Nonmuscle Myosin-2B Regulates Apical Cortical Mechanics, ZO-1 Dynamics and Cell Size in MDCK Epithelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Model
2.2. Genome Engineering
2.3. Transfection and Exogenous Expression of Proteins and siRNA-Mediated Depletion
2.4. Immunofluorescence Microscopy Analysis
2.5. Atomic Force Microscopy (AFM)-Based Force Measurements
2.6. Fluorescence Recovery After Photobleaching (FRAP) Experiments
2.7. Immunoblot Analysis
2.8. Lumen Morphogenesis
2.9. Quantification and Statistical Analysis
3. Results
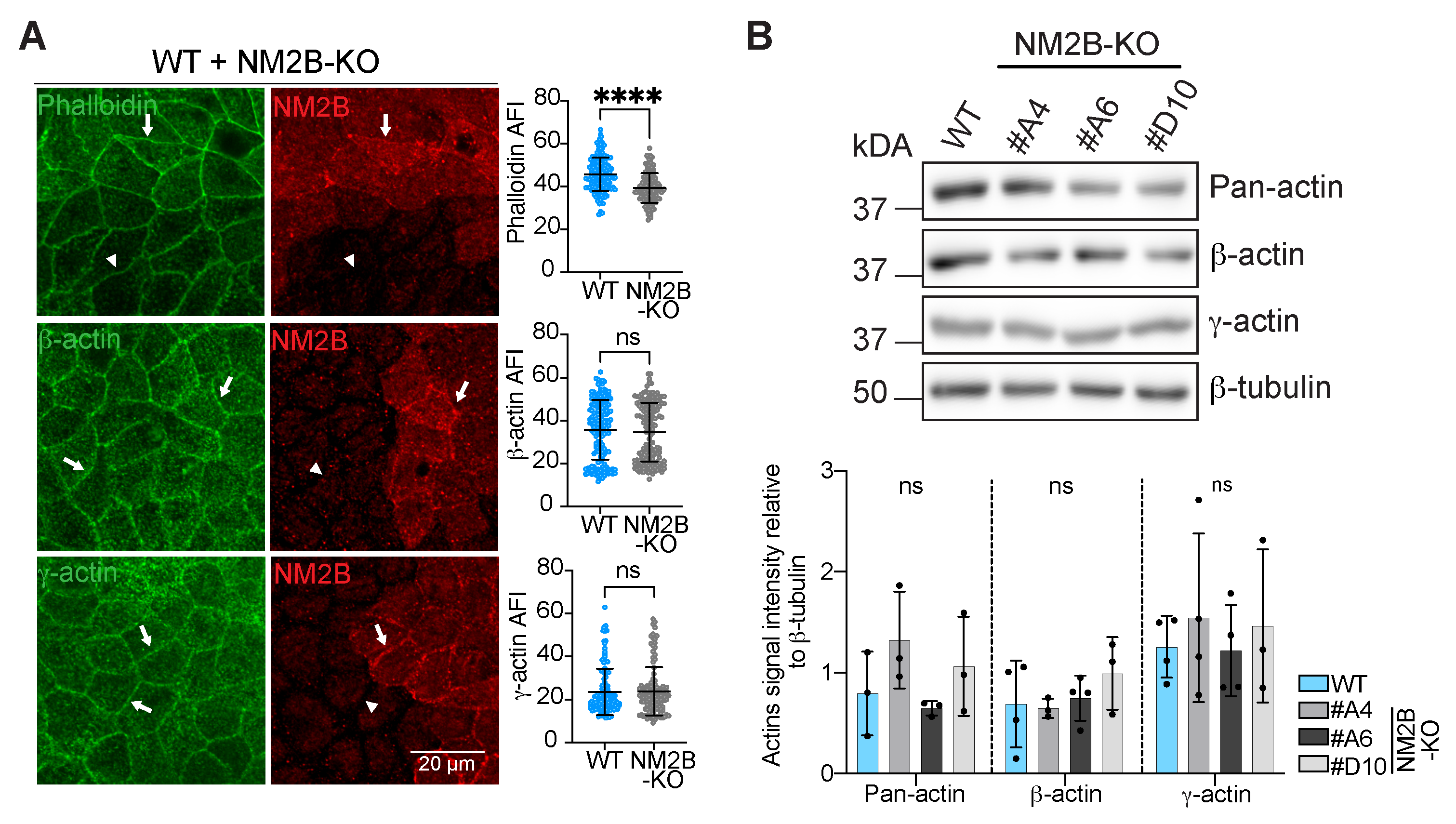
3.1. The Knock-Out of NM2B Results in Decreased Phalloidin and E-Cadherin Junctional Labeling
3.2. The Knock-Out of NM2B Reduces Apical Membrane Cortex Stiffness but Does Not Perturb TJ Membrane Tortuosity
3.3. The KO of NM2B Promotes Increased ZO-1 Exchange and Decreased Accumulation of ZO-1 at TJs
3.4. The KO of Either NM2B or γ-Actin Increases Cell Size in Cells Grown as Monolayers in 2D or Cysts in 3D
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AJ | Adherens Junction |
| IB | Immunoblot |
| IF | Immunofluorescence Microscopy |
| KO | Knock-Out |
| MDCK | Madin–Darby Canine Kidney |
| NM2 | Nonmuscle Myosin-2 |
| SD | Standard Deviation |
| TJ | Tight Junction |
| ZO | Zonula Occludens |
References
- Cartagena-Rivera, A.X.; Logue, J.S.; Waterman, C.M.; Chadwick, R.S. Actomyosin Cortical Mechanical Properties in Nonadherent Cells Determined by Atomic Force Microscopy. Biophys. J. 2016, 110, 2528–2539. [Google Scholar] [CrossRef] [PubMed]
- Chugh, P.; Paluch, E.K. The actin cortex at a glance. J. Cell Sci. 2018, 131, jcs186254. [Google Scholar] [CrossRef] [PubMed]
- Cartagena-Rivera, A.X.; Van Itallie, C.M.; Anderson, J.M.; Chadwick, R.S. Apical surface supracellular mechanical properties in polarized epithelium using noninvasive acoustic force spectroscopy. Nat. Commun. 2017, 8, 1030. [Google Scholar] [CrossRef] [PubMed]
- Milberg, O.; Shitara, A.; Ebrahim, S.; Masedunskas, A.; Tora, M.; Tran, D.T.; Chen, Y.; Conti, M.A.; Adelstein, R.S.; Ten Hagen, K.G.; et al. Concerted actions of distinct nonmuscle myosin II isoforms drive intracellular membrane remodeling in live animals. J. Cell Biol. 2017, 216, 1925–1936. [Google Scholar] [CrossRef] [PubMed]
- Bruckner, B.R.; Janshoff, A. Elastic properties of epithelial cells probed by atomic force microscopy. Biochim. Biophys. Acta 2015, 1853, 3075–3082. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Gundersen, G.G. Beyond polymer polarity: How the cytoskeleton builds a polarized cell. Nat. Rev. Mol. Cell Biol. 2008, 9, 860–873. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Takeichi, M. Adherens junction: Molecular architecture and regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a002899. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, L.S.; Fanning, A.S. Regulation of epithelial permeability by the actin cytoskeleton. Cytoskeleton 2011, 68, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Buckley, A.; Turner, J.R. Cell Biology of Tight Junction Barrier Regulation and Mucosal Disease. Cold Spring Harb. Perspect. Biol. 2018, 10, a029314. [Google Scholar] [CrossRef] [PubMed]
- Charras, G.; Yap, A.S. Tensile Forces and Mechanotransduction at Cell-Cell Junctions. Curr. Biol. 2018, 28, R445–R457. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.I.; Lechuga, S.; Marino-Melendez, A.; Naydenov, N.G. Unique and redundant functions of cytoplasmic actins and nonmuscle myosin II isoforms at epithelial junctions. Ann. N. Y. Acad. Sci. 2022, 1515, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, S.; Higashi, T.; Furuse, M. ZO-1 Knockout by TALEN-Mediated Gene Targeting in MDCK Cells: Involvement of ZO-1 in the Regulation of Cytoskeleton and Cell Shape. PLoS ONE 2014, 9, e104994. [Google Scholar] [CrossRef] [PubMed]
- Tang, V.W. Cell-cell adhesion interface: Orthogonal and parallel forces from contraction, protrusion, and retraction. F1000Research 2018, 7, F1000 Faculty Rev-1544. [Google Scholar] [CrossRef] [PubMed]
- Lynn, K.S.; Peterson, R.J.; Koval, M. Ruffles and spikes: Control of tight junction morphology and permeability by claudins. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183339. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Weber, C.R.; Turner, J.R. The tight junction protein complex undergoes rapid and continuous molecular remodeling at steady state. J. Cell Biol. 2008, 181, 683–695. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Marchiando, A.M.; Weber, C.R.; Raleigh, D.R.; Wang, Y.; Shen, L.; Turner, J.R. MLCK-dependent exchange and actin binding region-dependent anchoring of ZO-1 regulate tight junction barrier function. Proc. Natl. Acad. Sci. USA 2010, 107, 8237–8241. [Google Scholar] [CrossRef] [PubMed]
- Paschoud, S.; Yu, D.; Pulimeno, P.; Jond, L.; Turner, J.R.; Citi, S. Cingulin and paracingulin show similar dynamic behaviour, but are recruited independently to junctions. Mol. Membr. Biol. 2011, 28, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Mauperin, M.; Sun, Y.; Glandorf, T.; Oswald, T.A.; Klatt, N.; Geil, B.; Mutero-Maeda, A.; Méan, I.; Jond, L.; Janshoff, A.; et al. A feedback circuitry involving gamma-actin, beta-actin and non-muscle myosin 2A controls tight junction and apical cortex mechanics. figshare. Dataset. Nat. Commun. 2025, 16, 2514. [Google Scholar] [CrossRef] [PubMed]
- Simons, M.; Wang, M.; McBride, O.W.; Kawamoto, S.; Yamakawa, K.; Gdula, D.; Adelstein, R.S.; Weir, L. Human nonmuscle myosin heavy chains are encoded by two genes located on different chromosomes. Circ. Res. 1991, 69, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Maupin, P.; Phillips, C.L.; Adelstein, R.S.; Pollard, T.D. Differential localization of myosin-II isozymes in human cultured cells and blood cells. J. Cell Sci. 1994, 107 Pt 11, 3077–3090. [Google Scholar] [CrossRef] [PubMed]
- Tullio, A.N.; Accili, D.; Ferrans, V.J.; Yu, Z.X.; Takeda, K.; Grinberg, A.; Westphal, H.; Preston, Y.A.; Adelstein, R.S. Nonmuscle myosin II-B is required for normal development of the mouse heart. Proc. Natl. Acad. Sci. USA 1997, 94, 12407–12412. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Kovacs, M.; Hu, A.; Limouze, J.; Harvey, E.V.; Sellers, J.R. Kinetic mechanism of non-muscle myosin IIB: Functional adaptations for tension generation and maintenance. J. Biol. Chem. 2003, 278, 27439–27448. [Google Scholar] [CrossRef] [PubMed]
- Golomb, E.; Ma, X.; Jana, S.S.; Preston, Y.A.; Kawamoto, S.; Shoham, N.G.; Goldin, E.; Conti, M.A.; Sellers, J.R.; Adelstein, R.S. Identification and characterization of nonmuscle myosin II-C, a new member of the myosin II family. J. Biol. Chem. 2004, 279, 2800–2808. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.I.; Bachar, M.; Babbin, B.A.; Adelstein, R.S.; Nusrat, A.; Parkos, C.A. A unique role for nonmuscle myosin heavy chain IIA in regulation of epithelial apical junctions. PLoS ONE 2007, 2, e658. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Ma, X.; Liu, C.; Adelstein, R.S. Replacement of nonmuscle myosin II-B with II-A rescues brain but not cardiac defects in mice. J. Biol. Chem. 2007, 282, 22102–22111. [Google Scholar] [CrossRef] [PubMed]
- Babbin, B.A.; Koch, S.; Bachar, M.; Conti, M.A.; Parkos, C.A.; Adelstein, R.S.; Nusrat, A.; Ivanov, A.I. Non-muscle myosin IIA differentially regulates intestinal epithelial cell restitution and matrix invasion. Am. J. Pathol. 2009, 174, 436–448. [Google Scholar] [CrossRef] [PubMed]
- Billington, N.; Wang, A.; Mao, J.; Adelstein, R.S.; Sellers, J.R. Characterization of three full-length human nonmuscle myosin II paralogs. J. Biol. Chem. 2013, 288, 33398–33410. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A.; Takagi, Y.; Billington, N.; Sun, S.A.; Hong, D.K.; Homsher, E.; Wang, A.; Sellers, J.R. Kinetic characterization of nonmuscle myosin IIb at the single molecule level. J. Biol. Chem. 2013, 288, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Melli, L.; Billington, N.; Sun, S.A.; Bird, J.E.; Nagy, A.; Friedman, T.B.; Takagi, Y.; Sellers, J.R. Bipolar filaments of human nonmuscle myosin 2-A and 2-B have distinct motile and mechanical properties. eLife 2018, 7, e32871. [Google Scholar] [CrossRef] [PubMed]
- Naydenov, N.G.; Feygin, A.; Wang, D.; Kuemmerle, J.F.; Harris, G.; Conti, M.A.; Adelstein, R.S.; Ivanov, A.I. Nonmuscle Myosin IIA Regulates Intestinal Epithelial Barrier in vivo and Plays a Protective Role During Experimental Colitis. Sci. Rep. 2016, 6, 24161. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Manzanares, M.; Ma, X.; Adelstein, R.S.; Horwitz, A.R. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat. Rev. Mol. Cell Biol. 2009, 10, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Ma, X.; Conti, M.A.; Adelstein, R.S. Distinct and redundant roles of the non-muscle myosin II isoforms and functional domains. Biochem. Soc. Trans. 2011, 39, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Shutova, M.S.; Svitkina, T.M. Common and Specific Functions of Nonmuscle Myosin II Paralogs in Cells. Biochemistry 2018, 83, 1459–1468. [Google Scholar] [CrossRef] [PubMed]
- Sellers, J.R.; Heissler, S.M. Nonmuscle myosin-2 isoforms. Curr. Biol. 2019, 29, R275–R278. [Google Scholar] [CrossRef] [PubMed]
- Quintanilla, M.A.; Hammer, J.A.; Beach, J.R. Non-muscle myosin 2 at a glance. J. Cell Sci. 2023, 136, jcs260890. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Casado, M.; Asensio-Juarez, G.; Talayero, V.C.; Vicente-Manzanares, M. Engines of change: Nonmuscle myosin II in mechanobiology. Curr. Opin. Cell Biol. 2024, 87, 102344. [Google Scholar] [CrossRef] [PubMed]
- Shutova, M.S.; Spessott, W.A.; Giraudo, C.G.; Svitkina, T. Endogenous species of mammalian nonmuscle myosin IIA and IIB include activated monomers and heteropolymers. Curr. Biol. 2014, 24, 1958–1968. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, S.; Fujita, T.; Millis, B.A.; Kozin, E.; Ma, X.; Kawamoto, S.; Baird, M.A.; Davidson, M.; Yonemura, S.; Hisa, Y.; et al. NMII forms a contractile transcellular sarcomeric network to regulate apical cell junctions and tissue geometry. Curr. Biol. 2013, 23, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Smutny, M.; Cox, H.L.; Leerberg, J.M.; Kovacs, E.M.; Conti, M.A.; Ferguson, C.; Hamilton, N.A.; Parton, R.G.; Adelstein, R.S.; Yap, A.S. Myosin II isoforms identify distinct functional modules that support integrity of the epithelial zonula adherens. Nat. Cell Biol. 2010, 12, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Efimova, N.; Svitkina, T.M. Branched actin networks push against each other at adherens junctions to maintain cell-cell adhesion. J. Cell Biol. 2018, 217, 1827–1845. [Google Scholar] [CrossRef] [PubMed]
- Heuze, M.L.; Sankara Narayana, G.H.N.; D’Alessandro, J.; Cellerin, V.; Dang, T.; Williams, D.S.; Van Hest, J.C.; Marcq, P.; Mege, R.M.; Ladoux, B. Myosin II isoforms play distinct roles in adherens junction biogenesis. eLife 2019, 8, e46599. [Google Scholar] [CrossRef] [PubMed]
- Rouaud, F.; Huang, W.; Flinois, A.; Jain, K.; Vasileva, E.; Di Mattia, T.; Mauperin, M.; Parry, D.A.D.; Dugina, V.; Chaponnier, C.; et al. Cingulin and paracingulin tether myosins-2 to junctions to mechanoregulate the plasma membrane. J. Cell Biol. 2023, 322, e202208065. [Google Scholar] [CrossRef] [PubMed]
- Vasileva, E.; Spadaro, D.; Rouaud, F.; King, J.M.; Flinois, A.; Shah, J.; Sluysmans, S.; Mean, I.; Jond, L.; Turner, J.R.; et al. Cingulin binds to the ZU5 domain of scaffolding protein ZO-1 to promote its extended conformation, stabilization, and tight junction accumulation. J. Biol. Chem. 2022, 298, 101797. [Google Scholar] [CrossRef] [PubMed]
- Rouaud, F.; Mauperin, M.; Mutero-Maeda, A.; Citi, S. Cingulin-nonmuscle myosin interaction plays a role in epithelial morphogenesis and cingulin nanoscale organization. J. Cell Sci. 2024, 137, jcs262353. [Google Scholar] [CrossRef] [PubMed]
- Hutter, J.K.; Bechhoefer, J. Calibration of atomic-force microscope tips. Rev. Sci. Instr. 1993, 64, 1868–1873. [Google Scholar] [CrossRef]
- Hubrich, H.; Mey, I.P.; Bruckner, B.R.; Muhlenbrock, P.; Nehls, S.; Grabenhorst, L.; Oswald, T.; Steinem, C.; Janshoff, A. Viscoelasticity of Native and Artificial Actin Cortices Assessed by Nanoindentation Experiments. Nano Lett. 2020, 20, 6329–6335. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Okano, T.; Ma, X.; Adelstein, R.S.; Kelley, M.W. Myosin II regulates extension, growth and patterning in the mammalian cochlear duct. Development 2009, 136, 1977–1986. [Google Scholar] [CrossRef] [PubMed]
- Dugina, V.; Zwaenepoel, I.; Gabbiani, G.; Clement, S.; Chaponnier, C. Beta and gamma-cytoplasmic actins display distinct distribution and functional diversity. J. Cell Sci. 2009, 122, 2980–2988. [Google Scholar] [CrossRef] [PubMed]
- Nehls, S.; Noding, H.; Karsch, S.; Ries, F.; Janshoff, A. Stiffness of MDCK II Cells Depends on Confluency and Cell Size. Biophys. J. 2019, 116, 2204–2211. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Takeda, K.; Singh, A.; Yu, Z.X.; Zerfas, P.; Blount, A.; Liu, C.; Towbin, J.A.; Schneider, M.D.; Adelstein, R.S.; et al. Conditional ablation of nonmuscle myosin II-B delineates heart defects in adult mice. Circ. Res. 2009, 105, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Levuschkina, Y.G.; Dugina, V.B.; Shagieva, G.S.; Boichuk, S.V.; Eremin, I.I.; Khromova, N.V.; Kopnin, P.B. Induction of Fibroblast-to-Myofibroblast Differentiation by Changing Cytoplasmic Actin Ratio. Biochemistry 2025, 90, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Norstrom, M.F.; Smithback, P.A.; Rock, R.S. Unconventional processive mechanics of non-muscle myosin IIB. J. Biol. Chem. 2010, 285, 26326–26334. [Google Scholar] [CrossRef] [PubMed]
- Odenwald, M.A.; Choi, W.; Kuo, W.T.; Singh, G.; Sailer, A.; Wang, Y.; Shen, L.; Fanning, A.S.; Turner, J.R. The scaffolding protein ZO-1 coordinates actomyosin and epithelial apical specializations in vitro and in vivo. J. Biol. Chem. 2018, 293, 17317–17335. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, D.; Le, S.; Laroche, T.; Mean, I.; Jond, L.; Yan, J.; Citi, S. Tension-Dependent Stretching Activates ZO-1 to Control the Junctional Localization of Its Interactors. Curr. Biol. 2017, 27, 3783–3795.e8. [Google Scholar] [CrossRef] [PubMed]
- Beutel, O.; Maraspini, R.; PomboGarcia, K.; MartinLemaitre, C.; Honigmann, A. Phase Separation of Zonula Occludens Proteins Drives Formation of Tight Junctions. Cell 2019, 179, 923–936.e11. [Google Scholar] [CrossRef] [PubMed]
- Schwayer, C.; Shamipour, S.; Pranjic-Ferscha, K.; Schauer, A.; Balda, M.; Tada, M.; Matter, K.; Heisenberg, C.P. Mechanosensation of Tight Junctions Depends on ZO-1 Phase Separation and Flow. Cell 2019, 179, 937–952.e18. [Google Scholar] [CrossRef] [PubMed]
- Nietmann, P.; Kaub, K.; Suchenko, A.; Stenz, S.; Warnecke, C.; Balasubramanian, M.K.; Janshoff, A. Cytosolic actin isoforms form networks with different rheological properties that indicate specific biological function. Nat. Commun. 2023, 14, 7989. [Google Scholar] [CrossRef] [PubMed]
- Truong Quang, B.A.; Peters, R.; Cassani, D.A.D.; Chugh, P.; Clark, A.G.; Agnew, M.; Charras, G.; Paluch, E.K. Extent of myosin penetration within the actin cortex regulates cell surface mechanics. Nat. Commun. 2021, 12, 6511. [Google Scholar] [CrossRef] [PubMed]
- Cardellini, P.; Davanzo, G.; Citi, S. Tight junctions in early amphibian development: Detection of junctional cingulin from the 2-cell stage and its localization at the boundary of distinct membrane domains in dividing blastomeres in low calcium. Dev. Dyn. 1996, 207, 104–113. [Google Scholar] [CrossRef]
- Yano, T.; Kanoh, H.; Tamura, A.; Tsukita, S. Apical cytoskeletons and junctional complexes as a combined system in epithelial cell sheets. Ann. N. Y. Acad. Sci. 2017, 1405, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Cordenonsi, M.; D’Atri, F.; Hammar, E.; Parry, D.A.D.; Kendrick-Jones, J.; Shore, D.; Citi, S. Cingulin Contains Globular and Coiled-coil Domains and Interacts with ZO-1, ZO-2, ZO-3, and Myosin. J. Cell Biol. 1999, 147, 1569–1582. [Google Scholar] [CrossRef] [PubMed]
- Van Itallie, C.M.; Fanning, A.S.; Bridges, A.; Anderson, J.M. ZO-1 stabilizes the tight junction solute barrier through coupling to the perijunctional cytoskeleton. Mol. Biol. Cell 2009, 20, 3930–3940. [Google Scholar] [CrossRef] [PubMed]
- Baranwal, S.; Naydenov, N.G.; Harris, G.; Dugina, V.; Morgan, K.G.; Chaponnier, C.; Ivanov, A.I. Nonredundant roles of cytoplasmic beta- and gamma-actin isoforms in regulation of epithelial apical junctions. Mol. Biol. Cell 2012, 23, 3542–3553. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, A.C. The regulation of cell size. Cell 2013, 154, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Rhind, N. Cell-size control. Curr. Biol. 2021, 31, R1414–R1420. [Google Scholar] [CrossRef] [PubMed]
- Lengefeld, J.; Zatulovskiy, E. Editorial: Cell size regulation: Molecular mechanisms and physiological importance. Front. Cell Dev. Biol. 2023, 11, 1219294. [Google Scholar] [CrossRef] [PubMed]
- Cadart, C.; Monnier, S.; Grilli, J.; Saez, P.J.; Srivastava, N.; Attia, R.; Terriac, E.; Baum, B.; Cosentino-Lagomarsino, M.; Piel, M. Size control in mammalian cells involves modulation of both growth rate and cell cycle duration. Nat. Commun. 2018, 9, 3275. [Google Scholar] [CrossRef] [PubMed]
- Marshall, W.F.; Young, K.D.; Swaffer, M.; Wood, E.; Nurse, P.; Kimura, A.; Frankel, J.; Wallingford, J.; Walbot, V.; Qu, X.; et al. What determines cell size? BMC Biol. 2012, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Dugina, V.; Shagieva, G.; Khromova, N.; Kopnin, P. Divergent impact of actin isoforms on cell cycle regulation. Cell Cycle 2018, 17, 2610–2621. [Google Scholar] [CrossRef] [PubMed]
- Shagieva, G.S.; Alieva, I.B.; Chaponnier, C.; Dugina, V.B. Divergent Impact of Actin Isoforms on Division of Epithelial Cells. Biochemistry 2020, 85, 1072. [Google Scholar] [CrossRef] [PubMed]
- Salbreux, G.; Charras, G.; Paluch, E. Actin cortex mechanics and cellular morphogenesis. Trends Cell Biol. 2012, 22, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Shawky, J.H.; Balakrishnan, U.L.; Stuckenholz, C.; Davidson, L.A. Multiscale analysis of architecture, cell size and the cell cortex reveals cortical F-actin density and composition are major contributors to mechanical properties during convergent extension. Development 2018, 145, dev161281. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Jana, S.S.; Conti, M.A.; Kawamoto, S.; Claycomb, W.C.; Adelstein, R.S. Ablation of nonmuscle myosin II-B and II-C reveals a role for nonmuscle myosin II in cardiac myocyte karyokinesis. Mol. Biol. Cell 2010, 21, 3952–3962. [Google Scholar] [CrossRef] [PubMed]
- Tullio, A.N.; Bridgman, P.C.; Tresser, N.J.; Chan, C.C.; Conti, M.A.; Adelstein, R.S.; Hara, Y. Structural abnormalities develop in the brain after ablation of the gene encoding nonmuscle myosin II-B heavy chain. J. Comp. Neurol. 2001, 433, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Yang, T.; Wei, S.; DeWan, A.T.; Morell, R.J.; Elfenbein, J.L.; Fisher, R.A.; Leal, S.M.; Smith, R.J.; Friderici, K.H. Mutations in the gamma-actin gene (ACTG1) are associated with dominant progressive deafness (DFNA20/26). Am. J. Hum. Genet. 2003, 73, 1082–1091. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.J.; Huang, Y.; Zhang, L.; Yan, K.; Qiu, C.; He, Y.; Liu, Q.; Zhu, C.; Morin, M.; Moreno-Pelayo, M.A.; et al. Cingulin regulates hair cell cuticular plate morphology and is required for hearing in human and mouse. EMBO Mol. Med. 2023, 15, e17611. [Google Scholar] [CrossRef] [PubMed]
- Belyantseva, I.A.; Perrin, B.J.; Sonnemann, K.J.; Zhu, M.; Stepanyan, R.; McGee, J.; Frolenkov, G.I.; Walsh, E.J.; Friderici, K.H.; Friedman, T.B.; et al. Gamma-actin is required for cytoskeletal maintenance but not development. Proc. Natl. Acad. Sci. USA 2009, 106, 9703–9708. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, L.; Sun, Y.; Liu, Q.; Chen, J.; Qian, X.; Gao, X.; Zhu, G.J.; Wan, G. A human-specific cytotoxic neopeptide generated by the deafness gene Cingulin. J. Genet. Genom. 2024, 51, 1215–1227. [Google Scholar] [CrossRef] [PubMed]
- Sluysmans, S.; Mean, I.; Xiao, T.; Boukhatemi, A.; Ferreira, F.; Jond, L.; Mutero, A.; Chang, C.J.; Citi, S. PLEKHA5, PLEKHA6 and PLEKHA7 bind to PDZD11 to target the Menkes ATPase ATP7A to the cell periphery and regulate copper homeostasis. Mol. Biol. Cell 2021, 32, ar34. [Google Scholar] [CrossRef] [PubMed]



| Genotype, Clone | T0 (mN/m) | (N/m) | β |
|---|---|---|---|
| WT | 0.534 ± 0.122 | 0.116 ± 0.058 | 0.455 ± 0.073 |
| NM2B-KO A4 | 0.522 ± 0.118 | 0.029 ± 0.017 | 0.638 ± 0.059 |
| NM2B-KO A6 | 0.551 ± 0.146 | 0.034 ± 0.016 | 0.616 ± 0.060 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maupérin, M.; Klatt, N.; Glandorf, T.; Di Mattia, T.; Méan, I.; Janshoff, A.; Citi, S. Nonmuscle Myosin-2B Regulates Apical Cortical Mechanics, ZO-1 Dynamics and Cell Size in MDCK Epithelial Cells. Cells 2025, 14, 1138. https://doi.org/10.3390/cells14151138
Maupérin M, Klatt N, Glandorf T, Di Mattia T, Méan I, Janshoff A, Citi S. Nonmuscle Myosin-2B Regulates Apical Cortical Mechanics, ZO-1 Dynamics and Cell Size in MDCK Epithelial Cells. Cells. 2025; 14(15):1138. https://doi.org/10.3390/cells14151138
Chicago/Turabian StyleMaupérin, Marine, Niklas Klatt, Thomas Glandorf, Thomas Di Mattia, Isabelle Méan, Andreas Janshoff, and Sandra Citi. 2025. "Nonmuscle Myosin-2B Regulates Apical Cortical Mechanics, ZO-1 Dynamics and Cell Size in MDCK Epithelial Cells" Cells 14, no. 15: 1138. https://doi.org/10.3390/cells14151138
APA StyleMaupérin, M., Klatt, N., Glandorf, T., Di Mattia, T., Méan, I., Janshoff, A., & Citi, S. (2025). Nonmuscle Myosin-2B Regulates Apical Cortical Mechanics, ZO-1 Dynamics and Cell Size in MDCK Epithelial Cells. Cells, 14(15), 1138. https://doi.org/10.3390/cells14151138







