High-Speed-Ventral-Plane Videography Identifies Specific Gait Pattern Changes in Cuprizone-Induced Demyelination in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
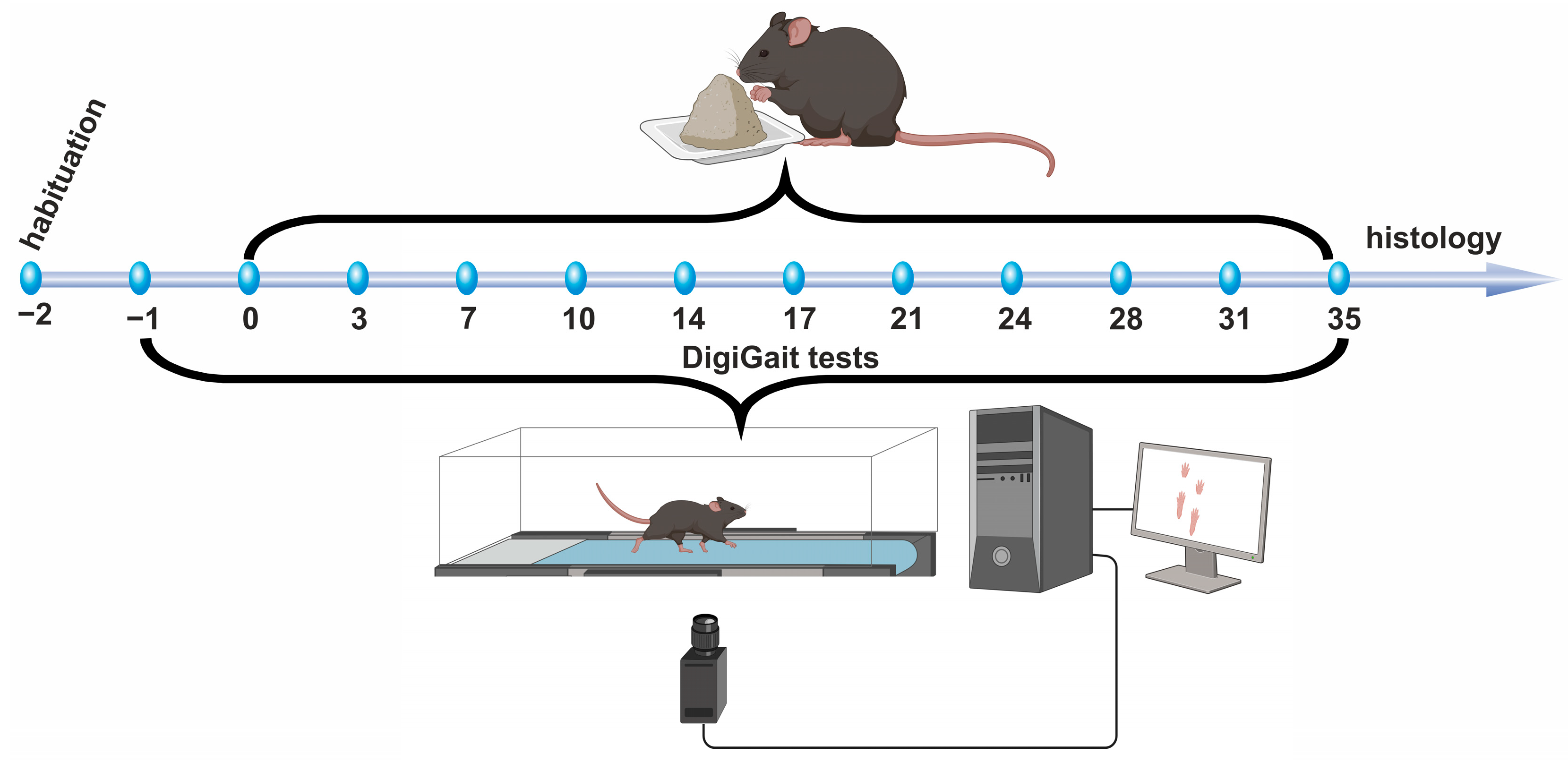
2.2. Experimental Design
2.3. High Speed Ventral Plane Videography and Evaluation
- Stride time
- Stance phase
- Brake phase
- Propel Phase
- Midline Distance
- Overlap Distance
- Stance width
- Swing time
- Paw Angle
- Sum Paw Angle
2.4. Determination of Body Weight and Brain Weight
2.5. Tissue Preparation and Immunohistochemistry
2.6. Selected Regions of Interest (ROI)
2.7. Planimetric Analyses
2.8. Statistics
3. Results
3.1. Body Weight Development
3.2. Brain Weight
3.3. DigiGait Test—Analysis of Gait Parameters
3.3.1. Stride Time
3.3.2. Stance Phase
3.3.3. Brake Phase
3.3.4. Propel Phase
3.3.5. Midline Distance
3.3.6. Overlap Distance
3.3.7. Stance Width
3.3.8. Swing Time
3.3.9. Paw Angle
3.3.10. Sum Paw Angle
3.4. Histology and Immunohistology
3.4.1. Anti-PLP-Immunohistochemistry and Planimetric Analysis
3.4.2. Anti-IBA1-Immunohistochemistry and Planimetric Analysis
Corpus Callosum (CC)
Motor Cortex
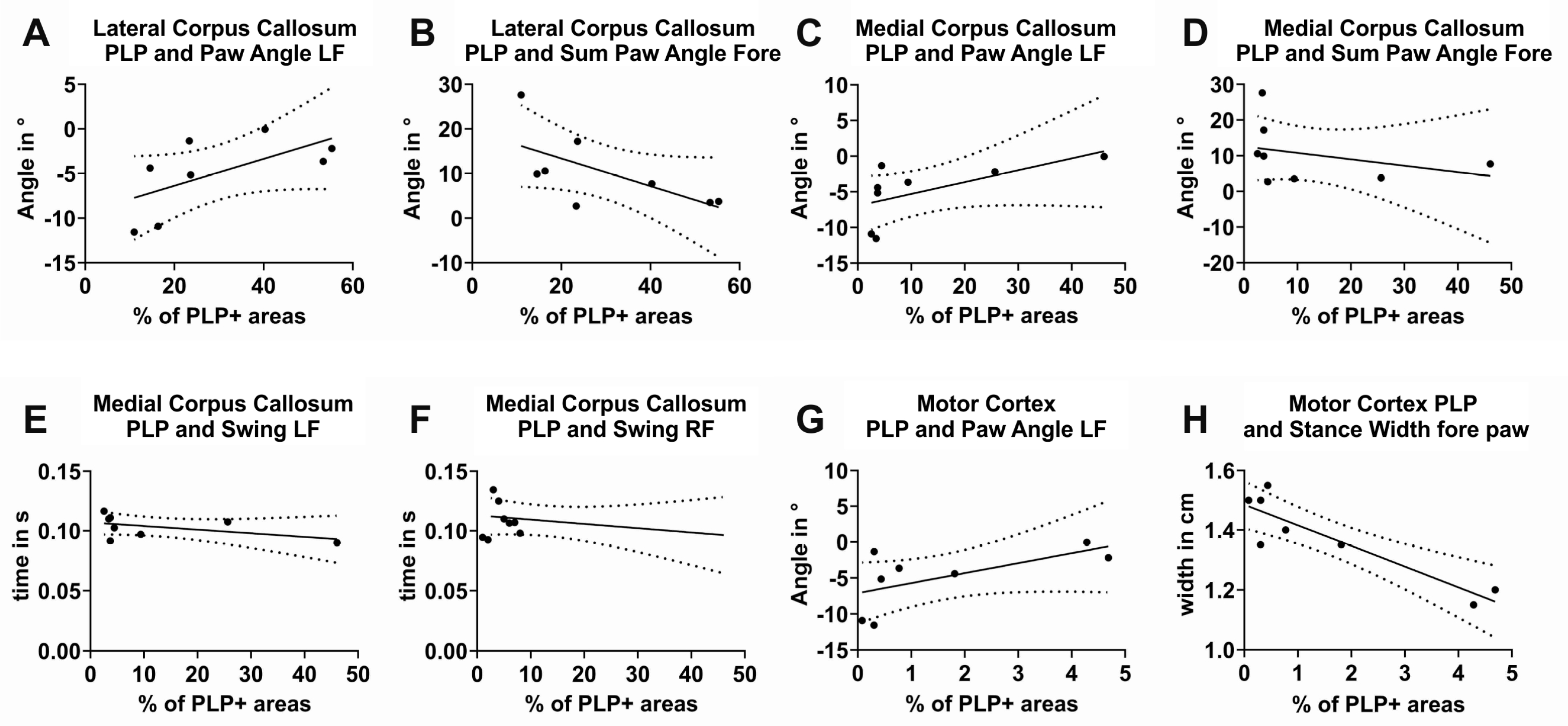
3.5. Correlation of Gait Parameters with Histological Findings
3.5.1. Correlation of the Relative Staining Intensity of PLP-Positive Areas in the Anterior Lateral CC with Gait Parameters
3.5.2. Correlation of the Relative Staining Intensity of PLP-Positive Areas in the Posterior Medial CC with Gait Parameters
3.5.3. Correlation of the Relative Staining Intensity of PLP-Positive Areas in the MC with Gait Parameters
4. Discussion
4.1. Successful Induction of Demyelination by Cuprizone Intoxication
4.2. Brain Weight and Ratio Brain Weight/Body Weight
4.3. Gait
4.4. Stride Time
4.5. Stance Phase
4.6. Brake Phase
4.7. Propel Phase
4.8. Midline Distance
4.9. Overlap Distance
4.10. Paw Angle and Sum Paw Angle
4.11. Stance Width
4.12. Neuroinflammatory Reactions in Demyelinated Structures
4.13. Correlation of Gait Parameters with Histological Measurements
4.14. Outlook and Critical Notes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dilokthornsakul, P.; Valuck, R.J.; Nair, K.V.; Corboy, J.R.; Allen, R.R.; Campbell, J.D. Multiple Sclerosis Prevalence in the United States Commercially Insured Population. Neurology 2016, 86, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Dobson, R.; Giovannoni, G. Multiple Sclerosis—A Review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef]
- Popescu, B.F.G.; Pirko, I.; Lucchinetti, C.F. Pathology of Multiple Sclerosis: Where Do We Stand? Continuum 2013, 19, 901–921. [Google Scholar] [CrossRef] [PubMed]
- Jakimovski, D.; Bittner, S.; Zivadinov, R.; Morrow, S.A.; Benedict, R.H.; Zipp, F.; Weinstock-Guttman, B. Multiple Sclerosis. Lancet 2024, 403, 183–202. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Hu, W.; Tang, L.; Ma, X.; Liao, J.; Yu, Z.; Qi, M.; Chen, B.; Li, J. Meta-Analysis of the Selected Genetic Variants in Immune-Related Genes and Multiple Sclerosis Risk. Mol. Neurobiol. 2024, 61, 8175–8187. [Google Scholar] [CrossRef]
- Goldenberg, M.M. Multiple Sclerosis Review. P T A Peer-Rev. J. Formul. Manag. 2012, 37, 175–184. [Google Scholar]
- Medana, I.M.; Esiri, M.M. Axonal Damage: A Key Predictor of Outcome in Human CNS Diseases. Brain 2003, 126, 515–530. [Google Scholar] [CrossRef]
- Spillers, N.J.; Luther, P.M.; Talbot, N.C.; Kidder, E.J.; Doyle, C.A.; Lutfallah, S.C.; Derouen, A.G.; Tirumala, S.; Ahmadzadeh, S.; Shekoohi, S.; et al. A Comparative Review of Typical and Atypical Optic Neuritis: Advancements in Treatments, Diagnostics, and Prognosis. Cureus 2024, 16, e56094. [Google Scholar] [CrossRef]
- Meyer-Moock, S.; Feng, Y.-S.; Maeurer, M.; Dippel, F.-W.; Kohlmann, T. Systematic Literature Review and Validity Evaluation of the Expanded Disability Status Scale (EDSS) and the Multiple Sclerosis Functional Composite (MSFC) in Patients with Multiple Sclerosis. BMC Neurol. 2014, 14, 58. [Google Scholar] [CrossRef]
- Motl, R.W.; Cohen, J.A.; Benedict, R.; Phillips, G.; LaRocca, N.; Hudson, L.D.; Rudick, R.; Multiple Sclerosis Outcome Assessments Consortium. Validity of the Timed 25-Foot Walk as an Ambulatory Performance Outcome Measure for Multiple Sclerosis. Mult. Scler. 2017, 23, 704–710. [Google Scholar] [CrossRef]
- Feys, P.; Lamers, I.; Francis, G.; Benedict, R.; Phillips, G.; LaRocca, N.; Hudson, L.D.; Rudick, R.; Multiple Sclerosis Outcome Assessments Consortium. The Nine-Hole Peg Test as a Manual Dexterity Performance Measure for Multiple Sclerosis. Mult. Scler. 2017, 23, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, M.G.; Piperno, R.; Simoncini, L.; Bonato, P.; Tonini, A.; Giannini, S. Gait Abnormalities in Minimally Impaired Multiple Sclerosis Patients. Mult. Scler. 1999, 5, 363–368. [Google Scholar] [CrossRef]
- Cameron, M.H.; Nilsagard, Y. Balance, Gait, and Falls in Multiple Sclerosis. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 159, pp. 237–250. ISBN 978-0-444-63916-5. [Google Scholar]
- Comber, L.; Galvin, R.; Coote, S. Gait Deficits in People with Multiple Sclerosis: A Systematic Review and Meta-Analysis. Gait Posture 2017, 51, 25–35. [Google Scholar] [CrossRef]
- Kalron, A.; Givon, U. Gait Characteristics According to Pyramidal, Sensory and Cerebellar EDSS Subcategories in People with Multiple Sclerosis. J. Neurol. 2016, 263, 1796–1801. [Google Scholar] [CrossRef]
- Mazumder, R.; Murchison, C.; Bourdette, D.; Cameron, M. Falls in People with Multiple Sclerosis Compared with Falls in Healthy Controls. PLoS ONE 2014, 9, e107620. [Google Scholar] [CrossRef]
- Kalron, A.; Dvir, Z.; Achiron, A. Effect of a Cognitive Task on Postural Control in Patients with a Clinically Isolated Syndrome Suggestive of Multiple Sclerosis. Eur. J. Phys. Rehabil. Med. 2011, 47, 579–586. [Google Scholar] [PubMed]
- Huisinga, J.M.; Schmid, K.K.; Filipi, M.L.; Stergiou, N. Gait Mechanics Are Different Between Healthy Controls and Patients with Multiple Sclerosis. J. Appl. Biomech. 2013, 29, 303–311. [Google Scholar] [CrossRef]
- Coca-Tapia, M.; Cuesta-Gómez, A.; Molina-Rueda, F.; Carratalá-Tejada, M. Gait Pattern in People with Multiple Sclerosis: A Systematic Review. Diagnostics 2021, 11, 584. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.L.; Brauer, S.G. Walking Impairment in Patients with Multiple Sclerosis: The Impact of Complex Motor and Non-Motor Symptoms across the Disability Spectrum. Aust. J. Gen. Pr. 2022, 51, 215–219. [Google Scholar] [CrossRef]
- Suarez-Cedeno, G.; Mehanna, R. Movement Disorders in Multiple Sclerosis and Other Demyelinating Diseases: A Retrospective Review from a Tertiary Academic Center. Neurologist 2021, 26, 161–166. [Google Scholar] [CrossRef]
- Denissen, S.; De Cock, A.; Meurrens, T.; Vleugels, L.; Van Remoortel, A.; Gebara, B.; D’Haeseleer, M.; D’Hooghe, M.B.; Van Schependom, J.; Nagels, G. The Impact of Cognitive Dysfunction on Locomotor Rehabilitation Potential in Multiple Sclerosis. J. Cent. Nerv. Syst. Dis. 2019, 11, 117957351988404. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, B.H.D.S.; De Andrade, P.H.S.; Luvizutto, G.J. Does Non-Invasive Brain Stimulation Improve Spatiotemporal Gait Parameters in People with Multiple Sclerosis? A Systematic Review and Meta-Analysis. J. Bodyw. Mov. Ther. 2024, 37, 350–359. [Google Scholar] [CrossRef]
- Giovannoni, G.; Boyko, A.; Correale, J.; Edan, G.; Freedman, M.S.; Montalban, X.; Rammohan, K.; Stefoski, D.; Yamout, B.; Leist, T.; et al. Long-Term Follow-up of Patients with Relapsing Multiple Sclerosis from the CLARITY/CLARITY Extension Cohort of CLASSIC-MS: An Ambispective Study. Mult. Scler. 2023, 29, 719–730. [Google Scholar] [CrossRef]
- Kipp, M.; Nyamoya, S.; Hochstrasser, T.; Amor, S. Multiple Sclerosis Animal Models: A Clinical and Histopathological Perspective. Brain Pathol. 2017, 27, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Behrangi, N.; Fischbach, F.; Kipp, M. Mechanism of Siponimod: Anti-Inflammatory and Neuroprotective Mode of Action. Cells 2019, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Chrzanowski, U.; Schmitz, C.; Horn-Bochtler, A.; Nack, A.; Kipp, M. Evaluation Strategy to Determine Reliable Demyelination in the Cuprizone Model. Metab. Brain Dis. 2019, 34, 681–685. [Google Scholar] [CrossRef]
- Zendedel, A.; Krauspe, B.; Kipp, M.; Fragoulis, A.; Denecke, B.; Clarner, T.; Beyer, C.; Nyamoya, S.; Teske, N.; Scheld, M. Mitochondrial Impairment in Oligodendroglial Cells Induces Cytokine Expression and Signaling. J. Mol. Neurosci. 2018, 67, 265–275. [Google Scholar] [CrossRef]
- Goldberg, J.; Clarner, T.; Beyer, C.; Kipp, M. Anatomical Distribution of Cuprizone-Induced Lesions in C57BL6 Mice. J. Mol. Neurosci. 2015, 57, 166–175. [Google Scholar] [CrossRef]
- Groebe, A.; Clarner, T.; Baumgartner, W.; Dang, J.; Beyer, C.; Kipp, M. Cuprizone Treatment Induces Distinct Demyelination, Astrocytosis, and Microglia Cell Invasion or Proliferation in the Mouse Cerebellum. Cerebellum 2009, 8, 163–174. [Google Scholar] [CrossRef]
- Pott, F.; Gingele, S.; Clarner, T.; Dang, J.; Baumgartner, W.; Beyer, C.; Kipp, M. Cuprizone Effect on Myelination, Astrogliosis and Microglia Attraction in the Mouse Basal Ganglia. Brain Res. 2009, 1305, 137–149. [Google Scholar] [CrossRef]
- Herder, V.; Hansmann, F.; Stangel, M.; Skripuletz, T.; Baumgärtner, W.; Beineke, A. Lack of Cuprizone-Induced Demyelination in the Murine Spinal Cord despite Oligodendroglial Alterations Substantiates the Concept of Site-Specific Susceptibilities of the Central Nervous System: Scientific Correspondence. Neuropathol. Appl. Neurobiol. 2011, 37, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Yakimov, V.; Rühling, S.; Fischbach, F.; Nikolova, E.; Joost, S.; Kaddatz, H.; Greiner, T.; Frenz, J.; Holzmann, C.; et al. High Speed Ventral Plane Videography as a Convenient Tool to Quantify Motor Deficits during Pre-Clinical Experimental Autoimmune Encephalomyelitis. Cells 2019, 8, 1439. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Fegg, F.N.; Kaddatz, H.; Rühling, S.; Frenz, J.; Denecke, B.; Amor, S.; Ponsaerts, P.; Hochstrasser, T.; Kipp, M. Focal White Matter Lesions Induce Long-Lasting Axonal Degeneration, Neuroinflammation and Behavioral Deficits. Neurobiol. Dis. 2021, 155, 105371. [Google Scholar] [CrossRef]
- Beecken, M.; Baumann, L.; Vankriekelsvenne, E.; Manzhula, K.; Greiner, T.; Heinig, L.; Schauerte, S.; Kipp, M.; Joost, S. The Cuprizone Mouse Model: A Comparative Study of Cuprizone Formulations from Different Manufacturers. Int. J. Mol. Sci. 2023, 24, 10564. [Google Scholar] [CrossRef]
- Kipp, M. How to Use the Cuprizone Model to Study De- and Remyelination. Int. J. Mol. Sci. 2024, 25, 1445. [Google Scholar] [CrossRef]
- Kale, A.; Amende, I.; Meyer, G.P.; Crabbe, J.C.; Hampton, T.G. Ethanol’s Effects on Gait Dynamics in Mice Investigated by Ventral Plane Videography. Alcohol. Clin. Exp. Res. 2004, 28, 1839–1848. [Google Scholar] [CrossRef]
- Vankriekelsvenne, E.; Chrzanowski, U.; Manzhula, K.; Greiner, T.; Wree, A.; Hawlitschka, A.; Llovera, G.; Zhan, J.; Joost, S.; Schmitz, C.; et al. Transmembrane Protein 119 Is Neither a Specific nor a Reliable Marker for Microglia. Glia 2022, 70, 1170–1190. [Google Scholar] [CrossRef]
- Sidman, R.L.; Angevine, J.B.; Pierce, E.T. Atlas of the Mouse Brain and Spinal Cord; Harvard University Press: Cambridge, MA, USA, 1971; ISBN 978-0-674-05225-3. [Google Scholar]
- Toomey, L.M.; Papini, M.; Lins, B.; Wright, A.J.; Warnock, A.; McGonigle, T.; Hellewell, S.C.; Bartlett, C.A.; Anyaegbu, C.; Fitzgerald, M. Cuprizone Feed Formulation Influences the Extent of Demyelinating Disease Pathology. Sci. Rep. 2021, 11, 22594. [Google Scholar] [CrossRef]
- Amlerova, Z.; Chmelova, M.; Anderova, M.; Vargova, L. Reactive Gliosis in Traumatic Brain Injury: A Comprehensive Review. Front. Cell. Neurosci. 2024, 18, 1335849. [Google Scholar] [CrossRef]
- Fallier-Becker, P.; Bonzheim, I.; Pfeiffer, F. Cuprizone Feeding Induces Swollen Astrocyte Endfeet. Pflug. Arch.-Eur. J. Physiol. 2022, 474, 1275–1283. [Google Scholar] [CrossRef]
- Kesterson, J.W.; Carlton, W.W. Histopathologic Ond Enzyme Histochemical Observations of the Cuprizone-Induced Brain Edema. Exp. Mol. Pathol. 1971, 15, 82–96. [Google Scholar] [CrossRef]
- Morishita, T.; Uehara, K.; Funase, K. Changes in Interhemispheric Inhibition from Active to Resting Primary Motor Cortex during a Fine-Motor Manipulation Task. J. Neurophysiol. 2012, 107, 3086–3094. [Google Scholar] [CrossRef]
- Wahl, M.; Lauterbach-Soon, B.; Hattingen, E.; Jung, P.; Singer, O.; Volz, S.; Klein, J.C.; Steinmetz, H.; Ziemann, U. Human Motor Corpus Callosum: Topography, Somatotopy, and Link between Microstructure and Function. J. Neurosci. 2007, 27, 12132–12138. [Google Scholar] [CrossRef] [PubMed]
- Wahl, M.; Ziemann, U. The Human Motor Corpus Callosum. Rev. Neurosci. 2008, 19, 451–466. [Google Scholar] [CrossRef] [PubMed]
- Moretti, M.; Carlucci, G.; Di Carlo, A.; Fonda, C.; Prieto, M.; Mugnai, S.; Bracco, L.; Piccini, C.; Pracucci, G.; Inzitari, D. Corpus Callosum Atrophy Is Associated with Gait Disorders in Patients with Leukoaraiosis. Neurol. Sci. 2005, 26, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Richmond, S.B.; Peterson, D.S.; Fling, B.W. Bridging the Callosal Gap in Gait: Corpus Callosum White Matter Integrity’s Role in Lower Limb Coordination. Brain Imaging Behav. 2022, 16, 1552–1562. [Google Scholar] [CrossRef]
- Lu, Y.; Lin, Z.; Li, M.; Zhuang, Y.; Nie, B.; Lei, J.; Zhao, Y.; Zhao, H. Three-Phase Enriched Environment Improves Post-Stroke Gait Dysfunction via Facilitating Neuronal Plasticity in the Bilateral Sensorimotor Cortex: A Multimodal MRI/PET Analysis in Rats. Neurosci. Bull. 2023, 40, 719–731. [Google Scholar] [CrossRef]
- Levitt, J.J.; Zhang, F.; Vangel, M.; Nestor, P.G.; Rathi, Y.; Kubicki, M.; Shenton, M.E.; O’Donnell, L.J. The Organization of Frontostriatal Brain Wiring in Healthy Subjects Using a Novel Diffusion Imaging Fiber Cluster Analysis. Cereb. Cortex 2021, 31, 5308–5318. [Google Scholar] [CrossRef]
- Reed, J.; Grillakis, A.; Kline, A.; Ahmed, A.E.; Byrnes, K.R. Gait Analysis in a Rat Model of Traumatic Brain Injury. Behav. Brain Res. 2021, 405, 113210. [Google Scholar] [CrossRef]
- Clarke, K.; Still, J. Gait Analysis in the Mouse. Physiol. Behav. 1999, 66, 723–729. [Google Scholar] [CrossRef]
- Okuma, Y. Freezing of Gait in Parkinson’s Disease. J. Neurol. 2006, 253, vii27–vii32. [Google Scholar] [CrossRef]
- Lambert, C.S.; Philpot, R.M.; Engberg, M.E.; Johns, B.E.; Kim, S.H.; Wecker, L. Gait Analysis and the Cumulative Gait Index (CGI): Translational Tools to Assess Impairments Exhibited by Rats with Olivocerebellar Ataxia. Behav. Brain Res. 2014, 274, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Lubjuhn, J.; Gastens, A.; Von Wilpert, G.; Bargiotas, P.; Herrmann, O.; Murikinati, S.; Rabie, T.; Marti, H.; Amende, I.; Hampton, T.G.; et al. Functional Testing in a Mouse Stroke Model Induced by Occlusion of the Distal Middle Cerebral Artery. J. Neurosci. Methods 2009, 184, 95–103. [Google Scholar] [CrossRef]
- Hampton, T.; Stasko, M.; Kale, A.; Amende, I.; Costa, A. Gait Dynamics in Trisomic Mice: Quantitative Neurological Traits of Down Syndrome. Physiol. Behav. 2004, 82, 381–389. [Google Scholar] [CrossRef]
- Muir, G.D.; Whishaw, I.Q. Ground Reaction Forces in Locomoting Hemi-Parkinsonian Rats: A Definitive Test for Impairments and Compensations. Exp. Brain Res. 1999, 126, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, M.; Pérez Gómez, A.A.; Carroll, R.J.; Lawley, K.S.; Amstalden, K.A.Z.; Welsh, C.J.; Threadgill, D.W.; Brinkmeyer-Langford, C. Baseline Gait and Motor Function Predict Long-Term Severity of Neurological Outcomes of Viral Infection. Int. J. Mol. Sci. 2023, 24, 2843. [Google Scholar] [CrossRef]
- Powell, E.; Anch, A.M.; Dyche, J.; Bloom, C.; Richter, R.R. The Splay Angle. Physiol. Behav. 1999, 67, 819–821. [Google Scholar] [CrossRef] [PubMed]
- Abeyratne, E.; Reshamwala, R.; Shelper, T.; Liu, X.; Zaid, A.; Mahalingam, S.; Taylor, A. Altered Spatial and Temporal Gait Parameters in Mice Infected with Ross River Virus. mSphere 2021, 6, e0065921. [Google Scholar] [CrossRef]
- Jenkyn, T.R.; Hunt, M.A.; Jones, I.C.; Giffin, J.R.; Birmingham, T.B. Toe-out Gait in Patients with Knee Osteoarthritis Partially Transforms External Knee Adduction Moment into Flexion Moment during Early Stance Phase of Gait: A Tri-Planar Kinetic Mechanism. J. Biomech. 2008, 41, 276–283. [Google Scholar] [CrossRef]
- Jenkyn, T.R.; Nicol, A.C. A Multi-Segment Kinematic Model of the Foot with a Novel Definition of Forefoot Motion for Use in Clinical Gait Analysis during Walking. J. Biomech. 2007, 40, 3271–3278. [Google Scholar] [CrossRef]
- Umansky, D.; Hagen, K.M.; Chu, T.H.; Pathiyil, R.K.; Alzahrani, S.; Ousman, S.S.; Midha, R. Functional Gait Assessment Using Manual, Semi-Automated and Deep Learning Approaches Following Standardized Models of Peripheral Nerve Injury in Mice. Biomolecules 2022, 12, 1355. [Google Scholar] [CrossRef]
- Akula, S.K.; McCullough, K.B.; Weichselbaum, C.; Dougherty, J.D.; Maloney, S.E. The Trajectory of Gait Development in Mice. Brain Behav. 2020, 10, e01636. [Google Scholar] [CrossRef]
- Guillot, T.S.; Asress, S.A.; Richardson, J.R.; Glass, J.D.; Miller, G.W. Treadmill Gait Analysis Does Not Detect Motor Deficits in Animal Models of Parkinson’s Disease or Amyotrophic Lateral Sclerosis. J. Mot. Behav. 2008, 40, 568–577. [Google Scholar] [CrossRef][Green Version]
- Barnett, M.H.; Prineas, J.W. Relapsing and Remitting Multiple Sclerosis: Pathology of the Newly Forming Lesion. Ann. Neurol. 2004, 55, 458–468. [Google Scholar] [CrossRef]
- Rüther, B.J.; Scheld, M.; Dreymueller, D.; Clarner, T.; Kress, E.; Brandenburg, L.; Swartenbroekx, T.; Hoornaert, C.; Ponsaerts, P.; Fallier-Becker, P.; et al. Combination of Cuprizone and Experimental Autoimmune Encephalomyelitis to Study Inflammatory Brain Lesion Formation and Progression. Glia 2017, 65, 1900–1913. [Google Scholar] [CrossRef]
- Samtani, G.; Kim, S.; Michaud, D.; Hillhouse, A.E.; Szule, J.A.; Konganti, K.; Li, J. Brain Region Dependent Molecular Signatures and Myelin Repair Following Chronic Demyelination. Front. Cell. Neurosci. 2023, 17, 1169786. [Google Scholar] [CrossRef]
- Scheld, M.; Rüther, B.J.; Große-Veldmann, R.; Ohl, K.; Tenbrock, K.; Dreymüller, D.; Fallier-Becker, P.; Zendedel, A.; Beyer, C.; Clarner, T.; et al. Neurodegeneration Triggers Peripheral Immune Cell Recruitment into the Forebrain. J. Neurosci. 2016, 36, 1410–1415. [Google Scholar] [CrossRef]
- Vega-Riquer, J.M.; Mendez-Victoriano, G.; Morales-Luckie, R.A.; Gonzalez-Perez, O. Five Decades of Cuprizone, an Updated Model to Replicate Demyelinating Diseases. CN 2019, 17, 129–141. [Google Scholar] [CrossRef]
- Clarner, T.; Diederichs, F.; Berger, K.; Denecke, B.; Gan, L.; Van Der Valk, P.; Beyer, C.; Amor, S.; Kipp, M. Myelin Debris Regulates Inflammatory Responses in an Experimental Demyelination Animal Model and Multiple Sclerosis Lesions. Glia 2012, 60, 1468–1480. [Google Scholar] [CrossRef]
- Gudi, V.; Gingele, S.; Skripuletz, T.; Stangel, M. Glial Response during Cuprizone-Induced de- and Remyelination in the CNS: Lessons Learned. Front. Cell. Neurosci. 2014, 8, 73. [Google Scholar] [CrossRef]
- O’Loughlin, E.; Madore, C.; Lassmann, H.; Butovsky, O. Microglial Phenotypes and Functions in Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2018, 8, a028993. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Tian, N.-X.; Bai, Q.-Y.; Chen, Q.; Sun, X.-H.; Wang, Y. Gait Assessment of Pain and Analgesics: Comparison of the DigiGaitTM and CatWalkTM Gait Imaging Systems. Neurosci. Bull. 2019, 35, 401–418. [Google Scholar] [CrossRef]
- Pitzer, C.; Kurpiers, B.; Eltokhi, A. Gait Performance of Adolescent Mice Assessed by the CatWalk XT Depends on Age, Strain and Sex and Correlates with Speed and Body Weight. Sci. Rep. 2021, 11, 21372. [Google Scholar] [CrossRef]
- Li, C.X.; Kapoor, E.; Chen, W.; Ward, L.M.; Lee, D.D.; Titus, A.; Reardon, K.M.; Lee, J.-M.; Yuede, C.M.; Landsness, E.C. Manual Assessment of Cylinder Rearing Behavior Is More Sensitive than Automated Gait Evaluations in Young, Male Mice Post-Stroke of the Forepaw Somatosensory Cortex. J. Stroke Cerebrovasc. Dis. 2025, 34, 108325. [Google Scholar] [CrossRef]
- Broom, L.; Ellison, B.A.; Worley, A.; Wagenaar, L.; Sörberg, E.; Ashton, C.; Bennett, D.A.; Buchman, A.S.; Saper, C.B.; Shih, L.C.; et al. A Translational Approach to Capture Gait Signatures of Neurological Disorders in Mice and Humans. Sci. Rep. 2017, 7, 3225. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Charles, J.P.; Akay, T.; Hutchinson, J.R.; Blemker, S.S. Are Mice Good Models for Human Neuromuscular Disease? Comparing Muscle Excursions in Walking between Mice and Humans. Skelet. Muscle 2017, 7, 26. [Google Scholar] [CrossRef]
- Badea, A.; Ali-Sharief, A.A.; Johnson, G.A. Morphometric Analysis of the C57BL/6J Mouse Brain. NeuroImage 2007, 37, 683–693. [Google Scholar] [CrossRef]
- DeFelipe, J. The Evolution of the Brain, the Human Nature of Cortical Circuits, and Intellectual Creativity. Front. Neuroanat. 2011, 5, 11068. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Maurenbrecher, H.; Schaer, A.; Becsek, B.; Awai Easthope, C.; Chatzipirpiridis, G.; Ergeneman, O.; Pané, S.; Nelson, B.J. Human Gait-Labeling Uncertainty and a Hybrid Model for Gait Segmentation. Front. Neurosci. 2022, 16, 976594. [Google Scholar] [CrossRef]
- Maas, S.A.; Göcking, T.; Stojan, R.; Voelcker-Rehage, C.; Kutz, D.F. Synchronization of Neurophysiological and Biomechanical Data in a Real-Time Virtual Gait Analysis System (GRAIL): A Proof-of-Principle Study. Sensors 2024, 24, 3779. [Google Scholar] [CrossRef]
- Socie, M.J.; Motl, R.W.; Pula, J.H.; Sandroff, B.M.; Sosnoff, J.J. Gait Variability and Disability in Multiple Sclerosis. Gait Posture 2013, 38, 51–55. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giesler, P.; Kipp, M.; Hawlitschka, A. High-Speed-Ventral-Plane Videography Identifies Specific Gait Pattern Changes in Cuprizone-Induced Demyelination in Mice. Cells 2025, 14, 969. https://doi.org/10.3390/cells14130969
Giesler P, Kipp M, Hawlitschka A. High-Speed-Ventral-Plane Videography Identifies Specific Gait Pattern Changes in Cuprizone-Induced Demyelination in Mice. Cells. 2025; 14(13):969. https://doi.org/10.3390/cells14130969
Chicago/Turabian StyleGiesler, Paula, Markus Kipp, and Alexander Hawlitschka. 2025. "High-Speed-Ventral-Plane Videography Identifies Specific Gait Pattern Changes in Cuprizone-Induced Demyelination in Mice" Cells 14, no. 13: 969. https://doi.org/10.3390/cells14130969
APA StyleGiesler, P., Kipp, M., & Hawlitschka, A. (2025). High-Speed-Ventral-Plane Videography Identifies Specific Gait Pattern Changes in Cuprizone-Induced Demyelination in Mice. Cells, 14(13), 969. https://doi.org/10.3390/cells14130969







