Dual Nature of Neutrophil Extracellular Traps (NETs)—From Cancer’s Ally to Therapeutic Target
Abstract
1. Introduction
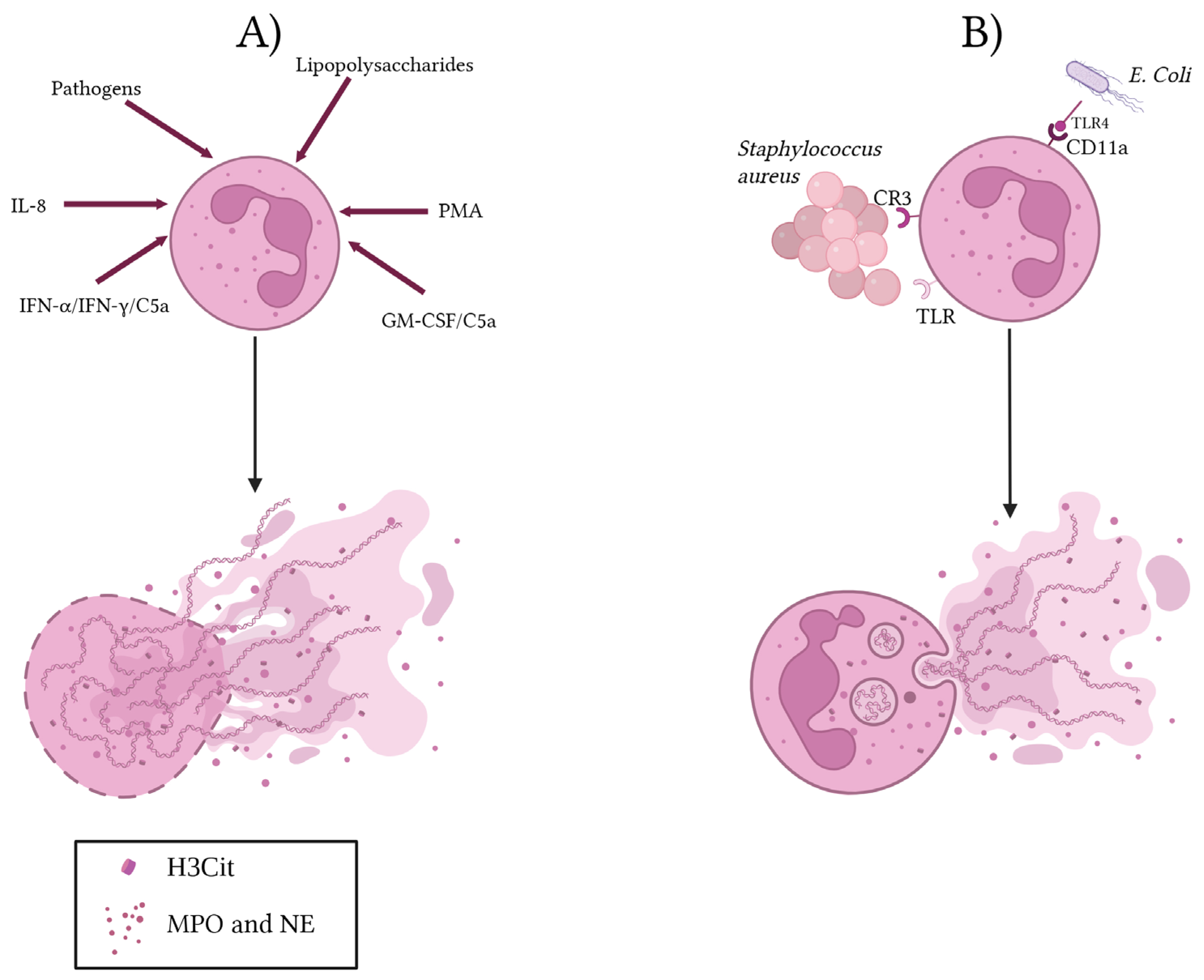
2. NET Production
3. NETs in Cancer
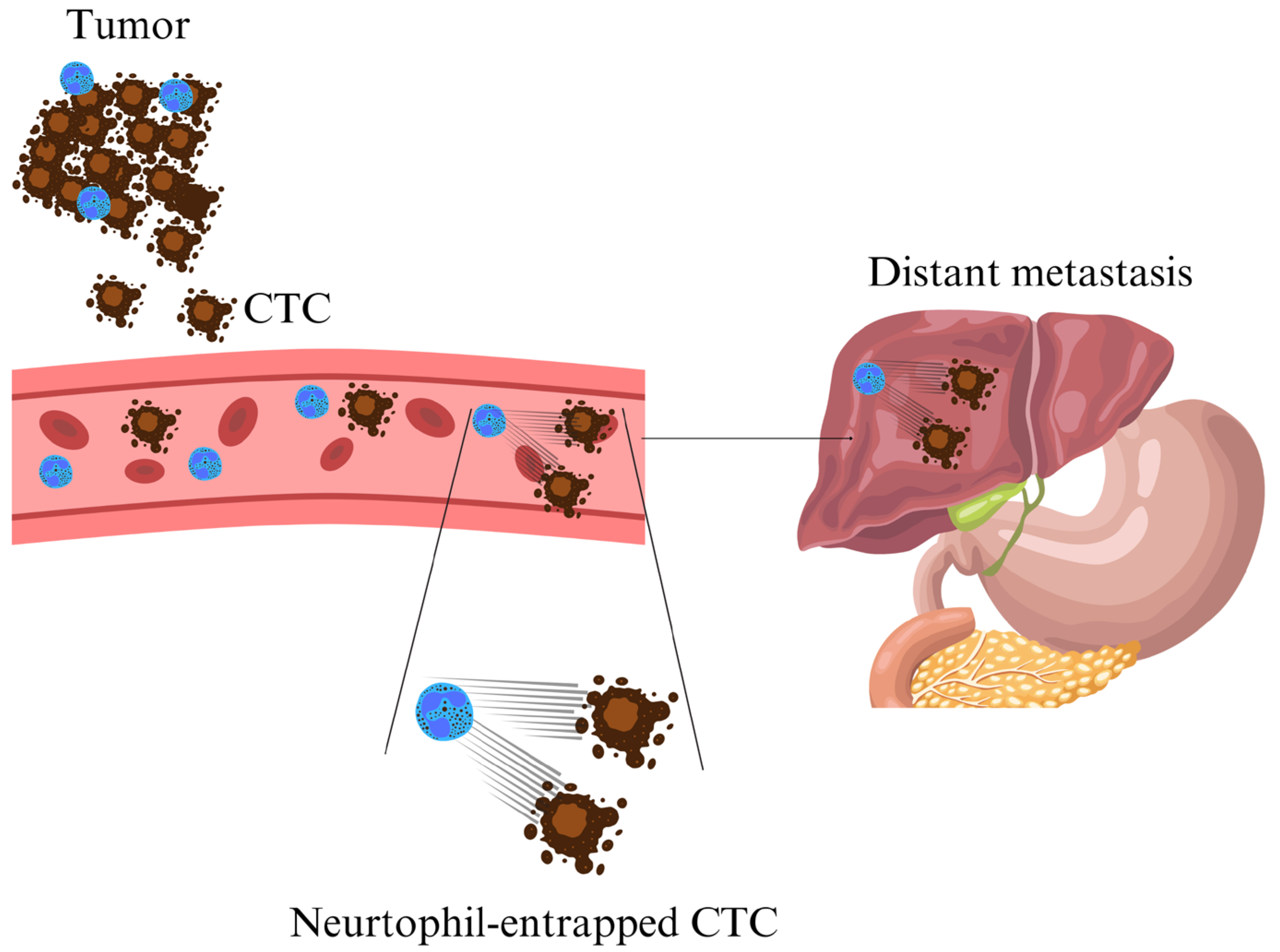
3.1. NETs in Cancer Progression and Metastasis
3.2. NET Protection of Cancer Cells
3.3. Prognostic Role of NET Markers in Cancer
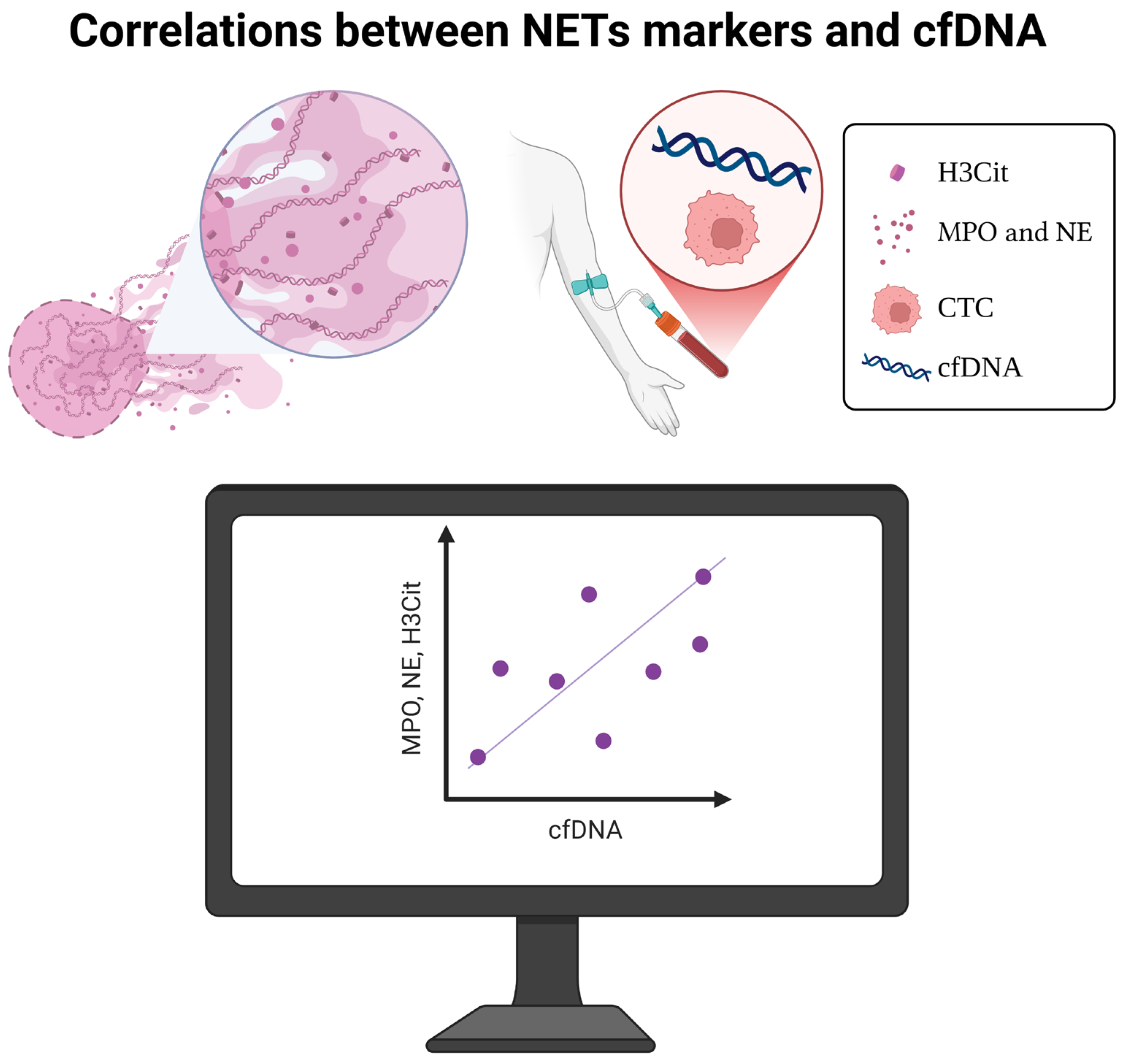
4. Correlations Between NET Markers and cfDNA
5. NETs in Cancer Therapy
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NETs | Neutrophil Extracellular Traps |
| H3Cit | Citrullinated Histone H3 |
| NE | Neutrophil Elastase |
| MPO | Myeloperoxidase |
| cfDNA | Circulating Free DNA |
| CTCs | Circulating Tumor Cells |
| TEPs | Tumor-Educated Platelets |
| circ-mRNA | Circulating mRNA |
| PFS | Progression-Free Survival |
| OS | Overall Survival |
| PMA | Phorbol 12-Myristate 13-Acetate |
| IL-8 | Interleukin-8 |
| GM-CSF | Granulocyte–Macrophage Colony-Stimulating Factor |
| IFN-α/IFN-γ | Interferon Alpha/Gamma |
| NOX | NADPH Oxidase |
| ROS | Reactive Oxygen Species |
| CR3 | Complement Receptor 3 |
| TLR/TLR4 | Toll-like Receptor/Toll-like Receptor 4 |
| CD11a | Cluster of Differentiation 11a |
| TANs | Tumor-Associated Neutrophils |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| GC | Gastric Cancer |
| VTE | Venous Thromboembolism |
| VE-cadherin | Vascular Endothelial Cadherin |
| β-catenin | Beta-Catenin |
| CXCR1/CXCR2 | C-X-C Motif Chemokine Receptor 1/2 |
| NK cells | Natural Killer Cells |
| PMN-MDSCs | Polymorphonuclear Myeloid-Derived Suppressor Cells |
| CTL | Cytotoxic T Lymphocyte |
| PD-1/CTLA-4 | Programmed Death-1/Cytotoxic T-Lymphocyte Antigen 4 |
| NSCLC | Non-Small Cell Lung Cancer |
| LUAD | Lung Adenocarcinoma |
| TME | Tumor Microenvironment |
| NETRS | NETosis Risk Score |
| mCRC | Metastatic Colorectal Cancer |
| SLE | Systemic Lupus Erythematosus |
| HGSOC | High-Grade Serous Ovarian Cancer |
| G-CSF | Granulocyte Colony-Stimulating Factor |
| PAD4 | Peptidylarginine Deiminase 4 |
| PGE1/PGE2 | Prostaglandin E1/E2 |
| LLC | Lewis Lung Carcinoma |
| rhDNase | Recombinant Human DNase |
| DNAse | Deoxyribonuclease |
References
- Cancer International Agency for Research on Cancer (IARC), This Information Available for Registry on Global Cancer Observatory. Available online: https://gco.iarc.fr/ (accessed on 10 March 2025).
- Kang, B.J.; Ra, S.W.; Lee, K.; Lim, S.; Son, S.H.; Ahn, J.J.; Kim, B.C. Circulating Tumor Cell Number Is Associated with Primary Tumor Volume in Patients with Lung Adenocarcinoma. Tuberc. Respir. Dis. 2020, 83, 61–70. [Google Scholar] [CrossRef]
- Kim, H.; Heo, C.M.; Oh, J.; Chung, H.H.; Lee, E.M.; Park, J.; Lee, S.-H.; Lee, K.H.; Lee, K.T.; Lee, J.K.; et al. Clinical Significance of Circulating Tumor Cells after Chemotherapy in Unresectable Pancreatic Ductal Adenocarcinoma. Transl. Oncol. 2022, 16, 101321. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, C.R.; Faugeroux, V.; Michiels, S.; Pailler, E.; Facchinetti, F.; Ou, D.; Bluthgen, M.V.; Pannet, C.; Ngo-Camus, M.; Bescher, G.; et al. A Prospective Examination of Circulating Tumor Cell Profiles in Non-Small-Cell Lung Cancer Molecular Subgroups. Ann. Oncol. 2017, 28, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Groot Koerkamp, B.; Rahbari, N.N.; Büchler, M.W.; Koch, M.; Weitz, J. Circulating Tumor Cells and Prognosis of Patients with Resectable Colorectal Liver Metastases or Widespread Metastatic Colorectal Cancer: A Meta-Analysis. Ann. Surg. Oncol. 2013, 20, 2156–2165. [Google Scholar] [CrossRef]
- Huang, X.; Gao, P.; Song, Y.; Sun, J.; Chen, X.; Zhao, J.; Liu, J.; Xu, H.; Wang, Z. Relationship between Circulating Tumor Cells and Tumor Response in Colorectal Cancer Patients Treated with Chemotherapy: A Meta-Analysis. BMC Cancer 2014, 14, 976. [Google Scholar] [CrossRef]
- Qian, C.; Cai, R.; Zhang, W.; Wang, J.; Hu, X.; Zhang, Y.; Jiang, B.; Yuan, H.; Liu, F. Neutrophil-Lymphocyte Ratio and Circulating Tumor Cells Counts Predict Prognosis in Gastrointestinal Cancer Patients. Front. Oncol. 2021, 11, 710704. [Google Scholar] [CrossRef]
- Gao, F.; Cui, Y.; Jiang, H.; Sui, D.; Wang, Y.; Jiang, Z.; Zhao, J.; Lin, S. Circulating Tumor Cell Is a Common Property of Brain Glioma and Promotes the Monitoring System. Oncotarget 2016, 7, 71330–71340. [Google Scholar] [CrossRef]
- Zhang, X.; Li, H.; Yu, X.; Li, S.; Lei, Z.; Li, C.; Zhang, Q.; Han, Q.; Li, Y.; Zhang, K.; et al. Analysis of Circulating Tumor Cells in Ovarian Cancer and Their Clinical Value as a Biomarker. Cell. Physiol. Biochem. 2018, 48, 1983–1994. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Geng, Q.; Wang, L.; Huang, J.; Liao, M.; Li, Y.; Ding, Z.; Yang, S.; Zhao, H.; Shen, Q.; et al. Value of Folate Receptor-Positive Circulating Tumour Cells in the Clinical Management of Indeterminate Lung Nodules: A Non-Invasive Biomarker for Predicting Malignancy and Tumour Invasiveness. EBioMedicine 2019, 41, 236–243. [Google Scholar] [CrossRef]
- Hochmair, M.J.; Buder, A.; Schwab, S.; Burghuber, O.C.; Prosch, H.; Hilbe, W.; Cseh, A.; Fritz, R.; Filipits, M. Liquid-Biopsy-Based Identification of EGFR T790M Mutation-Mediated Resistance to Afatinib Treatment in Patients with Advanced EGFR Mutation-Positive NSCLC, and Subsequent Response to Osimertinib. Target Oncol. 2019, 14, 75–83. [Google Scholar] [CrossRef]
- Qian, H.; Zhang, Y.; Xu, J.; He, J.; Gao, W. Progress and Application of Circulating Tumor Cells in Non-Small Cell Lung Cancer. Mol. Ther. Oncolytics 2021, 22, 72–84. [Google Scholar] [CrossRef]
- Zhang, Q.; Nong, J.; Wang, J.; Yan, Z.; Yi, L.; Gao, X.; Liu, Z.; Zhang, H.; Zhang, S. Isolation of Circulating Tumor Cells and Detection of EGFR Mutations in Patients with Non-Small-Cell Lung Cancer. Oncol. Lett. 2019, 17, 3799–3807. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.; Zu, Y. Detecting Circulating Tumor Cells: Current Challenges and New Trends. Theranostics 2013, 3, 377–394. [Google Scholar] [CrossRef] [PubMed]
- Pantel, K.; Alix-Panabières, C. Cell Lines from Circulating Tumor Cells. Oncoscience 2015, 2, 815–816. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Mei, C.; Nan, X.; Hui, L. Evaluation and Comparison of in Vitro Degradation Kinetics of DNA in Serum, Urine and Saliva: A Qualitative Study. Gene 2016, 590, 142–148. [Google Scholar] [CrossRef]
- Kamat, A.A.; Bischoff, F.Z.; Dang, D.; Baldwin, M.F.; Han, L.Y.; Lin, Y.G.; Merritt, W.M.; Landen, C.N.; Lu, C.; Gershenson, D.M.; et al. Circulating Cell-Free DNA: A Novel Biomarker for Response to Therapy in Ovarian Carcinoma. Cancer Biol. Ther. 2006, 5, 1369–1374. [Google Scholar] [CrossRef]
- Parkinson, C.A.; Gale, D.; Piskorz, A.M.; Biggs, H.; Hodgkin, C.; Addley, H.; Freeman, S.; Moyle, P.; Sala, E.; Sayal, K.; et al. Exploratory Analysis of TP53 Mutations in Circulating Tumour DNA as Biomarkers of Treatment Response for Patients with Relapsed High-Grade Serous Ovarian Carcinoma: A Retrospective Study. PLoS Med. 2016, 13, e1002198. [Google Scholar] [CrossRef]
- Yang, J.; Gong, Y.; Lam, V.K.; Shi, Y.; Guan, Y.; Zhang, Y.; Ji, L.; Chen, Y.; Zhao, Y.; Qian, F.; et al. Deep Sequencing of Circulating Tumor DNA Detects Molecular Residual Disease and Predicts Recurrence in Gastric Cancer. Cell Death Dis. 2020, 11, 346. [Google Scholar] [CrossRef]
- Žilovič, D.; Čiurlienė, R.; Sabaliauskaitė, R.; Jarmalaitė, S. Future Screening Prospects for Ovarian Cancer. Cancers 2021, 13, 3840. [Google Scholar] [CrossRef]
- Phallen, J.; Sausen, M.; Adleff, V.; Leal, A.; Hruban, C.; White, J.; Anagnostou, V.; Fiksel, J.; Cristiano, S.; Papp, E.; et al. Direct Detection of Early-Stage Cancers Using Circulating Tumor DNA. Sci. Transl. Med. 2017, 9, eaan2415. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Douville, C.; Cohen, J.D.; Yen, T.-T.; Kinde, I.; Sundfelt, K.; Kjær, S.K.; Hruban, R.H.; Shih, I.-M.; et al. Evaluation of Liquid from the Papanicolaou Test and Other Liquid Biopsies for the Detection of Endometrial and Ovarian Cancers. Sci. Transl. Med. 2018, 10, eaap8793. [Google Scholar] [CrossRef] [PubMed]
- Giannopoulou, L.; Chebouti, I.; Pavlakis, K.; Kasimir-Bauer, S.; Lianidou, E.S. RASSF1A Promoter Methylation in High-Grade Serous Ovarian Cancer: A Direct Comparison Study in Primary Tumors, Adjacent Morphologically Tumor Cell-Free Tissues and Paired Circulating Tumor DNA. Oncotarget 2017, 8, 21429–21443. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yu, L.; Luo, X.; Huang, L.; Li, Q.-S.; Shao, X.-S.; Liu, Y.; Fan, Y.; Yang, G.-Z. Detection of OPCML Methylation, a Possible Epigenetic Marker, from Free Serum Circulating DNA to Improve the Diagnosis of Early-Stage Ovarian Epithelial Cancer. Oncol. Lett. 2017, 14, 217–223. [Google Scholar] [CrossRef]
- Nicolazzo, C.; Barault, L.; Caponnetto, S.; De Renzi, G.; Belardinilli, F.; Bottillo, I.; Bargiacchi, S.; Macagno, M.; Grammatico, P.; Giannini, G.; et al. True Conversions from RAS Mutant to RAS Wild-Type in Circulating Tumor DNA from Metastatic Colorectal Cancer Patients as Assessed by Methylation and Mutational Signature. Cancer Lett. 2021, 507, 89–96. [Google Scholar] [CrossRef]
- Szczerba, B.M.; Castro-Giner, F.; Vetter, M.; Krol, I.; Gkountela, S.; Landin, J.; Scheidmann, M.C.; Donato, C.; Scherrer, R.; Singer, J.; et al. Neutrophils Escort Circulating Tumour Cells to Enable Cell Cycle Progression. Nature 2019, 566, 553–557. [Google Scholar] [CrossRef]
- Demkow, U. Neutrophil Extracellular Traps (NETs) in Cancer Invasion, Evasion and Metastasis. Cancers 2021, 13, 4495. [Google Scholar] [CrossRef] [PubMed]
- Pruchniak, M.P.; Demkow, U. Potent NETosis Inducers Do Not Show Synergistic Effects in Vitro. Cent. Eur. J. Immunol. 2019, 44, 51–58. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil Extracellular Traps Kill Bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Islam, M.M.; Takeyama, N. Role of Neutrophil Extracellular Traps in Health and Disease Pathophysiology: Recent Insights and Advances. Int. J. Mol. Sci. 2023, 24, 15805. [Google Scholar] [CrossRef]
- Vorobjeva, N.V.; Chernyak, B.V. NETosis: Molecular Mechanisms, Role in Physiology and Pathology. Biochemistry 2020, 85, 1178–1190. [Google Scholar] [CrossRef]
- Yousefi, S.; Mihalache, C.; Kozlowski, E.; Schmid, I.; Simon, H.U. Viable Neutrophils Release Mitochondrial DNA to Form Neutrophil Extracellular Traps. Cell Death Differ. 2009, 16, 1438–1444. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, S.; Urosevic, M.; Daryadel, A.; Oberholzer, P.A.; Baumann, C.; Fey, M.F.; Dummer, R.; Simon, H.-U.; Yousefi, S. Induction of Genes Mediating Interferon-Dependent Extracellular Trap Formation during Neutrophil Differentiation. J. Biol. Chem. 2004, 279, 44123–44132. [Google Scholar] [CrossRef]
- Amini, P.; Stojkov, D.; Felser, A.; Jackson, C.B.; Courage, C.; Schaller, A.; Gelman, L.; Soriano, M.E.; Nuoffer, J.-M.; Scorrano, L.; et al. Neutrophil Extracellular Trap Formation Requires OPA1-Dependent Glycolytic ATP Production. Nat. Commun. 2018, 9, 2958. [Google Scholar] [CrossRef]
- Caielli, S.; Athale, S.; Domic, B.; Murat, E.; Chandra, M.; Banchereau, R.; Baisch, J.; Phelps, K.; Clayton, S.; Gong, M.; et al. Oxidized Mitochondrial Nucleoids Released by Neutrophils Drive Type I Interferon Production in Human Lupus. J. Exp. Med. 2016, 213, 697–713. [Google Scholar] [CrossRef]
- Behnen, M.; Möller, S.; Brozek, A.; Klinger, M.; Laskay, T. Extracellular Acidification Inhibits the ROS-Dependent Formation of Neutrophil Extracellular Traps. Front. Immunol. 2017, 8, 184. [Google Scholar] [CrossRef]
- Spielman, L.J.; Gibson, D.L.; Klegeris, A. Incretin Hormones Regulate Microglia Oxidative Stress, Survival and Expression of Trophic Factors. Eur. J. Cell Biol. 2017, 96, 240–253. [Google Scholar] [CrossRef]
- Lodge, K.M.; Cowburn, A.S.; Li, W.; Condliffe, A.M. The Impact of Hypoxia on Neutrophil Degranulation and Consequences for the Host. Int. J. Mol. Sci. 2020, 21, 1183. [Google Scholar] [CrossRef] [PubMed]
- Naffah de Souza, C.; Breda, L.C.D.; Khan, M.A.; de Almeida, S.R.; Câmara, N.O.S.; Sweezey, N.; Palaniyar, N. Alkaline pH Promotes NADPH Oxidase-Independent Neutrophil Extracellular Trap Formation: A Matter of Mitochondrial Reactive Oxygen Species Generation and Citrullination and Cleavage of Histone. Front. Immunol. 2017, 8, 1849. [Google Scholar] [CrossRef] [PubMed]
- Branitzki-Heinemann, K.; Möllerherm, H.; Völlger, L.; Husein, D.M.; de Buhr, N.; Blodkamp, S.; Reuner, F.; Brogden, G.; Naim, H.Y.; von Köckritz-Blickwede, M. Formation of Neutrophil Extracellular Traps under Low Oxygen Level. Front. Immunol. 2016, 7, 518. [Google Scholar] [CrossRef]
- Nadesalingam, A.; Chen, J.H.K.; Farahvash, A.; Khan, M.A. Hypertonic Saline Suppresses NADPH Oxidase-Dependent Neutrophil Extracellular Trap Formation and Promotes Apoptosis. Front. Immunol. 2018, 9, 359. [Google Scholar] [CrossRef] [PubMed]
- Domingo-Gonzalez, R.; Martínez-Colón, G.J.; Smith, A.J.; Smith, C.K.; Ballinger, M.N.; Xia, M.; Murray, S.; Kaplan, M.J.; Yanik, G.A.; Moore, B.B. Inhibition of Neutrophil Extracellular Trap Formation after Stem Cell Transplant by Prostaglandin E2. Am. J. Respir. Crit. Care Med. 2016, 193, 186–197. [Google Scholar] [CrossRef]
- Ravindran, M.; Khan, M.A.; Palaniyar, N. Neutrophil Extracellular Trap Formation: Physiology, Pathology, and Pharmacology. Biomolecules 2019, 9, 365. [Google Scholar] [CrossRef]
- Pilsczek, F.H.; Salina, D.; Poon, K.K.H.; Fahey, C.; Yipp, B.G.; Sibley, C.D.; Robbins, S.M.; Green, F.H.Y.; Surette, M.G.; Sugai, M.; et al. A Novel Mechanism of Rapid Nuclear Neutrophil Extracellular Trap Formation in Response to Staphylococcus Aureus. J. Immunol. 2010, 185, 7413–7425. [Google Scholar] [CrossRef]
- Yipp, B.G.; Kubes, P. NETosis: How Vital Is It? Blood 2013, 122, 2784–2794. [Google Scholar] [CrossRef] [PubMed]
- Jorch, S.K.; Kubes, P. An Emerging Role for Neutrophil Extracellular Traps in Noninfectious Disease. Nat. Med. 2017, 23, 279–287. [Google Scholar] [CrossRef]
- Cristinziano, L.; Modestino, L.; Loffredo, S.; Varricchi, G.; Braile, M.; Ferrara, A.L.; de Paulis, A.; Antonelli, A.; Marone, G.; Galdiero, M.R. Anaplastic Thyroid Cancer Cells Induce the Release of Mitochondrial Extracellular DNA Traps by Viable Neutrophils. J. Immunol. 2020, 204, 1362–1372. [Google Scholar] [CrossRef]
- Stojkov, D.; Amini, P.; Oberson, K.; Sokollik, C.; Duppenthaler, A.; Simon, H.-U.; Yousefi, S. ROS and Glutathionylation Balance Cytoskeletal Dynamics in Neutrophil Extracellular Trap Formation. J. Cell Biol. 2017, 216, 4073–4090. [Google Scholar] [CrossRef]
- Wells, M.; Mikesh, M.; Gordon, V. Structure-Preserving Fixation Allows Scanning Electron Microscopy to Reveal Biofilm Microstructure and Interactions with Immune Cells. J. Microsc. 2024, 293, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Pastrana, T.; García-Hernández, M.J.; Carrizosa-Esquivel, A.M.; Camacho-Martínez, F.M. Evaluation of 25 Years of Phototherapy for Treating Psoriasis at a Teaching Hospital in Southern Spain. An. Bras. Dermatol. 2015, 90, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Tjioe, M.; Gerritsen, M.J.P.; Juhlin, L.; van de Kerkhof, P.C.M. Treatment of Vitiligo Vulgaris with Narrow Band UVB (311 Nm) for One Year and the Effect of Addition of Folic Acid and Vitamin B12. Acta Derm. Venereol. 2002, 82, 369–372. [Google Scholar] [CrossRef]
- Stein, K.R.; Pearce, D.J.; Feldman, S.R. Targeted UV Therapy in the Treatment of Psoriasis. J. Dermatolog. Treat. 2008, 19, 141–145. [Google Scholar] [CrossRef]
- Ramos-Martínez, E.; Hernández-González, L.; Ramos-Martínez, I.; Pérez-Campos Mayoral, L.; López-Cortés, G.I.; Pérez-Campos, E.; Mayoral Andrade, G.; Hernández-Huerta, M.T.; José, M.V. Multiple Origins of Extracellular DNA Traps. Front. Immunol. 2021, 12, 621311. [Google Scholar] [CrossRef] [PubMed]
- Poh, X.Y.; Loh, F.K.; Friedland, J.S.; Ong, C.W.M. Neutrophil-Mediated Immunopathology and Matrix Metalloproteinases in Central Nervous System-Tuberculosis. Front. Immunol. 2021, 12, 788976. [Google Scholar] [CrossRef]
- Pérez-Figueroa, E.; Álvarez-Carrasco, P.; Ortega, E.; Maldonado-Bernal, C. Neutrophils: Many Ways to Die. Front. Immunol. 2021, 12, 631821. [Google Scholar] [CrossRef]
- Na, J.; Lee, C.-H.; Chung, J.-K.; Youn, H. Overexpression of Both Human Sodium Iodide Symporter (NIS) and BRG1-Bromodomain Synergistically Enhances Radioiodine Sensitivity by Stabilizing P53 through NPM1 Expression. Int. J. Mol. Sci. 2023, 24, 2761. [Google Scholar] [CrossRef]
- Monticolo, F.; Palomba, E.; Chiusano, M.L. Identification of Novel Potential Genes Involved in Cancer by Integrated Comparative Analyses. Int. J. Mol. Sci. 2020, 21, 9560. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, S.; Jing, Y.; Zou, W. Neutrophil Extracellular Traps in Intracerebral Hemorrhage: Implications for Pathogenesis and Therapeutic Targets. Metab. Brain Dis. 2023, 38, 2505–2520. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Y.; Huang, J.; Cao, X.; An, Q.; Peng, Y.; Zhao, Y.; Luo, Y. A Variety of Death Modes of Neutrophils and Their Role in the Etiology of Autoimmune Diseases. Immunol. Rev. 2024, 321, 280–299. [Google Scholar] [CrossRef]
- Azzouz, D.; Palaniyar, N. How Do ROS Induce NETosis? Oxidative DNA Damage, DNA Repair, and Chromatin Decondensation. Biomolecules 2024, 14, 1307. [Google Scholar] [CrossRef] [PubMed]
- Azzouz, D.; Palaniyar, N. ApoNETosis: Discovery of a Novel Form of Neutrophil Death with Concomitant Apoptosis and NETosis. Cell Death Dis. 2018, 9, 839. [Google Scholar] [CrossRef] [PubMed]
- Azzouz, D.; Khan, M.A.; Sweezey, N.; Palaniyar, N. Two-in-One: UV Radiation Simultaneously Induces Apoptosis and NETosis. Cell Death Discov. 2018, 4, 51. [Google Scholar] [CrossRef]
- Oklu, R.; Sheth, R.A.; Wong, K.H.K.; Jahromi, A.H.; Albadawi, H. Neutrophil Extracellular Traps Are Increased in Cancer Patients but Does Not Associate with Venous Thrombosis. Cardiovasc. Diagn. Ther. 2017, 7, S140–S149. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Gan, T.; Zhou, J.; Hu, F.; Hao, N.; Yuan, B.; Chen, Y.; Zhang, M. Extracellular RNAs from Lung Cancer Cells Activate Epithelial Cells and Induce Neutrophil Extracellular Traps. Int. J. Oncol. 2019, 55, 69–80. [Google Scholar] [CrossRef]
- Richardson, J.J.R.; Hendrickse, C.; Gao-Smith, F.; Thickett, D.R. Neutrophil Extracellular Trap Production in Patients with Colorectal Cancer In Vitro. Int. J. Inflam. 2017, 2017, 4915062. [Google Scholar] [CrossRef] [PubMed]
- Masucci, M.T.; Minopoli, M.; Del Vecchio, S.; Carriero, M.V. The Emerging Role of Neutrophil Extracellular Traps (NETs) in Tumor Progression and Metastasis. Front. Immunol. 2020, 11, 1749. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Franco, M.M.; Leon-Rodriguez, E.; Torres-Ruiz, J.J.; Gómez-Martín, D.; Angles-Cano, E.; de la Luz Sevilla-González, M. Neutrophil Extracellular Traps Associate with Clinical Stages in Breast Cancer. Pathol. Oncol. Res. 2020, 26, 1781–1785. [Google Scholar] [CrossRef]
- Xu, D.; Lin, Y.; Shen, J.; Zhang, J.; Wang, J.; Zhang, Y.; Zhang, H.; Ning, L.; Liu, P.; Li, S.; et al. Overproduced Bone Marrow Neutrophils in Collagen-Induced Arthritis Are Primed for NETosis: An Ignored Pathological Cell Involving Inflammatory Arthritis. Cell Prolif. 2020, 53, e12824. [Google Scholar] [CrossRef] [PubMed]
- Wolach, O.; Sellar, R.S.; Martinod, K.; Cherpokova, D.; McConkey, M.; Chappell, R.J.; Silver, A.J.; Adams, D.; Castellano, C.A.; Schneider, R.K.; et al. Increased Neutrophil Extracellular Trap Formation Promotes Thrombosis in Myeloproliferative Neoplasms. Sci. Transl. Med. 2018, 10, eaan8292. [Google Scholar] [CrossRef]
- Warnatsch, A.; Ioannou, M.; Wang, Q.; Papayannopoulos, V. Neutrophil Extracellular Traps License Macrophages for Cytokine Production in Atherosclerosis. Science 2015, 349, 316–320. [Google Scholar] [CrossRef]
- Wang, Z.; Moresco, P.; Yan, R.; Li, J.; Gao, Y.; Biasci, D.; Yao, M.; Pearson, J.; Hechtman, J.F.; Janowitz, T.; et al. Carcinomas Assemble a Filamentous CXCL12-Keratin-19 Coating That Suppresses T Cell-Mediated Immune Attack. Proc. Natl. Acad. Sci. USA 2022, 119, e2119463119. [Google Scholar] [CrossRef]
- van Puffelen, J.H.; Keating, S.T.; Oosterwijk, E.; van der Heijden, A.G.; Netea, M.G.; Joosten, L.A.B.; Vermeulen, S.H. Trained Immunity as a Molecular Mechanism for BCG Immunotherapy in Bladder Cancer. Nat. Rev. Urol. 2020, 17, 513–525. [Google Scholar] [CrossRef]
- Usher, N.T.; Chang, S.; Howard, R.S.; Martinez, A.; Harrison, L.H.; Santosham, M.; Aronson, N.E. Association of BCG Vaccination in Childhood With Subsequent Cancer Diagnoses: A 60-Year Follow-up of a Clinical Trial. JAMA Netw. Open 2019, 2, e1912014. [Google Scholar] [CrossRef]
- Spicer, J.D.; McDonald, B.; Cools-Lartigue, J.J.; Chow, S.C.; Giannias, B.; Kubes, P.; Ferri, L.E. Neutrophils Promote Liver Metastasis via Mac-1-Mediated Interactions with Circulating Tumor Cells. Cancer Res. 2012, 72, 3919–3927. [Google Scholar] [CrossRef]
- Samarendra, H.; Jones, K.; Petrinic, T.; Silva, M.A.; Reddy, S.; Soonawalla, Z.; Gordon-Weeks, A. A Meta-Analysis of CXCL12 Expression for Cancer Prognosis. Br. J. Cancer 2017, 117, 124–135. [Google Scholar] [CrossRef]
- Peng, Z.; Liu, C.; Victor, A.R.; Cao, D.-Y.; Veiras, L.C.; Bernstein, E.A.; Khan, Z.; Giani, J.F.; Cui, X.; Bernstein, K.E.; et al. Tumors Exploit CXCR4hiCD62Llo Aged Neutrophils to Facilitate Metastatic Spread. Oncoimmunology 2021, 10, 1870811. [Google Scholar] [CrossRef]
- Park, S.J.; Bejar, R. Clonal Hematopoiesis in Cancer. Exp. Hematol. 2020, 83, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Ochando, J.; Mulder, W.J.M.; Madsen, J.C.; Netea, M.G.; Duivenvoorden, R. Trained Immunity-Basic Concepts and Contributions to Immunopathology. Nat. Rev. Nephrol. 2023, 19, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, M.; Tsuchiya, M.; Watanabe-Fukunaga, R.; Nagata, S. Multiple Elements in the Promoter of Granulocyte Colony-Stimulating Factor Gene Regulate Its Constitutive Expression in Human Carcinoma Cells. J. Biol. Chem. 1990, 265, 5897–5902. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Joosten, L.A.B.; Latz, E.; Mills, K.H.G.; Natoli, G.; Stunnenberg, H.G.; O’Neill, L.A.J.; Xavier, R.J. Trained Immunity: A Program of Innate Immune Memory in Health and Disease. Science 2016, 352, aaf1098. [Google Scholar] [CrossRef]
- Netea, M.G.; Domínguez-Andrés, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.A.B.; van der Meer, J.W.M.; Mhlanga, M.M.; Mulder, W.J.M.; et al. Defining Trained Immunity and Its Role in Health and Disease. Nat. Rev. Immunol. 2020, 20, 375–388. [Google Scholar] [CrossRef]
- Moorlag, S.J.C.F.M.; Rodriguez-Rosales, Y.A.; Gillard, J.; Fanucchi, S.; Theunissen, K.; Novakovic, B.; de Bont, C.M.; Negishi, Y.; Fok, E.T.; Kalafati, L.; et al. BCG Vaccination Induces Long-Term Functional Reprogramming of Human Neutrophils. Cell Rep. 2020, 33, 108387. [Google Scholar] [CrossRef] [PubMed]
- Mitroulis, I.; Ruppova, K.; Wang, B.; Chen, L.-S.; Grzybek, M.; Grinenko, T.; Eugster, A.; Troullinaki, M.; Palladini, A.; Kourtzelis, I.; et al. Modulation of Myelopoiesis Progenitors Is an Integral Component of Trained Immunity. Cell 2018, 172, 147–161.e12. [Google Scholar] [CrossRef]
- McNerney, M.E.; Godley, L.A.; Le Beau, M.M. Therapy-Related Myeloid Neoplasms: When Genetics and Environment Collide. Nat. Rev. Cancer 2017, 17, 513–527. [Google Scholar] [CrossRef]
- McCracken, J.M.; Allen, L.-A.H. Regulation of Human Neutrophil Apoptosis and Lifespan in Health and Disease. J. Cell Death 2014, 7, 15–23. [Google Scholar] [CrossRef]
- Martin, C.; Burdon, P.C.E.; Bridger, G.; Gutierrez-Ramos, J.C.; Williams, T.J.; Rankin, S.M. Chemokines Acting via CXCR2 and CXCR4 Control the Release of Neutrophils from the Bone Marrow and Their Return Following Senescence. Immunity 2003, 19, 583–593. [Google Scholar] [CrossRef]
- Mackey, J.B.G.; Coffelt, S.B.; Carlin, L.M. Neutrophil Maturity in Cancer. Front. Immunol. 2019, 10, 1912. [Google Scholar] [CrossRef]
- Liu, K.; Sun, E.; Lei, M.; Li, L.; Gao, J.; Nian, X.; Wang, L. BCG-Induced Formation of Neutrophil Extracellular Traps Play an Important Role in Bladder Cancer Treatment. Clin. Immunol. 2019, 201, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ebert, B.L. CHIP (Clonal Hematopoiesis of Indeterminate Potential): Potent and Newly Recognized Contributor to Cardiovascular Risk. Circulation 2018, 138, 666–668. [Google Scholar] [CrossRef]
- Koelwyn, G.J.; Newman, A.A.C.; Afonso, M.S.; van Solingen, C.; Corr, E.M.; Brown, E.J.; Albers, K.B.; Yamaguchi, N.; Narke, D.; Schlegel, M.; et al. Myocardial Infarction Accelerates Breast Cancer via Innate Immune Reprogramming. Nat. Med. 2020, 26, 1452–1458. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, E.; Sanz, J.; Dunn, J.L.; Khan, N.; Mendonça, L.E.; Pacis, A.; Tzelepis, F.; Pernet, E.; Dumaine, A.; Grenier, J.-C.; et al. BCG Educates Hematopoietic Stem Cells to Generate Protective Innate Immunity against Tuberculosis. Cell 2018, 172, 176–190.e19. [Google Scholar] [CrossRef] [PubMed]
- Kalafati, L.; Kourtzelis, I.; Schulte-Schrepping, J.; Li, X.; Hatzioannou, A.; Grinenko, T.; Hagag, E.; Sinha, A.; Has, C.; Dietz, S.; et al. Innate Immune Training of Granulopoiesis Promotes Anti-Tumor Activity. Cell 2020, 183, 771–785.e12. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.; Fontanillas, P.; Flannick, J.; Manning, A.; Grauman, P.V.; Mar, B.G.; Lindsley, R.C.; Mermel, C.H.; Burtt, N.; Chavez, A.; et al. Age-Related Clonal Hematopoiesis Associated with Adverse Outcomes. N. Engl. J. Med. 2014, 371, 2488–2498. [Google Scholar] [CrossRef]
- Huh, S.J.; Liang, S.; Sharma, A.; Dong, C.; Robertson, G.P. Transiently Entrapped Circulating Tumor Cells Interact with Neutrophils to Facilitate Lung Metastasis Development. Cancer Res. 2010, 70, 6071–6082. [Google Scholar] [CrossRef]
- Gray, R.D.; Hardisty, G.; Regan, K.H.; Smith, M.; Robb, C.T.; Duffin, R.; Mackellar, A.; Felton, J.M.; Paemka, L.; McCullagh, B.N.; et al. Delayed Neutrophil Apoptosis Enhances NET Formation in Cystic Fibrosis. Thorax 2018, 73, 134–144. [Google Scholar] [CrossRef]
- Geering, B.; Stoeckle, C.; Conus, S.; Simon, H.-U. Living and Dying for Inflammation: Neutrophils, Eosinophils, Basophils. Trends Immunol. 2013, 34, 398–409. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-Derived Suppressor Cells as Regulators of the Immune System. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef]
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of Tumor-Associated Neutrophil Phenotype by TGF-Beta: “N1” versus “N2” TAN. Cancer Cell 2009, 16, 183–194. [Google Scholar] [CrossRef]
- Diamantopoulou, Z.; Castro-Giner, F.; Schwab, F.D.; Foerster, C.; Saini, M.; Budinjas, S.; Strittmatter, K.; Krol, I.; Seifert, B.; Heinzelmann-Schwarz, V.; et al. The Metastatic Spread of Breast Cancer Accelerates during Sleep. Nature 2022, 607, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Christ, A.; Günther, P.; Lauterbach, M.A.R.; Duewell, P.; Biswas, D.; Pelka, K.; Scholz, C.J.; Oosting, M.; Haendler, K.; Baßler, K.; et al. Western Diet Triggers NLRP3-Dependent Innate Immune Reprogramming. Cell 2018, 172, 162–175.e14. [Google Scholar] [CrossRef]
- Casbon, A.-J.; Reynaud, D.; Park, C.; Khuc, E.; Gan, D.D.; Schepers, K.; Passegué, E.; Werb, Z. Invasive Breast Cancer Reprograms Early Myeloid Differentiation in the Bone Marrow to Generate Immunosuppressive Neutrophils. Proc. Natl. Acad. Sci. USA 2015, 112, E566–E575. [Google Scholar] [CrossRef] [PubMed]
- Casanova-Acebes, M.; Pitaval, C.; Weiss, L.A.; Nombela-Arrieta, C.; Chèvre, R.; A-González, N.; Kunisaki, Y.; Zhang, D.; van Rooijen, N.; Silberstein, L.E.; et al. Rhythmic Modulation of the Hematopoietic Niche through Neutrophil Clearance. Cell 2013, 153, 1025–1035. [Google Scholar] [CrossRef]
- Brostjan, C.; Oehler, R. The Role of Neutrophil Death in Chronic Inflammation and Cancer. Cell Death Discov. 2020, 6, 26. [Google Scholar] [CrossRef]
- Bousounis, P.; Bergo, V.; Trompouki, E. Inflammation, Aging and Hematopoiesis: A Complex Relationship. Cells 2021, 10, 1386. [Google Scholar] [CrossRef] [PubMed]
- Altznauer, F.; Martinelli, S.; Yousefi, S.; Thürig, C.; Schmid, I.; Conway, E.M.; Schöni, M.H.; Vogt, P.; Mueller, C.; Fey, M.F.; et al. Inflammation-Associated Cell Cycle–Independent Block of Apoptosis by Survivin in Terminally Differentiated Neutrophils. J. Exp. Med. 2004, 199, 1343–1354. [Google Scholar] [CrossRef] [PubMed]
- Adrover, J.M.; McDowell, S.A.C.; He, X.-Y.; Quail, D.F.; Egeblad, M. NETworking with Cancer: The Bidirectional Interplay between Cancer and Neutrophil Extracellular Traps. Cancer Cell 2023, 41, 505–526. [Google Scholar] [CrossRef] [PubMed]
- Adrover, J.M.; Aroca-Crevillén, A.; Crainiciuc, G.; Ostos, F.; Rojas-Vega, Y.; Rubio-Ponce, A.; Cilloniz, C.; Bonzón-Kulichenko, E.; Calvo, E.; Rico, D.; et al. Programmed “disarming” of the Neutrophil Proteome Reduces the Magnitude of Inflammation. Nat. Immunol. 2020, 21, 135–144. [Google Scholar] [CrossRef]
- Yang, C.; Sun, W.; Cui, W.; Li, X.; Yao, J.; Jia, X.; Li, C.; Wu, H.; Hu, Z.; Zou, X. Procoagulant Role of Neutrophil Extracellular Traps in Patients with Gastric Cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 14075–14086. [Google Scholar]
- Li, J.-C.; Zou, X.-M.; Yang, S.-F.; Jin, J.-Q.; Zhu, L.; Li, C.-J.; Yang, H.; Zhang, A.-G.; Zhao, T.-Q.; Chen, C.-Y. Neutrophil Extracellular Traps Participate in the Development of Cancer-Associated Thrombosis in Patients with Gastric Cancer. World J. Gastroenterol. 2022, 28, 3132–3149. [Google Scholar] [CrossRef]
- Park, J.; Wysocki, R.W.; Amoozgar, Z.; Maiorino, L.; Fein, M.R.; Jorns, J.; Schott, A.F.; Kinugasa-Katayama, Y.; Lee, Y.; Won, N.H.; et al. Cancer Cells Induce Metastasis-Supporting Neutrophil Extracellular DNA Traps. Sci. Transl. Med. 2016, 8, 361ra138. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, H.; Tan, S.; Dong, Q.; Fan, X.; Wang, Y.; Zhang, H.; He, J. The Role of Neutrophil Extracellular Traps in Cancer Progression, Metastasis and Therapy. Exp. Hematol. Oncol. 2022, 11, 99. [Google Scholar] [CrossRef]
- Masucci, M.T.; Minopoli, M.; Carriero, M.V. Tumor Associated Neutrophils. Their Role in Tumorigenesis, Metastasis, Prognosis and Therapy. Front. Oncol. 2019, 9, 1146. [Google Scholar] [CrossRef]
- Yazdani, H.O.; Roy, E.; Comerci, A.J.; van der Windt, D.J.; Zhang, H.; Huang, H.; Loughran, P.; Shiva, S.; Geller, D.A.; Bartlett, D.L.; et al. Neutrophil Extracellular Traps Drive Mitochondrial Homeostasis in Tumors to Augment Growth. Cancer Res. 2019, 79, 5626–5639. [Google Scholar] [CrossRef] [PubMed]
- Shaul, M.E.; Fridlender, Z.G. Neutrophils as Active Regulators of the Immune System in the Tumor Microenvironment. J. Leukoc. Biol. 2017, 102, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Kaltenmeier, C.; Simmons, R.L.; Tohme, S.; Yazdani, H.O. Neutrophil Extracellular Traps (NETs) in Cancer Metastasis. Cancers 2021, 13, 6131. [Google Scholar] [CrossRef]
- Erpenbeck, L.; Schön, M.P. Neutrophil Extracellular Traps: Protagonists of Cancer Progression? Oncogene 2017, 36, 2483–2490. [Google Scholar] [CrossRef]
- McDonald, B.; Spicer, J.; Giannais, B.; Fallavollita, L.; Brodt, P.; Ferri, L.E. Systemic Inflammation Increases Cancer Cell Adhesion to Hepatic Sinusoids by Neutrophil Mediated Mechanisms. Int. J. Cancer 2009, 125, 1298–1305. [Google Scholar] [CrossRef] [PubMed]
- Cools-Lartigue, J.; Spicer, J.; McDonald, B.; Gowing, S.; Chow, S.; Giannias, B.; Bourdeau, F.; Kubes, P.; Ferri, L. Neutrophil Extracellular Traps Sequester Circulating Tumor Cells and Promote Metastasis. J. Clin. Investig. 2013, 123, 3446–3458. [Google Scholar] [CrossRef]
- Najmeh, S.; Cools-Lartigue, J.; Rayes, R.F.; Gowing, S.; Vourtzoumis, P.; Bourdeau, F.; Giannias, B.; Berube, J.; Rousseau, S.; Ferri, L.E.; et al. Neutrophil Extracellular Traps Sequester Circulating Tumor Cells via Β1-Integrin Mediated Interactions. Int. J. Cancer 2017, 140, 2321–2330. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Q.; Zhang, X.; Liu, X.; Zhou, B.; Chen, J.; Huang, D.; Li, J.; Li, H.; Chen, F.; et al. DNA of Neutrophil Extracellular Traps Promotes Cancer Metastasis via CCDC25. Nature 2020, 583, 133–138. [Google Scholar] [CrossRef]
- Snoderly, H.T.; Boone, B.A.; Bennewitz, M.F. Neutrophil Extracellular Traps in Breast Cancer and beyond: Current Perspectives on NET Stimuli, Thrombosis and Metastasis, and Clinical Utility for Diagnosis and Treatment. Breast Cancer Res. 2019, 21, 145. [Google Scholar] [CrossRef]
- Saffarzadeh, M.; Juenemann, C.; Queisser, M.A.; Lochnit, G.; Barreto, G.; Galuska, S.P.; Lohmeyer, J.; Preissner, K.T. Neutrophil Extracellular Traps Directly Induce Epithelial and Endothelial Cell Death: A Predominant Role of Histones. PLoS ONE 2012, 7, e32366. [Google Scholar] [CrossRef]
- Pieterse, E.; Rother, N.; Garsen, M.; Hofstra, J.M.; Satchell, S.C.; Hoffmann, M.; Loeven, M.A.; Knaapen, H.K.; van der Heijden, O.W.H.; Berden, J.H.M.; et al. Neutrophil Extracellular Traps Drive Endothelial-to-Mesenchymal Transition. Arter. Thromb. Vasc. Biol. 2017, 37, 1371–1379. [Google Scholar] [CrossRef]
- Alfaro, C.; Teijeira, A.; Oñate, C.; Pérez, G.; Sanmamed, M.F.; Andueza, M.P.; Alignani, D.; Labiano, S.; Azpilikueta, A.; Rodriguez-Paulete, A.; et al. Tumor-Produced Interleukin-8 Attracts Human Myeloid-Derived Suppressor Cells and Elicits Extrusion of Neutrophil Extracellular Traps (NETs). Clin. Cancer Res. 2016, 22, 3924–3936. [Google Scholar] [CrossRef] [PubMed]
- Bertini, R.; Allegretti, M.; Bizzarri, C.; Moriconi, A.; Locati, M.; Zampella, G.; Cervellera, M.N.; Di Cioccio, V.; Cesta, M.C.; Galliera, E.; et al. Noncompetitive Allosteric Inhibitors of the Inflammatory Chemokine Receptors CXCR1 and CXCR2: Prevention of Reperfusion Injury. Proc. Natl. Acad. Sci. USA 2004, 101, 11791–11796. [Google Scholar] [CrossRef]
- Spiegel, A.; Brooks, M.W.; Houshyar, S.; Reinhardt, F.; Ardolino, M.; Fessler, E.; Chen, M.B.; Krall, J.A.; DeCock, J.; Zervantonakis, I.K.; et al. Neutrophils Suppress Intraluminal NK Cell-Mediated Tumor Cell Clearance and Enhance Extravasation of Disseminated Carcinoma Cells. Cancer Discov. 2016, 6, 630–649. [Google Scholar] [CrossRef]
- Teijeira, Á.; Garasa, S.; Gato, M.; Alfaro, C.; Migueliz, I.; Cirella, A.; de Andrea, C.; Ochoa, M.C.; Otano, I.; Etxeberria, I.; et al. CXCR1 and CXCR2 Chemokine Receptor Agonists Produced by Tumors Induce Neutrophil Extracellular Traps That Interfere with Immune Cytotoxicity. Immunity 2020, 52, 856–871.e8. [Google Scholar] [CrossRef]
- Flemming, A. Tumours Use NETs as Physical Shields. Nat. Rev. Immunol. 2020, 20, 352–353. [Google Scholar] [CrossRef]
- Ireland, A.S.; Oliver, T.G. Neutrophils Create an ImpeNETrable Shield between Tumor and Cytotoxic Immune Cells. Immunity 2020, 52, 729–731. [Google Scholar] [CrossRef] [PubMed]
- Cristinziano, L.; Modestino, L.; Antonelli, A.; Marone, G.; Simon, H.-U.; Varricchi, G.; Galdiero, M.R. Neutrophil Extracellular Traps in Cancer. Semin. Cancer Biol. 2022, 79, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Thålin, C.; Lundström, S.; Seignez, C.; Daleskog, M.; Lundström, A.; Henriksson, P.; Helleday, T.; Phillipson, M.; Wallén, H.; Demers, M. Citrullinated Histone H3 as a Novel Prognostic Blood Marker in Patients with Advanced Cancer. PLoS ONE 2018, 13, e0191231. [Google Scholar] [CrossRef]
- Yousefi, S.; Simon, D.; Stojkov, D.; Karsonova, A.; Karaulov, A.; Simon, H.-U. In Vivo Evidence for Extracellular DNA Trap Formation. Cell Death Dis. 2020, 11, 300. [Google Scholar] [CrossRef]
- Jin, W.; Xu, H.-X.; Zhang, S.-R.; Li, H.; Wang, W.-Q.; Gao, H.-L.; Wu, C.-T.; Xu, J.-Z.; Qi, Z.-H.; Li, S.; et al. Tumor-Infiltrating NETs Predict Postsurgical Survival in Patients with Pancreatic Ductal Adenocarcinoma. Ann. Surg. Oncol. 2019, 26, 635–643. [Google Scholar] [CrossRef]
- Agassi, R.; Czeiger, D.; Shaked, G.; Avriel, A.; Sheynin, J.; Lavrenkov, K.; Ariad, S.; Douvdevani, A. Measurement of Circulating Cell-Free DNA Levels by a Simple Fluorescent Test in Patients with Breast Cancer. Am. J. Clin. Pathol. 2015, 143, 18–24. [Google Scholar] [CrossRef]
- Yoo, D.; Floyd, M.; Winn, M.; Moskowitz, S.M.; Rada, B. NET Formation Induced by Pseudomonas Aeruginosa Cystic Fibrosis Isolates Measured as Release of Myeloperoxidase-DNA and Neutrophil Elastase-DNA Complexes. Immunol. Lett. 2014, 160, 186–194. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Y.; Ma, C.; Sun, H.; Wei, X.; Li, M.; Wei, W.; Zhang, F.; Yang, F.; Wang, H.; et al. Diagnostic, Therapeutic Predictive, and Prognostic Value of Neutrophil Extracellular Traps in Patients With Gastric Adenocarcinoma. Front. Oncol. 2020, 10, 1036. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, X.; Guan, M.; Yu, F.; Lai, F. In-Depth Single-Cell and Bulk-RNA Sequencing Developed a NETosis-Related Gene Signature Affects Non-Small-Cell Lung Cancer Prognosis and Tumor Microenvironment: Results from over 3,000 Patients. Front. Oncol. 2023, 13, 1282335. [Google Scholar] [CrossRef] [PubMed]
- Pastor, B.; Abraham, J.-D.; Pisareva, E.; Sanchez, C.; Kudriavstev, A.; Tanos, R.; Mirandola, A.; Mihalovičová, L.; Pezzella, V.; Adenis, A.; et al. Association of Neutrophil Extracellular Traps with the Production of Circulating DNA in Patients with Colorectal Cancer. iScience 2022, 25, 103826. [Google Scholar] [CrossRef] [PubMed]
- Pisareva, E.; Mihalovičová, L.; Pastor, B.; Kudriavtsev, A.; Mirandola, A.; Mazard, T.; Badiou, S.; Maus, U.; Ostermann, L.; Weinmann-Menke, J.; et al. Neutrophil Extracellular Traps Have Auto-Catabolic Activity and Produce Mononucleosome-Associated Circulating DNA. Genome Med. 2022, 14, 135. [Google Scholar] [CrossRef]
- Wang, M.; Lv, X.; Wang, Y.; Li, Y.; Li, H.; Shen, Z.; Zhao, L. Biomarkers of Peripheral Blood Neutrophil Extracellular Traps in the Diagnosis and Progression of Malignant Tumors. Cancer Med. 2024, 13, e6935. [Google Scholar] [CrossRef]
- Kim, J.G.; Kim, S.I.; Song, S.H.; Gu, J.-Y.; Lee, M.; Kim, H.K. Diagnostic and Prognostic Role of Circulating Neutrophil Extracellular Trap Markers and Prekallikrein in Patients with High-Grade Serous Ovarian Cancer. Front. Oncol. 2022, 12, 992056. [Google Scholar] [CrossRef]
- Tomás-Pérez, S.; Oto, J.; Aghababyan, C.; Herranz, R.; Cuadros-Lozano, A.; González-Cantó, E.; Mc Cormack, B.; Arrés, J.; Castaño, M.; Cana, F.; et al. Increased Levels of NETosis Biomarkers in High-Grade Serous Ovarian Cancer Patients’ Biofluids: Potential Role in Disease Diagnosis and Management. Front. Immunol. 2023, 14, 1111344. [Google Scholar] [CrossRef]
- Modestino, L.; Cristinziano, L.; Poto, R.; Ventrici, A.; Trocchia, M.; Ferrari, S.M.; Fallahi, P.; Paparo, S.R.; Marone, G.; Antonelli, A.; et al. Neutrophil Extracellular Traps and Neutrophil-Related Mediators in Human Thyroid Cancer. Front. Immunol. 2023, 14, 1167404. [Google Scholar] [CrossRef]
- Wannberg, F.; Hjalmar, V.; Ng, H.; Johansson, C.; Probert, F.; Phillipson, M.; Åberg, M.; Gordon, M.; Mackman, N.; Rosell, A.; et al. Plasma H3Cit-DNA Discriminates Between Cancer and Inflammation in a Cohort of Patients with Unspecific Cancer Symptoms. Inflammation 2024, 48, 760–769. [Google Scholar] [CrossRef]
- Tohme, S.; Yazdani, H.O.; Al-Khafaji, A.B.; Chidi, A.P.; Loughran, P.; Mowen, K.; Wang, Y.; Simmons, R.L.; Huang, H.; Tsung, A. Neutrophil Extracellular Traps Promote the Development and Progression of Liver Metastases after Surgical Stress. Cancer Res. 2016, 76, 1367–1380. [Google Scholar] [CrossRef]
- Hollmén, M.; Karaman, S.; Schwager, S.; Lisibach, A.; Christiansen, A.J.; Maksimow, M.; Varga, Z.; Jalkanen, S.; Detmar, M. G-CSF Regulates Macrophage Phenotype and Associates with Poor Overall Survival in Human Triple-Negative Breast Cancer. Oncoimmunology 2016, 5, e1115177. [Google Scholar] [CrossRef]
- Wu, L.; Saxena, S.; Awaji, M.; Singh, R.K. Tumor-Associated Neutrophils in Cancer: Going Pro. Cancers 2019, 11, 564. [Google Scholar] [CrossRef]
- Shishikura, K.; Horiuchi, T.; Sakata, N.; Trinh, D.-A.; Shirakawa, R.; Kimura, T.; Asada, Y.; Horiuchi, H. Prostaglandin E2 Inhibits Neutrophil Extracellular Trap Formation through Production of Cyclic AMP. Br. J. Pharmacol. 2016, 173, 319–331. [Google Scholar] [CrossRef]
- Salazar-Gonzalez, H.; Zepeda-Hernandez, A.; Melo, Z.; Saavedra-Mayorga, D.E.; Echavarria, R. Neutrophil Extracellular Traps in the Establishment and Progression of Renal Diseases. Medicina 2019, 55, 431. [Google Scholar] [CrossRef]
- Saffarzadeh, M. Neutrophil Extracellular Traps as a Drug Target to Counteract Chronic and Acute Inflammation. Curr. Pharm. Biotechnol. 2019, 19, 1196–1202. [Google Scholar] [CrossRef]
- Quan, J.M.; Tiddens, H.A.W.M.; Sy, J.P.; McKenzie, S.G.; Montgomery, M.D.; Robinson, P.J.; Wohl, M.E.B.; Konstan, M.W. A Two-Year Randomized, Placebo-Controlled Trial of Dornase Alfa in Young Patients with Cystic Fibrosis with Mild Lung Function Abnormalities. J. Pediatr. 2001, 139, 813–820. [Google Scholar] [CrossRef]
- Koenen, R.R. Neutrophil Extracellular Traps as Therapeutic Targets for Inflammatory Disease. Am. J. Pharmacol. Toxicol. 2015, 9, 200–202. [Google Scholar] [CrossRef][Green Version]
- Knight, J.S.; Subramanian, V.; O’Dell, A.A.; Yalavarthi, S.; Zhao, W.; Smith, C.K.; Hodgin, J.B.; Thompson, P.R.; Kaplan, M.J. Peptidylarginine Deiminase Inhibition Disrupts NET Formation and Protects against Kidney, Skin and Vascular Disease in Lupus-Prone MRL/Lpr Mice. Ann. Rheum. Dis. 2015, 74, 2199–2206. [Google Scholar] [CrossRef]
- Jung, H.S.; Gu, J.; Kim, J.-E.; Nam, Y.; Song, J.W.; Kim, H.K. Cancer Cell-Induced Neutrophil Extracellular Traps Promote Both Hypercoagulability and Cancer Progression. PLoS ONE 2019, 14, e0216055. [Google Scholar] [CrossRef]
- Hakkim, A.; Fürnrohr, B.G.; Amann, K.; Laube, B.; Abed, U.A.; Brinkmann, V.; Herrmann, M.; Voll, R.E.; Zychlinsky, A. Impairment of Neutrophil Extracellular Trap Degradation Is Associated with Lupus Nephritis. Proc. Natl. Acad. Sci. USA 2010, 107, 9813–9818. [Google Scholar] [CrossRef]
- Gonzalez-Aparicio, M.; Alfaro, C. Influence of Interleukin-8 and Neutrophil Extracellular Trap (NET) Formation in the Tumor Microenvironment: Is There a Pathogenic Role? J. Immunol. Res. 2019, 2019, 6252138. [Google Scholar] [CrossRef]
- Cedervall, J.; Zhang, Y.; Huang, H.; Zhang, L.; Femel, J.; Dimberg, A.; Olsson, A.-K. Neutrophil Extracellular Traps Accumulate in Peripheral Blood Vessels and Compromise Organ Function in Tumor-Bearing Animals. Cancer Res. 2015, 75, 2653–2662. [Google Scholar] [CrossRef]
- Brinkmann, V. Neutrophil Extracellular Traps in the Second Decade. J. Innate Immun. 2018, 10, 414–421. [Google Scholar] [CrossRef]
- Boone, B.A.; Murthy, P.; Miller-Ocuin, J.; Doerfler, W.R.; Ellis, J.T.; Liang, X.; Ross, M.A.; Wallace, C.T.; Sperry, J.L.; Lotze, M.T.; et al. Chloroquine Reduces Hypercoagulability in Pancreatic Cancer through Inhibition of Neutrophil Extracellular Traps. BMC Cancer 2018, 18, 678. [Google Scholar] [CrossRef]
- Cools-Lartigue, J.; Spicer, J.; Najmeh, S.; Ferri, L. Neutrophil Extracellular Traps in Cancer Progression. Cell. Mol. Life Sci. 2014, 71, 4179–4194. [Google Scholar] [CrossRef]
- Shak, S.; Capon, D.J.; Hellmiss, R.; Marsters, S.A.; Baker, C.L. Recombinant Human DNase I Reduces the Viscosity of Cystic Fibrosis Sputum. Proc. Natl. Acad. Sci. USA 1990, 87, 9188–9192. [Google Scholar] [CrossRef]
- Fuchs, H.J.; Borowitz, D.S.; Christiansen, D.H.; Morris, E.M.; Nash, M.L.; Ramsey, B.W.; Rosenstein, B.J.; Smith, A.L.; Wohl, M.E.; The Pulmozyme Study Group. Effect of Aerosolized Recombinant Human DNase on Exacerbations of Respiratory Symptoms and on Pulmonary Function in Patients with Cystic Fibrosis. N. Engl. J. Med. 1994, 331, 637–642. [Google Scholar] [CrossRef]
- Shah, P.I.; Bush, A.; Canny, G.J.; Colin, A.A.; Fuchs, H.J.; Geddes, D.M.; Johnson, C.A.; Light, M.C.; Scott, S.F.; Tullis, D.E. Recombinant Human DNase I in Cystic Fibrosis Patients with Severe Pulmonary Disease: A Short-Term, Double-Blind Study Followed by Six Months Open-Label Treatment. Eur. Respir. J. 1995, 8, 954–958. [Google Scholar] [CrossRef] [PubMed]
- Wilmott, R.W.; Amin, R.S.; Colin, A.A.; DeVault, A.; Dozor, A.J.; Eigen, H.; Johnson, C.; Lester, L.A.; McCoy, K.; McKean, L.P.; et al. Aerosolized Recombinant Human DNase in Hospitalized Cystic Fibrosis Patients with Acute Pulmonary Exacerbations. Am. J. Respir. Crit. Care Med. 1996, 153, 1914–1917. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.C.; Manzi, S.; Yarboro, C.; Rairie, J.; Mcinnes, I.; Averthelyi, D.; Sinicropi, D.; Hale, V.G.; Balow, J.; Austin, H.; et al. Recombinant Human Dnase I (rhDNase) in Patients with Lupus Nephritis. Lupus 1999, 8, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Macanovic, M.; Sinicropi, D.; Shak, S.; Baughman, S.; Thiru, S.; Lachmann, P.J. The Treatment of Systemic Lupus Erythematosus (SLE) in NZB/W F1 Hybrid Mice; Studies with Recombinant Murine DNase and with Dexamethasone. Clin. Exp. Immunol. 1996, 106, 243–252. [Google Scholar] [CrossRef]
- Patutina, O.; Mironova, N.; Ryabchikova, E.; Popova, N.; Nikolin, V.; Kaledin, V.; Vlassov, V.; Zenkova, M. Inhibition of Metastasis Development by Daily Administration of Ultralow Doses of RNase A and DNase I. Biochimie 2011, 93, 689–696. [Google Scholar] [CrossRef]
- Sugihara, S.; Yamamoto, T.; Tanaka, H.; Kambara, T.; Hiraoka, T.; Miyauchi, Y. Deoxyribonuclease Treatment Prevents Blood-Borne Liver Metastasis of Cutaneously Transplanted Tumour Cells in Mice. Br. J. Cancer 1993, 67, 66–70. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buszka, K.; Dompe, C.; Derwich, K.; Pieścikowska, I.; Nowicki, M.; Budna-Tukan, J. Dual Nature of Neutrophil Extracellular Traps (NETs)—From Cancer’s Ally to Therapeutic Target. Cells 2025, 14, 1200. https://doi.org/10.3390/cells14151200
Buszka K, Dompe C, Derwich K, Pieścikowska I, Nowicki M, Budna-Tukan J. Dual Nature of Neutrophil Extracellular Traps (NETs)—From Cancer’s Ally to Therapeutic Target. Cells. 2025; 14(15):1200. https://doi.org/10.3390/cells14151200
Chicago/Turabian StyleBuszka, Karolina, Claudia Dompe, Kinga Derwich, Izabela Pieścikowska, Michał Nowicki, and Joanna Budna-Tukan. 2025. "Dual Nature of Neutrophil Extracellular Traps (NETs)—From Cancer’s Ally to Therapeutic Target" Cells 14, no. 15: 1200. https://doi.org/10.3390/cells14151200
APA StyleBuszka, K., Dompe, C., Derwich, K., Pieścikowska, I., Nowicki, M., & Budna-Tukan, J. (2025). Dual Nature of Neutrophil Extracellular Traps (NETs)—From Cancer’s Ally to Therapeutic Target. Cells, 14(15), 1200. https://doi.org/10.3390/cells14151200






