Exosomes as Future Therapeutic Tools and Targets for Corneal Diseases
Abstract
1. Introduction
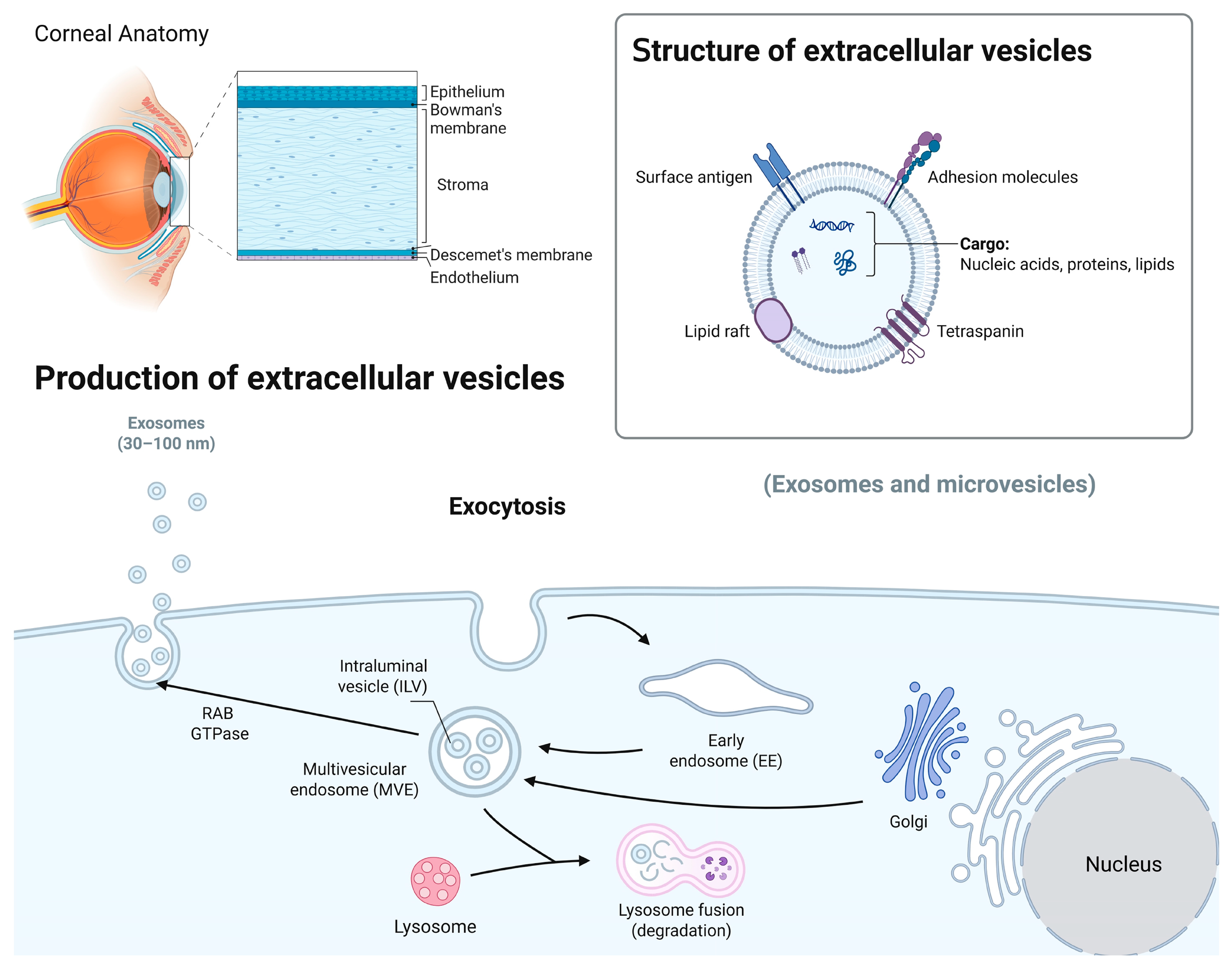
2. Overview of Exosomes
3. Exosomal Cargos
4. Exosome Isolation and Characterization
5. Dual Role of Exosomes in Cell Communication and Therapy as Tools and Targets
5.1. Exosomes as Treatment Tools and Biomarkers in Corneal Diseases
5.1.1. Exosomal miRNAs and Their Therapeutic Potential
5.1.2. MSC-Derived Exosome-Based Therapy
5.1.3. Modified-Exosome-Based Therapy
5.1.4. Circulating Exos in Biological Fluids as Biomarkers and Messengers
5.2. Exosomes as Treatment Targets—Inhibition of Exo Biogenesis or Secretion
6. Challenges and Opportunities of Exosomes’ Clinical Translation
7. Discussion and Perspectives
| Challenge | Conventional Delivery (e.g., Eye Drop) | Exosome-Based Delivery |
|---|---|---|
| Bioavailability | Rapid tear clearance, <5% [190] | Slightly improved but still not cleared unless modified [191] |
| Penetration | Poor corneal and conjunctival penetration [192] | Enhanced via vesicle fusion and receptor-mediated uptake [182] |
| Target Specificity | Largely non-specific | Surface modification allows targeted delivery [183] |
| Stability of Therapeutics | Susceptible to degradation [193] | Cargo protected within lipid bilayer [184] |
| Manufacturing | Simple and scalable | Technically demanding, not yet standardized [194] |
| Regulatory Pathway | Well-defined | Still evolving and undefined [189] |
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dziasko, M.A.; Daniels, J.T. Anatomical features and cell-cell interactions in the human limbal epithelial stem cell niche. Ocul. Surf. 2016, 14, 322–330. [Google Scholar] [CrossRef]
- Matic, M.; Petrov, I.N.; Chen, S.; Wang, C.; Dimitrijevich, S.D.; Wolosin, J.M. Stem cells of the corneal epithelium lack connexins and metabolite transfer capacity. Differentiation 1997, 61, 251–260. [Google Scholar] [CrossRef]
- Dogru, M.; Chen, M.; Shimmura, S.; Tsubota, K. Corneal epithelium and stem cells. In Corneal Surgery, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 25–31. [Google Scholar]
- Thomasy, S.M.; Leonard, B.C.; Greiner, M.A.; Skeie, J.M.; Raghunathan, V.K. Squishy matters—Corneal mechanobiology in health and disease. Prog. Retin. Eye Res. 2024, 99, 101234. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, H.; Liu, Y.; Chen, S.; Cai, S.; Zhu, Y.; Guo, P. The limbal epithelial progenitors in the limbal niche environment. Int. J. Med. Sci. 2016, 13, 835–840. [Google Scholar] [CrossRef]
- Espana, E.M.; Kawakita, T.; Romano, A.; Di Pascuale, M.; Smiddy, R.; Liu, C.Y.; Tseng, S.C. Stromal niche controls the plasticity of limbal and corneal epithelial differentiation in a rabbit model of recombined tissue. Investig. Ophthalmol. Vis. Sci. 2003, 44, 5130–5135. [Google Scholar] [CrossRef]
- Bourne, R.; Steinmetz, J.D.; Flaxman, S.; Briant, P.S.; Taylor, H.R.; Resnikoff, S.; Casson, R.J.; Abdoli, A.; Abu-Gharbieh, E.; Afshin, A.; et al. Trends in prevalence of blindness and distance and near vision impairment over 30 years: An analysis for the global burden of disease study. Lancet Glob. Health 2021, 9, e130–e143. [Google Scholar] [CrossRef]
- Oie, Y.; Nishida, K. Regenerative medicine for the cornea. Biomed. Res. Int. 2013, 2013, 428247. [Google Scholar] [CrossRef]
- Biber, J.M.; Holland, E.J.; Neff, K.D. Management of ocular stem cell disease. Int. Ophthalmol. Clin. 2010, 50, 25–34. [Google Scholar] [CrossRef]
- Mason, S.L.; Stewart, R.M.; Kearns, V.R.; Williams, R.L.; Sheridan, C.M. Ocular epithelial transplantation: Current uses and future potential. Regen. Med. 2011, 6, 767–782. [Google Scholar] [CrossRef]
- Shoham, A.; Hadziahmetovic, M.; Dunaief, J.L.; Mydlarski, M.B.; Schipper, H.M. Oxidative stress in diseases of the human cornea. Free Radic. Biol. Med. 2008, 45, 1047–1055. [Google Scholar] [CrossRef]
- Stoorvogel, W.; Kleijmeer, M.J.; Geuze, H.J.; Raposo, G. The biogenesis and functions of exosomes. Traffic 2002, 3, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Whiteside, T.L. Tumor-derived exosomes and their role in cancer progression. Adv. Clin. Chem. 2016, 74, 103–141. [Google Scholar] [CrossRef] [PubMed]
- Maas, S.L.N.; Breakefield, X.O.; Weaver, A.M. Extracellular vesicles: Unique intercellular delivery vehicles. Trends Cell Biol. 2017, 27, 172–188. [Google Scholar] [CrossRef]
- Tkach, M.; Théry, C. Communication by extracellular vesicles: Where we are and where we need to go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Han, K.Y.; Tran, J.A.; Chang, J.H.; Azar, D.T.; Zieske, J.D. Potential role of corneal epithelial cell-derived exosomes in corneal wound healing and neovascularization. Sci. Rep. 2017, 7, 40548. [Google Scholar] [CrossRef] [PubMed]
- Leszczynska, A.; Kulkarni, M.; Ljubimov, A.V.; Saghizadeh, M. Exosomes from normal and diabetic human corneolimbal keratocytes differentially regulate migration, proliferation and marker expression of limbal epithelial cells. Sci. Rep. 2018, 8, 15173. [Google Scholar] [CrossRef]
- Hess, C.; Sadallah, S.; Hefti, A.; Landmann, R.; Schifferli, A., Jr. Ectosomes released by human neutrophils are specialized functional units. J. Immunol. 1999, 163, 4564–4573. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Wang, L.; Hu, L.; Zhou, X.; Xiong, Z.; Zhang, C.; Shehada, H.M.A.; Hu, B.; Song, J.; Chen, L. Exosomes secreted by human adipose mesenchymal stem cells promote scarless cutaneous repair by regulating extracellular matrix remodelling. Sci. Rep. 2017, 7, 13321. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- Tiwari, A.; Singh, A.; Verma, S.; Stephenson, S.; Bhowmick, T.; Sangwan, V.S. Mini Review: Current trends and understanding of exosome therapeutic potential in corneal diseases. Front. Pharmacol. 2021, 12, 684712. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, H.; Gahan, P.B. Exosomes in immune regulation. Noncoding RNA 2021, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Kugeratski, F.G.; Kalluri, R. Exosomes as mediators of immune regulation and immunotherapy in cancer. FEBS J. 2021, 288, 10–35. [Google Scholar] [CrossRef]
- Sánchez-Alonso, S.; Alcaraz-Serna, A.; Sánchez-Madrid, F.; Alfranca, A. Extracellular vesicle-mediated immune regulation of tissue remodeling and angiogenesis after myocardial infarction. Front. Immunol. 2018, 9, 2799. [Google Scholar] [CrossRef]
- Askenase, P.W. Exosomes provide unappreciated carrier effects that assist transfers of their miRNAs to targeted cells; I. They are ‘The Elephant in the Room’. RNA Biol. 2021, 18, 2038–2053. [Google Scholar] [CrossRef]
- Zheng, D.; Huo, M.; Li, B.; Wang, W.; Piao, H.; Wang, Y.; Zhu, Z.; Li, D.; Wang, T.; Liu, K. The role of exosomes and exosomal microRNA in cardiovascular disease. Front. Cell Dev. Biol. 2020, 8, 616161. [Google Scholar] [CrossRef]
- Li, S.P.; Lin, Z.X.; Jiang, X.Y.; Yu, X.Y. Exosomal cargo-loading and synthetic exosome-mimics as potential therapeutic tools. Acta Pharmacol. Sin. 2018, 39, 542–551. [Google Scholar] [CrossRef]
- Balaj, L.; Lessard, R.; Dai, L.; Cho, Y.-J.; Pomeroy, S.L.; Breakefield, X.O.; Skog, J. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat. Commun. 2011, 2, 180. [Google Scholar] [CrossRef]
- Pan, B.T.; Johnstone, R.M. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: Selective externalization of the receptor. Cell 1983, 33, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Harding, C.; Heuser, J.; Stahl, P. Endocytosis and intracellular processing of transferrin and colloidal gold-transferrin in rat reticulocytes: Demonstration of a pathway for receptor shedding. Eur. J. Cell Biol. 1984, 35, 256–263. [Google Scholar]
- Yue, B.; Yang, H.; Wang, J.; Ru, W.; Wu, J.; Huang, Y.; Lan, X.; Lei, C.; Chen, H. Exosome biogenesis, secretion and function of exosomal miRNAs in skeletal muscle myogenesis. Cell Prolif. 2020, 53, e12857. [Google Scholar] [CrossRef] [PubMed]
- Jella, K.K.; Nasti, T.H.; Li, Z.; Malla, S.R.; Buchwald, Z.S.; Khan, M.K. Exosomes, their biogenesis and role in inter-cellular communication, tumor microenvironment and cancer immunotherapy. Vaccines 2018, 6, 69. [Google Scholar] [CrossRef] [PubMed]
- Isaac, R.; Reis, F.C.G.; Ying, W.; Olefsky, J.M. Exosomes as mediators of intercellular crosstalk in metabolism. Cell Metab. 2021, 33, 1744–1762. [Google Scholar] [CrossRef]
- Théry, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef]
- Malenica, M.; Vukomanović, M.; Kurtjak, M.; Masciotti, V.; Dal Zilio, S.; Greco, S.; Lazzarino, M.; Krušić, V.; Perčić, M.; Jelovica Badovinac, I.; et al. Perspectives of microscopy methods for morphology characterisation of extracellular vesicles from human biofluids. Biomedicines 2021, 9, 603. [Google Scholar] [CrossRef]
- Trams, E.G.; Lauter, C.J.; Salem, N., Jr.; Heine, U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim. Biophys. Acta 1981, 645, 63–70. [Google Scholar] [CrossRef]
- Johnstone, R.M. Revisiting the road to the discovery of exosomes. Blood Cells Mol. Dis. 2005, 34, 214–219. [Google Scholar] [CrossRef]
- Xie, S.; Zhang, Q.; Jiang, L. Current knowledge on exosome biogenesis, cargo-sorting mechanism and therapeutic implications. Membranes 2022, 12, 498. [Google Scholar] [CrossRef]
- Krylova, S.V.; Feng, D. The machinery of exosomes: Biogenesis, release, and uptake. Int. J. Mol. Sci. 2023, 24, 1337. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of exosome composition. Cell 2019, 177, 428–445.e418. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Moita, C.; van Niel, G.; Kowal, J.; Vigneron, J.; Benaroch, P.; Manel, N.; Moita, L.F.; Théry, C.; Raposo, G. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J. Cell Sci. 2013, 126, 5553–5565. [Google Scholar] [CrossRef] [PubMed]
- Baietti, M.F.; Zhang, Z.; Mortier, E.; Melchior, A.; Degeest, G.; Geeraerts, A.; Ivarsson, Y.; Depoortere, F.; Coomans, C.; Vermeiren, E.; et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 2012, 14, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Villarroya-Beltri, C.; Baixauli, F.; Mittelbrunn, M.; Fernández-Delgado, I.; Torralba, D.; Moreno-Gonzalo, O.; Baldanta, S.; Enrich, C.; Guerra, S.; Sánchez-Madrid, F. ISGylation controls exosome secretion by promoting lysosomal degradation of MVB proteins. Nat. Commun. 2016, 7, 13588. [Google Scholar] [CrossRef]
- Jin, H.; Tang, Y.; Yang, L.; Peng, X.; Li, B.; Fan, Q.; Wei, S.; Yang, S.; Li, X.; Wu, B.; et al. Rab GTPases: Central coordinators of membrane trafficking in cancer. Front. Cell Dev. Biol. 2021, 9, 648384. [Google Scholar] [CrossRef]
- Südhof, T.C.; Rothman, J.E. Membrane fusion: Grappling with SNARE and SM proteins. Science 2009, 323, 474–477. [Google Scholar] [CrossRef]
- Lee, Y.J.; Shin, K.J.; Chae, Y.C. Regulation of cargo selection in exosome biogenesis and its biomedical applications in cancer. Exp. Mol. Med. 2024, 56, 877–889. [Google Scholar] [CrossRef]
- Modani, S.; Tomar, D.; Tangirala, S.; Sriram, A.; Mehra, N.K.; Kumar, R.; Khatri, D.K.; Singh, P.K. An updated review on exosomes: Biosynthesis to clinical applications. J. Drug Target. 2021, 29, 925–940. [Google Scholar] [CrossRef]
- Cheng, K.; Kalluri, R. Guidelines for clinical translation and commercialization of extracellular vesicles and exosomes based therapeutics. Extracell. Vesicle 2023, 2, 100029. [Google Scholar] [CrossRef]
- Ramos, T.; Parekh, M.; Kaye, S.B.; Ahmad, S. Epithelial cell-derived extracellular vesicles trigger the differentiation of two epithelial cell lines. Int. J. Mol. Sci. 2022, 23, 1718. [Google Scholar] [CrossRef] [PubMed]
- Braunsperger, M.V.; Martin, G.; Herzig, T.; Kußberger, I.; Gießl, A.; Steimle, S.; Schlötzer-Schrehardt, U.; Schlunck, G.; Reinhard, T.; Polisetti, N. Proteomic insights into human limbal epithelial progenitor-derived small extracellular vesicles. Stem Cell Rev. Rep. 2025. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Guo, S.; Ren, X.; Wu, Z.; Liu, S.; Yao, X. Current strategies for exosome cargo loading and targeting delivery. Cells 2023, 12, 1416. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Dang, W.; Zhang, S.; Yue, W.; Yang, L.; Zhai, X.; Yan, Q.; Lu, J. The role of exosomal noncoding RNAs in cancer. Mol. Cancer 2019, 18, 37. [Google Scholar] [CrossRef] [PubMed]
- van den Boorn, J.G.; Dassler, J.; Coch, C.; Schlee, M.; Hartmann, G. Exosomes as nucleic acid nanocarriers. Adv. Drug Deliv. Rev. 2013, 65, 331–335. [Google Scholar] [CrossRef]
- Todorova, D.; Simoncini, S.; Lacroix, R.; Sabatier, F.; Dignat-George, F. Extracellular vesicles in angiogenesis. Circ. Res. 2017, 120, 1658–1673. [Google Scholar] [CrossRef]
- Jung, H.H.; Kim, J.Y.; Lim, J.E.; Im, Y.H. Cytokine profiling in serum-derived exosomes isolated by different methods. Sci. Rep. 2020, 10, 14069. [Google Scholar] [CrossRef]
- Hyvärinen, K.; Holopainen, M.; Skirdenko, V.; Ruhanen, H.; Lehenkari, P.; Korhonen, M.; Käkelä, R.; Laitinen, S.; Kerkelä, E. Mesenchymal stromal cells and their extracellular vesicles enhance the anti-inflammatory phenotype of regulatory macrophages by downregulating the production of interleukin (IL)-23 and IL-22. Front. Immunol. 2018, 9, 771. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L. Circulating exosomal miRNA as diagnostic biomarkers of neurodegenerative diseases. Front. Mol. Neurosci. 2020, 13, 53. [Google Scholar] [CrossRef]
- Sun, Z.; Yang, S.; Zhou, Q.; Wang, G.; Song, J.; Li, Z.; Zhang, Z.; Xu, J.; Xia, K.; Chang, Y.; et al. Emerging role of exosome-derived long non-coding RNAs in tumor microenvironment. Mol. Cancer 2018, 17, 82. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Ma, J.; Sun, T.; Zhou, Q.; Wang, W.; Wang, G.; Wu, P.; Wang, H.; Jiang, L.; et al. Exosomal circRNAs: Biogenesis, effect and application in human diseases. Mol. Cancer 2019, 18, 116. [Google Scholar] [CrossRef]
- Skotland, T.; Sandvig, K.; Llorente, A. Lipids in exosomes: Current knowledge and the way forward. Prog. Lipid Res. 2017, 66, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Mathivanan, S.; Fahner, C.J.; Reid, G.E.; Simpson, R.J. ExoCarta 2012: Database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012, 40, D1241–D1244. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Li, A.; Hu, J.; Feng, L.; Liu, L.; Shen, Z. Recent developments in isolating methods for exosomes. Front. Bioeng. Biotechnol. 2023, 10, 1100892. [Google Scholar] [CrossRef]
- Ter-Ovanesyan, D.; Norman, M.; Lazarovits, R.; Trieu, W.; Lee, J.H.; Church, G.M.; Walt, D.R. Framework for rapid comparison of extracellular vesicle isolation methods. eLife 2021, 10, 70725. [Google Scholar] [CrossRef]
- Kim, K.; Park, J.; Jung, J.H.; Lee, R.; Park, J.H.; Yuk, J.M.; Hwang, H.; Yeon, J.H. Cyclic tangential flow filtration system for isolation of extracellular vesicles. APL Bioeng. 2021, 5, 016103. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.K.; Whiteside, T.L. Immunoaffinity-based isolation of melanoma cell-derived and T cell-derived exosomes from plasma of melanoma patients. Methods Mol. Biol. 2021, 2265, 305–321. [Google Scholar] [CrossRef] [PubMed]
- Kamei, N.; Nishimura, H.; Matsumoto, A.; Asano, R.; Muranaka, K.; Fujita, M.; Takeda, M.; Hashimoto, H.; Takeda-Morishita, M. Comparative study of commercial protocols for high recovery of high-purity mesenchymal stem cell-derived extracellular vesicle isolation and their efficient labeling with fluorescent dyes. Nanomedicine 2021, 35, 102396. [Google Scholar] [CrossRef]
- Guo, J.; Wu, C.; Lin, X.; Zhou, J.; Zhang, J.; Zheng, W.; Wang, T.; Cui, Y. Establishment of a simplified dichotomic size-exclusion chromatography for isolating extracellular vesicles toward clinical applications. J. Extracell. Vesicles 2021, 10, e12145. [Google Scholar] [CrossRef]
- Lai, J.J.; Chau, Z.L.; Chen, S.Y.; Hill, J.J.; Korpany, K.V.; Liang, N.W.; Lin, L.H.; Lin, Y.H.; Liu, J.K.; Liu, Y.C.; et al. Exosome processing and characterization approaches for research and technology development. Adv. Sci. 2022, 9, e2103222. [Google Scholar] [CrossRef]
- Livshits, M.A.; Khomyakova, E.; Evtushenko, E.G.; Lazarev, V.N.; Kulemin, N.A.; Semina, S.E.; Generozov, E.V.; Govorun, V.M. Isolation of exosomes by differential centrifugation: Theoretical analysis of a commonly used protocol. Sci. Rep. 2015, 5, 17319. [Google Scholar] [CrossRef] [PubMed]
- Shu, S.; Allen, C.L.; Benjamin-Davalos, S.; Koroleva, M.; MacFarland, D.; Minderman, H.; Ernstoff, M.S. A rapid exosome isolation using ultrafiltration and size exclusion chromatography (REIUS) method for exosome isolation from melanoma cell lines. Methods Mol. Biol. 2021, 2265, 289–304. [Google Scholar] [CrossRef]
- Guan, S.; Yu, H.; Yan, G.; Gao, M.; Sun, W.; Zhang, X. Characterization of urinary exosomes purified with size exclusion chromatography and ultracentrifugation. J. Proteome Res. 2020, 19, 2217–2225. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.K.; Tsai, T.H.; Lee, C.H. Regulation of exosomes as biologic medicines: Regulatory challenges faced in exosome development and manufacturing processes. Clin. Transl. Sci. 2024, 17, e13904. [Google Scholar] [CrossRef] [PubMed]
- Muller, L.; Muller-Haegele, S.; Mitsuhashi, M.; Gooding, W.; Okada, H.; Whiteside, T.L. Exosomes isolated from plasma of glioma patients enrolled in a vaccination trial reflect antitumor immune activity and might predict survival. Oncoimmunology 2015, 4, e1008347. [Google Scholar] [CrossRef]
- Ludwig, N.; Whiteside, T.L.; Reichert, T.E. Challenges in exosome isolation and analysis in health and disease. Int. J. Mol. Sci. 2019, 20, 4684. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Lu, Y.; Luo, X.; Huang, Y.; Xie, T.; Pilarsky, C.; Dang, Y.; Zhang, J. Microfluidic technology for the isolation and analysis of exosomes. Micromachines 2022, 13, 1571. [Google Scholar] [CrossRef]
- Kang, Y.T.; Hadlock, T.; Jolly, S.; Nagrath, S. Extracellular vesicles on demand (EVOD) chip for screening and quantification of cancer-associated extracellular vesicles. Biosens. Bioelectron. 2020, 168, 112535. [Google Scholar] [CrossRef]
- Tang, Y.T.; Huang, Y.Y.; Zheng, L.; Qin, S.H.; Xu, X.P.; An, T.X.; Xu, Y.; Wu, Y.S.; Hu, X.M.; Ping, B.H.; et al. Comparison of isolation methods of exosomes and exosomal RNA from cell culture medium and serum. Int. J. Mol. Med. 2017, 40, 834–844. [Google Scholar] [CrossRef]
- Gardiner, C.; Di Vizio, D.; Sahoo, S.; Théry, C.; Witwer, K.W.; Wauben, M.; Hill, A.F. Techniques used for the isolation and characterization of extracellular vesicles: Results of a worldwide survey. J. Extracell. Vesicles 2016, 5, 32945. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, Q.; Cheng, L.; Wang, Y.; Li, M.; Yang, Q.; Hu, L.; Lou, D.; Li, J.; Dong, X.; et al. Exosome detection via the ultrafast-isolation system: EXODUS. Nat. Methods 2021, 18, 212–218. [Google Scholar] [CrossRef]
- Serrano-Pertierra, E.; Oliveira-Rodríguez, M.; Rivas, M.; Oliva, P.; Villafani, J.; Navarro, A.; Blanco-López, M.C.; Cernuda-Morollón, E. Characterization of plasma-derived extracellular vesicles isolated by different methods: A comparison study. Bioengineering 2019, 6, 8. [Google Scholar] [CrossRef]
- Logozzi, M.; Mizzoni, D.; Di Raimo, R.; Fais, S. Exosomes: A source for new and old biomarkers in cancer. Cancers 2020, 12, 2566. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.I.; Park, J.; Zhu, Y.; Wang, X.; Han, Y.; Zhang, D. Recent advances in extracellular vesicles for therapeutic cargo delivery. Exp. Mol. Med. 2024, 56, 836–849. [Google Scholar] [CrossRef] [PubMed]
- Hamzah, R.N.; Alghazali, K.M.; Biris, A.S.; Griffin, R.J. Exosome traceability and cell source dependence on composition and cell-cell cross talk. Int. J. Mol. Sci. 2021, 22, 5346. [Google Scholar] [CrossRef] [PubMed]
- Roszkowski, S. Therapeutic potential of mesenchymal stem cell-derived exosomes for regenerative medicine applications. Clin. Exp. Med. 2024, 24, 46. [Google Scholar] [CrossRef]
- Olejarz, W.; Kubiak-Tomaszewska, G.; Chrzanowska, A.; Lorenc, T. Exosomes in angiogenesis and anti-angiogenic therapy in cancers. Int. J. Mol. Sci. 2020, 21, 5840. [Google Scholar] [CrossRef]
- Jucker, M.; Walker, L.C. Pathogenic protein seeding in Alzheimer disease and other neurodegenerative disorders. Ann. Neurol. 2011, 70, 532–540. [Google Scholar] [CrossRef]
- ElShelmani, H.; Wride, M.A.; Saad, T.; Rani, S.; Kelly, D.J.; Keegan, D. The role of deregulated microRNAs in age-related macular degeneration pathology. Transl. Vis. Sci. Technol. 2021, 10, 12. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, J.; Zhang, L.; Jiang, G.; Wang, B.; Jiang, L. Extracellular vesicle-derived miR-26b-5p is up-regulated in the serum of patients with diabetic retinopathy. Comb. Chem. High. Throughput Screen. 2022, 25, 877–882. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, M.; Cui, C.; Zhao, Y.; Sun, X.; Wang, Y.; Liu, C.; Wu, H.; Zhong, X.; et al. Different Exosomal microRNA Profile in Aquaporin-4 Antibody Positive Neuromyelitis Optica Spectrum Disorders. Front. Immunol. 2020, 11, 1064. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wei, X. Exosome-based crosstalk in glaucoma pathogenesis: A focus on oxidative stress and neuroinflammation. Front. Immunol. 2023, 14, 1202704. [Google Scholar] [CrossRef]
- Yeung, V.; Boychev, N.; Farhat, W.; Ntentakis, D.P.; Hutcheon, A.E.K.; Ross, A.E.; Ciolino, J.B. Extracellular vesicles in corneal fibrosis/scarring. Int. J. Mol. Sci. 2022, 23, 5921. [Google Scholar] [CrossRef]
- Samaeekia, R.; Rabiee, B.; Putra, I.; Shen, X.; Park, Y.J.; Hematti, P.; Eslani, M.; Djalilian, A.R. Effect of human corneal mesenchymal stromal cell-derived exosomes on corneal epithelial wound healing. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5194–5200. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Khare, D.; Poe, A.J.; Amador, C.; Ghiam, S.; Fealy, A.; Ebrahimi, S.; Shadrokh, O.; Song, X.Y.; Santiskulvong, C.; et al. MicroRNA and protein cargos of human limbal epithelial cell-derived exosomes and their regulatory roles in limbal stromal cells of diabetic and non-diabetic corneas. Cells 2023, 12, 2524. [Google Scholar] [CrossRef]
- Desjardins, P.; Berthiaume, R.; Couture, C.; Le-Bel, G.; Roy, V.; Gros-Louis, F.; Moulin, V.J.; Proulx, S.; Chemtob, S.; Germain, L.; et al. Impact of exosomes released by different corneal cell types on the wound healing properties of human corneal epithelial cells. Int. J. Mol. Sci. 2022, 23, 12201. [Google Scholar] [CrossRef]
- Wu, S.; Su, W.; Wang, K.; Li, H.; Huang, S.; Tie, S.; Tan, M. Milk-derived exosome as delivery system for lutein encapsulation in alleviating dry eye disease. Chem. Eng. J. 2024, 486, 149898. [Google Scholar] [CrossRef]
- Yi, S.; Kim, J.; Kim, M.J.; Yae, C.G.; Kim, K.H.; Kim, H.K. Development of human amniotic epithelial cell-derived extracellular vesicles as cell-free therapy for dry eye disease. Ocul. Surf. 2024, 34, 370–380. [Google Scholar] [CrossRef]
- Liu, J.; Gao, J.; Lu, P.; Wang, Y.; Xing, S.; Yan, Y.; Han, R.; Hao, P.; Li, X. Mesenchymal stem cell-derived exosomes as drug carriers for delivering miRNA-29b to ameliorate inflammation in corneal injury via activating autophagy. Investig. Ophthalmol. Vis. Sci. 2024, 65, 16. [Google Scholar] [CrossRef]
- Khorrami-Nejad, M.; Hashemian, H.; Majdi, A.; Jadidi, K.; Aghamollaei, H.; Hadi, A. Application of stem cell-derived exosomes in anterior segment eye diseases: A comprehensive update review. Ocul. Surf. 2025, 36, 209–219. [Google Scholar] [CrossRef]
- Chan, S.M.; Tsai, C.; Lee, T.P.; Huang, Z.R.; Huang, W.H.; Lin, C.T. Therapeutic potential of umbilical cord MSC-derived exosomes in a severe dry eye rat model: Enhancing corneal protection and modulating inflammation. Biomedicines 2025, 13, 1174. [Google Scholar] [CrossRef]
- Massoumi, H.; Amin, S.; Soleimani, M.; Momenaei, B.; Ashraf, M.J.; Guaiquil, V.H.; Hematti, P.; Rosenblatt, M.I.; Djalilian, A.R.; Jalilian, E. Extracellular-vesicle-based therapeutics in neuro-ophthalmic disorders. Int. J. Mol. Sci. 2023, 24, 9006. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, C.; Chen, V.T.; Herbst, P.; Zhang, R.; Elfert, A.; Krishan, A.; Azar, D.T.; Chang, J.H.; Hu, W.Y.; Kremsmayer, T.P.; et al. Target specification and therapeutic potential of extracellular vesicles for regulating corneal angiogenesis, lymphangiogenesis, and nerve repair. Ocul. Surf. 2024, 34, 459–476. [Google Scholar] [CrossRef]
- Ciferri, M.C.; Quarto, R.; Tasso, R. Extracellular vesicles as biomarkers and therapeutic tools: From pre-clinical to clinical applications. Biology 2021, 10, 359. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zhang, T.; Ma, H.; Pan, Y.; Wang, S.; Liu, X.; Dai, X.; Zheng, Y.; Lee, L.P.; Liu, F. Discovering the secret of diseases by incorporated tear exosomes analysis. ACS Nano 2022, 16, 11720–11732. [Google Scholar] [CrossRef] [PubMed]
- Aqrawi, L.A.; Galtung, H.K.; Vestad, B.; Øvstebø¸, R.; Thiede, B.; Rusthen, S.; Young, A.; Guerreiro, E.M.; Utheim, T.P.; Chen, X.; et al. Identification of potential saliva and tear biomarkers in primary Sjögren’s syndrome, utilising the extraction of extracellular vesicles and proteomics analysis. Arthritis Res. Ther. 2017, 19, 14. [Google Scholar] [CrossRef]
- Amorim, M.; Martins, B.; Caramelo, F.; Gonçalves, C.; Trindade, G.; Simão, J.; Barreto, P.; Marques, I.; Leal, E.C.; Carvalho, E.; et al. Putative biomarkers in tears for diabetic retinopathy diagnosis. Front. Med. 2022, 9, 873483. [Google Scholar] [CrossRef]
- Hefley, B.S.; Deighan, C.; Vasini, B.; Khan, A.; Hjortdal, J.; Riaz, K.M.; Liu, Y.; Karamichos, D. Revealing the presence of tear extracellular vesicles in keratoconus. Exp. Eye Res. 2022, 224, 109242. [Google Scholar] [CrossRef]
- Li, J.; Jiang, X.; Wang, K. Exosomal miRNA: An alternative mediator of cell-to-cell communication. ExRNA 2019, 1, 31. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Li, L.; Li, M.; Guo, C.; Yao, J.; Mi, S. Exosome and exosomal microRNA: Trafficking, sorting, and function. Genom. Proteom. Bioinform. 2015, 13, 17–24. [Google Scholar] [CrossRef]
- Rani, V.; Sengar, R.S. Biogenesis and mechanisms of microRNA-mediated gene regulation. Biotechnol. Bioeng. 2022, 119, 685–692. [Google Scholar] [CrossRef]
- Dexheimer, P.J.; Cochella, L. MicroRNAs: From mechanism to organism. Front. Cell Dev. Biol. 2020, 8, 409. [Google Scholar] [CrossRef]
- Li, C.; Ni, Y.Q.; Xu, H.; Xiang, Q.Y.; Zhao, Y.; Zhan, J.K.; He, J.Y.; Li, S.; Liu, Y.S. Roles and mechanisms of exosomal non-coding RNAs in human health and diseases. Signal Transduct. Target. Ther. 2021, 6, 383. [Google Scholar] [CrossRef]
- Yu, Y.; Hou, K.; Ji, T.; Wang, X.; Liu, Y.; Zheng, Y.; Xu, J.; Hou, Y.; Chi, G. The role of exosomal microRNAs in central nervous system diseases. Mol. Cell Biochem. 2021, 476, 2111–2124. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Liang, Q.; Xu, Z.; Cai, Y.; Peng, B.; Li, J.; Zhang, W.; Kang, F.; Hong, Q.; Yan, Y.; et al. Current understanding of exosomal microRNAs in glioma immune regulation and therapeutic responses. Front. Immunol. 2021, 12, 813747. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lu, F.B.; Chen, D.Z.; Wu, J.L.; Hu, E.D.; Xu, L.M.; Zheng, M.H.; Li, H.; Huang, Y.; Jin, X.Y.; et al. BMSCs-derived miR-223-containing exosomes contribute to liver protection in experimental autoimmune hepatitis. Mol. Immunol. 2018, 93, 38–46. [Google Scholar] [CrossRef]
- Klingeborn, M.; Dismuke, W.M.; Bowes Rickman, C.; Stamer, W.D. Roles of exosomes in the normal and diseased eye. Prog. Retin. Eye Res. 2017, 59, 158–177. [Google Scholar] [CrossRef] [PubMed]
- Winkler, M.A.; Dib, C.; Ljubimov, A.V.; Saghizadeh, M. Targeting miR-146a to treat delayed wound healing in human diabetic organ-cultured corneas. PLoS ONE 2014, 9, e114692. [Google Scholar] [CrossRef]
- Stunf Pukl, S. MicroRNA of epithelial to mesenchymal transition in Fuchs’ endothelial corneal dystrophy. Genes 2022, 13, 1711. [Google Scholar] [CrossRef]
- Kulkarni, M.; Leszczynska, A.; Wei, G.; Winkler, M.A.; Tang, J.; Funari, V.A.; Deng, N.; Liu, Z.; Punj, V.; Deng, S.X.; et al. Genome-wide analysis suggests a differential microRNA signature associated with normal and diabetic human corneal limbus. Sci. Rep. 2017, 7, 3448. [Google Scholar] [CrossRef]
- Boomiraj, H.; Mohankumar, V.; Lalitha, P.; Devarajan, B. Human corneal microRNA expression profile in fungal keratitis. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7939–7946. [Google Scholar] [CrossRef] [PubMed]
- Matthaei, M.; Hu, J.; Kallay, L.; Eberhart, C.G.; Cursiefen, C.; Qian, J.; Lackner, E.M.; Jun, A.S. Endothelial cell microRNA expression in human late-onset Fuchs’ dystrophy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 216–225. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, F.; Jiang, Y.; Wang, Y.; Li, Z.; Shi, X.; Zhu, Y.; Wang, H.; Zhang, Z. Roles of exosomes in ocular diseases. Int. J. Nanomed. 2020, 15, 10519–10538. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Song, W.; Teng, L.; Huang, Y.; Liu, J.; Peng, Y.; Lu, X.; Yuan, J.; Zhao, X.; Zhao, Q.; et al. MiRNA 24-3p-rich exosomes functionalized DEGMA-modified hyaluronic acid hydrogels for corneal epithelial healing. Bioact. Mater. 2023, 25, 640–656. [Google Scholar] [CrossRef]
- Xu, W.; Fei, X.; Cui, Z.; Pan, D.; Liu, Y.; Liu, T. DNMT1 driven by mouse amniotic fluid mesenchymal stem cell exosomes improved corneal cryoinjury via inducing microRNA-33 promoter DNA hypermethylation modification in corneal epithelium cells. Hum. Cell 2024, 37, 1091–1106. [Google Scholar] [CrossRef]
- Altug, B.; Soykan, M.N.; Eyubova, S.; Eker Sariboyaci, A.; Dogan, C.; Ozalp, O.; Atalay, E. Crosstalk among miR-29 α-SMA, and TGFβ1/β3 in melatonin-induced exosome (Mel-prExo) treated human limbal mesenchymal stem cells (hLMSCs): An insight into scarless healing of the cornea. Biofactors 2024, 50, 1287–1297. [Google Scholar] [CrossRef]
- Wang, F.; Wang, D.; Song, M.; Zhou, Q.; Liao, R.; Wang, Y. MiRNA-155-5p reduces corneal epithelial permeability by remodeling epithelial tight junctions during corneal wound healing. Curr. Eye Res. 2020, 45, 904–913. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Zheng, Q.; Luo, H.; Li, X.; Chen, Z.; Song, Z.; Zhou, G.; Hong, C. Exosomal miR-19a from adipose-derived stem cells suppresses differentiation of corneal keratocytes into myofibroblasts. Aging 2020, 12, 4093–4110. [Google Scholar] [CrossRef]
- Wang, G.; Zhu, Y.; Liu, Y.; Yang, M.; Zeng, L. Mesenchymal stem cells-derived exosomal miR-223-3p alleviates ocular surface damage and inflammation by downregulating Fbxw7 in dry eye models. Investig. Ophthalmol. Vis. Sci. 2024, 65, 1. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Wu, G.; Qi, P.; Zhang, Y.; Liu, Z.; Yu, Y.; Ye, X.; Li, Y.; Yang, D.; et al. Umbilical cord mesenchymal stem cell-derived small extracellular vesicles deliver miR-21 to promote corneal epithelial wound healing through PTEN/PI3K/Akt pathway. Stem Cells Int. 2022, 2022, 1252557. [Google Scholar] [CrossRef]
- Golchin, A.; Hosseinzadeh, S.; Ardeshirylajimi, A. The exosomes released from different cell types and their effects in wound healing. J. Cell Biochem. 2018, 119, 5043–5052. [Google Scholar] [CrossRef] [PubMed]
- Louie, H.H.; Mugisho, O.O.; Chamley, L.W.; Rupenthal, I.D. Extracellular vesicles as biomarkers and therapeutics for inflammatory eye diseases. Mol. Pharm. 2023, 20, 23–40. [Google Scholar] [CrossRef]
- Marote, A.; Teixeira, F.G.; Mendes-Pinheiro, B.; Salgado, A.J. MSCs-derived exosomes: Cell-secreted nanovesicles with regenerative potential. Front. Pharmacol. 2016, 7, 231. [Google Scholar] [CrossRef]
- Jin, Q.H.; Kim, H.K.; Na, J.Y.; Jin, C.; Seon, J.K. Anti-inflammatory effects of mesenchymal stem cell-conditioned media inhibited macrophages activation in vitro. Sci. Rep. 2022, 12, 4754. [Google Scholar] [CrossRef] [PubMed]
- Ong, H.S.; Riau, A.K.; Yam, G.H.; Yusoff, N.Z.B.M.; Han, E.J.Y.; Goh, T.W.; Lai, R.C.; Lim, S.K.; Mehta, J.S. Mesenchymal stem cell exosomes as immunomodulatory therapy for corneal scarring. Int. J. Mol. Sci. 2023, 24, 7456. [Google Scholar] [CrossRef]
- Galindo, S.; de la Mata, A.; López-Paniagua, M.; Herreras, J.M.; Pérez, I.; Calonge, M.; Nieto-Miguel, T. Subconjunctival injection of mesenchymal stem cells for corneal failure due to limbal stem cell deficiency: State of the art. Stem Cell Res. Ther. 2021, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Buono, L.; Scalabrin, S.; De Iuliis, M.; Tanzi, A.; Grange, C.; Tapparo, M.; Nuzzi, R.; Bussolati, B. Mesenchymal stem cell-derived extracellular vesicles protect human corneal endothelial cells from endoplasmic reticulum stress-mediated apoptosis. Int. J. Mol. Sci. 2021, 22, 4930. [Google Scholar] [CrossRef] [PubMed]
- Shojaati, G.; Khandaker, I.; Funderburgh, M.L.; Mann, M.M.; Basu, R.; Stolz, D.B.; Geary, M.L.; Dos Santos, A.; Deng, S.X.; Funderburgh, J.L. Mesenchymal stem cells reduce corneal fibrosis and inflammation via extracellular vesicle-mediated delivery of miRNA. Stem Cells Transl. Med. 2019, 8, 1192–1201. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, D.; Liu, D.; Fan, Z.; Zhang, H.; Liu, O.; Ding, G.; Gao, R.; Zhang, C.; Ding, Y.; et al. Allogeneic mesenchymal stem cell treatment alleviates experimental and clinical Sjögren syndrome. Blood 2012, 120, 3142–3151. [Google Scholar] [CrossRef]
- Weng, J.; He, C.; Lai, P.; Luo, C.; Guo, R.; Wu, S.; Geng, S.; Xiangpeng, A.; Liu, X.; Du, X. Mesenchymal stromal cells treatment attenuates dry eye in patients with chronic graft-versus-host disease. Mol. Ther. 2012, 20, 2347–2354. [Google Scholar] [CrossRef]
- Kistenmacher, S.; Schwämmle, M.; Martin, G.; Ulrich, E.; Tholen, S.; Schilling, O.; Gießl, A.; Schlötzer-Schrehardt, U.; Bucher, F.; Schlunck, G.; et al. Enrichment, characterization, and proteomic profiling of small extracellular vesicles derived from human limbal mesenchymal stromal cells and melanocytes. Cells 2024, 13, 623. [Google Scholar] [CrossRef]
- Ortiz-Melo, M.T.; Campos, J.E.; Sánchez-Guzmán, E.; Herrera-Aguirre, M.E.; Castro-Muñozledo, F. Regulation of corneal epithelial differentiation: miR-141-3p promotes the arrest of cell proliferation and enhances the expression of terminal phenotype. PLoS ONE 2024, 19, e0315296. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, S.; Dong, Y.; Jin, X.; Wang, J.; Zhang, H. Tetrahedral DNA-based functional microRNA-21 delivery system: Application to corneal epithelial wound healing. Adv. Health Mater. 2024, 13, e2304381. [Google Scholar] [CrossRef] [PubMed]
- Bahadorani, M.; Nasiri, M.; Dellinger, K.; Aravamudhan, S.; Zadegan, R. Engineering exosomes for therapeutic applications: Decoding biogenesis, content modification, and cargo loading strategies. Int. J. Nanomed. 2024, 19, 7137–7164. [Google Scholar] [CrossRef]
- Wiklander, O.P.B.; Mamand, D.R.; Mohammad, D.K.; Zheng, W.; Jawad Wiklander, R.; Sych, T.; Zickler, A.M.; Liang, X.; Sharma, H.; Lavado, A.; et al. Antibody-displaying extracellular vesicles for targeted cancer therapy. Nat. Biomed. Eng. 2024, 8, 1453–1468. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Niu, Z.; Galli, V.; Howe, N.; Zhao, Y.; Wiklander, O.P.B.; Zheng, W.; Wiklander, R.J.; Corso, G.; Davies, C.; et al. Extracellular vesicles engineered to bind albumin demonstrate extended circulation time and lymph node accumulation in mouse models. J. Extracell. Vesicles 2022, 11, e12248. [Google Scholar] [CrossRef]
- Banskota, S.; Raguram, A.; Suh, S.; Du, S.W.; Davis, J.R.; Choi, E.H.; Wang, X.; Nielsen, S.C.; Newby, G.A.; Randolph, P.B.; et al. Engineered virus-like particles for efficient in vivo delivery of therapeutic proteins. Cell 2022, 185, 250–265.e216. [Google Scholar] [CrossRef]
- Liang, X.; Gupta, D.; Xie, J.; Van Wonterghem, E.; Van Hoecke, L.; Hean, J.; Niu, Z.; Ghaeidamini, M.; Wiklander, O.P.B.; Zheng, W.; et al. Engineering of extracellular vesicles for efficient intracellular delivery of multimodal therapeutics including genome editors. Nat. Commun. 2025, 16, 4028. [Google Scholar] [CrossRef]
- Vahab, S.A.; V, V.K.; Kumar, V.S. Exosome-based drug delivery systems for enhanced neurological therapeutics. Drug Deliv. Transl. Res. 2025, 15, 1121–1138. [Google Scholar] [CrossRef]
- Wahlgren, J.; De L Karlson, T.; Brisslert, M.; Vaziri Sani, F.; Telemo, E.; Sunnerhagen, P.; Valadi, H. Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Res. 2012, 40, e130. [Google Scholar] [CrossRef]
- Lozano, V.; Martín, C.; Blanco, N.; Alcalde, I.; Fernandez-Vega Cueto, L.; Merayo-Lloves, J.; Quirós, L.M. Exosomes released by corneal stromal cells show molecular alterations in keratoconus patients and induce different cellular behavior. Biomedicines 2022, 10, 2348. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; He, X.; Liu, R.; Ruan, Q. Accelerating corneal wound healing using exosome-mediated targeting of NF-κB c-Rel. Inflamm. Regen. 2023, 43, 6. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, S.H.; Koh, A.; Kim, K.W. EGF-conditioned M1 macrophages convey reduced inflammation into corneal endothelial cells through exosomes. Heliyon 2024, 10, e26800. [Google Scholar] [CrossRef]
- Choudhery, M.S.; Arif, T.; Mahmood, R.; Harris, D.T. Stem cell-based acellular therapy: Insight into biogenesis, bioengineering and therapeutic applications of exosomes. Biomolecules 2024, 14, 792. [Google Scholar] [CrossRef]
- Yang, G.; Waheed, S.; Wang, C.; Shekh, M.; Li, Z.; Wu, J. Exosomes and their bioengineering strategies in the cutaneous wound healing and related complications: Current knowledge and future perspectives. Int. J. Biol. Sci. 2023, 19, 1430–1454. [Google Scholar] [CrossRef]
- Wang, S.; Hou, Y.; Li, X.; Song, Z.; Sun, B.; Zhang, H. Comparison of exosomes derived from induced pluripotent stem cells and mesenchymal stem cells as therapeutic nanoparticles for treatment of corneal epithelial defects. Aging 2020, 12, 19546–19562. [Google Scholar] [CrossRef]
- Millán Cotto, H.A.; Pathrikar, T.V.; Hakim, B.; Baby, H.M.; Zhang, H.; Zhao, P.; Ansaripour, R.; Amini, R.; Carrier, R.L.; Bajpayee, A.G. Cationic-motif-modified exosomes for mRNA delivery to retinal photoreceptors. J. Mater. Chem. B 2024, 12, 7384–7400. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, K.; Chen, J.; Tan, S. Breakthrough in large-scale production of iPSCs-derived exosomes to promote clinical applications. Front. Bioeng. Biotechnol. 2023, 11, 1257186. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Gui, Y.; Liu, W.; Li, C.; Yang, Y. Precise molecular profiling of circulating exosomes using a metal-organic framework-based sensing interface and an enzyme-based electrochemical logic platform. Anal. Chem. 2022, 94, 875–883. [Google Scholar] [CrossRef]
- Mitchell, M.I.; Ma, J.; Carter, C.L.; Loudig, O. Circulating exosome cargoes contain functionally diverse cancer biomarkers: From biogenesis and function to purification and potential translational utility. Cancers 2022, 14, 3350. [Google Scholar] [CrossRef]
- Torrano, V.; Royo, F.; Peinado, H.; Loizaga-Iriarte, A.; Unda, M.; Falcón-Perez, J.M.; Carracedo, A. Vesicle-MaNiA: Extracellular vesicles in liquid biopsy and cancer. Curr. Opin. Pharmacol. 2016, 29, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Willms, A.; Müller, C.; Julich, H.; Klein, N.; Schwab, R.; Güsgen, C.; Richardsen, I.; Schaaf, S.; Krawczyk, M.; Lammert, F.; et al. Tumour-associated circulating microparticles: A novel liquid biopsy tool for screening and therapy monitoring of colorectal carcinoma and other epithelial neoplasia. Oncotarget 2016, 7, 30867–30875. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liu, S.; Zhao, X.; Zhao, P.; Jia, Q.; Ma, H.; Lin, Q. Role of tear exosomes in the spread of herpes simplex virus type 1 in recurrent herpes simplex keratitis. Eye 2023, 37, 3180–3185. [Google Scholar] [CrossRef]
- Yuana, Y.; Sturk, A.; Nieuwland, R. Extracellular vesicles in physiological and pathological conditions. Blood Rev. 2013, 27, 31–39. [Google Scholar] [CrossRef]
- Catalano, M.; O’Driscoll, L. Inhibiting extracellular vesicles formation and release: A review of EV inhibitors. J. Extracell. Vesicles 2020, 9, 1703244. [Google Scholar] [CrossRef]
- Jorfi, S.; Ansa-Addo, E.A.; Kholia, S.; Stratton, D.; Valley, S.; Lange, S.; Inal, J. Inhibition of microvesiculation sensitizes prostate cancer cells to chemotherapy and reduces docetaxel dose required to limit tumor growth in vivo. Sci. Rep. 2015, 5, 13006. [Google Scholar] [CrossRef]
- Matsumoto, A.; Takahashi, Y.; Nishikawa, M.; Sano, K.; Morishita, M.; Charoenviriyakul, C.; Saji, H.; Takakura, Y. Accelerated growth of B16BL6 tumor in mice through efficient uptake of their own exosomes by B16BL6 cells. Cancer Sci. 2017, 108, 1803–1810. [Google Scholar] [CrossRef] [PubMed]
- Tramontano, A.F.; O’Leary, J.; Black, A.D.; Muniyappa, R.; Cutaia, M.V.; El-Sherif, N. Statin decreases endothelial microparticle release from human coronary artery endothelial cells: Implication for the Rho-kinase pathway. Biochem. Biophys. Res. Commun. 2004, 320, 34–38. [Google Scholar] [CrossRef]
- Datta, A.; Kim, H.; Lal, M.; McGee, L.; Johnson, A.; Moustafa, A.A.; Jones, J.C.; Mondal, D.; Ferrer, M.; Abdel-Mageed, A.B. Manumycin A suppresses exosome biogenesis and secretion via targeted inhibition of Ras/Raf/ERK1/2 signaling and hnRNP H1 in castration-resistant prostate cancer cells. Cancer Lett. 2017, 408, 73–81. [Google Scholar] [CrossRef]
- Guo, B.B.; Bellingham, S.A.; Hill, A.F. The neutral sphingomyelinase pathway regulates packaging of the prion protein into exosomes. J. Biol. Chem. 2015, 290, 3455–3467. [Google Scholar] [CrossRef]
- Shamseddine, A.A.; Airola, M.V.; Hannun, Y.A. Roles and regulation of neutral sphingomyelinase-2 in cellular and pathological processes. Adv. Biol. Regul. 2015, 57, 24–41. [Google Scholar] [CrossRef] [PubMed]
- Hagemann, A.; Altrogge, P.K.; Kehrenberg, M.C.A.; Diehl, D.; Jung, D.; Weber, L.; Bachmann, H.S. Analyzing the postulated inhibitory effect of Manumycin A on farnesyltransferase. Front. Chem. 2022, 10, 967947. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, W.; Yao, Q.; Zhang, H.; Dong, G.; Zhang, M.; Liu, Y.; Chen, J.K.; Dong, Z. Exosome production and its regulation of EGFR during wound healing in renal tubular cells. Am. J. Physiol. Ren. Physiol. 2017, 312, F963–F970. [Google Scholar] [CrossRef]
- Oh, H.J.; Shin, Y.; Chung, S.; Hwang, D.W.; Lee, D.S. Convective exosome-tracing microfluidics for analysis of cell-non-autonomous neurogenesis. Biomaterials 2017, 112, 82–94. [Google Scholar] [CrossRef]
- Latham, S.L.; Chaponnier, C.; Dugina, V.; Couraud, P.O.; Grau, G.E.; Combes, V. Cooperation between β- and γ-cytoplasmic actions in the mechanical regulation of endothelial microparticle formation. FASEB J. 2013, 27, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Irep, N.; Inci, K.; Tokgun, P.E.; Tokgun, O. Exosome inhibition improves response to first-line therapy in small cell lung cancer. J. Cell Mol. Med. 2024, 28, e18138. [Google Scholar] [CrossRef]
- Blanco-Agudín, N.; Ye, S.; Alcalde, I.; Corte-Torres, M.D.; Galarreta, D.; Caro-Magdaleno, M.; Fernández-Vega, I.; Fernández-Vega Cueto, L.; Merayo-Lloves, J.; Quirós, L.M. Corneal stromal cells from patients with keratoconus exhibit alterations in the ESCRT-dependent machinery responsible for multivesicular body formation. Exp. Eye Res. 2025, 252, 110260. [Google Scholar] [CrossRef]
- Gandham, S.; Su, X.; Wood, J.; Nocera, A.L.; Alli, S.C.; Milane, L.; Zimmerman, A.; Amiji, M.; Ivanov, A.R. Technologies and standardization in research on extracellular vesicles. Trends Biotechnol. 2020, 38, 1066–1098. [Google Scholar] [CrossRef]
- Dua, H.S.; Freitas, R.; Mohammed, I.; Ting, D.S.J.; Said, D.G. The pre-Descemet’s layer (Dua’s layer, also known as the Dua-Fine layer and the pre-posterior limiting lamina layer): Discovery, characterisation, clinical and surgical applications, and the controversy. Prog. Retin. Eye Res. 2023, 97, 101161. [Google Scholar] [CrossRef]
- EL Andaloussi, S.; Mäger, I.; Breakefield, X.O.; Wood, M.J. Extracellular vesicles: Biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 2013, 12, 347–357. [Google Scholar] [CrossRef]
- Robbins, B.T.; Montreuil, K.A.; Kundu, N.; Kumar, P.; Agrahari, V. Corneal treatment, repair, and regeneration: Exosomes at rescue. Pharmaceutics 2024, 16, 1424. [Google Scholar] [CrossRef]
- Armstrong, J.P.K.; Stevens, M.M. Strategic design of extracellular vesicle drug delivery systems. Adv. Drug Deliv. Rev. 2018, 130, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhou, X.; Wei, M.; Gao, X.; Zhao, L.; Shi, R.; Sun, W.; Duan, Y.; Yang, G.; Yuan, L. In vitro and in vivo RNA inhibition by CD9-HuR functionalized exosomes encapsulated with miRNA or CRISPR/dCas9. Nano Lett. 2019, 19, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, Y.; Gao, Q.; Ping, D.; Wang, Y.; Wu, W.; Lin, X.; Fang, Y.; Zhang, J.; Shao, A. The role of exosomal microRNAs and oxidative stress in neurodegenerative diseases. Oxid. Med. Cell Longev. 2020, 2020, 3232869. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.D.; Lai, J.-Y. Synthesis, bioactive properties, and biomedical applications of intrinsically therapeutic nanoparticles for disease treatment. Chem. Eng. J. 2022, 435, 134970. [Google Scholar] [CrossRef]
- Lamichhane, T.N.; Raiker, R.S.; Jay, S.M. Exogenous DNA loading into extracellular vesicles via electroporation is size-dependent and enables limited gene delivery. Mol. Pharm. 2015, 12, 3650–3657. [Google Scholar] [CrossRef]
- Xu, M.; Yang, Q.; Sun, X.; Wang, Y. Recent advancements in the loading and modification of therapeutic exosomes. Front. Bioeng. Biotechnol. 2020, 8, 586130. [Google Scholar] [CrossRef]
- Fujita, M.; Hatta, T.; Ikka, T.; Onishi, T. The urgent need for clear and concise regulations on exosome-based interventions. Stem Cell Rep. 2024, 19, 1517–1519. [Google Scholar] [CrossRef]
- Hughes, P.M.; Olejnik, O.; Chang-Lin, J.E.; Wilson, C.G. Topical and systemic drug delivery to the posterior segments. Adv. Drug Deliv. Rev. 2005, 57, 2010–2032. [Google Scholar] [CrossRef]
- Karpman, D.; Ståhl, A.L.; Arvidsson, I. Extracellular vesicles in renal disease. Nat. Rev. Nephrol. 2017, 13, 545–562. [Google Scholar] [CrossRef]
- Gaudana, R.; Ananthula, H.K.; Parenky, A.; Mitra, A.K. Ocular drug delivery. AAPS J. 2010, 12, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Lener, T.; Gimona, M.; Aigner, L.; Börger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Del Portillo, H.A.; et al. Applying extracellular vesicles based therapeutics in clinical trials—An ISEV position paper. J. Extracell. Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]


| Origin of Exos | Model | Results |
|---|---|---|
| Corneal epithelial cells (CECs) | Mouse in vitro | Increased fibroblast and human umbilical vein endothelial cell proliferation [17] Transformed keratocytes to myofibroblasts [17] |
| Limbal epithelial cells (LECs) | Human in vitro | Induced wound closure and proliferation in primary LSCs [96] |
| Limbal stromal cells (LSCs) | Human in vitro | Induced wound closure in primary LECs and organ-cultured corneas [18] |
| Corneal stromal MSCs | Human in vitro | Induced wound closure and proliferation in human CECs [95] |
| Mouse in vitro | Induced wound closure and proliferation in mouse cornea [95] | |
| Human CECs, corneal fibroblasts, and endothelial cells | Human in vitro | Induced wound closure and proliferation in human CECs [97] |
| Human in vitro | Enhanced proliferation and migration of primary human LECs in dry eye disease model (high osmotic stress) [99] | |
| Mouse in vitro | Enhanced tear production in dry eye mouse model | |
| Bone marrow-derived MSCs | Human in vitro | Induced wound closure and proliferation in human CECs [100] |
| Mouse in vivo | Enhanced wound healing of mice with corneal injury [100] | |
| Adipose-derived MSCs | Rabbit in vitro | Enhanced wound healing in rabbit CECs [125] |
| Mouse amniotic fluid MSCs | Mouse—in vitro | Enhanced wound healing in mouse cryoinjured cornea [126] |
| Human umbilical cord MSCs | Rat—in vitro | Subconjunctival injection promoted the healing of rat corneal damage [131] |
| Human—in vitro | Increased proliferation and migration of human CECs [131] | |
| Embryonic stem cell-derived MSCs | Rat—in vitro | Enhanced corneal healing and reduced corneal scarring caused by irregular phototherapeutic keratectomy in rats [136] |
| Human—in vitro | Human corneal epithelial cells [136] | |
| Human bone marrow-derived MSCs | Human—in vitro | Decreased ER stress-related genes and apoptosis in human corneal endothelial cells [138] |
| Corneal stromal stem cells | Mouse—in vitro | Normal tissue morphology and prevention of scarring in mouse damaged corneas [138] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gamez, J.; Zha, D.; Ebrahimi, S.M.; White, S.; Ljubimov, A.V.; Saghizadeh, M. Exosomes as Future Therapeutic Tools and Targets for Corneal Diseases. Cells 2025, 14, 959. https://doi.org/10.3390/cells14130959
Gamez J, Zha D, Ebrahimi SM, White S, Ljubimov AV, Saghizadeh M. Exosomes as Future Therapeutic Tools and Targets for Corneal Diseases. Cells. 2025; 14(13):959. https://doi.org/10.3390/cells14130959
Chicago/Turabian StyleGamez, Joshua, Daxian Zha, Shaghaiegh M. Ebrahimi, Seok White, Alexander V. Ljubimov, and Mehrnoosh Saghizadeh. 2025. "Exosomes as Future Therapeutic Tools and Targets for Corneal Diseases" Cells 14, no. 13: 959. https://doi.org/10.3390/cells14130959
APA StyleGamez, J., Zha, D., Ebrahimi, S. M., White, S., Ljubimov, A. V., & Saghizadeh, M. (2025). Exosomes as Future Therapeutic Tools and Targets for Corneal Diseases. Cells, 14(13), 959. https://doi.org/10.3390/cells14130959







