Future Perspectives and Conclusions from Animal Models of CHI3L1-Related Inflammation-Associated Cancer
Abstract
1. Introduction
2. Basic Biological Role of CHI3L1
3. Potential Role of CHI3L1 in Inflammation-Associated Cancer Development
4. Animal Models of CHI3L1-Related Cancer
4.1. CHI3L1 Overexpression Models
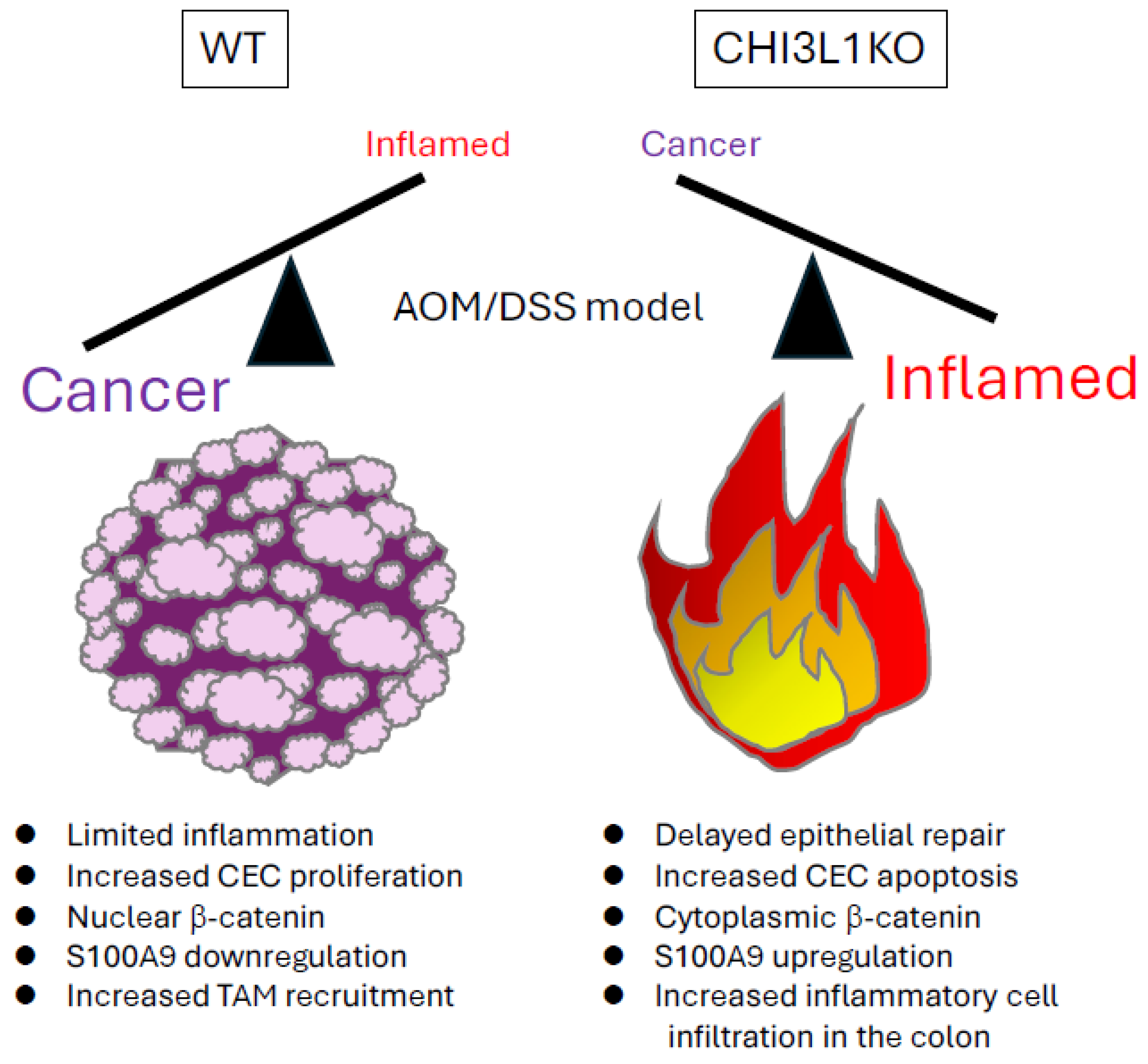
4.2. CHI3L1 Knockout Models
4.3. Xenograft and Syngeneic Tumor Models
4.4. CHI3L1-Driven Inflammation-Associated Cancer Models
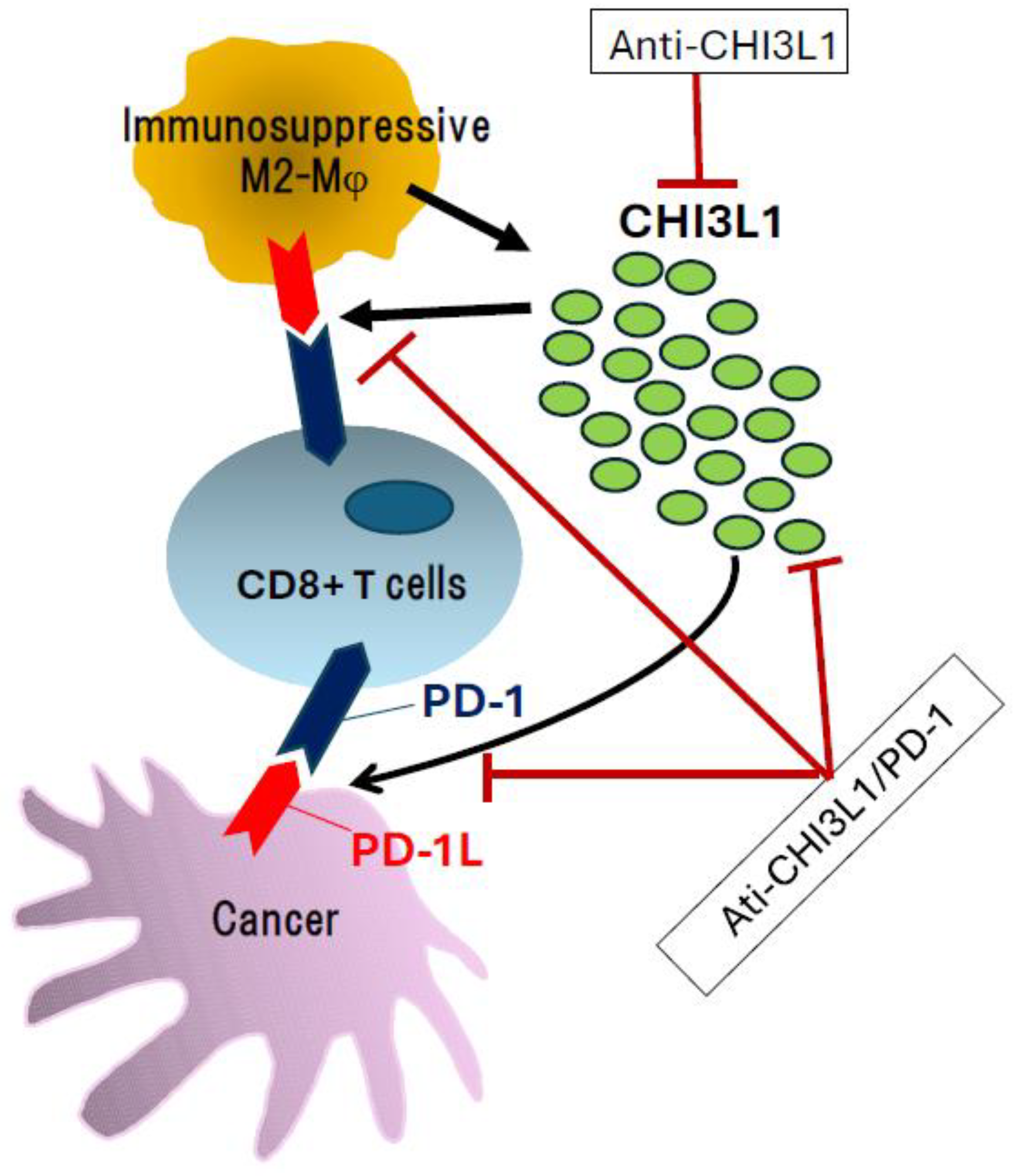
5. Translational Implications as Clinical Biomarkers and Potential Therapeutic Target
6. Future Perspectives and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Virchow, R.L.K.; Chance, F. Cellular Pathology as Based upon Physiological and Pathological History; Kessinger Publishing: Whitefish, MT, USA, 1863. [Google Scholar]
- Privitera, G.; Williams, J.J.; De Salvo, C. The importance of Th2 immune responses in mediating the progression of gastritis-associated metaplasia to gastric cancer. Cancers 2024, 16, 522. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Kakizoe, T. Synergistic interaction between Helicobacter pylori gastritis and diet in gastric cancer. Lancet Oncol. 2001, 2, 88–94. [Google Scholar] [CrossRef]
- Shan, S.C.; Itzkowitz, S.H. Colorectal cancer in inflammatory bowel disease: Mechanisms and management. Gastroenterology 2022, 162, 715–730. [Google Scholar]
- Francescone, R.; Hou, V.; Grivennikov, S.I. Cytokines, IBD, and colitis-associated cancer. Inflamm. Bowel Dis. 2015, 21, 409–418. [Google Scholar] [CrossRef]
- Dougan, M.; Li, D.; Neuberg, D.; Mihm, M.; George, P.; Wong, K.K.; Dranoff, G. A dual role for the immune response in a mouse of inflammation-associated lung cancer. J. Clin. Investig. 2011, 121, 2436–2446. [Google Scholar] [CrossRef]
- Schroedl, C.; Kalhan, R. Incidence, treatment options, and outcomes of lung cancer in patients with chronic obstructive pulmonary disease. Curr. Opin. Pulm. Med. 2012, 18, 131–137. [Google Scholar] [CrossRef]
- Khalaf, K.; Hana, D.; Chou, J.T.; Singh, C.; Mackiewicz, A.; Kaczmarek, M. Aspects of the tumor microenvironment involved in immune resistance and drug resistance. Front. Immunol. 2021, 12, 656364. [Google Scholar] [CrossRef]
- Muthusami, S.; Ramachandran, I.K.; Babu, K.N.; Krishnamoorthy, S.; Guruswamy, A.; Queimado, L.; Chaudhuri, G.; Ramachandran, I. Role of inflammation in the development of colorectal cancer. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 77–90. [Google Scholar]
- Nagatani, K.; Wang, S.; Llado, V.; Lau, C.W.; Li, Z.; Mizoguchi, A.; Nagler, C.R.; Shibata, Y.; Reinecker, H.C.; Mora, J.R.; et al. Chitin microparticles for the control of intestinal inflammation. Inflamm. Bowel Dis. 2012, 18, 1698–1710. [Google Scholar] [CrossRef]
- Ellen Ali Komi, D.; Sharmal, L.; Dela Cruz, C.S. Chitin and its effect on inflammatory and immune responses. Clin. Rev. Allergy Immunol. 2018, 54, 213–223. [Google Scholar] [CrossRef]
- Reese, T.A.; Liang, H.E.; Tager, A.M.; Luster, A.D.; Van Rooijen, N.; Voehringer, D.; Locksley, R.M. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature 2007, 447, 92–96. [Google Scholar] [CrossRef]
- Hakala, B.E.; White, C.; Recjkies, A.D. Human cartilage gp-39, a major secretory product of articular chondrocytes and synovial cells, is a member of a chitinase protein family. J. Biol. Chem. 1993, 268, 25803–25810. [Google Scholar] [CrossRef]
- Boot, R.G.; Blommaart, E.F.; Swart, E.; Ghauharali-van der Vlugt, K.; Bijl, N.; Moe, C.; Place, A.; Aerts, J.M. Identification of a novel acidic mammalian chitinase distinct from chitotriosidase. J. Biol. Chem. 2001, 276, 6770–6778. [Google Scholar] [CrossRef]
- Zhu, Z.; Zheng, T.; Hormer, R.J.; Kim, Y.K.; Chen, N.Y.; Cohn, L.; Hamid, Q.; Elias, J.A. Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science 2004, 304, 1678–1682. [Google Scholar] [CrossRef]
- Van Aalten, D.M.; Komander, D.; Synstad, B.; Gaseidnes, S.; Peter, M.G.; Eijsink, V.G. Structural insights into the catalytic mechanism of a family 18 exo-chitinase. Proc. Natl. Acad. Sci. USA 2000, 98, 8979–8984. [Google Scholar] [CrossRef]
- Lee, C.G.; Da Silva, C.A.; Dela Cruz, C.S.; Ahangarl, F.; Ma, B.; Kang, M.J.; He, C.H.; Takyar, S.; Elias, J.A. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu. Rev. Physiol. 2011, 73, 479–501. [Google Scholar] [CrossRef]
- Johansen, J.S.; Jensen, B.V.; Roslind, A.; Nielsen, D.; Price, P.A. Serum YKL-40: A new prognostic biomarker in cancer and inflammation. Cancer Epidermiol. Biomark. Prev. 2006, 15, 194–202. [Google Scholar] [CrossRef]
- Johansen, J.S. Studies on serum YKL-40 as a biomarker in disease with inflammation, tissue remodeling, fibroses and cancer. Dan. Med. Bull. 2006, 53, 172–209. [Google Scholar]
- Zhao, T.; Su, Z.; Li, Y.; Zhang, X.; You, Q. Chitinase-3 like-protein-1 function and its role in diseases. Signal Transduct. Target Ther. 2020, 5, 201. [Google Scholar] [CrossRef]
- He, C.H.; Lee, C.G.; Ma, B.; Kamle, S.; Choi, A.M.K.; Elias, J.A. N-Glycosylation regulates chitinase 3-like-1 and IL-13 ligand binding to IL-13 receptor α2. Am. J. Respir. Cell Mol. Biol. 2020, 63, 386–395. [Google Scholar] [CrossRef]
- He, C.H.; Lee, C.G.; Dela Cruz, C.S.; Lee, C.M.; Zhou, Y.; Ahangari, F.; Ma, B.; Herzong, E.L.; Rosenberg, S.A.; Li, Y.; et al. Chitinase 3-like-1 regulates cellular and tissue responses via IL-13 receptor α2. Cell Rep. 2013, 4, 830–841. [Google Scholar] [CrossRef] [PubMed]
- Kawada, M.; Seno, H.; Kanda, K.; Nakanishi, Y.; Akitake, R.; Komekado, H.; Kawada, K.; Sakai, Y.; Mizoguchi, E.; Chiba, T. Chitinase 3-like 1 promotes macrophage recruitment and angiogenesis in colorectal cancer. Oncogene 2012, 31, 3111–3123. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, S.; Jia, Z.; Hu, Y.; Cao, D.; Yang, M.; Liu, L.; Gao, L.; Qiu, S.; Yan, W.; et al. YKL-40 derived from infiltrating macrophages cooperates with GDF15 to establish an immune suppressive microenvironment in gallbladder cancer. Cancer Lett. 2023, 563, 216184. [Google Scholar] [CrossRef]
- Lee, C.G.; Hartl, D.; Lee, G.R.; Koller, B.; Matsuura, H.; Da Silva, C.A.; Sohn, M.H.; Cohn, L.; Homer, R.J.; Kozhich, A.A.; et al. Role of breast regression protein 39(BRP-39)/chitinase 3-like-1 in Th2 and IL-13-induced tissue responses and apoptosis. J. Exp. Med. 2009, 206, 1149–1166. [Google Scholar] [CrossRef]
- Yu, J.E.; Jeon, S.H.; Kim, M.J.; Kim, D.H.; Koo, J.K.; Kim, T.H.; Kim, B.; Yoon, J.Y.; Lim, Y.S.; Park, S.R.; et al. Anti-Chitinase-3-like 1 antibody attenuated atopic dermatitis-like skin inflammation through inhibition of STAT3-dependent CXCL8 expression. Br. J. Pharmacol. 2024, 181, 3232–3245. [Google Scholar] [CrossRef]
- Mizoguchi, E. Chitinase 3-like-1 exacerbates intestinal inflammation by enhancing bacterial adhesion and invasion in colonic epithelial cells. Gastroenterology 2006, 130, 398–411. [Google Scholar] [CrossRef]
- Renkema, G.H.; Boot, R.G.; Au, F.L.; Donker-Koopman, W.E.; Strijland, A.; Muijsers, A.O.; Hrebicek, M.; Aerts, J.M. Chitotriosidase, a chitinase, and the 39-kDa human cartilage glycoprotein, a chitin-binding lectin, are homologues of family 18 glycosyl hydrolases secreted by human macrophages. Eur. J. Biochem. 1998, 25, 504–509. [Google Scholar] [CrossRef]
- Houston, D.R.; Recklies, A.D.; Krupa, J.C.; van Aalten, D.M. Structure and ligand-induced glycoprotein from human articular chondrocytes. J. Biol. Chem. 2003, 278, 30206–30212. [Google Scholar] [CrossRef]
- Low, D.; Subramaniam, R.; Lin, L.; Aomatsu, T.; Mizoguchi, A.; Ng, A.; DeGruttola, A.K.; Lee, C.G.; Elias, J.A.; Andoh, A.; et al. Chitinase 3-like 1 induces survival and proliferation of intestinal epithelial cells during chronic inflammation and colitis-associated cancer by regulating S100A9. Oncotarget 2015, 6, 36535–36550. [Google Scholar] [CrossRef]
- Morera, E.; Steinhauser, S.S.; Budkova, Z.; Ingthorsson, S.; Kricker, J.; Krueger, A.; Traustadottir, G.A.; Gudjonsson, T. YKL-40/CHI3L1 facilitates migration and invasion in HER2 overexpressing breast epithelial progenitor cells and generates a niche for capillary-like network formation. Vitr. Cell Dev. Biol. Anim. 2019, 55, 836–853. [Google Scholar] [CrossRef]
- Tiriveedhi, V.; Upadhya, G.A.; Busch, R.A.; Gunter, K.L.; Dines, J.N.; Knolhoff, B.L.; Jia, J.; Sarma, N.J.; Ramachandran, S.; Anderson, C.D.; et al. Protective role of bortezomib in steatotic liver ischemia/reperfusion injury through abrogation of MMP activation and YKL-40 expression. Transpl. Immunol. 2014, 30, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Ji, X.; Mo, Z.; Zhou, Y. Serum YKL-40 positively correlates with MMP-9 and CRP in patients with acute ST segment elevation myocardial infarction following emergency treatment. Medicine 2019, 98, e17950. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J.; Yoon, C.M.; Nam, M.; Kim, D.H.; Choi, J.M.; Lee, C.G.; Elias, J.A. Role of chitinase 3-like -1 in interleukin-18-induced pulmonary type 1, type 2, and type 17 inflammation; Alveolar distraction; and airway fibrosis in the murine lung. Am. J. Respir. Cell Mol. Biol. 2025, 53, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; He, C.H.; Yang, D.S.; Nguyen, T.; Cao, Y.; Kamle, S.; Lee, C.M.; Gochuico, B.R.; Gahl, W.A.; Shea, B.S.; et al. Galectin-3 interacts with the CHI3L1 axis and contributes to Hermansky-Pudlak syndrome lung disease. J. Immunol. 2015, 200, 2140–2153. [Google Scholar] [CrossRef]
- Geng, B.; Pan, J.; Zhao, T.; Ji, J.; Zhang, C.; Che, Y.; Yang, J.; Shi, H.; Li, J.; Zhou, H.; et al. Chitinase 3-like 1-CD44 interaction promotes metastasis and epithelial-to-mesenchymal transition through β-catenin/Erk/Akt signaling in gastric cancer. J. Exp. Clin. Cancer Res. 2018, 37, 208. [Google Scholar] [CrossRef]
- Ma, B.; Akosman, B.; Kamle, S.; Lee, C.M.; He, C.H.; Koo, J.S.; Lee, C.G.; Elias, J.A. CHI3L1 regulates PD-L1 and anti-CHI3L1-PD-1 antibody elicits synergistic antitumor responses. J. Clin. Investig. 2021, 131, e137750. [Google Scholar] [CrossRef]
- Subramaniam, R.; Mizoguchi, A.; Mizoguchi, E. Mechanistic roles of epithelial and immune cell signaling during the development of colitis-associated cancer. Cancer Res. Front. 2016, 2, 1–21. [Google Scholar] [CrossRef]
- Bouvet, G.F.; Bulka, O.; Criati, A.; Sognigbe, L.; St-Pierre, G.; Masse, C.; Sato, S.; Berthiaume, Y. Peripheral blood mononuclear cell response to YKL-40 and Galectin-3 in cystic fibrosis. Cytokine 2021, 146, 155635. [Google Scholar] [CrossRef]
- Kzhyshkowska, J.; Yin, S.; Liu, T.; Riabov, V.; Mitrofanova, I. Role of chitinase-like proteins in cancer. Biol. Chem. 2016, 397, 231–247. [Google Scholar] [CrossRef]
- Song, Y.; Jiang, W.; Afridi, S.K.; Wang, T.; Zhu, F.; Xu, H.; Nazir, F.H.; Liu, C.; Wang, Y.; Long, Y.; et al. Astrocyte-derived CHI3L1 signaling impairs neurogenesis and cognition in the demyelinated hippocampus. Cell Rep. 2024, 43, 114226. [Google Scholar] [CrossRef]
- Karin, M.; Greten, F.R. NF-kappaB: Linking inflammation and immunity to cancer development and progression. Nat. Rev. Immunol. 2005, 5, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.; Charles, K.A.; Mantovani, A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 2005, 7, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Baussano, I.; Franceschi, S.; Plummer, M. Infection transmission and chronic disease models in the study of infection-associated cancer. Br. J. Cancer 2014, 110, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Beydoun, A.S.; Stabenau, K.A.; Altman, K.W.; Johnston, N. Cancer risk in Barrett’s esophagus: A clinical review. Int. J. Mol. Sci. 2023, 24, 6018. [Google Scholar] [CrossRef]
- Alalman, O.; Sakhat, G.; Alam, E.; Mallat, H.; Chalouhi, M. Latent chronic osteomyelitis presenting decades after initial trauma: A case report and literature review. Cureus 2024, 16, e61789. [Google Scholar] [CrossRef]
- Roelen, N.; Crouchet, E.; Braumert, T.F. Liver fibrosis: Mechanistic concepts and therapeutic perspectives. Cells 2020, 9, 875. [Google Scholar] [CrossRef]
- Cazacu, I.M.; Farkas, N.; Garami, A.; Balasko, M.; Mosdosi, B.; Alizadeh, H.; Gyongyl, Z.; Rakonczay, Z., Jr.; Vigh, E.; Habon, T.; et al. Pancreatitis-associated genes and pancreatic cancer risk: A systematic review and meta-analysis. Pancreas 2018, 47, 1078–1086. [Google Scholar] [CrossRef]
- Ullman, T.A.; Itzkowitz, S.H. Intestinal inflammation and cancer. Gastroenterology 2011, 140, 1807–1816. [Google Scholar] [CrossRef]
- Marchesi, J.R.; Adams, D.H.; Fava, F.; Hermes, G.D.; Hirschfield, G.M.; Hold, G.; Quraish, M.N.; Kinross, J.; Smidt, H.; Tuohy, K.M.; et al. The gut microbiota and host health: A new clinical frontier. Gut 2016, 65, 330–339. [Google Scholar] [CrossRef]
- Baldini, C.; Fulvio, G.; La Rocca, G.; Ferro, F. Update on the pathophysiology and treatment of primary Sjogren syndrome. Nat. Rev. Rheumatol. 2024, 20, 473–491. [Google Scholar] [CrossRef]
- Park, J.; Morley, T.S.; Kim, M.; Clegg, D.J.; Scherer, P.E. Obesity and cancer—Mechanisms underlying tumour progression and recurrence. Nat. Rev. Endocrinol. 2014, 10, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, N.M.; Gucalp, A.; Dannenberg, A.J.; Hudis, C.A. Obesity and cancer mechanisms: Tumor microenvironment and inflammation. J. Clin. Cancer 2016, 34, 4270–4276. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Montegut, L.; Keep, O.; Zitvogel, L. The danger theory of immunity revised. Nat. Rev. Immunol. 2024, 24, 912–928. [Google Scholar] [CrossRef] [PubMed]
- El Tekle, G.; Garrett, W.S. Bacteria in cancer initiation, promotion and progression. Nat. Rev. Cancer 2023, 23, 600–618. [Google Scholar] [CrossRef]
- Mossman, B.T.; Lippmann, M.; Hesterberg, T.W.; Kelsey, K.T.; Barchowsky, A.; Bonner, J.C. Pulmonary endpoints (lung carcinomas and asbestosis) following inhalation exposure to asbestos. J. Toxicol. Environ. Health B. Crit. Rev. 2011, 14, 76–121. [Google Scholar] [CrossRef]
- Bade, B.C.; Dela Cruz, C.S. Lung cancer 2020: Epidemiology, etiology, and prevention. Clin. Chest Med. 2020, 41, 1–24. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Cerrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Song, M.; Chan, A.T. Environmental factors, gut microbiota, and colorectal cancer prevention. Clin. Gastroenterol. Hapatol. 2019, 17, 275–289. [Google Scholar] [CrossRef]
- Song, M.; Chan, A.T.; Sun, J. Influence of the gut microbiome, diet, and environment on risk of colorectal cancer. Gastroenterology 2020, 158, 322–340. [Google Scholar] [CrossRef]
- Ngwa, W.; Irabor, O.C.; Schoenfeld, J.D.; Hesser, J.; Demaria, S.; Forment, S.C. Using immunotherapy to boost the abscopal effect. Nat. Rev. Cancer 2018, 18, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.E.; Yeo, I.J.; Han, S.B.; Yun, J.; Kim, B.; Yong, Y.J.; Lim, Y.S.; Kim, T.H.; Son, D.J.; Hong, J.T. Significance of chitinase 3-like protein 1 in the pathogenesis of inflammatory diseases and cancer. Exp. Mol. Med. 2024, 56, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, G.; Rath, B.; Burghuber, O. Chitinase-3-like-1/YKL-40 as marker of circulating tumor cells. Transl. Lung Cancer Res. 2015, 4, 287–291. [Google Scholar] [PubMed]
- Allin, K.H.; Bojesen, S.E.; Johansen, J.S.; Nordestgaard, B.G. Cancer risk by combined levels of YKL-40 and C-reactive protein in the general population. Br. J. Cancer 2012, 106, 199–205. [Google Scholar] [CrossRef]
- Watanabe, K.; Shiga, K.; Maeda, A.; Harata, S.; Yanagita, T.; Suzuki, T.; Ushigome, H.; Maeda, Y.; Hrokawa, T.; Ogawa, R.; et al. Chitinase 3-like 1 secreted from cancer-associated fiblobrasts promotes tumor angiogenesis via interleukin-8 secretion in colorectal cancer. Int. J. Oncol. 2022, 60, 3. [Google Scholar] [CrossRef]
- Qiu, Q.C.; Wang, L.; Jin, S.S.; Liu, G.F.; Liu, J.; Ma, L.; Mao, R.F.; Ma, Y.Y.; Zhao, N.; Chen, M.; et al. CHI3L1 promotes tumor progression by activating TGF-β signaling pathway in hepatocellular carcinoma. Sci. Rep. 2018, 8, 15029. [Google Scholar] [CrossRef]
- Wang, S.; Chen, S.; Jin, M.; Huang, W.; Jiang, Z.; Yang, J.; Zhang, Y.; Wu, H.; Hu, Y.; He, W.; et al. Diagnostic and prognostic value of serum Chitinase 3-like protein 1 in hepatocellular carcinoma. J. Clin. Lab. Anal. 2022, 36, e24234. [Google Scholar] [CrossRef]
- Grigoryeva, E.S.; Kokova, D.A.; Gratchev, A.N.; Cherdyntsev, E.S.; Buldakov, M.A.; Kzhyshkowska, J.G.; Cherdyntseva, N.V. Smoking-related DNA adducts as potential diagnostic markers of lung cancer: New perspectives. Exp. Oncol. 2015, 37, 5–12. [Google Scholar] [CrossRef]
- Yamanaka, K.; Koma, Y.I.; Urakami, S.; Takahashi, R.; Nagamata, S.; Omori, M.; Torigoe, R.; Yokoo, H.; Nakanishi, T.; Ishihara, N.; et al. YKL-40/integrin β4 axis induced by the interaction between cancer cells and tumor-associated macrophages is involved in the progression of high-grade serous ovarian carcinoma. Int. J. Mol. Sci. 2024, 25, 10598. [Google Scholar] [CrossRef]
- Fichtner-Feigl, S.; Terabe, M.; Kitani, A.; Young, C.A.; Fuss, I.; Geissler, E.K.; Schlitt, H.J.; Berzofsky, J.A.; Strober, W. Restoration of tumor immunosurveillance via targeting of interleukin-13 receptor-alpha 2. Cancer Res. 2008, 68, 3467–3475. [Google Scholar] [CrossRef]
- Strober, W.; Kitani, A.; Fichtner-Feigl, S.; Fuss, I.J. The signaling function of the IL-13R alpha 2 receptor in the development of gastrointestinal fibrosis and cancer surveillance. Curr. Mol. Med. 2009, 9, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, X.; Shi, M.; Han, Y.; Liu, K.; Wang, H.; Sun, S.; Yang, B.; Gao, Z.; Qu, M.; et al. YKL-40 inhibits melanoma progression and metastasis by inducing immune cell infiltration in a mouse model. Sci. Rep. 2025, 15, 7426. [Google Scholar] [CrossRef]
- Knogh, M.; Christensen, I.; Bouwhuls, M.; Johansen, J.S.; Norgaard, P.; Schmidt, H.; Hansson, J.S.; Suciu, S.; Eggermont, A.M.; Bastholt, L.; et al. Prognostic and predictive value of YKL-40 in stage IIB-III melanoma. Melanoma Res. 2016, 26, 367–376. [Google Scholar]
- Scmidt, H.; Johansen, J.S.; Gehl, J.; Geertsen, P.F.; Fode, K.; von der Maase, H. Elevated serum level of YKL-40 is an independent prognostic factor for poor survival in patients with metastatic melanoma. Cancer 2006, 106, 1130–1139. [Google Scholar] [CrossRef]
- Low, D.; DeGruttola, A.K.; Poltrak, A.; Mizoguchi, A.; Mino-Kenudson, M.; Mizoguchi, E. High endogenous expression of chitinase 3-like 1 and excessive epithelial proliferation with colonic tumor formation of MOLF/EiJ mice. PLoS ONE 2015, 10, e0139149. [Google Scholar] [CrossRef]
- Ma, B.; Kamle, S.; Akosman, B.; Khan, H.; Lee, C.M.; Lee, C.G.; Elias, J.A. CHI3L1 enhances melanoma lung metastasis via regulation of T cell co-stimulators and CTLA4-4/B7 axis. Front. Immunol. 2022, 13, 1056397. [Google Scholar] [CrossRef]
- Park, K.R.; Yan, H.M.; Yoo, K.; Ham, Y.W.; Han, S.B.; Hong, J.T. Chitinase 3 like 1 suppresses the stability and activity of p53 to promote lung tumorigenesis. Cell Commun. Signal. 2020, 18, 5. [Google Scholar] [CrossRef]
- Malinda, K.M.; Ponce, L.; Kleinman, H.K.; Shackelton, L.M.; Millis, A.J. Gp38k, a protein synthesized by vascular smooth muscle cells, stimulates directional migration of human umbilical vein endothelial cells. Exp. Cell Res. 1999, 250, 168–173. [Google Scholar] [CrossRef]
- Recklies, A.D.; White, C.; Ling, H. The chitinase 3-like protein human cartilage glycoprotein 39 (HC-gp39) stimulates proliferation of human connective-tissue cells and activate both extracellular signal-regulated kinase- and protein B-mediated signaling pathways. Biochem. J. 2002, 365, 119–126. [Google Scholar] [CrossRef]
- Ling, H.; Recklies, A.D. The chitinase 3-like protein human cartilage glycoprotein 39 inhibits cellular responses to the inflammatory cytokines interleukin-1 and tumor necrosis factor-alpha. Biochem. J. 2004, 380, 651–659. [Google Scholar] [CrossRef]
- Yang, P.S.; Yu, M.H.; Hou, Y.; Chang, C.; Lin, S.C.; Kuo, I.Y.; Su, P.C.; Cheng, H.C.; Su, W.C.; Shan, Y.S.; et al. Targeting protumor factor chitinase-3-like-1 secreted by Rab37 vesicles for cancer immunotherapy. Theranostics 2022, 340, 340–361. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, S.; Wang, Q.; Zhang, X. Tumor-recruited M2 macrophages promote gastric and breast cancer metastasis via M2macrophage-secreted CHI3L1 protein. J. Hematol. Oncol. 2017, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Yu, H.; Zhou, D.; Fan, X.; Duan, Y.; Tan, Y.; Lang, M.; Shao, G. Cancer stem cell-derived CHI3L1 activates the MAF/CTLA4 signaling pathway to promote immune escape in triple-negative breast cancer. J. Transl. Med. 2023, 21, 721. [Google Scholar] [CrossRef] [PubMed]
- Libreros, S.; Garcia-Areas, R.; Keating, P.; Carrio, R.; Iragavarapu-Charyulu, V.L. Exploring the role of CHI3L1in “pre-metastatic” lungs of mammary tumor-bearing mice. Front. Physiol. 2013, 4, 392. [Google Scholar] [CrossRef]
- Libreros, S.; Garcia-Areas, R.; Shibata, Y.; Carrio, R.; Torroella-Kouri, M.; Iragavarapu-Charyulu, V. Induction of proinflammatory mediators by CHI3L1 is reduced by chitin treatment: Decreased tumor metastasis in a breast cancer model. Int. J. Cancer 2012, 131, 377–386. [Google Scholar] [CrossRef]
- Ma, J.Y.; Li, R.H.; Huang, K.; Tan, G.; Li, C.; Zhi, F.C. Increased expression and possible role of chitinase 3-like-1 in a colitis-associated carcinoma model. World J. Gastroenterol. 2014, 20, 15736–15744. [Google Scholar] [CrossRef]
- Yan, C.; Ding, X.; Wu, L.; Yu, M.; Qu, P.; Du, H. Stat3 downstream gene product chitinase 3-like 1 is a potential biomarker of inflammation-induced lung cancer in multiple mouse lung tumor models and humans. PLoS ONE 2013, 6, e61964. [Google Scholar] [CrossRef]
- Yu, J.E.; Yeo, I.J.; Son, D.J.; Yun, J.; Han, S.B.; Hong, J.T. Anti-Chi3L1 antibody suppresses lung tumor growth and metastasis through inhibition of M2 polarization. Mol. Oncol. 2022, 1, 2214–2234. [Google Scholar] [CrossRef]
- Keenan, J.; Aitchison, A.; Frizelle, F.A.; Hock, B.D. Detection of chitinase 3-like 1 in symptomatic primary care patient faecal samples is not a reliable biomarker of colonic lesions. Asian Pac. Cancer Prev. 2023, 24, 2289–2293. [Google Scholar] [CrossRef]
- Chen, C.C.; Pekow, J.; Llado, V.; Kanneganti, M.; Lau, C.W.; Mizoguchi, A.; Mino-Kenudson, M.; Bissonnette, M.; Mizoguchi, E. Chitinase 3-like-1 expression in colonic epithelial cells as a potentially novel marker for colitis-associated neoplasia. Am. J. Pathol. 2011, 179, 1494–1503. [Google Scholar] [CrossRef]
- De Robertis, M.; Greco, M.R.; Cardone, R.A.; Mazza, T.; Marzano, F.; Mehterov, N.; Kazakova, M.; Belev, N.; Tullo, A.; Pesole, G.; et al. Upregulation of YKL-40 promotes metastatic phenotype and correlates with poor prognosis and therapy response in patients with colorectal cancer. Cells 2022, 11, 2568. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sheng, Z.; Yang, W.; Cai, Y. Elevated serum concentration of chitinase 3-like 1 is an independent prognostic biomarker for poor survival in lung cancer patients. Cell Physiol. Biochem. 2016, 38, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.J.; Ge, Y.S.; Xu, G.L.; Jia, W.D.; Liu, W.F.; Li, J.S.; Liu, W.B. The expression of chitinase 3-like 1: A novel prognostic predictor for hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2013, 139, 1043–1054. [Google Scholar] [CrossRef]
- Böckelmann, L.C.; Felix, T.; Calabrò, S.; Schumacher, U. YKL-40 protein expression in human tumor samples and human tumor cell line xenografts: Implications for its use in tumor models. Cell Oncol. 2021, 44, 1183–1195. [Google Scholar] [CrossRef]
- Zhu, C.B.; Chen, L.L.; Tian, J.J.; Su, L.; Wang, C.; Gai, Z.T.; Du, W.J.; Ma, G.L. Elevated serum YKL-40 level predicts poor prognosis in hepatocellular carcinoma after surgery. Annu. Surg. Oncol. 2012, 19, 817–825. [Google Scholar] [CrossRef]
- Bi, J.; Lau, S.H.; Lv, Z.L.; Xie, D.; Li, W.; Lai, Y.R.; Zhong, J.M.; Wu, H.Q.; Su, Q.; He, Y.L.; et al. Overexpression of YKL-40 is an independent prognostic marker in gastric cancer. Hum. Pathol. 2009, 40, 1790–1797. [Google Scholar] [CrossRef]
- Johansen, A.Z.; Novitski, S.I.; Hjaltelin, J.X.; Theile, S.; Boisen, M.K.; Brunak, S.; Madsen, D.H.; Nielsen, D.L.; Chen, I.M. Plasma YKL-40 is associated with prognosis in patients with metastatic pancreatic cancer receiving immune checkpoint inhibitors in combination with radiotherapy. Front. Immunol. 2023, 14, 1228907. [Google Scholar] [CrossRef]
- Schultz, N.A.; Christensen, I.J.; Werner, J.; Giese, N.; Jensen, B.V.; Larsen, O.; Bjerregaard, J.K.; Pfeiffer, P.; Calatayud, D.; Nielsen, S.E.; et al. Diagnostic and Prognostic Impact of Circulating YKL-40, IL-6, and CA 19.9 in Patients with Pancreatic Cancer. PLoS ONE 2013, 8, e67059. [Google Scholar] [CrossRef]
- Palmquist, C.; Dehlendorff, C.; Calatayud, D.; Hansen, C.P.; Hasselby, J.P.; Johansen, J.S. Prediction of Unresectability and Prognosis in Patients Undergoing Surgery on Suspicion of Pancreatic Cancer Using Carbohydrate Antigen 19-9, Interleukin 6, and YKL-40. Pancreas 2020, 49, 53–61. [Google Scholar] [CrossRef]
- Shao, R.; Cao, Q.J.; Arenas, R.B.; Bigelow, C.; Bentley, B.; Yan, W. Breast cancer expression of YKL-40 correlates with tumour grade, poor differentiation, and other cancer markers. Br. J. Cancer 2011, 105, 1203–1209. [Google Scholar] [CrossRef]
- Yamac, D.; Ozturk, B.; Coskun, U.; Tekin, E.; Sancak, B.; Yildiz, R.; Atalay, C. Serum YKL-40 levels as a prognostic factor in patients with locally advanced breast cancer. Adv. Ther. 2008, 25, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Jensen, B.V.; Johansen, J.S.; Price, P.A. High levels of serum HER-2/neu and YKL-40 independently reflect aggressiveness of metastatic breast cancer. Clin. Cancer Res. 2003, 9, 4423–4434. [Google Scholar] [PubMed]
- Qin, Y.; Zhao, W. The Modeling Analysis and Effect of CHI3L1 and CD31-Marked Microvessel Density in the Occurrence and Development of Cervical Squamous Cell Carcinoma. Comput. Math. Methods Med. 2022, 2022, 3516335. [Google Scholar] [CrossRef]
- Ngernyuang, N.; Francescone, R.A.; Jearanaikoon, P.; Daduang, J.; Supoken, A.; Yan, W.; Shao, R.; Limpaiboon, T. Chitinase 3 like 1 is associated with tumor angiogenesis in cervical cancer. Int. J. Biochem. Cell Biol. 2014, 51, 45–52. [Google Scholar] [CrossRef]
- Ngernyuang, N.; Shao, R.; Suwannarurk, K.; Limpaiboon, T. Chitinase 3 like 1 (CHI3L1) promotes vasculogenic mimicry formation in cervical cancer. Pathology 2018, 50, 293–297. [Google Scholar] [CrossRef]
- Mitsuhashi, A.; Matsui, H.; Usui, H.; Nagai, Y.; Tate, S.; Unno, Y.; Hirashiki, K.; Seki, K.; Shozu, M. Serum YKL-40 as a marker for cervical adenocarcinoma. Annu. Oncol. 2009, 20, 71–77. [Google Scholar] [CrossRef]
- Roslind, A.; Palle, C.; Johansen, J.S.; Christensen, I.J.; Nielsen, H.J.; Mosgaard, B.J. Prognostic utility of serum YKL-40 in patients with cervical cancer. Scand. J. Clin. Lab. Investig. 2020, 80, 687–693. [Google Scholar] [CrossRef]
- Yang, G.F.; Cai, P.Y.; Li, X.M.; Deng, H.X.; He, W.P.; Xie, D. Expression and clinical significance of YKL-40 protein in epithelial ovarian cancer tissues. Ai Zheng 2009, 28, 142–145. [Google Scholar]
- Dupont, J.; Tanwar, M.K.; Thaler, H.T.; Fleisher, M.; Kauff, N.; Hensley, M.L.; Sabbatini, P.; Anderson, S.; Aghajanian, C.; Holland, E.C.; et al. Early detection and prognosis of ovarian cancer using serum YKL-40. Clin. Oncol. 2004, 22, 3330–3339. [Google Scholar] [CrossRef]
- Høgdall, E.V.; Ringsholt, M.; Høgdall, C.K.; Christensen, I.J.; Johansen, J.S.; Kjaer, S.K.; Blaakaer, J.; Ostenfeld-Møller, L.; Price, P.A.; Christensen, L.H. YKL-40 tissue expression and plasma levels in patients with ovarian cancer. BMC Cancer 2009, 9, 8. [Google Scholar] [CrossRef]
- Dehn, H.; Høgdall, E.V.; Johansen, J.S.; Jørgensen, M.; Price, P.A.; Engelholm, S.A.; Høgdall, C.K. Plasma YKL-40, as a prognostic tumor marker in recurrent ovarian cancer. Acta Obstet. Gynecol. Scand. 2003, 82, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.R.; Mishra, N.; Kant, R.; Fox, J.T.; Shoemaker, R.H.; Agarwal, C.; Raina, K.; Agarwal, R. Effect of nonsteroidal anti-inflammatory drugs (aspirin and naproxen) on inflammation-associated proteomic profiles in mouse plasma and prostate during TMPRSS2-ERG (fusion)-driven prostate carcinogenesis. Mol. Carcinog. 2024, 63, 1188–1204. [Google Scholar] [CrossRef] [PubMed]
- Tschirdewahn, S.; Reis, H.; Niedworok, C.; Nyirady, P.; Szendröi, A.; Schmid, K.W.; Shariat, S.F.; Kramer, G.; vom Dorp, F.; Rübben, H.; et al. Prognostic effect of serum and tissue YKL-40 levels in bladder cancer. Urol. Oncol. 2014, 32, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.E.; Chan, T.C.; Tian, Y.F.; Liang, P.I.; Shiue, Y.L.; Chen, Y.S.; He, H.L. High expression of Chitinase 3-like-1 is an unfavorable prognostic factor in urothelial carcinoma of upper urinary tract and urinary bladder. Urol. Oncol. 2019, 37, 299.e7–299.e18. [Google Scholar] [CrossRef]
- Yasar, O.; Akcay, T.; Obek, C.; Turegun, F.A. Diagnostic potential of YKL-40 in bladder cancer. Urol. Oncol. 2016, 34, 257.e19–257.e24. [Google Scholar] [CrossRef]
- Vaananen, T.; Kallio, J.; Vuolteenaho, K.; Ojala, A.; Luukkaala, T.; Hamalainen, M.; Tammela, T.; Kellokumpu-Lehtinen, P.L.; Moilanen, E. High YKL-40 is associated with poor survival in patients with renal cell carcinoma: A novel independent prognostic marker. Scand. J. Urol. 2017, 51, 367–372. [Google Scholar] [CrossRef]
| Major Causes | Possible Mechanisms | Examples | Ref. |
|---|---|---|---|
| Infections | Persistent local infection-based chronic inflammation, oxidative stress, and DNA damage induce cancer formation. | HCV, HBV → HCC EBV → Head and neck cancer HPV → Cervical cancer H. pylori → Gastric cancer | [42,43,44] |
| Tissue injuries | Recurrent tissue damage causes compensatory tissue growth and inflammation. | IBD → Colorectal cancer Barrett esophagus → Esophageal cancer LC → HCC Chronic pancreatitis → Pancreatic cancer | [45,46,47,48] |
| Autoimmune-related disorders | Autoimmune-associated inflammation causes tissue damage and tumor formation. | IBD → colorectal cancer Sjogren’s syndrome → Malignant lymphoma | [49,50,51] |
| Obesity | Excessive secretion of pro-inflammatory cytokines due to fatty tissue growth caused by obesity, creating a tumor-promoting environment. | Obesity → Breast cancer, colorectal cancer, pancreatic cancer | [52,53] |
| Dysbiosis | Intestinal dysbiosis overstimulates immune responses and promotes tumor formation. | Dysbiosis → HCC, colorectal cancer | [54,55] |
| Environmental factors | Additive exposure to harmful substances causes chronic inflammation and mutagenesis. | Asbestosis → Mesothelioma Silica → Lung cancer Tobacco smoke → Lung cancer, cancer of airways | [56,57] |
| Genetic factors | Promoting tumorigenesis due to abnormalities in the inflammatory signaling pathway(s). | IL-10 genetic mutation → EO- IBD | [58] |
| Aging | Aging-related decline in the immune system leads to chronic low levels of inflammation, creating a basis for cancer. | Aging → Prostate cancer, colon cancer | [59] |
| Micronutrient deficiency | Vitamin D or iron deficiency enhances inflammation. | Digestive cancer | [60,61] |
| Iatrogenic factors | Chemotherapy/radiation therapy induced inflammation | Combination therapy → Secondary lung cancer | [62] |
| Cancer | Samples (Relative, Increase, X-Fold) | Results | Ref. |
|---|---|---|---|
| Colon | Stool (<1.3-fold) | ・ Fecal CHI3L1 levels increase with progression of inflammation in CAC. ・Fecal CHI3L1 is not a reliable biomarker of colonic lesions in symptomatic primary care patient. | [50,90] |
| Tissue (<25-fold) | ・ Significantly increased CHI3L1 expression in non-dysplastic mucosa from patients with IBD who had dysplasia/adenocarcinoma compared with control individuals. | [91] | |
| Serum (<2-fold) | ・ Patients with high operative serum CHI3L1 concentration had significantly shorter survival than patients with normal CHI3L1. | [92] | |
| Lung | Tissue (<4-fold) Serum (<1.5-fold) | ・ CHI3L1 is highly expressed in lung cancer tissue and in the serum of patients with poor prognosis as well as animal models. | [88,93] |
| Liver | Tissue (<16-fold) | ・ Elevated tissue CHI3L1 in HCC compared to adjacent peritumoral tissues and further elevated in tumors with metastasis. ・Positive CHI3L1 expression was significantly associated with clinicopathological features in HCC. ・HCC patients with positive CHI3L1 expression were correlated with poor overall survival and disease-free survival. | [94] |
| Serum (<3-fold) | ・ Serum CHI3L1 may not be as reliable a biomarker for HCC. ・Serum CHI3L1 may act as an independent prognostic factor for overall and RFS in HCC patients receiving curative resection. | [95,96] | |
| Stomach | Tissue (<2.5-fold) | ・ CHI3L1 expression was significantly higher in gastric cancer tissues compared to adjacent nonneoplastic tissues. ・Elevated CHI3L1 was positively correlated with the poor prognosis and aggressive behavior of gastric cancer cells. | [97] |
| Serum (<4-fold) | ・ Elevated serum CHI3L1 was associated with invasion depth, lymph node status, and tumor stage in patients with gastric cancer. | [36] | |
| Pancreas | Plasma (<3-fold) | ・ Increased plasma CHI3L1 is related to shorter overall survival (OS) in mPC (metastatic pancreatic cancer) patients. ・Plasma CHI3L1 could be a biomarker in patients with mPC receiving ICIs (immune checkpoint inhibitors) with radiotherapy. ・Plasma CHI3L1 in combination with serum CA 19-9 and plasma IL-6 could be useful to identify a subgroup of low-stage PC patients. | [98,99,100] |
| Breast | Tissue | ・ Elevated expression levels of CHI3L1 correlate with tumor grade and poor differentiation. | [101] |
| Serum (<2-fold) | ・ Increased serum CHI3L1 levels in metastatic breast cancer patients. ・Elevated CHI3L1 levels in patients with locally advanced breast cancer. | [102,103] | |
| Cervix | Tissue (<3.4-fold) | ・ High levels of CHI3L1 in CSCC (cervical squamous cell carcinoma). ・Elevated CHI3L1 in invasive CxCa (cervical cancer). ・Elevated CHI3L1 mediates VM (vasculogenic mimicry) in CxCa. | [104,105,106,107,108] |
| Serum (<5.1-fold) | ・ Elevated serum CHI3L1 in both SCC (squamous cell carcinoma) and adenocarcinoma. ・Elevated serum CHI3L1 was associated with shorter RFS (recurrence-free survival) and OS (overall survival). ・Early changes in serum CHI3L1 levels may serve as a biomarker to monitor patients with CxCa after the operation and other therapies. | ||
| Ovary | Tissue (<38-fold) | ・ Tissue CHI3L1 was closely correlated with the clinical stage of EOC (epithelial ovarian cancer). ・Elevated tissue CHI3L1 correlated with significantly shorter overall survival time in OC patients. | [109] |
| Serum (<2-fold) | ・ Elevated serum CHI3L1 in OC patients regardless of tumor grade, histology, or patient age. ・The serum levels of CHI3L1 in early-stage patients may predict disease recurrence and survival. ・Elevated serum CHI3L1 in early-stage OC patients. | [110] | |
| Plasma (<18-fold) | ・ Plasma CHI3L1 levels are associated with the stage and prognosis in OC. ・Elevated plasma CHI3L1 at the time of relapse of OC. | [111,112] | |
| Prostate | Tissue (<5-fold) | ・ CHI3L1 is one of the seven significantly suppressed tumor promoting inflammatory molecules identified in the plasma and prostate sample after an NSAID-treated prostate cancer model. | [113] |
| Bladder | Tissue (<3.4-fold) | ・ Elevated tissue CHI3L1 was significantly associated with aggressive clinicopathological features in UTUC or UBUC. ・Elevated tissue CHI3L1 can serve as an independent prognostic factor for worse DSS and MFS in both UTUC and UBUC groups. | [114,115,116] |
| Serum (<2.2-fold) | ・ Elevated serum CHI3L1 in patients with BC (Bladder Cancer) associated with poor survival. ・Elevated serum CHI3L1 as an independent prognostic factors in BC. | ||
| Urine (<23.2-fold) | ・ Urine CHI3L1 levels can differentiate invasiveness in BC patients. | ||
| Kidney | Blood (<2.4-fold) | ・ High blood CHI3L1 levels are associated with poor survival in patients with renal cell carcinoma. | [117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizoguchi, E.; Wang, S. Future Perspectives and Conclusions from Animal Models of CHI3L1-Related Inflammation-Associated Cancer. Cells 2025, 14, 982. https://doi.org/10.3390/cells14130982
Mizoguchi E, Wang S. Future Perspectives and Conclusions from Animal Models of CHI3L1-Related Inflammation-Associated Cancer. Cells. 2025; 14(13):982. https://doi.org/10.3390/cells14130982
Chicago/Turabian StyleMizoguchi, Emiko, and Siyuan Wang. 2025. "Future Perspectives and Conclusions from Animal Models of CHI3L1-Related Inflammation-Associated Cancer" Cells 14, no. 13: 982. https://doi.org/10.3390/cells14130982
APA StyleMizoguchi, E., & Wang, S. (2025). Future Perspectives and Conclusions from Animal Models of CHI3L1-Related Inflammation-Associated Cancer. Cells, 14(13), 982. https://doi.org/10.3390/cells14130982






