ¶ |
| τa(50 mV), ms | 0.89 ± 0.13 | 0.8 ± 0.3 ¤ | 0.75 ± 0.10 ¤ | 1.2 ± 0.4 ¤ |
| V1/2, mV | −17 ± 2 | −16 ± 2 ¤ | −20 ± 2 ¤ | −5 ± 2 ¶ |
| k | 18 ± 2 | 18 ± 2 ¤ | 17 ± 2 ¤ | 17 ± 2 ¤ |
| Channel | HgTx1 | AgTx2 | ChTx | Ce1 | Ce4 | MeKTx11-1 |
|---|---|---|---|---|---|---|
| Kv(1.1-1.2)2 | 0.04 ± 0.01 | 0.25 ± 0.05 | 0.8 ± 0.2 | 0.014 ± 0.006 | 0.0328 ± 0.0009 | 0.13 ± 0.05 |
| Kv1.1 | 0.03 ± 0.02 *,¤ | 1.5 ± 0.7 *,¤ | >>100 *,¶ | 11 ±5 *,¶ | >300 #,¶ | 2110 ** |
| Kv1.2 | 0.02 ± 0.01 #,¤ | 21.5 ± 1.2 #,¶ | 1.0 ± 0.1 #,¤ | 0.010 ± 0.002 #,¤ | 0.030 ± 0.010 #,¤ | 0.19 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Efremenko, A.V.; Kryukova, E.V.; Kazakov, O.V.; Ignatova, A.A.; Shmatin, I.I.; Korabeynikova, V.N.; Toporova, V.A.; Yakimov, S.A.; Kirpichnikov, M.P.; Nekrasova, O.V.; et al. Heterochannels Kv(1.1-1.2)2 and Their Interactions with Pore Blockers. Cells 2025, 14, 1364. https://doi.org/10.3390/cells14171364
Efremenko AV, Kryukova EV, Kazakov OV, Ignatova AA, Shmatin II, Korabeynikova VN, Toporova VA, Yakimov SA, Kirpichnikov MP, Nekrasova OV, et al. Heterochannels Kv(1.1-1.2)2 and Their Interactions with Pore Blockers. Cells. 2025; 14(17):1364. https://doi.org/10.3390/cells14171364
Chicago/Turabian StyleEfremenko, Anastasija V., Elena V. Kryukova, Oleg V. Kazakov, Anastasia A. Ignatova, Ivan I. Shmatin, Varvara N. Korabeynikova, Victoria A. Toporova, Sergey A. Yakimov, Mikhail P. Kirpichnikov, Oksana V. Nekrasova, and et al. 2025. "Heterochannels Kv(1.1-1.2)2 and Their Interactions with Pore Blockers" Cells 14, no. 17: 1364. https://doi.org/10.3390/cells14171364
APA StyleEfremenko, A. V., Kryukova, E. V., Kazakov, O. V., Ignatova, A. A., Shmatin, I. I., Korabeynikova, V. N., Toporova, V. A., Yakimov, S. A., Kirpichnikov, M. P., Nekrasova, O. V., & Feofanov, A. V. (2025). Heterochannels Kv(1.1-1.2)2 and Their Interactions with Pore Blockers. Cells, 14(17), 1364. https://doi.org/10.3390/cells14171364








