Dysfunction and Metabolic Reprogramming of Gut Regulatory T Cells in HIV-Infected Immunological Non-Responders
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects and Sample Processing
2.2. ScRNA-Seq Library Construction, Read Mapping and Quality Control
2.3. ScRNA-Seq Data Processing
2.4. Cell Annotation and Subpopulation Re-Analysis
2.5. Cell Distribution Discrepancy
2.6. Differentially Expressed Gene (DEG) Analysis
2.7. Functional Scoring
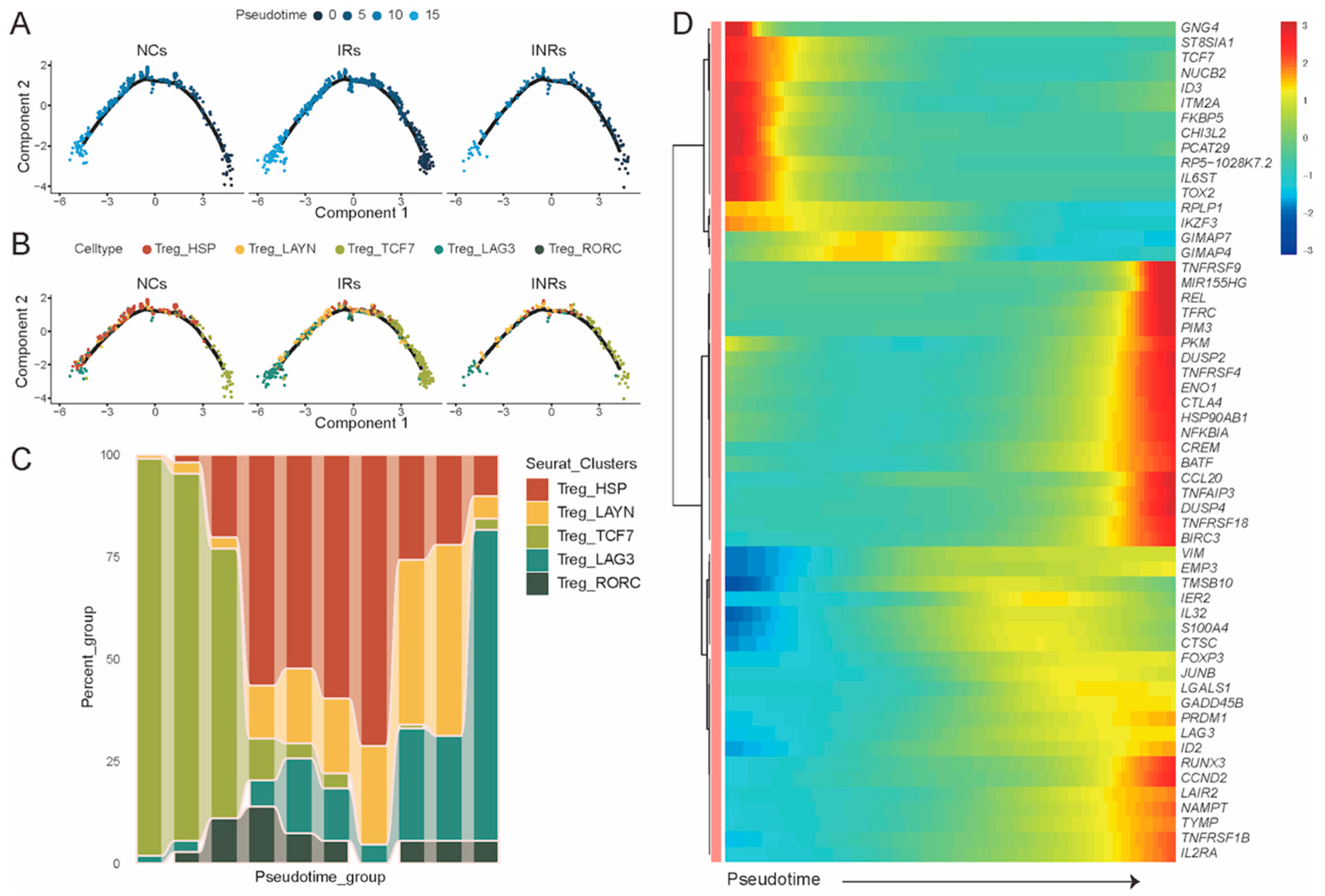
2.8. Trajectory Analysis
2.9. Faecal Microbiota Analysis
2.10. HIV DNA and CA HIV RNA Quantification
2.11. Detection of Plasma Short-Chain Fatty Acids
2.12. In Virto Stimulation
2.13. Detection of Cell Apoptosis and Cytokines
2.14. Detection of Cellular Energy Metabolism
2.15. Statistical Analysis
3. Results
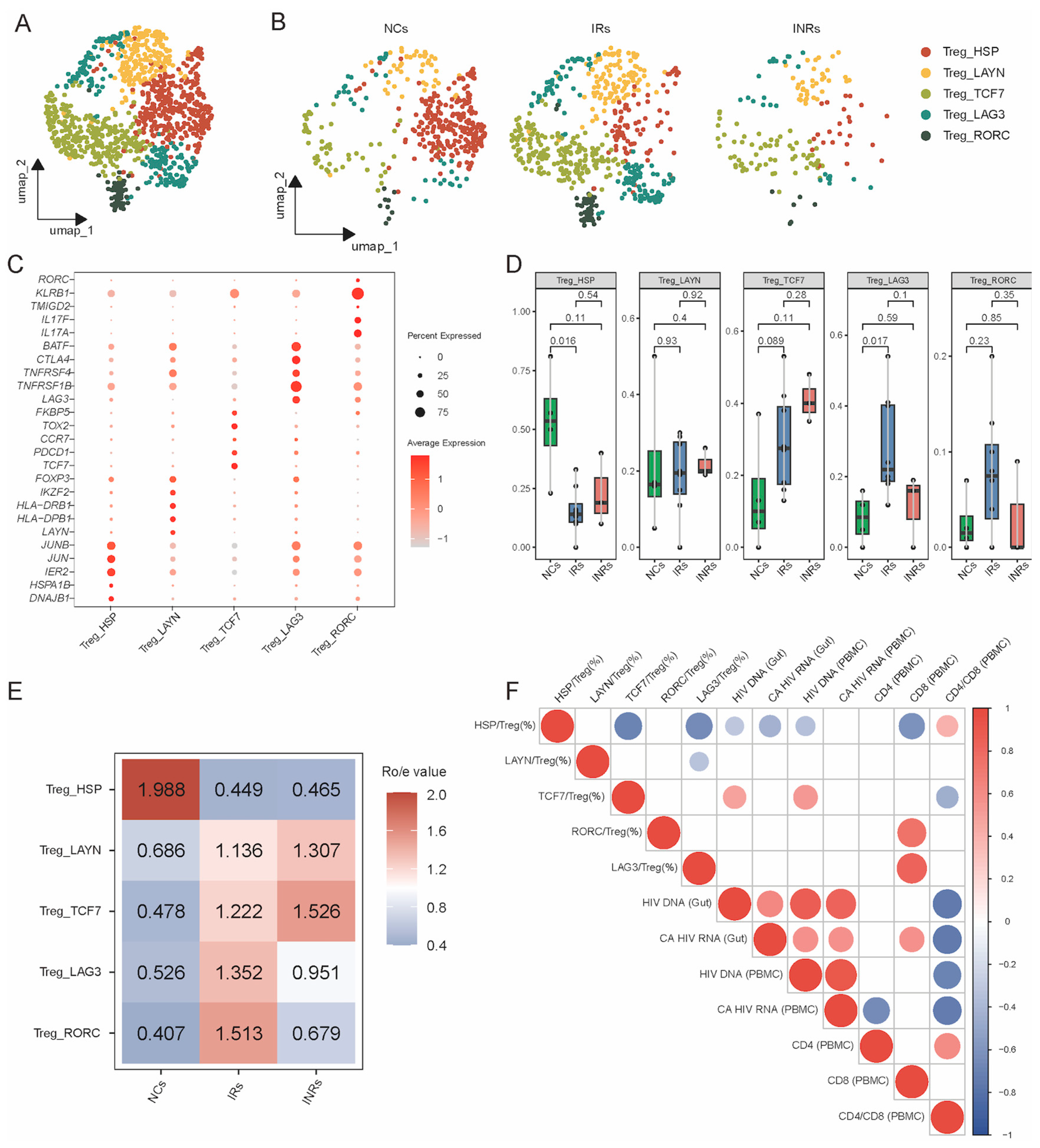
3.1. Marginal Increase but Dysfunction of Gut Tregs in INRs
3.2. Transcriptional Profiling of Gut Treg Subsets in NCs, IRs, and INRs
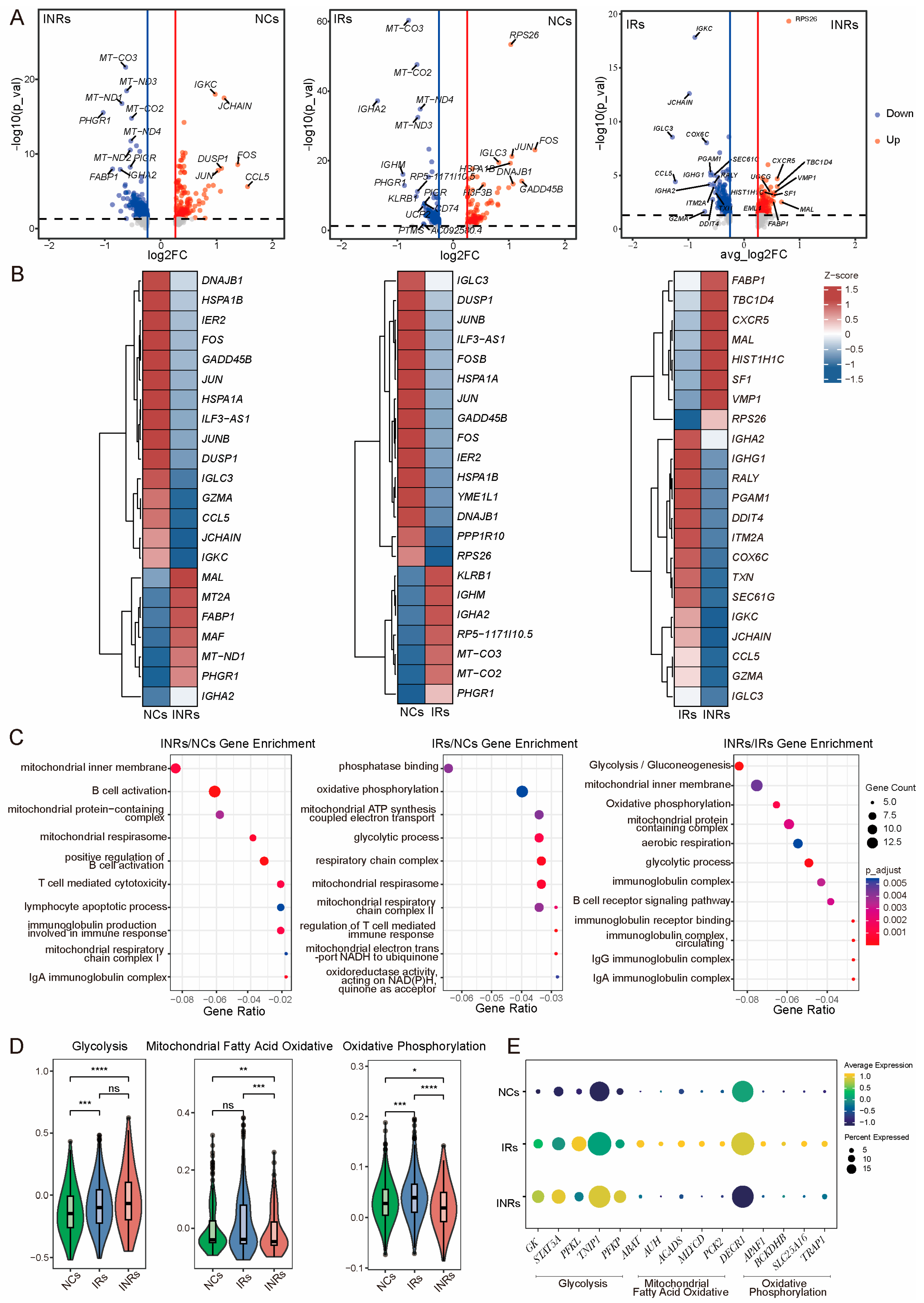
3.3. Reprogrammed Energy Metabolism of Gut Tregs in INRs
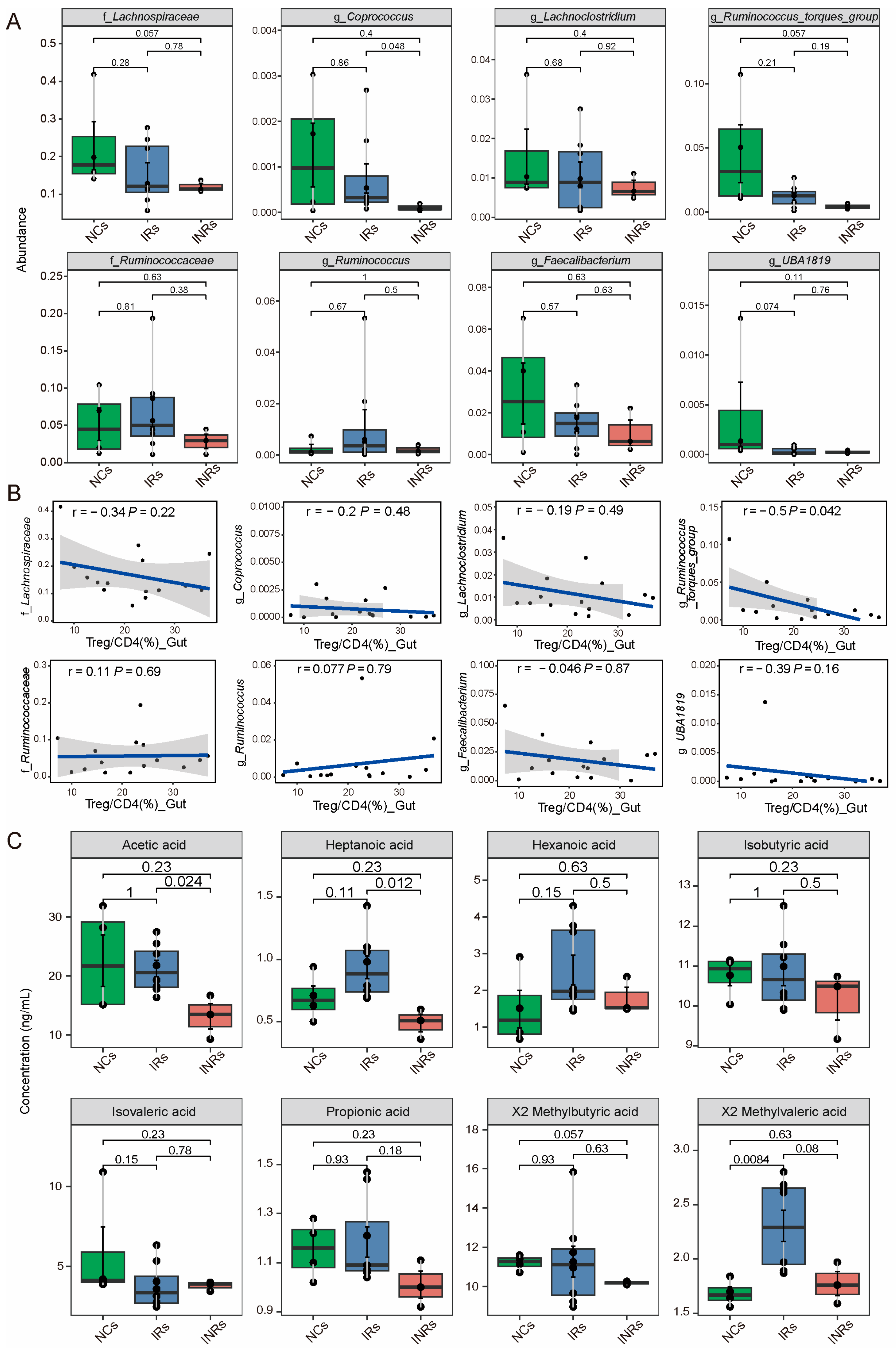
3.4. Reduced in the Abundance of SCFA-Producing Bacteria and SCFA Concentration in INRs
3.5. SCFA Stimulation Restores Treg Dysfunction
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foka, F.E.T.; Mufhandu, H.T. Current ARTs, Virologic Failure, and Implications for AIDS Management: A Systematic Review. Viruses 2023, 15, 1732. [Google Scholar] [CrossRef]
- Yang, X.; Su, B.; Zhang, X.; Liu, Y.; Wu, H.; Zhang, T. Incomplete immune reconstitution in HIV/AIDS patients on antiretroviral therapy: Challenges of immunological non-responders. J. Leukoc. Biol. 2020, 107, 597–612. [Google Scholar] [CrossRef]
- Zhao, H.; Feng, A.; Luo, D.; Yuan, T.; Lin, Y.-F.; Ling, X.; Zhong, H.; Li, J.; Li, L.; Zou, H. Factors associated with immunological non-response after ART initiation: A retrospective observational cohort study. BMC Infect. Dis. 2024, 24, 138. [Google Scholar] [CrossRef]
- Luo, S.; Wang, N. Human Immunodeficiency Virus/Acquired Immunodeficiency Syndrome Prevention and Control in China: Statistics, Challenges, and the Road Ahead. Infect. Dis. Immun. 2024, 4, 149–151. [Google Scholar] [CrossRef]
- Thompson, C.G.; Gay, C.L.; Kashuba, A.D.M. HIV Persistence in Gut-Associated Lymphoid Tissues: Pharmacological Challenges and Opportunities. AIDS Res. Hum. Retroviruses 2017, 33, 513–523. [Google Scholar] [CrossRef]
- Battegay, M.; Nüesch, R.; Hirschel, B.; Kaufmann, G.R. Immunological recovery and antiretroviral therapy in HIV-1 infection. Lancet Infect. Dis. 2006, 6, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.W.; Sereti, I. Residual immune dysfunction under antiretroviral therapy. Semin. Immunol. 2021, 51, 101471. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Kong, D.; Yang, X.; Zhang, T.; Kuang, Y.-Q. Mucosal-associated invariant T cells: A cryptic coordinator in HIV-infected immune reconstitution. J. Med. Virol. 2022, 94, 3043–3053. [Google Scholar] [CrossRef] [PubMed]
- Crum-Cianflone, N.F. HIV and the Gastrointestinal Tract. Infect. Dis. Clin. Pract. (Balt. Md) 2010, 18, 283–285. [Google Scholar] [CrossRef]
- Di Vincenzo, F.; Del Gaudio, A.; Petito, V.; Lopetuso, L.R.; Scaldaferri, F. Gut microbiota, intestinal permeability, and systemic inflammation: A narrative review. Intern. Emerg. Med. 2024, 19, 275–293. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.-D.; Serino, M.; Tilg, H.; Watson, A.; Wells, J.M. Intestinal permeability—A new target for disease prevention and therapy. BMC Gastroenterol. 2014, 14, 189. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.-Y.; Guo, Y.-T.; Wang, Z.-R.; Jiao, Y.-M.; Hu, Y.; Fan, L.-N.; Cheng, R.-Q.; Qu, M.-M.; Zhang, C.; Song, J.-W.; et al. Severe intestinal barrier damage in HIV-infected immunological non-responders. Heliyon 2023, 9, e20790. [Google Scholar] [CrossRef]
- Liu, J.; Ding, C.; Shi, Y.; Wang, Y.; Zhang, X.; Huang, L.; Fang, Q.; Shuai, C.; Gao, Y.; Wu, J. Advances in Mechanism of HIV-1 Immune Reconstitution Failure: Understanding Lymphocyte Subpopulations and Interventions for Immunological Nonresponders. J. Immunol. 2024, 212, 1609–1620. [Google Scholar] [CrossRef]
- Guo, Y.-T.; Guo, X.-Y.; Fan, L.-N.; Wang, Z.-R.; Qu, M.-M.; Zhang, C.; Fan, X.; Song, J.-W.; Xu, W.; Yang, B.-P.; et al. The Imbalance Between Intestinal Th17 and Treg Cells Is Associated with an Incomplete Immune Reconstitution During Long-Term Antiretroviral Therapy in Patients with HIV. Viral Immunol. 2023, 36, 331–342. [Google Scholar] [CrossRef]
- Ferrari, B.; Da Silva, A.C.; Liu, K.H.; Saidakova, E.V.; Korolevskaya, L.B.; Shmagel, K.V.; Shive, C.; Sanchez, G.P.; Retuerto, M.; Sharma, A.A.; et al. Gut-derived bacterial toxins impair memory CD4+ T cell mitochondrial function in HIV-1 infection. J. Clin. Investig. 2022, 132, e149571. [Google Scholar] [CrossRef]
- Sainz, T.; Diaz, L.; Rojo, D.; Clemente, M.I.; Barbas, C.; Gosalbes, M.J.; Jimenez-Hernandez, N.; Escosa, L.; Guillen, S.; Ramos, J.T.; et al. Targeting the Gut Microbiota of Vertically HIV-Infected Children to Decrease Inflammation and Immunoactivation: A Pilot Clinical Trial. Nutrients 2022, 14, 992. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M. Regulatory T cells and immune tolerance. Cell 2008, 133, 775–787. [Google Scholar] [CrossRef]
- Kleinman, A.J.; Sivanandham, R.; Pandrea, I.; Chougnet, C.A.; Apetrei, C. Regulatory T Cells As Potential Targets for HIV Cure Research. Front. Immunol. 2018, 9, 734. [Google Scholar] [CrossRef] [PubMed]
- Esmail Nia, G.; Mohammadi, M.; Sharifizadeh, M.; Ghalamfarsa, G.; Bolhassani, A. The role of T regulatory cells in the immunopathogenesis of HIV: Clinical implications. Braz. J. Infect. Dis. 2024, 28, 103866. [Google Scholar] [CrossRef]
- Min, B. Heterogeneity and Stability in Foxp3+ Regulatory T Cells. J. Interferon Cytokine Res. 2017, 37, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Shevyrev, D.; Tereshchenko, V. Treg Heterogeneity, Function, and Homeostasis. Front. Immunol. 2019, 10, 3100. [Google Scholar] [CrossRef]
- Omenetti, S.; Pizarro, T.T. The Treg/Th17 Axis: A Dynamic Balance Regulated by the Gut Microbiome. Front. Immunol. 2015, 6, 639. [Google Scholar] [CrossRef]
- Tan, J.; Taitz, J.; Sun, S.M.; Langford, L.; Ni, D.; Macia, L. Your Regulatory T Cells Are What You Eat: How Diet and Gut Microbiota Affect Regulatory T Cell Development. Front. Nutr. 2022, 9, 878382. [Google Scholar] [CrossRef]
- Cheng, H.; Guan, X.; Chen, D.; Ma, W. The Th17/Treg Cell Balance: A Gut Microbiota-Modulated Story. Microorganisms 2019, 7, 583. [Google Scholar] [CrossRef]
- Barbi, J.; Pardoll, D.; Pan, F. Treg functional stability and its responsiveness to the microenvironment. Immunol. Rev. 2014, 259, 115–139. [Google Scholar] [CrossRef] [PubMed]
- Okumura, R.; Takeda, K. Maintenance of intestinal homeostasis by mucosal barriers. Inflamm. Regen. 2018, 38, 5. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef]
- Cosovanu, C.; Neumann, C. The Many Functions of Foxp3(+) Regulatory T Cells in the Intestine. Front. Immunol. 2020, 11, 600973. [Google Scholar] [CrossRef]
- Oliver, A.J.; Huang, N.; Bartolome-Casado, R.; Li, R.; Koplev, S.; Nilsen, H.R.; Moy, M.; Cakir, B.; Polanski, K.; Gudiño, V.; et al. Single-cell integration reveals metaplasia in inflammatory gut diseases. Nature 2024, 635, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Schaefer, C.; Jiang, W.; Lu, Z.; Lee, J.; Sziraki, A.; Abdulraouf, A.; Wick, B.; Haeussler, M.; Li, Z.; et al. A panoramic view of cell population dynamics in mammalian aging. Science 2025, 387, eadn3949. [Google Scholar] [CrossRef]
- Pinglay, S.; Lalanne, J.-B.; Daza, R.M.; Kottapalli, S.; Quaisar, F.; Koeppel, J.; Garge, R.K.; Li, X.; Lee, D.S.; Shendure, J. Multiplex generation and single-cell analysis of structural variants in mammalian genomes. Science 2025, 387, eado5978. [Google Scholar] [CrossRef]
- Liu, H.; Li, Y.; Karsidag, M.; Tu, T.; Wang, P. Technical and Biological Biases in Bulk Transcriptomic Data Mining for Cancer Research. J. Cancer 2025, 16, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, G.; Tincati, C.; Silvestri, G. Microbial translocation in the pathogenesis of HIV infection and AIDS. Clin. Microbiol. Rev. 2013, 26, 2–18. [Google Scholar] [CrossRef]
- Nganou-Makamdop, K.; Talla, A.; Sharma, A.A.; Darko, S.; Ransier, A.; Laboune, F.; Chipman, J.G.; Beilman, G.J.; Hoskuldsson, T.; Fourati, S.; et al. Translocated microbiome composition determines immunological outcome in treated HIV infection. Cell 2021, 184, 3899–3914.e16. [Google Scholar] [CrossRef]
- Guo, X.; Wang, Z.; Qu, M.; Guo, Y.; Yu, M.; Hong, W.; Zhang, C.; Fan, X.; Song, J.; Xu, R.; et al. Abnormal blood microbiota profiles are associated with inflammation and immune restoration in HIV/AIDS individuals. mSystems 2023, 8, e0046723. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly, Y.M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef]
- Zeng, H.; Chi, H. Metabolic control of regulatory T cell development and function. Trends Immunol. 2015, 36, 3–12. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, W.; Qin, H.; Chen, Z.; Zhou, Y.; Zhou, Z.; Wang, J.; Wang, K. Astragalus polysaccharides alleviate DSS-induced ulcerative colitis in mice by restoring SCFA production and regulating Th17/Treg cell homeostasis in a microbiota-dependent manner. Carbohydr. Polym. 2025, 349, 122829. [Google Scholar] [CrossRef]
- van der Hee, B.; Wells, J.M. Microbial Regulation of Host Physiology by Short-chain Fatty Acids. Trends Microbiol. 2021, 29, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Gaardbo, J.C.; Hartling, H.J.; Ronit, A.; Springborg, K.; Gjerdrum, L.M.R.; Ralfkiær, E.; Thorsteinsson, K.; Ullum, H.; Andersen, Å.B.; Nielsen, S.D. Regulatory T cells in HIV-infected immunological nonresponders are increased in blood but depleted in lymphoid tissue and predict immunological reconstitution. J. Acquir. Immune Defic. Syndr. 2014, 66, 349–357. [Google Scholar] [CrossRef]
- Marchetti, G.; Gazzola, L.; Trabattoni, D.; Bai, F.; Ancona, G.; Ferraris, L.; Meroni, L.; Galli, M.; Clerici, M.; Gori, A.; et al. Skewed T-cell maturation and function in HIV-infected patients failing CD4+ recovery upon long-term virologically suppressive HAART. Aids 2010, 24, 1455–1460. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhen, C.; Guo, X.; Qu, M.; Zhang, C.; Song, J.; Fan, X.; Huang, H.; Xu, R.; Zhang, J.; et al. Landscape of gut mucosal immune cells showed gap of follicular or memory B cells into plasma cells in immunological non-responders. Clin. Transl. Med. 2024, 14, e1699. [Google Scholar] [CrossRef]
- Qu, M.-M.; Jiao, Y.-M. Think twice: How single-cell suspensions from solid tissues for single-cell transcriptomics are well prepared. Infect. Dis. Immun. 2025, 5, 72–74. [Google Scholar] [CrossRef]
- Hu, C.; Li, T.; Xu, Y.; Zhang, X.; Li, F.; Bai, J.; Chen, J.; Jiang, W.; Yang, K.; Ou, Q.; et al. CellMarker 2.0: An updated database of manually curated cell markers in human/mouse and web tools based on scRNA-seq data. Nucleic Acids Res. 2022, 51, D870–D876. [Google Scholar] [CrossRef]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Raeber, M.E.; Caspar, D.P.; Zurbuchen, Y.; Guo, N.; Schmid, J.; Michler, J.; Martin, A.C.; Steiner, U.C.; Moor, A.E.; Koning, F.; et al. Interleukin-2 immunotherapy reveals human regulatory T cell subsets with distinct functional and tissue-homing characteristics. Immunity 2024, 57, 2232–2250.e10. [Google Scholar] [CrossRef]
- Zorn, E.; Nelson, E.A.; Mohseni, M.; Porcheray, F.; Kim, H.; Litsa, D.; Bellucci, R.; Raderschall, E.; Canning, C.; Soiffer, R.J.; et al. IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood 2006, 108, 1571–1579. [Google Scholar] [CrossRef] [PubMed]
- Luu, M.; Riester, Z.; Baldrich, A.; Reichardt, N.; Yuille, S.; Busetti, A.; Klein, M.; Wempe, A.; Leister, H.; Raifer, H.; et al. Microbial short-chain fatty acids modulate CD8(+) T cell responses and improve adoptive immunotherapy for cancer. Nat. Commun. 2021, 12, 4077. [Google Scholar] [CrossRef]
- Thome, C.D.; Tausche, P.; Hohenberger, K.; Yang, Z.; Krammer, S.; Trufa, D.I.; Sirbu, H.; Schmidt, J.; Finotto, S. Short-chain fatty acids induced lung tumor cell death and increased peripheral blood CD4+ T cells in NSCLC and control patients ex vivo. Front. Immunol. 2024, 15, 1328263. [Google Scholar] [CrossRef]
- Ramanan, D.; Pratama, A.; Zhu, Y.; Venezia, O.; Sassone-Corsi, M.; Chowdhary, K.; Galván-Peña, S.; Sefik, E.; Brown, C.; Gélineau, A.; et al. Regulatory T cells in the face of the intestinal microbiota. Nat. Rev. Immunol. 2023, 23, 749–762. [Google Scholar] [CrossRef]
- Sereti, I.; Verburgh, M.L.; Gifford, J.; Lo, A.; Boyd, A.; Verheij, E.; Verhoeven, A.; Wit, F.W.; van der Loeff, M.F.S.; Giera, M.; et al. Impaired gut microbiota-mediated short-chain fatty acid production precedes morbidity and mortality in people with HIV. Cell Rep. 2023, 42, 113336. [Google Scholar] [CrossRef]
- Kempkes, R.W.M.; Joosten, I.; Koenen, H.J.P.; He, X. Metabolic Pathways Involved in Regulatory T Cell Functionality. Front. Immunol. 2019, 10, 2839. [Google Scholar] [CrossRef]
- Yan, Y.; Huang, L.; Liu, Y.; Yi, M.; Chu, Q.; Jiao, D.; Wu, K. Metabolic profiles of regulatory T cells and their adaptations to the tumor microenvironment: Implications for antitumor immunity. J. Hematol. Oncol. 2022, 15, 104. [Google Scholar] [CrossRef]
- Seguí-Pérez, A.; Castillo-González, R.; Sancho-Temiño, L.; Cruz-Adalia, A. Newly identified cell types crucial for gut commensal tolerance. Trends Cell Biol. 2025, 35, 186–189. [Google Scholar] [CrossRef]
- Tariq, M.; Gallien, S.; Surenaud, M.; Wiedemann, A.; Jean-Louis, F.; Lacabaratz, C.; Zaragoza, J.L.L.; Zeitoun, J.-D.; Ysmail-Dalhouk, S.; Lelièvre, J.-D.; et al. Profound Defect of Amphiregulin Secretion by Regulatory T Cells in the Gut of HIV-Treated Patients. J. Immunol. 2022, 208, 2300–2308. [Google Scholar] [CrossRef]
- Wang, J.; Hou, Y.; Mu, L.; Yang, M.; Ai, X. Gut microbiota contributes to the intestinal and extraintestinal immune homeostasis by balancing Th17/Treg cells. Int. Immunopharmacol. 2024, 143, 113570. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, F.; Zhang, R.; Shen, Y.; Liu, L.; Wang, J.; Yang, J.; Tang, Q.; Xun, J.; Qi, T.; et al. Changes in intestinal microbiota in HIV-1-infected subjects following cART initiation: Influence of CD4+ T cell count. Emerg. Microbes Infect. 2018, 7, 113. [Google Scholar] [CrossRef]
- Brenchley, J.M.; Serrano-Villar, S. From dysbiosis to defense: Harnessing the gut microbiome in HIV/SIV therapy. Microbiome 2024, 12, 113. [Google Scholar] [CrossRef] [PubMed]
- d’Ettorre, G.; Rossi, G.; Scagnolari, C.; Andreotti, M.; Giustini, N.; Serafino, S.; Schietroma, I.; Scheri, G.C.; Fard, S.N.; Trinchieri, V.; et al. Probiotic supplementation promotes a reduction in T-cell activation, an increase in Th17 frequencies, and a recovery of intestinal epithelium integrity and mitochondrial morphology in ART-treated HIV-1-positive patients. Immun. Inflamm. Dis. 2017, 5, 244–260. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.M.; A Klase, Z.; DiNapoli, S.R.; Vujkovic-Cvijin, I.; Carmack, K.; Perkins, M.R.; Calantone, N.; Vinton, C.L.; E Riddick, N.; Gallagher, J.; et al. IL-21 and probiotic therapy improve Th17 frequencies, microbial translocation, and microbiome in ARV-treated, SIV-infected macaques. Mucosal Immunol. 2016, 9, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Villar, S.; Vázquez-Castellanos, J.F.; Vallejo, A.; Latorre, A.; Sainz, T.; Ferrando-Martínez, S.; Rojo, D.; Martínez-Botas, J.; del Romero, J.; Madrid, N.; et al. The effects of prebiotics on microbial dysbiosis, butyrate production and immunity in HIV-infected subjects. Mucosal Immunol. 2017, 10, 1279–1293. [Google Scholar] [CrossRef]
- Yang, Q.; Zaongo, S.D.; Zhu, L.; Yan, J.; Yang, J.; Ouyang, J. The Potential of Clostridium butyricum to Preserve Gut Health, and to Mitigate Non-AIDS Comorbidities in People Living with HIV. Probiotics Antimicrob. Proteins 2024, 16, 1465–1482. [Google Scholar] [CrossRef] [PubMed]
- Younes, S.-A.; Talla, A.; Ribeiro, S.P.; Saidakova, E.V.; Korolevskaya, L.B.; Shmagel, K.V.; Shive, C.L.; Freeman, M.L.; Panigrahi, S.; Zweig, S.; et al. Cycling CD4+ T cells in HIV-infected immune nonresponders have mitochondrial dysfunction. J. Clin. Investig. 2018, 128, 5083–5094. [Google Scholar] [CrossRef]
- Girard, A.P.; Vergnon-Miszczycha, D.; Depincé-Berger, A.-E.; Roblin, X.; Lutch, F.; Lambert, C.; Rochereau, N.; Bourlet, T.; Genin, C.; Paul, S. Brief Report: A High Rate of β7+ Gut-Homing Lymphocytes in HIV-Infected Immunological Nonresponders is Associated with Poor CD4 T-Cell Recovery During Suppressive HAART. J. Acquir. Immune Defic. Syndr. 2016, 72, 259–265. [Google Scholar] [CrossRef]
- Peng, Q.; Wang, H.; Wang, H.; Li, X.; Lu, X.; Liu, L.; Zhou, B.; Chen, Z. Imbalances of gut-homing CD4+ T-cell subsets in HIV-1-infected Chinese patients. J. Acquir. Immune Defic. Syndr. 2013, 64, 25–31. [Google Scholar] [CrossRef]
- Mudd, J.C.; Brenchley, J.M. Gut Mucosal Barrier Dysfunction, Microbial Dysbiosis, and Their Role in HIV-1 Disease Progression. J. Infect. Dis. 2016, 214, S58–S66. [Google Scholar] [CrossRef]
- Yero, A.; Farnos, O.; Rabezanahary, H.; Racine, G.; Estaquier, J.; Jenabian, M.A. Differential Dynamics of Regulatory T-Cell and Th17 Cell Balance in Mesenteric Lymph Nodes and Blood following Early Antiretroviral Initiation during Acute Simian Immunodeficiency Virus Infection. J. Virol. 2019, 93, e00371-19. [Google Scholar] [CrossRef]
- Moreno-Fernandez, M.E.; Presicce, P.; Chougnet, C.A. Homeostasis and function of regulatory T cells in HIV/SIV infection. J. Virol. 2012, 86, 10262–10269. [Google Scholar] [CrossRef]
- Valverde-Villegas, J.M.; Matte, M.C.C.; de Medeiros, R.M.; Chies, J.A.B. New Insights about Treg and Th17 Cells in HIV Infection and Disease Progression. J. Immunol. Res. 2015, 2015, 647916. [Google Scholar] [CrossRef] [PubMed]
- Rueda, C.M.; Velilla, P.A.; Chougnet, C.A.; Rugeles, M.T. Incomplete normalization of regulatory t-cell frequency in the gut mucosa of Colombian HIV-infected patients receiving long-term antiretroviral treatment. PLoS ONE 2013, 8, e71062. [Google Scholar] [CrossRef] [PubMed]
- Oldenhove, G.; de Heusch, M.; Urbain-Vansanten, G.; Urbain, J.; Maliszewski, C.; Leo, O.; Moser, M. CD4+ CD25+ regulatory T cells control T helper cell type 1 responses to foreign antigens induced by mature dendritic cells in vivo. J. Exp. Med. 2003, 198, 259–266. [Google Scholar] [CrossRef]
- Oparaugo, N.C.; Ouyang, K.; Nguyen, N.P.N.; Nelson, A.M.; Agak, G.W. Human Regulatory T Cells: Understanding the Role of Tregs in Select Autoimmune Skin Diseases and Post-Transplant Nonmelanoma Skin Cancers. Int. J. Mol. Sci. 2023, 24, 1527. [Google Scholar] [CrossRef]
- Ma, S.; Ming, Y.; Wu, J.; Cui, G. Cellular metabolism regulates the differentiation and function of T-cell subsets. Cell Mol. Immunol. 2024, 21, 419–435. [Google Scholar] [CrossRef]
- Huang, C.; Shen, Y.; Shen, M.; Fan, X.; Men, R.; Ye, T.; Yang, L. Glucose Metabolism Reprogramming of Regulatory T Cells in Concanavalin A-Induced Hepatitis. Front. Pharmacol. 2021, 12, 726128. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Luo, F.; Yu, H.; Yan, Y.; Gong, Y.; Xie, C.; Cheng, L. Flux Balance Analysis Reveals Potential Anti–HIV-1 Metabolic Targets. Infect. Dis. Immun. 2024, 4, 61–68. [Google Scholar] [CrossRef]
- Mann, E.R.; Lam, Y.K.; Uhlig, H.H. Short-chain fatty acids: Linking diet, the microbiome and immunity. Nat. Rev. Immunol. 2024, 24, 577–595. [Google Scholar] [CrossRef] [PubMed]
- Wahl, A.; Yao, W.; Liao, B.; Chateau, M.; Richardson, C.; Ling, L.; Franks, A.; Senthil, K.; Doyon, G.; Li, F.; et al. A germ-free humanized mouse model shows the contribution of resident microbiota to human-specific pathogen infection. Nat. Biotechnol. 2024, 42, 905–915. [Google Scholar] [CrossRef]


| NCs (n = 4) | IRs (n = 8) | INRs (n = 3) | p_Value | |
|---|---|---|---|---|
| Age (years) | 45 (39–46) | 40 (30–48) | 45 (40–49) | 0.497 |
| Gender (Male/Female) | 4/0 | 8/0 | 3/0 | |
| CD4+ T cell count (cells/µL) | 919 (844–980) | 861 (730–1006) | 135 (133–138) | 0.0206 * |
| CD8+ T cell count (cells/µL) | 471 (424–527) | 1033 (877–1370) | 482 (479–597) | 0.0015 ** |
| CD4/CD8 ratio | 2.01 (1.83–2.13) | 0.79 (0.73–0.92) | 0.27 (0.24–0.28) | <0.0001 **** |
| Nadir CD4+ T cell count (cells/µL) | - | 352 (299–382) | 24 (10–53) | 0.0121 * |
| ART duration (years) | - | 4 (3–6) | 4(3–5) | 0.63 |
| ART regimen (%) | 1 | |||
| 2NRTIs+1NNRTIs | - | 5 (62.5%) | 2 (66.7%) | |
| 2NRTIs+1INSTIs | - | 3 (37.5%) | 1 (33.3%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, M.; Qu, M.; Wang, Z.; Zhen, C.; Yang, B.; Zhang, Y.; Huang, H.; Zhang, C.; Song, J.; Fan, X.; et al. Dysfunction and Metabolic Reprogramming of Gut Regulatory T Cells in HIV-Infected Immunological Non-Responders. Cells 2025, 14, 1164. https://doi.org/10.3390/cells14151164
Yu M, Qu M, Wang Z, Zhen C, Yang B, Zhang Y, Huang H, Zhang C, Song J, Fan X, et al. Dysfunction and Metabolic Reprogramming of Gut Regulatory T Cells in HIV-Infected Immunological Non-Responders. Cells. 2025; 14(15):1164. https://doi.org/10.3390/cells14151164
Chicago/Turabian StyleYu, Minrui, Mengmeng Qu, Zerui Wang, Cheng Zhen, Baopeng Yang, Yi Zhang, Huihuang Huang, Chao Zhang, Jinwen Song, Xing Fan, and et al. 2025. "Dysfunction and Metabolic Reprogramming of Gut Regulatory T Cells in HIV-Infected Immunological Non-Responders" Cells 14, no. 15: 1164. https://doi.org/10.3390/cells14151164
APA StyleYu, M., Qu, M., Wang, Z., Zhen, C., Yang, B., Zhang, Y., Huang, H., Zhang, C., Song, J., Fan, X., Xu, R., Jiao, Y.-M., & Wang, F.-S. (2025). Dysfunction and Metabolic Reprogramming of Gut Regulatory T Cells in HIV-Infected Immunological Non-Responders. Cells, 14(15), 1164. https://doi.org/10.3390/cells14151164









