Nanotechnology-Based Delivery of CRISPR/Cas9 for Cancer Treatment: A Comprehensive Review
Abstract
1. Introduction
2. From Bacterial Defense to Gene Editing Revolution: The CRISPR-Cas9 Story
3. CRISPR-Cas9: A Bacterial Defense Mechanism Repurposed for Accurate Gene Editing

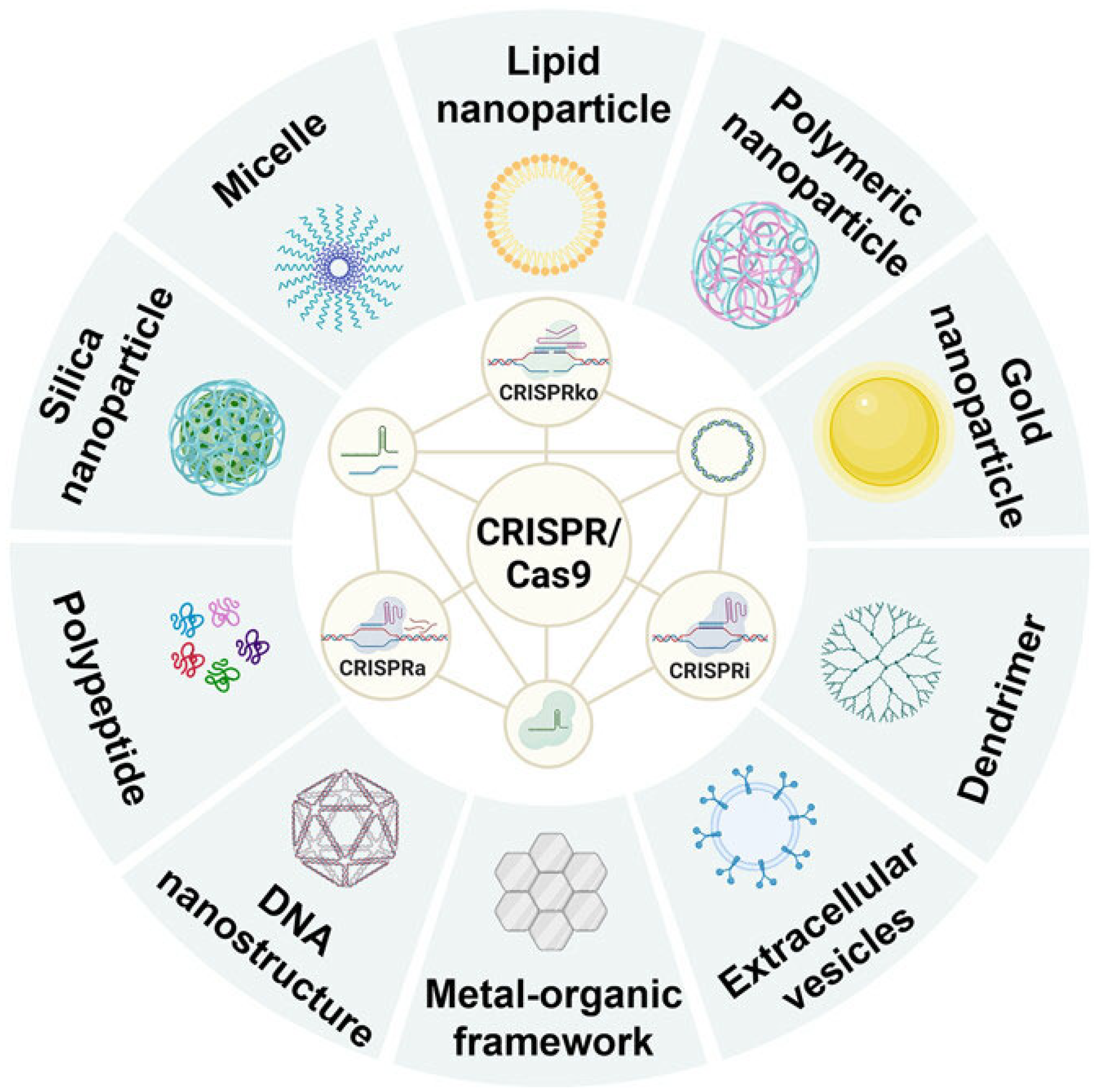
4. Nanotechnology-Based Delivery of CRISPR/Cas9 for Cancer Treatment
4.1. Lipid Nanoparticles (LNPs) for CRISPR/Cas9 Plasmid Delivery
4.2. Inorganic and Cationic Nanomaterials for CRISPR/Cas9 Plasmid Delivery
4.3. Barriers to CRISPR/Cas9 Delivery Systems: mRNA-Based CRISPR/Cas9 Systems
4.4. RNP-Based CRISPR/Cas9: Direct Administration for Improved Gene Editing
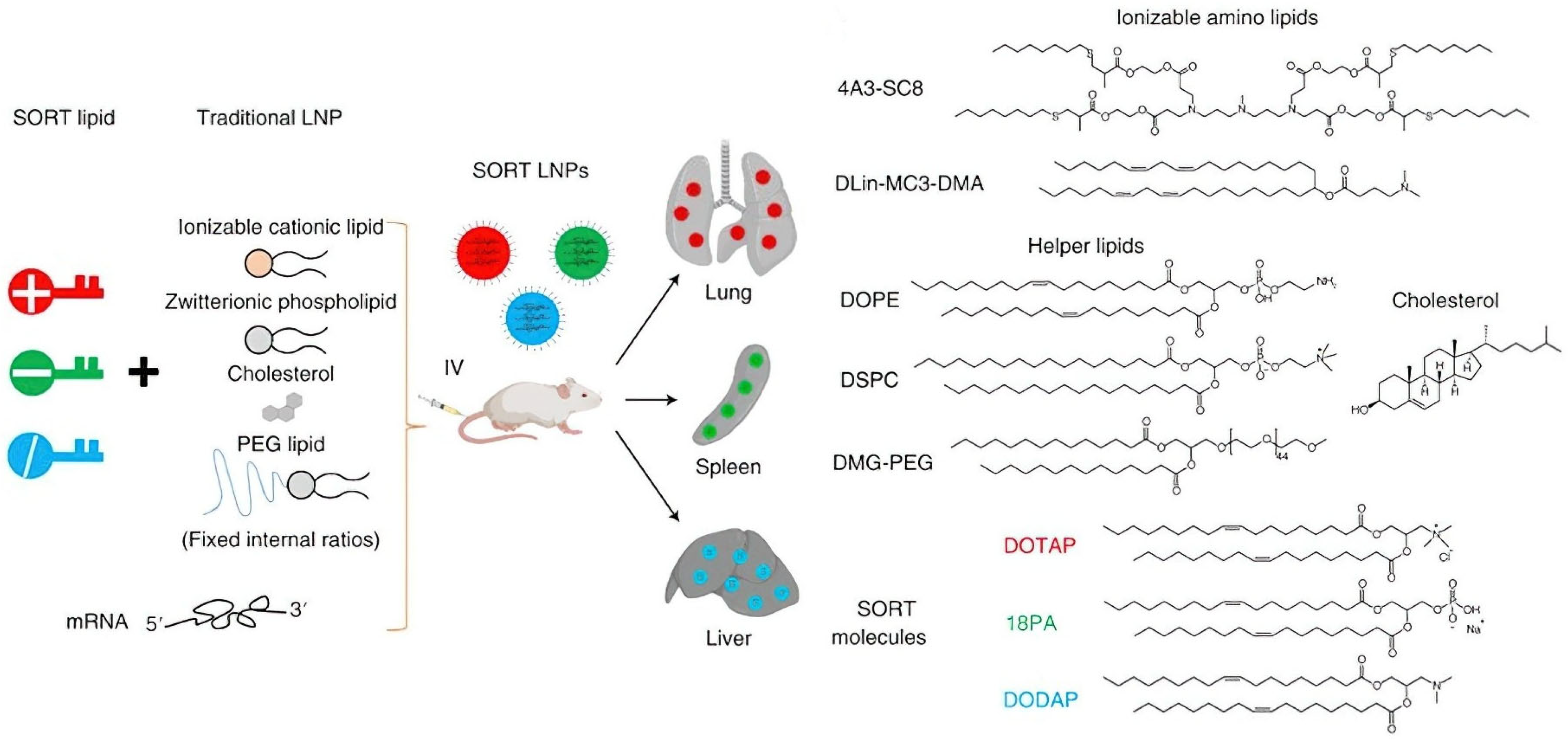
4.5. Lipid-Based Nanotechnology for Ribonucleoprotein Delivery
4.6. Polymer Nanocarriers for Improved CRISPR/Cas9 RNP Delivery
4.7. Inorganic Nanomaterials for Ribonucleoprotein Delivery
4.8. Extracellular Vesicles and Polymeric Micelles for Ribonucleoprotein Delivery
5. Clinical Applications
5.1. Challenges and Future Outlook on Clinical Translation
5.2. Future Direction
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A. Cancer Chemotherapy and beyond: Current Status, Drug Candidates, Associated Risks and Progress in Targeted Therapeutics. Genes Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef]
- Leng, G.; Duan, B.; Liu, J.; Li, S.; Zhao, W.; Wang, S.; Hou, G.; Qu, J. The Advancements and Prospective Developments in Anti-Tumor Targeted Therapy. Neoplasia 2024, 56, 101024. [Google Scholar] [CrossRef]
- Casotti, M.C.; Meira, D.D.; Zetum, A.S.S.; Campanharo, C.V.; da Silva, D.R.C.; Giacinti, G.M.; da Silva, I.M.; Moura, J.A.D.; Barbosa, K.R.M.; Altoé, L.S.C. Integrating Frontiers: A Holistic, Quantum and Evolutionary Approach to Conquering Cancer through Systems Biology and Multidisciplinary Synergy. Front. Oncol. 2024, 14, 1419599. [Google Scholar] [CrossRef] [PubMed]
- Naldi, I. An Innovative Epigenetic Strategy for Retinoblastoma Treatment. Ph.D. Thesis, Università degli Studi di Firenze, Firenze, Italy, 2017. [Google Scholar]
- Pandey, M.; Shah, S.K.; Gromiha, M.M. Databases and Computational Algorithms for Identifying Cancer Hotspot Residues and Mutations in Proteins. In Protein Mutations: Consequences on Structure, Functions, and Diseases; World Scientific: Singapore, 2025; pp. 229–259. [Google Scholar]
- Aljabali, A.A.A.; El-Tanani, M.; Tambuwala, M.M. Principles of CRISPR-Cas9 Technology: Advancements in Genome Editing and Emerging Trends in Drug Delivery. J. Drug Deliv. Sci. Technol. 2024, 92, 105338. [Google Scholar] [CrossRef]
- Li, H.; Yang, Y.; Hong, W.; Huang, M.; Wu, M.; Zhao, X. Applications of Genome Editing Technology in the Targeted Therapy of Human Diseases: Mechanisms, Advances and Prospects. Signal Transduct. Target. Ther. 2020, 5, 1. [Google Scholar] [CrossRef]
- Li, T.; Li, S.; Kang, Y.; Zhou, J.; Yi, M. Harnessing the Evolving CRISPR/Cas9 for Precision Oncology. J. Transl. Med. 2024, 22, 749. [Google Scholar] [CrossRef]
- Gupta, D.; Bhattacharjee, O.; Mandal, D.; Sen, M.K.; Dey, D.; Dasgupta, A.; Kazi, T.A.; Gupta, R.; Sinharoy, S.; Acharya, K. CRISPR-Cas9 System: A New-Fangled Dawn in Gene Editing. Life Sci. 2019, 232, 116636. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, M.; Ren, Y.; Xu, H.; Weng, S.; Ning, W.; Ge, X.; Liu, L.; Guo, C.; Duo, M. Recent Advances and Applications of CRISPR-Cas9 in Cancer Immunotherapy. Mol. Cancer 2023, 22, 35. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Y.; Liu, X.; Li, Z.; Dai, X. The Application of CRISPR/Cas9 Technology for Cancer Immunotherapy: Current Status and Problems. Front. Oncol. 2022, 11, 704999. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, L.; Liu, H.; Cheng, K. Delivery Strategies of the CRISPR-Cas9 Gene-Editing System for Therapeutic Applications. J. Control. Release 2017, 266, 17–26. [Google Scholar] [CrossRef]
- Karimian, A.; Azizian, K.; Parsian, H.; Rafieian, S.; Shafiei-Irannejad, V.; Kheyrollah, M.; Yousefi, M.; Majidinia, M.; Yousefi, B. CRISPR/Cas9 Technology as a Potent Molecular Tool for Gene Therapy. J. Cell. Physiol. 2019, 234, 12267–12277. [Google Scholar] [CrossRef]
- Xu, X.; Wan, T.; Xin, H.; Li, D.; Pan, H.; Wu, J.; Ping, Y. Delivery of CRISPR/Cas9 for Therapeutic Genome Editing. J. Gene Med. 2019, 21, e3107. [Google Scholar] [CrossRef]
- Aghamiri, S.; Talaei, S.; Ghavidel, A.A.; Zandsalimi, F.; Masoumi, S.; Hafshejani, N.H.; Jajarmi, V. Nanoparticles-Mediated CRISPR/Cas9 Delivery: Recent Advances in Cancer Treatment. J. Drug Deliv. Sci. Technol. 2020, 56, 101533. [Google Scholar] [CrossRef]
- Xu, X.; Liu, C.; Wang, Y.; Koivisto, O.; Zhou, J.; Shu, Y.; Zhang, H. Nanotechnology-Based Delivery of CRISPR/Cas9 for Cancer Treatment. Adv. Drug Deliv. Rev. 2021, 176, 113891. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, H.; Salehiabar, M.; Charmi, J.; Barsbay, M.; Ghaffarlou, M.; Razlighi, M.R.; Davaran, S.; Khalilov, R.; Sugiyama, M.; Nosrati, H. Harnessing Nanoparticles for the Efficient Delivery of the CRISPR/Cas9 System. Nano Today 2020, 34, 100895. [Google Scholar] [CrossRef]
- Chen, K.; Jiang, S.; Hong, Y.; Li, Z.; Wu, Y.-L.; Wu, C. Cationic Polymeric Nanoformulation: Recent Advances in Material Design for CRISPR/Cas9 Gene Therapy. Prog. Nat. Sci. Mater. Int. 2019, 29, 617–627. [Google Scholar] [CrossRef]
- Zhou, S.; Li, Y.; Wu, Q.; Gong, C. Nanotechnology-based CRISPR/Cas9 Delivery System for Genome Editing in Cancer Treatment. MedComm Biomater. Appl. 2024, 3, e70. [Google Scholar] [CrossRef]
- Ramos-Martín, F.; D’amelio, N. Drug Resistance: An Incessant Fight against Evolutionary Strategies of Survival. Microbiol. Res. 2023, 14, 507–542. [Google Scholar] [CrossRef]
- Mistry, A.; Tanga, S.; Maji, B. Nucleic Acid Editing. In Nucleic Acid Biology and Its Application in Human Diseases; Springer: Berlin/Heidelberg, Germany, 2023; pp. 365–416. [Google Scholar]
- Davies, K. Editing Humanity: The CRISPR Revolution and the New Era of Genome Editing; Simon and Schuster; Pegasus Books: New York, NY, USA, 2020; ISBN 1643133942. [Google Scholar]
- Hu, X.; Xu, B.; Chen, M.; Li, K.; Xiao, Y.; Liang, S.; Zhang, C.; Ma, H.; Song, H. Development and Assessment of Cutting-Edge Biotechnologies. J. Biosaf. Biosecur. 2024, 6, 51–63. [Google Scholar] [CrossRef]
- Loureiro, A.; da Silva, G.J. Crispr-Cas: Converting a Bacterial Defence Mechanism into a State-of-the-Art Genetic Manipulation Tool. Antibiotics 2019, 8, 18. [Google Scholar] [CrossRef]
- Ishino, Y.; Shinagawa, H.; Makino, K.; Amemura, M.; Nakata, A. Nucleotide Sequence of the Iap Gene, Responsible for Alkaline Phosphatase Isozyme Conversion in Escherichia Coli, and Identification of the Gene Product. J. Bacteriol. 1987, 169, 5429–5433. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, A.; Mahmoud, E.-H.M.; Al Deabel, R.; Kanwal, F.; Ahmad, Q.; Naeem, M.; Ahmad, I. CRISPR-Cas: Effectors, Mechanism, and Classification. In CRISPRized Horticulture Crops; Elsevier: Amsterdam, The Netherlands, 2024; pp. 37–50. [Google Scholar]
- Nidhi, S.; Anand, U.; Oleksak, P.; Tripathi, P.; Lal, J.A.; Thomas, G.; Kuca, K.; Tripathi, V. Novel CRISPR–Cas Systems: An Updated Review of the Current Achievements, Applications, and Future Research Perspectives. Int. J. Mol. Sci. 2021, 22, 3327. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Yadav, S.K. CRISPR-Cas for Genome Editing: Classification, Mechanism, Designing and Applications. Int. J. Biol. Macromol. 2023, 238, 124054. [Google Scholar] [CrossRef]
- Hanewich-Hollatz, M.H. Conditional Guide RNAs: Programmable Conditional Regulation of CRISPR/Cas Function Via Dynamic RNA Nanotechnolog; California Institute of Technology: Pasadena, CA, USA, 2020; ISBN 9798379856175. [Google Scholar]
- Chen, C.; Ma, Y.; Du, S.; Wu, Y.; Shen, P.; Yan, T.; Li, X.; Song, Y.; Zha, Z.; Han, X. Controlled CRISPR-Cas9 Ribonucleoprotein Delivery for Sensitized Photothermal Therapy. Small 2021, 17, 2101155. [Google Scholar] [CrossRef]
- Tao, W.; Cheng, X.; Sun, D.; Guo, Y.; Wang, N.; Ruan, J.; Hu, Y.; Zhao, M.; Zhao, T.; Feng, H. Synthesis of Multi-Branched Au Nanocomposites with Distinct Plasmon Resonance in NIR-II Window and Controlled CRISPR-Cas9 Delivery for Synergistic Gene-Photothermal Therapy. Biomaterials 2022, 287, 121621. [Google Scholar] [CrossRef]
- Fernandes, H.; Pastor, M.; Bochtler, M. Type II and Type V CRISPR Effector Nucleases from a Structural Biologist’s Perspective. Postepy. Biochem. 2016, 62, 315–326. [Google Scholar] [CrossRef]
- Chaudhary, E.; Chaudhary, A.; Sharma, S.; Tiwari, V.; Garg, M. Different Classes of CRISPR-Cas Systems. In Gene Editing in Plants: CRISPR-Cas and Its Applications; Springer: Berlin/Heidelberg, Germany, 2024; pp. 73–94. [Google Scholar]
- Chuang, C.-K.; Lin, W.-M. Points of View on the Tools for Genome/Gene Editing. Int. J. Mol. Sci. 2021, 22, 9872. [Google Scholar] [CrossRef]
- Palanivelu, P. Analyses of Homing Endonucleases and Mechanism of Action of CRISPR-Cas9 HNH Endonucleases. Int. J. Biochem. Res. Rev. 2020, 29, 1–25. [Google Scholar] [CrossRef]
- Palanivelu, P. Assessment and Analyses of Homing Endonucleases and Mechanism of Action of CRISPR-Cas9 HNH Endonucleases. Curr. Adv. Chem. Biochem. 2021, 1, 20–48. [Google Scholar]
- Su, T.; Liu, F.; Gu, P.; Jin, H.; Chang, Y.; Wang, Q.; Liang, Q.; Qi, Q. A CRISPR-Cas9 Assisted Non-Homologous End-Joining Strategy for One-Step Engineering of Bacterial Genome. Sci. Rep. 2016, 6, 37895. [Google Scholar] [CrossRef]
- Guo, T.; Feng, Y.-L.; Xiao, J.-J.; Liu, Q.; Sun, X.-N.; Xiang, J.-F.; Kong, N.; Liu, S.-C.; Chen, G.-Q.; Wang, Y. Harnessing Accurate Non-Homologous End Joining for Efficient Precise Deletion in CRISPR/Cas9-Mediated Genome Editing. Genome Biol. 2018, 19, 170. [Google Scholar] [CrossRef] [PubMed]
- van Kampen, S.J.; van Rooij, E. CRISPR Craze to Transform Cardiac Biology. Trends Mol. Med. 2019, 25, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, Y.; Zhao, X.; Huang, G.; Gong, J.; Li, H.; Wan, W.; Jia, C.; Chen, G.; Zhang, X. A Multifunctional Non-Viral Vector for the Delivery of MTH1-Targeted CRISPR/Cas9 System for Non-Small Cell Lung Cancer Therapy. Acta Biomater. 2022, 153, 481–493. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, G.; Xu, C.-F.; Luo, Y.-L.; Lu, Z.-D.; Wang, J. Systemic Delivery of CRISPR/Cas9 with PEG-PLGA Nanoparticles for Chronic Myeloid Leukemia Targeted Therapy. Biomater. Sci. 2018, 6, 1592–1603. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Dai, Q.; Yang, Q.; Bao, X.; Zhou, Y.; Zhong, H.; Wu, L.; Wang, T.; Zhang, Z.; Lu, Y. Therapeutic Nucleus-Access BNCT Drug Combined CD47-Targeting Gene Editing in Glioblastoma. J. Nanobiotechnol. 2022, 20, 102. [Google Scholar] [CrossRef]
- Li, C.; Yang, T.; Weng, Y.; Zhang, M.; Zhao, D.; Guo, S.; Hu, B.; Shao, W.; Wang, X.; Hussain, A.; et al. Ionizable lipid-assisted efficient hepatic delivery of gene editing elements for oncotherapy. Bioact. Mater. 2022, 9, 590–601. [Google Scholar] [CrossRef]
- Wuttke, S.; Braig, S.; Preiß, T.; Zimpel, A.; Sicklinger, J.; Bellomo, C.; Rädler, J.O.; Vollmar, A.M.; Bein, T. MOF Nanoparticles Coated by Lipid Bilayers and Their Uptake by Cancer Cells. Chem Commun. 2015, 51, 15752–15755. [Google Scholar] [CrossRef]
- Rabiee, N.; Bagherzadeh, M.; Heidarian Haris, M.; Ghadiri, A.M.; Matloubi Moghaddam, F.; Fatahi, Y.; Dinarvand, R.; Jarahiyan, A.; Ahmadi, S.; Shokouhimehr, M. Polymer-Coated NH2-UiO-66 for the Codelivery of DOX/PCRISPR. ACS Appl. Mater. Interfaces 2021, 13, 10796–10811. [Google Scholar] [CrossRef]
- Pyreddy, S.; Poddar, A.; Carraro, F.; Polash, S.A.; Dekiwadia, C.; Murdoch, B.; Nasa, Z.; Reddy, T.S.; Falcaro, P.; Shukla, R. Targeting telomerase utilizing zeolitic imidazole frameworks as non-viral gene delivery agents across different cancer cell types. Biomater. Adv. 2023, 149, 213420. [Google Scholar] [CrossRef]
- Poddar, A.; Conesa, J.J.; Liang, K.; Dhakal, S.; Reineck, P.; Bryant, G.; Pereiro, E.; Ricco, R.; Amenitsch, H.; Doonan, C. Encapsulation, Visualization and Expression of Genes with Biomimetically Mineralized Zeolitic Imidazolate Framework-8 (ZIF-8). Small 2019, 15, 1902268. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, X.; Wu, D.; Xin, H.; Chen, D.; Li, D.; Pan, H.; Zhou, C.; Ping, Y. Delivery of CRISPR/Cas9 Plasmids by Cationic Gold Nanorods: Impact of the Aspect Ratio on Genome Editing and Treatment of Hepatic Fibrosis. Chem. Mater. 2020, 33, 81–91. [Google Scholar] [CrossRef]
- Tang, H.; Xu, X.; Chen, Y.; Xin, H.; Wan, T.; Li, B.; Pan, H.; Li, D.; Ping, Y. Reprogramming the Tumor Microenvironment through Second-near-infrared-window Photothermal Genome Editing of PD-L1 Mediated by Supramolecular Gold Nanorods for Enhanced Cancer Immunotherapy. Adv. Mater. 2021, 33, 2006003. [Google Scholar] [CrossRef]
- Zhang, B.-C.; Luo, B.-Y.; Zou, J.-J.; Wu, P.-Y.; Jiang, J.-L.; Le, J.-Q.; Zhao, R.-R.; Chen, L.; Shao, J.-W. Co-Delivery of Sorafenib and CRISPR/Cas9 Based on Targeted Core–Shell Hollow Mesoporous Organosilica Nanoparticles for Synergistic HCC Therapy. ACS Appl. Mater. Interfaces 2020, 12, 57362–57372. [Google Scholar] [CrossRef]
- He, X.-Y.; Liu, B.-Y.; Peng, Y.; Zhuo, R.-X.; Cheng, S.-X. Multifunctional Vector for Delivery of Genome Editing Plasmid Targeting β-Catenin to Remodulate Cancer Cell Properties. ACS Appl. Mater. Interfaces 2018, 11, 226–237. [Google Scholar] [CrossRef]
- He, X.; Ren, X.; Peng, Y.; Zhang, J.; Ai, S.; Liu, B.; Xu, C.; Cheng, S. Aptamer/Peptide-functionalized Genome-editing System for Effective Immune Restoration through Reversal of PD-L1-mediated Cancer Immunosuppression. Adv. Mater. 2020, 32, 2000208. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Yi, K.; Hu, H.; Shao, D.; Li, M. Coassembly of Nucleus-Targeting Gold Nanoclusters with CRISPR/Cas9 for Simultaneous Bioimaging and Therapeutic Genome Editing. J. Mater. Chem. B 2021, 9, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Aktaş, Y.; Yemisci, M.; Andrieux, K.; Gürsoy, R.N.; Alonso, M.J.; Fernandez-Megia, E.; Novoa-Carballal, R.; Quiñoá, E.; Riguera, R.; Sargon, M.F. Development and Brain Delivery of Chitosan-PEG Nanoparticles Functionalized with the Monoclonal Antibody OX26. Bioconjug Chem. 2005, 16, 1503–1511. [Google Scholar] [CrossRef]
- Li, Q.; Lv, X.; Tang, C.; Yin, C. Co-Delivery of Doxorubicin and CRISPR/Cas9 or RNAi-Expressing Plasmid by Chitosan-Based Nanoparticle for Cancer Therapy. Carbohydr. Polym. 2022, 287, 119315. [Google Scholar] [CrossRef]
- Menjoge, A.R.; Kannan, R.M.; Tomalia, D.A. Dendrimer-Based Drug and Imaging Conjugates: Design Considerations for Nanomedical Applications. Drug Discov. Today 2010, 15, 171–185. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, J.; Yu, T.; Chen, C.; Cheng, Q.; Sengupta, K.; Huang, Y.; Li, H.; Liu, C.; Wang, Y.; et al. Adaptive Amphiphilic Dendrimer-Based Nanoassemblies as Robust and Versatile SiRNA Delivery Systems. Angew. Chem. Int. Ed. Engl. 2014, 53, 11822–11827. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, Y.; Li, S.; Guan, X.; Jiang, X. In Situ Reprogramming of Tumor-Associated Macrophages with Internally and Externally Engineered Exosomes. Angew. Chem. Int. Ed. Engl. 2023, 62, e202217089. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Cutlar, L.; Gao, Y.; Wang, W.; O’Keeffe-Ahern, J.; McMahon, S.; Duarte, B.; Larcher, F.; Rodriguez, B.J.; Greiser, U. The Transition from Linear to Highly Branched Poly (β-Amino Ester) s: Branching Matters for Gene Delivery. Sci. Adv. 2016, 2, e1600102. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, R.A.; Serra, A.; Coelho, J.F.J.; Faneca, H. Poly (β-Amino Ester)-Based Gene Delivery Systems: From Discovery to Therapeutic Applications. J. Control. Release 2019, 310, 155–187. [Google Scholar] [CrossRef]
- Xu, X.; Tang, H.; Guo, J.; Xin, H.; Ping, Y. A dual-specific CRISPR-Cas nanosystem for precision therapeutic editing of liver disorders. Signal Transduct. Target. Ther. 2022, 7, 269. [Google Scholar] [CrossRef]
- Liu, J.; Chang, J.; Jiang, Y.; Meng, X.; Sun, T.; Mao, L.; Xu, Q.; Wang, M. Fast and Efficient CRISPR/Cas9 Genome Editing In Vivo Enabled by Bioreducible Lipid and Messenger RNA Nanoparticles. Adv. Mater. 2019, 31, e1902575. [Google Scholar] [CrossRef]
- Rosenblum, D.; Gutkin, A.; Kedmi, R.; Ramishetti, S.; Veiga, N.; Jacobi, A.M.; Schubert, M.S.; Friedmann-Morvinski, D.; Cohen, Z.R.; Behlke, M.A.; et al. CRISPR-Cas9 genome editing using targeted lipid nanoparticles for cancer therapy. Sci. Adv. 2020, 6, eabc9450. [Google Scholar] [CrossRef]
- Farbiak, L.; Cheng, Q.; Wei, T.; Álvarez-Benedicto, E.; Johnson, L.T.; Lee, S.; Siegwart, D.J. All-In-One Dendrimer-Based Lipid Nanoparticles Enable Precise HDR-Mediated Gene Editing In Vivo. Adv. Mater. 2021, 33, 2006619. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, G.; Yu, X.; Wei, T.; Farbiak, L.; Johnson, L.T.; Taylor, A.M.; Xu, J.; Hong, Y.; Zhu, H.; et al. Enhancing CRISPR/Cas gene editing through modulating cellular mechanical properties for cancer therapy. Nat. Nanotechnol. 2022, 17, 777–787. [Google Scholar] [CrossRef]
- Abbasi, S.; Uchida, S.; Toh, K.; Tockary, T.A.; Dirisala, A.; Hayashi, K.; Fukushima, S.; Kataoka, K. Co-encapsulation of Cas9 mRNA and guide RNA in polyplex micelles enables genome editing in mouse brain. J. Control. Release 2021, 332, 260–268. [Google Scholar] [CrossRef]
- Usman, W.M.; Pham, T.C.; Kwok, Y.Y.; Vu, L.T.; Ma, V.; Peng, B.; Chan, Y.S.; Wei, L.; Chin, S.M.; Azad, A.; et al. Efficient RNA drug delivery using red blood cell extracellular vesicles. Nat. Commun. 2018, 9, 2359. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, X.; Wei, M.; Gao, X.; Zhao, L.; Shi, R.; Sun, W.; Duan, Y.; Yang, G.; Yuan, L. In Vitro and in Vivo RNA Inhibition by CD9-HuR Functionalized Exosomes Encapsulated with MiRNA or CRISPR/DCas9. Nano Lett. 2018, 19, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Walther, J.; Wilbie, D.; Tissingh, V.S.J.; Öktem, M.; Van Der Veen, H.; Lou, B.; Mastrobattista, E. Impact of Formulation Conditions on Lipid Nanoparticle Characteristics and Functional Delivery of CRISPR RNP for Gene Knock-out and Correction. Pharmaceutics 2022, 14, 213. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Wei, T.; Farbiak, L.; Johnson, L.T.; Dilliard, S.A.; Siegwart, D.J. Selective Organ Targeting (SORT) Nanoparticles for Tissue-Specific MRNA Delivery and CRISPR–Cas Gene Editing. Nat. Nanotechnol. 2020, 15, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Liu, J.; Chen, X.; Mao, L.; Wang, M. Orthogonal Chemical Activation of Enzyme-Inducible CRISPR/Cas9 for Cell-Selective Genome Editing. J. Am. Chem. Soc. 2022, 144, 22272–22280. [Google Scholar] [CrossRef]
- Ruan, W.; Jiao, M.; Xu, S.; Ismail, M.; Xie, X.; An, Y.; Guo, H.; Qian, R.; Shi, B.; Zheng, M. Brain-Targeted CRISPR/Cas9 Nanomedicine for Effective Glioblastoma Therapy. J. Control. Release 2022, 351, 739–751. [Google Scholar] [CrossRef]
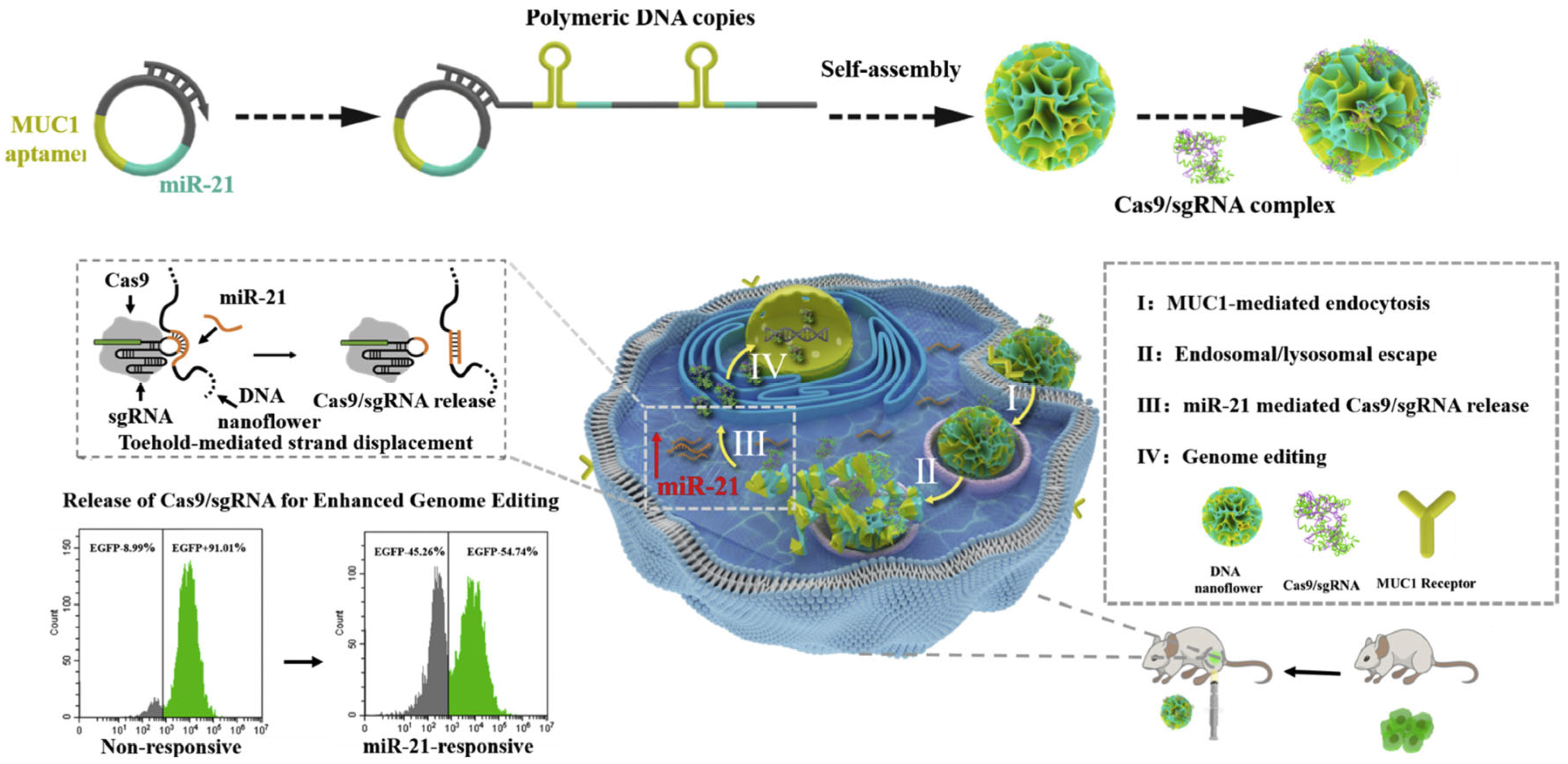
- Shi, J.; Yang, X.; Li, Y.; Wang, D.; Liu, W.; Zhang, Z.; Liu, J.; Zhang, K. MicroRNA-Responsive Release of Cas9/SgRNA from DNA Nanoflower for Cytosolic Protein Delivery and Enhanced Genome Editing. Biomaterials 2020, 256, 120221. [Google Scholar] [CrossRef]
- Tong, P.-H.; Zhu, L.; Zang, Y.; Li, J.; He, X.-P.; James, T.D. Metal–Organic Frameworks (MOFs) as Host Materials for the Enhanced Delivery of Biomacromolecular Therapeutics. Chem. Commun. 2021, 57, 12098–12110. [Google Scholar] [CrossRef]
- Pu, Y.; Yin, H.; Dong, C.; Xiang, H.; Wu, W.; Zhou, B.; Du, D.; Chen, Y.; Xu, H. Sono-Controllable and ROS-Sensitive CRISPR-Cas9 Genome Editing for Augmented/Synergistic Ultrasound Tumor Nanotherapy. Adv. Mater. 2021, 33, 2104641. [Google Scholar] [CrossRef]
- Luther, D.C.; Huang, R.; Jeon, T.; Zhang, X.; Lee, Y.-W.; Nagaraj, H.; Rotello, V.M. Delivery of Drugs, Proteins, and Nucleic Acids Using Inorganic Nanoparticles. Adv. Drug Deliv. Rev. 2020, 156, 188–213. [Google Scholar] [CrossRef]
- Hii, A.R.K.; Qi, X.; Wu, Z. Advanced Strategies for CRISPR/Cas9 Delivery and Applications in Gene Editing, Therapy, and cCancer Detection Using Nanoparticles and Nanocarriers. J. Mater. Chem. B 2024, 12, 1467–1489. [Google Scholar] [CrossRef]
- Li, X.; Pan, Y.; Chen, C.; Gao, Y.; Liu, X.; Yang, K.; Luan, X.; Zhou, D.; Zeng, F.; Han, X.; et al. Hypoxia-Responsive Gene Editing to Reduce Tumor Thermal Tolerance for Mild-Photothermal Therapy. Angew. Chem. Int. Ed. Engl. 2021, 60, 21200–21204. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhou, M.; Abbas, G.; Li, C.; Cui, M.; Zhang, X.E.; Wang, D.B. A Cancer Cell Membrane-Derived Biomimetic Nanocarrier for Synergistic Photothermal/Gene Therapy by Efficient Delivery of CRISPR/Cas9 and Gold Nanorods. Adv. Healthc. Mater. 2022, 11, e2201038. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jeong, Y.K.; Cho, C.S.; Lee, S.; Sohn, C.H.; Kim, J.H.; Jeong, Y.; Jo, D.H.; Bae, S.; Lee, H. Enhancement of Gene Editing and Base Editing with Therapeutic Ribonucleoproteins through in Vivo Delivery Based on Absorptive Silica Nanoconstruct. Adv. Healthc. Mater. 2023, 12, 2201825. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pan, Y.; Chen, C.; Li, Z.; Du, S.; Luan, X.; Gao, Y.; Han, X.; Song, Y. Multistage-responsive Gene Editing to Sensitize Ion-interference Enhanced Carbon Monoxide Gas Therapy. Small 2022, 18, 2204244. [Google Scholar] [CrossRef]
- Brezgin, S.; Danilik, O.; Yudaeva, A.; Kachanov, A.; Kostyusheva, A.; Karandashov, I.; Ponomareva, N.; Zamyatnin, A.A., Jr.; Parodi, A.; Chulanov, V. Basic Guide for Approaching Drug Delivery with Extracellular Vesicles. Int. J. Mol. Sci. 2024, 25, 10401. [Google Scholar] [CrossRef]
- Le Saux, S.; Aubert-Pouëssel, A.; Mohamed, K.E.; Martineau, P.; Guglielmi, L.; Devoisselle, J.-M.; Legrand, P.; Chopineau, J.; Morille, M. Interest of Extracellular Vesicles in Regards to Lipid Nanoparticle Based Systems for Intracellular Protein Delivery. Adv. Drug Deliv. Rev. 2021, 176, 113837. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Zhao, Z.; Meng, Q.; Yu, Y.; Sun, J.; Yang, Z.; Chen, Y.; Li, J.; Ma, T. Engineered Exosomes with Ischemic Myocardium-targeting Peptide for Targeted Therapy in Myocardial Infarction. J. Am. Heart Assoc. 2018, 7, e008737. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Q.; Lu, Q. Engineering ARMMs for improved intracellular delivery of CRISPR-Cas9. Extracell. Vesicle. 2025, 5, 100082. [Google Scholar] [CrossRef]
- Zhang, C.; Ren, H.; Liu, G.; Li, J.; Wang, X.; Zhang, Y. Effective Genome Editing Using CRISPR-Cas9 Nanoflowers. Adv. Healthc. Mater. 2022, 11, 2102365. [Google Scholar] [CrossRef]
- Schoen, S.; Kilinc, M.S.; Lee, H.; Guo, Y.; Degertekin, F.L.; Woodworth, G.F.; Arvanitis, C. Towards Controlled Drug Delivery in Brain Tumors with Microbubble-Enhanced Focused Ultrasound. Adv. Drug Deliv. Rev. 2022, 180, 114043. [Google Scholar] [CrossRef]
- Kofoed, R.H.; Aubert, I. Focused Ultrasound Gene Delivery for the Treatment of Neurological Disorders. Trends Mol. Med. 2024, 30, 263–277. [Google Scholar] [CrossRef]
- Navarro-Marchal, S.A.; GrinÌ án-Lisan, C.; Entrena, J.M.; Ruiz-Alcalá, G.; Tristán-Manzano, M.; Martin, F.; Pérez-Victoria, I.; Peula-Garciá, J.M.; Marchal, J.A. Anti-CD44-Conjugated Olive Oil Liquid Nanocapsules for Targeting Pancreatic Cancer Stem Cells. Biomacromolecules 2021, 22, 1374–1388. [Google Scholar] [CrossRef]
- McAndrews, K.M.; Xiao, F.; Chronopoulos, A.; LeBleu, V.S.; Kugeratski, F.G.; Kalluri, R. Exosome-Mediated Delivery of CRISPR/Cas9 for Targeting of Oncogenic KrasG12D in Pancreatic Cancer. Life Sci. Alliance 2021, 4, e202000875. [Google Scholar] [CrossRef]


| S.No. | Nanoparticles | Biocompatibility | Cellular Uptake/Endosomal Escape | Gene-Editing Efficiency | Key Advantages | Major Limitations |
|---|---|---|---|---|---|---|
| 1 | Lipid nanoparticles (LNP) | High | High | High | Proven clinical track-record (e.g., NTLA-2001); protects DNA; ligand-targetable | Possible immune activation; nuclear entry required; size-linked toxicity |
| 2 | LNPs-RNPs | High | High | Very high | Rapid editing; low off-target risk; >80% KO in vivo with SORT | Endosomal escape dose-limiting; off-target liver/spleen accumulation |
| 3 | mRNA + sgRNA (mainly LNP/iLNP) | High | Moderate–High | High | No nuclear step; fast; modified bases ↓ immunogenicity | mRNA instability and bulk; co-packaging challenge |
| 4 | Polymeric NPs (PBAE, dendrimer, chitosan)-plasmid | Moderate–High | Moderate–High | Moderate–High | Tunable charge/degradation; large payloads | Dose-dependent cytotoxicity; reproducibility |
| 5 | Polymeric NPs-RNP | High | High | High–Very high | Stable in blood; DNA-nanocages allow precise loading | Polymer-RNP stability and complement activation |
| 6 | Inorganic NPs (AuNP, CuS) | Moderate | High | High | Photothermal NIR release; tunable size/shape | Hepatic/splenic deposition; cost (Au) |
| 7 | MOF/ZIF | Moderate–High | Moderate | Moderate | Highly porous; stimuli-responsive; membrane-coatable | Early stage; potential metal-ion toxicity |
| 8 | Silica nanostructures/HMSN | High | Moderate–High | High | Huge loading; robust; TME-responsive | Complex surface chemistry; optimize escape |
| 9 | Extracellular vesicles (EVs) | Very high | Moderate | Moderate | Immune-silent; crosses BBB; intrinsic tropism | Low yield/loading heterogeneity; upscale cost |
| 10 | Polymeric micelles | High | Moderate | Moderate | Simple self-assembly; co-delivery friendly | Limited core size for RNP; premature unpacking |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rauf, M.A.; Rao, A.; Sivasoorian, S.S.; Iyer, A.K. Nanotechnology-Based Delivery of CRISPR/Cas9 for Cancer Treatment: A Comprehensive Review. Cells 2025, 14, 1136. https://doi.org/10.3390/cells14151136
Rauf MA, Rao A, Sivasoorian SS, Iyer AK. Nanotechnology-Based Delivery of CRISPR/Cas9 for Cancer Treatment: A Comprehensive Review. Cells. 2025; 14(15):1136. https://doi.org/10.3390/cells14151136
Chicago/Turabian StyleRauf, Mohd Ahmar, Afifa Rao, Siva Sankari Sivasoorian, and Arun K. Iyer. 2025. "Nanotechnology-Based Delivery of CRISPR/Cas9 for Cancer Treatment: A Comprehensive Review" Cells 14, no. 15: 1136. https://doi.org/10.3390/cells14151136
APA StyleRauf, M. A., Rao, A., Sivasoorian, S. S., & Iyer, A. K. (2025). Nanotechnology-Based Delivery of CRISPR/Cas9 for Cancer Treatment: A Comprehensive Review. Cells, 14(15), 1136. https://doi.org/10.3390/cells14151136








