The Effect of Ursodeoxycholic Acid (UDCA) on Serum Expression of miR-34a and miR-506 in Patients with Chronic Cholestatic Liver Diseases
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Cell Culture
2.3. Single-Cell Sequencing
2.4. Immunocytochemistry
2.5. ELISA Analyses
2.6. Isolation of RNA and Quantification miRNA and mRNA
2.7. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tanaka, A.; Ma, X.; Takahashi, A.; Vierling, J.M. Primary biliary cholangitis. Lancet 2024, 404, 1053–1066. [Google Scholar] [CrossRef] [PubMed]
- Dyson, J.K.; Beuers, U.; Jones, D.E.J.; Lohse, A.W.; Hudson, M. Primary sclerosing cholangitis. Lancet 2018, 391, 2547–2559. [Google Scholar] [CrossRef] [PubMed]
- Lindor, K.D. New treatment strategies for primary sclerosing cholangitis. Dig Dis. 2011, 29, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Lindor, K.D.; Dickson, E.R.; Baldus, W.P.; Jorgensen, R.A.; Ludwig, J.; Murtaugh, P.A.; Harrison, J.M.; Wiesner, R.H.; Anderson, M.L.; Lange, S.M.; et al. Ursodeoxycholic acid in the treatment of primary biliary cirrhosis. Gastroenterology 1994, 106, 1284–1290. [Google Scholar] [CrossRef]
- Poupon, R.; Chretien, Y.; Poupon, R.E.; Ballet, F.; Calmus, Y.; Darnis, F. Is ursodeoxycholic acid an effective treatment for primary biliary cirrhosis? Lancet 1987, 1, 834–836. [Google Scholar] [CrossRef]
- Jopson, L.; Dyson, J.K.; Jones, D.E. Understanding and Treating Fatigue in Primary Biliary Cirrhosis and Primary Sclerosing Cholangitis. Clin. Liver Dis. 2016, 20, 131–142. [Google Scholar] [CrossRef]
- Ljubuncic, P.; Fuhrman, B.; Oiknine, J.; Aviram, M.; Bomzon, A. Effect of deoxycholic acid and ursodeoxycholic acid on lipid peroxidation in cultured macrophages. Gut 1996, 39, 475–478. [Google Scholar] [CrossRef]
- Paumgartner, G.; Beuers, U. Ursodeoxycholic acid in cholestatic liver disease: Mechanisms of action and therapeutic use revisited. Hepatology 2002, 36, 525–531. [Google Scholar] [CrossRef]
- Padgett, K.A.; Lan, R.Y.; Leung, P.C.; Lleo, A.; Dawson, K.; Pfeiff, J.; Mao, T.K.; Coppel, R.L.; Ansari, A.A.; Gershwin, M.E. Primary biliary cirrhosis is associated with altered hepatic microRNA expression. J. Autoimmun. 2009, 32, 246–253. [Google Scholar] [CrossRef]
- Qin, B.; Huang, F.; Liang, Y.; Yang, Z.; Zhong, R. Analysis of altered microRNA expression profiles in peripheral blood mononuclear cells from patients with primary biliary cirrhosis. J. Gastroenterol. Hepatol. 2013, 28, 543–550. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Huo, W.; He, L.; Li, B.; Wang, H.; Meng, F.; Duan, C.; Zhou, B.; Wu, J.; et al. The Role of miRNA and Long Noncoding RNA in Cholestatic Liver Diseases. Am. J. Pathol. 2024, 194, 879–893. [Google Scholar] [CrossRef]
- Cermelli, S.; Ruggieri, A.; Marrero, J.A.; Ioannou, G.N.; Beretta, L. Circulating microRNAs in patients with chronic hepatitis C and non-alcoholic fatty liver disease. PLoS ONE 2011, 6, e23937. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, J.; He, L.; Zhang, F. MicroRNA-34a Promotes EMT and Liver Fibrosis in Primary Biliary Cholangitis by Regulating TGF-beta1/smad Pathway. J. Immunol. Res. 2021, 2021, 6890423. [Google Scholar] [CrossRef]
- Ostrycharz, E.; Wasik, U.; Kempinska-Podhorodecka, A.; Banales, J.M.; Milkiewicz, P.; Milkiewicz, M. Melatonin Protects Cholangiocytes from Oxidative Stress-Induced Proapoptotic and Proinflammatory Stimuli via miR-132 and miR-34. Int. J. Mol Sci. 2020, 21, 9667. [Google Scholar] [CrossRef] [PubMed]
- Banales, J.M.; Saez, E.; Uriz, M.; Sarvide, S.; Urribarri, A.D.; Splinter, P.; Tietz Bogert, P.S.; Bujanda, L.; Prieto, J.; Medina, J.F.; et al. Up-regulation of microRNA 506 leads to decreased Cl−/HCO3− anion exchanger 2 expression in biliary epithelium of patients with primary biliary cirrhosis. Hepatology 2012, 56, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Erice, O.; Munoz-Garrido, P.; Vaquero, J.; Perugorria, M.J.; Fernandez-Barrena, M.G.; Saez, E.; Santos-Laso, A.; Arbelaiz, A.; Jimenez-Aguero, R.; Fernandez-Irigoyen, J.; et al. MicroRNA-506 promotes primary biliary cholangitis-like features in cholangiocytes and immune activation. Hepatology 2018, 67, 1420–1440. [Google Scholar] [CrossRef]
- Jay, T.R.; von Saucken, V.E.; Landreth, G.E. TREM2 in Neurodegenerative Diseases. Mol Neurodegener. 2017, 12, 56. [Google Scholar] [CrossRef]
- Colonna, M. The biology of TREM receptors. Nat. Rev. Immunol. 2023, 23, 580–594. [Google Scholar] [CrossRef]
- Seki, E.; Schnabl, B. Role of innate immunity and the microbiota in liver fibrosis: Crosstalk between the liver and gut. J. Physiol. 2012, 590, 447–458. [Google Scholar] [CrossRef]
- Ramachandran, P. Iredale, J.P. Macrophages: Central regulators of hepatic fibrogenesis and fibrosis resolution. J. Hepatol. 2012, 56, 1417–1419. [Google Scholar] [CrossRef]
- Labiano, I.; Agirre-Lizaso, A.; Olaizola, P.; Echebarria, A.; Huici-Izagirre, M.; Olaizola, I.; Esparza-Baquer, A.; Sharif, O.; Hijona, E.; Milkiewicz, P.; et al. TREM-2 plays a protective role in cholestasis by acting as a negative regulator of inflammation. J. Hepatol. 2022, 77, 991–1004. [Google Scholar] [CrossRef]
- Ma, K.; Guo, S.; Li, J.; Wei, T.; Liang, T. Biological and clinical role of TREM2 in liver diseases. Hepatol. Commun. 2024, 8, e0578. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Zhao, Y.; Lukiw, W.J. Deficits in the miRNA-34a-regulated endogenous TREM2 phagocytosis sensor-receptor in Alzheimer’s disease (AD); an update. Front. Aging Neurosci. 2014, 6, 116. [Google Scholar] [CrossRef] [PubMed]
- Doggrell, S.A. TACE inhibition: A new approach to treating inflammation. Expert. Opin. Investig. Drugs. 2002, 11, 1003–1006. [Google Scholar] [CrossRef] [PubMed]
- Buryova, H.; Chalupsky, K.; Zbodakova, O.; Kanchev, I.; Jirouskova, M.; Gregor, M.; Sedlacek, R. Liver protective effect of ursodeoxycholic acid includes regulation of ADAM17 activity. BMC Gastroenterol. 2013, 13, 155. [Google Scholar] [CrossRef] [PubMed]
- Kazankov, K.; Barrera, F.; Moller, H.J.; Bibby, B.M.; Vilstrup, H.; George, J.; Gronbaek, H. Soluble CD163, a macrophage activation marker, is independently associated with fibrosis in patients with chronic viral hepatitis B and C. Hepatology 2014, 60, 521–530. [Google Scholar] [CrossRef]
- Kazankov, K.; Moller, H.J.; Lange, A.; Birkebaek, N.H.; Holland-Fischer, P.; Solvig, J.; Horlyck, A.; Kristensen, K.; Rittig, S.; Handberg, A.; et al. The macrophage activation marker sCD163 is associated with changes in NAFLD and metabolic profile during lifestyle intervention in obese children. Pediatr. Obes. 2015, 10, 226–233. [Google Scholar] [CrossRef]
- Kazankov, K.; Tordjman, J.; Moller, H.J.; Vilstrup, H.; Poitou, C.; Bedossa, P.; Bouillot, J.L.; Clement, K.; Gronbaek, H. Macrophage activation marker soluble CD163 and non-alcoholic fatty liver disease in morbidly obese patients undergoing bariatric surgery. J. Gastroenterol. Hepatol. 2015, 30, 1293–1300. [Google Scholar] [CrossRef]
- Sandahl, T.D.; Gronbaek, H.; Moller, H.J.; Stoy, S.; Thomsen, K.L.; Dige, A.K.; Agnholt, J.; Hamilton-Dutoit, S.; Thiel, S.; Vilstrup, H. Hepatic macrophage activation and the LPS pathway in patients with alcoholic hepatitis: A prospective cohort study. Am. J. Gastroenterol. 2014, 109, 1749–1756. [Google Scholar] [CrossRef]
- Gronbaek, H.; Kreutzfeldt, M.; Kazankov, K.; Jessen, N.; Sandahl, T.; Hamilton-Dutoit, S.; Vilstrup, H.; Moller, J. Single-centre experience of the macrophage activation marker soluble (s)CD. Aliment. Pharmacol. Ther. 2016, 44, 1062–1070. [Google Scholar] [CrossRef]
- Bossen, L.; Lau, T.S.; Nielsen, M.B.; Nielsen, M.C.; Andersen, A.H.; Ott, P.; Becker, S.; Glerup, H.; Svenningsen, L.; Eivindson, M.; et al. The association between soluble CD163, disease severity, and ursodiol treatment in patients with primary biliary cholangitis. Hepatol. Commun. 2023, 7, e0068. [Google Scholar] [CrossRef]
- Bossen, L.; Rebora, P.; Bernuzzi, F.; Jepsen, P.; Gerussi, A.; Andreone, P.; Galli, A.; Terziroli, B.; Alvaro, D.; Labbadia, G.; et al. Soluble CD163 and mannose receptor as markers of liver disease severity and prognosis in patients with primary biliary cholangitis. Liver Int. 2020, 40, 1408–1414. [Google Scholar] [CrossRef]
- EASL Clinical Practice Guidelines: Management of cholestatic liver diseases. J. Hepatol. 2009, 51, 237–267. [CrossRef]
- Andrews, T.S.; Nakib, D.; Perciani, C.T.; Ma, X.Z.; Liu, L.; Winter, E.; Camat, D.; Chung, S.W.; Lumanto, P.; Manuel, J.; et al. Single-cell, single-nucleus, and spatial transcriptomics characterization of the immunological landscape in the healthy and PSC human liver. J. Hepatol. 2024, 80, 730–743. [Google Scholar] [CrossRef] [PubMed]
- MacParland, S.A.; Liu, J.C.; Ma, X.Z.; Innes, B.T.; Bartczak, A.M.; Gage, B.K.; Manuel, J.; Khuu, N.; Echeverri, J.; Linares, I.; et al. Single cell RNA sequencing of human liver reveals distinct intrahepatic macrophage populations. Nat. Commun. 2018, 9, 4383. [Google Scholar] [CrossRef] [PubMed]
- Wasik, U.; Milkiewicz, M.; Kempinska-Podhorodecka, A.; Milkiewicz, P. Protection against oxidative stress mediated by the Nrf2/Keap1 axis is impaired in Primary Biliary Cholangitis. Sci Rep. 2017, 7, 44769. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.E.; Ferreira, D.M.; Afonso, M.B.; Borralho, P.M.; Machado, M.V.; Cortez-Pinto, H.; Rodrigues, C.M. miR-34a/SIRT1/p53 is suppressed by ursodeoxycholic acid in the rat liver and activated by disease severity in human non-alcoholic fatty liver disease. J. Hepatol. 2013, 58, 119–125. [Google Scholar] [CrossRef]
- Mueller, M.; Castro, R.E.; Thorell, A.; Marschall, H.U.; Auer, N.; Herac, M.; Rodrigues, C.M.P.; Trauner, M. Ursodeoxycholic acid: Effects on hepatic unfolded protein response, apoptosis and oxidative stress in morbidly obese patients. Liver Int. 2018, 38, 523–531. [Google Scholar] [CrossRef]
- Feuerbach, D.; Schindler, P.; Barske, C.; Joller, S.; Beng-Louka, E.; Worringer, K.A.; Kommineni, S.; Kaykas, A.; Ho, D.J.; Ye, C.; et al. ADAM17 is the main sheddase for the generation of human triggering receptor expressed in myeloid cells (hTREM2) ectodomain and cleaves TREM2 after Histidine 157. Neurosci. Lett. 2017, 660, 109–114. [Google Scholar] [CrossRef]
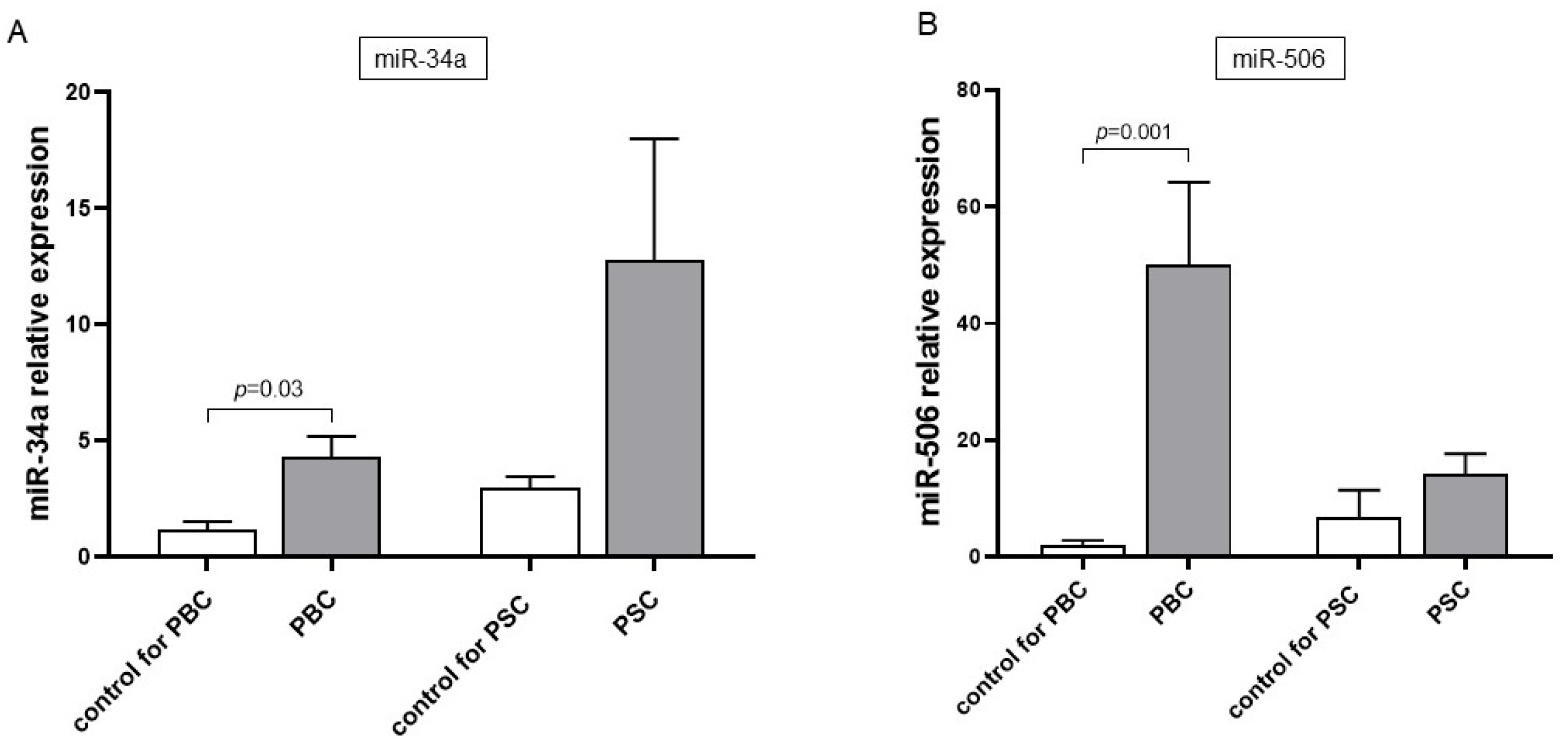


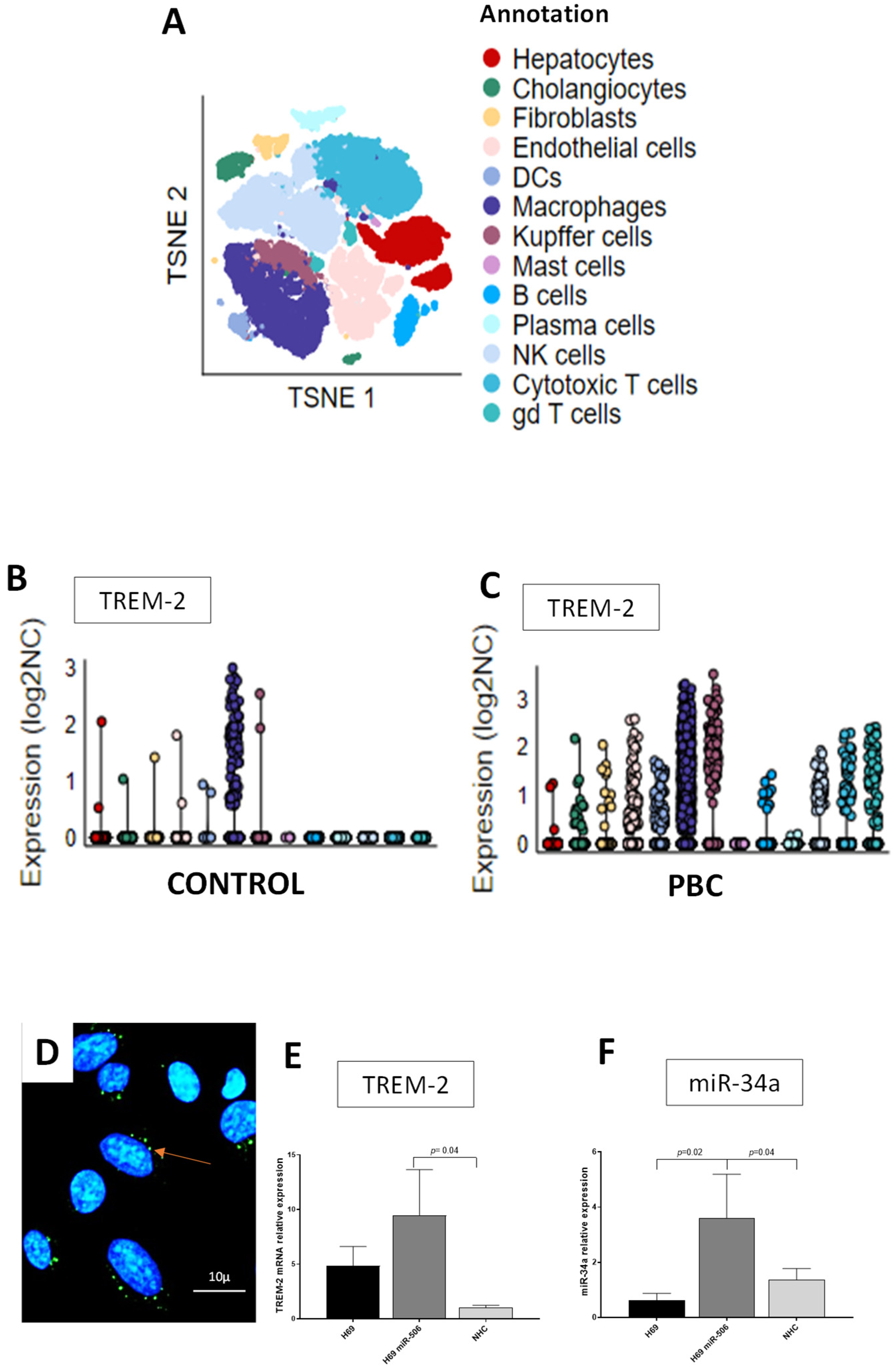

| Parameter | PBC | PSC | ||||
|---|---|---|---|---|---|---|
| Before UDCA (n = 27) | After UDCA (n = 11) | p Before vs. After | Before UDCA (n = 17) | After UDCA (n = 10) | p Before vs. After | |
| Gender (Male/Female) | 2/25 | 1/10 | 0.8 | 11/6 | 7/3 | 0.7 |
| Age (years) | 54 ± 11 | 58 ± 9 | NS | 36 ± 10 | 42 ± 9 | NS |
| GGTP (IU/L, NR male < 60, female < 35) | 287 ± 59 | 98 ± 16 | 0.003 | 498 ± 104 | 259 ± 78 | 0.007 |
| Bilirubin (mg/dL, NR 0.2–1.1) | 1.40 ± 0.5 | 0.77 ± 0.2 | 0.23 | 2.0 ± 0.8 | 0.67 ± 0.1 | 0.14 |
| ALP (IU/L, NR 30–120) | 240 ± 25 | 148 ± 18 | 0.0006 | 329 ± 69 | 289 ± 71 | 0.17 |
| AST (IU/L, NR 5–35) | 99 ± 37 | 41 ± 6 | 0.11 | 128 ± 53 | 55 ± 9 | 0.18 |
| Parameter | Control for PSC (n = 9) | Control for PBC (n = 16) | p PBC vs. PSC |
|---|---|---|---|
| Gender (Female/Male) | 2/7 | 15/1 | 0.0002 |
| Age (years) | 35 ± 1.2 | 52 ± 3.7 | 0.004 |
| GGTP (IU/I, NR male < 60, female < 35) | WNR | WNR | |
| Bilirubin (mg/dL, NR: 0.2–1.1) | WNR | WNR | |
| ALP (IU/L, NR 0.2–1.1) | WNR | WNR | |
| AST (IU/L, NR 5–35) | WNR | WNR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cielica, E.; Łaba, A.; Milkiewicz, P.; Kruk, B.; Kempinska-Podhorodecka, A.; Kłos, P.; Rodrigues, P.M.; Val, B.; Perugorria, M.J.; Banales, J.M.; et al. The Effect of Ursodeoxycholic Acid (UDCA) on Serum Expression of miR-34a and miR-506 in Patients with Chronic Cholestatic Liver Diseases. Cells 2025, 14, 1137. https://doi.org/10.3390/cells14151137
Cielica E, Łaba A, Milkiewicz P, Kruk B, Kempinska-Podhorodecka A, Kłos P, Rodrigues PM, Val B, Perugorria MJ, Banales JM, et al. The Effect of Ursodeoxycholic Acid (UDCA) on Serum Expression of miR-34a and miR-506 in Patients with Chronic Cholestatic Liver Diseases. Cells. 2025; 14(15):1137. https://doi.org/10.3390/cells14151137
Chicago/Turabian StyleCielica, Eliza, Alicja Łaba, Piotr Milkiewicz, Beata Kruk, Agnieszka Kempinska-Podhorodecka, Patrycja Kłos, Pedro M. Rodrigues, Beatriz Val, Maria J. Perugorria, Jesus M. Banales, and et al. 2025. "The Effect of Ursodeoxycholic Acid (UDCA) on Serum Expression of miR-34a and miR-506 in Patients with Chronic Cholestatic Liver Diseases" Cells 14, no. 15: 1137. https://doi.org/10.3390/cells14151137
APA StyleCielica, E., Łaba, A., Milkiewicz, P., Kruk, B., Kempinska-Podhorodecka, A., Kłos, P., Rodrigues, P. M., Val, B., Perugorria, M. J., Banales, J. M., & Milkiewicz, M. (2025). The Effect of Ursodeoxycholic Acid (UDCA) on Serum Expression of miR-34a and miR-506 in Patients with Chronic Cholestatic Liver Diseases. Cells, 14(15), 1137. https://doi.org/10.3390/cells14151137







