Exploring OR2H1-Mediated Sperm Chemotaxis: Development and Application of a Novel Microfluidic Device
Abstract
1. Introduction
2. Materials and Methods
2.1. Sperm Samples: Ethical Approval and Biological Preparation
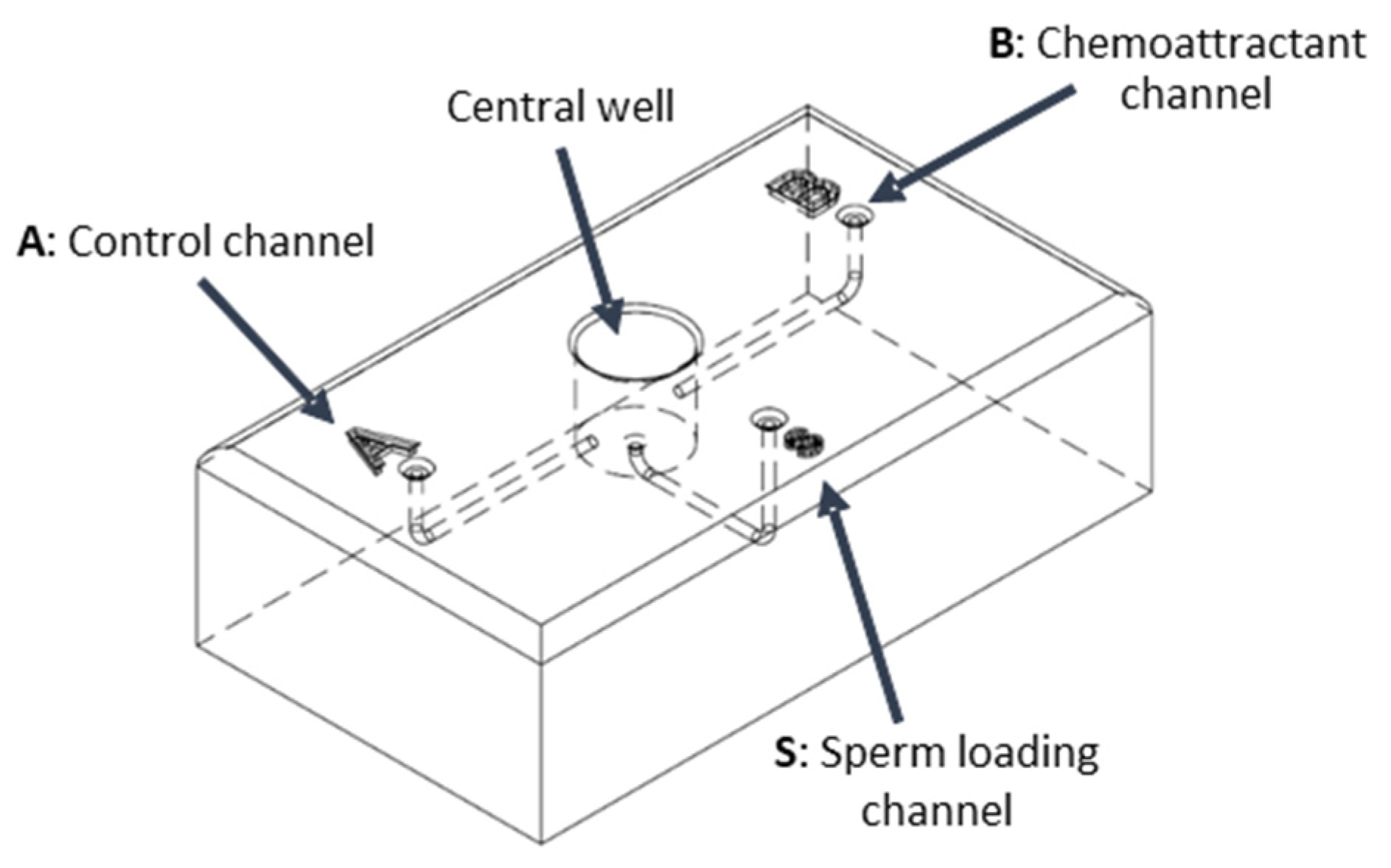
2.2. Design and Validation of the Sperm Device
- ➢
- Priming: Introduce 100 μL of capacitation medium (HAM1x supplemented with 0.6 mg/mL BSA and 0.2 mg/mL NaHCO3-Sigma-Aldrich, St. Louis, MO, USA) into the three peripheral wells (S, A, and B). This step primes the device, filling the central well and eliminating air pockets within the channels.
- ➢
- Gradient Formation: Add 10 μL of the designated chemoattractant solution to well B and 10 μL of the control solution (capacitation medium) to well A. Allow a 20 s period for the establishment of a chemoattractant gradient, extending from each well through its respective channel (channel B or A) and into the central well.
- ➢
- Sperm Introduction: Carefully introduce 40 μL of sperm pre-incubated in a capacitation medium into the designated sperm well, S.
- ➢
- Sperm Collection and Analysis: Following a 30 min incubation at 37 °C, collect 20 μL aliquots of migrated sperm from the two collection wells (B and A). Quantify the collected sperm using an optical microscope.
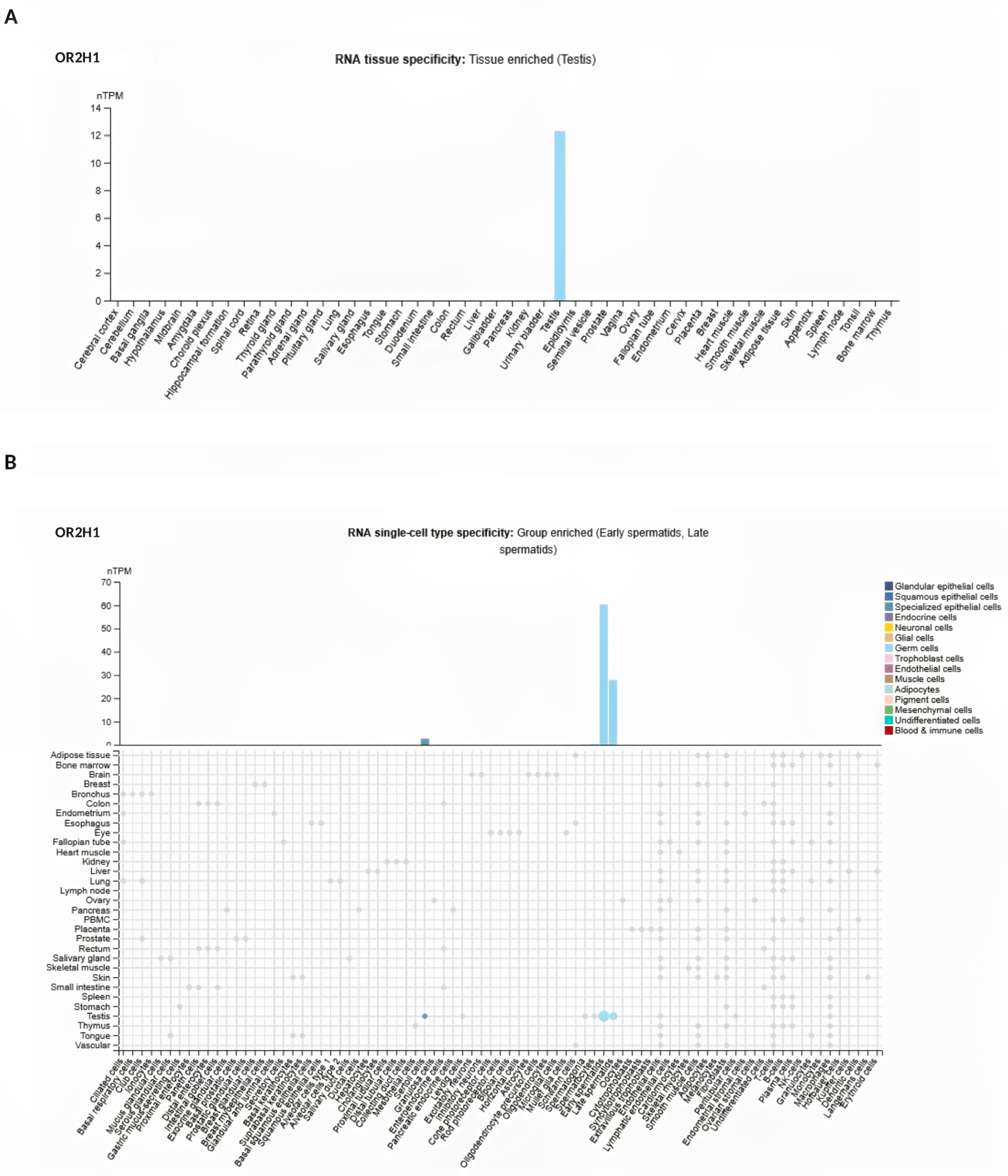
2.3. Analysis of OR2H1 from Atlas Database
2.4. Measurement of Calcium Changes
2.5. Evaluation of Sperm Motility Parameters by CASA
2.6. Statistical Analysis
3. Results
3.1. Sperm Device Development and Validation
3.2. Analysis of OR2H1 Expression
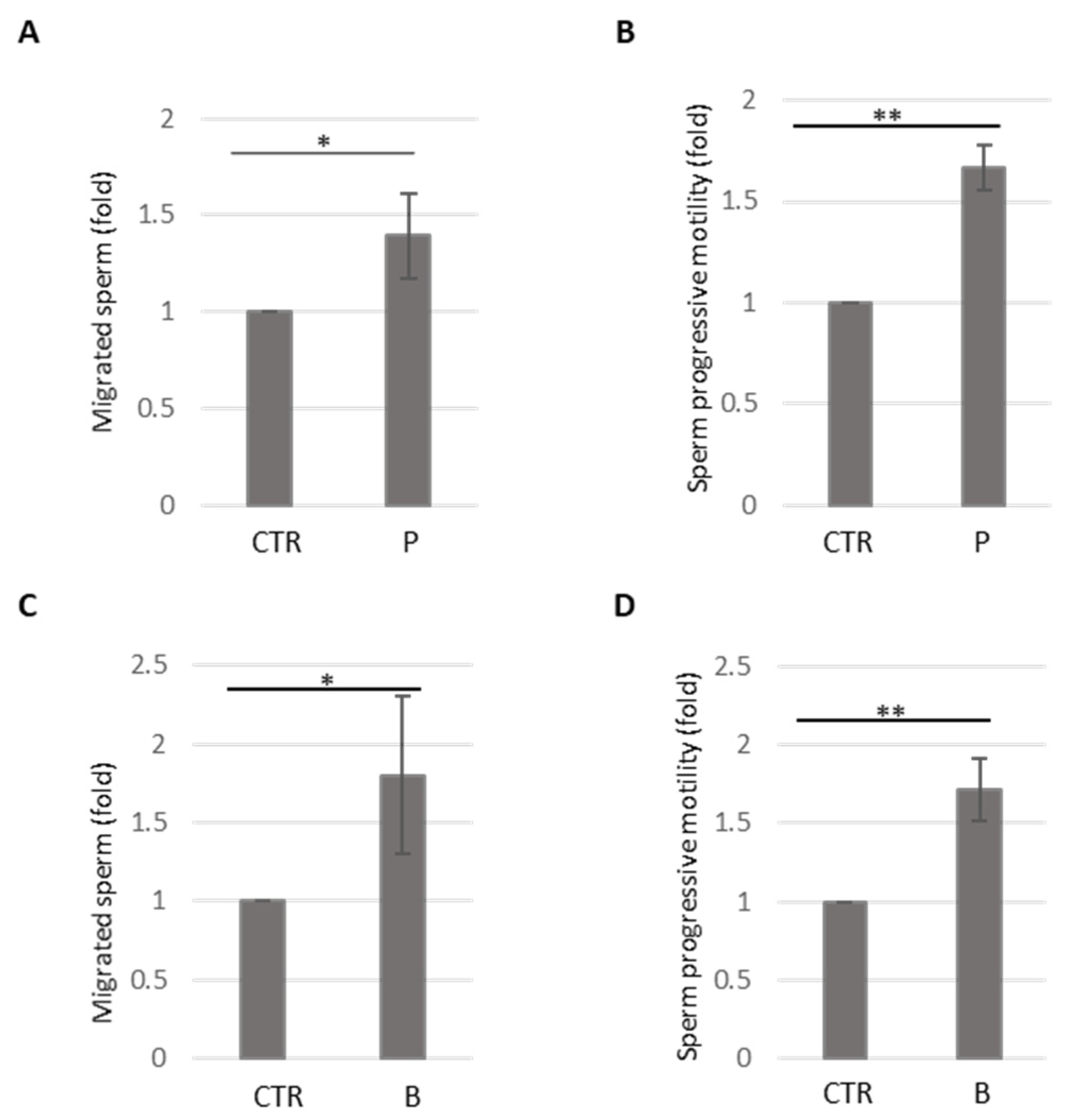
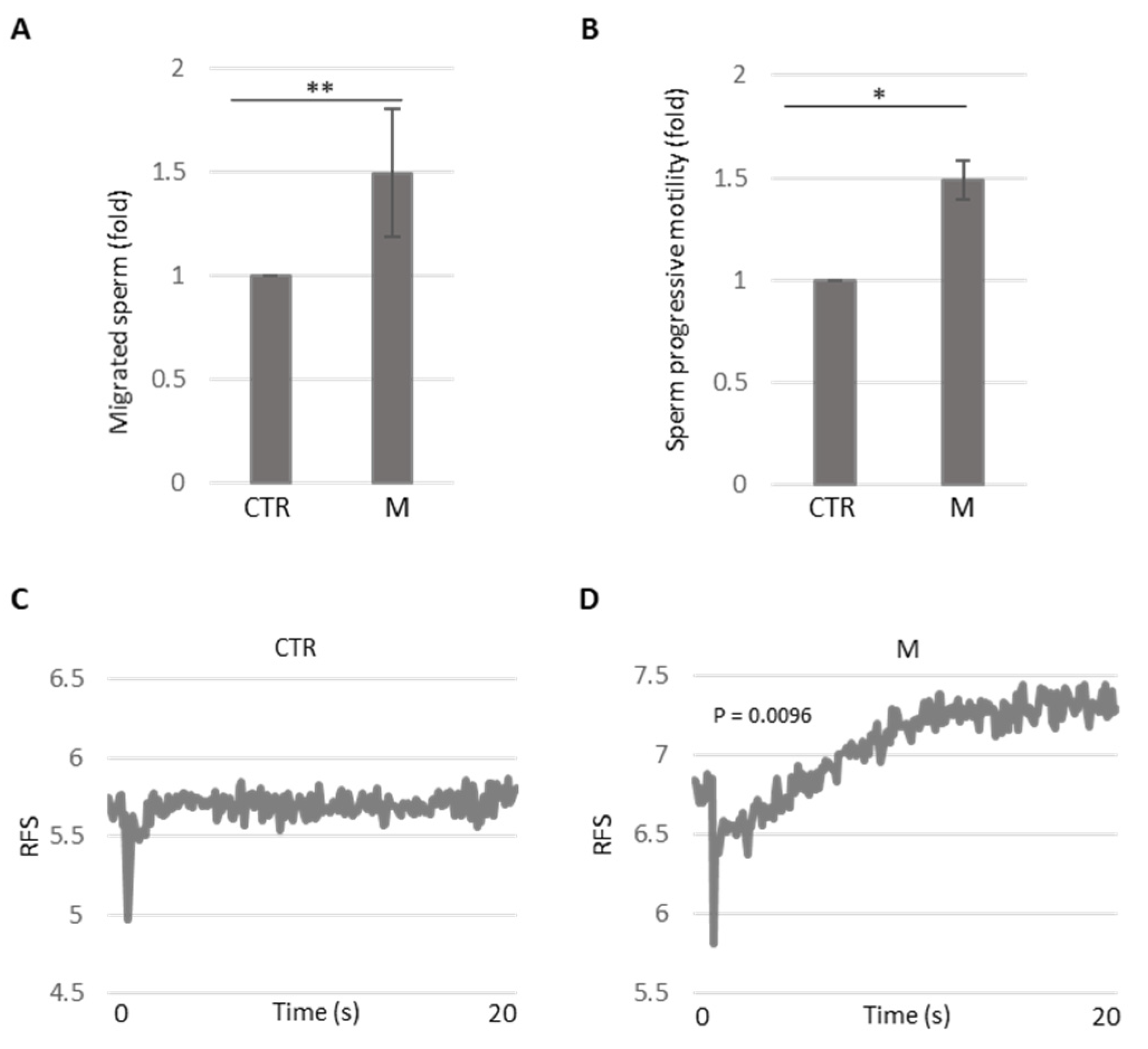
3.3. Analysis of Methional’s Effect on Sperm Migration and Activation
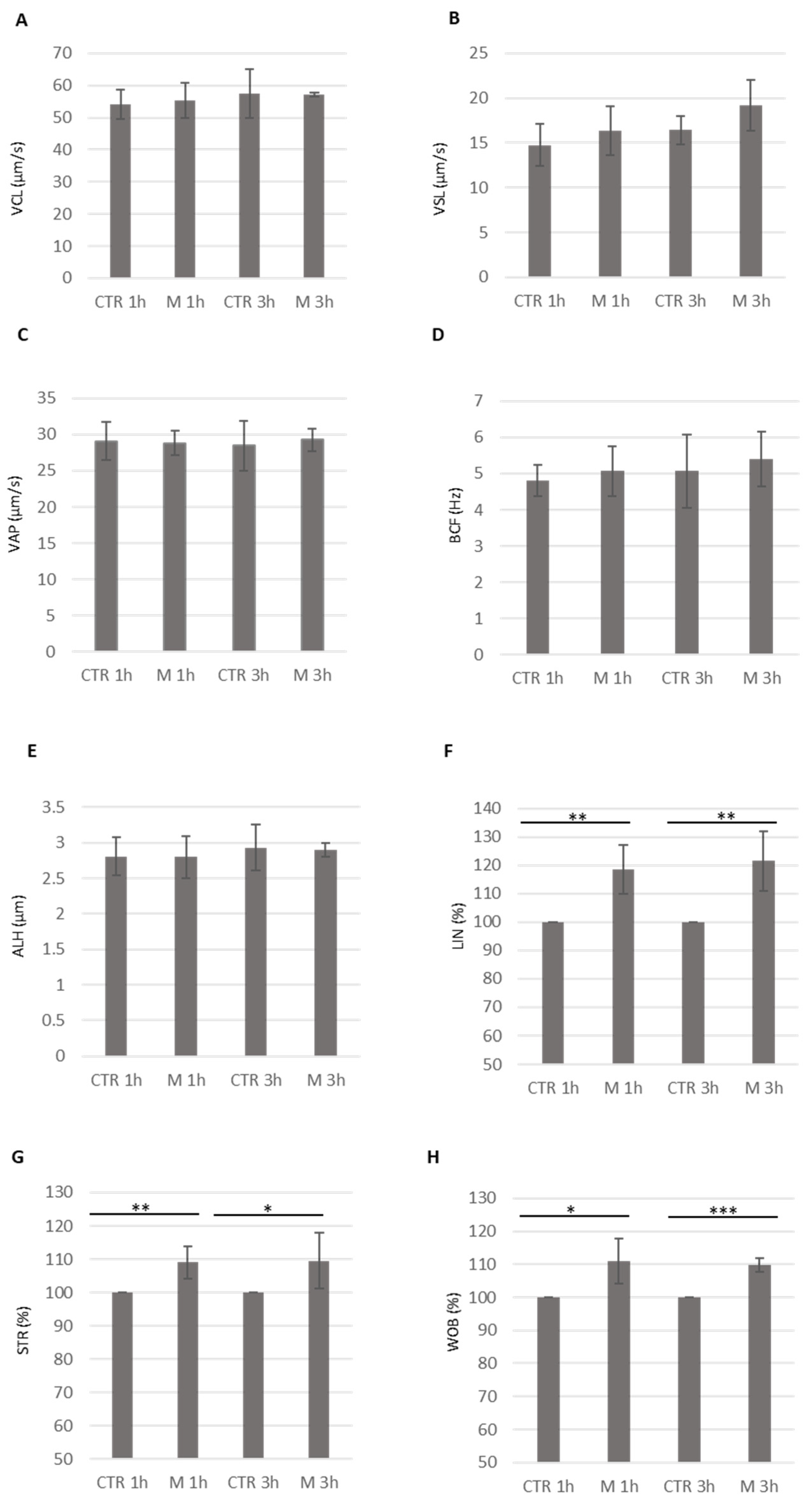
3.4. Analysis of Methional’s Effect on Sperm Kinematics Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koyama, S.; Amarie, D.; Soini, H.; Novotny, M.; Jacobson, S. Chemotaxis Assays of Mouse Sperm on Microfluidic Devices. Anal. Chem. 2006, 78, 3354–3359. [Google Scholar] [CrossRef] [PubMed]
- Penny, J.; Lymbery, R.; Evans, J.; Sherman, C.; Conlan, X. The Use of Microfluidic Devices in Studies of Differential Sperm Chemotaxis. Trends Biotechnol. 2022, 40, 1144–1147. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.; Maeng, J.; Hwang, S.; Ahn, Y. Design, Fabrication, and Testing of a Microfluidic Device for Thermotaxis and Chemotaxis Assays of Sperm. SLAS Technol. 2018, 23, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, S.; Sontakke, S.; Deekshith, K.; Parte, P.; Jadhav, S. Chemotactic Behavior of Spermatozoa Captured Using a Microfluidic Chip. Biomicrofluidics 2018, 12, 024112. [Google Scholar] [CrossRef]
- Berendsen, J.; Kruit, S.; Atak, N.; Willink, E.; Segerink, L. Flow-Free Microfluidic Device for Quantifying Chemotaxis in Spermatozoa. Anal. Chem. 2020, 92, 3302–3306. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, B.; Fu, Q.; Wu, J.; Liu, R. A Fully Integrated Biomimetic Microfluidic Device for Evaluation of Sperm Response to Thermotaxis and Chemotaxis. Lab Chip 2021, 21, 397–406. [Google Scholar] [CrossRef]
- Doostabadi, M.; Mangoli, E.; Marvast, L.; Dehghanpour, F.; Maleki, B.; Torkashvand, H.; Talebi, A. Microfluidic Devices Employing Chemo- and Thermotaxis for Sperm Selection Can Improve Sperm Parameters and Function in Patients with High DNA Fragmentation. Andrologia 2022, 54, e14623. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, R.; Yin, T.; Zou, W.; Tang, Y.; Ding, J.; Yang, J. Generation of Gradients on a Microfluidic Device: Toward a High-Throughput Investigation of Spermatozoa Chemotaxis. PLoS ONE 2015, 10, e0142555. [Google Scholar] [CrossRef]
- Xie, L.; Ma, R.; Han, C.; Su, K.; Zhang, Q.; Qiu, T.; Wang, L.; Huang, G.; Qiao, J.; Wang, J.; et al. Integration of Sperm Motility and Chemotaxis Screening with a Microchannel-Based Device. Clin. Chem. 2010, 56, 1270–1278. [Google Scholar] [CrossRef]
- Jahangiri, A.; Ziarati, N.; Dadkhah, E.; Bucak, M.; Rahimizadeh, P.; Shahverdi, A.; Gilani, M.; Topraggaleh, T. Microfluidics: The Future of Sperm Selection in Assisted Reproduction. Andrology 2023, 12, 1236–1252. [Google Scholar] [CrossRef]
- Maßberg, D.; Hatt, H. Human Olfactory Receptors: Novel Cellular Functions Outside of the Nose. Physiol. Rev. 2018, 98, 1739–1763. [Google Scholar] [CrossRef] [PubMed]
- Firestein, S. How the Olfactory System Makes Sense of Scents. Nature 2001, 413, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Feldmesser, E.; Olender, T.; Khen, M.; Yanai, I.; Ophir, R.; Lancet, D. Widespread Ectopic Expression of Olfactory Receptor Genes. BMC Genom. 2006, 7, 121. [Google Scholar] [CrossRef] [PubMed]
- De La Cruz, O.; Blekhman, R.; Zhang, X.; Nicolae, D.; Firestein, S.; Gilad, Y. A Signature of Evolutionary Constraint on a Subset of Ectopically Expressed Olfactory Receptor Genes. Mol. Biol. Evol. 2009, 26, 491–494. [Google Scholar] [CrossRef]
- Kang, N.N.; Koo, J.H. Olfactory Receptors in Non-Chemosensory Tissues. BMB Rep. 2012, 45, 612. [Google Scholar] [CrossRef]
- Flegel, C.; Manteniotis, S.; Osthold, S.; Hatt, H.; Gisselmann, G. Expression Profile of Ectopic Olfactory Receptors Determined by Deep Sequencing. PLoS ONE 2013, 8, e55368. [Google Scholar] [CrossRef]
- Foster, S.R.; Roura, E.; Thomas, W.G. Extrasensory Perception: Odorant and Taste Receptors Beyond the Nose and Mouth. Pharmacol. Ther. 2014, 142, 41–61. [Google Scholar] [CrossRef]
- Abaffy, T. Human Olfactory Receptors Expression and Their Role in Non-Olfactory Tissues: A Mini-Review. J. Pharm. Pharm. 2015, 6, 4. [Google Scholar] [CrossRef]
- Milardi, D.; Colussi, C.; Grande, G.; Vincenzoni, F.; Pierconti, F.; Mancini, F.; Baroni, S.; Castagnola, M.; Marana, R.; Pontecorvi, A. Olfactory receptors in semen and in the male tract: From proteome to proteins. Front. Endocrinol. 2018, 8, 379. [Google Scholar] [CrossRef]
- Flegel, C.; Vogel, F.; Hofreuter, A.; Schreiner, B.S.P.; Osthold, S.; Veitinger, S.; Becker, C.; Brockmeyer, N.H.; Muschol, M.; Wennemuth, G.; et al. Characterization of the olfactory receptors expressed in human spermatozoa. Front. Mol. Biosci. 2016, 2, 73. [Google Scholar] [CrossRef]
- Neuhaus, E.M.; Mashukova, A.; Barbour, J.; Wolters, D.; Hatt, H. Novel function of beta-arrestin2 in the nucleus of mature spermatozoa. J. Cell Sci. 2006, 119, 3047–3056. [Google Scholar] [CrossRef] [PubMed]
- Veitinger, T.; Riffell, J.R.; Veitinger, S.; Nascimento, J.M.; Triller, A.; Chandsawangbhuwana, C.; Schwane, K.; Geerts, A.; Wunde, F.; Berns, M.W.; et al. Chemosensory Ca2+ dynamics correlate with diverse behavioral phenotypes in human sperm. J. Biol. Chem. 2011, 286, 17311. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, N.; Yomogida, K.; Okabe, M.; Touhara, K. Functional characterization of a mouse testicular olfactory receptor and its role in chemosensing and in regulation of sperm motility. J. Cell Sci. 2004, 117, 5835–5845. [Google Scholar] [CrossRef] [PubMed]
- Spehr, M.; Gisselmann, G.; Poplawski, A.; Riffell, J.A.; Wetzel, C.H.; Zimmer, R.K.; Hatt, H. Identification of a testicular odorant receptor mediating human sperm chemotaxis. Science 2003, 299, 2054–2058. [Google Scholar] [CrossRef]
- Spehr, M.; Schwane, K.; Riffell, J.A.; Barbour, J.; Zimmer, R.K.; Neuhaus, E.M.; Hatt, H. Particulate adenylate cyclase plays a key role in human sperm olfactory receptor-mediated chemotaxis. J. Biol. Chem. 2004, 279, 40194–40203. [Google Scholar] [CrossRef]
- Ottaviano, G.; Zuccarello, D.; Menegazzo, M.; Perilli, L.; Marioni, G.; Frigo, A.C.; Staffieri, A.; Foresta, C. Human olfactory sensitivity for bourgeonal and male infertility: A preliminary investigation. Eur. Arch. Oto-Rhino-Laryngol. 2013, 270, 3079–3086. [Google Scholar] [CrossRef]
- Hartmann, C.; Triller, A.; Spehr, M.; Dittrich, R.; Hatt, H.; Buettner, A. Sperm-activating odorous substances in human follicular fluid and vaginal secretion: Identification by gas chromatography-olfactometry and Ca2+ imaging. ChemPlusChem 2013, 78, 695–702. [Google Scholar] [CrossRef]
- Teveroni, E.; Di Nicuolo, F.; Vergani, E.; Bruno, C.; Maulucci, G.; Bianchetti, G.; Astorri, A.L.; Grande, G.; Gervasoni, J.; Santucci, L.; et al. Short-chain fatty acids modulate sperm migration through olfactory receptor 51E2 activity. Int. J. Mol. Sci. 2022, 23, 12726. [Google Scholar] [CrossRef]
- Devigili, A.; Cattelan, S.; Gasparini, C. Sperm accumulation induced by the female reproductive fluid: Putative evidence of chemoattraction using a new tool. Cells 2021, 10, 2472. [Google Scholar] [CrossRef]
- Pujianto, D.A.; Zaini, M.; Salim, S.O.; Sisca. Progesterone increases the progressive motility of human sperm. J. Int. Dent. Med. Res. 2019, 12, 228–231. Available online: https://api.semanticscholar.org/CorpusID:150377470 (accessed on 10 March 2024).
- Teves, M.E.; Guidobaldi, H.A.; Uñates, D.R.; Sanchez, R.; Miska, W.; Publicover, S.J.; Garcia, A.A.M.; Giojalas, L.C.; Hansen, I.A. Molecular mechanism for human sperm chemotaxis mediated by progesterone. PLoS ONE 2009, 4, e8211. [Google Scholar] [CrossRef] [PubMed]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Karlsson, M.; Zhang, C.; Méar, L.; Zhong, W.; Digre, A.; Katona, B.; Sjöstedt, E.; Butler, L.; Odeberg, J.; Dusart, P.; et al. A single-cell type transcriptomics map of human tissues. Sci. Adv. 2021, 7, eabh2169. [Google Scholar] [CrossRef] [PubMed]
- Di, R.; Kim, J.; Martin, M.; Leustek, T.; Jhoo, J.; Ho, C.; Tumer, N. Enhancement of the primary flavor compound methional in potato by increasing the level of soluble methionine. J. Agric. Food Chem. 2003, 51, 5695–5702. [Google Scholar] [CrossRef] [PubMed]
- Amärita, F.; Fernández-Esplà, D.; Requena, T.; Peláez, C. Conversion of methionine to methional by Lactococcus lactis. FEMS Microbiol. Lett. 2001, 204, 189–195. [Google Scholar] [CrossRef]
- Gao, Y.; Lu, Y.; Teng, K.; Chen, M.; Zheng, H.; Zhu, Y.; Zhong, J. Complete genome sequence of Lactococcus lactis subsp. lactis CV56, a probiotic strain isolated from the vaginas of healthy women. J. Bacteriol. 2011, 193, 2886–2887. [Google Scholar] [CrossRef]
- Todorov, S.; Botes, M.; Danova, S.; Dicks, L. Probiotic properties of Lactococcus lactis ssp. lactis HV219, isolated from human vaginal secretions. J. Appl. Microbiol. 2007, 103, 227–236. [Google Scholar] [CrossRef]
- Van den Bergh, M.; Emiliani, S.; Biramane, J.; Vannin, A.S.; Englert, Y. A first prospective study of the individual straight line velocity of the spermatozoon and its influences on the fertilization rate after intracytoplasmic sperm injection. Hum. Reprod. 1998, 13, 3103–3107. [Google Scholar] [CrossRef]
- Jaiswal, B.; Tur-Kaspa, I.; Dor, J.; Mashiach, S.; Eisenbach, M. Human sperm chemotaxis: Is progesterone a chemoattractant? Biol. Reprod. 1999, 60, 1314–1319. [Google Scholar] [CrossRef]
- Pérez-Cerezales, S.; López-Cardona, A.; Gutiérrez-Adán, A. Progesterone effects on mouse sperm kinetics in conditions of viscosity. Reproduction 2016, 151, 501–507. [Google Scholar] [CrossRef]
- Hovhannisyan, H.; Grigoryan, G. A new sustainable symbiotic association of lactic acid cocci and bacilli for colonization/recolonization of vagina and prevention of bacterial vaginosis. Am. J. Biosci. 2014, 2, 84. [Google Scholar] [CrossRef]
- Brenker, C.; Goodwin, N.; Weyand, I.; Kashikar, N.D.; Naruse, M.; Krähling, M.; Muller, A.; Kaupp, U.M.; Strunker, T. The CatSper channel: A polymodal chemosensor in human sperm. EMBO J. 2012, 31, 1654–1665. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Luo, T.; Peng, Z.; Chen, H.; Zhang, J.; Zeng, X. OR1D2 receptor mediates bourgeonal-induced human CatSper activation in a G-protein dependent manner. bioRxiv 2019, 757880. [Google Scholar] [CrossRef]
- Punzón-Jiménez, P.; Labarta, E. The impact of the female genital tract microbiome in women’s health and reproduction: A review. J. Assist. Reprod. Genet. 2021, 38, 2519–2541. [Google Scholar] [CrossRef]
- Pay, A.; Turienzo, A.; Garcia, E.; Ortiz, J.; Lledó, B.; Hortal, M.; Cascales, A.; Morales, R.; Bernabeu, A.; Bernabeu, R. Vaginal microbiome dysbiosis influences partner’s sperm motility. Reprod. BioMed. Online 2024, 48, 104044. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Nicuolo, F.; Teveroni, E.; Devigili, A.; Gasparini, C.; Urbani, A.; Ghi, T.; Pontecorvi, A.; Milardi, D.; Mancini, F. Exploring OR2H1-Mediated Sperm Chemotaxis: Development and Application of a Novel Microfluidic Device. Cells 2025, 14, 944. https://doi.org/10.3390/cells14130944
Di Nicuolo F, Teveroni E, Devigili A, Gasparini C, Urbani A, Ghi T, Pontecorvi A, Milardi D, Mancini F. Exploring OR2H1-Mediated Sperm Chemotaxis: Development and Application of a Novel Microfluidic Device. Cells. 2025; 14(13):944. https://doi.org/10.3390/cells14130944
Chicago/Turabian StyleDi Nicuolo, Fiorella, Emanuela Teveroni, Alessandro Devigili, Clelia Gasparini, Andrea Urbani, Tullio Ghi, Alfredo Pontecorvi, Domenico Milardi, and Francesca Mancini. 2025. "Exploring OR2H1-Mediated Sperm Chemotaxis: Development and Application of a Novel Microfluidic Device" Cells 14, no. 13: 944. https://doi.org/10.3390/cells14130944
APA StyleDi Nicuolo, F., Teveroni, E., Devigili, A., Gasparini, C., Urbani, A., Ghi, T., Pontecorvi, A., Milardi, D., & Mancini, F. (2025). Exploring OR2H1-Mediated Sperm Chemotaxis: Development and Application of a Novel Microfluidic Device. Cells, 14(13), 944. https://doi.org/10.3390/cells14130944







