Modulation of Neurexins Alternative Splicing by Cannabinoid Receptors 1 (CB1) Signaling
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Primary Hippocampal Neuronal Cultures
2.3. Drugs
2.4. Hippocampal Slices Stimulation and Recordings
2.5. RT-PCR Analysis and Quantitative Real-Time PCR (RT-qPCR)
2.6. Western Blotting
2.7. CB1 Immunohistochemistry
2.8. Statistical Analysis
3. Results
3.1. Expression of Functional CB1 Receptors in Hippocampus
3.2. Expression of Nrxns Splice Variants Is Modulated by CB1 Receptors Signaling
3.3. SLM2 Implication in Nrxns Splicing Pattern at SS4 Site
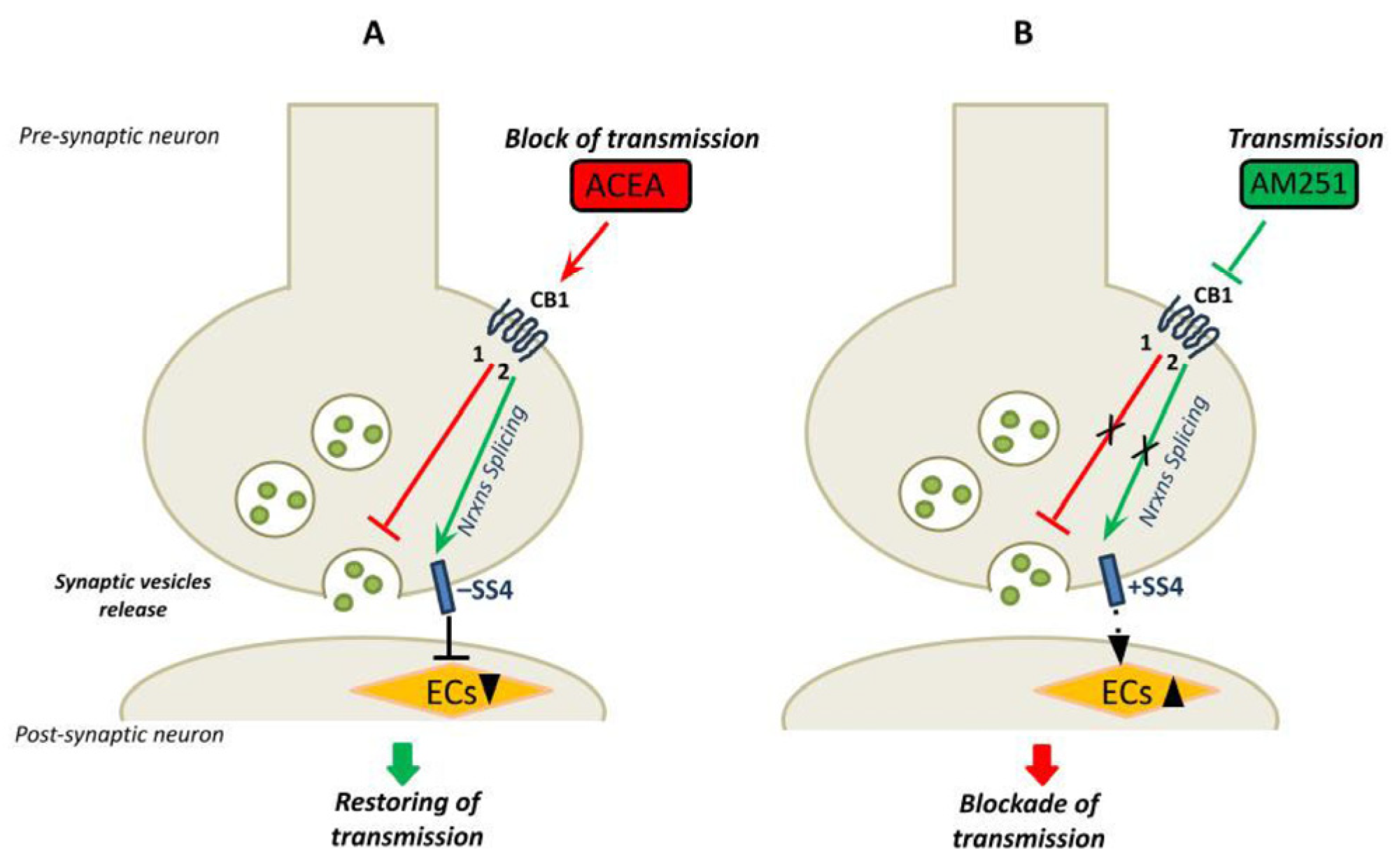
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Missler, M.; Sudhof, T.C.; Biederer, T. Synaptic cell adhesion. Cold Spring Harb. Perspect. Biol. 2012, 4, a005694. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tabuchi, K.; Sudhof, T.C. Structure and evolution of neurexin genes: Insight into the mechanism of alternative splicing. Genomics 2002, 79, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Ushkaryov, Y.A.; Petrenko, A.G.; Geppert, M.; Sudhof, T.C. Neurexins: Synaptic cell surface proteins related to the alpha-latrotoxin receptor and laminin. Science 1992, 257, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Sudhof, T.C. Synaptic Neurexin Complexes: A Molecular Code for the Logic of Neural Circuits. Cell 2017, 171, 745–769. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gomez, A.M.; Traunmuller, L.; Scheiffele, P. Neurexins: Molecular codes for shaping neuronal synapses. Nat. Rev. Neurosci. 2021, 22, 137–151. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schreiner, D.; Nguyen, T.M.; Russo, G.; Heber, S.; Patrignani, A.; Ahrne, E.; Scheiffele, P. Targeted combinatorial alternative splicing generates brain region-specific repertoires of neurexins. Neuron 2014, 84, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Boucard, A.A.; Ko, J.; Sudhof, T.C. High affinity neurexin binding to cell adhesion G-protein-coupled receptor CIRL1/latrophilin-1 produces an intercellular adhesion complex. J. Biol. Chem. 2012, 287, 9399–9413. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ko, J.; Fuccillo, M.V.; Malenka, R.C.; Sudhof, T.C. LRRTM2 functions as a neurexin ligand in promoting excitatory synapse formation. Neuron 2009, 64, 791–798. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Siddiqui, T.J.; Pancaroglu, R.; Kang, Y.; Rooyakkers, A.; Craig, A.M. LRRTMs and neuroligins bind neurexins with a differential code to cooperate in glutamate synapse development. J. Neurosci. 2010, 30, 7495–7506. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sugita, S.; Saito, F.; Tang, J.; Satz, J.; Campbell, K.; Sudhof, T.C. A stoichiometric complex of neurexins and dystroglycan in brain. J. Cell Biol. 2001, 154, 435–445. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matsuda, K.; Yuzaki, M. Cbln family proteins promote synapse formation by regulating distinct neurexin signaling pathways in various brain regions. Eur. J. Neurosci. 2011, 33, 1447–1461. [Google Scholar] [CrossRef] [PubMed]
- Uemura, T.; Lee, S.J.; Yasumura, M.; Takeuchi, T.; Yoshida, T.; Ra, M.; Taguchi, R.; Sakimura, K.; Mishina, M. Trans-synaptic interaction of GluRdelta2 and Neurexin through Cbln1 mediates synapse formation in the cerebellum. Cell 2010, 141, 1068–1079. [Google Scholar] [CrossRef] [PubMed]
- Boucard, A.A.; Chubykin, A.A.; Comoletti, D.; Taylor, P.; Sudhof, T.C. A splice code for trans-synaptic cell adhesion mediated by binding of neuroligin 1 to alpha- and beta-neurexins. Neuron 2005, 48, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Chih, B.; Gollan, L.; Scheiffele, P. Alternative splicing controls selective trans-synaptic interactions of the neuroligin-neurexin complex. Neuron 2006, 51, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Comoletti, D.; Flynn, R.E.; Boucard, A.A.; Demeler, B.; Schirf, V.; Shi, J.; Jennings, L.L.; Newlin, H.R.; Sudhof, T.C.; Taylor, P. Gene selection, alternative splicing, and post-translational processing regulate neuroligin selectivity for beta-neurexins. Biochemistry 2006, 45, 12816–12827. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, B.; Ushkaryov, Y.A.; Sudhof, T.C. Cartography of neurexins: More than 1000 isoforms generated by alternative splicing and expressed in distinct subsets of neurons. Neuron 1995, 14, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Shapiro-Reznik, M.; Jilg, A.; Lerner, H.; Earnest, D.J.; Zisapel, N. Diurnal rhythms in neurexins transcripts and inhibitory/excitatory synapse scaffold proteins in the biological clock. PLoS ONE 2012, 7, e37894. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Resnick, M.; Segall, A.; Rozic-Kotliroff, G.; Lupowitz, Z.; Zisapel, N. Alternative splicing of neurexins: A role for neuronal polypyrimidine tract binding protein. Neurosci. Lett. 2008, 439, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Iijima, T.; Wu, K.; Witte, H.; Hanno-Iijima, Y.; Glatter, T.; Richard, S.; Scheiffele, P. SAM68 regulates neuronal activity-dependent alternative splicing of neurexin-1. Cell 2011, 147, 1601–1614. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rozic, G.; Lupowitz, Z.; Zisapel, N. Exonal elements and factors involved in the depolarization-induced alternative splicing of neurexin 2. J. Mol. Neurosci. 2013, 50, 221–233. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cesari, E.; Farini, D.; Medici, V.; Ehrmann, I.; Guerra, M.; Testa, E.; Naro, C.; Geloso, M.C.; Pagliarini, V.; La Barbera, L.; et al. Differential expression of paralog RNA binding proteins establishes a dynamic splicing program required for normal cerebral cortex development. Nucleic Acids Res. 2024, 52, 4167–4184. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Liu, S.; Tian, M.; Zhang, W.; Zhu, T.; Li, D.; Wu, J.; Deng, H.; Jia, Y.; Xie, W.; et al. Activity-induced histone modifications govern Neurexin-1 mRNA splicing and memory preservation. Nat. Neurosci. 2017, 20, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Aoto, J.; Martinelli, D.C.; Malenka, R.C.; Tabuchi, K.; Sudhof, T.C. Presynaptic neurexin-3 alternative splicing trans-synaptically controls postsynaptic AMPA receptor trafficking. Cell 2013, 154, 75–88. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dai, J.; Liakath-Ali, K.; Golf, S.R.; Sudhof, T.C. Distinct neurexin-cerebellin complexes control AMPA- and NMDA-receptor responses in a circuit-dependent manner. Elife 2022, 11, e78649. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Castillo, P.E.; Younts, T.J.; Chavez, A.E.; Hashimotodani, Y. Endocannabinoid signaling and synaptic function. Neuron 2012, 76, 70–81. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beltramo, M.; Stella, N.; Calignano, A.; Lin, S.Y.; Makriyannis, A.; Piomelli, D. Functional role of high-affinity anandamide transport, as revealed by selective inhibition. Science 1997, 277, 1094–1097. [Google Scholar] [CrossRef] [PubMed]
- Cadas, H.; Gaillet, S.; Beltramo, M.; Venance, L.; Piomelli, D. Biosynthesis of an endogenous cannabinoid precursor in neurons and its control by calcium and cAMP. J. Neurosci. 1996, 16, 3934–3942. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cravatt, B.F.; Giang, D.K.; Mayfield, S.P.; Boger, D.L.; Lerner, R.A.; Gilula, N.B. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 1996, 384, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; Fontana, A.; Cadas, H.; Schinelli, S.; Cimino, G.; Schwartz, J.C.; Piomelli, D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature 1994, 372, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Cristino, L.; Bisogno, T.; Di Marzo, V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat. Rev. Neurol. 2020, 16, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Katona, I.; Freund, T.F. Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat. Med. 2008, 14, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Ohno-Shosaku, T.; Kano, M. Endocannabinoid-mediated retrograde modulation of synaptic transmission. Curr. Opin. Neurobiol. 2014, 29, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Parolaro, D.; Realini, N.; Vigano, D.; Guidali, C.; Rubino, T. The endocannabinoid system and psychiatric disorders. Exp. Neurol. 2010, 224, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Galve-Roperh, I.; Palazuelos, J.; Aguado, T.; Guzman, M. The endocannabinoid system and the regulation of neural development: Potential implications in psychiatric disorders. Eur. Arch. Psychiatry Clin. Neurosci. 2009, 259, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Augustin, S.M.; Lovinger, D.M. Functional Relevance of Endocannabinoid-Dependent Synaptic Plasticity in the Central Nervous System. ACS Chem. Neurosci. 2018, 9, 2146–2161. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anderson, G.R.; Aoto, J.; Tabuchi, K.; Foldy, C.; Covy, J.; Yee, A.X.; Wu, D.; Lee, S.J.; Chen, L.; Malenka, R.C.; et al. beta-Neurexins Control Neural Circuits by Regulating Synaptic Endocannabinoid Signaling. Cell 2015, 162, 593–606. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Farini, D.; Cesari, E.; Weatheritt, R.J.; La Sala, G.; Naro, C.; Pagliarini, V.; Bonvissuto, D.; Medici, V.; Guerra, M.; Di Pietro, C.; et al. A Dynamic Splicing Program Ensures Proper Synaptic Connections in the Developing Cerebellum. Cell Rep. 2020, 31, 107703. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.M.; Schreiner, D.; Xiao, L.; Traunmuller, L.; Bornmann, C.; Scheiffele, P. An alternative splicing switch shapes neurexin repertoires in principal neurons versus interneurons in the mouse hippocampus. Elife 2016, 5, e22757. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Blazquez, C.; Chiarlone, A.; Bellocchio, L.; Resel, E.; Pruunsild, P.; Garcia-Rincon, D.; Sendtner, M.; Timmusk, T.; Lutz, B.; Galve-Roperh, I.; et al. The CB(1) cannabinoid receptor signals striatal neuroprotection via a PI3K/Akt/mTORC1/BDNF pathway. Cell Death Differ. 2015, 22, 1618–1629. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ozaita, A.; Puighermanal, E.; Maldonado, R. Regulation of PI3K/Akt/GSK-3 pathway by cannabinoids in the brain. J. Neurochem. 2007, 102, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Li, J.; Li, C.J.; Lin, Y.Q.; Huang, C.H.; Zheng, X.; Song, X.C.; Tu, Z.C.; Li, X.J.; Yan, S. Differential development and electrophysiological activity in cultured cortical neurons from the mouse and cynomolgus monkey. Neural Regen. Res. 2021, 16, 2446–2452. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mody, M.; Cao, Y.; Cui, Z.; Tay, K.Y.; Shyong, A.; Shimizu, E.; Pham, K.; Schultz, P.; Welsh, D.; Tsien, J.Z. Genome-wide gene expression profiles of the developing mouse hippocampus. Proc. Natl. Acad. Sci. USA 2001, 98, 8862–8867. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ehrmann, I.; Dalgliesh, C.; Liu, Y.; Danilenko, M.; Crosier, M.; Overman, L.; Arthur, H.M.; Lindsay, S.; Clowry, G.J.; Venables, J.P.; et al. The tissue-specific RNA binding protein T-STAR controls regional splicing patterns of neurexin pre-mRNAs in the brain. PLoS Genet. 2013, 9, e1003474. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ehrmann, I.; Gazzara, M.R.; Pagliarini, V.; Dalgliesh, C.; Kheirollahi-Chadegani, M.; Xu, Y.; Cesari, E.; Danilenko, M.; Maclennan, M.; Lowdon, K.; et al. A SLM2 Feedback Pathway Controls Cortical Network Activity and Mouse Behavior. Cell Rep. 2016, 17, 3269–3280. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Danilenko, M.; Dalgliesh, C.; Pagliarini, V.; Naro, C.; Ehrmann, I.; Feracci, M.; Kheirollahi-Chadegani, M.; Tyson-Capper, A.; Clowry, G.J.; Fort, P.; et al. Binding site density enables paralog-specific activity of SLM2 and Sam68 proteins in Neurexin2 AS4 splicing control. Nucleic Acids Res. 2017, 45, 4120–4130. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ehrmann, I.; Fort, P.; Elliott, D.J. STARs in the CNS. Biochem. Soc. Trans. 2016, 44, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Iijima, T.; Iijima, Y.; Witte, H.; Scheiffele, P. Neuronal cell type-specific alternative splicing is regulated by the KH domain protein SLM1. J. Cell Biol. 2014, 204, 331–342. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Traunmuller, L.; Gomez, A.M.; Nguyen, T.M.; Scheiffele, P. Control of neuronal synapse specification by a highly dedicated alternative splicing program. Science 2016, 352, 982–986. [Google Scholar] [CrossRef] [PubMed]
- Graves, A.R.; Moore, S.J.; Bloss, E.B.; Mensh, B.D.; Kath, W.L.; Spruston, N. Hippocampal pyramidal neurons comprise two distinct cell types that are countermodulated by metabotropic receptors. Neuron 2012, 76, 776–789. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Piomelli, D. The molecular logic of endocannabinoid signalling. Nat. Rev. Neurosci. 2003, 4, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Lutz, B.; Marsicano, G.; Maldonado, R.; Hillard, C.J. The endocannabinoid system in guarding against fear, anxiety and stress. Nat. Rev. Neurosci. 2015, 16, 705–718. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gorecki, D.C.; Szklarczyk, A.; Lukasiuk, K.; Kaczmarek, L.; Simons, J.P. Differential seizure-induced and developmental changes of neurexin expression. Mol. Cell Neurosci. 1999, 13, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Rozic-Kotliroff, G.; Zisapel, N. Ca2+-dependent splicing of neurexin IIalpha. Biochem. Biophys. Res. Commun. 2007, 352, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Rozic, G.; Lupowitz, Z.; Piontkewitz, Y.; Zisapel, N. Dynamic changes in neurexins’ alternative splicing: Role of Rho-associated protein kinases and relevance to memory formation. PLoS ONE 2011, 6, e18579. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Burrone, J.; Murthy, V.N. Synaptic gain control and homeostasis. Curr. Opin. Neurobiol. 2003, 13, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Traunmuller, L.; Schulz, J.; Ortiz, R.; Feng, H.; Furlanis, E.; Gomez, A.M.; Schreiner, D.; Bischofberger, J.; Zhang, C.; Scheiffele, P. A cell-type-specific alternative splicing regulator shapes synapse properties in a trans-synaptic manner. Cell Rep. 2023, 42, 112173. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goosens, K.A. Hippocampal regulation of aversive memories. Curr. Opin. Neurobiol. 2011, 21, 460–466. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Freire-Cobo, C.; Wang, J. Dietary phytochemicals modulate experience-dependent changes in Neurexin gene expression and alternative splicing in mice after chronic variable stress exposure. Eur. J. Pharmacol. 2020, 883, 173362. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]






| Nrxns SS4 Splice Variants [19] | |
| Nrxn1 (+/−SS4) 318–228 bp | Fw: 5′-TGT TGG GAC AGA TGA CAT CGC C-3′ |
| Rv: 5′-GAG AGC TGG CCC TGG AAG GG-3′ | |
| Nrxn2 (+/−SS4) 270–180 bp | Fw: 5′-GTG CGC TTT ACT CGA AGT GGT G-3′ |
| Rv: 5′-CCC ATT GTA GTA GAG GCC GGA C-3′ | |
| Nrxn3 (+/−SS4) 261–171 bp | Fw: 5′-TTG TGC GCT TCA CCA GGA ATG-3′ |
| Rv: 5′-AGA GCC CAG AGA GTT GAC CTT G-3′ | |
| Nrxns Alpha/Beta [38] | |
| Nrxn1 (+/−SS4) α: 645–555 bp β: 699–609 bp | Fw (α): 5′-CAG CAC AAC CTG CCA AGA-3′ |
| Fw (β): 5′-CCT GGC CCT GAT CTG GAT AGT-3′ | |
| Rv (αβ): 5′-GAG AGC TGG CCC TGG AAG GG-3′ | |
| Nrxn2 (+/−SS4) α: 661–571 bp β: 597–507 bp | Fw (α): 5′-CAC CAC CTG CAC CGA AGA G-3′ |
| Fw (β): 5′-GTG CCC ATC GCC ATC AA-3′ | |
| Rv (αβ): 5′-CCC ATT GTA GTA GAG GCC GGA C-3′ | |
| Nrxn3 (+/−SS4) α: 584–494 bp β: 598–508 bp | Fw (α): 5′-CTG TGA CTGCTC CAT GAC ATC ATATT-3′ |
| Fw (β): 5′-AAGCACCACTCTGTGCCTATTTCT-3′ | |
| Rv (αβ): 5′-AGA GCC CAG AGA GTT GAC CTT G-3′ | |
| β-Actin 135 bp | Fw: 5′-CTG TCG AGT CGC GTC CAC-3′ |
| Rv: 5′-GCT TTG CAC ATG CCG GAG-3′ | |
| Slm2 71bp | Fw: 5′-TGATGGCGGAGAAGGACTCT-3′ |
| Rv: 5′-TTCTATTTCTCGGTTCACCAAGCG-3′ | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Innocenzi, E.; Sciamanna, G.; Zucchi, A.; Medici, V.; Cesari, E.; Farini, D.; Elliott, D.J.; Sette, C.; Grimaldi, P. Modulation of Neurexins Alternative Splicing by Cannabinoid Receptors 1 (CB1) Signaling. Cells 2025, 14, 972. https://doi.org/10.3390/cells14130972
Innocenzi E, Sciamanna G, Zucchi A, Medici V, Cesari E, Farini D, Elliott DJ, Sette C, Grimaldi P. Modulation of Neurexins Alternative Splicing by Cannabinoid Receptors 1 (CB1) Signaling. Cells. 2025; 14(13):972. https://doi.org/10.3390/cells14130972
Chicago/Turabian StyleInnocenzi, Elisa, Giuseppe Sciamanna, Alice Zucchi, Vanessa Medici, Eleonora Cesari, Donatella Farini, David J. Elliott, Claudio Sette, and Paola Grimaldi. 2025. "Modulation of Neurexins Alternative Splicing by Cannabinoid Receptors 1 (CB1) Signaling" Cells 14, no. 13: 972. https://doi.org/10.3390/cells14130972
APA StyleInnocenzi, E., Sciamanna, G., Zucchi, A., Medici, V., Cesari, E., Farini, D., Elliott, D. J., Sette, C., & Grimaldi, P. (2025). Modulation of Neurexins Alternative Splicing by Cannabinoid Receptors 1 (CB1) Signaling. Cells, 14(13), 972. https://doi.org/10.3390/cells14130972








