Calpain in Traumatic Brain Injury: From Cinderella to Central Player
Abstract
1. Introduction
2. Calpain Biology and TBI
2.1. Calpain and Regulation
2.2. Loss of Calcium Homeostasis Following TBI
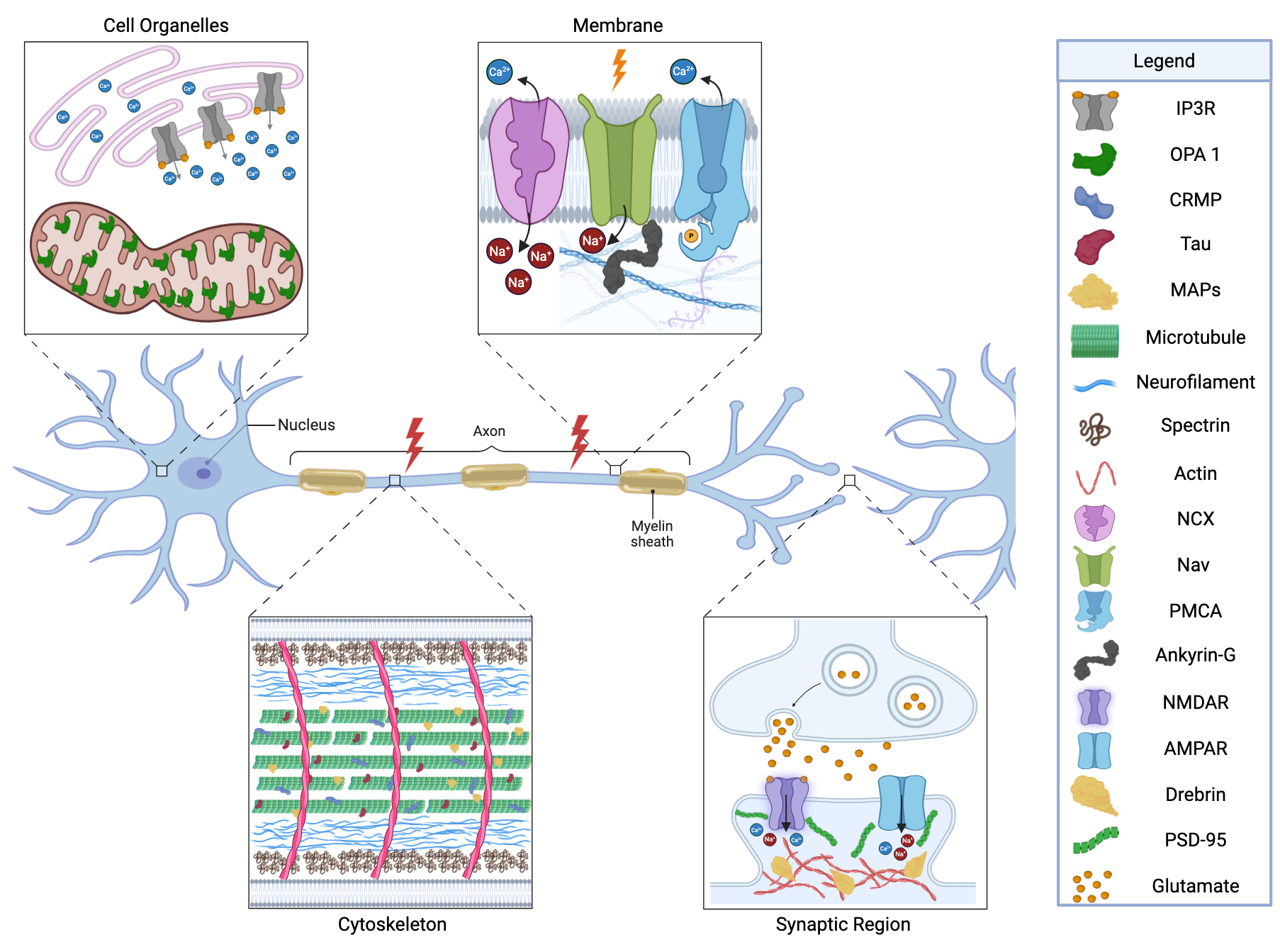
3. Calpain Substrates in the Traumatically Injured Brain
3.1. Cytoskeletal Substrates
3.1.1. Spectrin and ⍺II-Spectrin
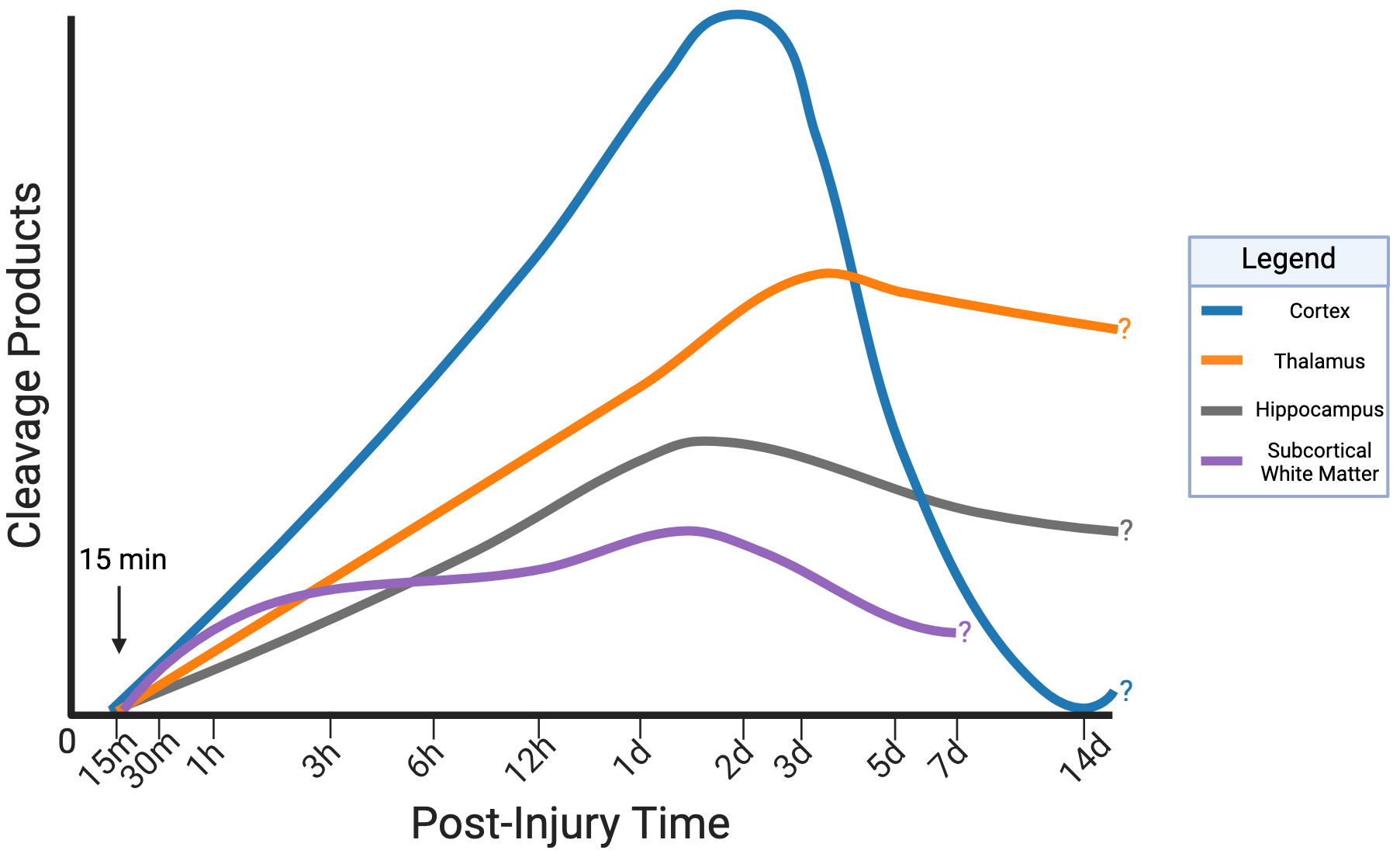
3.1.2. Spatiotemporal Pattern of ⍺II-Spectrin Cleavage
3.1.3. Neurofilaments
3.1.4. Microtubules and Microtubule-Associated Proteins (MAP’s)
3.1.5. Collapsin Response Mediator Proteins (CRMP’s)
3.2. Calpain Targets of the Membrane/Axolemma
3.2.1. Sodium Channels and Ankyrin-G
3.2.2. Failed Calcium Clearance Mechanisms
3.3. Synaptic Targets
3.3.1. Postsynaptic Scaffolds
3.3.2. Glutamate Receptors
3.4. Organelle Targets
3.4.1. Endoplasmic Reticulum
3.4.2. Mitochondria
3.5. Emerging Targets
4. Calpain in the Transition from Acute TBI to Chronic Neurodegeneration and SARM1
5. Translational Aspects of Calpain Biology
5.1. Biomarkers
5.2. Previous and Current Approaches of Calpain-Inhibitors
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TBI | Traumatic Brain Injury |
| LTP | Long-Term Potentiation |
| Refs. | References |
| AMPA(-R) | α-Amino-3-Hydroxy-5-Methyl-4-Isoxazolepropionic Acid (-Receptor) |
| NMDA(-R) | N-Methyl D-Aspartate (-Receptor) |
| VGCC | Voltage-Gated Calcium Channel |
| NCX | Sodium-Calcium Exchanger |
| mGluR | Metabotropic Glutamate Receptor |
| IP3(-R) | Inositol 1,4,5-Trisphosphate (-Receptor) |
| CICR | Calcium Induced Calcium Release |
| ER | Endoplasmic Reticulum |
| RyR | Ryanodine Receptor |
| MCU | Mitochondrial Calcium Uniporter |
| ROS | Reactive Oxygen Species |
| mNCX | Mitochondrial Sodium-Calcium Exchanger |
| SBDP | Spectrin Breakdown Product |
| SNTF | Spectrin N-Terminal Fragment |
| βsBDP | β-Spectrin Breakdown Product |
| CCI | Controlled Cortical Impact |
| I/A | Impact Acceleration Injury |
| CHI | Closed Head Injury |
| BOP | Blast Overpressure |
| FPI | Fluid Percussion Injury |
| Nf(-L, -M, -H) | Neurofilament (-light, -medium, -heavy) |
| MAP | Microtubule-Associated Protein |
| CRMP | Collapsing Response Mediator Protein |
| Kv | Voltage- Gated Potassium Channel |
| Nav | Voltage-Gated Sodium Channel |
| AIS | Axon Initial Segment |
| NOR | Nodes of Ranvier |
| PMCA | Plasma Membrane Calcium ATPase |
| NOR | Nucleolar Organizer Region |
| PSD(-95) | Post-Synaptic Density(-95) |
| Opa1 | Optic Atrophy 1 Protein |
| PTPN13 | Protein Tyrosine Phosphatase, Non-Receptor Type 13 |
| CTE | Chronic Traumatic Encephalopathy |
| AD | Alzheimer’s Disease |
| PD | Parkinson’s Disease |
| ALS | Amyotrophic Lateral Sclerosis |
| HD | Huntington’s Disease |
| MS | Multiple Sclerosis |
| SARM1 | Sterile Alpha TIR Motif-Containing Protein 1 |
| NAD+ | Nicotinamid Adenine Dinucleotide |
| GFAP | Glial Fibrillary Acidic Protein |
| UCH-L1 | Ubiquitin C-Terminal Hydrolase-L1 |
| FDA | Federal Drug Administration |
| mTBI | Mild Traumatic Brain Injury |
| CSF | Cerebrospinal Fluid |
| NSE | Neuron-Specific Enolase |
| S100B | Calcium Binding Protein 100B |
References
- Maas, A.I.R.; Menon, D.K.; Manley, G.T.; Abrams, M.; Åkerlund, C.; Andelic, N.; Aries, M.; Bashford, T.; Bell, M.J.; Bodien, Y.G.; et al. Traumatic brain injury: Progress and challenges in prevention, clinical care, and research. Lancet Neurol. 2022, 21, 1004–1060. [Google Scholar] [CrossRef]
- Maas, A.I.R.; Menon, D.K.; Adelson, P.D.; Andelic, N.; Bell, M.J.; Belli, A.; Bragge, P.; Brazinova, A.; Büki, A.; Chesnut, R.M.; et al. Traumatic brain injury: Integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017, 16, 987–1048. [Google Scholar] [CrossRef] [PubMed]
- Lisi, I.; Moro, F.; Mazzone, E.; Marklund, N.; Pischiutta, F.; Kobeissy, F.; Mao, X.; Corrigan, F.; Helmy, A.; Nasrallah, F.; et al. Exploiting blood-based biomarkers to align preclinical models with human traumatic brain injury. Brain 2024, 148, 1062–1080. [Google Scholar] [CrossRef]
- Saatman, K.E.; Duhaime, A.-C.; Bullock, R.; Maas, A.I.; Valadka, A.; Manley, G.T. Classification of Traumatic Brain Injury for Targeted Therapies. J. Neurotrauma 2008, 25, 719–738. [Google Scholar] [CrossRef] [PubMed]
- Wachtler, N.; O’brien, R.; Ehrlich, B.E.; McGuone, D. Exploring Calcium Channels as Potential Therapeutic Targets in Blast Traumatic Brain Injury. Pharmaceuticals 2025, 18, 223. [Google Scholar] [CrossRef]
- Weber, J.T. Altered Calcium Signaling Following Traumatic Brain Injury. Front. Pharmacol. 2012, 3, 60. [Google Scholar] [CrossRef]
- Kumar, A.; Loane, D.J. Neuroinflammation after traumatic brain injury: Opportunities for therapeutic intervention. Brain Behav. Immun. 2012, 26, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Ma, M. Role of calpains in the injury-induced dysfunction and degeneration of the mammalian axon. Neurobiol. Dis. 2013, 60, 61–79. [Google Scholar] [CrossRef]
- Kampfl, A.; Posmantur, R.; Zhao, X.; Schmutzhard, E.; Clifton, G.; Hayes, R. Mechanisms of Calpain Proteolysis Following Traumatic Brain Injury: Implications for Pathology and Therapy: A Review and Update. J. Neurotrauma 1997, 14, 121–134. [Google Scholar] [CrossRef]
- Wang, K.K.; Yang, Z.; Zhu, T.; Shi, Y.; Rubenstein, R.; Tyndall, J.A.; Manley, G.T. An update on diagnostic and prognostic biomarkers for traumatic brain injury. Expert Rev. Mol. Diagn. 2018, 18, 165–180. [Google Scholar] [CrossRef]
- Mahaman, Y.A.R.; Huang, F.; Afewerky, H.K.; Maibouge, T.M.S.; Ghose, B.; Wang, X. Involvement of calpain in the neuropathogenesis of Alzheimer’s disease. Med. Res. Rev. 2018, 39, 608–630. [Google Scholar] [CrossRef] [PubMed]
- Schultz, B.; Taday, J.; Menezes, L.; Cigerce, A.; Leite, M.C.; Gonçalves, C.-A. Calpain-Mediated Alterations in Astrocytes Before and During Amyloid Chaos in Alzheimer’s Disease. J. Alzheimer’s Dis. 2021, 84, 1415–1430. [Google Scholar] [CrossRef]
- Saatman, K.E.; Creed, J.; Raghupathi, R. Calpain as a Therapeutic Target in Traumatic Brain Injury. Neurotherapeutics 2010, 7, 31–42. [Google Scholar] [CrossRef]
- Baudry, M.; Luo, Y.L.; Bi, X. Calpain-2 Inhibitors as Therapy for Traumatic Brain Injury. Neurotherapeutics 2023, 20, 1592–1602. [Google Scholar] [CrossRef] [PubMed]
- Suryavanshi, P.; Baule, S.; Glykys, J. Trauma in Neonatal Acute Brain Slices Alters Calcium and Network Dynamics and Causes Calpain-Mediated Cell Death. Eneuro 2024, 11. [Google Scholar] [CrossRef]
- Guroff, G. A Neutral, Calcium-activated Proteinase from the Soluble Fraction of Rat Brain. J. Biol. Chem. 1964, 239, 149–155. [Google Scholar] [CrossRef]
- Goll, D.E.; Thompson, V.F.; Li, H.; Wei, W.; Cong, J. The Calpain System. Physiol. Rev. 2003, 83, 731–801. [Google Scholar] [CrossRef]
- Murachi, T.; Tanaka, K.; Hatanaka, M.; Murakami, T. Intracellular Ca2+-dependent protease (CALPAIN) and its high-molecular-weight endogenous inhibitor (CALPASTATIN). Adv. Enzym. Regul. 1981, 19, 407–424. [Google Scholar] [CrossRef]
- Spinozzi, S.; Albini, S.; Best, H.; Richard, I. Calpains for dummies: What you need to know about the calpain family. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2021, 1869, 140616. [Google Scholar] [CrossRef]
- Ono, Y.; Sorimachi, H. Calpains—An elaborate proteolytic system. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2012, 1824, 224–236. [Google Scholar] [CrossRef]
- E Croall, D.; Ersfeld, K. The calpains: Modular designs and functional diversity. Genome Biol. 2007, 8, 218. [Google Scholar] [CrossRef]
- Nakagawa, K.; Masumoto, H.; Sorimachi, H.; Suzuki, K. Dissociation of m-Calpain Subunits Occurs after Autolysis of the N-Terminus of the Catalytic Subunit, and Is Not Required for Activation. J. Biochem. 2001, 130, 605–611. [Google Scholar] [CrossRef]
- Moldoveanu, T.; Jia, Z.; Davies, P.L. Calpain Activation by Cooperative Ca2+ Binding at Two Non-EF-hand Sites. J. Biol. Chem. 2004, 279, 6106–6114. [Google Scholar] [CrossRef]
- Tompa, P.; Emori, Y.; Sorimachi, H.; Suzuki, K.; Friedrich, P. Domain III of Calpain Is a Ca2+-Regulated Phospholipid-Binding Domain. Biochem. Biophys. Res. Commun. 2001, 280, 1333–1339. [Google Scholar] [CrossRef]
- Hosfield, C.M.; Elce, J.S.; Davies, P.L.; Jia, Z. Crystal structure of calpain reveals the structural basis for Ca2+-dependent protease activity and a novel mode of enzyme activation. EMBO J. 1999, 18, 6880–6889. [Google Scholar] [CrossRef]
- Guttmann, R.P.; Baker, D.L.; Seifert, K.M.; Cohen, A.S.; Coulter, D.A.; Lynch, D.R. Specific proteolysis of the NR2 subunit at multiple sites by calpain. J. Neurochem. 2001, 78, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Zhou, Q.; Wang, Y.; Chishti, A.; Li, Q.Q.; Dayal, S.; Shiehzadegan, S.; Cheng, A.; Moore, C.; Bi, X.; et al. Deletion of the Capn1 Gene Results in Alterations in Signaling Pathways Related to Alzheimer’s Disease, Protein Quality Control and Synaptic Plasticity in Mouse Brain. Front. Genet. 2020, 11, 334. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Bi, X.; Baudry, M. Calpain-1 and Calpain-2 in the Brain: New Evidence for a Critical Role of Calpain-2 in Neuronal Death. Cells 2020, 9, 2698. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Liu, Y.; Wang, Y.; Bi, X.; Baudry, M. Different Patterns of Electrical Activity Lead to Long-term Potentiation by Activating Different Intracellular Pathways. J. Neurosci. 2015, 35, 621–633. [Google Scholar] [CrossRef]
- Baudry, M.; Wang, Y.; Bi, X.; Luo, Y.L.; Wang, Z.; Kamal, Z.; Shirokov, A.; Sullivan, E.; Lagasca, D.; Khalil, H.; et al. Identification and neuroprotective properties of NA-184, a calpain-2 inhibitor. Pharmacol. Res. Perspect. 2024, 12, e1181. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Lopez, D.; Lee, M.; Dayal, S.; Hurtado, A.; Bi, X.; Baudry, M. Protection against TBI-Induced Neuronal Death with Post-Treatment with a Selective Calpain-2 Inhibitor in Mice. J. Neurotrauma 2018, 35, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Duquette, P.M.; Lamarche-Vane, N. The calcium-activated protease calpain regulates netrin-1 receptor deleted in colorectal cancer-induced axon outgrowth in cortical neurons. J. Neurochem. 2019, 152, 315–332. [Google Scholar] [CrossRef] [PubMed]
- Gan-Or, Z.; Bouslam, N.; Birouk, N.; Lissouba, A.; Chambers, D.B.; Vérièpe, J.; Androschuk, A.; Laurent, S.B.; Rochefort, D.; Spiegelman, D.; et al. Mutations in CAPN1 Cause Autosomal-Recessive Hereditary Spastic Paraplegia. Am. J. Hum. Genet. 2016, 98, 1271. [Google Scholar] [CrossRef] [PubMed]
- Vinade, L.; Petersen, J.D.; Do, K.; Dosemeci, A.; Reese, T.S. Activation of calpain may alter the postsynaptic density structure and modulate anchoring of NMDA receptors. Synapse 2001, 40, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Raynaud, F.; Marcilhac, A.; Chebli, K.; Benyamin, Y.; Rossel, M. Calpain 2 expression pattern and sub-cellular localization during mouse embryogenesis. Int. J. Dev. Biol. 2008, 52, 383–388. [Google Scholar] [CrossRef]
- Franco, S.J.; Huttenlocher, A. Regulating cell migration: Calpains make the cut. J. Cell Sci. 2005, 118, 3829–3838. [Google Scholar] [CrossRef]
- Glading, A.; Bodnar, R.J.; Reynolds, I.J.; Shiraha, H.; Satish, L.; Potter, D.A.; Blair, H.C.; Wells, A. Epidermal Growth Factor Activates m-Calpain (Calpain II), at Least in Part, by Extracellular Signal-Regulated Kinase-Mediated Phosphorylation. Mol. Cell. Biol. 2004, 24, 2499–2512. [Google Scholar] [CrossRef]
- Adamec, E.; Mohan, P.; Vonsattel, J.P.; Nixon, R.A. Calpain activation in neurodegenerative diseases: Confocal immunofluorescence study with antibodies specifically recognizing the active form of calpain 2. Acta Neuropathol. 2002, 104, 92–104. [Google Scholar] [CrossRef]
- Ji, X.-Y.; Zheng, D.; Ni, R.; Wang, J.-X.; Shao, J.-Q.; Vue, Z.; Hinton, A.; Song, L.-S.; Fan, G.-C.; Chakrabarti, S.; et al. Sustained over-expression of calpain-2 induces age-dependent dilated cardiomyopathy in mice through aberrant autophagy. Acta Pharmacol. Sin. 2022, 43, 2873–2884. [Google Scholar] [CrossRef]
- Sorimachi, H.; Imajoh-Ohmi, S.; Emori, Y.; Kawasaki, H.; Ohno, S.; Minami, Y.; Suzuki, K. Molecular cloning of a novel mammalian calcium-dependent protease distinct from both m- and μ-types. J. Biol. Chem. 1989, 264, 20106–20111. [Google Scholar] [CrossRef]
- Beckmann, J.S.; Spencer, M. Calpain 3, the “gatekeeper” of proper sarcomere assembly, turnover and maintenance. Neuromuscul. Disord. 2008, 18, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Sáenz, A.; Leturcq, F.; Cobo, A.M.; Poza, J.J.; Ferrer, X.; Otaegui, D.; Camaño, P.; Urtasun, M.; Vílchez, J.; Gutiérrez-Rivas, E.; et al. LGMD2A: Genotype–phenotype correlations based on a large mutational survey on the calpain 3 gene. Brain 2005, 128, 732–742. [Google Scholar] [CrossRef]
- Schaefer, K.; Mahajan, M.; Gore, A.; Tsang, S.H.; Bassuk, A.G.; Mahajan, V.B. Calpain-5 gene expression in the mouse eye and brain. BMC Res. Notes 2017, 10, 602. [Google Scholar] [CrossRef]
- Mahajan, V.B.; Skeie, J.M.; Bassuk, A.G.; Fingert, J.H.; Braun, T.A.; Daggett, H.T.; Folk, J.C.; Sheffield, V.C.; Stone, E.M.; Barsh, G.S. Calpain-5 Mutations Cause Autoimmune Uveitis, Retinal Neovascularization, and Photoreceptor Degeneration. PLoS Genet. 2012, 8, e1003001. [Google Scholar] [CrossRef]
- Dear, T.; Boehm, T. Diverse mRNA expression patterns of the mouse calpain genes Capn5, Capn6 and Capn11 during development. Mech. Dev. 1999, 89, 201–209. [Google Scholar] [CrossRef]
- Tonami, K.; Kurihara, Y.; Aburatani, H.; Uchijima, Y.; Asano, T.; Kurihara, H. Calpain 6 Is Involved in Microtubule Stabilization and Cytoskeletal Organization. Mol. Cell. Biol. 2007, 27, 2548–2561. [Google Scholar] [CrossRef]
- Xiang, Y.; Li, F.; Wang, L.; Zheng, A.; Zuo, J.; Li, M.; Wang, Y.; Xu, Y.; Chen, C.; Chen, S.; et al. Decreased calpain 6 expression is associated with tumorigenesis and poor prognosis in HNSCC. Oncol. Lett. 2017, 13, 2237–2243. [Google Scholar] [CrossRef]
- Marion, A.; Dieudonné, F.; Patiño-Garcia, A.; Lecanda, F.; Marie, P.J.; Modrowski, D. Calpain-6 is an endothelin-1 signaling dependent protective factor in chemoresistant osteosarcoma. Int. J. Cancer 2011, 130, 2514–2525. [Google Scholar] [CrossRef]
- Paine, E.L.; Skalicky, J.J.; Whitby, F.G.; Mackay, D.R.; Ullman, K.S.; Hill, C.P.; I Sundquist, W. The Calpain-7 protease functions together with the ESCRT-III protein IST1 within the midbody to regulate the timing and completion of abscission. eLife 2023, 12, e84515. [Google Scholar] [CrossRef] [PubMed]
- Maemoto, Y.; Ono, Y.; Kiso, S.; Shibata, H.; Takahara, T.; Sorimachi, H.; Maki, M. Involvement of calpain-7 in epidermal growth factor receptor degradation via the endosomal sorting pathway. FEBS J. 2014, 281, 3642–3655. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.; Wu, D.; Ni, R.; Li, P.; Tian, Z.; Shui, Y.; Hu, H.; Wei, Q. Novel insights into the progression and prognosis of the calpain family members in hepatocellular carcinoma: A comprehensive integrated analysis. Front. Mol. Biosci. 2023, 10, 1162409. [Google Scholar] [CrossRef] [PubMed]
- Hata, S.; Abe, M.; Suzuki, H.; Kitamura, F.; Toyama-Sorimachi, N.; Abe, K.; Sakimura, K.; Sorimachi, H.; Barsh, G.S. Calpain 8/nCL-2 and Calpain 9/nCL-4 Constitute an Active Protease Complex, G-Calpain, Involved in Gastric Mucosal Defense. PLoS Genet. 2010, 6, e1001040. [Google Scholar] [CrossRef]
- Ma, H.; Fukiage, C.; Kim, Y.H.; Duncan, M.K.; Reed, N.A.; Shih, M.; Azuma, M.; Shearer, T.R. Characterization and Expression of Calpain 10: A Novel Ubiquitous Calpain with Nuclear Localization. J. Biol. Chem. 2001, 276, 28525–28531. [Google Scholar] [CrossRef]
- Hatta, T.; Iemura, S.-I.; Ohishi, T.; Nakayama, H.; Seimiya, H.; Yasuda, T.; Iizuka, K.; Fukuda, M.; Takeda, J.; Natsume, T.; et al. Calpain-10 regulates actin dynamics by proteolysis of microtubule-associated protein 1B. Sci. Rep. 2018, 8, 16756. [Google Scholar] [CrossRef]
- Ling, C.; Groop, L.; Del Guerra, S.; Lupi, R.; Maedler, K. Calpain-10 Expression Is Elevated in Pancreatic Islets from Patients with Type 2 Diabetes. PLoS ONE 2009, 4, e6558. [Google Scholar] [CrossRef]
- Ben-Aharon, I.; Brown, P.R.; Shalgi, R.; Eddy, E.M. Calpain 11 is unique to mouse spermatogenic cells. Mol. Reprod. Dev. 2006, 73, 767–773. [Google Scholar] [CrossRef]
- Bochner, R.; Samuelov, L.; Sarig, O.; Li, Q.; Adase, C.A.; Isakov, O.; Malchin, N.; Vodo, D.; Shayevitch, R.; Peled, A.; et al. Calpain 12 Function Revealed through the Study of an Atypical Case of Autosomal Recessive Congenital Ichthyosis. J. Investig. Dermatol. 2016, 137, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Dear, T.; Meier, N.T.; Hunn, M.; Boehm, T. Gene Structure, Chromosomal Localization, and Expression Pattern of Capn12, a New Member of the Calpain Large Subunit Gene Family. Genomics 2000, 68, 152–160. [Google Scholar] [CrossRef]
- Chukai, Y.; Sudo, T.; Fukuda, T.; Tomita, H.; Sugano, E.; Ozaki, T. Proteolysis of mitochondrial calpain-13 in cerebral ischemia-reperfusion injury. Biochem. Biophys. Rep. 2024, 39, 101768. [Google Scholar] [CrossRef]
- Davis, B.P.; Stucke, E.M.; Khorki, M.E.; Litosh, V.A.; Rymer, J.K.; Rochman, M.; Travers, J.; Kottyan, L.C.; Rothenberg, M.E. Eosinophilic esophagitis–linked calpain 14 is an IL-13–induced protease that mediates esophageal epithelial barrier impairment. J. Clin. Investig. 2016, 1, e86355. [Google Scholar] [CrossRef]
- Zha, C.; A Farah, C.; Holt, R.J.; Ceroni, F.; Al-Abdi, L.; Thuriot, F.; O Khan, A.; Helaby, R.; Lévesque, S.; Alkuraya, F.S.; et al. Biallelic variants in the small optic lobe calpain CAPN15 are associated with congenital eye anomalies, deafness and other neurodevelopmental deficits. Hum. Mol. Genet. 2020, 29, 3054–3063. [Google Scholar] [CrossRef]
- Zha, C.; Huang, A.; Kailasam, S.; Young, D.; Dufour, A.; Sossin, W.S.; Goymer, P. Identifying putative substrates of Calpain-15 in neurodevelopment. PLoS ONE 2025, 20, e0319489. [Google Scholar] [CrossRef]
- Yoder, M.W.; Wright, N.T.; Borzok, M.A. Calpain Regulation and Dysregulation—Its Effects on the Intercalated Disk. Int. J. Mol. Sci. 2023, 24, 11726. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.; Min, L.; Song, S.; Zhao, J.; Li, L.; Yang, C.; Shao, M.; Zhang, M.; Wu, H.; Zhang, J.; et al. Elevated Expression of Calpain-4 Predicts Poor Prognosis in Patients with Gastric Cancer after Gastrectomy. Int. J. Mol. Sci. 2016, 17, 1612. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Hata, S.; Kawabata, Y.; Sorimachi, H. Structure, Activation, and Biology of Calpain. Diabetes 2004, 53, S12–S18. [Google Scholar] [CrossRef]
- Schád, É.; Farkas, A.; Jékely, G.; Tompa, P.; Friedrich, P. A novel human small subunit of calpains. Biochem. J. 2002, 362, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Gennarelli, T.A.; Graham, D.I. Neuropathology of the Head Injuries. Semin. Clin. Neuropsychiatry 1998, 3, 160–175. [Google Scholar]
- Farkas, O.; Lifshitz, J.; Povlishock, J.T. Mechanoporation Induced by Diffuse Traumatic Brain Injury: An Irreversible or Reversible Response to Injury? J. Neurosci. 2006, 26, 3130–3140. [Google Scholar] [CrossRef]
- Wolf, J.A.; Stys, P.K.; Lusardi, T.; Meaney, D.; Smith, D.H. Traumatic Axonal Injury Induces Calcium Influx Modulated by Tetrodotoxin-Sensitive Sodium Channels. J. Neurosci. 2001, 21, 1923–1930. [Google Scholar] [CrossRef]
- Musyaju, S.; Modi, H.R.; Shear, D.A.; Scultetus, A.H.; Pandya, J.D. Time Course of Mitochondrial Antioxidant Markers in a Preclinical Model of Severe Penetrating Traumatic Brain Injury. Int. J. Mol. Sci. 2025, 26, 906. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Weber, J.T.; Liang, S.; Willoughby, K.A.; Sitterding, H.A.; Rzigalinski, B.A.; Ellis, E.F. NMDA Receptor Activation Contributes to a Portion of the Decreased Mitochondrial Membrane Potential and Elevated Intracellular Free Calcium in Strain-Injured Neurons. J. Neurotrauma 2002, 19, 1619–1629. [Google Scholar] [CrossRef]
- Hollmann, M.; Hartley, M.; Heinemann, S. Ca2 + Permeability of KA-AMPA—Gated Glutamate Receptor Channels Depends on Subunit Composition. Science 1991, 252, 851–853. [Google Scholar] [CrossRef]
- Burnashev, N.; Monyer, H.; Seeburg, P.; Sakmann, B. Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron 1992, 8, 189–198. [Google Scholar] [CrossRef]
- Isaac, J.T.; Ashby, M.C.; McBain, C.J. The Role of the GluR2 Subunit in AMPA Receptor Function and Synaptic Plasticity. Neuron 2007, 54, 859–871. [Google Scholar] [CrossRef]
- Shcherbatko, A.; Ono, F.; Mandel, G.; Brehm, P. Voltage-Dependent Sodium Channel Function Is Regulated Through Membrane Mechanics. Biophys. J. 1999, 77, 1945–1959. [Google Scholar] [CrossRef]
- Wang, J.A.; Lin, W.; Morris, T.; Banderali, U.; Juranka, P.F.; Morris, C.E. Membrane trauma and Na+leak from Nav1.6 channels. Am. J. Physiol. Physiol. 2009, 297, C823–C834. [Google Scholar] [CrossRef] [PubMed]
- Shahlaie, K.; Lyeth, B.G.; Gurkoff, G.G.; Muizelaar, J.P.; Berman, R.F. Neuroprotective Effects of Selective N-Type VGCC Blockade on Stretch-Injury-Induced Calcium Dynamics in Cortical Neurons. J. Neurotrauma 2010, 27, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Stys, P.; Waxman, S.; Ransom, B. Ionic mechanisms of anoxic injury in mammalian CNS white matter: Role of Na+ channels and Na+-Ca2+ exchanger. J. Neurosci. 1992, 12, 430–439. [Google Scholar] [CrossRef]
- Brittain, M.K.; Brustovetsky, T.; Sheets, P.L.; Brittain, J.M.; Khanna, R.; Cummins, T.R.; Brustovetsky, N. Delayed calcium dysregulation in neurons requires both the NMDA receptor and the reverse Na+/Ca2+ exchanger. Neurobiol. Dis. 2012, 46, 109–117. [Google Scholar] [CrossRef]
- Omelchenko, A.; Shrirao, A.B.; Bhattiprolu, A.K.; Zahn, J.D.; Schloss, R.S.; Dickson, S.; Meaney, D.F.; Boustany, N.N.; Yarmush, M.L.; Firestein, B.L. Dynamin and reverse-mode sodium calcium exchanger blockade confers neuroprotection from diffuse axonal injury. Cell Death Dis. 2019, 10, 727. [Google Scholar] [CrossRef] [PubMed]
- Chou, A.-C.; Ju, Y.-T.; Pan, C.-Y.; Obukhov, A.G. Calmodulin Interacts with the Sodium/Calcium Exchanger NCX1 to Regulate Activity. PLoS ONE 2015, 10, e0138856. [Google Scholar] [CrossRef] [PubMed]
- Masilamoni, G.J.; Smith, Y. Metabotropic glutamate receptors: Targets for neuroprotective therapies in Parkinson disease. Curr. Opin. Pharmacol. 2018, 38, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, F.; Bockaert, J.; Collingridge, G.; Conn, P.; Ferraguti, F.; Schoepp, D.; Wroblewski, J.; Pin, J. Metabotropic glutamate receptors: From the workbench to the bedside. Neuropharmacology 2011, 60, 1017–1041. [Google Scholar] [CrossRef]
- Chen, T.; Willoughby, K.A.; Ellis, E.F. Group I Metabotropic Receptor Antagonism Blocks Depletion of Calcium Stores and Reduces Potentiated Capacitative Calcium Entry in Strain-Injured Neurons and Astrocytes. J. Neurotrauma 2004, 21, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Orem, B.C.; Rajaee, A.; Stirling, D.P. IP3R-mediated intra-axonal Ca2+ release contributes to secondary axonal degeneration following contusive spinal cord injury. Neurobiol. Dis. 2020, 146, 105123. [Google Scholar] [CrossRef]
- Orem, B.C.; Pelisch, N.; Williams, J.; Nally, J.M.; Stirling, D.P. Intracellular calcium release through IP 3 R or RyR contributes to secondary axonal degeneration. Neurobiol. Dis. 2017, 106, 235–243. [Google Scholar] [CrossRef]
- Tully, K.; Treistman, S.N.; Plotkin, J.L.; Shen, W.; Rafalovich, I.; Sebel, L.E.; Day, M.; Chan, C.S.; Surmeier, D.J.; Behringer, E.J.; et al. Distinct Intracellular Calcium Profiles Following Influx Through N- Versus L-Type Calcium Channels: Role of Ca2+-Induced Ca2+ Release. J. Neurophysiol. 2004, 92, 135–143. [Google Scholar] [CrossRef][Green Version]
- Kopil, C.M.; Siebert, A.P.; Foskett, J.K.; Neumar, R.W. Calpain-cleaved type 1 inositol 1,4,5-trisphosphate receptor impairs ER Ca2+ buffering and causes neurodegeneration in primary cortical neurons. J. Neurochem. 2012, 123, 147–158. [Google Scholar] [CrossRef]
- Cheng, G.; Fu, L.; Zhang, H.-Y.; Wang, Y.-M.; Zhang, L.-M.; Zhang, J.-N. The role of mitochondrial calcium uniporter in neuroprotection in traumatic brain injury. Med. Hypotheses 2013, 80, 115–117. [Google Scholar] [CrossRef]
- Xiong, Y.; Peterson, P.; Lee, C. Effect of N-Acetylcysteine on Mitochondrial Function Following Traumatic Brain Injury in Rats. J. Neurotrauma 1999, 16, 1067–1082. [Google Scholar] [CrossRef]
- Susin, S.A.; Zamzami, N.; Kroemer, G. Mitochondria as regulators of apoptosis: Doubt no more. Biochim. Biophys. Acta (BBA)-Bioenerg. 1998, 1366, 151–165. [Google Scholar] [CrossRef]
- Singh, I.N.; Sullivan, P.G.; Deng, Y.; Mbye, L.H.; Hall, E.D. Time Course of Post-Traumatic Mitochondrial Oxidative Damage and Dysfunction in a Mouse Model of Focal Traumatic Brain Injury: Implications for Neuroprotective Therapy. J. Cereb. Blood Flow Metab. 2006, 26, 1407–1418. [Google Scholar] [CrossRef] [PubMed]
- Mira, R.G.; Quintanilla, R.A.; Cerpa, W. Mild Traumatic Brain Injury Induces Mitochondrial Calcium Overload and Triggers the Upregulation of NCLX in the Hippocampus. Antioxidants 2023, 12, 403. [Google Scholar] [CrossRef]
- Staal, J.A.; Dickson, T.C.; Gasperini, R.; Liu, Y.; Foa, L.; Vickers, J.C. Initial calcium release from intracellular stores followed by calcium dysregulation is linked to secondary axotomy following transient axonal stretch injury. J. Neurochem. 2010, 112, 1147–1155. [Google Scholar] [CrossRef]
- Shirao, T.; González-Billault, C. Actin filaments and microtubules in dendritic spines. J. Neurochem. 2013, 126, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, P.N.; Griffin, J.W.; Price, D.L. Control of axonal caliber by neurofilament transport. J. Cell Biol. 1984, 99, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Krebs, A.; Goldie, K.N.; Hoenger, A. Structural rearrangements in tubulin following microtubule formation. Embo Rep. 2005, 6, 227–232. [Google Scholar] [CrossRef]
- Jenkins, S.M.; Bennett, V. Ankyrin-G coordinates assembly of the spectrin-based membrane skeleton, voltage-gated sodium channels, and L1 CAMs at Purkinje neuron initial segments. J. Cell Biol. 2001, 155, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Hotulainen, P.; Hoogenraad, C.C. Actin in dendritic spines: Connecting dynamics to function. J. Cell Biol. 2010, 189, 619–629. [Google Scholar] [CrossRef]
- Xu, K.; Zhong, G.; Zhuang, X. Actin, Spectrin, and Associated Proteins Form a Periodic Cytoskeletal Structure in Axons. Science 2013, 339, 452–456. [Google Scholar] [CrossRef]
- Saatman, K.E.; Bozyczko-Coyne, D.; Marcy, V.; Siman, R.; McIntosh, T.K. Prolonged Calpain-mediated Spectrin Breakdown Occurs Regionally Following Experimental Brain Injury in the Rat. J. Neuropathol. Exp. Neurol. 1996, 55, 850–860. [Google Scholar] [CrossRef]
- Wang, K.K.W. Calpain and caspase: Can you tell the difference? Trends Neurosci. 2000, 23, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Thompson, B.; Gao, X.; Hall, E. Temporal relationship of peroxynitrite-induced oxidative damage, calpain-mediated cytoskeletal degradation and neurodegeneration after traumatic brain injury. Exp. Neurol. 2007, 205, 154–165. [Google Scholar] [CrossRef]
- McGinn, M.J.; Kelley, B.J.; Akinyi, L.; Oli, M.W.; Liu, M.C.; Hayes, R.L.; Wang, K.K.; Povlishock, J.T. Biochemical, Structural, and Biomarker Evidence for Calpain-Mediated Cytoskeletal Change After Diffuse Brain Injury Uncomplicated by Contusion. J. Neuropathol. Exp. Neurol. 2009, 68, 241–249. [Google Scholar] [CrossRef]
- Büki, A.; Siman, R.; Trojanowski, J.Q.; Povlishock, J.T. The Role of Calpail-Mediated Spectrin Proteolysis in Traumatically Induced Axonal Injury. J. Neuropathol. Exp. Neurol. 1999, 58, 365–375. [Google Scholar] [CrossRef]
- Reeves, T.M.; Greer, J.E.; Vanderveer, A.S.; Phillips, L.L. Proteolysis of Submembrane Cytoskeletal Proteins Ankyrin-G and αII-Spectrin Following Diffuse Brain Injury: A Role in White Matter Vulnerability at Nodes of Ranvier. Brain Pathol. 2010, 20, 1055–1068. [Google Scholar] [CrossRef]
- Newcomb, J.K.; Kampfl, A.; Posmantur, R.M.; Zhao, X.; Pike, B.R.; Liu, S.-J.; Clifton, G.L.; Hayes, R.L. Immunohistochemical Study of Calpain-Mediated Breakdown Products to α-Spectrin Following Controlled Cortical Impact Injury in the Rat. J. Neurotrauma 1997, 14, 369–383. [Google Scholar] [CrossRef]
- Pike, B.R.; Zhao, X.; Newcomb, J.K.; Posmantur, R.M.; Wang, K.K.; Hayes, R.L. Regional calpain and caspase-3 proteolysis of α-spectrin after traumatic brain injury. NeuroReport 1998, 9, 2437–2442. [Google Scholar] [CrossRef]
- Rubenstein, R.; Wang, K.K.; Chiu, A.; Grinkina, N.; Sharma, D.R.; Agarwal, S.; Lin, F.; Yang, Z. PrPC expression and calpain activity independently mediate the effects of closed head injury in mice. Behav. Brain Res. 2018, 340, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Valiyaveettil, M.; Alamneh, Y.A.; Wang, Y.; Arun, P.; Oguntayo, S.; Wei, Y.; Long, J.B.; Nambiar, M.P. Cytoskeletal protein α–II spectrin degradation in the brain of repeated blast exposed mice. Brain Res. 2014, 1549, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Pike, B.R.; Flint, J.; Dutta, S.; Johnson, E.; Wang, K.K.W.; Hayes, R.L. Accumulation of non-erythroid αII-spectrin and calpain-cleaved αII-spectrin breakdown products in cerebrospinal fluid after traumatic brain injury in rats. J. Neurochem. 2001, 78, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Mondello, S.; Robicsek, S.A.; Gabrielli, A.; Brophy, G.M.; Papa, L.; Tepas, J.; Robertson, C.; Buki, A.; Scharf, D.; Jixiang, M.; et al. αII-Spectrin Breakdown Products (SBDPs): Diagnosis and Outcome in Severe Traumatic Brain Injury Patients. J. Neurotrauma 2010, 27, 1203–1213. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.L.; Singh, I.N.; Wang, J.A.; Hall, E.D. Time courses of post-injury mitochondrial oxidative damage and respiratory dysfunction and neuronal cytoskeletal degradation in a rat model of focal traumatic brain injury. Neurochem. Int. 2017, 111, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, J.M.; Quarrington, R.D.; Krieg, J.L.; Kaukas, L.; Turner, R.J.; Leonard, A.; Jones, C.F.; Corrigan, F. Evaluating the effect of post-traumatic hypoxia on the development of axonal injury following traumatic brain injury in sheep. Brain Res. 2023, 1817, 148475. [Google Scholar] [CrossRef]
- Siman, R.; Cui, H.; Wewerka, S.S.; Hamel, L.; Smith, D.H.; Zwank, M.D. Serum SNTF, a Surrogate Marker of Axonal Injury, Is Prognostic for Lasting Brain Dysfunction in Mild TBI Treated in the Emergency Department. Front. Neurol. 2020, 11, 249. [Google Scholar] [CrossRef]
- Siman, R.; Giovannone, N.; Hanten, G.; Wilde, E.A.; McCauley, S.R.; Hunter, J.V.; Li, X.; Levin, H.S.; Smith, D.H. Evidence That the Blood Biomarker SNTF Predicts Brain Imaging Changes and Persistent Cognitive Dysfunction in Mild TBI Patients. Front. Neurol. 2013, 4, 71158. [Google Scholar] [CrossRef]
- Siman, R.; Shahim, P.; Tegner, Y.; Blennow, K.; Zetterberg, H.; Smith, D.H. Serum SNTF Increases in Concussed Professional Ice Hockey Players and Relates to the Severity of Postconcussion Symptoms. J. Neurotrauma 2015, 32, 1294–1300. [Google Scholar] [CrossRef]
- Kobeissy, F.H.; Liu, M.C.; Yang, Z.; Zhang, Z.; Zheng, W.; Glushakova, O.; Mondello, S.; Anagli, J.; Hayes, R.L.; Wang, K.K.W. Erratum to: Degradation of βII-Spectrin Protein by Calpain-2 and Caspase-3 Under Neurotoxic and Traumatic Brain Injury Conditions. Mol. Neurobiol. 2017, 55, 898–900. [Google Scholar] [CrossRef]
- Burton, P.R.; Wentz, M.A. Neurofilaments are prominent in bullfrog olfactory axons but are rarely seen in those of the tiger salamander, Ambystoma tigrinum. J. Comp. Neurol. 1992, 317, 396–406. [Google Scholar] [CrossRef]
- Shaw, G.; Yang, C.; Zhang, L.; Cook, P.; Pike, B.; Hill, W.D. Characterization of the bovine neurofilament NF-M protein and cDNA sequence, and identification of in vitro and in vivo calpain cleavage sites. Biochem. Biophys. Res. Commun. 2004, 325, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Kahn, O.I.; Dominguez, S.L.; Glock, C.; Hayne, M.; Vito, S.; Ghosh, A.S.; Adrian, M.; Burgess, B.L.; Meilandt, W.J.; Friedman, B.A.; et al. Secreted neurofilament light chain after neuronal damage induces myeloid cell activation and neuroinflammation. Cell Rep. 2025, 44, 115382. [Google Scholar] [CrossRef] [PubMed]
- Schlaepfer, W.W.; Lee, C.; Lee, V.M.; Zimmerman, U.P. An Immunoblot Study of Neurofilament Degradation In Situ and During Calcium-Activated Proteolysis. J. Neurochem. 1985, 44, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Kamakura, K.; Ishiura, S.; Suzuki, K.; Sugita, H.; Toyokura, Y. Calcium-Activated neutral protease in the peripheral nerve, which requires μM order Ca2+, and its effect on the neurofilament triplet. J. Neurosci. Res. 1985, 13, 391–403. [Google Scholar] [CrossRef]
- Kapitein, L.C.; Hoogenraad, C.C. Building the Neuronal Microtubule Cytoskeleton. Neuron 2015, 87, 492–506. [Google Scholar] [CrossRef]
- Baas, P.W.; Deitch, J.S.; Black, M.M.; Banker, G.A. Polarity orientation of microtubules in hippocampal neurons: Uniformity in the axon and nonuniformity in the dendrite. Proc. Natl. Acad. Sci. USA 1988, 85, 8335–8339. [Google Scholar] [CrossRef]
- Yau, K.W.; Schätzle, P.; Tortosa, E.; Pagès, S.; Holtmaat, A.; Kapitein, L.C.; Hoogenraad, C.C. Dendrites In Vitro and In Vivo Contain Microtubules of Opposite Polarity and Axon Formation Correlates with Uniform Plus-End-Out Microtubule Orientation. J. Neurosci. 2016, 36, 1071–1085. [Google Scholar] [CrossRef]
- Binder, L.I.; Frankfurter, A.; Rebhun, L.I. The distribution of tau in the mammalian central nervous system. J. Cell Biol. 1985, 101, 1371–1378. [Google Scholar] [CrossRef]
- Maccioni, R.B.; Cambiazo, V. Role of microtubule-associated proteins in the control of microtubule assembly. Physiol. Rev. 1995, 75, 835–864. [Google Scholar] [CrossRef]
- Bloom, G.S.; A Schoenfeld, T.; Vallee, R.B. Widespread distribution of the major polypeptide component of MAP 1 (microtubule-associated protein 1) in the nervous system. J. Cell Biol. 1984, 98, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Serbest, G.; Burkhardt, M.F.; Siman, R.; Raghupathi, R.; Saatman, K.E. Temporal Profiles of Cytoskeletal Protein Loss following Traumatic Axonal Injury in Mice. Neurochem. Res. 2007, 32, 2006–2014. [Google Scholar] [CrossRef]
- Billger, M.; Wallin, M.; Karlsson, J.-O. Proteolysis of tubulin and microtubule-associated proteins 1 and 2 by calpain I and II. Difference in sensitivity of assembled and disassembled microtubules. Cell Calcium 1988, 9, 33–44. [Google Scholar] [CrossRef]
- Yoshimura, N.; Tsukahara, I.; Murachi, T. Calpain and calpastatin in porcine retina. Identification and action on microtubule-associated proteins. Biochem. J. 1984, 223, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.N.; Ramaswamy, S.; Tuzio, H.; Shiekh, A.M.; Fenko, M.D.; Wisniewski, H.M.; Howard, R.G. Micromolar Ca2+ requiring protease from human platelets: Purification, partial characterization and effect on the cytoskeletal proteins. Life Sci. 1987, 40, 593–604. [Google Scholar] [CrossRef]
- Malik, M.N.; Fenko, M.D.; Iqbal, K.; Wisniewski, H.M. Purification and characterization of two forms of Ca2+-activated neutral protease from calf brain. J. Biol. Chem. 1983, 258, 8955–8962. [Google Scholar] [CrossRef]
- Yadikar, H.; Johnson, C.; Pafundi, N.; Mouhawasse, E.; Nguyen, L.; Torres, I.; Kurup, M.; Yang, Z.; Kobeissy, F.; Yost, R.; et al. Novel Peptidomic Approach for Identification of Low and High Molecular Weight Tauopathy Peptides Following Calpain Digestion, and Primary Culture Neurotoxic Challenges. Int. J. Mol. Sci. 2019, 20, 5213. [Google Scholar] [CrossRef]
- Yang, L.; Ksiezak-Reding, H. Calpain-Induced Proteolysis of Normal Human Tau and Tau Associated with Paired Helical Filaments. Eur. J. Biochem. 1995, 233, 9–17. [Google Scholar] [CrossRef]
- Johnson, G.V.; Jope, R.S.; Binder, L.I. Proteolysis of tau by calpain. Biochem. Biophys. Res. Commun. 1989, 163, 1505–1511. [Google Scholar] [CrossRef]
- Girouard, M.-P.; Simas, T.; Hua, L.; Morquette, B.; Khazaei, M.R.; Unsain, N.; Johnstone, A.D.; Rambaldi, I.; Sanz, R.L.; Di Raddo, M.-E.; et al. Collapsin Response Mediator Protein 4 (CRMP4) Facilitates Wallerian Degeneration and Axon Regeneration following Sciatic Nerve Injury. Eneuro 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ottens, A.K.; Sadasivan, S.; Kobeissy, F.H.; Fang, T.; Hayes, R.L.; Wang, K.K. Calpain-mediated collapsin response mediator protein-1, -2, and -4 proteolysis after neurotoxic and traumatic brain injury. J. Neurotrauma 2007, 24, 460–472. Available online: http://www.ncbi.nlm.nih.gov/pubmed/17402852 (accessed on 1 August 2025). [CrossRef]
- Hou, S.T.; Jiang, S.X.; Desbois, A.; Huang, D.; Kelly, J.; Tessier, L.; Karchewski, L.; Kappler, J. Calpain-Cleaved Collapsin Response Mediator Protein-3 Induces Neuronal Death after Glutamate Toxicity and Cerebral Ischemia. J. Neurosci. 2006, 26, 2241–2249. [Google Scholar] [CrossRef] [PubMed]
- Bevers, M.B.; Lawrence, E.; Maronski, M.; Starr, N.; Amesquita, M.; Neumar, R.W. Knockdown of m-calpain increases survival of primary hippocampal neurons following NMDA excitotoxicity. J. Neurochem. 2009, 108, 1237–1250. [Google Scholar] [CrossRef]
- Kuba, H.; Yamada, R.; Ishiguro, G.; Adachi, R. Redistribution of Kv1 and Kv7 enhances neuronal excitability during structural axon initial segment plasticity. Nat. Commun. 2015, 6, 8815. [Google Scholar] [CrossRef] [PubMed]
- Waxman, S.G.; Ritchie, J.M. Molecular dissection of the myelinated axon. Ann. Neurol. 1993, 33, 121–136. [Google Scholar] [CrossRef] [PubMed]
- A Black, J.; Renganathan, M.; Waxman, S.G. Sodium channel Nav1.6 is expressed along nonmyelinated axons and it contributes to conduction. Mol. Brain Res. 2002, 105, 19–28. [Google Scholar] [CrossRef]
- Juhaszova, M.; Church, P.; Blaustein, M.P.; Stanley, E.F. Location of calcium transporters at presynaptic terminals. Eur. J. Neurosci. 2000, 12, 839–846. [Google Scholar] [CrossRef]
- Song, H.; McEwan, P.P.; Ameen-Ali, K.E.; Tomasevich, A.; Kennedy-Dietrich, C.; Palma, A.; Arroyo, E.J.; Dolle, J.-P.; Johnson, V.E.; Stewart, W.; et al. Concussion leads to widespread axonal sodium channel loss and disruption of the node of Ranvier. Acta Neuropathol. 2022, 144, 967–985. [Google Scholar] [CrossRef] [PubMed]
- Iwata, A.; Stys, P.K.; Wolf, J.A.; Chen, X.-H.; Taylor, A.G.; Meaney, D.F.; Smith, D.H. Traumatic Axonal Injury Induces Proteolytic Cleavage of the Voltage-Gated Sodium Channels Modulated by Tetrodotoxin and Protease Inhibitors. J. Neurosci. 2004, 24, 4605–4613. [Google Scholar] [CrossRef]
- von Reyn, C.R.; Spaethling, J.M.; Mesfin, M.N.; Ma, M.; Neumar, R.W.; Smith, D.H.; Siman, R.; Meaney, D.F. Calpain Mediates Proteolysis of the Voltage-Gated Sodium Channel α-Subunit. J. Neurosci. 2009, 29, 10350–10356. [Google Scholar] [CrossRef] [PubMed]
- Arratia, L.M.; Bermudes-Contreras, J.D.; Juarez-Monroy, J.A.; Romero-Macías, E.A.; Luna-Rojas, J.C.; López-Hidalgo, M.; Vega, A.V.; Zamorano-Carrillo, A. Experimental and computational evidence that Calpain-10 binds to the carboxy terminus of NaV1.2 and NaV1.6. Sci. Rep. 2024, 14, 6761. [Google Scholar] [CrossRef]
- Bano, D.; Young, K.W.; Guerin, C.J.; LeFeuvre, R.; Rothwell, N.J.; Naldini, L.; Rizzuto, R.; Carafoli, E.; Nicotera, P. Cleavage of the Plasma Membrane Na+/Ca2+ Exchanger in Excitotoxicity. Cell 2005, 120, 275–285. [Google Scholar] [CrossRef]
- Brustovetsky, T.; Bolshakov, A.; Brustovetsky, N. Calpain activation and Na+/Ca2+exchanger degradation occur downstream of calcium deregulation in hippocampal neurons exposed to excitotoxic glutamate. J. Neurosci. Res. 2009, 88, 1317–1328. [Google Scholar] [CrossRef] [PubMed]
- Araújo, I.M.; Carreira, B.P.; Pereira, T.; Santos, P.F.; Soulet, D.; Inácio, Â.; A Bahr, B.; Carvalho, A.P.; Ambrósio, A.F.; Carvalho, C.M. Changes in calcium dynamics following the reversal of the sodium-calcium exchanger have a key role in AMPA receptor-mediated neurodegeneration via calpain activation in hippocampal neurons. Cell Death Differ. 2007, 14, 1635–1646. [Google Scholar] [CrossRef] [PubMed]
- Pottorf, W.J.; Johanns, T.M.; Derrington, S.M.; Strehler, E.E.; Enyedi, A.; Thayer, S.A. Glutamate-induced protease-mediated loss of plasma membrane Ca2+ pump activity in rat hippocampal neurons. J. Neurochem. 2006, 98, 1646–1656. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.M.; Weinberg, R.J. Ultrastructure of Synapses in the Mammalian Brain. Cold Spring Harb. Perspect. Biol. 2012, 4, a005587. [Google Scholar] [CrossRef]
- Sheng, M.; Kim, E. The Postsynaptic Organization of Synapses. Cold Spring Harb. Perspect. Biol. 2011, 3, a005678. [Google Scholar] [CrossRef]
- Mueller, B.D.; A Merrill, S.; Watanabe, S.; Liu, P.; Niu, L.; Singh, A.; Maldonado-Catala, P.; Cherry, A.; Rich, M.S.; Silva, M.; et al. CaV1 and CaV2 calcium channels mediate the release of distinct pools of synaptic vesicles. eLife 2023, 12, e81407. [Google Scholar] [CrossRef]
- Bartschat, D.K.; Blaustein, M.P. Calcium-activated potassium channels in isolated presynaptic nerve terminals from rat brain. J. Physiol. 1985, 361, 441–457. [Google Scholar] [CrossRef]
- Parsons, T.D.; Obaid, A.L.; Salzberg, B.M. Aminoglycoside antibiotics block voltage-dependent calcium channels in intact vertebrate nerve terminals. J. Gen. Physiol. 1992, 99, 491–504. [Google Scholar] [CrossRef]
- Meir, A.; Ginsburg, S.; Butkevich, A.; Kachalsky, S.G.; Kaiserman, I.; Ahdut, R.; Demirgoren, S.; Rahamimoff, R. Ion Channels in Presynaptic Nerve Terminals and Control of Transmitter Release. Physiol. Rev. 1999, 79, 1019–1088. [Google Scholar] [CrossRef]
- Nelson, J.C.; Stavoe, A.K.; Colón-Ramos, D.A. The actin cytoskeleton in presynaptic assembly. Cell Adhes. Migr. 2013, 7, 379–387. [Google Scholar] [CrossRef]
- Lu, X.; Rong, Y.; Bi, R.; Baudry, M. Calpain-mediated truncation of rat brain AMPA receptors increases their Triton X-100 solubility. Brain Res. 2000, 863, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Rong, Y.; Baudry, M. Calpain-mediated degradation of PSD-95 in developing and adult rat brain. Neurosci. Lett. 2000, 286, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Gascón, S.; Sobrado, M.; Roda, J.M.; Rodríguez-Peña, A.; Díaz-Guerra, M. Excitotoxicity and focal cerebral ischemia induce truncation of the NR2A and NR2B subunits of the NMDA receptor and cleavage of the scaffolding protein PSD-95. Mol. Psychiatry 2007, 13, 99–114. [Google Scholar] [CrossRef]
- Chimura, T.; Launey, T.; Yoshida, N.; Norris, C.M. Calpain-Mediated Degradation of Drebrin by Excitotoxicity In vitro and In vivo. PLoS ONE 2015, 10, e0125119. [Google Scholar] [CrossRef]
- Bi, X.; Rong, Y.; Chen, J.; Dang, S.; Wang, Z.; Baudry, M. Calpain-mediated regulation of NMDA receptor structure and function. Brain Res. 1998, 790, 245–253. [Google Scholar] [CrossRef]
- Bi, X.; Chen, J.; Dang, S.; Wenthold, R.J.; Tocco, G.; Baudry, M. Characterization of Calpain-Mediated Proteolysis of GluR1 Subunits of α-Amino-3-Hydroxy-5-Methylisoxazole-4-Propionate Receptors in Rat Brain. J. Neurochem. 1997, 68, 1484–1494. [Google Scholar] [CrossRef]
- Simpkins, K.L.; Guttmann, R.P.; Dong, Y.; Chen, Z.; Sokol, S.; Neumar, R.W.; Lynch, D.R. Selective Activation Induced Cleavage of the NR2B Subunit by Calpain. J. Neurosci. 2003, 23, 11322–11331. [Google Scholar] [CrossRef]
- Guttmann, R.P.; Sokol, S.; Baker, D.L.; Simpkins, K.L.; Dong, Y.; Lynch, D.R. Proteolysis of the N-Methyl-d-Aspartate Receptor by Calpain In Situ. J. Pharmacol. Exp. Ther. 2002, 302, 1023–1030. [Google Scholar] [CrossRef]
- Hayashi, K.; Ishikawa, R.; Ye, L.-H.; He, X.-L.; Takata, K.; Kohama, K.; Shirao, T. Modulatory Role of Drebrin on the Cytoskeleton within Dendritic Spines in the Rat Cerebral Cortex. J. Neurosci. 1996, 16, 7161–7170. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wong, T.P.; Chery, N.; Gaertner, T.; Wang, Y.T.; Baudry, M. Calpain-Mediated mGluR1α Truncation: A Key Step in Excitotoxicity. Neuron 2007, 53, 399–412. [Google Scholar] [CrossRef]
- Magnusson, A.; Haug, L.S.; Walaas, S.; Østvold, A.C. Calcium-induced degradation of the Inositol (1,4,5)-trisphosphate receptor/Ca2+-channel. FEBS Lett. 1993, 323, 229–232. [Google Scholar] [CrossRef]
- Jahani-Asl, A.; Pilon-Larose, K.; Xu, W.; MacLaurin, J.G.; Park, D.S.; McBride, H.M.; Slack, R.S. The Mitochondrial Inner Membrane GTPase, Optic Atrophy 1 (Opa1), Restores Mitochondrial Morphology and Promotes Neuronal Survival following Excitotoxicity. J. Biol. Chem. 2011, 286, 4772–4782. [Google Scholar] [CrossRef] [PubMed]
- Tangmansakulchai, K.; Abubakar, Z.; Kitiyanant, N.; Suwanjang, W.; Leepiyasakulchai, C.; Govitrapong, P.; Chetsawang, B. Calpastatin overexpression reduces oxidative stress-induced mitochondrial impairment and cell death in human neuroblastoma SH-SY5Y cells by decreasing calpain and calcineurin activation, induction of mitochondrial fission and destruction of mitochondrial fusion. Mitochondrion 2016, 30, 151–161. [Google Scholar] [CrossRef]
- Wang, Y.; Hall, R.A.; Lee, M.; Kamgar-Parsi, A.; Bi, X.; Baudry, M. The tyrosine phosphatase PTPN13/FAP-1 links calpain-2, TBI and tau tyrosine phosphorylation. Sci. Rep. 2017, 7, 11771. [Google Scholar] [CrossRef]
- Wang, Y.; Brazdzionis, J.; Dong, F.; Patchana, T.; Ghanchi, H.; Podkovik, S.; Wiginton, J.G.; Marino, M.; Duong, J.; Wacker, M.; et al. P13BP, a Calpain-2-Mediated Breakdown Product of PTPN13, Is a Novel Blood Biomarker for Traumatic Brain Injury. J. Neurotrauma 2021, 38, 3077–3085. [Google Scholar] [CrossRef]
- Mufson, E.J.; He, B.; Ginsberg, S.D.; Carper, B.A.; Bieler, G.S.; Crawford, F.C.; Alvarez, V.E.; Huber, B.R.; Stein, T.D.; McKee, A.C.; et al. Gene Profiling of Nucleus Basalis Tau Containing Neurons in Chronic Traumatic Encephalopathy: A Chronic Effects of Neurotrauma Consortium Study. J. Neurotrauma 2018, 35, 1260–1271. [Google Scholar] [CrossRef]
- Rao, M.V.; McBrayer, M.K.; Campbell, J.; Kumar, A.; Hashim, A.; Sershen, H.; Stavrides, P.H.; Ohno, M.; Hutton, M.; Nixon, R.A. Specific Calpain Inhibition by Calpastatin Prevents Tauopathy and Neurodegeneration and Restores Normal Lifespan in Tau P301L Mice. J. Neurosci. 2014, 34, 9222–9234. [Google Scholar] [CrossRef]
- Liu, M.; Wang, L.; Gao, J.; Dong, Q.; Perry, G.; Ma, X.; Wang, X.; Avila, J. Inhibition of Calpain Protects Against Tauopathy in Transgenic P301S Tau Mice. J. Alzheimer’s Dis. 2019, 69, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Elce, J.S.; E Hamos, J.; A Nixon, R. Widespread activation of calcium-activated neutral proteinase (calpain) in the brain in Alzheimer disease: A potential molecular basis for neuronal degeneration. Proc. Natl. Acad. Sci. USA 1993, 90, 2628–2632. [Google Scholar] [CrossRef] [PubMed]
- Fà, M.; Zhang, H.; Staniszewski, A.; Saeed, F.; Shen, L.W.; Schiefer, I.T.; Siklos, M.I.; Tapadar, S.; Litosh, V.A.; Libien, J.; et al. Novel Selective Calpain 1 Inhibitors as Potential Therapeutics in Alzheimer’s Disease. J. Alzheimer’s Dis. 2016, 49, 707–721. [Google Scholar] [CrossRef]
- Crocker, S.J.; Smith, P.D.; Jackson-Lewis, V.; Lamba, W.R.; Hayley, S.P.; Grimm, E.; Callaghan, S.M.; Slack, R.S.; Melloni, E.; Przedborski, S.; et al. Inhibition of Calpains Prevents Neuronal and Behavioral Deficits in an MPTP Mouse Model of Parkinson’s Disease. J. Neurosci. 2003, 23, 4081–4091. [Google Scholar] [CrossRef]
- Yamashita, T.; Hideyama, T.; Hachiga, K.; Teramoto, S.; Takano, J.; Iwata, N.; Saido, T.C.; Kwak, S. A role for calpain-dependent cleavage of TDP-43 in amyotrophic lateral sclerosis pathology. Nat. Commun. 2012, 3, 1307. [Google Scholar] [CrossRef]
- Gafni, J.; Ellerby, L.M. Calpain Activation in Huntington’s Disease. J. Neurosci. 2002, 22, 4842–4849. [Google Scholar] [CrossRef] [PubMed]
- Imam, S.A.; Guyton, M.K.; Haque, A.; Vandenbark, A.; Tyor, W.R.; Ray, S.K.; Banik, N.L. Increased calpain correlates with Th1 cytokine profile in PBMCs from MS patients. J. Neuroimmunol. 2007, 190, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Trager, N.; Smith, A.; Iv, G.W.; Azuma, M.; Inoue, J.; Beeson, C.; Haque, A.; Banik, N.L. Effects of a novel orally administered calpain inhibitor SNJ-1945 on immunomodulation and neurodegeneration in a murine model of multiple sclerosis. J. Neurochem. 2014, 130, 268–279. [Google Scholar] [CrossRef]
- Gerdts, J.; Brace, E.; Sasaki, Y.; DiAntonio, A.; Milbrandt, J. SARM1 activation triggers axon degeneration locally via NAD+ destruction. Science 2015, 348, 453–457. [Google Scholar] [CrossRef]
- Gerdts, J.; Summers, D.W.; Sasaki, Y.; DiAntonio, A.; Milbrandt, J. Sarm1-Mediated Axon Degeneration Requires Both SAM and TIR Interactions. J. Neurosci. 2013, 33, 13569–13580. [Google Scholar] [CrossRef]
- Osterloh, J.M.; Yang, J.; Rooney, T.M.; Fox, A.N.; Adalbert, R.; Powell, E.H.; Sheehan, A.E.; Avery, M.A.; Hackett, R.; Logan, M.A.; et al. dSarm/Sarm1 Is Required for Activation of an Injury-Induced Axon Death Pathway. Science 2012, 337, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Henninger, N.; Bouley, J.; Sikoglu, E.M.; An, J.; Moore, C.M.; King, J.A.; Bowser, R.; Freeman, M.R.; Brown, R.H., Jr. Attenuated traumatic axonal injury and improved functional outcome after traumatic brain injury in mice lacking Sarm1. Brain 2016, 139, 1094–1105. [Google Scholar] [CrossRef]
- Miyamoto, T.; Kim, C.; Chow, J.; Dugas, J.C.; DeGroot, J.; Bagdasarian, A.L.; Thottumkara, A.P.; Larhammar, M.; Calvert, M.E.; Fox, B.M.; et al. SARM1 is responsible for calpain-dependent dendrite degeneration in mouse hippocampal neurons. J. Biol. Chem. 2024, 300, 105630. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, X.; Lv, X.; Qian, J. The role of spectrin breakdown products in patients with traumatic brain injury: A systematic review and meta-analysis. Neurol. Sci. 2022, 44, 1171–1183. [Google Scholar] [CrossRef]
- Yue, J.K.; Yuh, E.L.; Korley, F.K.; A Winkler, E.A.; Sun, X.; Puffer, R.C.; Deng, H.; Choy, W.; Chandra, A.; Taylor, S.R.; et al. Association between plasma GFAP concentrations and MRI abnormalities in patients with CT-negative traumatic brain injury in the TRACK-TBI cohort: A prospective multicentre study. Lancet Neurol. 2019, 18, 953–961. [Google Scholar] [CrossRef] [PubMed]
- DiGiorgio, A.M.; Wittenberg, B.A.; Crutcher, C.L.; Kennamer, B.; Greene, C.S.; Velander, A.J.; Wilson, J.D.; Tender, G.C.; Culicchia, F.; Hunt, J.P. The Impact of Drug and Alcohol Intoxication on Glasgow Coma Scale Assessment in Patients with Traumatic Brain Injury. World Neurosurg. 2020, 135, e664–e670. [Google Scholar] [CrossRef]
- Gan, Z.S.; Stein, S.C.; Swanson, R.; Guan, S.; Garcia, L.; Mehta, D.; Smith, D.H. Blood Biomarkers for Traumatic Brain Injury: A Quantitative Assessment of Diagnostic and Prognostic Accuracy. Front. Neurol. 2019, 10, 446. [Google Scholar] [CrossRef]
- Kobeissy, F.; Arja, R.D.; Munoz, J.C.; Shear, D.A.; Gilsdorf, J.; Zhu, J.; Yadikar, H.; Haskins, W.; Tyndall, J.A.; Wang, K.K. The game changer: UCH-L1 and GFAP-based blood test as the first marketed in vitro diagnostic test for mild traumatic brain injury. Expert Rev. Mol. Diagn. 2024, 24, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Papa, L.; Brophy, G.M.; Welch, R.D.; Lewis, L.M.; Braga, C.F.; Tan, C.N.; Ameli, N.J.; Lopez, M.A.; Haeussler, C.A.; Giordano, D.I.M.; et al. Time Course and Diagnostic Accuracy of Glial and Neuronal Blood Biomarkers GFAP and UCH-L1 in a Large Cohort of Trauma Patients With and Without Mild Traumatic Brain Injury. JAMA Neurol. 2016, 73, 551–560. [Google Scholar] [CrossRef]
- Welch, R.D.; Bazarian, J.J.; Chen, J.Y.; Chandran, R.; Datwyler, S.A.; McQuiston, B.; Caudle, K. A high-performance core laboratory GFAP/UCH-L1 test for the prediction of intracranial injury after mild traumatic brain injury. Am. J. Emerg. Med. 2024, 89, 129–134. [Google Scholar] [CrossRef]
- Bazarian, J.J.; Biberthaler, P.; Welch, R.D.; Lewis, L.M.; Barzo, P.; Bogner-Flatz, V.; Brolinson, P.G.; Büki, A.; Chen, J.Y.; Christenson, R.H.; et al. Serum GFAP and UCH-L1 for prediction of absence of intracranial injuries on head CT (ALERT-TBI): A multicentre observational study. Lancet Neurol. 2018, 17, 782–789. [Google Scholar] [CrossRef]
- Syzdykbayev, M.; Kazymov, M.; Aubakirov, M.; Kurmangazina, A.; Kairkhanov, E.; Kazangapov, R.; Bryzhakhina, Z.; Imangazinova, S.; Sheinin, A. A Modern Approach to the Treatment of Traumatic Brain Injury. Medicines 2024, 11, 10. [Google Scholar] [CrossRef]
- Ghiam, M.K.; Patel, S.D.; Hoffer, A.; Selman, W.R.; Hoffer, B.J.; Hoffer, M.E. Drug Repurposing in the Treatment of Traumatic Brain Injury. Front. Neurosci. 2021, 15, 635483. [Google Scholar] [CrossRef] [PubMed]
- Bains, M.; Cebak, J.E.; Gilmer, L.K.; Barnes, C.C.; Thompson, S.N.; Geddes, J.W.; Hall, E.D. Pharmacological analysis of the cortical neuronal cytoskeletal protective efficacy of the calpain inhibitor SNJ-1945 in a mouse traumatic brain injury model. J. Neurochem. 2013, 125, 125–132. [Google Scholar] [CrossRef]
- Thompson, S.N.; Carrico, K.M.; Mustafa, A.G.; Bains, M.; Hall, E.D. A Pharmacological Analysis of the Neuroprotective Efficacy of the Brain- and Cell-Permeable Calpain Inhibitor MDL-28170 in the Mouse Controlled Cortical Impact Traumatic Brain Injury Model. J. Neurotrauma 2010, 27, 2233–2243. [Google Scholar] [CrossRef] [PubMed]
- Cuerrier, D.; Moldoveanu, T.; Inoue, J.; Davies, P.L.; Campbell, R.L. Calpain Inhibition by α-Ketoamide and Cyclic Hemiacetal Inhibitors Revealed by X-ray Crystallography, Biochemistry 2006, 45, 7446–7452. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ortega-Vilain, A.-C.; Patil, G.S.; Chu, D.-L.; Foreman, J.E.; Eveleth, D.D.; Powers, J.C. Novel Peptidyl α-Keto Amide Inhibitors of Calpains and Other Cysteine Proteases. J. Med. Chem. 1996, 39, 4089–4098. [Google Scholar] [CrossRef] [PubMed]


| Name | Gene | Tissue Distribution | Physiological Function | Associated Pathology | Refs. |
|---|---|---|---|---|---|
| Calpain-1 * | CAPN1 | Ubiquitous; high in brain, muscle | Synaptic plasticity, learning (LTP), axon growth | Hereditary spastic paraplegia; Spinocerebellar ataxia | [26,27,29,31,32,33] |
| Calpain-2 * | CAPN2 | Ubiquitous; high in brain, heart | Cytoskeletal regulation, cell migration, embryogenesis, cell proliferation | Neurodegenerative diseases; Cardiomyopathy | [34,35,36,37,38,39] |
| Calpain-3 * | CAPN3 | Skeletal muscle | Muscle repair and maintenance, Sarcomere remodeling | Limb-girdle muscular dystrophy type 2A (LGMD2A) | [40,41,42] |
| Calpain-5 | CAPN5 | Ubiquitous; high in retina, brain | Signal transduction | Autosomal dominant neovascular inflammatory vitreoretinopathy (ADNIV) | [43,44] |
| Calpain-6 | CAPN6 | Placenta; embryonic muscle | No proteolytic activity, involved in cytoskeletal dynamics | Cancer progression | [45,46,47,48] |
| Calpain-7 | CAPN7 | Ubiquitous | Cell division, endosomal trafficking/receptor turnover | Hepatocellular carcinoma (HCC) | [49,50,51] |
| Calpain-8 * | CAPN8 | Gastro- intestinal; stomach | Cell migration and proliferation, maintenance of gastric mucosal integrity | Inflammatory gastric diseases | [52] |
| Calpain-9 * | CAPN9 | Gastro- intestinal | Cell migration and proliferation, maintenance of gastric mucosal integrity | Inflammatory gastric diseases | [52] |
| Calpain-10 | CAPN10 | Ubiquitous; high in pancreas, brain | Cytoskeletal remodeling, insulin secretion in pancreatic β-cells | Type 2 diabetes mellitus (T2DM) | [53,54,55] |
| Calpain-11 * | CAPN11 | Testis | Cytoskeletal remodeling, spermatogenesis, meiosis and sperm functional processes | Unknown | [56] |
| Calpain-12 * | CAPN12 | Skin; hair follicles | Epidermal ontogenesis, hair follicle cycling | Congenital ichthyosis | [57,58] |
| Calpain-13 * | CAPN13 | Ubiquitous | Unknown | Cerebral ischemia- reperfusion injury | [59] |
| Calpain-14 * | CAPN14 | Esophagus | Unknown, potentially epithelial barrier regulation | Eosinophilic esophagitis (EoE) | [60] |
| Calpain-15 | CAPN15 | Ubiquitous | Unknown, potentially neurodevelopment | Congenital eye anomalies and other neurodevelopmental deficits | [61,62] |
| Calpain-16 | CAPN16 | Ubiquitous | Unknown, presumed to lack proteolytic function as it only encodes the N-terminal half of the catalytic domain | Unknown | [63] |
| Calpain-4/ Calpain Small Subunit 1 | CAPN4/ CAPNS1 | Ubiquitous | Regulatory subunit | Cancer progression | [20,64] |
| Calpain Small Subunit 2 | CAPNS2 | Ubiquitous | Unknown | Unknown | [65,66] |
| Biomarkers | Sample Matrix | Detection Window | Calpain Specificity | Sensitivity/ Specificity | Application | Refs. |
|---|---|---|---|---|---|---|
| GFAP/ UCH-L1 | Blood (serum) | Within 12h post-TBI | Not applicable | High/ moderate | FDA approved clinical biomarker for mTBI CT necessity assessment | [196,197,198] |
| SBDP145 | CSF (primarily); blood (serum) | 6h-7d post-TBI | High | Moderate/ not applicable | Preclinical/ clinical studies | [191] |
| SBDP150 | CSF (primarily) | 6h-5d post-TBI | Low (also generated by caspase-3) | Low- moderate/ not applicable | Preclinical/ clinical studies | [191] |
| SNTF | Blood (serum/ plasma) | 1h-6d post-TBI | High | Low- moderate/ High | Preclinical/ clinical studies | [115,116,117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schallerer, C.; Neuschmid, S.; Ehrlich, B.E.; McGuone, D. Calpain in Traumatic Brain Injury: From Cinderella to Central Player. Cells 2025, 14, 1253. https://doi.org/10.3390/cells14161253
Schallerer C, Neuschmid S, Ehrlich BE, McGuone D. Calpain in Traumatic Brain Injury: From Cinderella to Central Player. Cells. 2025; 14(16):1253. https://doi.org/10.3390/cells14161253
Chicago/Turabian StyleSchallerer, Carla, Stephan Neuschmid, Barbara E. Ehrlich, and Declan McGuone. 2025. "Calpain in Traumatic Brain Injury: From Cinderella to Central Player" Cells 14, no. 16: 1253. https://doi.org/10.3390/cells14161253
APA StyleSchallerer, C., Neuschmid, S., Ehrlich, B. E., & McGuone, D. (2025). Calpain in Traumatic Brain Injury: From Cinderella to Central Player. Cells, 14(16), 1253. https://doi.org/10.3390/cells14161253







