A Backwards Approach to GD2 Immunofluorescence in Human Neuroblastoma Tissue Samples: From Staining to Slicing
Abstract
1. Introduction
2. Materials and Methods
2.1. Source of Samples
2.2. Antibodies
2.3. Antibody Validation Strategy
2.4. Immunofluorescence Staining
2.5. Tissue Processing After Immunofluorescence Staining Completion
2.5.1. Dehydration and Paraffin Embedment
2.5.2. Tissue Slicing
2.5.3. Deparaffinization, Rehydration and Mounting
2.6. Imaging
2.6.1. Three-Dimensional Imaging
2.6.2. Two-Dimensional Imaging
2.7. Image Processing and Analysis
3. Results
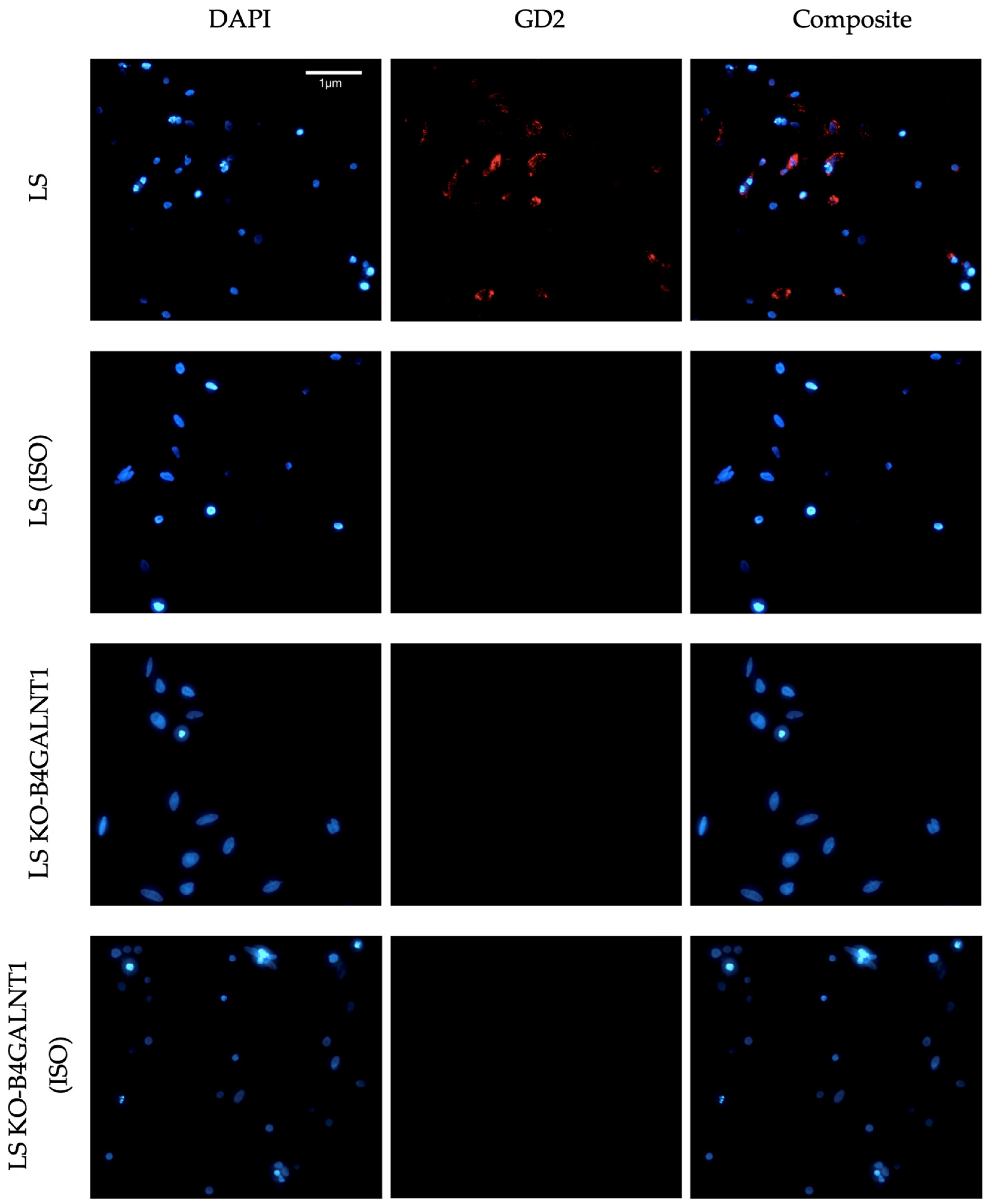
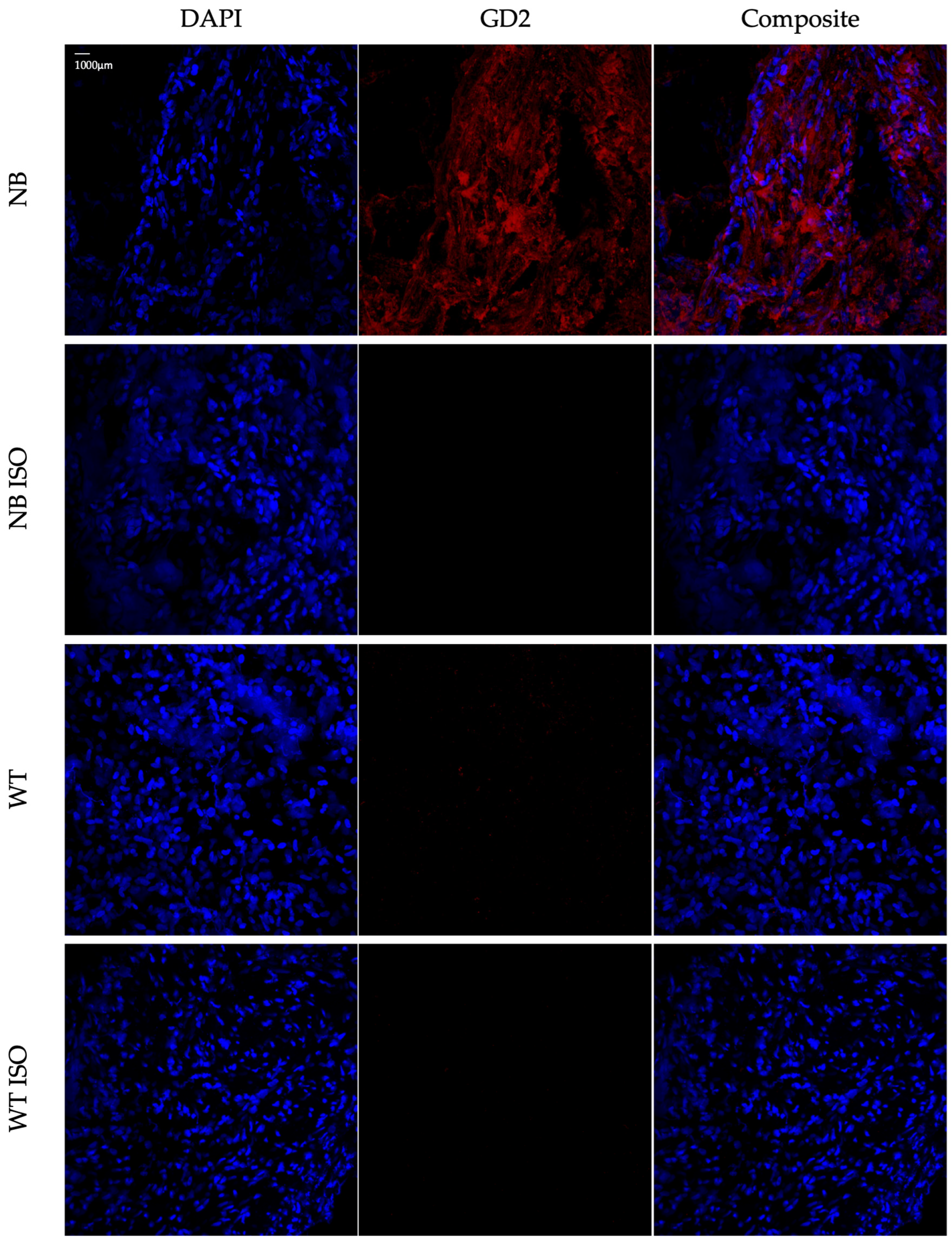
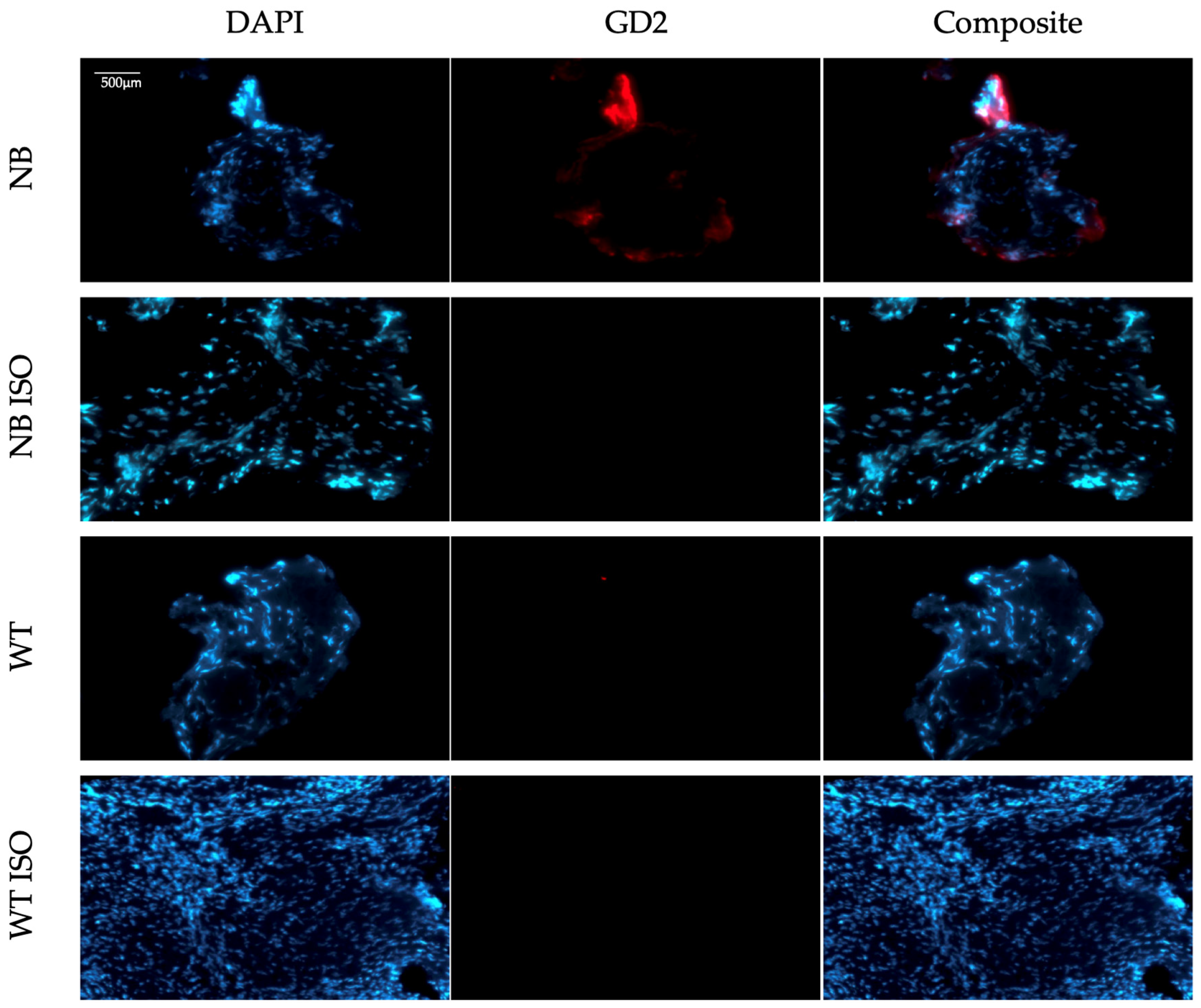
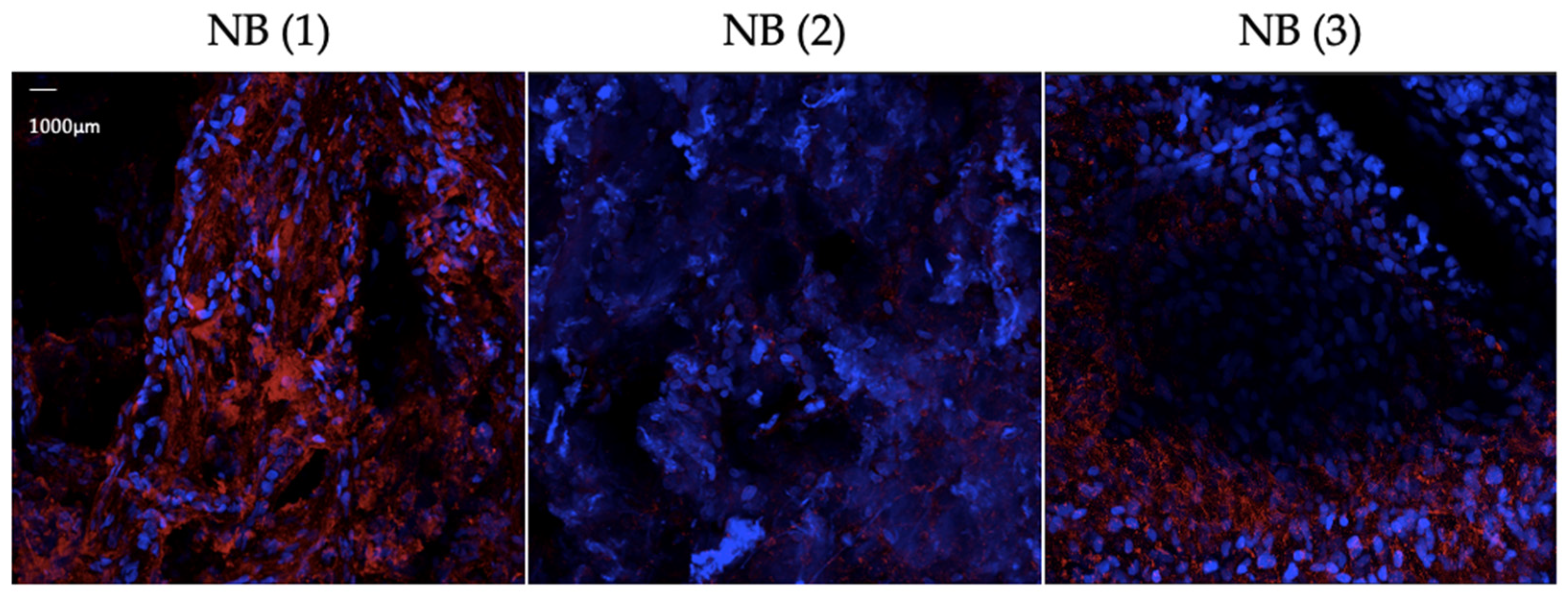
3.1. Results from Antibody Validation
3.2. Results from Protocol Establishment and Validation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NB | Neuroblastoma |
| mAb | Monoclonal antibody |
| CAR | Chimeric antigen receptor-expressing |
| FFPE | Formalin-fixed paraffin-embedded |
| IF | Immunofluorescence |
| AF | Alexa Fluor |
| KO | Knock out |
| RT | Room temperature |
| FBS | Fetal bovine serum |
| (D)PBS | (Dulbecco’s) phosphate-buffered saline |
| BSA | Bovine serum albumin |
| DAPI | 4′,6-diamidino-2-phenylindole |
| SOP | Standard operating protocol |
| WT | Wilms tumor |
| GFP | Green fluorescent protein |
| IHC | Immunohistochemistry |
| FC | Flow cytometry |
References
- Machy, P.; Mortier, E.; Birklé, S. Biology of GD2 ganglioside: Implications for cancer immunotherapy. Front. Pharmacol. 2023, 14, 1249929. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, S.; Brennan, K.; Willison, H. Heteromeric glycolipid complexes as modulators of autoantibody and lectin binding. Prog. Lipid Res. 2010, 49, 87–95. [Google Scholar] [CrossRef]
- Paret, C.; Ustjanzew, A.; Ersali, S.; Seidmann, L.; Jennemann, R.; Ziegler, N.; El Malki, K.; Russo, A.; Wingerter, A.; Ortmüller, F.; et al. GD2 Expression in Medulloblastoma and Neuroblastoma for Personalized Immunotherapy: A Matter of Subtype. Cancers 2022, 14, 6051. [Google Scholar] [CrossRef]
- Peggion, S.; Najem, S.; Kolman, J.P.; Reinshagen, K.; Raluy, L.P. Revisiting Neuroblastoma: Nrf2, NF-κB and Phox2B as a Promising Network in Neuroblastoma. Curr. Issues Mol. Biol. 2024, 46, 3193–3208. [Google Scholar] [CrossRef]
- Pennington, B.; Ren, S.; Barton, S.; Bacelar, M.; Edwards, S.J. Dinutuximab Beta for Treating Neuroblastoma: An Evidence Review Group and Decision Support Unit Perspective of a NICE Single Technology Appraisal. PharmacoEconomics 2018, 37, 985–993. [Google Scholar] [CrossRef]
- Del Bufalo, F.; De Angelis, B.; Caruana, I.; Del Baldo, G.; De Ioris, M.A.; Serra, A.; Mastronuzzi, A.; Cefalo, M.G.; Pagliara, D.; Amicucci, M.; et al. GD2-CART01 for Relapsed or Refractory High-Risk Neuroblastoma. N. Engl. J. Med. 2023, 388, 1284–1295. [Google Scholar] [CrossRef]
- Burchill, S.A.; Beiske, K.; Shimada, H.; Ambros, P.F.; Seeger, R.; Tytgat, G.A.; Brock, P.R.; Haber, M.; Park, J.R.; Berthold, F. Recommendations for the standardization of bone marrow disease assessment and reporting in children with neuroblastoma on behalf of the International Neuroblastoma Response Criteria Bone Marrow Working Group. Cancer 2016, 123, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Kramer, K.; Gerald, W.L.; Kushner, B.H.; Larson, S.M.; Hameed, M.; Cheung, N.-K.V. Disialo-ganglioside GD2 loss following monoclonal antibody therapy is rare in neuroblastoma. Med. Pediatr. Oncol. 2001, 36, 194–196. [Google Scholar] [CrossRef]
- Schumacher-Kuckelkorn, R.; Hero, B.; Ernestus, K.; Berthold, F. Lacking immunocytological GD2 expression in neuroblastoma: Report of 3 cases. Pediatr. Blood Cancer 2005, 45, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Erber, R.; Kailayangiri, S.; Huebner, H.; Ruebner, M.; Hartmann, A.; Häberle, L.; Meyer, J.; Völkl, S.; Mackensen, A.; Landgraf, L.; et al. Variable Expression of the Disialoganglioside GD2 in Breast Cancer Molecular Subtypes. Cancers 2021, 13, 5577. [Google Scholar] [CrossRef]
- Nazha, B.; Inal, C.; Owonikoko, T.K. Disialoganglioside GD2 Expression in Solid Tumors and Role as a Target for Cancer Therapy. Front. Oncol. 2020, 10, 1000. [Google Scholar] [CrossRef]
- O’Boyle, K.P.; Freeman, K.; Kalisiak, A.; Agregado, A.; Scheinberg, D.A. Patterns of Ganglioside Expression in B Cell Neoplasms. Leuk. Lymphoma 1996, 21, 255–266. [Google Scholar] [CrossRef]
- Seitz, C.M.; Flaadt, T.; Mezger, M.; Lang, A.-M.; Michaelis, S.; Katz, M.; Syring, D.; Joechner, A.; Rabsteyn, A.; Siebert, N.; et al. Immunomonitoring of Stage IV Relapsed Neuroblastoma Patients Undergoing Haploidentical Hematopoietic Stem Cell Transplantation and Subsequent GD2 (ch14.18/CHO) Antibody Treatment. Front. Immunol. 2021, 12, 690467. [Google Scholar] [CrossRef]
- Furukawa, K.; Takamiya, K.; Furukawa, K. β1,4-N-acetylgalactosaminyltransferase—GM2/GD2 synthase: A key enzyme to control the synthesis of brain-enriched complex gangliosides. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2002, 1573, 356–362. [Google Scholar] [CrossRef]
- Zeiss MP In Vivo Platform. Available online: https://www.umif.de/systems/14-zeiss-mp-in-vivo-platform (accessed on 4 July 2024).
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Zaqout, S.; Becker, L.-L.; Kaindl, A.M. Immunofluorescence Staining of Paraffin Sections Step by Step. Front. Neuroanat. 2020, 14, 582218. [Google Scholar] [CrossRef] [PubMed]
- Daneshtalab, N.; Doré, J.J.; Smeda, J.S. Troubleshooting tissue specificity and antibody selection: Procedures in immunohistochemical studies. J. Pharmacol. Toxicol. Methods 2010, 61, 127–135. [Google Scholar] [CrossRef]
- Aygün, Z.; Batur, Ş.; Emre, Ş.; Celkan, T.; Özman, O.; Comunoglu, N. Frequency of ALK and GD2 Expression in Neuroblastoma. Fetal Pediatr. Pathol. 2019, 38, 326–334. [Google Scholar] [CrossRef]
- Baker, M. 1,500 scientists lift the lid on reproducibility. Nature 2016, 533, 452–454. [Google Scholar] [CrossRef] [PubMed]
- Mebratie, D.Y.; Dagnaw, G.G. Review of immunohistochemistry techniques: Applications, current status, and future perspectives. Semin. Diagn. Pathol. 2024, 41, 154–160. [Google Scholar] [CrossRef]
- Kailayangiri, S.; Altvater, B.; Meltzer, J.; Pscherer, S.; Luecke, A.; Dierkes, C.; Titze, U.; Leuchte, K.; Landmeier, S.; Hotfilder, M.; et al. The ganglioside antigen GD2 is surface-expressed in Ewing sarcoma and allows for MHC-independent immune targeting. Br. J. Cancer 2012, 106, 1123–1133. [Google Scholar] [CrossRef]
- Zhong, E.; Brogi, E.; D’aLfonso, T.M.; Wen, H.; Frosina, D.B.; Cheung, N.-K.; Jungbluth, A.A.; Ross, D.S. Expression Analysis of GD2 by Immunohistochemistry in Invasive Breast Carcinoma: Clinical and Pathologic Correlation. Appl. Immunohistochem. Mol. Morphol. 2021, 30, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Kailayangiri, S.; Altvater, B.; Lesch, S.; Balbach, S.; Göttlich, C.; Kühnemundt, J.; Mikesch, J.-H.; Schelhaas, S.; Jamitzky, S.; Meltzer, J.; et al. EZH2 Inhibition in Ewing Sarcoma Upregulates GD2 Expression for Targeting with Gene-Modified T Cells. Mol. Ther. 2019, 27, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, M.; Kholodenko, I.; Kalinovsky, D.; Shamanskaya, T.; Doronin, I.; Konovalov, D.; Mironov, A.; Kuzmin, D.; Nikitin, D.; Deyev, S.; et al. RNA Sequencing-Based Identification of Ganglioside GD2-Positive Cancer Phenotype. Biomedicines 2020, 8, 142. [Google Scholar] [CrossRef] [PubMed]
- Kailayangiri, S.; Altvater, B.; Farwick, N.; Meltzer, J.; Hartmann, W.; Rossig, C. Protocol for assessing GD2 on formalin-fixed paraffin-embedded tissue sections using immunofluorescence staining. STAR Protoc. 2024, 5, 103199. [Google Scholar] [CrossRef]
- Terzic, T.; Cordeau, M.; Herblot, S.; Teira, P.; Cournoyer, S.; Beaunoyer, M.; Peuchmaur, M.; Duval, M.; Sartelet, H. Expression of Disialoganglioside (GD2) in Neuroblastic Tumors: A Prognostic Value for Patients Treated With Anti-GD2 Immunotherapy. Pediatr. Dev. Pathol. 2017, 21, 355–362. [Google Scholar] [CrossRef]
| Reagent | Step Duration |
|---|---|
| 70% isopropanol | 50 min |
| 70% isopropanol | 60 min |
| 80% isopropanol | 60 min |
| 85% isopropanol | 60 min |
| 90% isopropanol | 60 min |
| 90% isopropanol | 60 min |
| 96% isopropanol | 60 min |
| 96% isopropanol | 60 min |
| 100% isopropanol 1 | 90 min |
| 100% isopropanol | 90 min |
| Wax bath with paraffin 2 (56 °C) | 120 min |
| Wax bath with paraffin 2 (56 °C) | 180 min |
| Objective | 20× W Plan-APOCHROMAT Corr DIC Refractive index: 1.33–1.36 Numerical Aperture: 1.0 Working Distance (mm): 2.4 | ||
| Detectors | Confocal unit: −32× GaAsP −2× PMT (UV and NIR) −Airyscan 2 | ||
| Laser lines (nm) | SpectraPhysics InSight X3+ | 680–1030 (tunable) | 1040 (fixed) |
| Filters for fluorescence | For 32+2 channel spectral detection: tunable between 380 and 900 nm For eyepiece: Filter system (em.-color, dye): excitation | beamsplitter | emission • 49 (blue; AF405, DAPI): BP 365 | FT 395 | BP 445/50 • 38 (green; AF488, GFP): BP 470/40 | FT 495 | BP 525/50 • 43 (red; AF568, mCherry): BP 550/25 | FT 570 | BP 605/70 For Airyscan: 1. BP 420-480 + BP 495-550 2. BP 420-480 + BP 570-630 3. BP 420-500 + LP 605 4. BP 465-505 + BP 525-585 5. BP 495-550 + BP 570-630 6. BP 495-560 + LP 660 7. BP 570-620 + LP 660 | ||
| UV-VIS illumination | HXP 120 V | ||
| Halogen lamp | 100 W 12 V | ||
| Actively damped optical table | Newport Smart Table UT2 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peggion, S.; Volz, C.; Trochimiuk, M.; Bley, I.A.; Ramos, J.; Reinshagen, K.; Pagerols Raluy, L. A Backwards Approach to GD2 Immunofluorescence in Human Neuroblastoma Tissue Samples: From Staining to Slicing. Cells 2025, 14, 1462. https://doi.org/10.3390/cells14181462
Peggion S, Volz C, Trochimiuk M, Bley IA, Ramos J, Reinshagen K, Pagerols Raluy L. A Backwards Approach to GD2 Immunofluorescence in Human Neuroblastoma Tissue Samples: From Staining to Slicing. Cells. 2025; 14(18):1462. https://doi.org/10.3390/cells14181462
Chicago/Turabian StylePeggion, Sara, Clara Volz, Magdalena Trochimiuk, Isabelle Ariane Bley, Júlia Ramos, Konrad Reinshagen, and Laia Pagerols Raluy. 2025. "A Backwards Approach to GD2 Immunofluorescence in Human Neuroblastoma Tissue Samples: From Staining to Slicing" Cells 14, no. 18: 1462. https://doi.org/10.3390/cells14181462
APA StylePeggion, S., Volz, C., Trochimiuk, M., Bley, I. A., Ramos, J., Reinshagen, K., & Pagerols Raluy, L. (2025). A Backwards Approach to GD2 Immunofluorescence in Human Neuroblastoma Tissue Samples: From Staining to Slicing. Cells, 14(18), 1462. https://doi.org/10.3390/cells14181462








