The Role of Cytokine Gene Polymorphisms in Rehabilitation Outcome After Traumatic Brain Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Assessment
2.2. Rehabilitation Protocol
2.3. Additional Clinical Considerations
2.4. Genetic Analysis
2.5. Statistical Analysis
3. Results
3.1. Cytokine Genetic Characterization
3.2. Outcome of the Rehabilitation Treatment
3.3. Co-Segregation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Iaccarino, C.; Gerosa, A.; Viaroli, E. Epidemiology of Traumatic Brain Injury. In Traumatic Brain Injury: Science, Practice, Evidence and Ethics; Springer: Berlin/Heidelberg, Germany, 2021; pp. 3–11. [Google Scholar] [CrossRef]
- Capizzi, A.; Woo, J.; Verduzco-Gutierrez, M. Traumatic Brain Injury: An Overview of Epidemiology, Pathophysiology, and Medical Management. Med. Clin. N. Am. 2020, 104, 213–238. [Google Scholar] [CrossRef] [PubMed]
- Lakshmipathy, D.; Rangarajan, S.; Barreau, A.; Lu, J.; Kleinberg, G.; Lucke-Wold, B. Genetic Contributions to Recovery following Brain Trauma: A Narrative Review. Front. Biosci. (Landmark Ed.) 2024, 29, 103. [Google Scholar] [CrossRef]
- Galgano, M.; Toshkezi, G.; Qiu, X.; Russell, T.; Chin, L.; Zhao, L.R. Traumatic Brain Injury: Current Treatment Strategies and Future Endeavors. Cell Transplant. 2017, 26, 1118–1130. [Google Scholar] [CrossRef]
- Kumar, J.; Patel, T.; Sugandh, F.; Dev, J.; Kumar, U.; Adeeb, M.; Kachhadia, M.P.; Puri, P.; Prachi, F.; Zaman, M.U.; et al. Innovative Approaches and Therapies to Enhance Neuroplasticity and Promote Recovery in Patients With Neurological Disorders: A Narrative Review. Cureus 2023, 15, e41914. [Google Scholar] [CrossRef]
- Hanafy, S.; Xiong, C.; Chan, V.; Sutton, M.; Escobar, M.; Colantonio, A.; Mollayeva, T. Comorbidity in traumatic brain injury and functional outcomes: A systematic review. Eur. J. Phys. Rehabil. Med. 2021, 57, 535–550. [Google Scholar] [CrossRef]
- Cáceres, E.; Olivella, J.C.; Di Napoli, M.; Raihane, A.S.; Divani, A.A. Immune Response in Traumatic Brain Injury. Curr. Neurol. Neurosci. Rep. 2024, 24, 593–609. [Google Scholar] [CrossRef] [PubMed]
- Freire, M.A.M.; Rocha, G.S.; Bittencourt, L.O.; Falcao, D.; Lima, R.R.; Cavalcanti, J.R.L.P. Cellular and Molecular Pathophysiology of Traumatic Brain Injury: What Have We Learned So Far? Biology 2023, 12, 1139. [Google Scholar] [CrossRef] [PubMed]
- Waters, R.J.; Murray, G.D.; Teasdale, G.M.; Stewart, J.; Day, I.; Lee, R.J.; Nicoll, J.A. Cytokine gene polymorphisms and outcome after traumatic brain injury. J. Neurotrauma 2013, 30, 1710–1716. [Google Scholar] [CrossRef]
- Goyal, L.; Singh, S. Neurological Manifestations Following Traumatic Brain Injury: Role of Behavioral, Neuroinflammation, Excitotoxicity, Nrf-2 and Nitric Oxide. CNS Neurol. Disord. Drug Targets 2025, 24, 47–59. [Google Scholar] [CrossRef]
- Lassarén, P.; Lindblad, C.; Frostell, A.; Carpenter, K.L.H.; Guilfoyle, M.R.; Hutchinson, P.J.A.; Helmy, A.; Thelin, E.P. Systemic inflammation alters the neuroinflammatory response: A prospective clinical trial in traumatic brain injury. J. Neuroinflamm. 2021, 18, 221. [Google Scholar] [CrossRef]
- Vedantam, A.; Brennan, J.; Levin, H.S.; McCarthy, J.J.; Dash, P.K.; Redell, J.B.; Yamal, J.M.; Robertson, C.S. Early versus Late Profiles of Inflammatory Cytokines after Mild Traumatic Brain Injury and Their Association with Neuropsychological Outcomes. J. Neurotrauma 2021, 38, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Porro, C.; Cianciulli, A.; Panaro, M.A. The Regulatory Role of IL-10 in Neurodegenerative Diseases. Biomolecules 2020, 10, 1017. [Google Scholar] [CrossRef] [PubMed]
- Bidwell, J.; Keen, L.; Gallagher, G.; Kimberly, R.; Huizinga, T.; McDermott, M.F.; Oksenberg, J.; McNicholl, J.; Pociot, F.; Hardt, C.; et al. Cytokine gene polymorphism in human disease: On-line databases. Genes Immun. 1999, 1, 3–19. [Google Scholar] [CrossRef]
- Maat, M.P.M.; Bladbjerg, E.M.; Hjelmborg, J.B.; Bathum, L.; Jespersen, J.; Christensen, K. Genetic influence on inflammation variables in the elderly. Arter. Thromb. Vasc. Biol. 2024, 24, 2168–2173. [Google Scholar] [CrossRef]
- Frugier, T.; Morganti-Kossmann, M.C.; O’Reilly, D.; McLean, C.A. In situ detection of inflammatory mediators in post mortem human brain tissue after traumatic injury. J. Neurotrauma 2010, 27, 497–507. [Google Scholar] [CrossRef]
- Pereira, D.S.; Mateo, E.C.; de Queiroz, B.Z.; Assumpção, A.M.; Miranda, A.S.; Felício, D.C.; Rocha, N.P.; da Cruz dos Anjos, D.M.; Pereira, D.A.; Teixeira, A.L.; et al. TNF-α, IL6, and IL10 polymorphisms and the effect of physical exercise on inflammatory parameters and physical performance in elderly women. Age 2013, 35, 2455–2463. [Google Scholar] [CrossRef][Green Version]
- Dubois, B.; Slachevsky, A.; Litvan, I.; Pillon, B. The FAB: A Frontal Assessment Battery at bedside. Neurology 2000, 55, 1621–1626. [Google Scholar] [CrossRef] [PubMed]
- Derchi, C.C.; Arcuri, P.; Comanducci, A.; Caronni, A.; Pagliari, C.; Viganò, A.; Volpato, E.; Navarro, J.; Trimarchi, P.D. Italian translation and cultural adaptation of the Agitated Behavior Scale (ABS-I) in patients with acquired brain injuries. J. Rehabil. Med. 2024, 56, jrm11663. [Google Scholar] [CrossRef]
- Fi, M.; Dw, B. Functional evaluation: The Barthel index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar]
- Tinetti, M.E. Performance-oriented assessment of mobility problems in elderly patients. J. Am. Geriatr. Soc. 1986, 34, 119–126. [Google Scholar] [CrossRef]
- Wilson, L.; Boase, K.; Nelson, L.D.; Temkin, N.R.; Giacino, J.T.; Markowitz, A.J.; Maas, A.; Menon, D.K.; Teasdale, G.; Manley, G.T. A Manual for the Glasgow Outcome Scale-Extended Interview. J. Neurotrauma 2021, 38, 2435–2446. [Google Scholar] [CrossRef]
- Hammond, F.M.; Ketchum, J.M.; Patni, V.V.; Nejadnik, B.; Bates, D.; Weintraub, A.H. Determining the Minimally Clinically Important Difference for the Disability Rating Scale in Persons With Chronic Traumatic Brain Injury. Neurotrauma Rep. 2023, 4, 447–457. [Google Scholar] [CrossRef]
- Levin, H.S.; O’Donnell, V.M.; Grossman, R.G. The Galveston Orientation and Amnesia Test: A practical scale to assess cognition after head injury. J. Nerv. Ment. Dis. 1979, 167, 675–684. [Google Scholar] [CrossRef]
- Flannery, J. Using the levels of cognitive functioning assessment scale with patients with traumatic brain injury in an acute care setting. Rehabil. Nurs. 1998, 23, 88–94. [Google Scholar] [CrossRef]
- Shi, Y.Y.; He, L. SHEsis, a powerful software platform for analyses of linkage disequilibrium, haplotype construction, and genetic association at polymorphism loci. Cell Res. 2005, 15, 97–98. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.; He, Z.; Tang, W.; Li, T.; Zeng, Z.; He, L.; Shi, Y. A partition-ligation-combination-subdivision EM algorithm for haplotype inference with multiallelic markers: Update of the SHEsis (http://analysis.bio-x.cn). Cell Res. 2009, 4, 519–523. [Google Scholar] [CrossRef]
- Duan, R.; Wang, N.; Shang, Y.; Li, H.; Liu, Q.; Li, L.; Zhao, X. TNF-α (G-308A) Polymorphism, Circulating Levels of TNF-α and IGF-1: Risk Factors for Ischemic Stroke-An Updated Meta-Analysis. Front. Aging Neurosci. 2022, 14, 831910. [Google Scholar] [CrossRef]
- Ghaith, H.S.; Nawar, A.A.; Gabra, M.D.; Abdelrahman, M.E.; Nafady, M.H.; Bahbah, E.I.; Ebada, M.A.; Ashraf, G.M.; Negida, A.; Barreto, G.E. A Literature Review of Traumatic Brain Injury Biomarkers. Mol. Neurobiol. 2022, 7, 4141–4158. [Google Scholar] [CrossRef]
- Jeong, P.; Kim, E.J.; Kim, E.G.; Byun, S.S.; Kim, C.S.; Kim, W.J. Association of bladder tumors and GA genotype of -308 nucleotide in tumor necrosis factor-alpha promoter with greater tumor necrosis factor-alpha expression. Urology 2004, 64, 1052–1056. [Google Scholar] [CrossRef]
- Liang, J.; Su, Y.; Wang, N.; Wang, X.; Hao, L.; Ren, C. A meta-analysis of the association between inflammatory cytokine polymorphism and neonatal sepsis. PLoS ONE 2024, 19, e0301859. [Google Scholar] [CrossRef]
- Sadowski, K.; Zając, W.; Milanowski, Ł.; Koziorowski, D.; Figura, M. Exploring Fecal Microbiota Transplantation for Modulating Inflammation in Parkinson’s Disease: A Review of Inflammatory Markers and Potential Effects. Int. J. Mol. Sci. 2024, 25, 7741. [Google Scholar] [CrossRef]
- Bounder, G.; Jouimyi, M.R.; Boura, H.; Touati, E.; Michel, V.; Badre, W.; Jouhadi, H.; Kadi, M.; Eljihad, M.; Benomar Het, a.l. Associations of the -238(G/A) and -308(G/A) TNF-α Promoter Polymorphisms and TNF-α Serum Levels with the Susceptibility to Gastric Precancerous Lesions and Gastric Cancer Related to Helicobacter pylori Infection in a Moroccan Population. Asian Pac. J. Cancer Prev. 2020, 21, 1623–1629. [Google Scholar] [CrossRef]
- Alsbrook, D.L.; Di Napoli, M.; Bhatia, K.; Biller, J.; Andalib, S.; Hinduja, A.; Rodrigues, R.; Rodriguez, M.; Sabbagh, S.Y.; Selim, M.; et al. Neuroinflammation in Acute Ischemic and Hemorrhagic Stroke. Curr. Neurol. Neurosci. Rep. 2023, 23, 407–431. [Google Scholar] [CrossRef]
- Doğanyiğit, Z.; Erbakan, K.; Akyuz, E.; Polat, A.K.; Arulsamy, A.; Shaikh, M.F. The Role of Neuroinflammatory Mediators in the Pathogenesis of Traumatic Brain Injury: A Narrative Review. ACS Chem. Neurosci. 2022, 13, 1835–1848. [Google Scholar] [CrossRef]
- Ross, S.A.; Halliday, M.I.; Campbell, G.C.; Byrnes, D.P.; Rowlands, B.J. The presence of tumour necrosis factor in CSF and plasma after severe head injury. Br. J. Neurosurg. 1994, 8, 419–425. [Google Scholar] [CrossRef]
- Tiainen, K.; Hurme, M.; Hervonen, A.; Luukkaala, T.; Jylhä, M. Inflammatory markers and physical performance among nonagenarians. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 658–663. [Google Scholar] [CrossRef]
- Visser, M.; Goodpaster, B.H.; Kritchevsky, S.B.; Newman, A.B.; Nevitt, M.; Rubin, S.M.; Simonsick, E.M.; Harris, T.B. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60A, 324–333. [Google Scholar] [CrossRef]
- Gallucci, M.; Amici, G.P.; Ongaro, F.; Gajo, G.B.; De Angeli, S.; Forloni, G.L.; Albani, D.; Prato, F.; Polito, L.; Zanardo, A.; et al. Associations of theplasma interleukin-6 (IL-6) levels with disability and mortality in the elderly in the Treviso Longeva (TRELONG) Study. Arch. Gerontol. Geriatr. 2007, 44 (Suppl. 1), 193–198. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.S.; Garcia, D.M.; Narciso, F.M.; Santos, M.L.; Dias, J.M.; Queiroz, B.Z.; Souza, E.R.; Nóbrega, O.T.; Pereira, L.S. Effects of 174 G/C polymorphism in the promoter region of the interleukin-6 gene on plasma IL-6 levels and muscle strength in elderly women. Braz. J. Med. Biol. Res. 2011, 44, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Dalla Libera, A.L.; Regner, A.; de Paoli, J.; Centenaro, L.; Martins, T.T.; Simon, D. IL-6 polymorphism associated with fatal outcome in patients with severe traumatic brain injury. Brain Inj. 2011, 25, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Sinh, S.; Monsoori, N.; Mukhopadhyay, A.; Sharma, B. Effect of IL-6-174 G/C polymorphism in predicting disability and functional outcome in patients with severe traumatic brain injury (STBI). J. Neurosurg. 2014, 122, 673. [Google Scholar]
- Ciryam, P.; Gerzanich, V.; Simard, J.M. Interleukin-6 in Traumatic Brain Injury: A Janus-Faced Player in Damage and Repair. J. Neurotrauma 2023, 40, 2249–2269. [Google Scholar] [CrossRef]
- Ooi, S.Z.Y.; Spencer, R.J.; Hodgson, M.; Mehta, S.; Phillips, N.L.; Preest, G.; Manivannan, S.; Wise, M.P.; Galea, J.; Zaben, M. Interleukin-6 as a prognostic biomarker of clinical outcomes after traumatic brain injury: A systematic review. Neurosurg. Rev. 2022, 45, 3035–3054. [Google Scholar] [CrossRef]
- Ferreira, R.C.; Freitag, D.F.; Cutler, A.J.; Howson, J.M.; Rainbow, D.B.; Smyth, D.J.; Kaptoge, S.; Clarke, P.; Boreham, C.; Coulson, R.M.; et al. Functional IL6R 358Ala Allele Impairs Classical IL-6 Receptor Signaling and Influences Risk of Diverse Inflammatory Diseases. PLoS Genet. 2013, 9, e1003444. [Google Scholar] [CrossRef]
- Zakharyan, R.; Petrek, M.; Arakelyan, A.; Mrazek, F.; Atshemyan, S.; Boyajyan, A. Interleukin-6 promoter polymorphism and plasma levels in patients with schizophrenia. Tissue Antigens 2012, 80, 136–142. [Google Scholar] [CrossRef]
- Nakahara, Y.; Kouro, T.; Motoyama, S.; Miura, M.; Fujita, K.; Igarashi, Y.; Higashijima, N.; Matsuo, N.; Himuro, H.; Wei, F.; et al. Circulating IL-6 and not its circulating signaling components sIL-6R and sgp130 demonstrate clinical significance in NSCLC patients treated with immune checkpoint inhibitors. Front. Cell Dev. Biol. 2024, 11, 1324898. [Google Scholar] [CrossRef]
- Calderone, A.; Latella, D.; Cardile, D.; Gangemi, A.; Corallo, F.; Rifici, C.; Quartarone, A.; Calabrò, R.S. The Role of Neuroinflammation in Shaping Neuroplasticity and Recovery Outcomes Following Traumatic Brain Injury: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 11708. [Google Scholar] [CrossRef]
- Bian, A.L.; Hu, H.Y.; Rong, Y.D.; Wang, J.; Wang, J.X.; Zhou, X.Z. A study on relationship between elderly sarcopenia and inflammatory factors IL-6 and TNF-α. Eur. J. Med. Res. 2017, 22, 25. [Google Scholar] [CrossRef]
- Stratos, I.; Behrendt, A.K.; Anselm, C.; Gonzalez, A.; Mittlmeier, T.; Vollmar, B. Inhibition of TNF-α Restores Muscle Force, Inhibits Inflammation, and Reduces Apoptosis of Traumatized Skeletal Muscles. Cells 2022, 11, 2397. [Google Scholar] [CrossRef] [PubMed]
- Kummer, K.K.; Zeidler, M.; Kalpachidou, T.; Kress, M. Role of IL-6 in the regulation of neuronal development, survival and function. Cytokine 2021, 144, 155582. [Google Scholar] [CrossRef] [PubMed]
- McKee, C.A.; Lukens, J.R. Emerging Roles for the Immune System in Traumatic Brain Injury. Front. Immunol. 2016, 7, 556. [Google Scholar] [CrossRef]
- Simon, D.W.; McGeachy, M.J.; Bayır, H.; Clark, R.S.; Loane, D.J.; Kochanek, P.M. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat. Rev. Neurol. 2017, 13, 171–191. [Google Scholar] [CrossRef]
| Genotype | n (%) | HWE p-Value | |
|---|---|---|---|
| IL1A-2231 G/A | G G | 14 (50.0) | 0.79 |
| A G | 12 (42.9) | ||
| A A | 2 (7.1) | ||
| IL-1B-3 1G/A | G G | 7 (25.0) | 0.23 |
| G A | 17 (60.7) | ||
| A A | 4 (14.3) | ||
| IL-6-174 G/C | G G | 13 (46.4) | 0.51 |
| G C | 11 (39.3) | ||
| C C | 4 (14.3) | ||
| IL-6R 1073 A/C | A A | 10 (35.7) | 0.45 |
| A C | 15 (53.6) | ||
| C C | 3 (10.7) | ||
| IL-10-1082 T/C | T T | 10 (35.7) | 0.28 |
| T C | 11 (39.3) | ||
| C C | 7 (25.0) | ||
| IL10-592 G/T | G G | 21 (75.0) | 0.45 |
| G T | 7 (25.0) | ||
| TNF-α-238 G/A | G G | 26 (92.9) | 0.84 |
| G A | 2 (7.1) | ||
| TNF-α-308 G/A | G G | 23 (82.1) | 0.60 |
| G A | 5 (17.9) |
| Score | Delta Values | |||
|---|---|---|---|---|
| Mean (sd) | Mean | 95%CI | p-Value | |
| GOSE T0 | 3.7 (0.5) | 2.0 | 1.65:2.35 | <0.001 |
| GOSE T1 | 5.7 (1.2) | |||
| FAB T0 | 10.6 (5.5) | 3.3 | 2.01:4.58 | <0.001 |
| FAB T1 | 13.9 (5.4) | |||
| ABS T0 | 22.9 (6.9) | −5.8 | −7.8:−3.8 | <0.001 |
| ABS T1 | 17.2 (4.1) | |||
| DRS T0 | 14.8 (4.5) | −9.7 | −11.5:−7.9 | <0.001 |
| DRS T1 | 5.10 (3.9) | |||
| BI T0 | 22.9 (25.2) | 63.2 | 52.9:73.5 | <0.001 |
| BI T1 | 86.1 (19.9) | |||
| POMA T0 | 10.4 (9.9) | 14.7 | 11.1:18.4 | <0.001 |
| POMA T1 | 25.1 (6.0) | |||
| (n of subjects) yes/no | success rate | 95% CI | ||
| Presence of PTCS at T0 according to GOAT | 16/12 | 11/16 | 41.3%:88.9% | |
| Presence of PTCS at T1 according to GOAT | 5/11 | |||
| Delta GOSE | ||||
|---|---|---|---|---|
| Beta Value | 95%CI | p-Value | ||
| (Intercept) | 1.56 | −2.52 | 5.64 | 0.43 |
| Age | −0.01 | −0.03 | 0.01 | 0.27 |
| GOSE at admission | 0.58 | 0.39 | 1.47 | 0.16 |
| Sex [M vs. F] | −0.01 | −0.99 | 0.96 | 0.97 |
| Systemic infections [yes vs no] | 0.11 | −0.75 | 0.97 | 0.79 |
| NSAIDs and corticosteroids [yes vs no] | −0.58 | −1.64 | 0.48 | 0.27 |
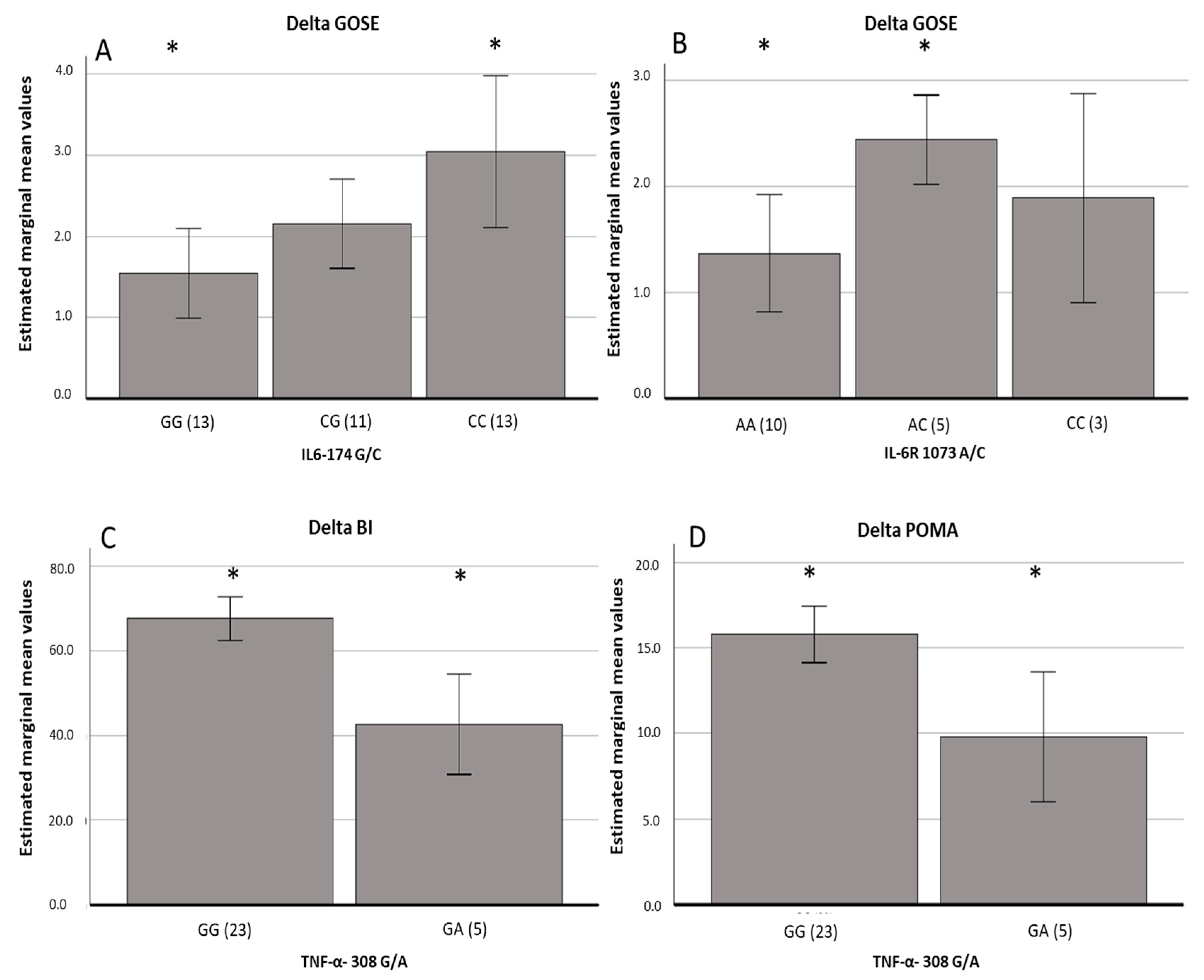
| IL-6-174 [GC vs. CC] | −0.89 | −1.95 | 0.17 | 0.09 |
| IL-6-174 [GG vs. CC] | −1.49 | −2.74 | −0.25 | 0.02 |
| (Intercept) | −0.36 | 1.55 | −0.23 | 0.82 |
| Age | 0.01 | 0.01 | 0.51 | 0.62 |
| GOSE at admission | 0.42 | 0.40 | 1.04 | 0.31 |
| Sex [M vs. F] | −0.11 | 0.45 | −0.25 | 0.80 |
| Systemic infections [yes vs. no] | 0.07 | 0.39 | 0.18 | 0.86 |
| NSAIDs and corticosteroids [yes vs. no] | 0.32 | 0.41 | 0.77 | 0.45 |
| IL-6R 1073 [AC vs. AA] | 1.07 | 0.37 | 2.91 | 0.01 |
| IL-6R 1073 [CC vs. AA] | 0.52 | 0.59 | 0.87 | 0.39 |
| (Intercept) | 91.02 | 68.99 | 113.05 | <0.001 |
| Age | −0.59 | −0.91 | −0.28 | <0.001 |
| BI at admission | −0.70 | −0.91 | −0.50 | <0.001 |
| Sex [M vs. F] | −4.55 | −18.86 | 9.76 | 0.52 |
| Systemic infections [yes vs. no] | −1.70 | −14.36 | 10.95 | 0.78 |
| NSAIDs and corticosteroids [yes vs. no] | −2.72 | −16.31 | 10.85 | 0.68 |
| TNF-alpha 308 [GA vs. GG] | −24.95 | −38.81 | −11.09 | 0.001 |
| (Intercept) | 23.01 | 15.95 | 30.07 | <0.001 |
| Age | −0.13 | −0.24 | −0.05 | 0.02 |
| POMA admission | −0.77 | −0.96 | −0.57 | <0.001 |
| Sex [M vs. F] | 1.53 | −3.19 | 6.24 | 0.51 |
| Systemic infections [yes vs. no] | −3.46 | −7.67 | 0.74 | 0.10 |
| NSAIDs and corticosteroids [yes vs. no] | 2.27 | −2.03 | 6.58 | 0.28 |
| TNF-alpha 308 [GA vs. GG] | −5.99 | −10.45 | −1.55 | 0.01 |
| Allelic Co-Segregation Association with Delta GOSE | |||
|---|---|---|---|
| IL-6-174 G/C | IL-6R 1073 A/C | Beta Value | p-Value |
| C | A | 0.22 | 0.47 |
| G | A | −0.66 | 0.004 |
| G | C | 0.09 | 0.74 |
| C | C | 1.11 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerini, F.R.; Agliardi, C.; Zanzottera, M.; Caronni, A.; Antolini, L.; Derchi, C.C.; Atzori, T.; Bolognesi, E.; Navarro, J.; Clerici, M.; et al. The Role of Cytokine Gene Polymorphisms in Rehabilitation Outcome After Traumatic Brain Injury. Cells 2025, 14, 1056. https://doi.org/10.3390/cells14141056
Guerini FR, Agliardi C, Zanzottera M, Caronni A, Antolini L, Derchi CC, Atzori T, Bolognesi E, Navarro J, Clerici M, et al. The Role of Cytokine Gene Polymorphisms in Rehabilitation Outcome After Traumatic Brain Injury. Cells. 2025; 14(14):1056. https://doi.org/10.3390/cells14141056
Chicago/Turabian StyleGuerini, Franca Rosa, Cristina Agliardi, Milena Zanzottera, Antonio Caronni, Laura Antolini, Chiara Camilla Derchi, Tiziana Atzori, Elisabetta Bolognesi, Jorge Navarro, Mario Clerici, and et al. 2025. "The Role of Cytokine Gene Polymorphisms in Rehabilitation Outcome After Traumatic Brain Injury" Cells 14, no. 14: 1056. https://doi.org/10.3390/cells14141056
APA StyleGuerini, F. R., Agliardi, C., Zanzottera, M., Caronni, A., Antolini, L., Derchi, C. C., Atzori, T., Bolognesi, E., Navarro, J., Clerici, M., & Comanducci, A. (2025). The Role of Cytokine Gene Polymorphisms in Rehabilitation Outcome After Traumatic Brain Injury. Cells, 14(14), 1056. https://doi.org/10.3390/cells14141056







