Epigenetic Modifiers to Treat Retinal Degenerative Diseases
Abstract
1. Introduction
2. Materials and Methods
3. Results
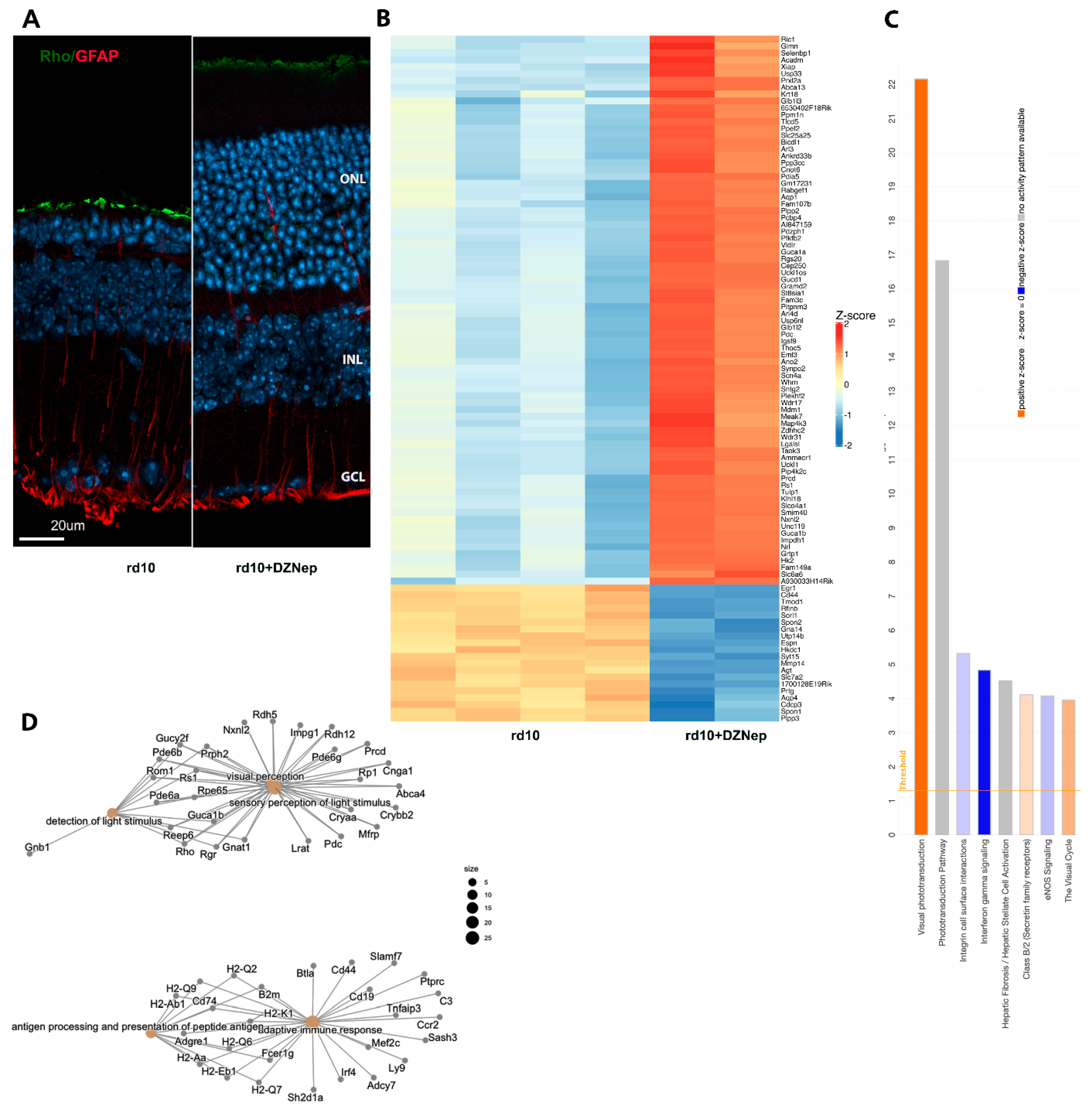
3.1. Treatment of rd10 Mouse Model of RP with EZH2 Inhibitors Leads to Retina Neuroprotection
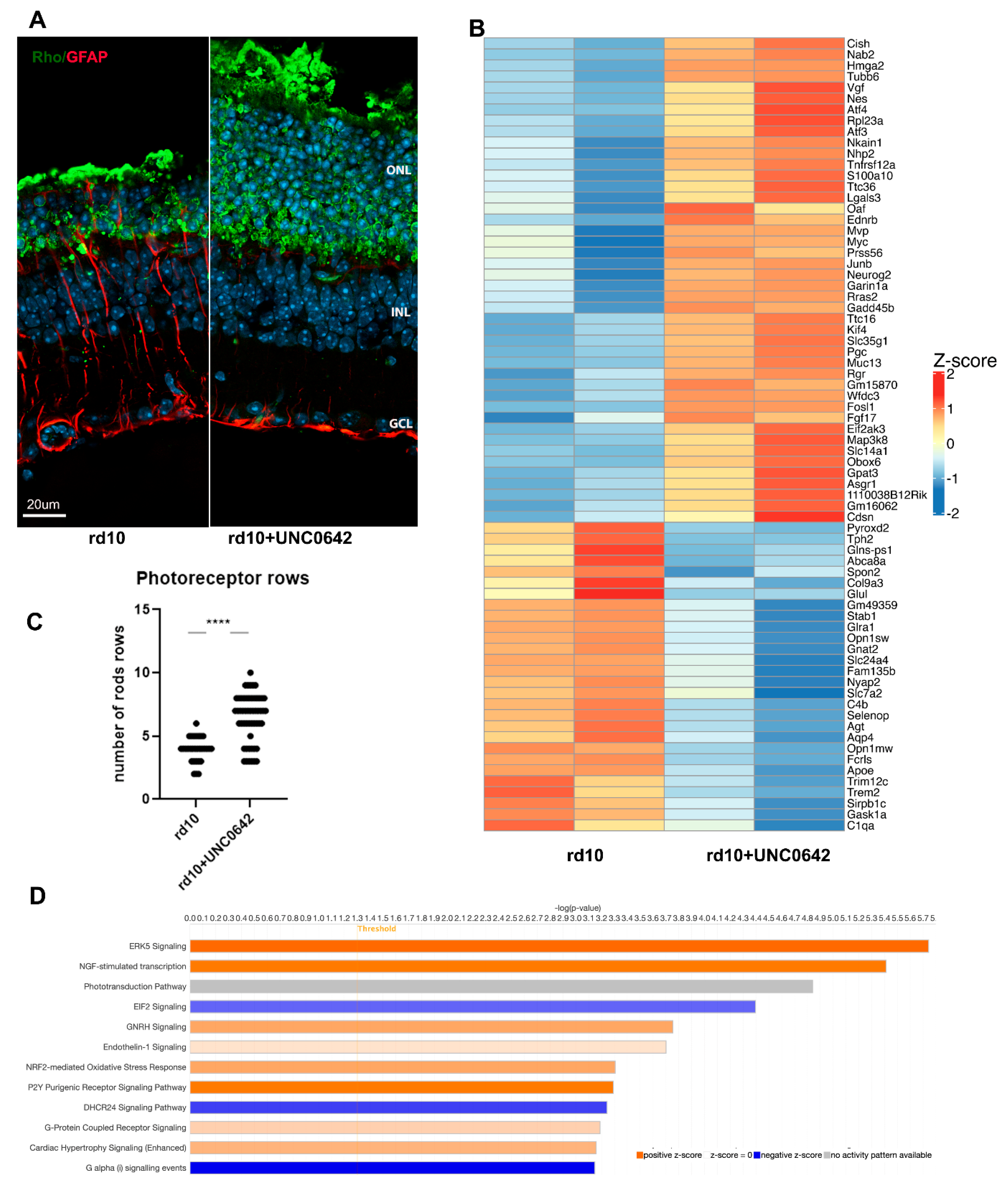
3.2. Treatment of rd10 Mouse Model of RP with G9A/GLP Inhibitor Leads to Partial Retina Neuroprotection
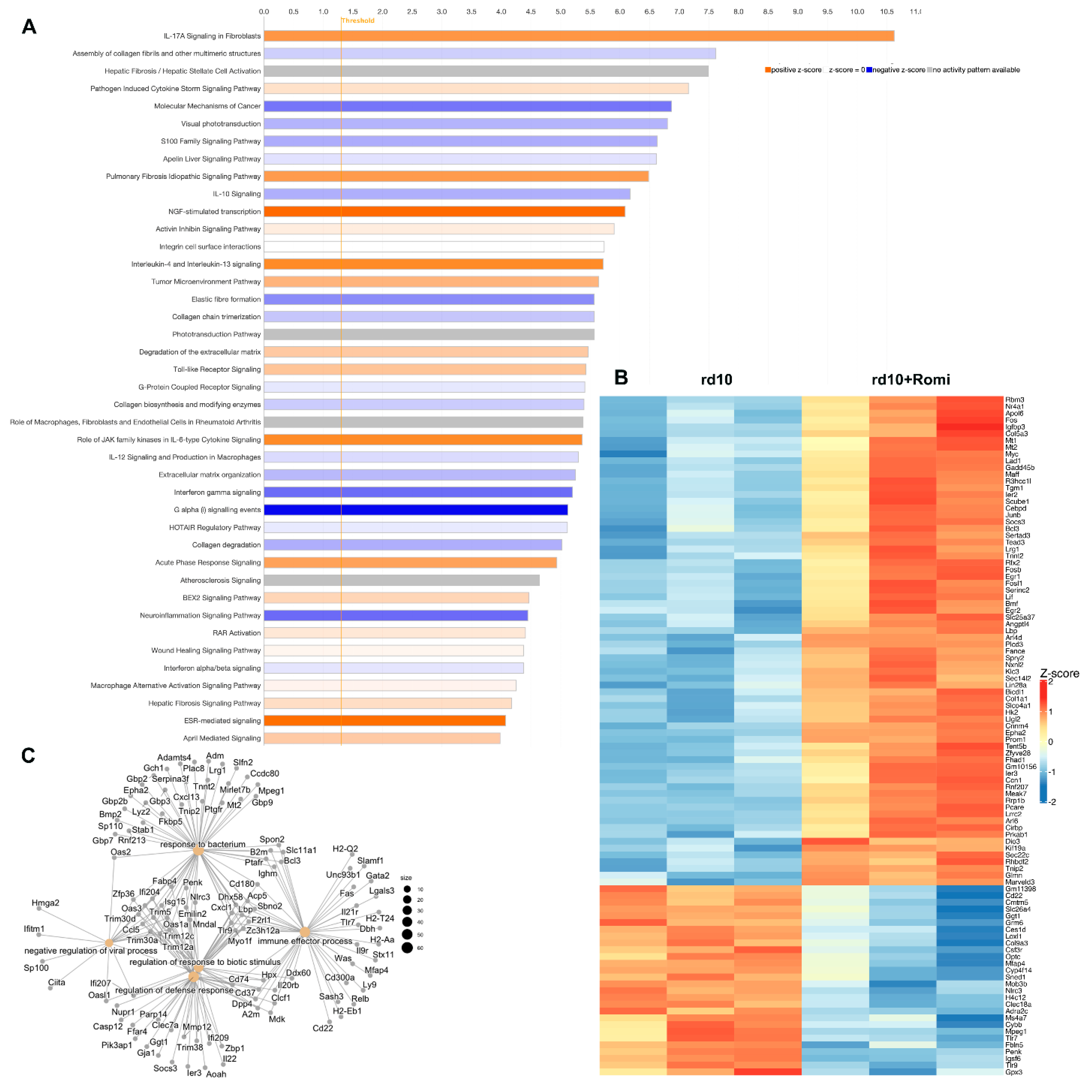
3.3. RNA-Seq of Retina of rd10 Mice Treated with HDAC1 Inhibitor
3.4. Comparison of Action of Different Inhibitors of Chromatin Compaction
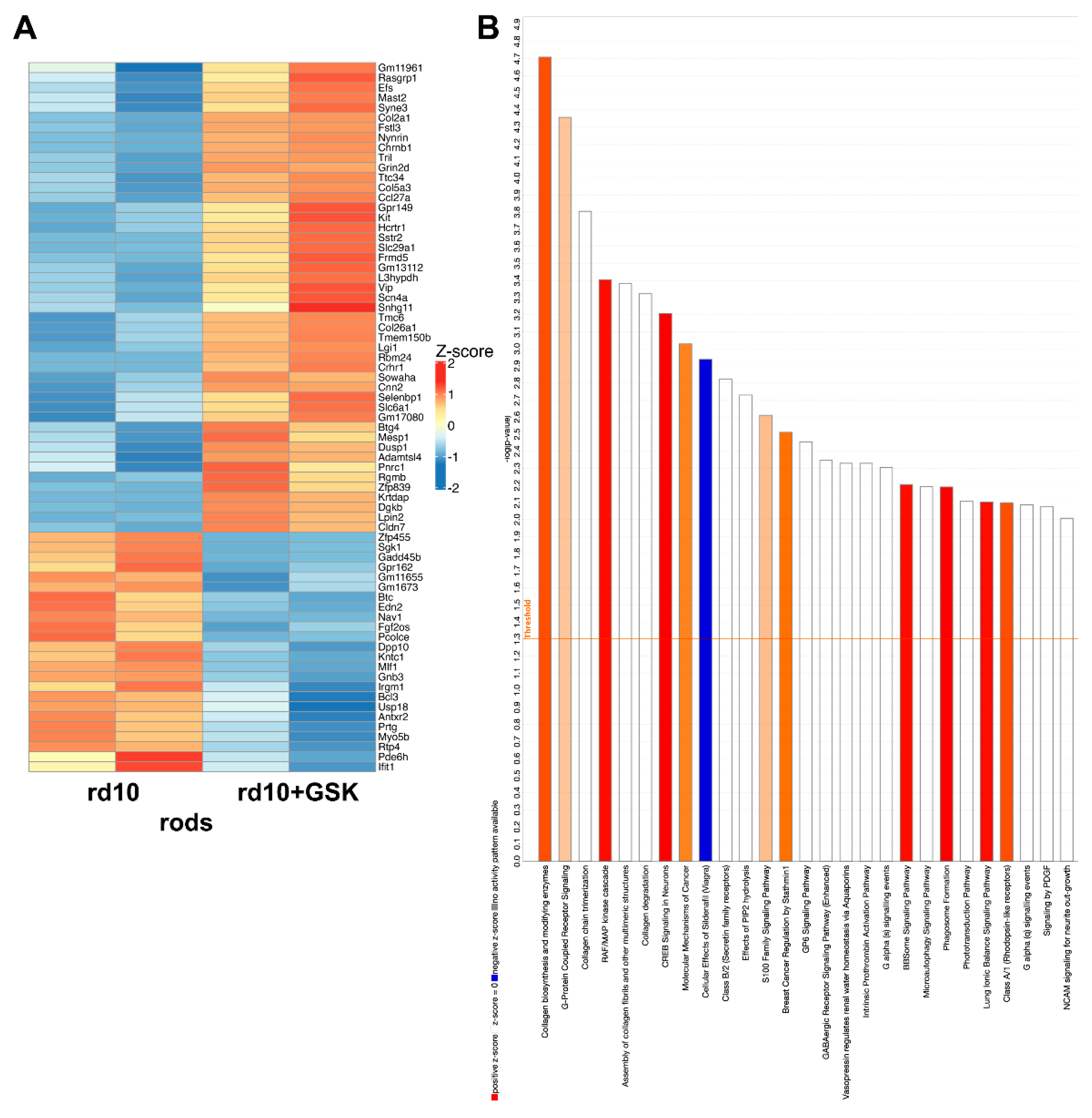
3.5. Changes in Gene Expression of Isolated Rod Photoreceptor in rd10 Mice Treated with GSK2879552
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Solovei, I.; Kreysing, M.; Lanctôt, C.; Kösem, S.; Peichl, L.; Cremer, T.; Guck, J.; Joffe, B. Nuclear architecture of rod photoreceptor cells adapts to vision in mammalian evolution. Cell 2009, 137, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Popova, E.Y.; Grigoryev, S.A.; Fan, Y.; Skoultchi, A.I.; Zhang, S.S.; Barnstable, C.J. Developmentally regulated linker histone H1c promotes heterochromatin condensation and mediates structural integrity of rod photoreceptors in mouse retina. J. Biol. Chem. 2013, 288, 17895–17907. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hughes, A.E.; Enright, J.M.; Myers, C.A.; Shen, S.Q.; Corbo, J.C. Cell Type-Specific Epigenomic Analysis Reveals a Uniquely Closed Chromatin Architecture in Mouse Rod Photoreceptors. Sci. Rep. 2017, 7, 43184. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Popova, E.Y.; Xu, X.; DeWan, A.T.; Salzberg, A.C.; Berg, A.; Hoh, J.; Zhang, S.S.; Barnstable, C.J.; Englert, C. Stage and gene specific signatures defined by histones H3K4me2 and H3K27me3 accompany mammalian retina maturation in vivo. PLoS ONE 2012, 7, e46867. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bovolenta, P.; Cisneros, E. Retinitis pigmentosa: Cone photoreceptors starving to death. Nat. Neurosci. 2009, 12, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Hejtmancik, J.F.; Daiger, S.P. Understanding the genetic architecture of human retinal degenerations. Proc. Natl. Acad. Sci. USA 2020, 117, 3904–3906. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hanany, M.; Rivolta, C.; Sharon, D. Worldwide carrier frequency and genetic prevalence of autosomal recessive inherited retinal diseases. Proc. Natl. Acad. Sci. USA 2020, 117, 2710–2716. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dias, M.F.; Joo, K.; Kemp, J.A.; Fialho, S.L.; da Silva Cunha, A.; Woo, S.J., Jr.; Kwon, Y.J. Molecular genetics and emerging therapies for retinitis pigmentosa: Basic research and clinical perspectives. Prog. Retin. Eye Res. 2018, 63, 107–131. [Google Scholar] [CrossRef] [PubMed]
- Guadagni, V.; Novelli, E.; Piano, I.; Gargini, C.; Strettoi, E. Pharmacological approaches to retinitis pigmentosa: A laboratory perspective. Prog. Retin. Eye Res. 2015, 48, 62–81. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J.L.; Pierce, E.A.; Laster, A.M.; Daiger, S.P.; Birch, D.G.; Ash, J.D.; Iannaccone, A.; Flannery, J.G.; Sahel, J.A.; Zack, D.J.; et al. Inherited Retinal Degenerations: Current Landscape and Knowledge Gaps. Transl. Vis. Sci. Technol. 2018, 7, 6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Popova, E.Y.; Kawasawa, Y.I.; Zhang, S.S.; Barnstable, C.J. Inhibition of epigenetic modifiers LSD1 and HDAC1 blocks rod photoreceptor death in mouse models of Retinitis Pigmentosa. J. Neurosci. 2021, 41, 6775–6792. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Nam, H.J.; Lee, W.; Yim, H.Y.; Ahn, J.Y.; Park, S.W.; Shin, H.-J.R.; Yu, R.; Won, K.-J.; Bae, J.-S.; et al. PKCα-LSD1-NF-κB-Signaling Cascade Is Crucial for Epigenetic Control of the Inflammatory Response. Mol. Cell. 2018, 69, 398–411.e6. [Google Scholar] [CrossRef] [PubMed]
- Kiernan, R.; Brès, V.; Ng, R.W.; Coudart, M.P.; El Messaoudi, S.; Sardet, C.; Jin, D.-Y.; Emiliani, S.; Benkirane, M. Post-activation turn-off of NF-kappa B-dependent transcription is regulated by acetylation of p65. J. Biol. Chem. 2003, 278, 2758–2766. [Google Scholar] [CrossRef] [PubMed]
- Furumai, R.; Ito, A.; Ogawa, K.; Maeda, S.; Saito, A.; Nishino, N.; Horinouchi, S.; Yoshida, M. Histone deacetylase inhibitors block nuclear factor-κB-dependent transcription by interfering with RNA polymerase II recruitment. Cancer Sci. 2011, 102, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Xiao, L.; Liu, Y.; Wang, Y.; Cheng, L.; Zhang, J.; Yan, N.; Chen, D. DZNep inhibits H3K27me3 deposition and delays retinal degeneration in the rd1 mice. Cell Death Dis. 2018, 9, 310. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, J.; Zhang, M.; Zhang, X.; Fan, L.; Liu, P.; Yu, L.; Cao, X.; Qiu, S.; Xu, Y. EZH2 inhibitor DZNep modulates microglial activation and protects against ischaemic brain injury after experimental stroke. Eur. J. Pharmacol. 2019, 857, 172452. [Google Scholar] [CrossRef] [PubMed]
- Mbefo, M.; Berger, A.; Schouwey, K.; Gérard, X.; Kostic, C.; Beryozkin, A.; Sharon, D.; Dolfuss, H.; Munier, F.; Tran, H.V.; et al. Enhancer of Zeste Homolog 2 (EZH2) Contributes to Rod Photoreceptor Death Process in Several Forms of Retinal Degeneration and Its Activity Can Serve as a Biomarker for Therapy Efficacy. Int. J. Mol. Sci. 2021, 22, 9331. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Griñán-Ferré, C.; Marsal-García, L.; Bellver-Sanchis, A.; Kondengaden, S.M.; Turga, R.C.; Vázquez, S.; Pallàs, M. Pharmacological inhibition of G9a/GLP restores cognition and reduces oxidative stress, neuroinflammation and β-Amyloid plaques in an early-onset Alzheimer’s disease mouse model. Aging 2019, 11, 11591–11608. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, Y.; Lee, H.M.; Xiong, Y.; Sciaky, N.; Hulbert, S.W.; Cao, X.; Everitt, J.I.; Jin, J.; Roth, B.L.; Jiang, Y.-H. Targeting the histone methyltransferase G9a activates imprinted genes and improves survival of a mouse model of Prader-Willi syndrome. Nat. Med. 2017, 23, 213–222. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barnstable, C.J. Monoclonal antibodies which recognize different cell types in the rat retina. Nature 1980, 286, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, M.; Cheng, H.; Zhu, D.; Brzezinski, J.A.; Khanna, R.; Filippova, E.; Oh, E.C.T.; Jing, Y.; Linares, J.-L.; Brooks, M.; et al. Targeting of GFP to newborn rods by Nrl promoter and temporal expression profiling of flow-sorted photoreceptors. Proc. Natl. Acad. Sci. USA 2006, 103, 3890–3895. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Feodorova, Y.; Koch, M.; Bultman, S.; Michalakis, S.; Solovei, I. Quick and reliable method for retina dissociation and separation of rod photoreceptor perikarya from adult mice. MethodsX 2015, 2, 39–46. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Popova, E.Y.; Kawasawa, Y.I.; Leung, M.; Barnstable, C.J. Temporal changes in mouse hippocampus transcriptome after pilocarpine-induced seizures. Front Neurosci. 2024, 18, 1384805. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Popova, E.Y.; Pinzon-Guzman, C.; Salzberg, A.C.; Zhang, S.S.; Barnstable, C.J. LSD1-Mediated Demethylation of H3K4me2 Is Required for the Transition from Late Progenitor to Differentiated Mouse Rod Photoreceptor. Mol. Neurobiol. 2016, 53, 4563–4581. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferreira, R.C.; Popova, E.Y.; James, J.; Briones, M.R.; Zhang, S.S.; Barnstable, C.J. Histone Deacetylase 1 Is Essential for Rod Photoreceptor Differentiation by Regulating Acetylation at Histone H3 Lysine 9 and Histone H4 Lysine 12 in the Mouse Retina. J. Biol. Chem. 2017, 292, 2422–2440. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, S.E.; Xiong, Y.; Jang, M.A.; Park, K.S.; Donahue, M.; Velez, J.; Jin, J.; Jiang, Y.-H. Newly developed oral bioavailable EHMT2 inhibitor as a potential epigenetic therapy for Prader-Willi syndrome. Mol. Ther. 2024, 32, 2662–2675. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aldiri, I.; Xu, B.; Wang, L.; Chen, X.; Hiler, D.; Griffiths, L.; Valentine, M.; Shirinifard, A.; Thiagarajan, S.; Sablauer, A.; et al. The Dynamic Epigenetic Landscape of the Retina During Development, Reprogramming, and Tumorigenesis. Neuron 2017, 94, 550–568.e10. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karademir, D.; Todorova, V.; Ebner, L.J.A.; Samardzija, M.; Grimm, C. Single-cell RNA sequencing of the retina in a model of retinitis pigmentosa reveals early responses to degeneration in rods and cones. BMC Biol. 2022, 20, 86. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kaur, P.; Verma, S.; Kushwaha, P.P.; Gupta, S. EZH2 and NF-κB: A context-dependent crosstalk and transcriptional regulation in cancer. Cancer Lett. 2023, 560, 216143. [Google Scholar] [CrossRef] [PubMed]
- Weng, H.R.; Taing, K.; Chen, L.; Penney, A. EZH2 Methyltransferase Regulates Neuroinflammation and Neuropathic Pain. Cells 2023, 12, 1058. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Luu, J.; Kallestad, L.; Hoang, T.; Lewandowski, D.; Dong, Z.; Blackshaw, S.; Palczewski, K. Epigenetic hallmarks of age-related macular degeneration are recapitulated in a photosensitive mouse model. Hum. Mol. Genet. 2020, 29, 2611–2624. [Google Scholar] [CrossRef] [PubMed]
- Trifunović, D.; Arango-Gonzalez, B.; Comitato, A.; Barth, M.; Del Amo, E.M.; Kulkarni, M.; Sahaboglu, A.; Hauck, S.M.; Urtti, A.; Arsenijevic, Y.; et al. HDAC inhibition in the cpfl1 mouse protects degenerating cone photoreceptors in vivo. Hum. Mol. Genet. 2016, 25, 4462–4472. [Google Scholar] [CrossRef] [PubMed]
- Trifunović, D.; Petridou, E.; Comitato, A.; Marigo, V.; Ueffing, M.; Paquet-Durand, F. Primary Rod and Cone Degeneration Is Prevented by HDAC Inhibition. Adv. Exp. Med. Biol. 2018, 1074, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Yan, J.; Xu, W.; Paquet-Durand, F.; Hu, Z.; Jiao, K. HDAC inhibition delays photoreceptor loss in. PeerJ 2023, 11, e15659. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mota, M.; Porrini, V.; Parrella, E.; Benarese, M.; Bellucci, A.; Rhein, S.; Schwaninger, M.; Pizzi, M. Neuroprotective epi-drugs quench the inflammatory response and microglial/macrophage activation in a mouse model of permanent brain ischemia. J. Neuroinflammation 2020, 17, 361. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schioppa, T.; Nguyen, H.O.; Tiberio, L.; Sozio, F.; Gaudenzi, C.; Passari, M.; Del Prete, A.; Bosisio, D.; Salvi, V. Inhibition of Class I Histone Deacetylase Activity Blocks the Induction of TNFAIP3 Both Directly and Indirectly via the Suppression of Endogenous TNF-α. Int. J. Mol. Sci. 2022, 23, 9752. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sundar, J.C.; Munezero, D.; Bryan-Haring, C.; Saravanan, T.; Jacques, A.; Ramamurthy, V. Rhodopsin signaling mediates light-induced photoreceptor cell death in rd10 mice through a transducin-independent mechanism. Hum. Mol. Genet. 2020, 29, 394–406. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Weh, E.; Scott, K.; Wubben, T.J.; Besirli, C.G. Dark-reared rd10 mice experience rapid photoreceptor degeneration with short exposure to room-light during in vivo retinal imaging. Exp. Eye Res. 2022, 215, 108913. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barabino, A.; Plamondon, V.; Abdouh, M.; Chatoo, W.; Flamier, A.; Hanna, R.; Zhou, S.; Motoyama, N.; Hébert, M.; Lavoie, J.; et al. Loss of Bmi1 causes anomalies in retinal development and degeneration of cone photoreceptors. Development 2016, 143, 1571–1584. [Google Scholar] [CrossRef] [PubMed]
- Popova, E.Y.; Barnstable, C.J. Insights Into the Epigenetics of Retinal Development and Diseases In Epigenetics and Regeneration; Academic Press: Cambridge, MA, USA, 2019; pp. 355–384. [Google Scholar]
- Kerenyi, M.A.; Shao, Z.; Hsu, Y.J.; Guo, G.; Luc, S.; O’Brien, K.; Fujiwara, Y.; Peng, C.; Nguyen, M.; Orkin, S.H.; et al. Histone demethylase Lsd1 represses hematopoietic stem and progenitor cell signatures during blood cell maturation. Elife 2013, 2, e00633. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popova, E.Y.; Schneper, L.; Sebastian, A.; Albert, I.; Tombran-Tink, J.; Barnstable, C.J. Epigenetic Modifiers to Treat Retinal Degenerative Diseases. Cells 2025, 14, 961. https://doi.org/10.3390/cells14130961
Popova EY, Schneper L, Sebastian A, Albert I, Tombran-Tink J, Barnstable CJ. Epigenetic Modifiers to Treat Retinal Degenerative Diseases. Cells. 2025; 14(13):961. https://doi.org/10.3390/cells14130961
Chicago/Turabian StylePopova, Evgenya Y., Lisa Schneper, Aswathy Sebastian, Istvan Albert, Joyce Tombran-Tink, and Colin J. Barnstable. 2025. "Epigenetic Modifiers to Treat Retinal Degenerative Diseases" Cells 14, no. 13: 961. https://doi.org/10.3390/cells14130961
APA StylePopova, E. Y., Schneper, L., Sebastian, A., Albert, I., Tombran-Tink, J., & Barnstable, C. J. (2025). Epigenetic Modifiers to Treat Retinal Degenerative Diseases. Cells, 14(13), 961. https://doi.org/10.3390/cells14130961






