Systematic Comparison of Temperature Effects on Antibody Performance via Automated Image Analysis: A Key for Primary Ciliary Dyskinesia Diagnostic
Abstract
1. Introduction
2. Materials and Methods
2.1. Nasal Samples
2.2. Immunofluorescence Microscopy
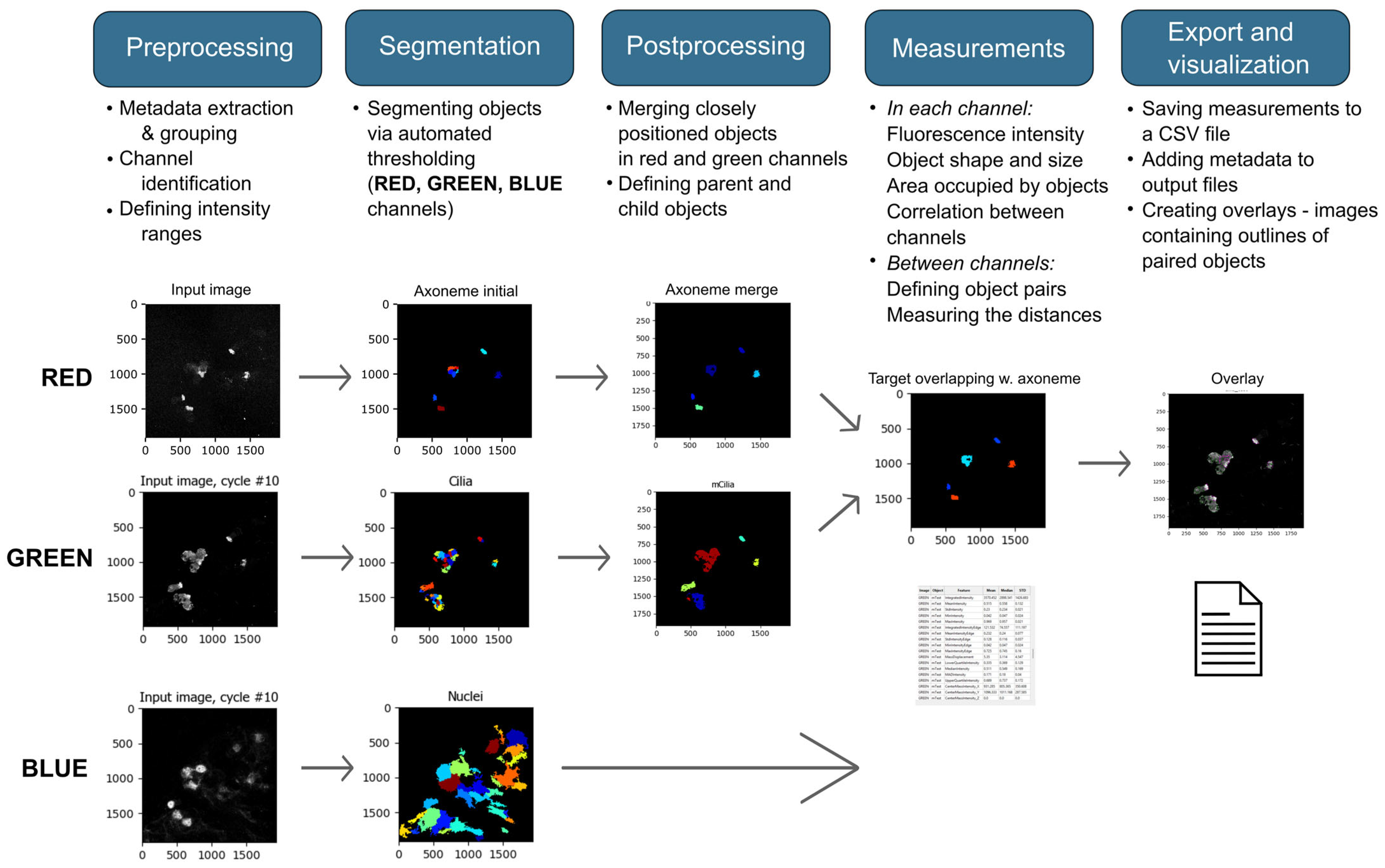
2.3. Image Analysis
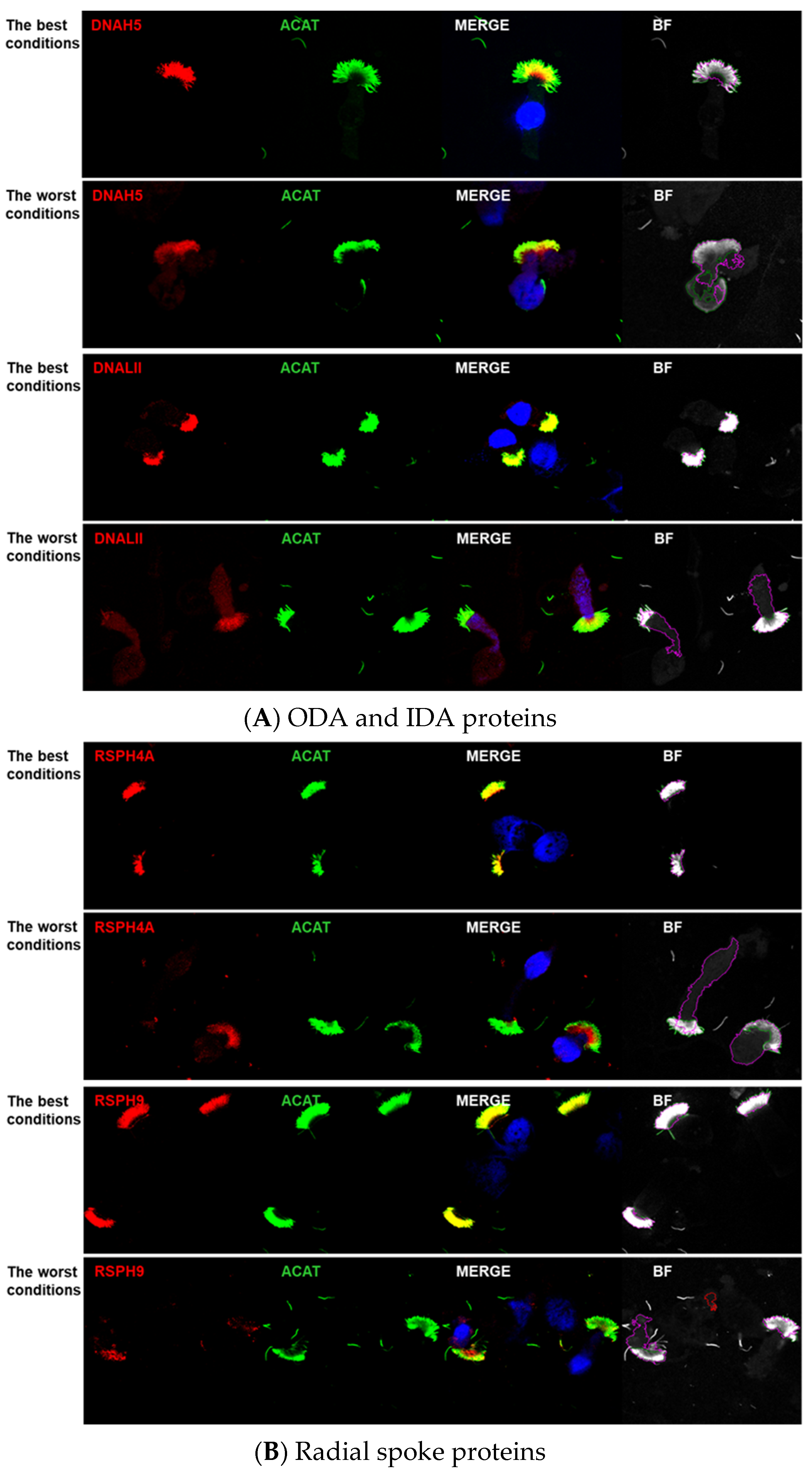
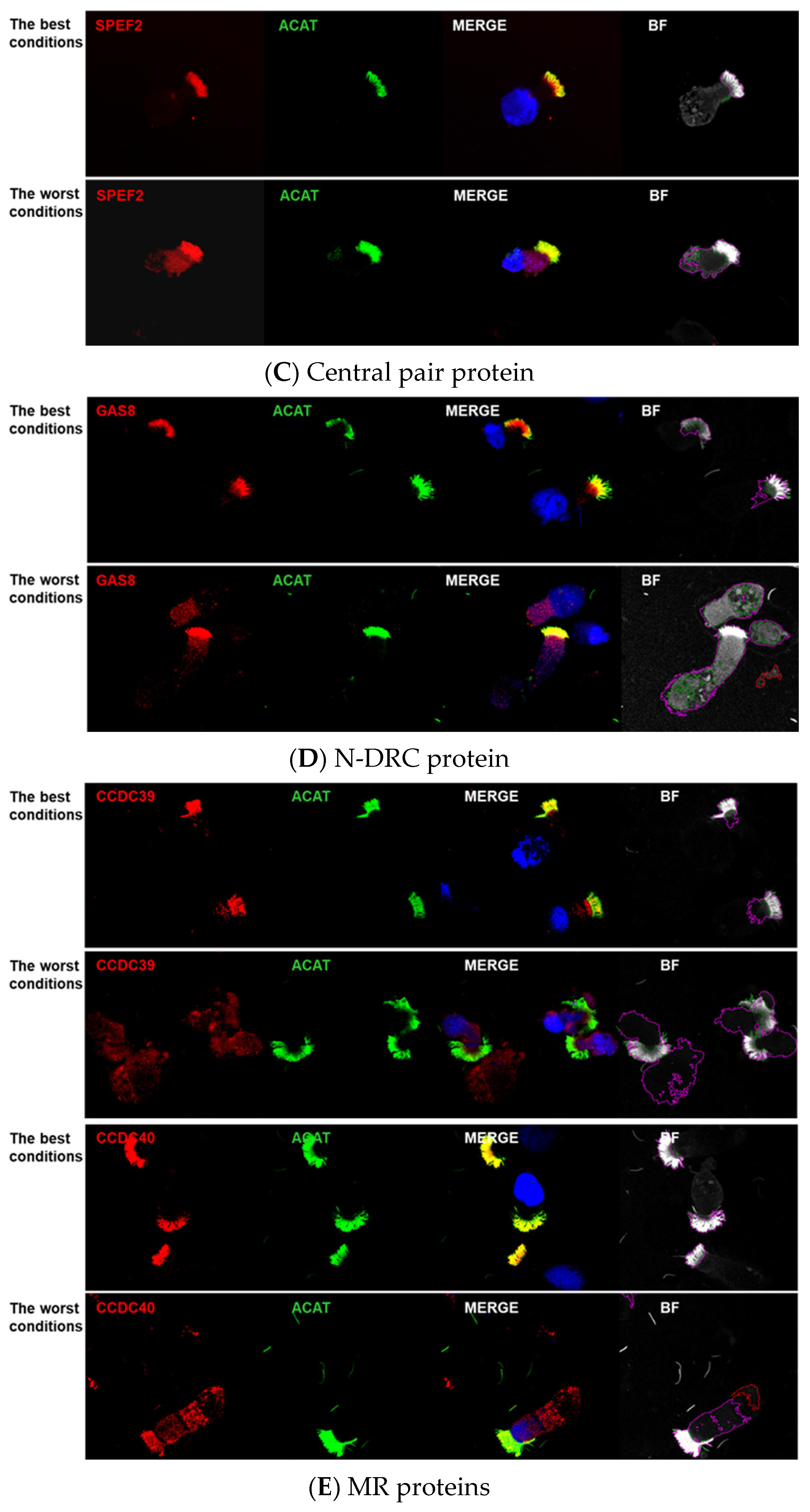
3. Results
Image Analyses Using CellProfiler
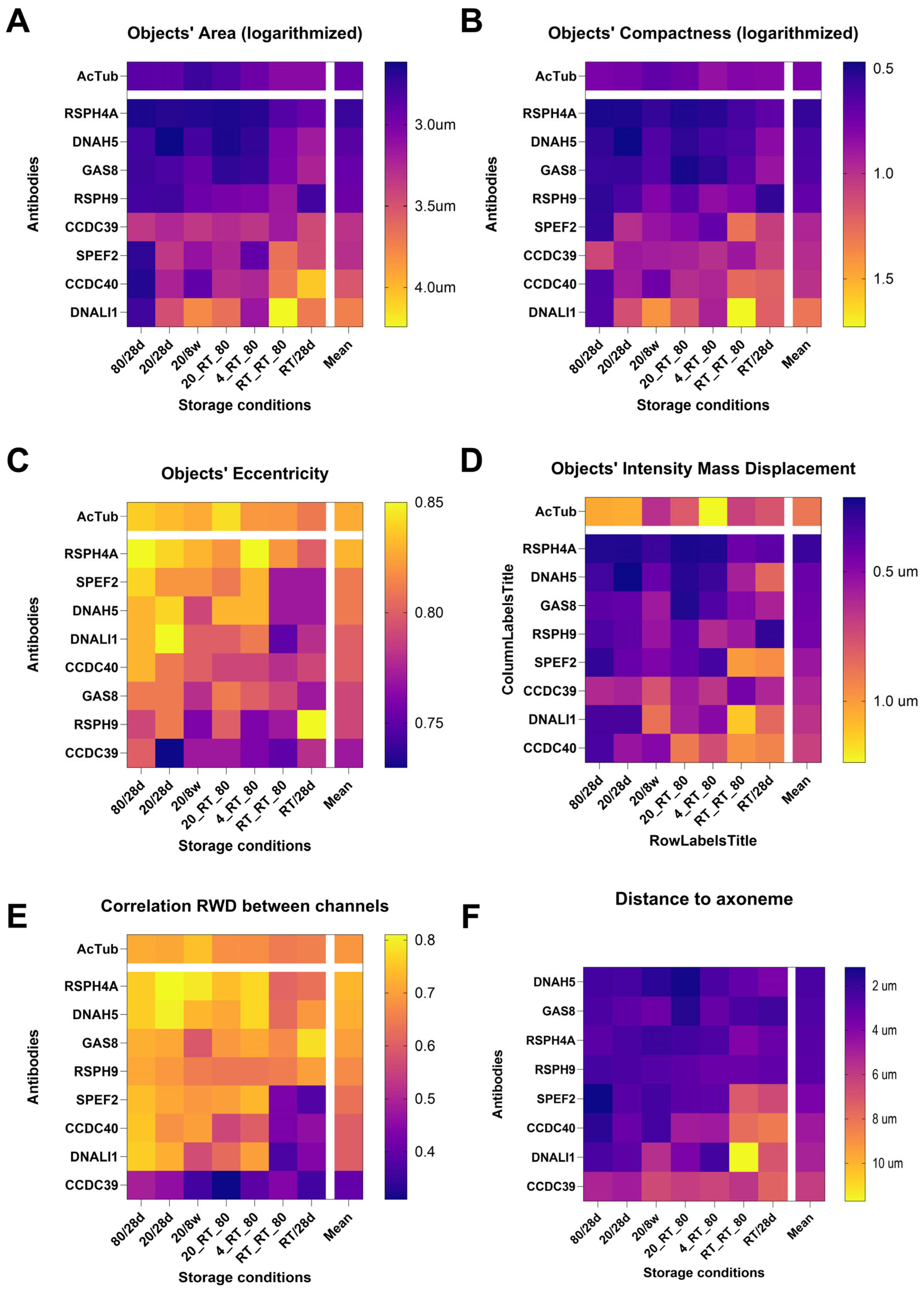
4. Channel Parameters
4.1. Area Characteristics
4.2. Intensity Parameters
4.3. Analysis Between Channels
4.3.1. Pairing of Tested Antibodies with the Axoneme Marker
4.3.2. Correlation Characteristics
4.3.3. Distance Between the Object Pairs
4.3.4. Integrated Performance Metrics
4.3.5. Color Switch of the Antibodies
5. Discussion
5.1. Slide Storage Condition
5.2. Epitope Sensitivity to Storage Conditions
5.3. Interindividual Differences
5.4. Limitations of the Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knowles, M.R.; Daniels, L.A.; Davis, S.D.; Zariwala, M.A.; Leigh, M.W. Primary Ciliary Dyskinesia. Recent Advances in Diagnostics, Genetics, and Characterization of Clinical Disease. Am. J. Respir. Crit. Care Med. 2013, 188, 913–922. [Google Scholar] [CrossRef]
- Despotes, K.A.; Zariwala, M.A.; Davis, S.D.; Ferkol, T.W. Primary Ciliary Dyskinesia: A Clinical Review. Cells 2024, 13, 974. [Google Scholar] [CrossRef]
- Wallmeier, J.; Nielsen, K.G.; Kuehni, C.E.; Lucas, J.S.; Leigh, M.W.; Zariwala, M.A.; Omran, H. Motile Ciliopathies. Nat. Rev. Dis. Primers. 2020, 6, 77. [Google Scholar] [CrossRef]
- Hannah, W.B.; Seifert, B.A.; Truty, R.; Zariwala, M.A.; Ameel, K.; Zhao, Y.; Nykamp, K.; Gaston, B. The Global Prevalence and Ethnic Heterogeneity of Primary Ciliary Dyskinesia Gene Variants: A Genetic Database Analysis. Lancet Respir. Med. 2022, 10, 459–468. [Google Scholar] [CrossRef]
- Wee, W.B.; Gatt, D.; Seidl, E.; Santyr, G.; To, T.; Dell, S.D. Estimates of Primary Ciliary Dyskinesia Prevalence: A Scoping Review. ERJ Open Res. 2024, 10, 00989–02023. [Google Scholar] [CrossRef] [PubMed]
- Legendre, M.; Zaragosi, L.-E.; Mitchison, H.M. Motile Cilia and Airway Disease. Semin. Cell Dev. Biol. 2021, 110, 19–33. [Google Scholar] [CrossRef]
- Goutaki, M.; Shoemark, A. Diagnosis of Primary Ciliary Dyskinesia. Clin. Chest Med. 2022, 43, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Raidt, J.; Riepenhausen, S.; Pennekamp, P.; Olbrich, H.; Amirav, I.; Athanazio, R.A.; Aviram, M.; Balinotti, J.E.; Bar-On, O.; Bode, S.F.N.; et al. Analyses of 1236 Genotyped Primary Ciliary Dyskinesia Individuals Identify Regional Clusters of Distinct DNA Variants and Significant Genotype-Phenotype Correlations. Eur. Respir. J. 2024, 64, 2301769. [Google Scholar] [CrossRef] [PubMed]
- Kuehni, C.E.; Frischer, T.; Strippoli, M.-P.F.; Maurer, E.; Bush, A.; Nielsen, K.G.; Escribano, A.; Lucas, J.S.A.; Yiallouros, P.; Omran, H.; et al. Factors Influencing Age at Diagnosis of Primary Ciliary Dyskinesia in European Children. Eur. Respir. J. 2010, 36, 1248–1258. [Google Scholar] [CrossRef] [PubMed]
- Lucas, J.S.; Barbato, A.; Collins, S.A.; Goutaki, M.; Behan, L.; Caudri, D.; Dell, S.; Eber, E.; Escudier, E.; Hirst, R.A.; et al. European Respiratory Society Guidelines for the Diagnosis of Primary Ciliary Dyskinesia. Eur. Respir. J. 2017, 49, 1601090. [Google Scholar] [CrossRef] [PubMed]
- Baz-Redón, N.; Rovira-Amigo, S.; Fernández-Cancio, M.; Castillo-Corullón, S.; Cols, M.; Caballero-Rabasco, M.A.; Asensio, Ó.; Martín de Vicente, C.; Martínez-Colls, M.D.; Torrent-Vernetta, A.; et al. Immunofluorescence Analysis as a Diagnostic Tool in a Spanish Cohort of Patients with Suspected Primary Ciliary Dyskinesia. J. Clin. Med. 2020, 9, 3603. [Google Scholar] [CrossRef]
- Shoemark, A.; Frost, E.; Dixon, M.; Ollosson, S.; Kilpin, K.; Patel, M.; Scully, J.; Rogers, A.V.; Mitchison, H.M.; Bush, A.; et al. Accuracy of Immunofluorescence in the Diagnosis of Primary Ciliary Dyskinesia. Am. J. Respir. Crit. Care Med. 2017, 196, 94–101. [Google Scholar] [CrossRef]
- Müller, L.; Savas, S.T.; Tschanz, S.A.; Stokes, A.; Escher, A.; Nussbaumer, M.; Bullo, M.; Kuehni, C.E.; Blanchon, S.; Jung, A.; et al. A Comprehensive Approach for the Diagnosis of Primary Ciliary Dyskinesia—Experiences from the First 100 Patients of the PCD-UNIBE Diagnostic Center. Diagnostics 2021, 11, 1540. [Google Scholar] [CrossRef] [PubMed]
- Jeanson, L.; Thomas, L.; Copin, B.; Coste, A.; Sermet-Gaudelus, I.; Dastot-Le Moal, F.; Duquesnoy, P.; Montantin, G.; Collot, N.; Tissier, S.; et al. Mutations in GAS8, a Gene Encoding a Nexin-Dynein Regulatory Complex Subunit, Cause Primary Ciliary Dyskinesia with Axonemal Disorganization. Hum. Mutat. 2016, 37, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Omran, H.; Loges, N.T. Immunofluorescence Staining of Ciliated Respiratory Epithelial Cells. Methods Cell Biol. 2009, 91, 123–133. [Google Scholar] [CrossRef]
- Bush, A.; Chodhari, R.; Collins, N.; Copeland, F.; Hall, P.; Harcourt, J.; Hariri, M.; Hogg, C.; Lucas, J.; Mitchison, H.M.; et al. Primary Ciliary Dyskinesia: Current State of the Art. Arch. Dis. Child 2007, 92, 1136–1140. [Google Scholar] [CrossRef]
- Bukowy-Bieryłło, Z.; Daca-Roszak, P.; Jurczak, J.; Przystałowska-Macioła, H.; Jaksik, R.; Witt, M.; Ziętkiewicz, E. In Vitro Differentiation of Ciliated Cells in ALI-Cultured Human Airway Epithelium—The Framework for Functional Studies on Airway Differentiation in Ciliopathies. Eur. J. Cell Biol. 2022, 101, 151189. [Google Scholar] [CrossRef]
- Stirling, D.R.; Swain-Bowden, M.J.; Lucas, A.M.; Carpenter, A.E.; Cimini, B.A.; Goodman, A. CellProfiler 4: Improvements in Speed, Utility and Usability. BMC Bioinform. 2021, 22, 433. [Google Scholar] [CrossRef] [PubMed]
- Merveille, A.-C.; Davis, E.E.; Becker-Heck, A.; Legendre, M.; Amirav, I.; Bataille, G.; Belmont, J.; Beydon, N.; Billen, F.; Clément, A.; et al. CCDC39 Is Required for Assembly of Inner Dynein Arms and the Dynein Regulatory Complex and for Normal Ciliary Motility in Humans and Dogs. Nat. Genet 2011, 43, 72–78. [Google Scholar] [CrossRef]
- Becker-Heck, A.; Zohn, I.E.; Okabe, N.; Pollock, A.; Lenhart, K.B.; Sullivan-Brown, J.; McSheene, J.; Loges, N.T.; Olbrich, H.; Haeffner, K.; et al. The Coiled-Coil Domain Containing Protein CCDC40 Is Essential for Motile Cilia Function and Left-Right Axis Formation. Nat. Genet. 2011, 43, 79–84. [Google Scholar] [CrossRef]
- Wilken, A.; Höben, I.M.; Wolter, A.; Loges, N.T.; Olbrich, H.; Aprea, I.; Dworniczak, B.; Raidt, J.; Omran, H. Primary Ciliary Dyskinesia Associated Disease-Causing Variants in CCDC39 and CCDC40 Cause Axonemal Absence of Inner Dynein Arm Heavy Chains DNAH1, DNAH6, and DNAH7. Cells 2024, 13, 1200. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, B.; Collet, S.; Eloy, P.; Rombaux, P. Secondary Ciliary Dyskinesia in Upper Respiratory Tract. Acta Otorhinolaryngol. Belg. 2000, 54, 309–316. [Google Scholar] [PubMed]
- Jorissen, M.; Willems, T. The Secondary Nature of Ciliary (Dis)Orientation in Secondary and Primary Ciliary Dyskinesia. Acta Otolaryngol. 2004, 124, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Fliegauf, M.; Olbrich, H.; Horvath, J.; Wildhaber, J.H.; Zariwala, M.A.; Kennedy, M.; Knowles, M.R.; Omran, H. Mislocalization of DNAH5 and DNAH9 in Respiratory Cells from Patients with Primary Ciliary Dyskinesia. Am. J. Respir. Crit. Care Med. 2005, 171, 1343–1349. [Google Scholar] [CrossRef] [PubMed]
| Group | Conditions (Temperature and Duration Time) | |||
|---|---|---|---|---|
| 1 | −80_28 d (best conditions) | −80 °C 28 days | ||
| 2 | −20_28 d | −20 °C 28 days | ||
| 3 | −20_8 w | −20 °C 8 weeks | ||
| 4 | −20/RT/−80 | −20 °C 11 days | RT 3 days | −80 °C 14 days |
| 5 | 4/RT/−80 | 4 °C 11 days | RT 3 days | −80 °C 14 days |
| 6 | RT/RT/−80 | RT 11 days | RT 3 days | −80 °C 14 days |
| 7 | RT_28 d (worst conditions) | RT 28 days | ||
| Storage Condition | −80_28 d | −20_28 d | −20/RT/-80 | −20_8w | 4/RT/−80 | RT/RT/−80 | RT_28 d |
|---|---|---|---|---|---|---|---|
| Mean_mTest_Correlation_RWC_RED_GREEN | 1 | 2 | 5 | 4 | 3 | 6 | 7 |
| Mean_mTest_AreaShape_Compactness | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Mean_mTest_AreaShape_Area | 1 | 2 | 4 | 3 | 5 | 6 | 7 |
| Mean_mTest_Area_Eccentricity | 1 | 2 | 3 | 5 | 4 | 6 | 7 |
| Mean_mTest_Intensity_Mass_Displacement | 1 | 3 | 2 | 5 | 4 | 7 | 6 |
| Mean_mTest_Distance_Centroid_mCilia | 1 | 2 | 4 | 3 | 5 | 7 | 6 |
| Overall Performance Rank: | 1.0 | 2.2 | 3.5 | 4.0 | 4.3 | 6.3 | 6.7 |
| RSPH4A | DNAH5 | GAS8 | RSPH9 | SPEF2 | CCDC40 | DNALI1 | CCDC39 | |
|---|---|---|---|---|---|---|---|---|
| Mean_mTest_Correlation_RWC_RED_GREEN | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Mean_mTest_AreaShape_Compactness | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Mean_mTest_AreaShape_Aarea | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Mean_mTest_Area_Eccentricity | 3 | 6 | 4 | 2 | 1 | 7 | 5 | 8 |
| Mean_mTest_Intensity_Mass_Displacement | 1 | 2 | 3 | 4 | 5 | 8 | 7 | 6 |
| Mean_mTest_Distance_Centroid_mCilia | 4 | 1 | 2 | 3 | 5 | 6 | 7 | 8 |
| Overall Performance Rank: | 1.8 | 2.5 | 3.0 | 3.5 | 4.3 | 6.5 | 6.7 | 7.7 |
| Antibody | All Storage Conditions | All Conditions Except Prolonged RT |
|---|---|---|
| RSPH4A | 1.8 | 1.5 |
| DNAH5 | 2.5 | 2.2 |
| GAS8 | 3.0 | 4.2 |
| RSPH9 | 3.5 | 4.5 |
| SPEF2 | 4.3 | 4.2 |
| CCDC40 | 6.5 | 5.7 |
| DNALI1 | 6.7 | 6.0 |
| CCDC39 | 7.7 | 7.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Przystalowska-Maciola, H.; Dabrowska, M.; Zietkiewicz, E.; Zuzanna, B.-B. Systematic Comparison of Temperature Effects on Antibody Performance via Automated Image Analysis: A Key for Primary Ciliary Dyskinesia Diagnostic. Cells 2025, 14, 1236. https://doi.org/10.3390/cells14161236
Przystalowska-Maciola H, Dabrowska M, Zietkiewicz E, Zuzanna B-B. Systematic Comparison of Temperature Effects on Antibody Performance via Automated Image Analysis: A Key for Primary Ciliary Dyskinesia Diagnostic. Cells. 2025; 14(16):1236. https://doi.org/10.3390/cells14161236
Chicago/Turabian StylePrzystalowska-Maciola, Hanna, Malgorzata Dabrowska, Ewa Zietkiewicz, and Bukowy-Bieryllo Zuzanna. 2025. "Systematic Comparison of Temperature Effects on Antibody Performance via Automated Image Analysis: A Key for Primary Ciliary Dyskinesia Diagnostic" Cells 14, no. 16: 1236. https://doi.org/10.3390/cells14161236
APA StylePrzystalowska-Maciola, H., Dabrowska, M., Zietkiewicz, E., & Zuzanna, B.-B. (2025). Systematic Comparison of Temperature Effects on Antibody Performance via Automated Image Analysis: A Key for Primary Ciliary Dyskinesia Diagnostic. Cells, 14(16), 1236. https://doi.org/10.3390/cells14161236










