Cu2+ and Zn2+ Ions Affecting Biochemical Paths and DNA Methylation of Rye (Secale cereale L.) Anther Culture Influencing Plant Regeneration Efficiency
Abstract
1. Introduction
2. Materials and Methods
2.1. Donor Plants’ Growing Conditions and Plant Regeneration
2.2. DNA Extraction and Methylation-Sensitive Amplified Fragment Length Polymorphism (metAFLP) Procedure
2.3. Quantifying Variation Based on the metAFLP Marker System
2.4. Determination of Glutathione and S-adenosyl-L-methionine Amount in Leaves
2.5. Determination of the Content of Phytohormones in Leaves
2.6. Determination of Polyamine Amount in Leaves
2.7. Statistical Analysis
3. Results
3.1. MetAFLP-Based Primary Analyses
3.2. Principal Coordinates Analyses Based on the metAFLP Markers
3.3. MetAFLP Quantitative Characteristics
3.4. Biochemical Characteristics
3.5. Plant Regeneration Efficiency
3.6. Analysis of Variance
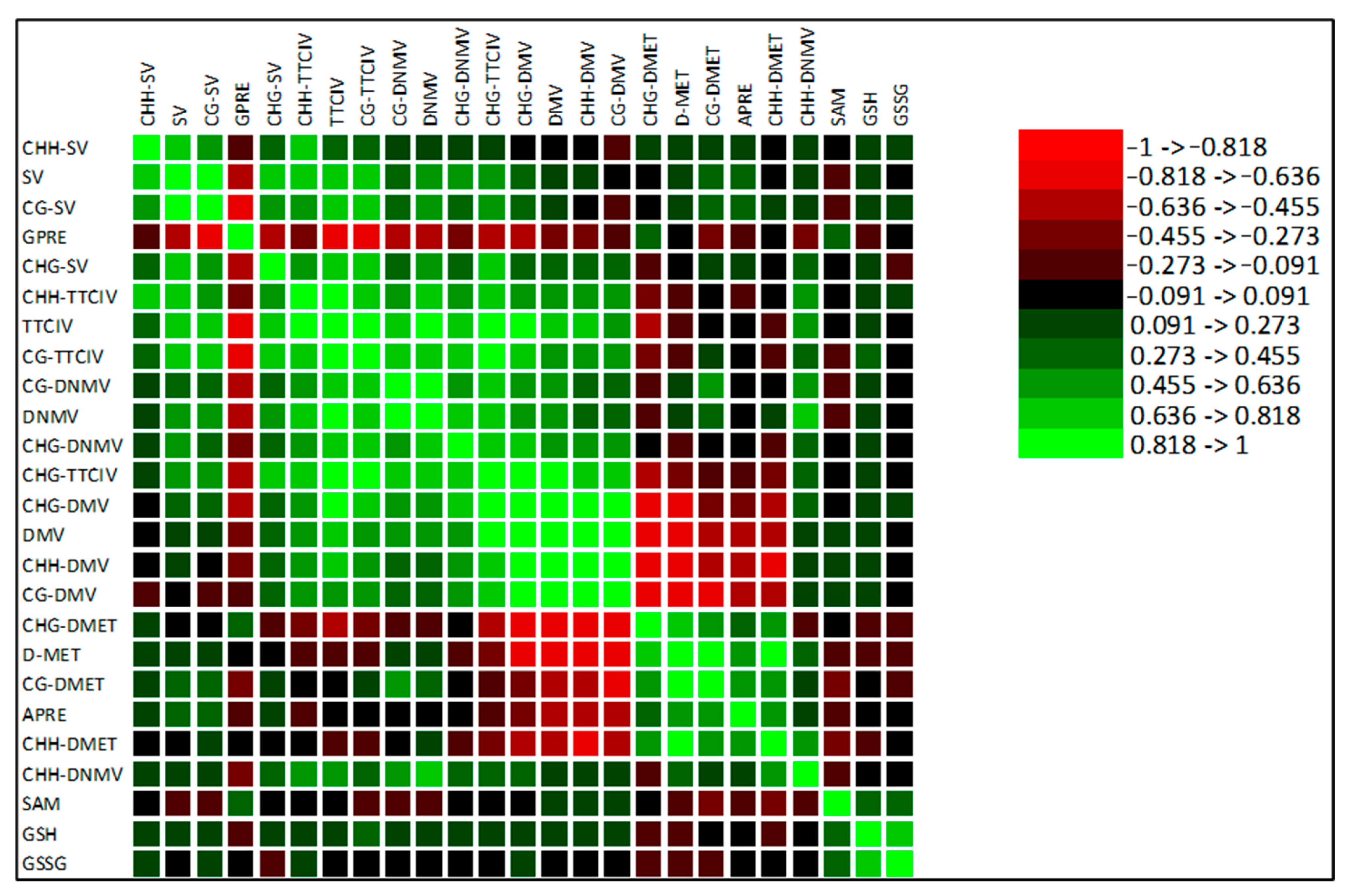
3.7. Ridge Regression Analysis
3.8. Analysis of Amino Acid Sequence Related to Sulfur-Containing Metabolites and Polyamines Requiring Metal Ions for Their Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAs | Amino acids |
| ABA | Abscisic acid |
| ADC-1 | Arginine decarboxylase-1 |
| AHC | Agglomerative hierarchical analysis |
| AIH | Agmatine iminohydrolase |
| APRE | Albino plant regeneration efficiency |
| Asn | Aspargine |
| Cad | Cadaverine |
| CG, CHG, and CHH | Methylation context |
| COX | Cytochrome c oxidase |
| CuAOx | Copper amine oxidase |
| cZ | cis-zeatin |
| cZR | cis-zeatin riboside |
| DMV | Demethylation |
| DNMV | De novo methylation |
| ETC | Electron transport chain |
| Gln | Glutamine |
| GPRE | Green plant regeneration efficiency |
| GSH | Glutathione (reduced form) |
| GSSG | Glutathione disulfide (oxidized form) |
| HPLC | High-pressure liquid chromatography |
| IAA | Indole-3-acetic acid |
| IM | Induction medium |
| IPA | Isopentenyladenine |
| JA | Jasmonic acid |
| Lys | Lysine |
| metAFLP | Methylation-sensitive amplified fragment length polymorphism |
| MRM | Multiple reaction monitoring |
| NAA | Naphtylo acetic acid |
| NEM | N-ethylmaleimide |
| ODC | Ornitine decarboxylase |
| PA | Polyamine |
| PCoA | Principal coordinated analysis |
| PCR | Polymerase chain reaction |
| PLP | Pyridoxal phosphate |
| PQH2 | Plastoquinol |
| PSI, PSII | Photosystems I and II |
| Put | Putrescine |
| ROS | Reactive oxygen species |
| RP-HPLC | Reverse-phase high-pressure liquid chromatography |
| SA | Salicylic acid |
| SAM | S-adenosyl-L-methionine |
| Ser | Serine |
| SOD | Superoxide dismutase |
| Spd | Spermidine |
| SPE | Solid-phase extraction |
| Spm | Spermine |
| SV | Sequence variation |
| TCA | Trichloroacetic acid |
| TCIV | Tissue culture-induced variation |
| tZ | trans-zeatin |
| tZR | trans-zeatin riboside |
| UPGMA | Unweighted pair group method with arithmetic mean |
References
- Marcińska, I.; Czyczyło-Mysza, I.; Skrzypek, E.; Warchoł, M.; Zieliński, K.; Dubas, E. Obtaining of winter rye (Secale cereale L. ssp. cereale) haploid embryos through hybridization with maize (Zea mays L.). Cereal Res. Commun. Cereal Res. Commun. 2018, 46, 521–532. [Google Scholar] [CrossRef]
- Zimny, J.; Michalski, K. Development of in vitro culture techniques for advancement of rye (Secale cereale L.) breeding. Acta Biol. Cracoviensia Ser. Bot. 2019, 61, 7–15. [Google Scholar] [CrossRef]
- Popelka, J.; Altpeter, F. Interactions between genotypes and culture media components for improved in vitro response of rye (Secale cereale L.) inbred lines. Plant Cell Rep. 2001, 20, 575–582. [Google Scholar] [CrossRef]
- Luo, P.; Zhao, Z.; Yang, F.; Zhang, L.; Li, S.; Qiao, Y.; Zhang, L.; Yang, M.; Zhou, X.; Zhao, L.; et al. Stress-Induced Autophagy Is Essential for Microspore Cell Fate Transition to the Initial Cell of Androgenesis. Plant Cell Environ. 2025, 48, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Mydy, L.S.; Chigumba, D.N.; Kersten, R.D. Plant Copper Metalloenzymes As Prospects for New Metabolism Involving Aromatic Compounds. Front. Plant Sci. 2021, 12, 692108. [Google Scholar] [CrossRef]
- Moffatt, B.A.; Weretilnyk, E.A. Sustaining S-adenosyl-l-methionine-dependent methyltransferase activity in plant cells. Physiol. Plant. 2001, 113, 435–442. [Google Scholar] [CrossRef]
- Pérez-Pérez, Y.; Berenguer, E.; Carneros, E.; Testillano, P.S. Increase of histone acetylation by suberoylanilide hydroxamic acid enhances microspore reprogramming and expression of somatic embryogenesis transcription factors in Brassica napus. Plant Sci. 2025, 351, 112318. [Google Scholar] [CrossRef]
- Berenguer, E.; Bárány, I.; Solís, M.-T.; Pérez-Pérez, Y.; Risueño, M.C.; Testillano, P.S. Inhibition of Histone H3K9 Methylation by BIX-01294 Promotes Stress-Induced Microspore Totipotency and Enhances Embryogenesis Initiation. Front. Plant Sci. 2017, 8, 1161. [Google Scholar] [CrossRef]
- Tenhola-Roininen, T.; Tanhuanpää, P.; Immonen, S. The effect of cold and heat treatments on the anther culture response of diverse rye genotypes. Euphytica 2005, 145, 1–9. [Google Scholar] [CrossRef]
- Immonen, S.; Anttila, H. Media Composition and Anther Plating for Production of Androgenetic Green Plants from Cultivated Rye (Secale cereale L.). J. Plant Physiol. 2000, 156, 204–210. [Google Scholar] [CrossRef]
- Guo, Y.D.; Pulli, S. Isolated microspore culture and plant regeneration in rye (Secale cereale L.). Plant Cell Rep 2000, 19, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Warchoł, M.; Juzoń, K.; Dziurka, K.; Czyczyło-Mysza, I.; Kapłoniak, K.; Marcińska, I.; Skrzypek, E. The effect of zinc, copper, and silver ions on oat (Avena sativa L.) androgenesis. Plants 2021, 10, 248. [Google Scholar] [CrossRef] [PubMed]
- Echavarri, B.; Soriano, M.; Cistué, L.; Vallés, M.P.; Castillo, A.M. Zinc sulphate improved microspore embryogenesis in barley. Plant Cell Tissue Organ Cult. 2008, 93, 295–301. [Google Scholar] [CrossRef]
- Bednarek, P.T.; Orłowska, R.; Mańkowski, D.R.; Zimny, J.; Kowalczyk, K.; Nowak, M.; Zebrowski, J. Glutathione and copper ions as critical factors of green plant regeneration efficiency of triticale in vitro anther culture. Front. Plant Sci. 2022, 13, 926305. [Google Scholar] [CrossRef]
- Makowska, K.; Oleszczuk, S.; Zimny, J. The effect of copper on plant regeneration in barley microspore culture. Czech J. Genet. Plant Breed. 2017, 53, 17–22. [Google Scholar] [CrossRef]
- Mansilla, N.; Racca, S.; Gras, D.E.; Gonzalez, D.H.; Welchen, E. The Complexity of Mitochondrial Complex IV: An Update of Cytochrome c Oxidase Biogenesis in Plants. Int. J. Mol. Sci. 2018, 19, 662. [Google Scholar] [CrossRef]
- Schertl, P.; Braun, H.-P. Respiratory electron transfer pathways in plant mitochondria. Front. Plant Sci. 2014, 5, 163. [Google Scholar] [CrossRef]
- Pattyn, J.; Vaughan-Hirsch, J.; Van de Poel, B. The regulation of ethylene biosynthesis: A complex multilevel control circuitry. New Phytol. 2021, 229, 770–782. [Google Scholar] [CrossRef]
- Van de Poel, B.; Bulens, I.; Oppermann, Y.; Hertog, M.L.A.T.M.; Nicolai, B.M.; Sauter, M.; Geeraerd, A.H. S-adenosyl-l-methionine usage during climacteric ripening of tomato in relation to ethylene and polyamine biosynthesis and transmethylation capacity. Physiol. Plant. 2013, 148, 176–188. [Google Scholar] [CrossRef]
- Maraschin, S.F.; de Priester, W.; Spaink, H.P.; Wang, M. Androgenic switch: An example of plant embryogenesis from the male gametophyte perspective. J. Exp. Bot. 2005, 56, 1711–1726. [Google Scholar] [CrossRef]
- Ouyang, Y.; Wu, Q.; Li, J.; Sun, S.; Sun, S. S-adenosylmethionine: A metabolite critical to the regulation of autophagy. Cell Prolif. 2020, 53, e12891. [Google Scholar] [CrossRef]
- Purnhauser, L.; Gyulai, G. Effect of copper on shoot and root regeneration in wheat, triticale, rape and tobacco tissue cultures. Plant Cell Tissue Organ Cult. 1993, 35, 131–139. [Google Scholar] [CrossRef]
- Ghaemi, M.; Sarrafi, A.; Alibert, G. The effects of silver nitrate, colchicine, cupric sulfate and genotype on the production of embryoids from anthers of tetraploid wheat (Triticum turgidum). Plant Cell Tissue Organ Cult. 1994, 36, 355–359. [Google Scholar] [CrossRef]
- Kothari-Chajer, A.; Sharma, M.; Kachhwaha, S.; Kothari, S.L. Micronutrient optimization results into highly improved in vitro plant regeneration in kodo (Paspalum scrobiculatum L.) and finger (Eleusine coracana (L.) Gaertn.) millets. Plant Cell Tissue Organ Cult. 2008, 94, 105–112. [Google Scholar] [CrossRef]
- Wojnarowiez, G.; Jacquard, C.; Devaux, P.; Sangwan, R.S.; Clement, C. Influence of copper sulfate on anther culture in barley (Hordeum vulgare L.). Plant Sci. 2002, 162, 843–847. [Google Scholar] [CrossRef]
- Nirwan, R.S.; Kothari, S.L. High Copper Levels Improve Callus Induction and Plant Regeneration in Sorghum bicolor (L.) Moench. In Vitr. Cell. Dev. Biol. Plant 2003, 39, 161–164. [Google Scholar] [CrossRef]
- Kumar Sahrawat, A.; Chand, S. Stimulatory Effect of Copper on Plant Regeneration in Indica Rice (Oryza sativa L.). J. Plant Physiol. 1999, 154, 517–522. [Google Scholar] [CrossRef]
- Immonen, S.; Anttila, H. Cold pretreatment to enhance green plant regeneration from rye anther culture. Plant Cell Tissue Organ Cult. 1999, 57, 121–127. [Google Scholar] [CrossRef]
- Immonen, S.; Tenhola-Roininen, T. Protocol for rye anther culture. In Doubled Haploid Production in Crop Plants—Manual; Maluszynski, M., Kasha, K.J., Forster, B.P., Szarejko, I., Eds.; Springer: Dordrecht, The Netherlands, 2003; pp. 141–150. [Google Scholar]
- Orłowska, R.; Dynkowska, W.M.; Niedziela, A.; Zebrowski, J.; Zimny, J.; Androsiuk, P.; Bednarek, P.T. β-glucans, SAM, and GSH fluctuations in barley anther tissue culture conditions affect regenerants’ DNA methylation and GPRE. BMC Plant Biol. 2024, 24, 853. [Google Scholar] [CrossRef]
- Orłowska, R.; Zebrowski, J.; Zimny, J.; Androsiuk, P.; Bednarek, P.T. S-Adenosyl-L-Methionine and Cu(II) Impact Green Plant Regeneration Efficiency. Cells 2022, 11, 2700. [Google Scholar] [CrossRef]
- Wang, W.; Paschalidis, K.; Feng, J.-C.; Song, J.; Liu, J.-H. Polyamine Catabolism in Plants: A Universal Process With Diverse Functions. Front. Plant Sci. 2019, 10, 561. [Google Scholar] [CrossRef]
- Cona, A.; Rea, G.; Angelini, R.; Federico, R.; Tavladoraki, P. Functions of amine oxidases in plant development and defence. Trends Plant Sci. 2006, 11, 80–88. [Google Scholar] [CrossRef]
- Tavladoraki, P.; Cona, A.; Angelini, R. Copper-Containing Amine Oxidases and FAD-Dependent Polyamine Oxidases Are Key Players in Plant Tissue Differentiation and Organ Development. Front. Plant Sci. 2016, 7, 824. [Google Scholar] [CrossRef]
- Cheng, W.H.; Wang, F.L.; Cheng, X.Q.; Zhu, Q.H.; Sun, Y.Q.; Zhu, H.G.; Sun, J. Polyamine and Its Metabolite H2O2 Play a Key Role in the Conversion of Embryogenic Callus into Somatic Embryos in Upland Cotton (Gossypium hirsutum L.). Front. Plant Sci. 2015, 6, 1063. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Limwachiranon, J.; Li, L.; Zhang, L.; Xu, Y.; Fu, M.; Luo, Z. Hydrogen peroxide accelerated the lignification process of bamboo shoots by activating the phenylpropanoid pathway and programmed cell death in postharvest storage. Postharvest Biol. Technol. 2019, 153, 79–86. [Google Scholar] [CrossRef]
- Camacho-Fernández, C.; Seguí-Simarro, J.M.; Mir, R.; Boutilier, K.; Corral-Martínez, P. Cell Wall Composition and Structure Define the Developmental Fate of Embryogenic Microspores in Brassica napus. Front. Plant Sci. 2021, 12, 737139. [Google Scholar] [CrossRef] [PubMed]
- Górecka, K.; Kiszczak, W.; Krzyżanowska, D.M.; Kowalska, U.; Kapuścińska, A. Effect of polyamines on in vitro anther cultures of carrot (Daucus carota L.). Turk. J. Biol. 2014, 38, 593–600. [Google Scholar] [CrossRef]
- Chiancone, B.; Tassoni, A.; Bagni, N.; Germanà, M.A. Effect of polyamines on in vitro anther culture of Citrus clementina Hort. ex Tan. Plant Cell Tissue Organ Cult. 2006, 87, 145–153. [Google Scholar] [CrossRef]
- Sauter, M.; Moffatt, B.; Saechao, M.C.; Hell, R.; Wirtz, M. Methionine salvage and S-adenosylmethionine: Essential links between sulfur, ethylene and polyamine biosynthesis. Biochem. J. 2013, 451, 145–154. [Google Scholar] [CrossRef]
- Broadley, M.R.; White, P.J.; Hammond, J.P.; Zelko, I.; Lux, A. Zinc in plants. New Phytol. 2007, 173, 677–702. [Google Scholar] [CrossRef]
- Fariduddin, Q.; Saleem, M.; Khan, T.A.; Hayat, S. Zinc as a Versatile Element in Plants: An Overview on Its Uptake, Translocation, Assimilatory Roles, Deficiency and Toxicity Symptoms. In Microbial Biofertilizers and Micronutrient Availability: The Role of Zinc in Agriculture and Human Health; Khan, S.T., Malik, A., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 137–158. [Google Scholar]
- He, D.-G.; Yang, Y.-M.; Scott, K.J. Zinc deficiency and the formation of white structures in immature embryo cultures of wheat (Triticum aestivum L.). Plant Cell Tissue Organ Cult. 1991, 24, 9–12. [Google Scholar] [CrossRef]
- Kobayashi, A.; Sakamoto, A.; Kubo, K.; Rybka, Z.; Kanno, Y.; Takatsuji, H. Seven zinc-finger transcription factors are expressed sequentially during the development of anthers in petunia. Plant J. 1998, 13, 571–576. [Google Scholar] [CrossRef]
- Yanagisawa, S. Dof Domain Proteins: Plant-Specific Transcription Factors Associated with Diverse Phenomena Unique to Plants. Plant Cell Physiol. 2004, 45, 386–391. [Google Scholar] [CrossRef]
- Han, G.; Lu, C.; Guo, J.; Qiao, Z.; Sui, N.; Qiu, N.; Wang, B. C2H2 Zinc Finger Proteins: Master Regulators of Abiotic Stress Responses in Plants. Front. Plant Sci. 2020, 11, 115. [Google Scholar] [CrossRef]
- Wang, L.; Pei, Z.; Tian, Y.; He, C. OsLSD1, a rice zinc finger protein, regulates programmed cell death and callus differentiation. Mol. Plant Microbe Interact. 2005, 18, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Hossain, B.; Hirata, N.; Nagatomo, Y.; Akashi, R.; Takaki, H. Internal Zinc Accumulation Is Correlated with Increased Growth in Rice Suspension Culture. J. Plant Growth Regul. 1997, 16, 239–243. [Google Scholar] [CrossRef]
- Casanova-Sáez, R.; Mateo-Bonmatí, E.; Ljung, K. Auxin Metabolism in Plants. Cold Spring Harb Perspect. Biol. 2021, 13, a039867. [Google Scholar] [CrossRef] [PubMed]
- Enders, T.A.; Strader, L.C. Auxin activity: Past, present, and future. Am. J. Bot. 2015, 102, 180–196. [Google Scholar] [CrossRef]
- Sun, P.; Huang, Y.; Yang, X.; Liao, A.; Wu, J. The role of indole derivative in the growth of plants: A review. Front. Plant Sci. 2023, 13, 1120613. [Google Scholar] [CrossRef]
- Cakmak, I.; Brown, P.; Colmenero-Flores, J.M.; Husted, S.; Kutman, B.Y.; Nikolic, M.; Rengel, Z.; Schmidt, S.B.; Zhao, F.-J. Chapter 7—Micronutrients. This chapter is a revision of the third edition chapter by Broadley M., Brown P., Cakmak I., Rengel Z., Zhao F.J.; pp. 191–248. https://doi.org/10.1016/B978-0-12-384905-2.00007-8. © Elsevier Ltd.**The individual micronutrient sections were written as follows: Fe (M.N.), Mn (S.H. and S.B.S.), Cu (Z.R.), Zn (I.C.), Ni (P.B. and B.Y.K.), Mo (F-J.Z.), B (P.B. and I.C.), and Cl (J.M.C.F.); In Marschner’s Mineral Nutrition of Plants, 4th ed.; Rengel, Z., Cakmak, I., White, P.J., Eds.; Academic Press: San Diego, CA, USA, 2023; pp. 283–385. [Google Scholar]
- Gazarin, I.G.; Lagrimini, L.M.; Mellon, F.A.; Naldrett, M.J.; Ashby, G.A.; Thorneley, R.N.F. Identification of skatolyl hydroperoxide and its role in the peroxidase-catalysed oxidation of indol-3-yl acetic acid. Biochem. J. 1998, 333, 223–232. [Google Scholar] [CrossRef]
- Fridovich, I. Oxygen toxicity: A radical explanation. J. Exp. Biol. 1998, 201, 1203–1209. [Google Scholar] [CrossRef]
- Yruela, I. Copper in plants: Acquisition, transport and interactions. Funct. Plant Biol. 2009, 36, 409–430. [Google Scholar] [CrossRef]
- Hänsch, R.; Mendel, R.R. Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr. Opin. Plant Biol. 2009, 12, 259–266. [Google Scholar] [CrossRef]
- Broadley, M.; Brown, P.; Cakmak, I.; Rengel, Z.; Zhao, F. Chapter 7—Function of Nutrients: Micronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 191–248. [Google Scholar]
- Bhat, B.A.; Islam, S.T.; Ali, A.; Sheikh, B.A.; Tariq, L.; Islam, S.U.; Hassan Dar, T.U. Role of Micronutrients in Secondary Metabolism of Plants. In Plant Micronutrients: Deficiency and Toxicity Management; Aftab, T., Hakeem, K.R., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 311–329. [Google Scholar]
- Dumont, S.; Rivoal, J. Consequences of Oxidative Stress on Plant Glycolytic and Respiratory Metabolism. Front. Plant Sci. 2019, 10, 166. [Google Scholar] [CrossRef]
- Touraev, A.; Vicente, O.; Heberle-Bors, E. Initiation of microspore embryogenesis by stress. Trends Plant Sci. 1997, 2, 297–302. [Google Scholar] [CrossRef]
- Kothari, S.L.; Agarwal, K.; Kumar, S. Inorganic nutrient manipulation for highly improved in vitro plant regeneration in finger millet—Eleusine coracana (L.) Gaertn. In Vitro Cell. Dev. Biol.-Plant 2004, 40, 515–519. [Google Scholar] [CrossRef]
- Zhuang, J.J.; Xu, J. Increasing differentiation frequencies in wheat pollen callus. In Cell and Tissue Culture Techniques for Cereal Crop Improvement; Hu, H., Vega, M.R., Eds.; Science Press: Beijing, China, 1983; p. 431. [Google Scholar]
- Chu, C.C. The N6 medium and its applications to anther culture of cereal crops. In Proceedings of Symposium on Plant Tissue Culture; Hu, H., Ed.; Science Press: Beijing, China, 1978; pp. 43–50. [Google Scholar]
- Bednarek, P.T.; Orłowska, R.; Koebner, R.M.; Zimny, J. Quantification of the tissue-culture induced variation in barley (Hordeum vulgare L.). BMC Plant Biol. 2007, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Machczyńska, J.; Orłowska, R.; Zimny, J.; Bednarek, P.T. Extended metAFLP approach in studies of the tissue culture induced variation (TCIV) in case of triticale. Mol. Breed. 2014, 34, 845–854. [Google Scholar] [CrossRef]
- Orłowska, R.; Bednarek, P.T. Precise evaluation of tissue culture-induced variation during optimisation of in vitro regeneration regime in barley. Plant Mol. Biol. 2020, 103, 33–50. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research-an update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef]
- Giustarini, D.; Dalle-Donne, I.; Milzani, A.; Fanti, P.; Rossi, R. Analysis of GSH and GSSG after derivatization with N-ethylmaleimide. Nat. Protoc. 2013, 8, 1660–1669. [Google Scholar] [CrossRef]
- Żur, I.; Dubas, E.; Krzewska, M.; Waligorski, P.; Dziurka, M.; Janowiak, F. Hormonal requirements for effective induction of microspore embryogenesis in triticale (x Triticosecale Wittm.) anther cultures. Plant Cell Rep. 2015, 34, 47–62. [Google Scholar] [CrossRef]
- Juzoń, K.; Czyczyło-Mysza, I.; Marcińska, I.; Dziurka, M.; Waligórski, P.; Skrzypek, E. Polyamines in yellow lupin (Lupinus luteus L.) tolerance to soil drought. Acta Physiol. Plant. 2017, 39, 202. [Google Scholar] [CrossRef]
- Addinsoft XLSTAT Statistical and Data Analysis Solution; New York, NY, USA. 2022. Available online: https://www.xlstat.com (accessed on 1 January 2020).
- Bednarek, P.T.; Pachota, K.A.; Dynkowska, W.M.; Machczynska, J.; Orłowska, R. Understanding In Vitro Tissue Culture-Induced Variation Phenomenon in Microspore System. Int. J. Mol. Sci. 2021, 22, 7546. [Google Scholar] [CrossRef]
- Krzepiłko, A.; Prażak, R.; Matyszczuk, K.; Dyduch-Sieminska, M. The effect of zinc oxide nanoparticles on the growth and development of Stevia plants cultured in vitro. Acta Sci. Pol. Hortorum Cultus 2024, 23, 43–56. [Google Scholar] [CrossRef]
- Ahmad, N.; Alatar, A.A.; Faisal, M.; Khan, M.I.; Fatima, N.; Anis, M.; Hegazy, A.K. Effect of copper and zinc on the in vitro regeneration of Rauvolfia serpentina. Biol. Plant. 2015, 59, 11–17. [Google Scholar] [CrossRef]
- Grauda, D.; Mikelsone, A.; Lisina, N.; Zagata, K.; Ornicans, R.; Fokina, O.; Lapiņa, L.; Rashal, I. Anther culture effectiveness in producing doubled haploids of cereals. Proc. Latv. Acad. Sci. Sect. B Nat. Exact Appl. Sci. 2014, 68, 142–147. [Google Scholar] [CrossRef]
- Smirnoff, N.; Arnaud, D. Hydrogen peroxide metabolism and functions in plants. New Phytol. 2019, 221, 1197–1214. [Google Scholar] [CrossRef]
- Choudhary, A.; Kumar, A.; Kaur, N. ROS and oxidative burst: Roots in plant development. Plant Divers. 2020, 42, 33–43. [Google Scholar] [CrossRef]
- Gill, S.S.; Anjum, N.A.; Gill, R.; Yadav, S.; Hasanuzzaman, M.; Fujita, M.; Mishra, P.; Sabat, S.C.; Tuteja, N. Superoxide dismutase—Mentor of abiotic stress tolerance in crop plants. Environ. Sci. Pollut. Res. 2015, 22, 10375–10394. [Google Scholar] [CrossRef]
- Valero-Rubira, I.; Castillo, A.M.; Burrell, M.; Vallés, M.P. Microspore embryogenesis induction by mannitol and TSA results in a complex regulation of epigenetic dynamics and gene expression in bread wheat. Front. Plant Sci. 2022, 13, 1058421. [Google Scholar] [CrossRef]
- Greco, M.; Sáez, C.A.; Contreras, R.A.; Rodríguez-Rojas, F.; Bitonti, M.B.; Brown, M.T. Cadmium and/or copper excess induce interdependent metal accumulation, DNA methylation, induction of metal chelators and antioxidant defences in the seagrass Zostera marina. Chemosphere 2019, 224, 111–119. [Google Scholar] [CrossRef]
- Baldini, A.; Battaglia, F.; Perrella, G. The generation of novel epialleles in plants: The prospective behind re-shaping the epigenome. Front. Plant Sci. 2025, 16, 1544744. [Google Scholar] [CrossRef] [PubMed]
- Miguel, J.F. Influence of High Concentrations of Copper Sulfate on In Vitro Adventitious Organogenesis of Cucumis sativus L. Int. J. Plant Biol. 2023, 14, 974–985. [Google Scholar]
- Dahleen, L.S. Improved plant regeneration from barley callus cultures by increased copper levels. Plant Cell Tissue Organ Cult. 1995, 43, 267–269. [Google Scholar] [CrossRef]
- Orłowska, R.; Zimny, J.; Zebrowski, J.; Androsiuk, P.; Bednarek, P.T. An insight into tissue culture-induced variation origin shared between anther culture-derived triticale regenerants. BMC Plant Biol. 2024, 24, 43. [Google Scholar] [CrossRef]
- Yan, G.; Li, X.; Yang, J.; Li, Z.; Hou, J.; Rao, B.; Hu, Y.; Ma, L.; Wang, Y. Cost-Effective Production of ATP and S-Adenosylmethionine Using Engineered Multidomain Scaffold Proteins. Biomolecules 2021, 11, 1706. [Google Scholar] [CrossRef]
- Roeder, S.; Dreschler, K.; Wirtz, M.; Cristescu, S.M.; van Harren, F.J.M.; Hell, R.; Piechulla, B. SAM levels, gene expression of SAM synthetase, methionine synthase and ACC oxidase, and ethylene emission from N. suaveolens flowers. Plant Mol. Biol. 2009, 70, 535–546. [Google Scholar] [CrossRef]
- Chiang, P.K.; Gordon, R.K.; Tal, J.; Zeng, G.C.; Doctor, B.P.; Pardhasaradhi, K.; McCann, P.P. S-Adenosylmetionine and methylation. FASEB J. 1996, 10, 471–480. [Google Scholar] [CrossRef]
- Sarewicz, M.; Pintscher, S.; Pietras, R.; Borek, A.; Bujnowicz, Ł.; Hanke, G.; Cramer, W.A.; Finazzi, G.; Osyczka, A. Catalytic Reactions and Energy Conservation in the Cytochrome bc1 and b6f Complexes of Energy-Transducing Membranes. Chem. Rev. 2021, 121, 2020–2108. [Google Scholar] [CrossRef]
- Yamori, W.; Takahashi, S.; Makino, A.; Price, G.D.; Badger, M.R.; von Caemmerer, S. The roles of ATP synthase and the cytochrome b6/f complexes in limiting chloroplast electron transport and determining photosynthetic capacity. Plant Physiol. 2011, 155, 956–962. [Google Scholar] [CrossRef]
- Roberts, A.G.; Bowman, M.K.; Kramer, D.M. Certain Metal Ions Are Inhibitors of Cytochrome b6f Complex ‘Rieske’ Iron−Sulfur Protein Domain Movements. Biochemistry 2002, 41, 4070–4079. [Google Scholar] [CrossRef]
- Malone, L.A.; Proctor, M.S.; Hitchcock, A.; Hunter, C.N.; Johnson, M.P. Cytochrome b6f—Orchestrator of photosynthetic electron transfer. Biochim. Et Biophys. Acta (BBA)—Bioenerg. 2021, 1862, 148380. [Google Scholar] [CrossRef]
- Khan, N. Decoding phytohormone signaling in plant stress physiology: Insights, challenges, and future directions. Environ. Exp. Bot. 2025, 231, 106099. [Google Scholar] [CrossRef]
- Pasternak, T.P.; Steinmacher, D. Plant Growth Regulation in Cell and Tissue Culture In Vitro. Plants 2024, 13, 327. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Park, J.-S.; Deng, J.-H.; Bai, Y. Cytochrome c oxidase subunit IV is essential for assembly and respiratory function of the enzyme complex. J. Bioenerg. Biomembr. 2006, 38, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.-L.; Balabin, I.; Onuchic, J.N. Dynamics of Electron Transfer Pathways in Cytochrome c Oxidase. Biophys. J. 2004, 86, 1813–1819. [Google Scholar] [CrossRef] [PubMed]
- Dennerlein, S.; Rehling, P.; Richter-Dennerlein, R. Cytochrome c oxidase biogenesis—from translation to early assembly of the core subunit COX1. FEBS Lett. 2023, 597, 1569–1578. [Google Scholar] [CrossRef]
- Bunsupa, S.; Hanada, K.; Maruyama, A.; Aoyagi, K.; Komatsu, K.; Ueno, H.; Yamashita, M.; Sasaki, R.; Oikawa, A.; Saito, K.; et al. Molecular Evolution and Functional Characterization of a Bifunctional Decarboxylase Involved in Lycopodium Alkaloid Biosynthesis. Plant Physiol. 2016, 171, 2432–2444. [Google Scholar] [CrossRef]
- Foudouli, A.C.; Kyriakidis, D.A. Induction of ornithine decarboxylase activity by growth regulators in bean and corn plants. Plant Growth Regul. 1990, 9, 247–253. [Google Scholar] [CrossRef]
- Fariduddin, Q.; Varshney, P.; Yusuf, M.; Ahmad, A. Polyamines: Potent modulators of plant responses to stress. J. Plant Interact. 2013, 8, 1–16. [Google Scholar] [CrossRef]
- Janowitz, T.; Kneifel, H.; Piotrowski, M. Identification and characterization of plant agmatine iminohydrolase, the last missing link in polyamine biosynthesis of plants. FEBS Lett. 2003, 544, 258–261. [Google Scholar] [CrossRef]
- Planas-Portell, J.; Gallart, M.; Tiburcio, A.F.; Altabella, T. Copper-containing amine oxidases contribute to terminal polyamine oxidation in peroxisomes and apoplast of Arabidopsis thaliana. BMC Plant Biol. 2013, 13, 109. [Google Scholar] [CrossRef]
- Shimizu, Y.; Rai, A.; Okawa, Y.; Tomatsu, H.; Sato, M.; Kera, K.; Suzuki, H.; Saito, K.; Yamazaki, M. Metabolic diversification of nitrogen-containing metabolites by the expression of a heterologous lysine decarboxylase gene in Arabidopsis. Plant J. 2019, 100, 505–521. [Google Scholar] [CrossRef]


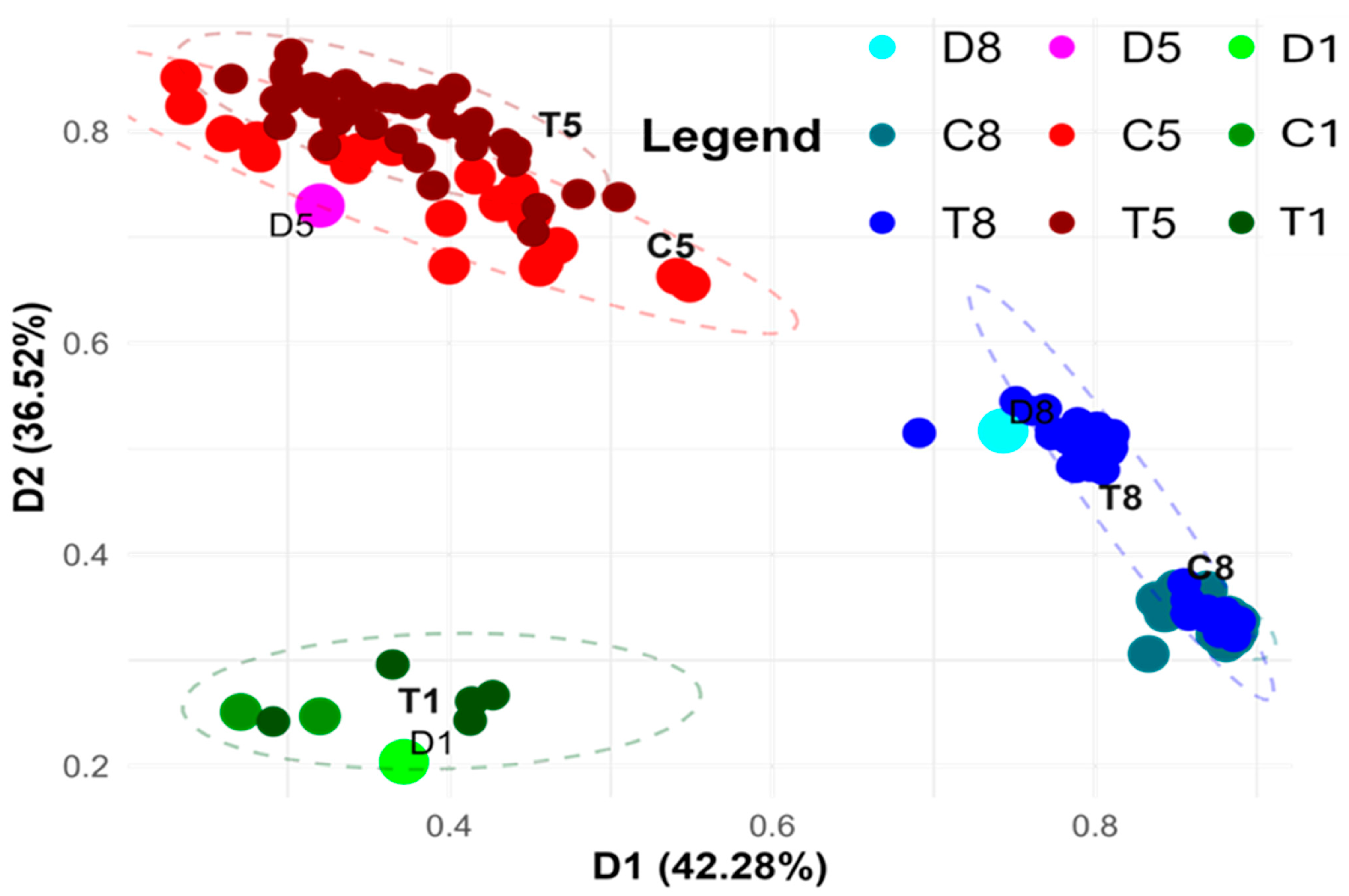


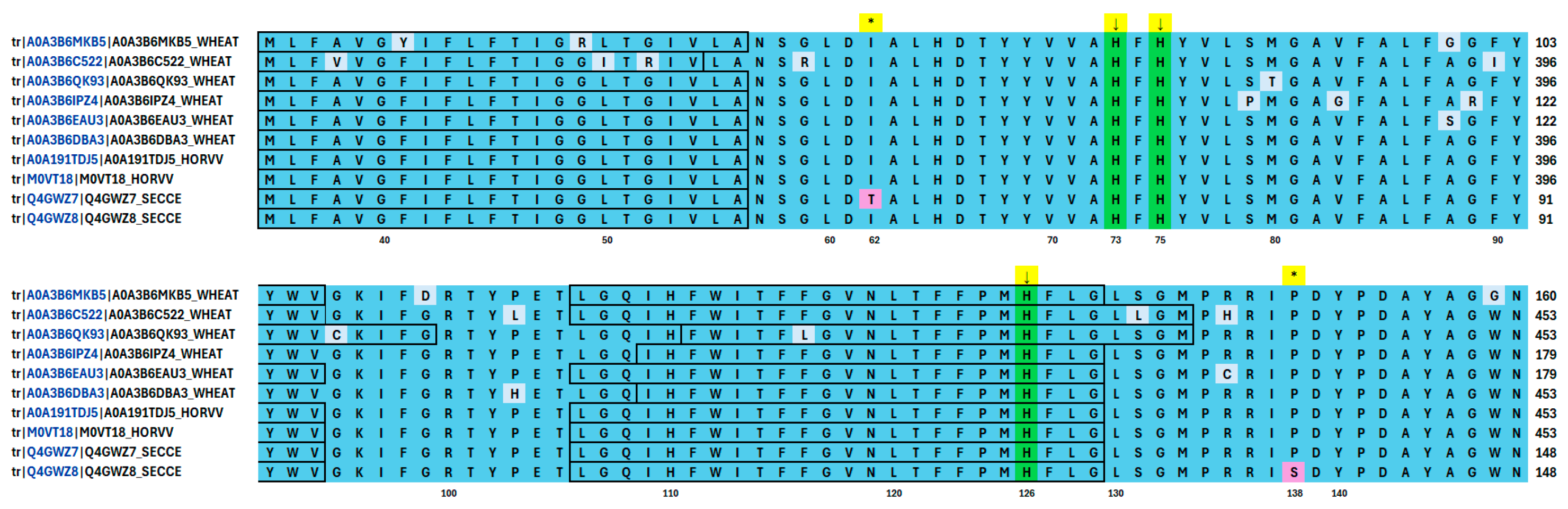

| Trial | In Vitro Anther Culture Conditions | Total Number of Regenerants | Regeneration Efficiency 1 | Number of Green Regenerants Employed in metAFLP | |||
|---|---|---|---|---|---|---|---|
| Cu (μM) | Zn (μM) | Albino | Green | APRE | GPRE | ||
| C1 | - | - | 40 | 6 | 12.90 | 1.94 | 2 |
| T1 | 5 | - | 20 | 13 | 5.97 | 3.88 | 5 |
| C5 | - | - | 1207 | 78 | 482.8 | 31.2 | 21 |
| T5 | 5 | - | 1314 | 63 | 503.45 | 24.14 | 39 |
| C8 | - | - | 371 | 140 | 403.26 | 142.17 | 22 |
| T8 | - | 150 | 392 | 133 | 296.27 | 99.25 | 48 |
| Band Characteristics | Matrix 1 | Trial | |||||
|---|---|---|---|---|---|---|---|
| C1 | T1 | C5 | T5 | C8 | T8 | ||
| No. bands | K | 261 | 274 | 303 | 301 | 273 | 281 |
| M | 86 | 80 | 164 | 168 | 91 | 127 | |
| No. private bands | K | 1 | 0 | 5 | 2 | 0 | 0 |
| M | 7 | 4 | 9 | 17 | 1 | 3 | |
| %P | K | 4.26 | 9.66 | 23.86 | 19.89 | 5.97 | 14.77 |
| M | 9.66 | 10.80 | 40.91 | 41.76 | 17.33 | 29.83 | |
| metAFLP Quantitative Characteristics | Regeneration | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sequence Contexts | Total | |||||||||||||||||||||||
| Samples | Statistics | Line | CHH | CHG | CG | |||||||||||||||||||
| SV * | DMV | DNMV | TCIV | D-MET | SV | DMV | DNMV | TCIV | D-MET | SV | DMV | DNMV | TCIV | D_MET | SV | DMV | DNMV | TCIV | D_MET | APRE | GPRE | |||
| Controls | R2adj | 0.22 | 0.72 | 0.31 | 0.35 | 0.16 | 0.39 | 0.83 | 0.14 | 0.64 | 0.66 | 0.47 | 0.89 | 0.56 | 0.56 | 0.49 | 0.13 | 0.85 | 0.57 | 0.57 | 0.50 | 0.22 | 0.93 | |
| F(Welch) | 8.42 | 259 | 5.48 | 5.05 | 3.17 | 9.46 | 161 | 8.67 | 64.52 | 29.75 | 15.7 | 3983 | 36.93 | 471.4 | 20.25 | 4.42 | 4049 | 13.17 | 41.01 | 7.20 | 1.54 | 567.7 | ||
| p | 0.07 | 0.00 | 0.11 | 0.13 | 0.19 | 0.06 | 0.00 | 0.06 | 0.00 | 0.02 | 0.02 | 0.00 | 0.01 | 0.00 | 0.02 | 0.14 | 0.00 | 0.04 | 0.01 | 0.09 | 0.36 | 0.00 | ||
| Games- Howell | 1 | A | C | C | A | A | B | B | C | A | C | B | B | A | ||||||||||
| 5 | A | B | B | A | B | A | B | B | A | B | B | B | B | |||||||||||
| 8 | B | A | A | A | A | A | A | A | A | A | A | A | C | |||||||||||
| Treated | R2adj | 0.19 | 0.91 | 0.13 | 0.32 | 0.48 | 0.37 | 0.94 | 0.56 | 0.80 | 0.66 | 0.72 | 0.95 | 0.37 | 0.72 | 0.54 | 0.56 | 0.98 | 0.41 | 0.71 | 0.67 | 0.71 | 0.69 | |
| F(Welch) | 14.5 | 216 | 8.32 | 33.07 | 151.59 | 25.2 | 458 | 96.95 | 273.5 | 52.09 | 132 | 207 | 42.70 | 118.85 | 124.41 | 62.1 | 2776 | 61.37 | 176.62 | 237.59 | 689.1 | 222.2 | ||
| p | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||
| Games- Howell | 1 | A | B | AB | C | A | AB | C | A | A | A | B | C | B | C | A | B | B | C | C | A | A | A | |
| 5 | B | A | B | B | C | B | B | B | B | C | C | A | B | B | C | C | A | B | B | C | C | B | ||
| 8 | A | A | A | A | B | A | A | C | C | B | A | B | A | A | B | A | A | A | A | B | B | C | ||
| Controls vs. Treated | R2adj | 0.51 | −0.11 | 0.59 | 0.52 | 0.55 | 0.43 | −0.14 | −0.19 | 0.40 | −0.19 | −0.19 | 0.41 | 0.47 | 0.26 | −0.02 | 0.57 | 0.52 | 0.45 | 0.59 | 0.25 | 0.08 | −0.19 | |
| F(Welch) | 7.48 | 0.78 | 3.30 | 4.09 | 3.44 | 5.41 | 0.26 | 0.03 | 3.95 | 0.03 | 0.06 | 14.3 | 6.60 | 7.71 | 0.61 | 8.37 | 15.7 | 2.73 | 6.33 | 1.23 | 0.00 | 0.15 | ||
| p | 0.11 | 0.42 | 0.31 | 0.25 | 0.30 | 0.16 | 0.66 | 0.89 | 0.22 | 0.88 | 0.82 | 0.02 | 0.13 | 0.04 | 0.54 | 0.12 | 0.01 | 0.32 | 0.18 | 0.45 | 1.00 | 0.72 | ||
| Games–Howell | 1 | T > C | T > C | T > C | ||||||||||||||||||||
| Controls vs. Treated | R2adj | −0.01 | 0.33 | −0.01 | −0.01 | 0.09 | −0.02 | 0.30 | −0.02 | 0.04 | 0.11 | 0.03 | 0.23 | 0.03 | 0.00 | 0.12 | −0.01 | 0.37 | 0.00 | 0.01 | 0.17 | 0.05 | −0.02 | |
| F(Welch) | 0.13 | 20.8 | 0.49 | 0.58 | 7.82 | 0.07 | 17.1 | 0.00 | 2.69 | 8.24 | 1.68 | 12.6 | 3.72 | 0.52 | 11.29 | 0.32 | 21.2 | 1.38 | 1.21 | 16.04 | 4.89 | 0.14 | ||
| p | 0.72 | 0.00 | 0.49 | 0.45 | 0.01 | 0.79 | 0.00 | 0.97 | 0.11 | 0.01 | 0.21 | 0.00 | 0.06 | 0.48 | 0.00 | 0.58 | 0.00 | 0.25 | 0.28 | 0.00 | 0.03 | 0.71 | ||
| Games–Howell | 5 | T > C | C > T | T > C | C > T | T > C | C > T | T > C | C > T | C > T | ||||||||||||||
| Controls vs. Treated | R2adj | 0.44 | 0.35 | −0.01 | 0.37 | 0.08 | 0.17 | 0.02 | 0.57 | 0.33 | 0.59 | 0.31 | 0.13 | 0.00 | 0.22 | 0.05 | 0.37 | 0.32 | 0.09 | 0.36 | −0.01 | 0.05 | 0.16 | |
| F(Welch) | 116 | 37.9 | 0.29 | 72.68 | 6.25 | 19.2 | 2.93 | 81.13 | 39.79 | 95.95 | 64.7 | 11.7 | 1.37 | 40.72 | 6.51 | 86.4 | 33.9 | 9.84 | 77.77 | 0.05 | 2.34 | 21.38 | ||
| p | 0.00 | 0.00 | 0.59 | 0.00 | 0.02 | 0.00 | 0.09 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.25 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.82 | 0.14 | 0.00 | ||
| Games–Howell | 8 | T > C | T > C | T > C | C > T | T > C | T > C | T > C | T > C | T > C | T > C | T > C | C > T | T > C | T > C | T > C | T > C | T > C | ||||||
| Sample | Statistics | Line | Biochemical Variables/Growth and Stress-Related Metabolites | Regeneration | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sulfur-Containing Metabolites | Polyamines | Phytohormones | ||||||||||||||||||
| SAM * | GSH | GSSG | Put | Cad | Spd | Spm | IAA | IPA | ABA | SA | JA | tZ | cZ | tZR | cZR | APRE | GPRE | |||
| Controls | R2adj | 0.14 | 0.13 | 0.02 | 0.01 | 0.02 | 0.05 | −0.02 | 0.04 | -0.01 | −0.04 | −0.02 | 0.25 | 0.08 | 0.12 | 0.05 | −0.02 | 0.22 | 0.93 | |
| F(Welch) | 3.71 | 2.55 | 0.99 | 0.97 | 1.11 | 1.73 | 0.46 | 0.67 | 0.62 | 0.00 | 0.23 | 5.34 | 1.61 | 1.74 | 1.66 | 0.31 | 1.54 | 567.70 | ||
| p | 0.16 | 0.24 | 0.47 | 0.48 | 0.45 | 0.33 | 0.67 | 0.58 | 0.60 | 1.00 | 0.81 | 0.12 | 0.35 | 0.33 | 0.35 | 0.76 | 0.36 | 0.00 | ||
| Games–Howell | 1 | A | ||||||||||||||||||
| 5 | B | |||||||||||||||||||
| 8 | C | |||||||||||||||||||
| Treated | R2adj | 0.16 | −0.02 | 0.03 | −0.02 | 0.26 | 0.08 | 0.33 | 0.01 | −0.13 | 0.15 | −0.13 | 0.17 | 0.04 | −0.09 | 0.16 | −0.08 | 0.71 | 0.69 | |
| F(Welch) | 6.89 | 0.22 | 1.61 | 0.20 | 0.90 | 12.87 | 17.27 | 1.09 | 0.07 | 2.45 | 0.09 | 2.67 | 1.35 | 0.31 | 2.50 | 0.41 | 689.14 | 222.25 | ||
| p | 0.02 | 0.81 | 0.26 | 0.83 | 0.46 | 0.02 | 0.00 | 0.36 | 0.94 | 0.12 | 0.91 | 0.10 | 0.29 | 0.74 | 0.12 | 0.67 | 0.00 | 0.00 | ||
| Games–Howell | 1 | B | AB | A | A | A | ||||||||||||||
| 5 | A | A | B | C | B | |||||||||||||||
| 8 | B | B | A | B | C | |||||||||||||||
| Controls vs. Treated | R2adj | −0.25 | 0.25 | −0.20 | 0.14 | −0.23 | 0.79 | 0.97 | NA | NA | NA | NA | NA | NA | NA | NA | NA | 0.08 | −0.19 | |
| F(Welch) | 0.02 | 1.24 | 0.19 | 1.50 | 0.43 | 11.98 | 97.77 | NA | NA | NA | NA | NA | NA | NA | NA | NA | 0.00 | 0.15 | ||
| p | 0.90 | 0.45 | 0.70 | 0.35 | 0.58 | 0.07 | 0.01 | NA | NA | NA | NA | NA | NA | NA | NA | NA | 1.00 | 0.72 | ||
| Games–Howell | 1 | NA | ||||||||||||||||||
| Controls vs. Treated | R2adj | 0.04 | 0.13 | 0.16 | −0.02 | −0.02 | 0.02 | 0.01 | −0.03 | −0.03 | −0.01 | 0.04 | −0.07 | 0.07 | 0.03 | −0.07 | −0.07 | 0.05 | −0.02 | |
| F(Welch) | 2.18 | 4.79 | 6.82 | 0.02 | 0.18 | 1.08 | 0.85 | 0.59 | 0.48 | 0.88 | 1.52 | 0.05 | 1.90 | 1.27 | 0.10 | 0.14 | 4.89 | 0.14 | ||
| p | 0.09 | 0.04 | 0.02 | 0.90 | 0.67 | 0.31 | 0.37 | 0.46 | 0.51 | 0.37 | 0.24 | 0.82 | 0.20 | 0.29 | 0.77 | 0.72 | 0.03 | 0.71 | ||
| Games–Howell | 5 | T > C | T > C | C > T | ||||||||||||||||
| Controls vs. Treated | R2adj | 0.03 | −0.01 | −0.02 | 0.12 | 0.01 | 0.17 | 0.18 | −0.05 | −0.03 | 0.08 | 0.00 | −0.04 | 0.10 | 0.00 | −0.05 | 0.13 | 0.05 | 0.16 | |
| F(Welch) | 2.45 | 0.43 | 0.30 | 4.87 | 1.45 | 6.86 | 7.33 | 0.18 | 0.46 | 2.46 | 1.06 | 0.32 | 2.74 | 0.86 | 0.08 | 3.30 | 2.34 | 21.38 | ||
| p | 0.13 | 0.52 | 0.59 | 0.04 | 0.24 | 0.02 | 0.01 | 0.68 | 0.51 | 0.14 | 0.32 | 0.59 | 0.12 | 0.37 | 0.78 | 0.10 | 0.14 | 0.00 | ||
| Games–Howell | 8 | T > C | T > C | T > C | T > C | |||||||||||||||
| Ridge Regression Analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Common | General (Basic) | General (Extended) | |||||||
| Models | APRE 1 | GPRE | APRE | GPRE | APRE | GPRE | |||
| Statistics | |||||||||
| Optimal lambda. | 5.56 | 0.640 | 8.93 | 1.24 | 1.57 | 3.0 | |||
| Classification | Intercept. | 331.0 | 129.0 | 328.88 | 128.54 | 0.74 | 109.02 | ||
| metAFLP characteristics | CHH_SV | −32.02 | 12.17 | −25.97 | 10.13 | −46.8 | 25.08 | ||
| CHH_DMV | −5.99 | 0 | 0 | 0 | 0 | 0 | |||
| CHH_DNMV | 30.26 | 0.75 | 30.65 | 0 | 72.83 | 0 | |||
| CG_SV | 30.97 | −15.42 | 34.28 | −14.14 | 86.39 | −20.99 | |||
| CG_DMV | −145.39 | 25.34 | −134.85 | 23.1 | −105.8 | 0 | |||
| CG_DNMV | 0 | −18.4 | 0 | −17.883 | −38.33 | −6.9 | |||
| CHG_SV | 24.28 | −7.87 | 21.31 | −8.53 | 0 | −6.81 | |||
| CHG_DMV | 0 | −34.71 | 0 | −29.84 | 0 | −15.95 | |||
| CHG_DNMV | 41.034 | 14.51 | 21.64 | 8.74 | 0 | 0 | |||
| Growth and Stress-Related Metabolites | Sulfur-containing metabolites 2 | SAM 2 | 0 | 377.73 | 0 | 357.34 | 1922.24 | 331.27 | |
| GSH | 4.3 | −2.93 | 0 | −1.16 | −7.53 | 0 | |||
| GSSG | 0 | 0.183 | 0 | 0 | 0 | 0.02 | |||
| Polyamines 3 | Put 3 | 0 | 0 | 0 | 0 | ||||
| Cad | 0 | 6.57 | 798.43 | 36.19 | |||||
| Spd | 0 | 1.0 | 0 | 0.74 | |||||
| Spm | 0.68 | 0 | 3.2 | 0.68 | |||||
| Phytohormones 4 | IAA 4 | −0.16 | −0.06 | ||||||
| ABA | 0.056 | 0 | |||||||
| SA | 0 | 0 | |||||||
| JA | 0 | 0 | |||||||
| tZ | −0.56 | 0 | |||||||
| cZ | −0.23 | 0 | |||||||
| tZR | −25.33 | 0 | |||||||
| cZR | 0 | 0 | |||||||
| IPA | 0 | 0 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dynkowska, W.M.; Orłowska, R.; Waligórski, P.; Bednarek, P.T. Cu2+ and Zn2+ Ions Affecting Biochemical Paths and DNA Methylation of Rye (Secale cereale L.) Anther Culture Influencing Plant Regeneration Efficiency. Cells 2025, 14, 1167. https://doi.org/10.3390/cells14151167
Dynkowska WM, Orłowska R, Waligórski P, Bednarek PT. Cu2+ and Zn2+ Ions Affecting Biochemical Paths and DNA Methylation of Rye (Secale cereale L.) Anther Culture Influencing Plant Regeneration Efficiency. Cells. 2025; 14(15):1167. https://doi.org/10.3390/cells14151167
Chicago/Turabian StyleDynkowska, Wioletta Monika, Renata Orłowska, Piotr Waligórski, and Piotr Tomasz Bednarek. 2025. "Cu2+ and Zn2+ Ions Affecting Biochemical Paths and DNA Methylation of Rye (Secale cereale L.) Anther Culture Influencing Plant Regeneration Efficiency" Cells 14, no. 15: 1167. https://doi.org/10.3390/cells14151167
APA StyleDynkowska, W. M., Orłowska, R., Waligórski, P., & Bednarek, P. T. (2025). Cu2+ and Zn2+ Ions Affecting Biochemical Paths and DNA Methylation of Rye (Secale cereale L.) Anther Culture Influencing Plant Regeneration Efficiency. Cells, 14(15), 1167. https://doi.org/10.3390/cells14151167






