Expression of Toll-like Receptors on Lymphocyte Subpopulations and Their Soluble Forms in Serum and Urine of Women with Endometriosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Characteristics
- Confirmed diagnosis of endometriosis by histopathological and clinical evaluation;
- Age ≥ 18 years;
- No immunosuppressive treatment within the past 12 months;
- No use of hormonal contraception (including intrauterine systems);
- Provision of written informed consent to participate in the study.
- Presence of uterine fibroids or adenomyosis;
- Active bacterial, viral, or fungal infections;
- Diabetes mellitus or other metabolic/endocrine disorders;
- Chronic wounds, severe allergic diseases, or significant systemic illnesses affecting the kidneys, gastrointestinal tract, heart, or lungs, as well as adherence to strict elimination diets;
- History of hematopoietic stem cell or organ transplantation;
- Active malignancy or autoimmune disorders;
- Pregnancy or breastfeeding;
- Use of immunosuppressive medications or participation in other clinical trials;
- Presence of CNS metastatic disease or diagnosed psychiatric disorders.
- Peritoneal endometriosis (PE);
- Ovarian endometriosis (OE);
- Deeply infiltrating endometriosis (DIE);
- Cesarean section scar endometriosis (CC).
2.2. Immunophenotyping
2.3. Determination of the Concentration of Soluble Forms of TLRs in the Tested Material
2.4. Statistical Analyses
3. Results
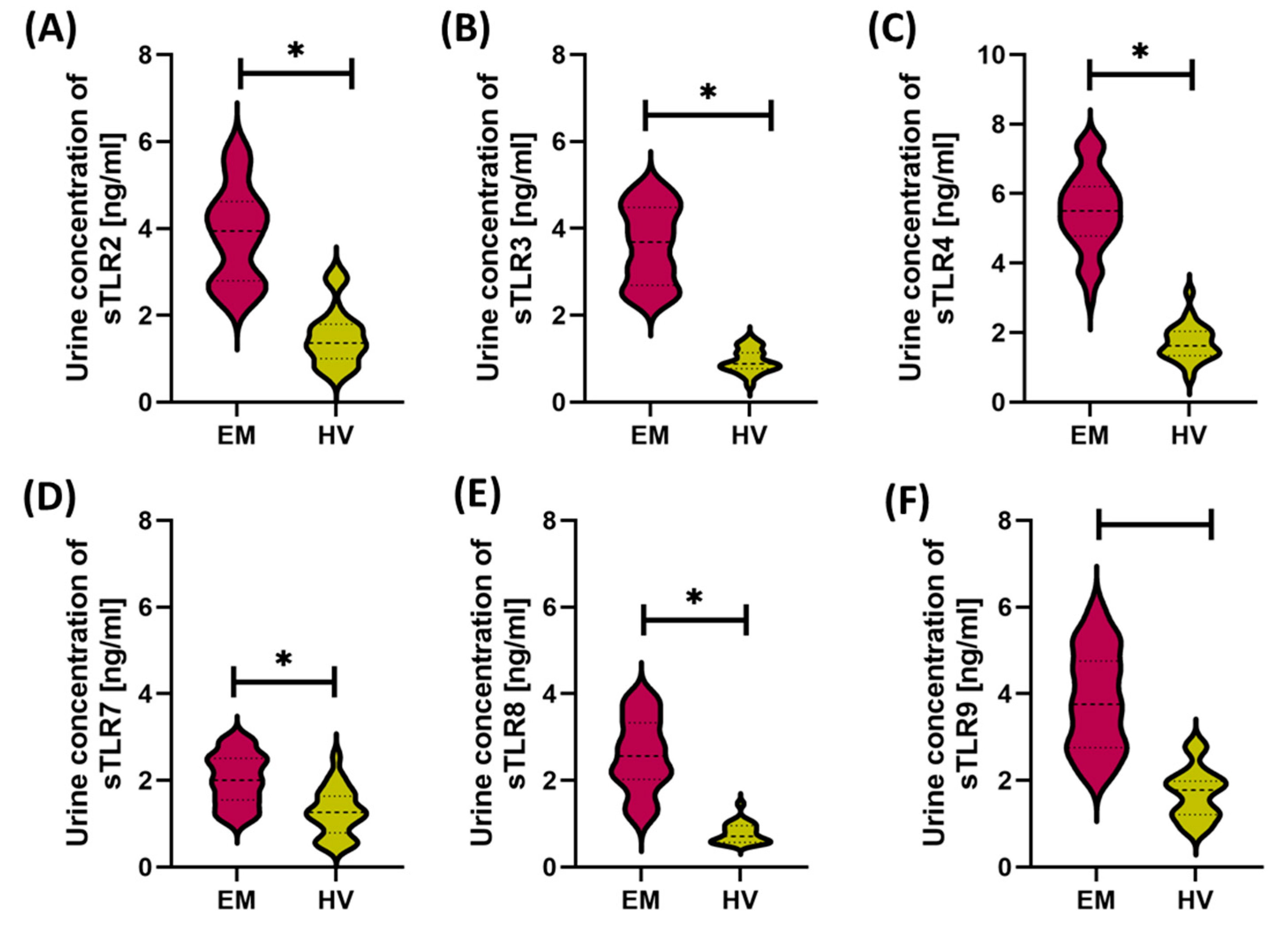
3.1. Comparison of Immunological and Biochemical Parameters Between Patients with Endometriosis and Healthy Women
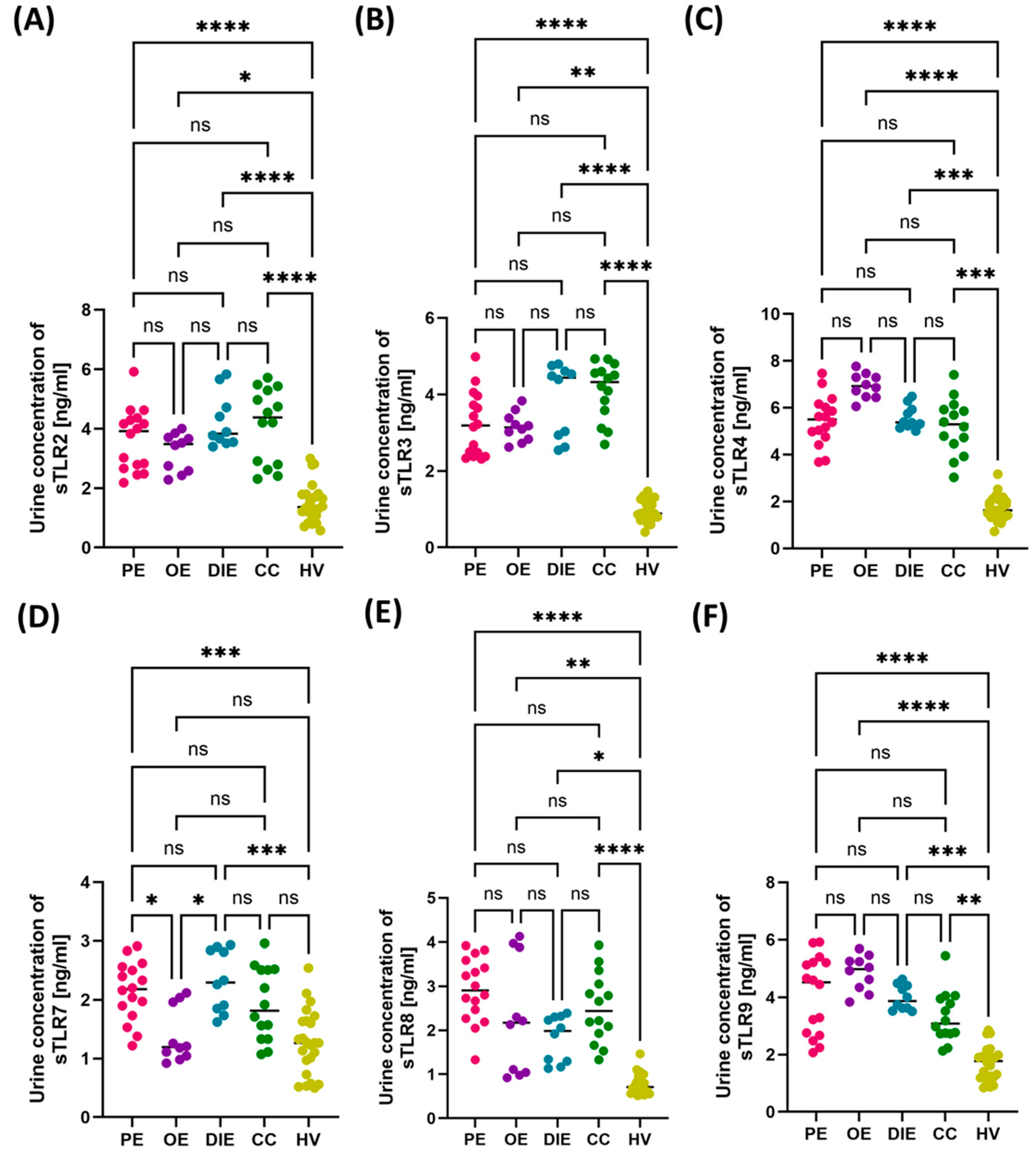
3.2. Comparison of Immunological and Biochemical Parameters in Patients with Different Forms of Endometriosis: Peritoneal, Ovarian, Deeply Infiltrating, and Cesarean Section Scar
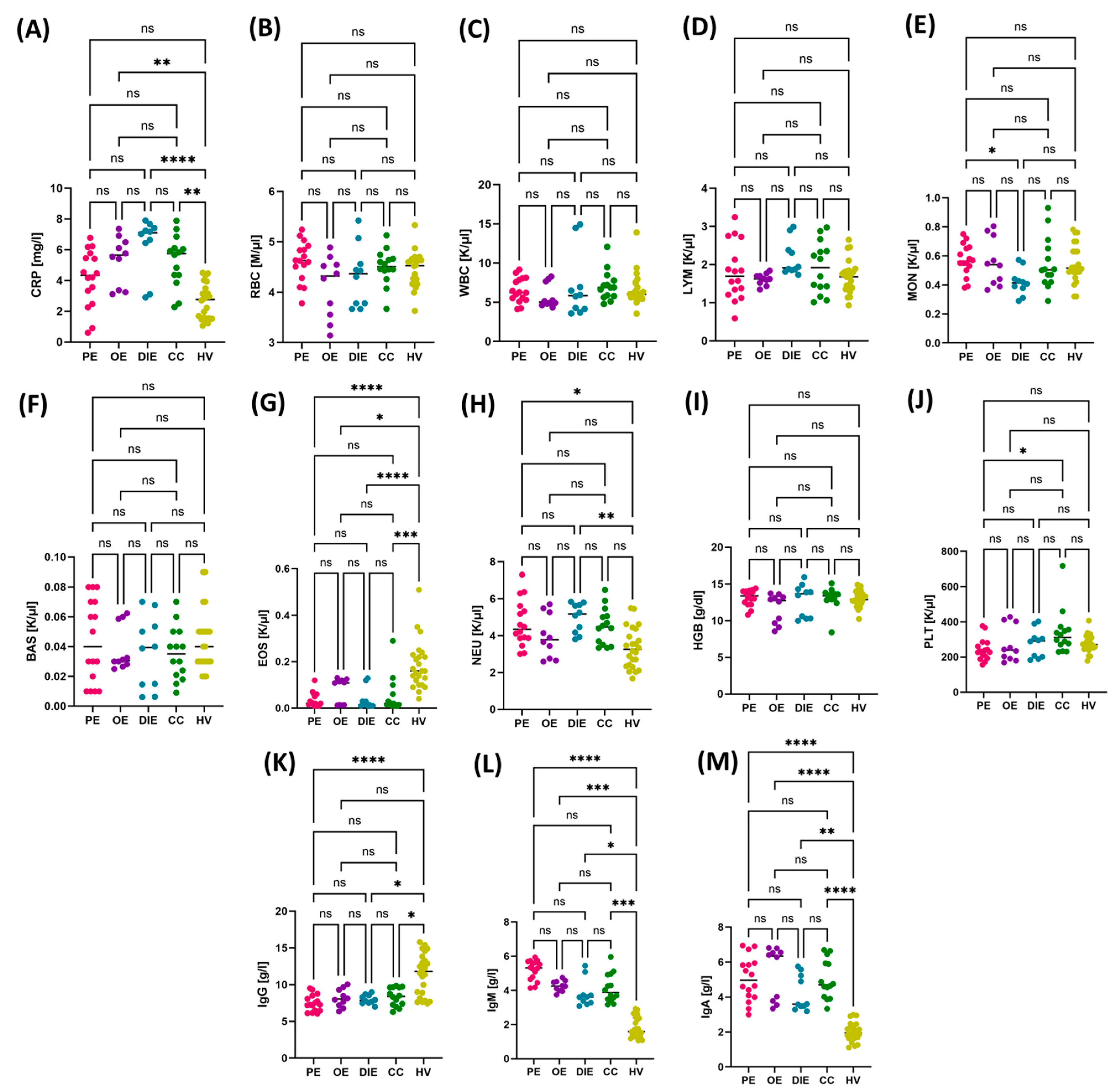
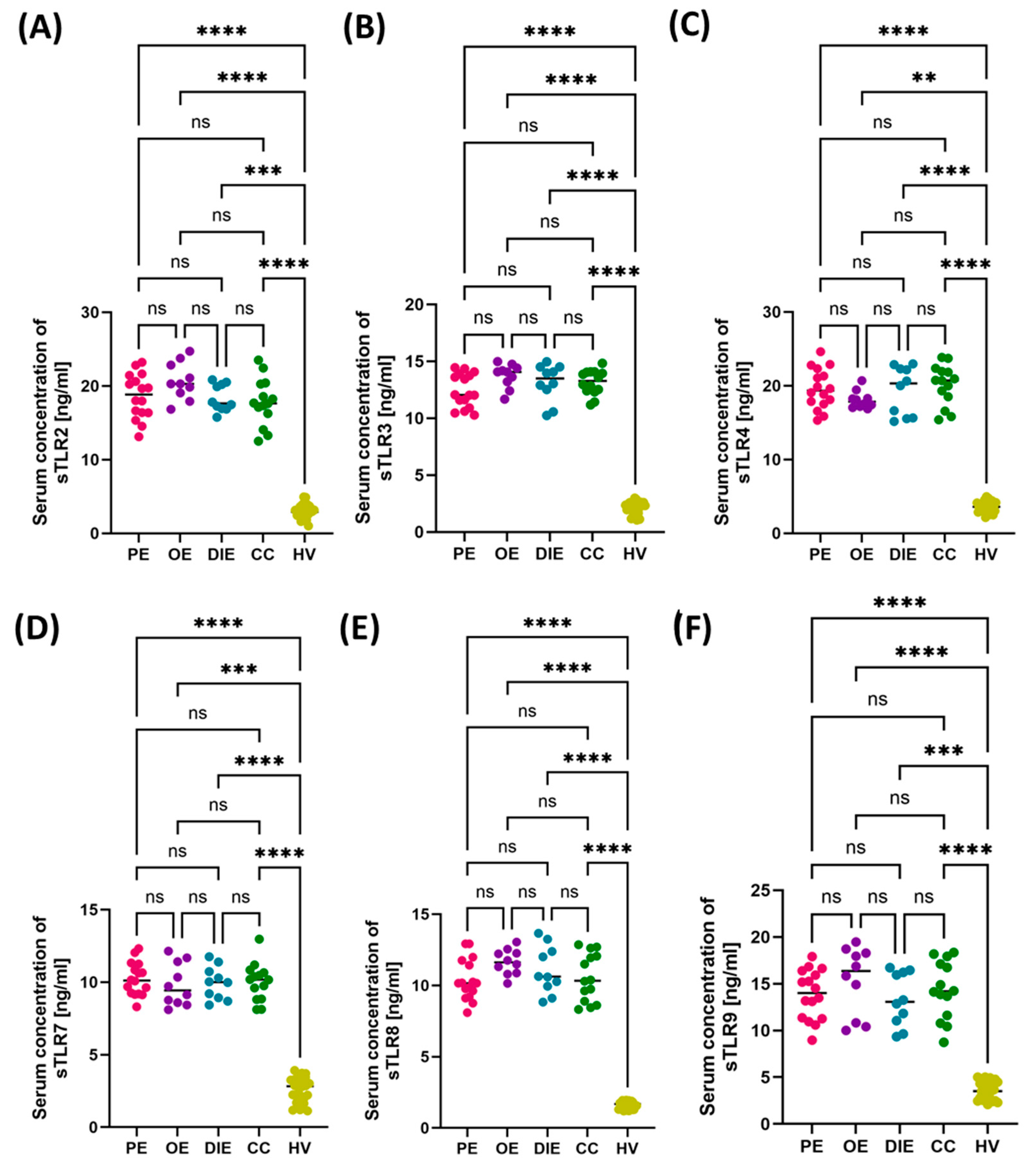
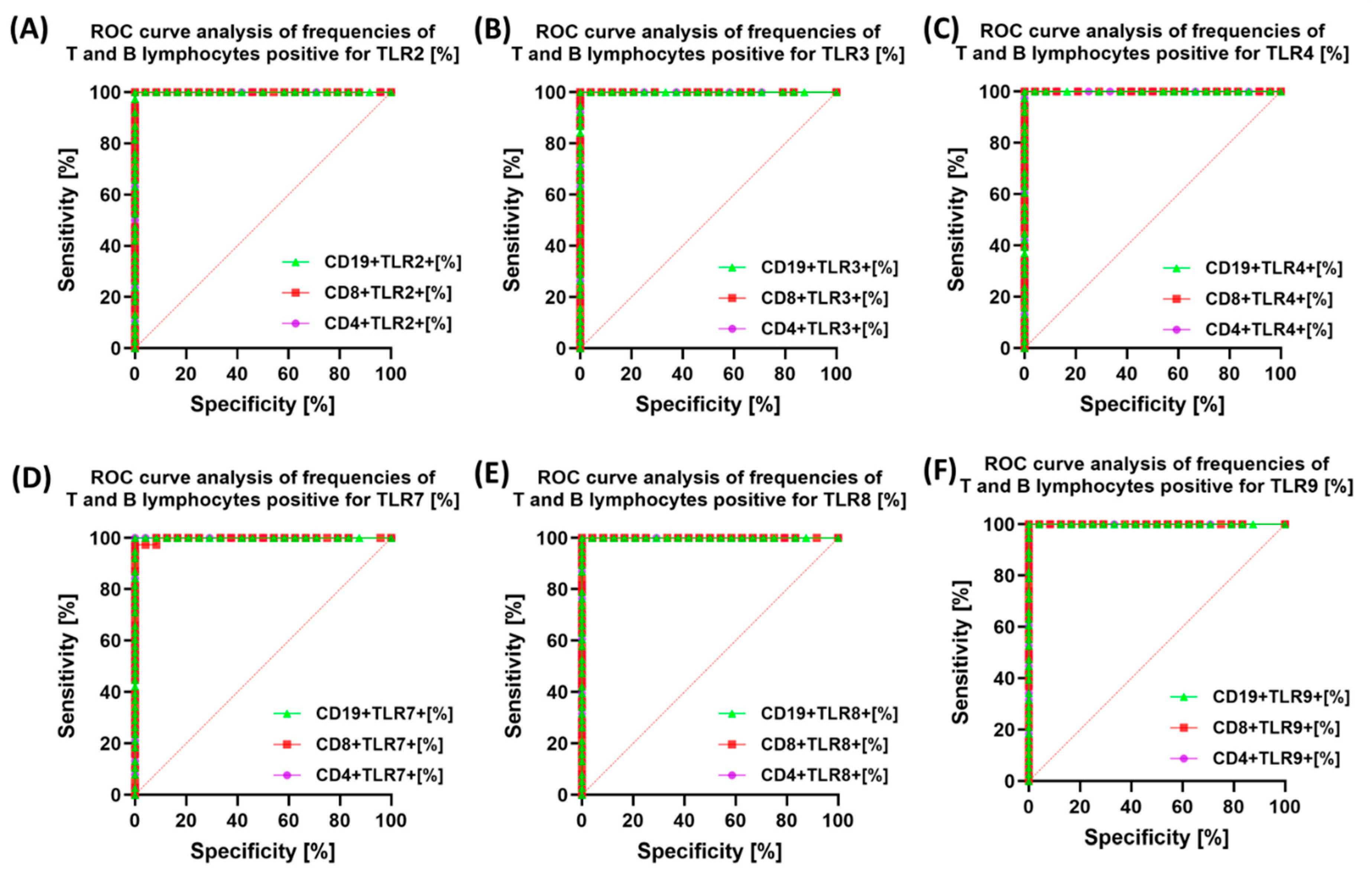
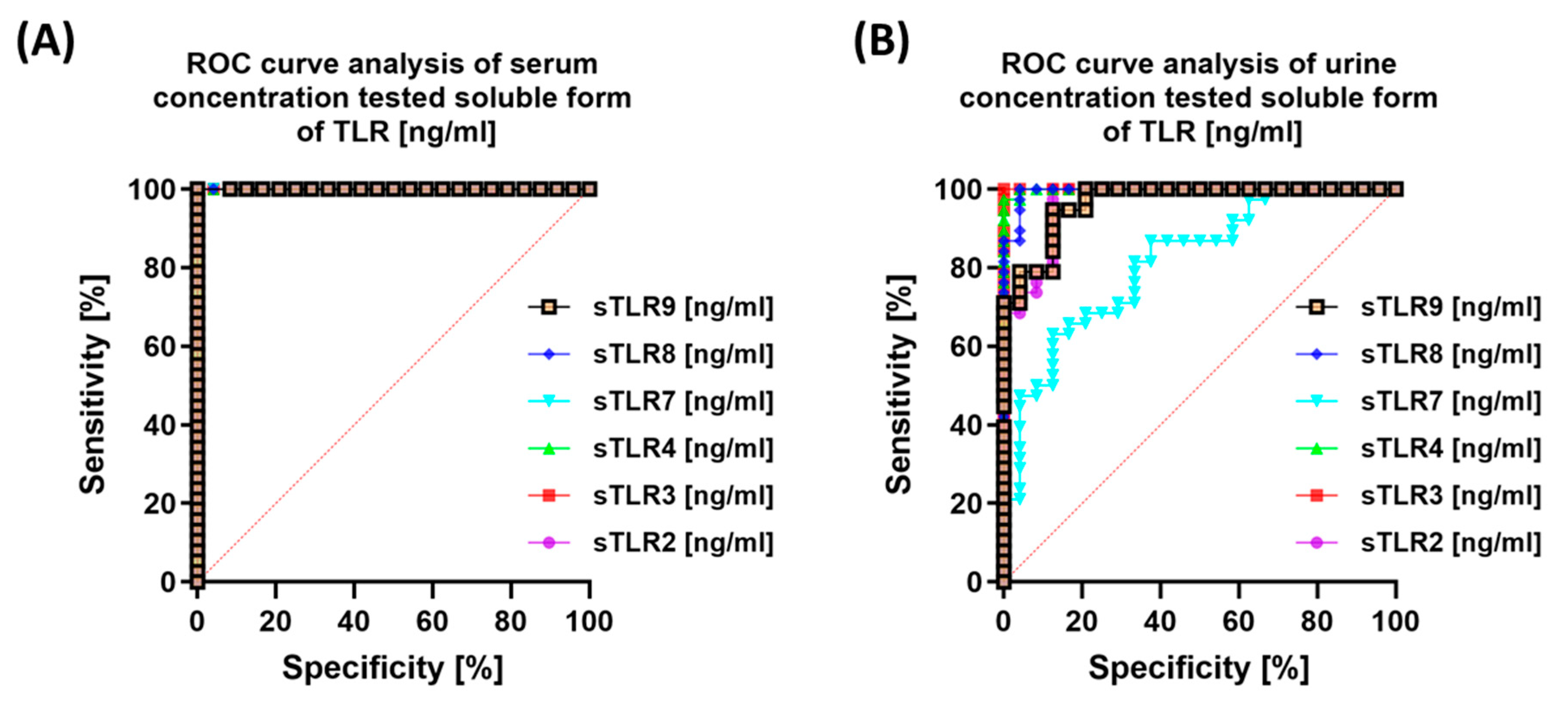
3.3. Diagnostic Value of TLR Expression and Their Soluble Forms in Endometriosis—ROC Curve Analysis
4. Discussion
- Peritoneal endometriosis (PE): Involves superficial lesions on the serosa of the abdominal and pelvic cavities. These lesions are typically multifocal, small, and vary in color (red, black, or white), often accompanied by local fibrosis and inflammatory infiltration. PE is most frequently diagnosed during laparoscopy and commonly manifests as chronic pelvic pain.
- Ovarian endometriosis (OE): Characterized by the presence of endometriotic ovarian cysts (so-called “chocolate cysts”) filled with thick, hemorrhagic content. These lesions contribute to structural damage to ovarian tissue, reduced ovarian reserve, and decreased fertility.
- Deeply infiltrating endometriosis (DIE): The most severe form, defined by lesions penetrating more than 5 mm into extraperitoneal tissues. DIE may affect the sacrotuberous ligaments, rectovaginal septum, bladder wall, intestines, and, rarely, the diaphragm or lungs. This form is typically associated with severe pelvic pain, dyspareunia, gastrointestinal symptoms, and infertility.
- Cesarean section scar endometriosis (CC): A less frequent form, occurring in the subcutaneous tissue or muscle layers of the anterior abdominal wall, usually in the region of surgical scars following cesarean delivery. It typically presents as cyclic pain localized to the scar area that intensifies during menstruation.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BAS | basophils |
| CC | cesarean section scar endometriosis |
| CD | cluster of differentiation |
| CD3 | T lymphocytes |
| CD4 | T helper lymphocytes |
| CD8 | cytotoxic T lymphocytes |
| CD19 | B lymphocytes |
| CD45 | total leukocytes |
| CRP | C-reactive protein |
| DAMPs | danger-associated molecular patterns |
| DIE | deeply infiltrating endometriosis |
| EM | endometriosis |
| EOS | eosinophils |
| HGB | hemoglobin |
| HV | healthy volunteers |
| IgA | immunoglobulin A |
| IgG | immunoglobulin G |
| IgM | immunoglobulin M |
| IL-6 | interleukin-6 |
| IL-8 | interleukin-8 |
| LPS | lipopolysaccharides |
| LYM | lymphocytes |
| MON | monocytes |
| MRI | magnetic resonance imaging |
| MyD88 | myeloid differentiation primary response 88 |
| NEU | neutrophils |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NK | natural killer cells |
| OE | ovarian endometriosis |
| PAMPs | pathogen-associated molecular patterns |
| PE | peritoneal endometriosis |
| PLT | platelets |
| PRRs | pattern recognition receptors |
| ROC | receiver operating characteristic |
| RBC | red blood cells |
| sTLR | soluble Toll-like receptor |
| STAT | signal transducer and activator of transcription |
| TLR | Toll-like receptor |
| TLRs | Toll-like receptors |
| TNF-α | tumor necrosis factor alpha |
| TRIF | TIR-domain-containing adapter-inducing interferon-β |
| WBC | white blood cells |
References
- Endometriosis. Available online: https://www.who.int/news-room/fact-sheets/detail/endometriosis (accessed on 26 March 2025).
- Parasar, P.; Ozcan, P.; Terry, K.L. Endometriosis: Epidemiology, Diagnosis and Clinical Management. Curr. Obstet. Gynecol. Rep. 2017, 6, 34–41. [Google Scholar] [CrossRef]
- Chmaj-Wierzchowska, K.; Kampioni, M.; Wilczak, M.; Opala, T. Do Inflammatory Factors Play a Significant Role in Etiopathogenesis of Endometrial Cysts? Part 1. Ann. Agric. Environ. Med. 2013, 20, 854–858. [Google Scholar]
- Malec-Milewska, M.; Horosz, B.; Sękowska, A.; Kolęda, I.; Kosson, D.; Jakiel, G. Pharmacological Treatment and Regional Anesthesia Techniques for Pain Management after Completion of Both Conservative and Surgical Treatment of Endometriosis and Pelvic Adhesions in Women with Chronic Pelvic Pain as a Mandated Treatment Strategy. Ann. Agric. Environ. Med. 2015, 22, 353–356. [Google Scholar] [CrossRef]
- Słabuszewska-Jóźwiak, A.; Ciebiera, M.; Baran, A.; Jakiel, G. Effectiveness of Laparoscopic Surgeries in Treating Infertility Related to Endometriosis. Ann. Agric. Environ. Med. 2015, 22, 329–331. [Google Scholar] [CrossRef] [PubMed]
- Santulli, P.; Tran, C.; Gayet, V.; Bourdon, M.; Maignien, C.; Marcellin, L.; Pocate-Cheriet, K.; Chapron, C.; de Ziegler, D. Oligo-Anovulation Is Not a Rarer Feature in Women with Documented Endometriosis. Fertil. Steril. 2018, 110, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sheng, S.; Pan, Z.; Zhao, L.; Yang, C.; Li, C.; Wang, F. Immune and Endocrine Regulation in Endometriosis: What We Know. J. Endometr. Uterine Disord. 2023, 4, 100049. [Google Scholar] [CrossRef]
- Chopyak, V.V.; Koval, H.D.; Havrylyuk, A.M.; Lishchuk-Yakymovych, K.A.; Potomkina, H.A.; Kurpisz, M.K. Immunopathogenesis of Endometriosis—A Novel Look at an Old Problem. Cent. Eur. J. Immunol. 2022, 47, 109–116. [Google Scholar] [CrossRef]
- Abramiuk, M.; Grywalska, E.; Małkowska, P.; Sierawska, O.; Hrynkiewicz, R.; Niedźwiedzka-Rystwej, P. The Role of the Immune System in the Development of Endometriosis. Cells 2022, 11, 2028. [Google Scholar] [CrossRef]
- Liu, H.; Li, J.; Guan, C.; Gao, W.; Li, Y.; Wang, J.; Yang, Y.; Du, Y. Endometriosis Is a Disease of Immune Dysfunction, Which Could Be Linked to Microbiota. Front. Genet. 2024, 15, 1386411. [Google Scholar] [CrossRef]
- Agostinis, C.; Balduit, A.; Mangogna, A.; Zito, G.; Romano, F.; Ricci, G.; Kishore, U.; Bulla, R. Immunological Basis of the Endometriosis: The Complement System as a Potential Therapeutic Target. Front. Immunol. 2021, 11, 599117. [Google Scholar] [CrossRef]
- Khan, K.N.; Kitajima, M.; Fujishita, A.; Nakashima, M.; Masuzaki, H. Toll-like Receptor System and Endometriosis. J. Obstet. Gynaecol. Res. 2013, 39, 1281–1292. [Google Scholar] [CrossRef]
- De Azevedo, B.C.; Mansur, F.; Podgaec, S. A Systematic Review of Toll-like Receptors in Endometriosis. Arch. Gynecol. Obstet. 2021, 304, 309–316. [Google Scholar] [CrossRef]
- Guo, B.; Chen, J.H.; Zhang, J.H.; Fang, Y.; Liu, X.J.; Zhang, J.; Zhu, H.Q.; Zhan, L. Pattern-Recognition Receptors in Endometriosis: A Narrative Review. Front. Immunol. 2023, 14, 1161606. [Google Scholar] [CrossRef]
- Almasi, M.Z.; Hosseini, E.; Jafari, R.; Aflatoonian, K.; Aghajanpour, S.; Ramazanali, F.; Moini, A.; Shahhoseini, M.; Afsharian, P.; Aflatoonian, R. Evaluation of Toll-like Receptor 3 (TLR3) Signaling Pathway Genes and Its Genetic Polymorphisms in Ectopic and Eutopic Endometrium of Women with Endometriosis. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 102153. [Google Scholar] [CrossRef]
- Jiang, C.; Liu, C.; Guo, J.; Chen, L.; Luo, N.; Qu, X.; Yang, W.; Ren, Q.; Cheng, Z. The Expression of Toll-like Receptors in Eutopic and Ectopic Endometrium and Its Implication in the Inflammatory Pathogenesis of Adenomyosis. Sci. Rep. 2017, 7, 7365. [Google Scholar] [CrossRef] [PubMed]
- Jafari, R.; Taghavi, S.A.; Amirchaghmaghi, E.; Yazdi, R.S.; Karimian, L.; Ashrafi, M.; Aflatoonian, R. Detailed Investigation of Downstream TLR Signaling in the Follicular Cells of Women with Endometriosis. J. Reprod. Infertil. 2020, 21, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Kusama, K.; Satoyoshi, A.; Azumi, M.; Yoshie, M.; Kojima, J.; Mizuno, Y.; Ono, M.; Nishi, H.; Kajihara, T.; Tamura, K. Toll-like Receptor Signaling Pathway Triggered by Inhibition of Serpin A1 Stimulates Production of Inflammatory Cytokines by Endometrial Stromal Cells. Front. Endocrinol. 2022, 13, 966455. [Google Scholar] [CrossRef]
- Khan, K.N.; Fujishita, A.; Hiraki, K.; Kitajima, M.; Nakashima, M.; Fushiki, S.; Kitawaki, J. Bacterial Contamination Hypothesis: A New Concept in Endometriosis. Reprod. Med. Biol. 2018, 17, 125–133. [Google Scholar] [CrossRef]
- Qin, R.; Tian, G.; Liu, J.; Cao, L. The Gut Microbiota and Endometriosis: From Pathogenesis to Diagnosis and Treatment. Front. Cell Infect. Microbiol. 2022, 12, 1069557. [Google Scholar] [CrossRef] [PubMed]
- Talwar, C.; Singh, V.; Kommagani, R. The Gut Microbiota: A Double-Edged Sword in Endometriosis. Biol. Reprod. 2022, 107, 881–901. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, C. Role of the Gut Microbiota in the Pathogenesis of Endometriosis: A Review. Front. Microbiol. 2024, 15, 1363455. [Google Scholar] [CrossRef]
- Malvezzi, H.; Cestari, B.A.; Mendes, H.; Hernandes, C.; Podgaec, S. Peritoneal Fluid Microbiota Profile of Patients with Deep Endometriosis. Microb. Pathog. 2025, 199, 107244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Mohd Sahardi, N.F.N.; Di, W.; Long, X.; Shafiee, M.N. The Gut–Endometrium Axis: Exploring the Role of Microbiome in the Pathogenesis and Treatment of Endometrial Cancer—A Narrative Review. Cancers 2025, 17, 1044. [Google Scholar] [CrossRef]
- Weber, I.; Sienko, A.; Urban, A.; Szwed, C.; Czajkowski, K.; Basta, P.; Sienko, J. Relationship between the Gut Microbiome and Endometriosis and Its Role in Pathogenesis, Diagnosis, and Treatment: A Systematic Review. Ginekol. Pol. 2024, 95, 893–901. [Google Scholar] [CrossRef]
- Uzuner, C.; Mak, J.; El-Assaad, F.; Condous, G. The Bidirectional Relationship between Endometriosis and Microbiome. Front. Endocrinol. 2023, 14, 1110824. [Google Scholar] [CrossRef]
- Hearn-Yeates, F.; Horne, A.W.; O’Mahony, S.M.; Saunders, P.T.K. Microbiome: The Impact of the Microbiota–Gut–Brain Axis on Endometriosis-Associated Symptoms: Mechanisms and Opportunities for Personalised Management Strategies. Reprod. Fertil. 2024, 5, e230085. [Google Scholar] [CrossRef] [PubMed]
- Greygoose, E.; Metharom, P.; Kula, H.; Seckin, T.K.; Seckin, T.A.; Ayhan, A.; Yu, Y. The Estrogen–Immune Interface in Endometriosis. Cells 2025, 14, 58. [Google Scholar] [CrossRef]
- Eisa, M.A.; Mansour, A.M.; Salama, S.A.; Elsadek, B.E.M.; Ashour, A.A.; Abdelghany, T.M. Estrogen/Estrogen Receptor Activation Protects against DEN-Induced Liver Fibrosis in Female Rats via Modulating TLR-4/NF-Kβ Signaling. Eur. J. Pharmacol. 2023, 960, 176165. [Google Scholar] [CrossRef]
- Zandieh, Z.; Amjadi, F.; Ashrafi, M.; Aflatoonian, A.; Fazeli, A.; Aflatoonian, R. The Effect of Estradiol and Progesterone on Toll Like Receptor Gene Expression in A Human Fallopian Tube Epithelial Cell Line. Cell J. 2016, 17, 678–691. [Google Scholar] [CrossRef]
- Gou, Y.; Li, X.; Li, P.; Zhang, H.; Xu, T.; Wang, H.; Wang, B.; Ma, X.; Jiang, X.; Zhang, Z. Estrogen Receptor β Upregulates CCL2 via NF-κB Signaling in Endometriotic Stromal Cells and Recruits Macrophages to Promote the Pathogenesis of Endometriosis. Hum. Reprod. 2019, 34, 646–658. [Google Scholar] [CrossRef] [PubMed]
- Huhtinen, K.; Desai, R.; Ståhle, M.; Salminen, A.; Handelsman, D.J.; Perheentupa, A.; Poutanen, M. Endometrial and Endometriotic Concentrations of Estrone and Estradiol Are Determined by Local Metabolism Rather than Circulating Levels. J. Clin. Endocrinol. Metab. 2012, 97, 4228–4235. [Google Scholar] [CrossRef]
- Su, L.; Sun, Y.; Ma, F.; Lü, P.; Huang, H.; Zhou, J. Progesterone Inhibits Toll-like Receptor 4-Mediated Innate Immune Response in Macrophages by Suppressing NF-κB Activation and Enhancing SOCS1 Expression. Immunol. Lett. 2009, 125, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Endometriosis. Available online: https://www.asrm.org/practice-guidance/coding-resources/coding/coding-summaries/endometriosis/ (accessed on 14 May 2025).
- Kedzia, M.; Basta, P.; Czajkowski, K.; Gogacz, M.; Spaczynski, R.; Mroczkowska, B.; Stojko, R.; Szaflik, T.; Szubert, M.; Szyllo, K.; et al. Guidelines of the Polish Society of Gynecologists and Obstetricians on the Management of Women with Endometriosis. Ginekol. Pol. 2024, 95, 729–758. [Google Scholar] [CrossRef] [PubMed]
- Kedzia, M.; Osinski, M.; Mantaj, U.; Wender-Ozegowska, E. Endometriosis Is Associated with an Increased Whole-Blood Thrombogenicity Detected by a Novel Automated Microchip Flow-Chamber System (T-TAS®). Ginekol. Pol. 2023, 94, 291–297. [Google Scholar] [CrossRef]
- Chen, Y.; Waseem, S.; Luo, L. Advances in the Diagnosis and Management of Endometriosis: A Comprehensive Review. Pathol. —Res. Pract. 2025, 266, 155813. [Google Scholar] [CrossRef]
- Duan, T.; Du, Y.; Xing, C.; Wang, H.Y.; Wang, R.-F. Toll-Like Receptor Signaling and Its Role in Cell-Mediated Immunity. Front. Immunol. 2022, 13, 812774. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Wu, K.-H.; Wu, H.-P. Unraveling the Complexities of Toll-like Receptors: From Molecular Mechanisms to Clinical Applications. Int. J. Mol. Sci. 2024, 25, 5037. [Google Scholar] [CrossRef]
- Marchionni, E.; Porpora, M.G.; Megiorni, F.; Piacenti, I.; Giovannetti, A.; Marchese, C.; Benedetti Panici, P.; Pizzuti, A. TLR4 T399I Polymorphism and Endometriosis in a Cohort of Italian Women. Diagnostics 2020, 10, 255. [Google Scholar] [CrossRef]
- Khan, K.N.; Akira, F.; Kitawaki, J.; Mori, T. Educational Lecture 1—Role of Innate and Adaptive Immunity in Endometriosis. J. Reprod. Immunol. 2022, 153, 103696. [Google Scholar] [CrossRef]
- Su, W.; Cui, H.; Wu, D.; Yu, J.; Ma, L.; Zhang, X.; Huang, Y.; Ma, C. Suppression of TLR4-MyD88 Signaling Pathway Attenuated Chronic Mechanical Pain in a Rat Model of Endometriosis. J. Neuroinflammation 2021, 18, 65. [Google Scholar] [CrossRef]
- Khan, K.N.; Kitajima, M.; Hiraki, K.; Yamaguchi, N.; Katamine, S.; Matsuyama, T.; Nakashima, M.; Fujishita, A.; Ishimaru, T.; Masuzaki, H. Escherichia Coli Contamination of Menstrual Blood and Effect of Bacterial Endotoxin on Endometriosis. Fertil. Steril. 2010, 94, 2860–2863.e3. [Google Scholar] [CrossRef] [PubMed]
- Campos, G.B.; Marques, L.M.; Rezende, I.S.; Barbosa, M.S.; Abrão, M.S.; Timenetsky, J. Mycoplasma Genitalium Can Modulate the Local Immune Response in Patients with Endometriosis. Fertil. Steril. 2018, 109, 549–560.e4. [Google Scholar] [CrossRef]
- Hayashi, C.; Chishima, F.; Sugitani, M.; Ichikawa, G.; Nakazawa-Watanabe, T.; Sugita, K.; Suzuki, M.; Nemoto, N.; Yamamoto, T. Relationship Between Toll-like Receptor-4 and mPGES-1 Gene Expression in Local Lesions of Endometriosis Patients. Am. J. Reprod. Immunol. 2013, 69, 231–239. [Google Scholar] [CrossRef]
- Latha, M.; Vaidya, S.; Movva, S.; Chava, S.; Govindan, S.; Govatati, S.; Banoori, M.; Hasan, Q.; Kodati, V.L. Molecular Pathogenesis of Endometriosis; Toll-Like Receptor-4 A896G (D299G) Polymorphism: A Novel Explanation. Genet. Test. Mol. Biomark. 2011, 15, 181–184. [Google Scholar] [CrossRef]
- Sobstyl, M.; Niedźwiedzka-Rystwej, P.; Grywalska, E.; Korona-Głowniak, I.; Sobstyl, A.; Bednarek, W.; Roliński, J. Toll-Like Receptor 2 Expression as a New Hallmark of Advanced Endometriosis. Cells 2020, 9, 1813. [Google Scholar] [CrossRef] [PubMed]
- Attaman, J.A.; Stanic, A.K.; Kim, M.; Lynch, M.P.; Rueda, B.R.; Styer, A.K. The Anti-Inflammatory Impact of Omega-3 Polyunsaturated Fatty Acids During the Establishment of Endometriosis-Like Lesions. Am. J. Reprod. Immunol. 2014, 72, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Kajihara, H.; Yamada, Y.; Kanayama, S.; Furukawa, N.; Noguchi, T.; Haruta, S.; Yoshida, S.; Sado, T.; Oi, H.; Kobayashi, H. New Insights into the Pathophysiology of Endometriosis: From Chronic Inflammation to Danger Signal. Gynecol. Endocrinol. 2011, 27, 73–79. [Google Scholar] [CrossRef]
- Henry, L.; Tremblay-Lemoine, P.-L.; Timmermans, M.; Ravet, S.; Munaut, C.; Nisolle, M.; Henry, L. The Endometrial Microbiota: Challenges and Prospects. Medicina 2023, 59, 1540. [Google Scholar] [CrossRef]
- Hernandes, C.; Gueuvoghlanian-Silva, B.Y.; Monnaka, V.U.; Ribeiro, N.M.; Pereira, W.d.O.; Podgaec, S. Regulatory T Cells Isolated from Endometriotic Peritoneal Fluid Express a Different Number of Toll-like Receptors. Einstein 2020, 18, eAO5294. [Google Scholar] [CrossRef]
- Xiao, F.; Liu, X.; Guo, S.-W. Platelets and Regulatory T Cells May Induce a Type 2 Immunity That Is Conducive to the Progression and Fibrogenesis of Endometriosis. Front. Immunol. 2020, 11, 610963. [Google Scholar] [CrossRef]
- Podgaec, S.; Rizzo, L.V.; Fernandes, L.F.C.; Baracat, E.C.; Abrao, M.S. CD4+CD25highFoxp3+ Cells Increased in the Peritoneal Fluid of Patients with Endometriosis. Am. J. Reprod. Immunol. 2012, 68, 301–308. [Google Scholar] [CrossRef]
- Peluso, C.; Christofolini, D.M.; Goldman, C.S.; Mafra, F.A.; Cavalcanti, V.; Barbosa, C.P.; Bianco, B. TYK2 Rs34536443 Polymorphism Is Associated with a Decreased Susceptibility to Endometriosis-Related Infertility. Hum. Immunol. 2013, 74, 93–97. [Google Scholar] [CrossRef]
- Klemmt, P.A.B.; Starzinski-Powitz, A. Molecular and Cellular Pathogenesis of Endometriosis. Curr. Women’s Health Rev. 2018, 14, 106–116. [Google Scholar] [CrossRef]
- Riccio, L.G.C.; Jeljeli, M.; Santulli, P.; Chouzenoux, S.; Doridot, L.; Nicco, C.; Reis, F.M.; Abrão, M.S.; Chapron, C.; Batteux, F. B Lymphocytes Inactivation by Ibrutinib Limits Endometriosis Progression in Mice. Hum. Reprod. 2019, 34, 1225–1234. [Google Scholar] [CrossRef] [PubMed]
- Kisovar, A.; Becker, C.M.; Granne, I.; Southcombe, J.H. The Role of CD8+ T Cells in Endometriosis: A Systematic Review. Front. Immunol. 2023, 14, 1225639. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.L.; Rosa, N.N.; Martins, C.; Ângelo-Dias, M.; Borrego, L.M.; Lima, J. The Role of NK and T Cells in Endometriosis. Int. J. Mol. Sci. 2024, 25, 10141. [Google Scholar] [CrossRef] [PubMed]
- Calmon, M.S.; Lemos, F.F.B.; Silva Luz, M.; Rocha Pinheiro, S.L.; de Oliveira Silva, L.G.; Correa Santos, G.L.; Rocha, G.R.; Freire de Melo, F. Immune Pathway through Endometriosis to Ovarian Cancer. World J. Clin. Oncol. 2024, 15, 496–522. [Google Scholar] [CrossRef]
- Park, M.; Kim, Y.S.; Song, H. Macrophages: A Double-Edged Sword in Female Reproduction and Disorders. Exp. Mol. Med. 2025, 57, 285–297. [Google Scholar] [CrossRef]
- Kobayashi, H.; Imanaka, S. Understanding the Molecular Mechanisms of Macrophage Polarization and Metabolic Reprogramming in Endometriosis: A Narrative Review. Reprod. Med. Biol. 2022, 21, e12488. [Google Scholar] [CrossRef]
- Panir, K.; Hull, M.L.; Greaves, E. Chapter 2—Macrophages in Endometriosis: They Came, They Saw, They Conquered. In Immunology of Endometriosis; Koga, K., Ed.; Reproductive Immunology; Academic Press: Cambridge, MA, USA, 2022; pp. 13–41. ISBN 978-0-12-820661-4. [Google Scholar]
- Ścieżyńska, A.; Komorowski, M.; Soszyńska, M.; Malejczyk, J. NK Cells as Potential Targets for Immunotherapy in Endometriosis. J. Clin. Med. 2019, 8, 1468. [Google Scholar] [CrossRef]
- Meggyes, M.; Szereday, L.; Bohonyi, N.; Koppan, M.; Szegedi, S.; Marics-Kutas, A.; Marton, M.; Totsimon, A.; Polgar, B. Different Expression Pattern of TIM-3 and Galectin-9 Molecules by Peripheral and Peritoneal Lymphocytes in Women with and without Endometriosis. Int. J. Mol. Sci. 2020, 21, 2343. [Google Scholar] [CrossRef]
- Sourial, S.; Tempest, N.; Hapangama, D.K. Theories on the Pathogenesis of Endometriosis. Int. J. Reprod. Med. 2014, 2014, 179515. [Google Scholar] [CrossRef]
- Ding, S.; Lin, Q.; Zhu, T.; Li, T.; Zhu, L.; Wang, J.; Zhang, X. Is There a Correlation between Inflammatory Markers and Coagulation Parameters in Women with Advanced Ovarian Endometriosis? BMC Women’s Health 2019, 19, 169. [Google Scholar] [CrossRef]
- El-Zayat, S.R.; Sibaii, H.; Mannaa, F.A. Toll-like Receptors Activation, Signaling, and Targeting: An Overview. Bull. Natl. Res. Cent. 2019, 43, 187. [Google Scholar] [CrossRef]
- Fakhry, N.; Gowily, A.; Okda, T.; Houssen, M. Serum Soluble Toll-like Receptor 2 and 4 as Diagnostic and Prognostic Biomarkers for Non-Hodgkin Lymphoma. Contemp. Oncol. 2020, 24, 157–162. [Google Scholar] [CrossRef]
- AlQallaf, H.; Hamada, Y.; Blanchard, S.; Shin, D.; Gregory, R.; Srinivasan, M. Differential Profiles of Soluble and Cellular Toll like Receptor (TLR)-2 and 4 in Chronic Periodontitis. PLoS ONE 2018, 13, e0200231. [Google Scholar] [CrossRef]
- Ten Oever, J.; Kox, M.; van de Veerdonk, F.L.; Mothapo, K.M.; Slavcovici, A.; Jansen, T.L.; Tweehuysen, L.; Giamarellos-Bourboulis, E.J.; Schneeberger, P.M.; Wever, P.C.; et al. The Discriminative Capacity of Soluble Toll-like Receptor (sTLR)2 and sTLR4 in Inflammatory Diseases. BMC Immunol. 2014, 15, 55. [Google Scholar] [CrossRef]
- Song, J.; Abraham, S.N. TLR Mediated Immune Responses in the Urinary Tract. Curr. Opin. Microbiol. 2008, 11, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhang, M.; Wang, S.; Han, Y.; Fang, X. Common and Specific Gene Signatures among Three Different Endometriosis Subtypes. PeerJ 2020, 8, e8730. [Google Scholar] [CrossRef] [PubMed]
- Imperiale, L.; Nisolle, M.; Noël, J.-C.; Fastrez, M. Three Types of Endometriosis: Pathogenesis, Diagnosis and Treatment. State of the Art. J. Clin. Med. 2023, 12, 994. [Google Scholar] [CrossRef] [PubMed]
- Arkoudis, N.-A.; Moschovaki-Zeiger, O.; Prountzos, S.; Spiliopoulos, S.; Kelekis, N. Caesarean-Section Scar Endometriosis (CSSE): Clinical and Imaging Fundamentals of an Underestimated Entity. Clin. Radiol. 2023, 78, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J.; Zhang, X. An Update on the Multifaceted Role of NF-kappaB in Endometriosis. Int. J. Biol. Sci. 2022, 18, 4400–4413. [Google Scholar] [CrossRef]
- Zdrojkowski, Ł.; Jasiński, T.; Ferreira-Dias, G.; Pawliński, B.; Domino, M. The Role of NF-κB in Endometrial Diseases in Humans and Animals: A Review. Int. J. Mol. Sci. 2023, 24, 2901. [Google Scholar] [CrossRef]
- Inui, H.; Kawakita, T.; Murayama, M.; Nakagawa, T.; Sasada, H.; Shinohara, A.; Aragaki, R.; Kagawa, T.; Kadota, Y.; Kato, T.; et al. Effects of STAT Inhibitors in Mouse Models of Endometriosis. Reprod. Sci. 2023, 30, 2449–2456. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.R.; Kasper, S.; Burns, K.A. An Emerging Role for Neutrophils in the Pathogenesis of Endometriosis. npj Womens Health 2025, 3, 9. [Google Scholar] [CrossRef]
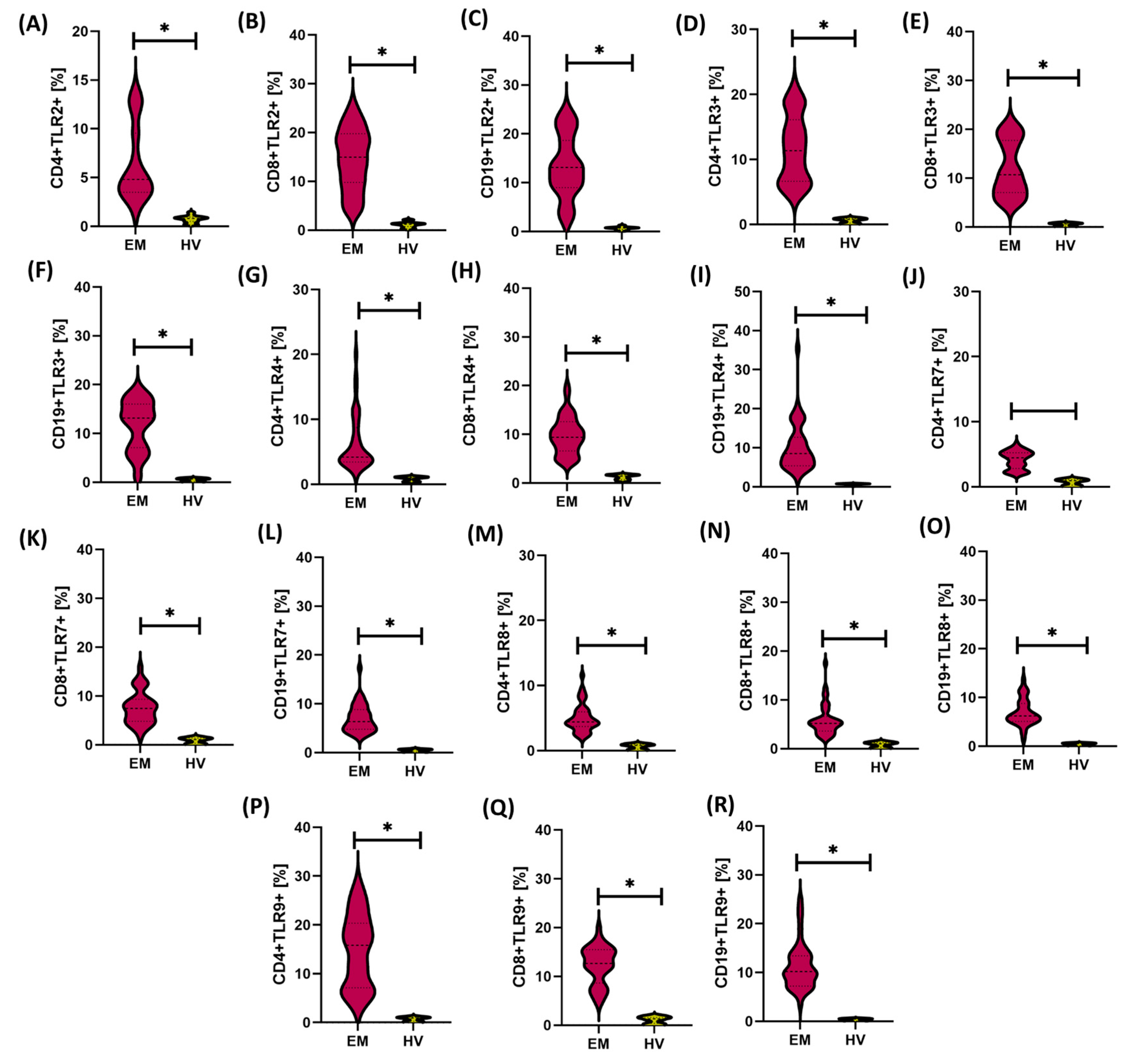
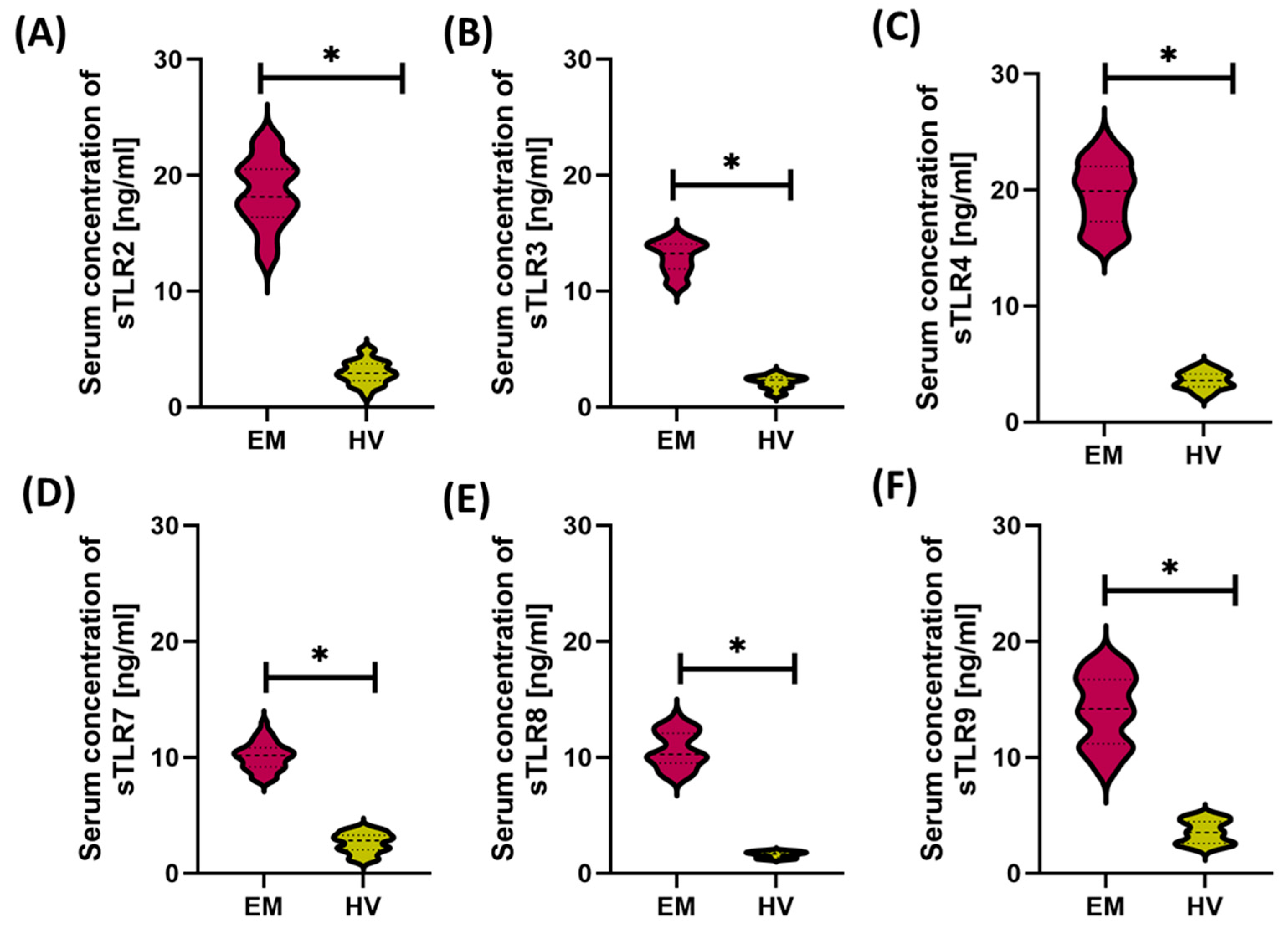
| Parameters | EM (n = 50) | HV (n = 24) | p-Value | |||
|---|---|---|---|---|---|---|
| Median | Q1–Q3 | Median | Q1–Q3 | |||
| Hematological, inflammatory, and immunoglobulin parameters | RBC [M/µL] | 4.52 | 4.10–4.71 | 4.53 | 4.18–4.64 | 0.850 |
| WBC [K/µL] | 6.10 | 5.04–7.73 | 6.01 | 5.71–7.15 | 0.718 | |
| LYM [K/µL] | 1.79 | 1.43–2.29 | 1.68 | 1.39–1.83 | 0.180 | |
| MON [K/µL] | 0.52 | 0.42–0.60 | 0.52 | 0.49–0.63 | 0.626 | |
| BAS [K/µL] | 0.03 | 0.02–0.06 | 0.04 | 0.03–0.05 | 0.367 | |
| EOS [K/µL] | 0.02 | 0.01–0.07 | 0.16 | 0.11–0.23 | <0.0001 * | |
| NEU [K/µL] | 4.43 | 3.68–5.43 | 3.26 | 2.38–4.16 | <0.0001 * | |
| HGB [g/dL] | 13.15 | 12.20–13.85 | 12.90 | 12.50–13.80 | 0.895 | |
| PLT [K/µL] | 256.55 | 206.75–335.00 | 270.5 | 249.00–302.50 | 0.562 | |
| CRP [mg/L] | 5.61 | 3.51–6.60 | 2.76 | 1.54–3.32 | <0.0001 * | |
| IgG [g/L] | 7.84 | 7.20–8.75 | 11.81 | 8.53–13.55 | <0,0001 * | |
| IgM [g/L] | 4.28 | 3.71–5.10 | 1.59 | 1.38–2.33 | <0,0001 * | |
| IgA [g/L] | 4.70 | 3.76–5.94 | 1.95 | 1.68–2.36 | <0,0001 * | |
| Immunophenotypic characterization of lymphocyte subpopulations | CD45+ [%] | 87.86 | 81.86–91.80 | 96.28 | 92.83–98.05 | <0.0001 * |
| CD3+ [%] | 66.02 | 59.95–74.20 | 72.34 | 70.70–76.54 | 0.0012 * | |
| CD4+ [%] | 41.84 | 27.20–51.37 | 47.49 | 44.77–49.84 | 0.0144 * | |
| CD8+ [%] | 20.51 | 17.31–28.39 | 27.23 | 25.88–28.52 | 0.0021 * | |
| CD19+ [%] | 11.27 | 10.17–14.35 | 12.70 | 11.82–13.69 | 0.032 * | |
| NK cells [%] | 9.24 | 4.26–17.72 | 7.90 | 7.67–10.89 | 0.042 * | |
| Group | n | Median Age (Years) | Age Range (Years) |
|---|---|---|---|
| Peritoneal endometriosis (PE) | 16 | 33 | 26–46 |
| Ovarian endometriosis (OE) | 10 | 35.5 | 26–47 |
| Deeply infiltrating endometriosis (DIE) | 10 | 36.5 | 27–47 |
| Cesarean section scar endometriosis (CC) | 14 | 34 | 24–42 |
| HV | 24 | 33 | 18–57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobstyl, A.; Mertowska, P.; Mertowski, S.; Tarkowski, R.; Dudziński, D.; Kotowski, M.; Bojarski, K.; Stelmach, B.; Chermuła, B.; Brązert, M.; et al. Expression of Toll-like Receptors on Lymphocyte Subpopulations and Their Soluble Forms in Serum and Urine of Women with Endometriosis. Cells 2025, 14, 1273. https://doi.org/10.3390/cells14161273
Sobstyl A, Mertowska P, Mertowski S, Tarkowski R, Dudziński D, Kotowski M, Bojarski K, Stelmach B, Chermuła B, Brązert M, et al. Expression of Toll-like Receptors on Lymphocyte Subpopulations and Their Soluble Forms in Serum and Urine of Women with Endometriosis. Cells. 2025; 14(16):1273. https://doi.org/10.3390/cells14161273
Chicago/Turabian StyleSobstyl, Anna, Paulina Mertowska, Sebastian Mertowski, Rafał Tarkowski, Dominik Dudziński, Michał Kotowski, Krzysztof Bojarski, Bogusława Stelmach, Błażej Chermuła, Maciej Brązert, and et al. 2025. "Expression of Toll-like Receptors on Lymphocyte Subpopulations and Their Soluble Forms in Serum and Urine of Women with Endometriosis" Cells 14, no. 16: 1273. https://doi.org/10.3390/cells14161273
APA StyleSobstyl, A., Mertowska, P., Mertowski, S., Tarkowski, R., Dudziński, D., Kotowski, M., Bojarski, K., Stelmach, B., Chermuła, B., Brązert, M., & Grywalska, E. (2025). Expression of Toll-like Receptors on Lymphocyte Subpopulations and Their Soluble Forms in Serum and Urine of Women with Endometriosis. Cells, 14(16), 1273. https://doi.org/10.3390/cells14161273






