New Approaches to Old Techniques in Cell Handling for Microscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Primary Cells and Cell Lines
2.2. Cell Culture
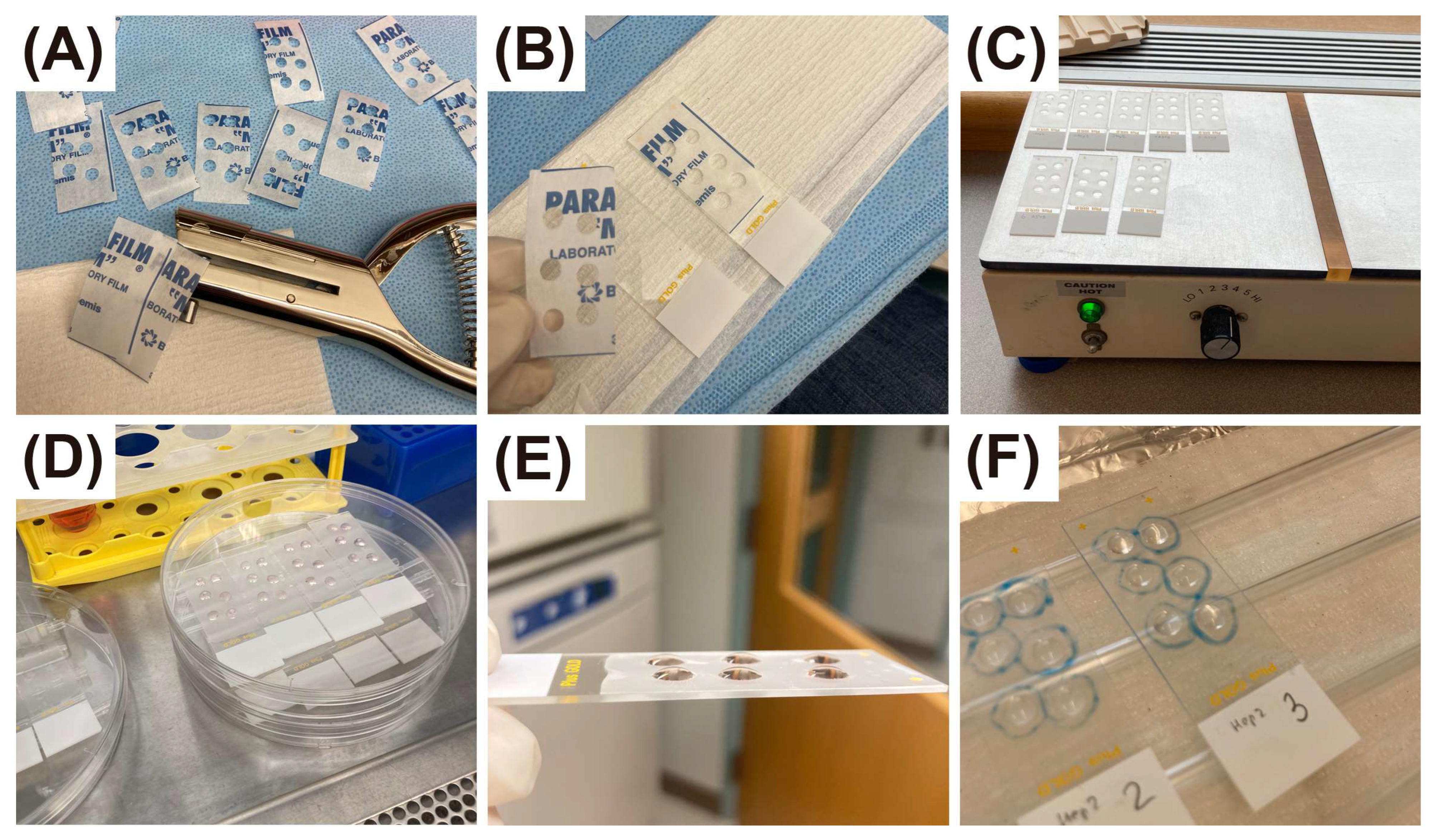
2.3. Preparation of Time-Series of Slides with Primary Cells in Suspension
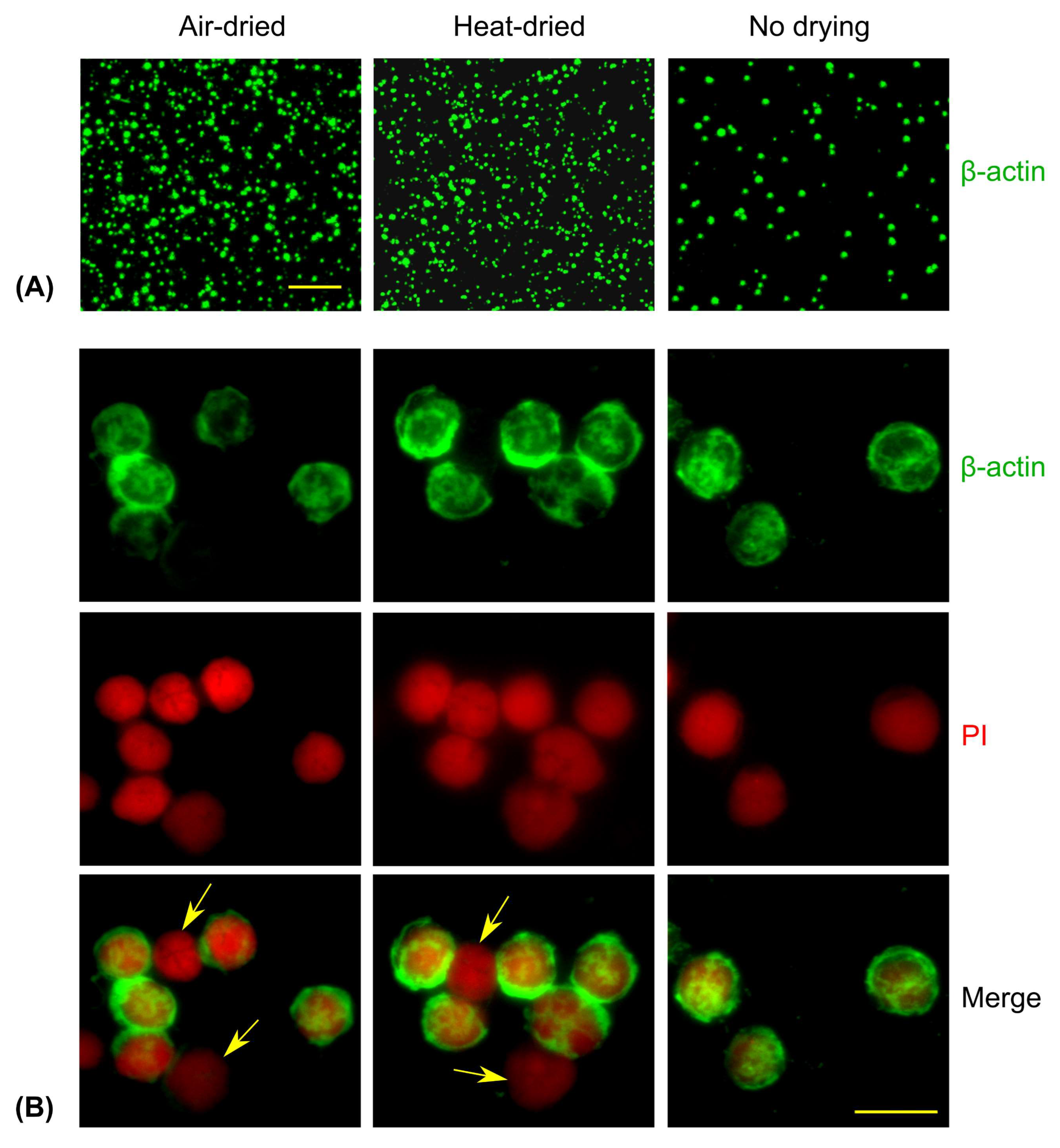
2.4. Comparison of Air-Dried, Heat-Dried, and Immediately Fixed Slides
- (a)
- Left to air-dry at room temperature for 10 min;
- (b)
- Heat-dried on a hot plate at low heat (55–60 °C) for 20 min;
- (c)
- Immediately fixed in fixation buffer (BD Pharmingen, San Diego, CA, USA, cat# 562574) for 50 min at 4 °C.
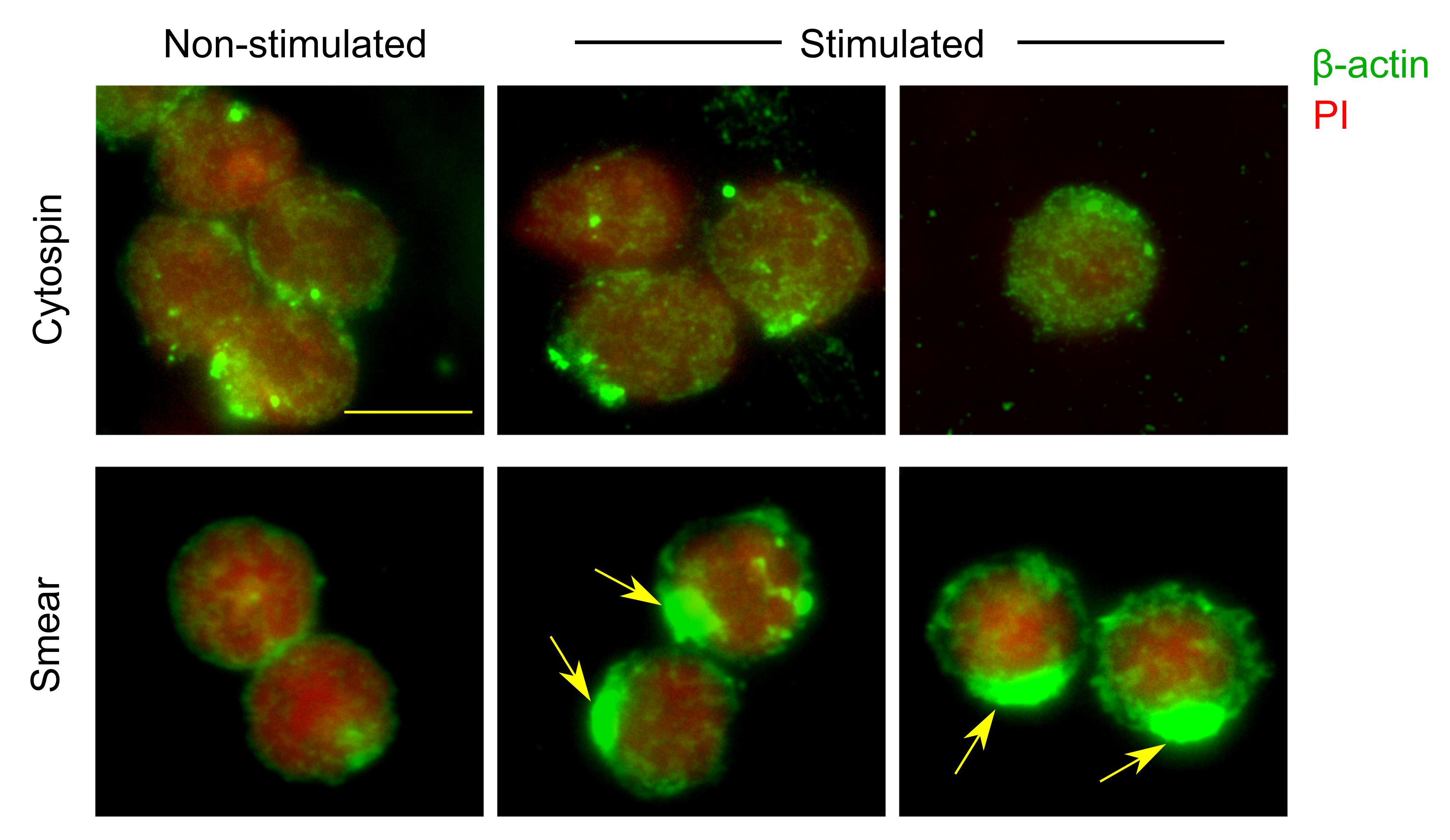
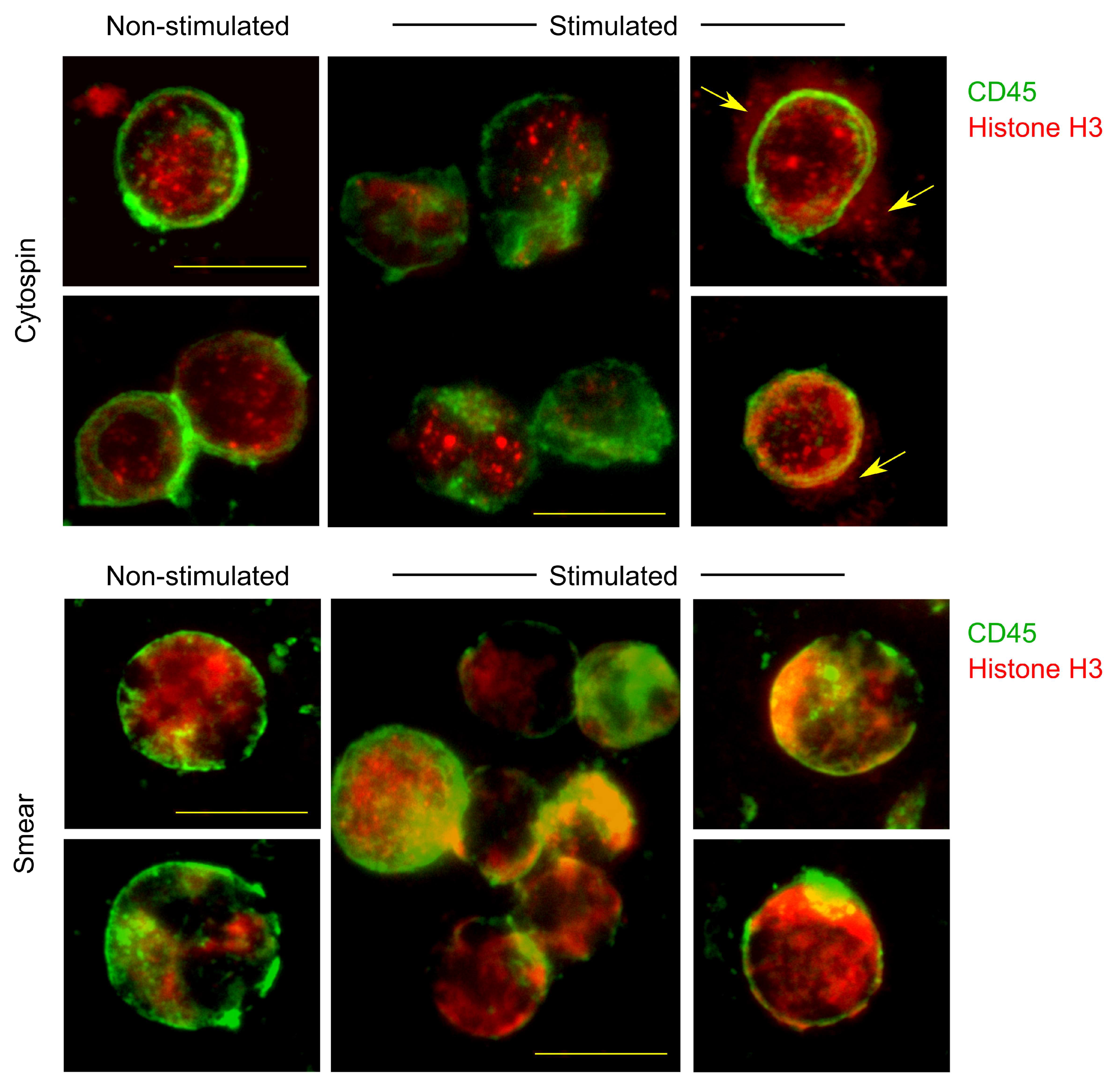
2.5. Comparison of Cytospin Preparation and Cell Smears
2.6. Preparation of Time-Series of Slides with Adherent Cells
2.7. Use of Generative Artificial Intelligence in Graphical Abstract
2.8. Data Handling and Statistics
3. Results
3.1. Comparison of Different Techniques for Slide Preparation
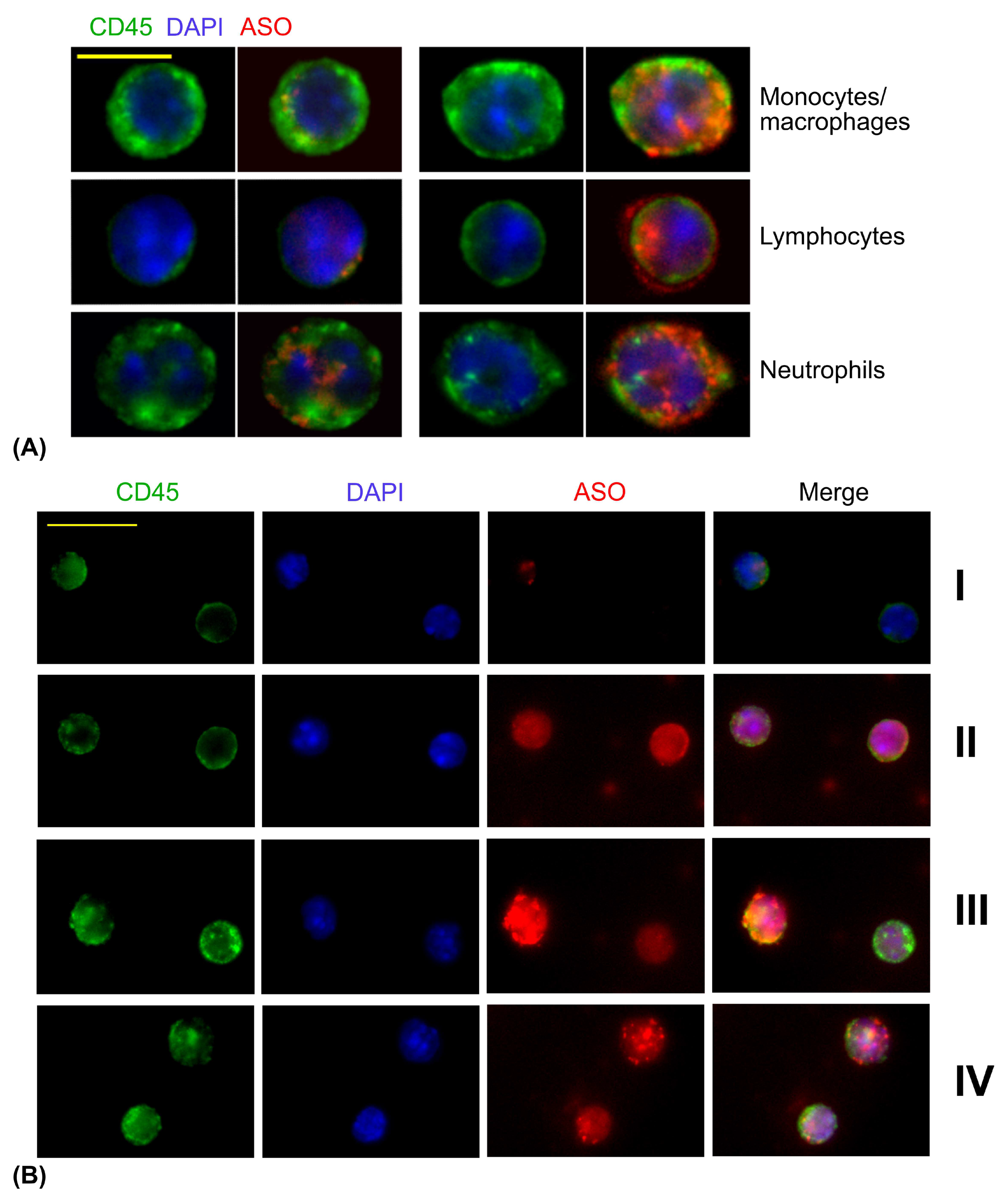
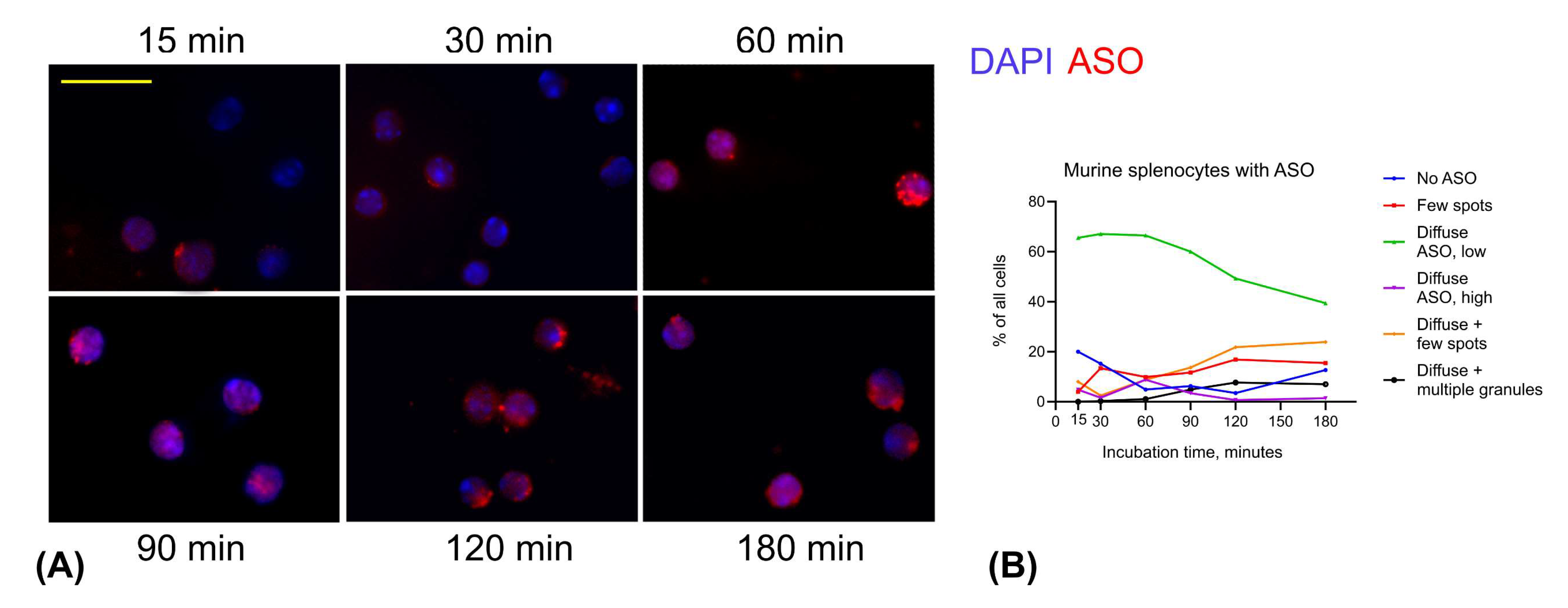
3.2. ASO Uptake in Primary Cells
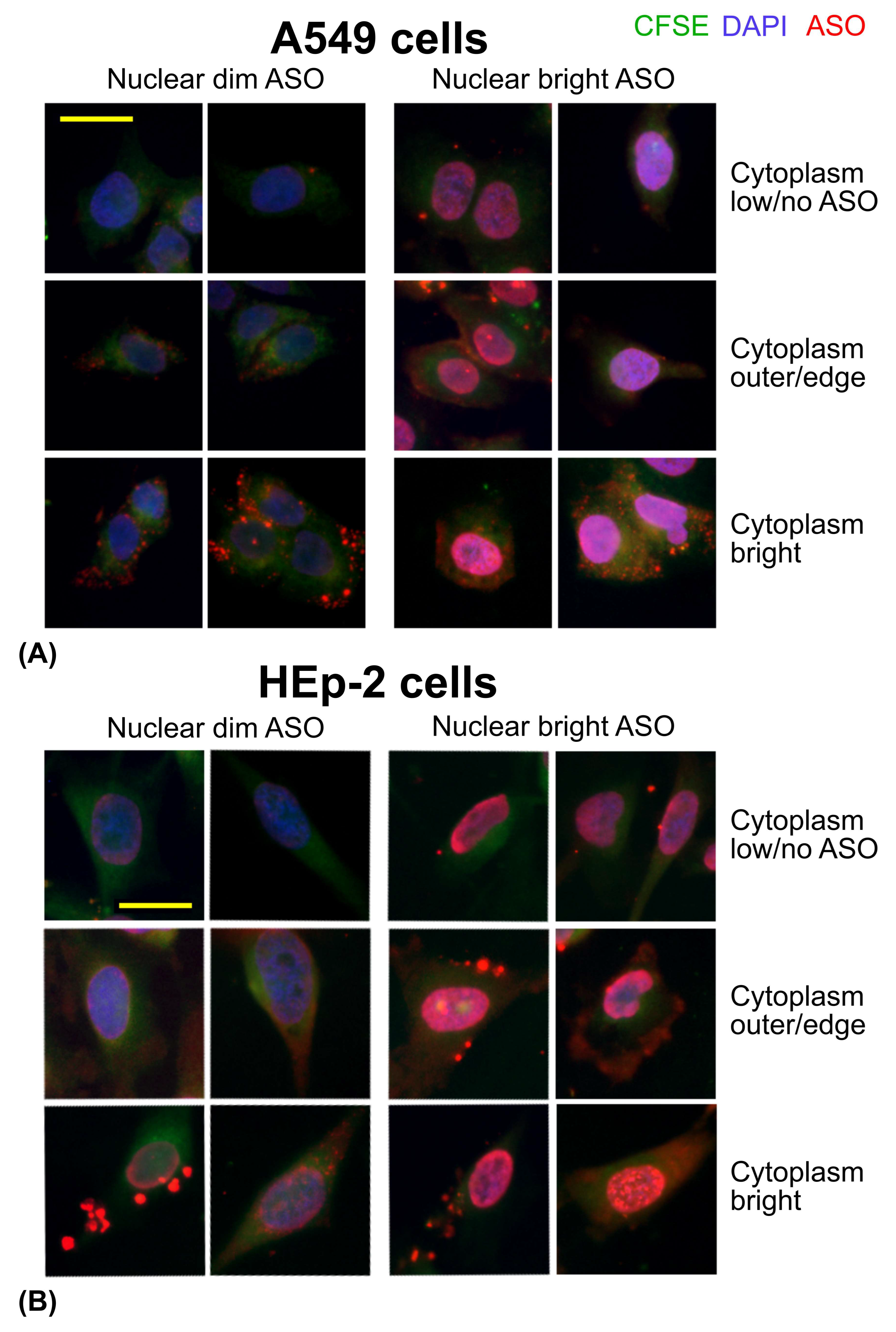
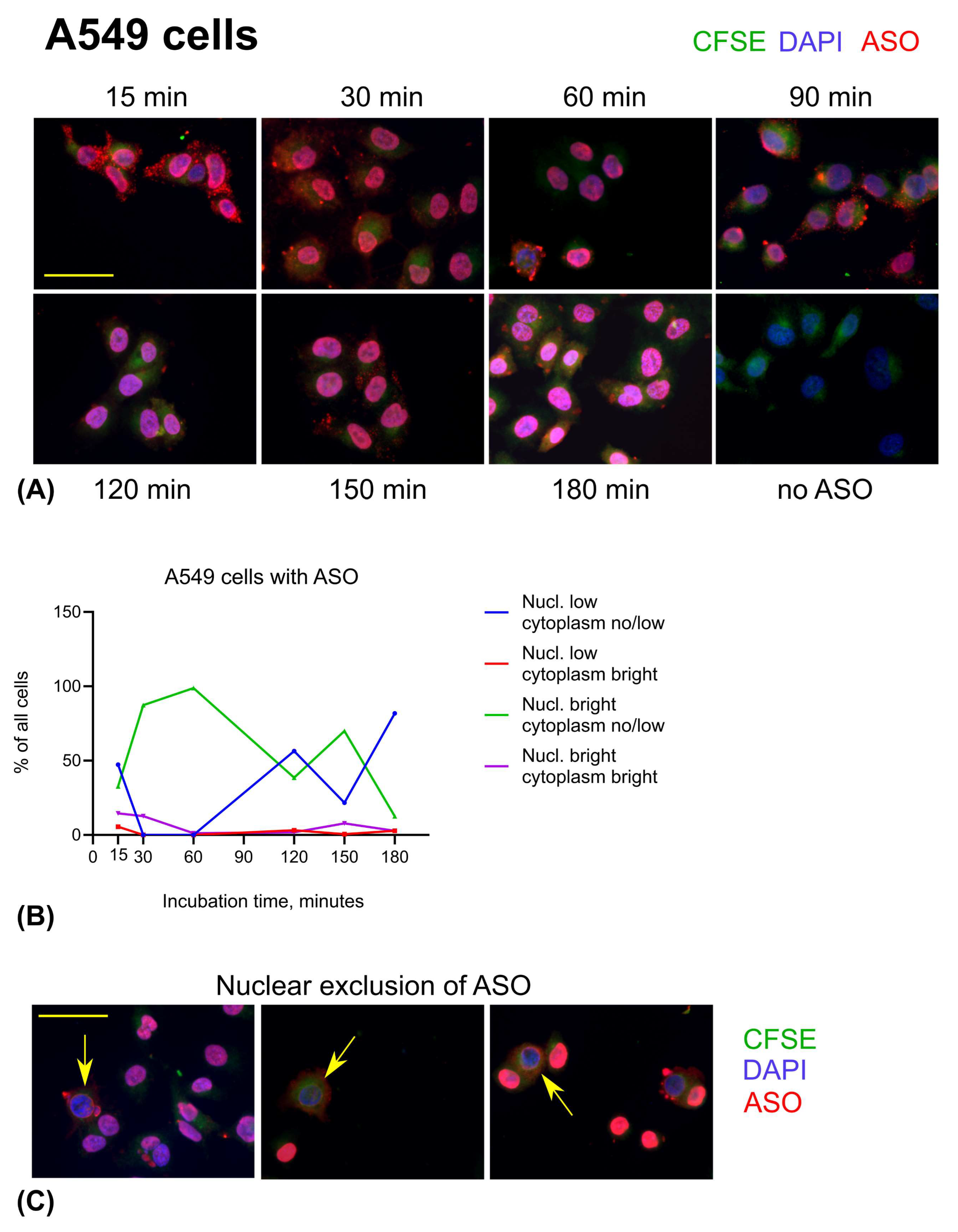
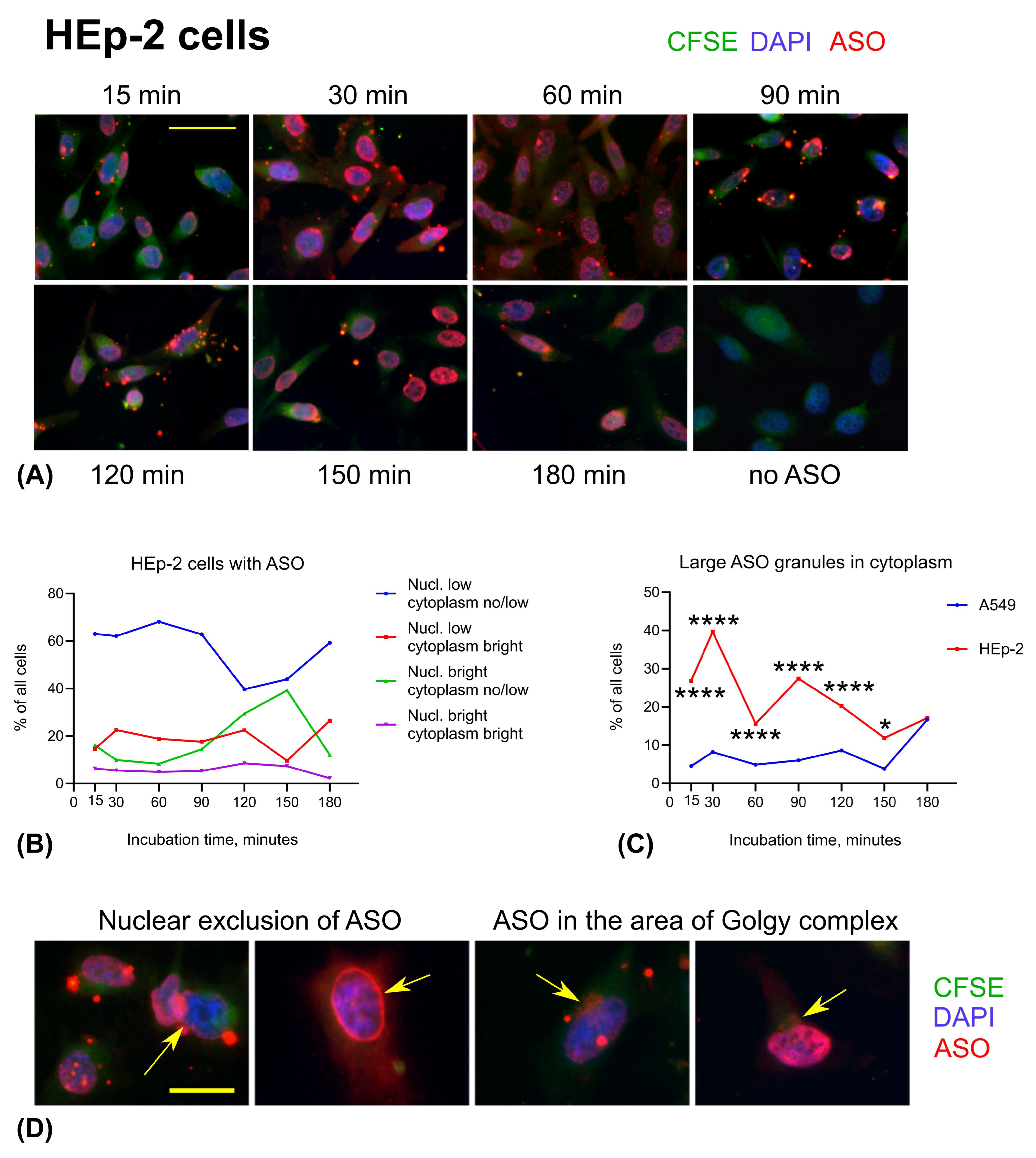
3.3. ASO Uptake in Cell Lines: A549 and HEp-2 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASO | Antisense oligonucleotides |
| FBS | Fetal bovine serum, heat-inactivated |
| NET | Neutrophil extracellular trap |
| PI | Propidium iodide |
| PMA | Phorbol 12-myristate 13-acetate |
References
- Qamar, I.; Rehman, S.; Mehdi, G.; Maheshwari, V.; Ansari, H.A.; Chauhan, S. Utility of Cytospin and Cell block Technology in Evaluation of Body Fluids and Urine Samples: A Comparative Study. J. Cytol. 2018, 35, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Koh, C.M. Preparation of cells for microscopy using cytospin. Methods Enzymol. 2013, 533, 235–240. [Google Scholar] [CrossRef] [PubMed]
- De Brauwer, E.I.; Jacobs, J.A.; Nieman, F.; Bruggeman, C.A.; Wagenaar, S.S.; Drent, M. Cytocentrifugation conditions affecting the differential cell count in bronchoalveolar lavage fluid. Anal. Quant. Cytol. Histol. 2000, 22, 416–422. [Google Scholar]
- Bratthauer, G.L. Processing of cytological specimens. Methods Mol. Biol. 2010, 588, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Maleki, S.; Dorokhova, O.; Sunkara, J.; Schlesinger, K.; Suhrland, M.; Oktay, M.H. Estrogen, progesterone, and HER-2 receptor immunostaining in cytology: The effect of varied fixation on human breast cancer cells. Diagn. Cytopathol. 2013, 41, 864–870. [Google Scholar] [CrossRef]
- Kshatriya, A.S.; Santwani, P.M. Comparison of FNAC smears, cytospin smears, and cellblocks of transthoracic guided FNAC of suspected lung tumor: A study of 100 cases. J. Cytol. 2016, 33, 141–144. [Google Scholar] [CrossRef]
- Dyken, P.R.; Shirley, S.; Trefz, J.; El Gammal, T. Comparison of cytocentrifugation and sedimentation techniques for CSF cytomorphology. Acta Cytol. 1980, 24, 167–170. [Google Scholar] [PubMed]
- Negorev, D.; Beier, U.H.; Zhang, T.; Quatromoni, J.G.; Bhojnagarwala, P.; Albelda, S.M.; Singhal, S.; Eruslanov, E.; Lohoff, F.W.; Levine, M.H.; et al. Human neutrophils can mimic myeloid-derived suppressor cells (PMN-MDSC) and suppress microbead or lectin-induced T cell proliferation through artefactual mechanisms. Sci. Rep. 2018, 8, 3135. [Google Scholar] [CrossRef]
- Koh, C.M. Preparation of cells for microscopy using chamber slides and coverslips. Methods Enzymol. 2013, 533, 241–247. [Google Scholar] [CrossRef]
- Akimova, T.; Levine, M.H.; Beier, U.H.; Hancock, W.W. Standardization, Evaluation, and Area-Under-Curve Analysis of Human and Murine Treg Suppressive Function. Methods Mol. Biol. 2016, 1371, 43–78. [Google Scholar] [CrossRef]
- Zhang, X.; Castanotto, D.; Liu, X.; Shemi, A.; Stein, C.A. Ammonium and arsenic trioxide are potent facilitators of oligonucleotide function when delivered by gymnosis. Nucleic Acids Res. 2018, 46, 3612–3624. [Google Scholar] [CrossRef] [PubMed]
- Samstag, Y.; Eibert, S.M.; Klemke, M.; Wabnitz, G.H. Actin cytoskeletal dynamics in T lymphocyte activation and migration. J. Leukoc. Biol. 2003, 73, 30–48. [Google Scholar] [CrossRef] [PubMed]
- Holsinger, L.J.; Graef, I.A.; Swat, W.; Chi, T.; Bautista, D.M.; Davidson, L.; Lewis, R.S.; Alt, F.W.; Crabtree, G.R. Defects in actin-cap formation in Vav-deficient mice implicate an actin requirement for lymphocyte signal transduction. Curr. Biol. 1998, 8, 563–572. [Google Scholar] [CrossRef]
- Nakayama, T.; Mihara, K.; Kawata, J.; Kimura, H.; Saitoh, H. Adhesion of suspension cells on a coverslip in serum-free conditions. Anal. Biochem. 2014, 466, 1–3. [Google Scholar] [CrossRef]
- Tsang, M.; Gantchev, J.; Ghazawi, F.M.; Litvinov, I.V. Protocol for adhesion and immunostaining of lymphocytes and other non-adherent cells in culture. Biotechniques 2017, 63, 230–233. [Google Scholar] [CrossRef]
- Wang, C.C.; Hwang, T.Z.; Lien, C.F.; Li, Y.; Lan, Y.Y. Serum Starvation-induced ROS Production Activates the ERK-AP-1-TfR1 Pathway to Up-regulate Survivin to Support Nasopharyngeal Carcinoma Cell Viability. Cancer Genom. Proteom. 2025, 22, 458–466. [Google Scholar] [CrossRef]
- Giannasi, C.; Niada, S.; Della Morte, E.; Casati, S.R.; De Palma, C.; Brini, A.T. Serum starvation affects mitochondrial metabolism of adipose-derived stem/stromal cells. Cytotherapy 2023, 25, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Charles, I.; Khalyfa, A.; Kumar, D.M.; Krishnamoorthy, R.R.; Roque, R.S.; Cooper, N.; Agarwal, N. Serum deprivation induces apoptotic cell death of transformed rat retinal ganglion cells via mitochondrial signaling pathways. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1330–1338. [Google Scholar] [CrossRef]
- Rashid, M.U.; Coombs, K.M. Serum-reduced media impacts on cell viability and protein expression in human lung epithelial cells. J. Cell. Physiol. 2019, 234, 7718–7724. [Google Scholar] [CrossRef]
- Jamur, M.C.; Oliver, C. Cell fixatives for immunostaining. Methods Mol. Biol. 2010, 588, 55–61. [Google Scholar] [CrossRef]
- Li, Y.; Almassalha, L.M.; Chandler, J.E.; Zhou, X.; Stypula-Cyrus, Y.E.; Hujsak, K.A.; Roth, E.W.; Bleher, R.; Subramanian, H.; Szleifer, I.; et al. The effects of chemical fixation on the cellular nanostructure. Exp. Cell. Res. 2017, 358, 253–259. [Google Scholar] [CrossRef]
- Wennberg, A. Simplified technique for culturing of cells on microscope slides. J. Dent. Res. 1976, 55, 920. [Google Scholar] [CrossRef]
- Sandoval-Mojica, A.F.; Hunter, W.B.; Aishwarya, V.; Bonilla, S.; Pelz-Stelinski, K.S. Antibacterial FANA oligonucleotides as a novel approach for managing the Huanglongbing pathosystem. Sci. Rep. 2021, 11, 2760. [Google Scholar] [CrossRef]
- Takahashi, M.; Li, H.; Zhou, J.; Chomchan, P.; Aishwarya, V.; Damha, M.J.; Rossi, J.J. Dual Mechanisms of Action of Self-Delivering, Anti-HIV-1 FANA Oligonucleotides as a Potential New Approach to HIV Therapy. Mol. Ther. Nucleic Acids 2019, 17, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Page, J.J.; Almanza, J.R.; Xiong, S.; Aishwarya, V.; Kroner, A. Self-delivering mRNA inhibitors of MK2 improve outcomes after spinal cord injury. J. Neuroimmunol. 2023, 379, 578103. [Google Scholar] [CrossRef] [PubMed]
- Pelisch, N.; Rosas Almanza, J.; Stehlik, K.E.; Aperi, B.V.; Kroner, A. Use of a Self-Delivering Anti-CCL3 FANA Oligonucleotide as an Innovative Approach to Target Inflammation after Spinal Cord Injury. eNeuro 2021, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Smaldone, G.; Beneduce, G.; Incoronato, M.; Pane, K.; Franzese, M.; Coppola, L.; Cordella, A.; Parasole, R.; Ripaldi, M.; Nassa, G.; et al. KCTD15 is overexpressed in human childhood B-cell acute lymphoid leukemia. Sci. Rep. 2019, 9, 20108. [Google Scholar] [CrossRef] [PubMed]
- Souleimanian, N.; Deleavey, G.F.; Soifer, H.; Wang, S.; Tiemann, K.; Damha, M.J.; Stein, C.A. Antisense 2′-Deoxy, 2′-Fluroarabino Nucleic Acids (2′F-ANAs) Oligonucleotides: In Vitro Gymnotic Silencers of Gene Expression Whose Potency Is Enhanced by Fatty Acids. Mol. Ther. Nucleic Acids 2012, 1, e43. [Google Scholar] [CrossRef]
- Soifer, H.S.; Koch, T.; Lai, J.; Hansen, B.; Hoeg, A.; Oerum, H.; Stein, C.A. Silencing of gene expression by gymnotic delivery of antisense oligonucleotides. Methods Mol. Biol. 2012, 815, 333–346. [Google Scholar] [CrossRef]
- Deprey, K.; Batistatou, N.; Kritzer, J.A. A critical analysis of methods used to investigate the cellular uptake and subcellular localization of RNA therapeutics. Nucleic Acids Res. 2020, 48, 7623–7639. [Google Scholar] [CrossRef]
- McMahon, M.A.; Rahdar, M.; Mukhopadhyay, S.; Bui, H.H.; Hart, C.; Damle, S.; Courtney, M.; Baughn, M.W.; Cleveland, D.W.; Bennett, C.F. GOLGA8 increases bulk antisense oligonucleotide uptake and activity in mammalian cells. Mol. Ther. Nucleic Acids 2023, 32, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Linnane, E.; Davey, P.; Zhang, P.; Puri, S.; Edbrooke, M.; Chiarparin, E.; Revenko, A.S.; Macleod, A.R.; Norman, J.C.; Ross, S.J. Differential uptake, kinetics and mechanisms of intracellular trafficking of next-generation antisense oligonucleotides across human cancer cell lines. Nucleic Acids Res. 2019, 47, 4375–4392. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.H.; Nichols, J.G.; Hsu, C.W.; Crooke, S.T. Hsc70 Facilitates Mannose-6-Phosphate Receptor-Mediated Intracellular Trafficking and Enhances Endosomal Release of Phosphorothioate-Modified Antisense Oligonucleotides. Nucleic Acid Ther. 2021, 31, 284–297. [Google Scholar] [CrossRef]
- Miller, C.M.; Tanowitz, M.; Donner, A.J.; Prakash, T.P.; Swayze, E.E.; Harris, E.N.; Seth, P.P. Receptor-Mediated Uptake of Phosphorothioate Antisense Oligonucleotides in Different Cell Types of the Liver. Nucleic Acid Ther. 2018, 28, 119–127. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartosh, Z.; Aishwarya, V.; Hancock, W.W.; Akimova, T. New Approaches to Old Techniques in Cell Handling for Microscopy. Cells 2025, 14, 1271. https://doi.org/10.3390/cells14161271
Bartosh Z, Aishwarya V, Hancock WW, Akimova T. New Approaches to Old Techniques in Cell Handling for Microscopy. Cells. 2025; 14(16):1271. https://doi.org/10.3390/cells14161271
Chicago/Turabian StyleBartosh, Zhanna, Veenu Aishwarya, Wayne W. Hancock, and Tatiana Akimova. 2025. "New Approaches to Old Techniques in Cell Handling for Microscopy" Cells 14, no. 16: 1271. https://doi.org/10.3390/cells14161271
APA StyleBartosh, Z., Aishwarya, V., Hancock, W. W., & Akimova, T. (2025). New Approaches to Old Techniques in Cell Handling for Microscopy. Cells, 14(16), 1271. https://doi.org/10.3390/cells14161271






