Pharmacoepigenomic Impact of Antihypertensive Drugs on miRNome and Proteome and Its Potential Influence on Health and Side Effects
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Cell Culture
2.3. Antihypertensive Drug Treatment
2.4. RNA Extraction and Small RNA Sequencing
2.5. Protein Isolation and Whole Proteome Analysis
2.6. Integrated Analysis of the Coexpression Network of miRNA Target Genes and Target Proteins from Proteome Data
3. Results
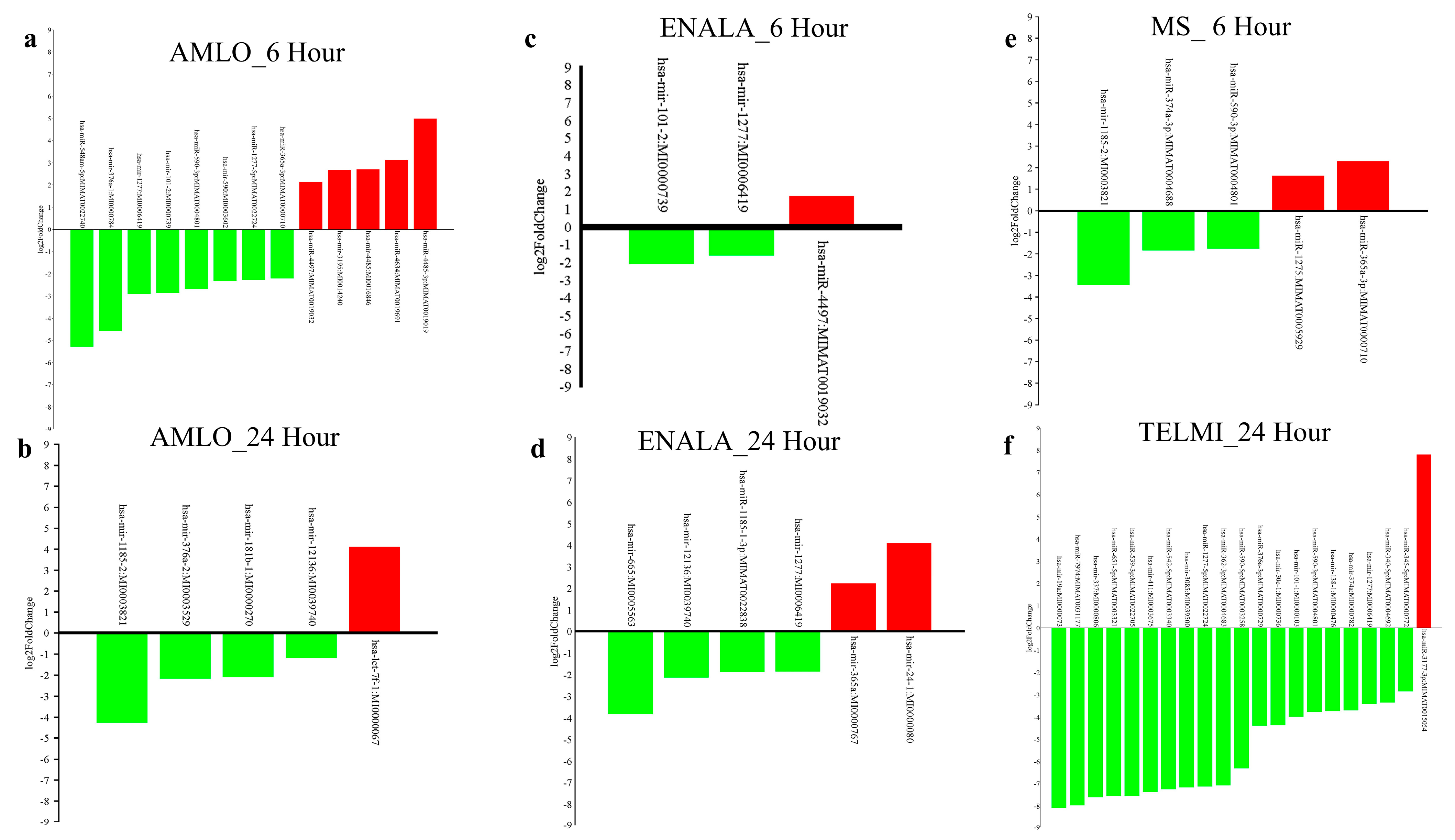
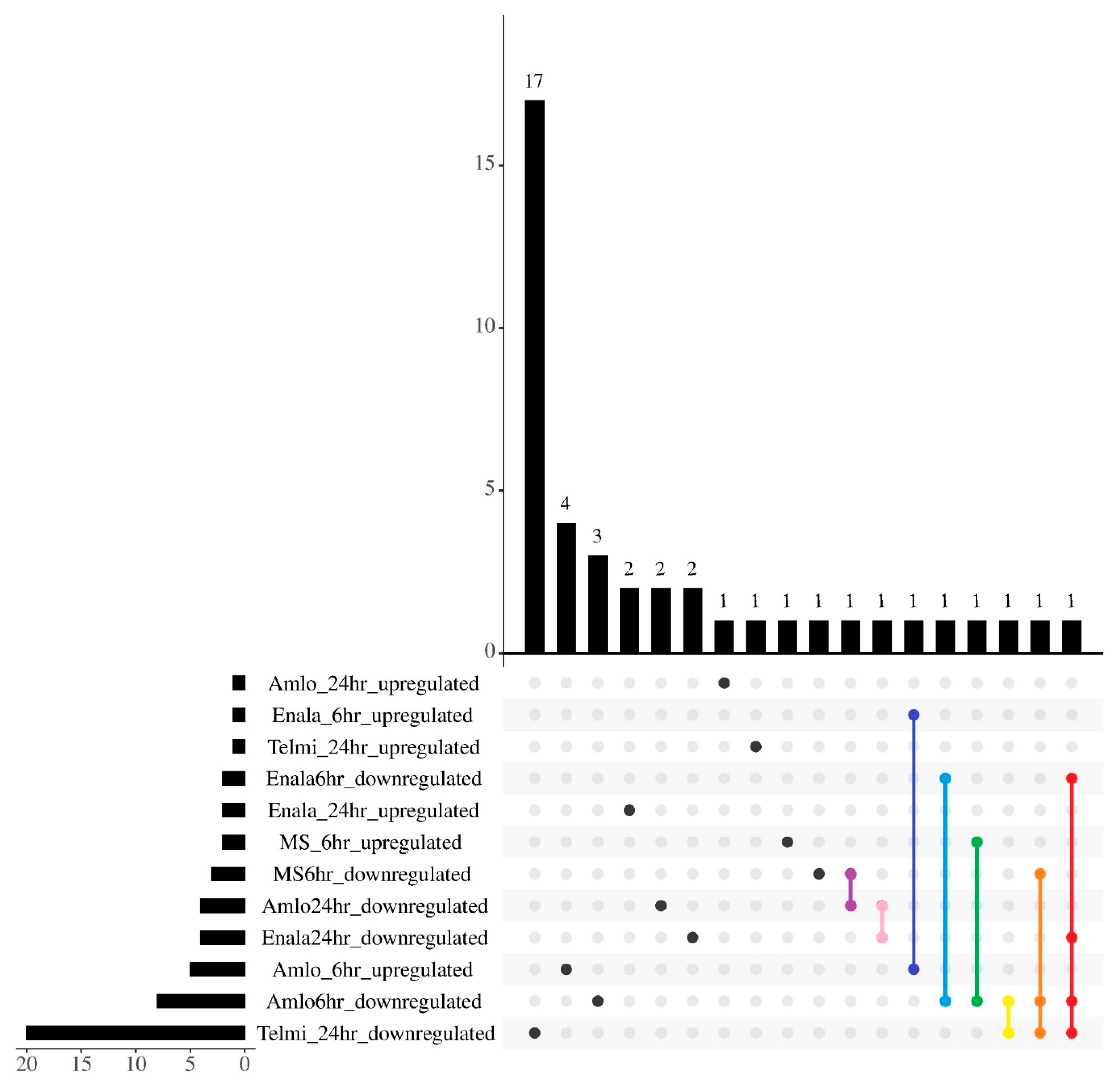
3.1. Differentially Expressed microRNAs
3.2. Differentially Expressed microRNAs and Their Target Genes
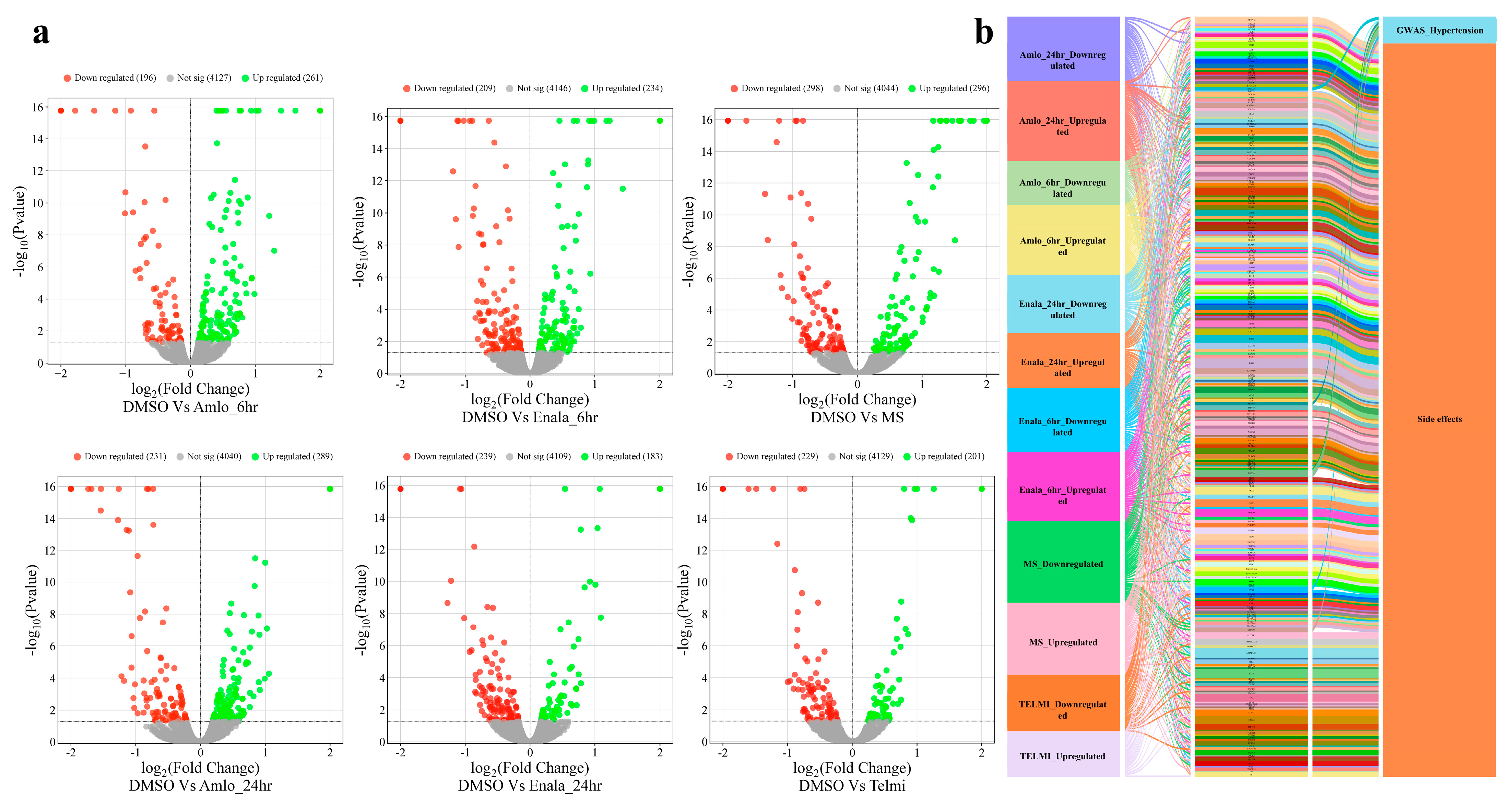
3.3. Proteomic Changes Following Treatment
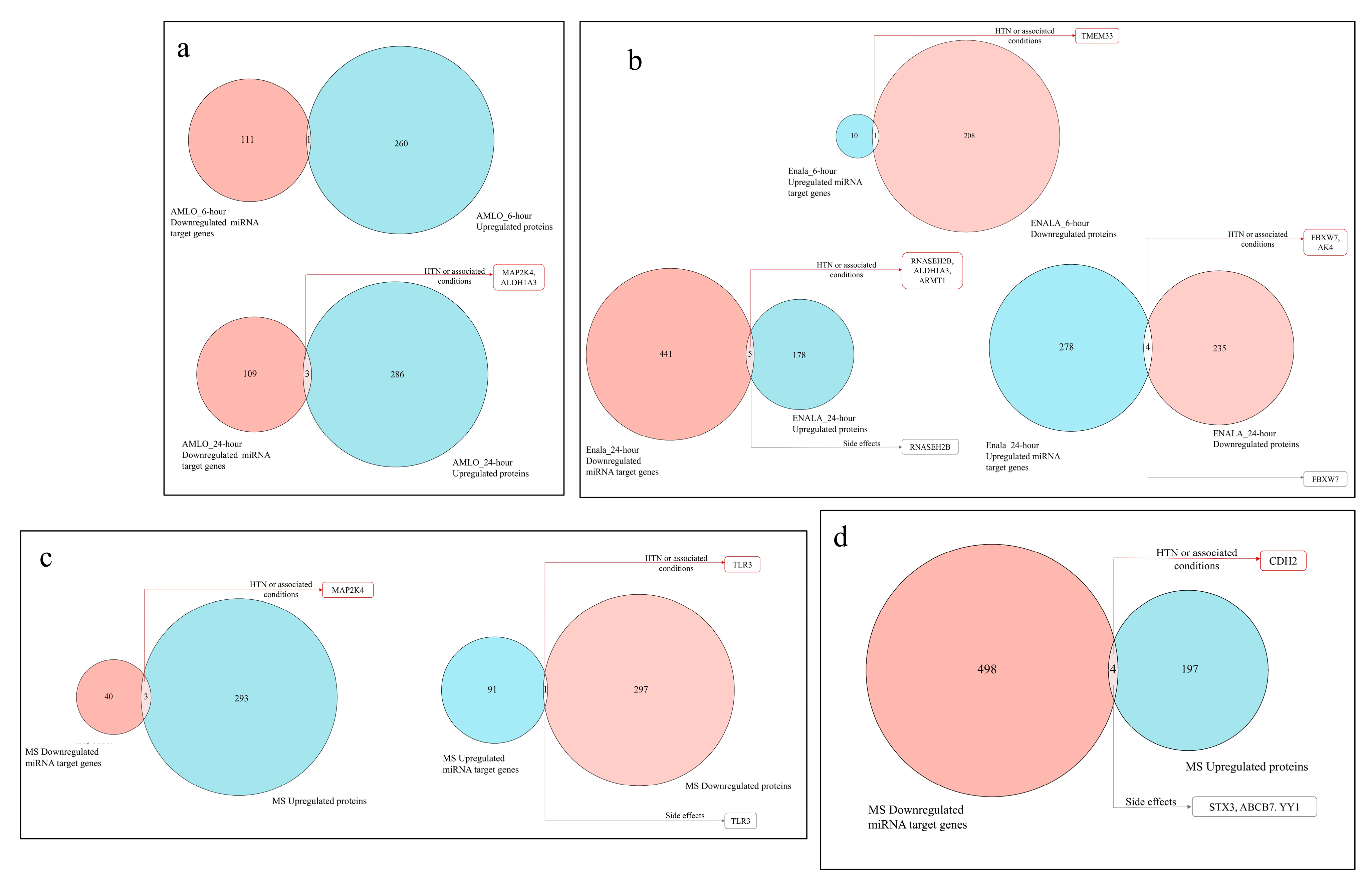
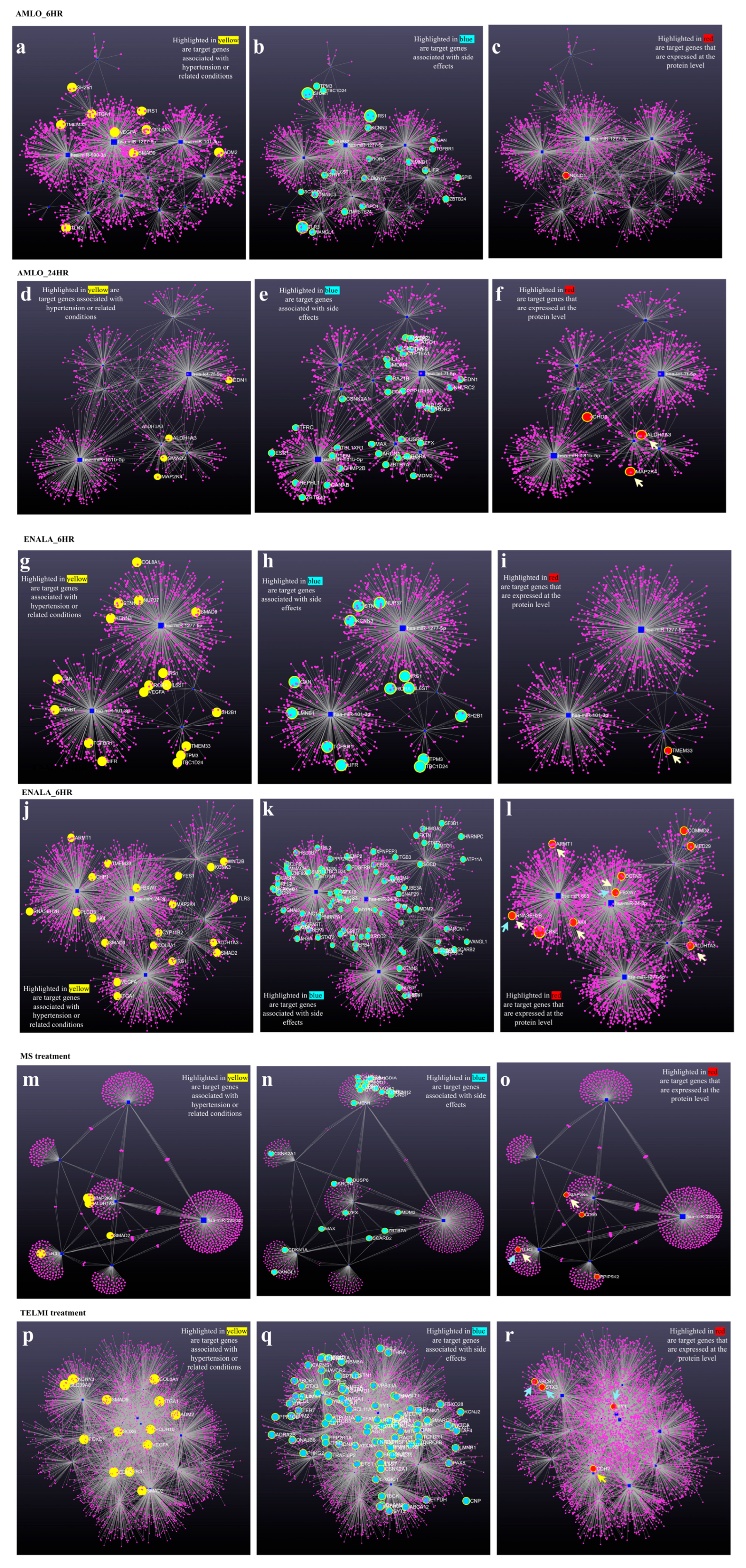
3.4. Coregulation of MiRNA Expression of Its Target Genes and Corresponding Proteomic Changes Following Treatment and Its Relationship to Hypertension HPO Terms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faraco, G.; Iadecola, C. Hypertension: A harbinger of stroke and dementia. Hypertension 2013, 62, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen, S.E. Hypertension and cardiovascular risk: General aspects. Pharmacol. Res. 2018, 129, 95–99. [Google Scholar] [CrossRef]
- Khalil, H.; Zeltser, R. Antihypertensive Medications. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Johnson, J.A. Pharmacogenomics of antihypertensive drugs: Past, present and future. Pharmacogenomics 2010, 11, 487–491. [Google Scholar] [CrossRef]
- Redon, J.; Mourad, J.J.; Schmieder, R.E.; Volpe, M.; Weiss, T.W. Why in 2016 are patients with hypertension not 100% controlled? A call to action. J. Hypertens. 2016, 34, 1480–1488. [Google Scholar] [CrossRef]
- Ettehad, D.; Emdin, C.A.; Kiran, A.; Anderson, S.G.; Callender, T.; Emberson, J.; Chalmers, J.; Rodgers, A.; Rahimi, K. Blood pressure lowering for prevention of cardiovascular disease and death: A systematic review and meta-analysis. Lancet 2016, 387, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Guilbert, J.J. The World Health Report 2002—Reducing Risks, Promoting Healthy Life. Educ. Health Chang. Learn. Pract. 2003, 16, 230. [Google Scholar]
- Gebreyohannes, E.A.; Bhagavathula, A.S.; Abebe, T.B.; Tefera, Y.G.; Abegaz, T.M. Adverse effects and non-adherence to antihypertensive medications in University of Gondar Comprehensive Specialized Hospital. Clin. Hypertens. 2019, 25, 1. [Google Scholar] [CrossRef]
- Nosa, U.S.; Kardela, W.; Bellatasie, R.; Ramadhani, W.A. The Incidence Side Effects of Antihypertensive Drugs in the Intensive Care Unit General Hospital Dr. M. Ddjamil Padang. Int. J. Pharm. Sci. Med. 2024, 9, 29–35. [Google Scholar] [CrossRef]
- Lennestål, R.; Otterblad Olausson, P.; Källén, B. Maternal use of antihypertensive drugs in early pregnancy and delivery outcome, notably the presence of congenital heart defects in the infants. Eur. J. Clin. Pharmacol. 2009, 65, 615–625. [Google Scholar] [CrossRef]
- Fitton, C.A.; Steiner, M.F.; Aucott, L.; Pell, J.P.; Mackay, D.F.; Fleming, M.; Mclay, J.S. In-utero exposure to antihypertensive medication and neonatal and child health outcomes: A systematic review. J. Hypertens. 2017, 35, 2123–2137. [Google Scholar] [CrossRef]
- He, L.; Liu, Y.; Widlansky, M.E.; Kriegel, A.J.; Qiu, Q.; Liang, M. microRNA and Hypertension. Hypertension 2025, 82, 181–184. [Google Scholar] [CrossRef]
- Kostiniuk, D.; Marttila, S.; Raitoharju, E. Circulatory miRNAs in essential hypertension. Atherosclerosis 2025, 401, 119069. [Google Scholar] [CrossRef]
- Bhass, S.; Banerjee, M. Pharmacoepigenomic impact of anti-hypertensive drugs on DNA methylation: A genomewide methylation study. Epigenomics, 2025; Submitted. [Google Scholar]
- Sticht, C.; De La Torre, C.; Parveen, A.; Gretz, N. miRWalk: An online resource for prediction of microRNA binding sites. PLoS ONE 2018, 13, e0206239. [Google Scholar] [CrossRef]
- Mohan, N.; Banerjee, M. Integrated Pharmacoepigenomic Analysis Uncovers the Impact of Antiseizure Medications on Developmental Pathways and the Protective Effect of Folic Acid. Int. J. Mol. Sci. 2025, 26, 7981. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Chen, M.; Huang, X.; Zhang, G.; Zeng, L.; Zhang, G.; Wu, S.; Wang, Y. SRplot: A free online platform for data visualization and graphing. PLoS ONE 2023, 18, e0294236. [Google Scholar] [CrossRef] [PubMed]
- Talapova, P.; Gargano, M.A.; Matentzoglu, N.; Coleman, B.; Addo-Lartey, E.B.; Anagnostopoulos, A.V.; Anderton, J.; Avillach, P. The Human Phenotype Ontology in 2024: Phenotypes around the world. Nucleic Acids Res. 2024, 52, D1333–D1346. [Google Scholar] [CrossRef]
- Chang, L.; Zhou, G.; Soufan, O.; Xia, J. miRNet 2.0: Network-based visual analytics for miRNA functional analysis and systems biology. Nucleic Acids Res. 2020, 48, W244–W251. [Google Scholar] [CrossRef]
- Li, D.; Shao, N.Y.; Moonen, J.R.; Zhao, Z.; Shi, M.; Otsuki, S.; Wang, L.; Nguyen, T.; Yan, E.; Marciano, D.P.; et al. ALDH1A3 Coordinates Metabolism with Gene Regulation in Pulmonary Arterial Hypertension. Circulation 2021, 143, 2074–2090. [Google Scholar] [CrossRef] [PubMed]
- Fardella, C.; Rodriguez-Portales, J.A. Intracellular calcium and blood pressure: Comparison between primary hyperparathyroidism and essential hypertension. J. Endocrinol. Investig. 1995, 18, 827–832. [Google Scholar] [CrossRef]
- Lindner, A.; Kenny, M.; Meacham, A.J. Effects of a Circulating Factor in Patients with Essential Hypertension on Intracellular Free Calcium in Normal Platelets. N. Engl. J. Med. 1987, 316, 509–513. [Google Scholar] [CrossRef]
- Gonzalez, J.M.; Suki, W.N. Cell calcium and arterial blood pressure. Semin. Nephrol. 1995, 15, 564–568. [Google Scholar] [CrossRef] [PubMed]
- TMEM33 Gene—GeneCards|TMM33 Protein|TMM33 Antibody. Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=TMEM33 (accessed on 4 July 2025).
- Arhatte, M.; Gunaratne, G.S.; El Boustany, C.; Kuo, I.Y.; Moro, C.; Duprat, F.; Plaisant, M.; Duval, H.; Li, D.; Picard, N.; et al. TMEM33 regulates intracellular calcium homeostasis in renal tubular epithelial cells. Nat. Commun. 2019, 10, 2024. [Google Scholar] [CrossRef] [PubMed]
- Savage, A.M.; Kurusamy, S.; Chen, Y.; Jiang, Z.; Chhabria, K.; MacDonald, R.B.; Kim, H.R.; Wilson, H.L.; van Eeden, F.J.; Armesilla, A.L.; et al. tmem33 is essential for VEGF-mediated endothelial calcium oscillations and angiogenesis. Nat. Commun. 2019, 10, 732. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, X.; Li, Y.; Zhang, S.; Wang, Y.; Wang, L.; Ma, W. High Expression of TMEM33 Predicts Poor Prognosis and Promotes Cell Proliferation in Cervical Cancer. Front. Genet. 2022, 13, 908807. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.F.; Zhang, C.; Li, Z.C.; Zhou, X.Y.; Jiang, J.Y.; Chen, D.D.; Zhang, Y.A.; Xiong, F.; Zhou, F.; Li, S. A novel role of Zebrafish TMEM33 in negative regulation of interferon production by two distinct mechanisms. PLoS Pathog. 2021, 17, e1009317. [Google Scholar] [CrossRef]
- Hazuková, R.; Zadák, Z.; Pleskot, M.; Zdráhal, P.; Pumprla, M.; Táborský, M. Oxidative DNA Damage and Arterial Hypertension in Light of Current ESC Guidelines. Int. J. Mol. Sci. 2024, 25, 12557. [Google Scholar] [CrossRef]
- Perry, J.J.P.; Ballard, G.D.; Albert, A.E.; Dobrolecki, L.E.; Malkas, L.H.; Hoelz, D.J. Human C6orf211 Encodes Armt1, a Protein Carboxyl Methyltransferase that Targets PCNA and Is Linked to the DNA Damage Response. Cell Rep. 2015, 10, 1288–1296. [Google Scholar] [CrossRef]
- Kruse, K.; Lee, Q.S.; Sun, Y.; Klomp, J.; Yang, X.; Huang, F.; Sun, M.Y.; Zhao, S.; Hong, Z.; Vogel, S.M.; et al. N-cadherin signaling via Trio assembles adherens junctions to restrict endothelial permeability. J. Cell Biol. 2019, 218, 299–316. [Google Scholar] [CrossRef]
- Viazzi, F.; Leoncini, G.; Ratto, E.; Parodi, A.; Falqui, V.; Conti, N.; Tomolillo, C.; Ravera, G.; Deferrari, G.; Pontremoli, R. Vascular Permeability, Blood Pressure, and Organ Damage in Primary Hypertension. Hypertens. Res. 2008, 31, 873–879. [Google Scholar] [CrossRef][Green Version]
- Williams, B.; Baker, A.Q.; Gallacher, B.; Lodwick, D. Angiotensin II Increases Vascular Permeability Factor Gene Expression by Human Vascular Smooth Muscle Cells. Hypertension 1995, 25, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Justina, V.D.; Giachini, F.R.; Priviero, F.; Webb, R.C. Double-stranded RNA and Toll-like receptor activation: A novel mechanism for blood pressure regulation. Clin. Sci. 2020, 134, 303–313. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhass, S.; Banerjee, M. Pharmacoepigenomic Impact of Antihypertensive Drugs on miRNome and Proteome and Its Potential Influence on Health and Side Effects. Cells 2025, 14, 1359. https://doi.org/10.3390/cells14171359
Bhass S, Banerjee M. Pharmacoepigenomic Impact of Antihypertensive Drugs on miRNome and Proteome and Its Potential Influence on Health and Side Effects. Cells. 2025; 14(17):1359. https://doi.org/10.3390/cells14171359
Chicago/Turabian StyleBhass, Samyukta, and Moinak Banerjee. 2025. "Pharmacoepigenomic Impact of Antihypertensive Drugs on miRNome and Proteome and Its Potential Influence on Health and Side Effects" Cells 14, no. 17: 1359. https://doi.org/10.3390/cells14171359
APA StyleBhass, S., & Banerjee, M. (2025). Pharmacoepigenomic Impact of Antihypertensive Drugs on miRNome and Proteome and Its Potential Influence on Health and Side Effects. Cells, 14(17), 1359. https://doi.org/10.3390/cells14171359





