Role of Kindlin-2-Expressing Extracellular Vesicles in the Invasiveness of Triple Negative Breast Cancer Tumor Cells
Abstract
1. Introduction
2. Methods
2.1. Cell Culture
2.2. Isolation and Characterization of Small Extracellular Vesicles (EVs)
2.3. Immunocytochemistry
2.4. Direct Stochastic Optical Reconstruction Microscopy (D-STORM)
2.5. Western Blot
2.6. Tumorsphere Invasion
2.7. Statistical Analysis
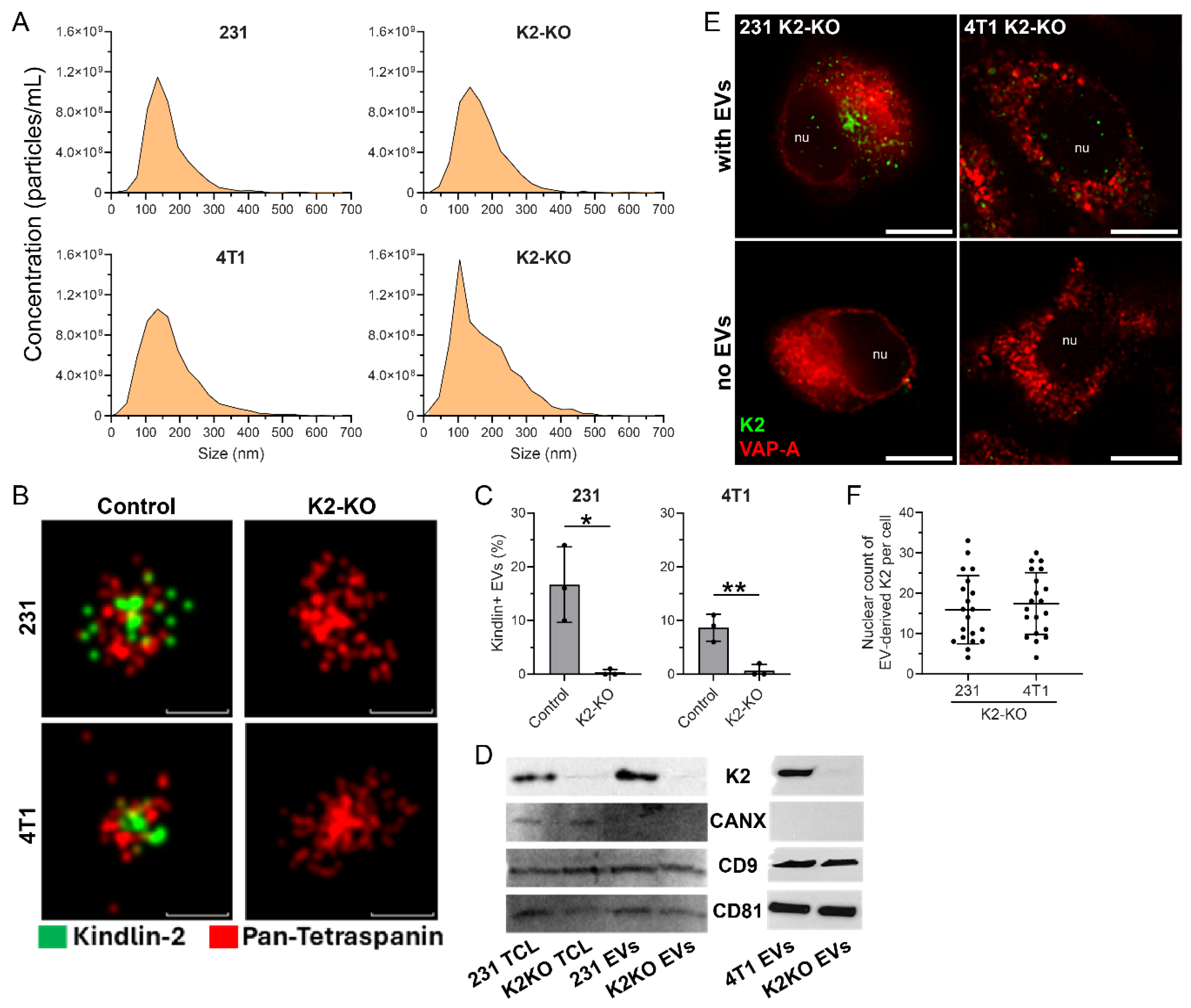
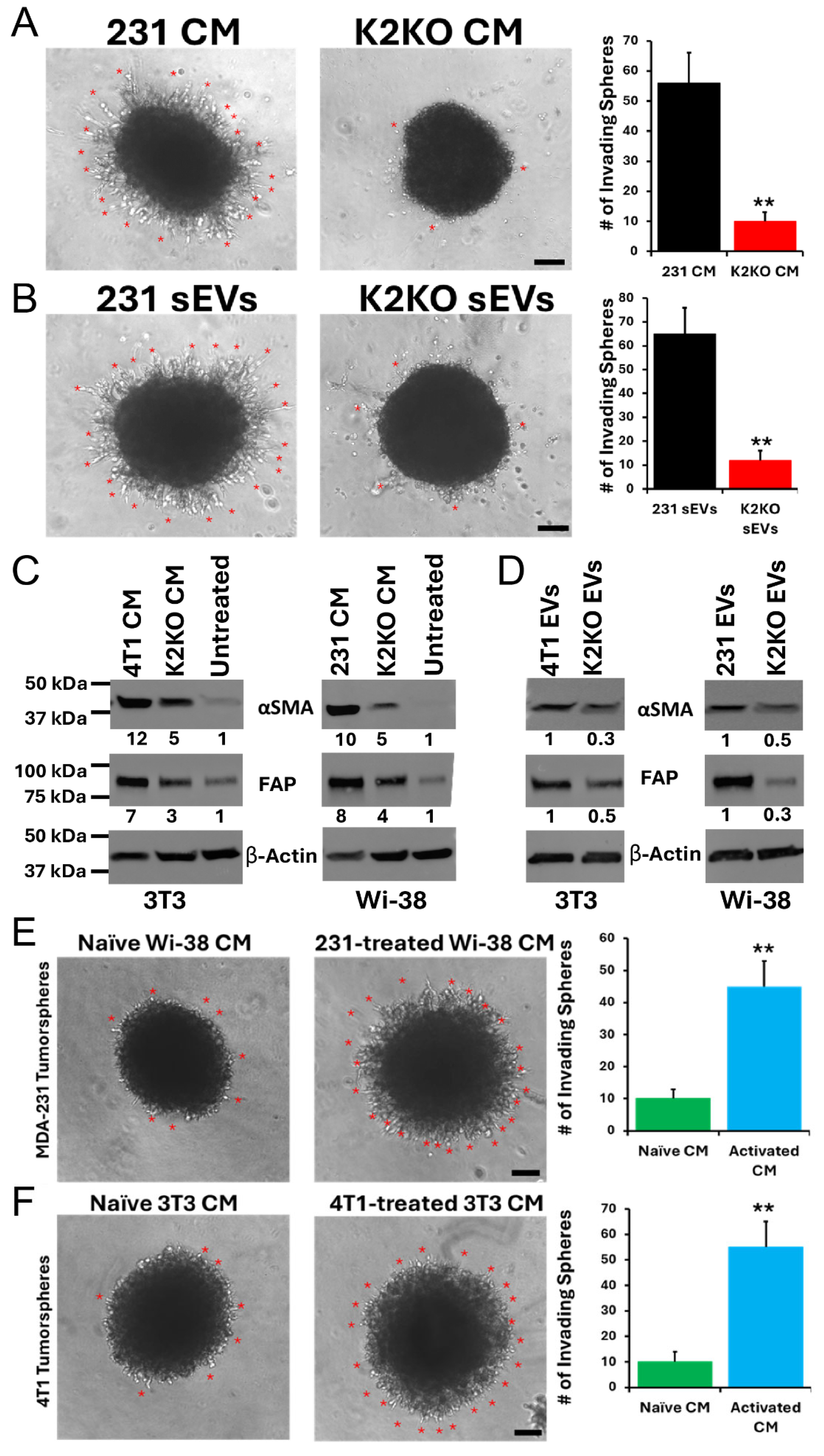
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.X.; Bos, P.D.; Massague, J. Metastasis: From dissemination to organ-specific colonization. Nat. Rev. Cancer 2009, 9, 274–284. [Google Scholar] [CrossRef]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA A Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; Van De Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Sorlie, T.; Perou, C.M.; Tibshirani, R.; Aas, T.; Geisler, S.; Johnsen, H.; Hastie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 2001, 98, 10869–10874. [Google Scholar] [CrossRef]
- Sørlie, T.; Tibshirani, R.; Parker, J.; Hastie, T.; Marron, J.S.; Nobel, A.; Deng, S.; Johnsen, H.; Pesich, R.; Geisler, S.; et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc. Natl. Acad. Sci. USA 2003, 100, 8418–8423. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.; O‘DRiscoll, L.; Théry, C.; Witwer, K.W. MISEV2023: An updated guide to EV research and applications. J. Extracell. Vesicles 2024, 13, e12416. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Xu, R.; Rai, A.; Chen, M.; Suwakulsiri, W.; Greening, D.W.; Simpson, R.J. Extracellular vesicles in cancer — implications for future improvements in cancer care. Nat. Rev. Clin. Oncol. 2018, 15, 617–638. [Google Scholar] [CrossRef]
- Adem, B.; Vieira, P.F.; Melo, S.A. Decoding the Biology of Exosomes in Metastasis. Trends Cancer 2020, 6, 20–30. [Google Scholar] [CrossRef]
- Corbeil, D.; Lorico, A. Exosomes, microvesicles, and their friends in solid tumors. Exosomes, microvesicles, and their friends in solid tumors. In Exosomes: A Clinical Compendium; Edelstein, L., Smythies, J., Quesenberry, P., Noble, D., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 39–80. [Google Scholar]
- Tkach, M.; Théry, C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Asao, T.; Tobias, G.C.; Lucotti, S.; Jones, D.R.; Matei, I.; Lyden, D. Extracellular vesicles and particles as mediators of long-range communication in cancer: Connecting biological function to clinical applications. Extracell. Vesicles Circ. Nucleic Acids 2023, 4, 461–485. [Google Scholar] [CrossRef] [PubMed]
- Al-Nedawi, K.; Meehan, B.; Micallef, J.; Lhotak, V.; May, L.; Guha, A.; Rak, J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 2008, 10, 619–624, Erratum in Nat. Cell Biol. 2008, 10, 752. [Google Scholar] [CrossRef]
- Beckler, M.D.; Higginbotham, J.N.; Franklin, J.L.; Ham, A.-J.; Halvey, P.J.; Imasuen, I.E.; Whitwell, C.; Li, M.; Liebler, D.C.; Coffey, R.J. Proteomic Analysis of Exosomes from Mutant KRAS Colon Cancer Cells Identifies Intercellular Transfer of Mutant KRAS. Mol. Cell. Proteom. 2013, 12, 343–355. [Google Scholar] [CrossRef]
- Peinado, H.; Alečković, M.; Lavotshkin, S.; Matei, I.; Costa-Silva, B.; Moreno-Bueno, G.; Hergueta-Redondo, M.; Williams, C.; García-Santos, G.; Ghajar, C.M.; et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 2012, 18, 883–891. [Google Scholar] [CrossRef]
- Qu, L.; Ding, J.; Chen, C.; Wu, Z.-J.; Liu, B.; Gao, Y.; Chen, W.; Liu, F.; Sun, W.; Li, X.-F.; et al. Exosome-transmitted lncARSR promotes sunitinib resistance in renal cancer by acting as a competing endogenous RNA. Cancer Cell 2016, 29, 653–668. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, J.; Li, X.; Wang, X.; Lin, Y.; Wang, X. Exosomes derived from hypoxic epithelial ovarian cancer cells deliver microRNAs to macrophages and elicit a tumor-promoted phenotype. Cancer Lett. 2018, 435, 80–91. [Google Scholar] [CrossRef]
- Ma, R.; Ji, T.; Chen, D.; Dong, W.; Zhang, H.; Yin, X.; Ma, J.; Liang, X.; Zhang, Y.; Shen, G.; et al. Tumor cell-derived microparticles polarize M2 tumor-associated macrophages for tumor progression. OncoImmunology 2016, 5, e1118599. [Google Scholar] [CrossRef]
- Skog, J.; Würdinger, T.; Van Rijn, S.; Meijer, D.H.; Gainche, L.; Curry, W.T., Jr.; Carter, B.S.; Krichevsky, A.M.; Breakefield, X.O. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008, 10, 1470–1476. [Google Scholar] [CrossRef]
- Luga, V.; Zhang, L.; Viloria-Petit, A.M.; Ogunjimi, A.A.; Inanlou, M.R.; Chiu, E.; Buchanan, M.; Hosein, A.N.; Basik, M.; Wrana, J.L. Exosomes Mediate Stromal Mobilization of Autocrine Wnt-PCP Signaling in Breast Cancer Cell Migration. Cell 2012, 151, 1542–1556. [Google Scholar] [CrossRef]
- Santos, M.F.; Rappa, G.; Karbanová, J.; Diana, P.; Cirrincione, G.; Carbone, D.; Manna, D.; Aalam, F.; Wang, D.; Vanier, C.; et al. HIV-1-induced nuclear invaginations mediated by VAP-A, ORP3, and Rab7 complex explain infection of activated T cells. Nat. Commun. 2023, 14, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.H.; Silke, B.; Lee, P.S.; Hilal, A. Haemodynamic dose-response effects of intravenous beta-blocking drugs with different ancillary properties in patients with coronary heart disease. Eur. Hear. J. 1982, 3, 564–569. [Google Scholar] [CrossRef]
- Santos, M.F.; Rappa, G.; Karbanová, J.; Fontana, S.; Di Bella, M.A.; Pope, M.R.; Parrino, B.; Cascioferro, S.M.; Vistoli, G.; Diana, P.; et al. Itraconazole inhibits nuclear delivery of extracellular vesicle cargo by disrupting the entry of late endosomes into the nucleoplasmic reticulum. J. Extracell. Vesicles 2021, 10, e12132. [Google Scholar] [CrossRef] [PubMed]
- Plow, E.F.; Das, M.; Bialkowska, K.; Sossey-Alaoui, K. Of Kindlins and Cancer. Discoveries 2016, 4, e59. [Google Scholar] [CrossRef]
- Rognoni, E.; Ruppert, R.; Fässler, R. The kindlin family: Functions, signaling properties and implications for human disease. J. Cell Sci. 2016, 129, 17–27. [Google Scholar] [CrossRef]
- Wang, W.; Kansakar, U.; Markovic, V.; Sossey-Alaoui, K. Role of Kindlin-2 in cancer progression and metastasis. Ann. Transl. Med. 2020, 8, 901. [Google Scholar] [CrossRef]
- Bledzka, K.; Bialkowska, K.; Sossey-Alaoui, K.; Vaynberg, J.; Pluskota, E.; Qin, J.; Plow, E.F. Kindlin-2 directly binds actin and regulates integrin outside-in signaling. J. Cell Biol. 2016, 213, 97–108. [Google Scholar] [CrossRef]
- Bledzka, K.; Liu, J.; Xu, Z.; Perera, H.D.; Yadav, S.P.; Bialkowska, K.; Qin, J.; Ma, Y.-Q.; Plow, E.F. Spatial Coordination of Kindlin-2 with Talin Head Domain in Interaction with Integrin β Cytoplasmic Tails. J. Biol. Chem. 2012, 287, 24585–24594. [Google Scholar] [CrossRef]
- Pluskota, E.; Ma, Y.; Bledzka, K.M.; Bialkowska, K.; Soloviev, D.A.; Szpak, D.; Podrez, E.A.; Fox, P.L.; Hazen, S.L.; Dowling, J.J.; et al. Kindlin-2 regulates hemostasis by controlling endothelial cell–surface expression of ADP/AMP catabolic enzymes via a clathrin-dependent mechanism. Blood 2013, 122, 2491–2499. [Google Scholar] [CrossRef]
- Verrillo, C.E.; Quaglia, F.; Shields, C.D.; Lin, S.; Kossenkov, A.V.; Tang, H.; Speicher, D.; Naranjo, N.M.; Testa, A.; Kelly, W.K.; et al. Expression of the αVβ3 integrin affects prostate cancer sEV cargo and density and promotes sEV pro-tumorigenic activity in vivo through a GPI-anchored receptor, NgR2. J. Extracell. Vesicles 2024, 13, e12482. [Google Scholar] [CrossRef]
- Sossey-Alaoui, K.; Pluskota, E.; Szpak, D.; Plow, E.F. The Kindlin2-p53-SerpinB2 signaling axis is required for cellular senescence in breast cancer. Cell Death Dis. 2019, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Yousafzai, N.A.; El Khalki, L.; Wang, W.; Szpendyk, J.; Sossey-Alaoui, K. Kindlin-2 regulates the oncogenic activities of integrins and TGF-β in triple-negative breast cancer progression and metastasis. Oncogene 2024, 43, 3291–3305. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chaudhary, R.; Szpendyk, J.; El Khalki, L.; Yousafzai, N.A.; Chan, R.; Desai, A.; Sossey-Alaoui, K. Kindlin-2–Mediated Hematopoiesis Remodeling Regulates Triple-Negative Breast Cancer Immune Evasion. Mol. Cancer Res. 2025, 23, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Rappa, G.; Santos, M.F.; Green, T.M.; Karbanová, J.; Hassler, J.; Bai, Y.; Barsky, S.H.; Corbeil, D.; Lorico, A. Nuclear transport of cancer extracellular vesicle-derived biomaterials through nuclear envelope invagination-associated late endosomes. Oncotarget 2017, 8, 14443–14461. [Google Scholar] [CrossRef]
- Rana, P.S.; Wang, W.; Markovic, V.; Szpendyk, J.; Chan, E.R.; Sossey-Alaoui, K. The WAVE2/miR-29/Integrin-β1 Oncogenic Signaling Axis Promotes Tumor Growth and Metastasis in Triple-negative Breast Cancer. Cancer Res. Commun. 2023, 3, 160–174. [Google Scholar] [CrossRef]
- Santos, M.F.; Rappa, G.; Karbanová, J.; Kurth, T.; Corbeil, D.; Lorico, A. VAMP-associated protein-A and oxysterol-binding protein–related protein 3 promote the entry of late endosomes into the nucleoplasmic reticulum. J. Biol. Chem. 2018, 293, 13834–13848. [Google Scholar] [CrossRef]
- Hoshino, A.; Costa-Silva, B.; Shen, T.-L.; Rodrigues, G.; Hashimoto, A.; Mark, M.T.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef]
- Peinado, H.; Zhang, H.; Matei, I.R.; Costa-Silva, B.; Hoshino, A.; Rodrigues, G.; Psaila, B.; Kaplan, R.N.; Bromberg, J.F.; Kang, Y.; et al. Pre-metastatic niches: Organ-specific homes for metastases. Nat. Rev. Cancer 2017, 17, 302–317. [Google Scholar] [CrossRef]
- Santner, S.J.; Dawson, P.J.; Tait, L.; Soule, H.D.; Eliason, J.; Mohamed, A.N.; Wolman, S.R.; Heppner, G.H.; Miller, F.R. Malignant MCF10CA1 Cell Lines Derived from Premalignant Human Breast Epithelial MCF10AT Cells. Breast Cancer Res. Treat. 2001, 65, 101–110. [Google Scholar] [CrossRef]
- Aslakson, C.J.; Miller, F.R. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res. 1992, 52, 1399–1405. [Google Scholar]
- Wendt, M.K.; A Smith, J.; Schiemann, W.P. Transforming growth factor-β-induced epithelial–mesenchymal transition facilitates epidermal growth factor-dependent breast cancer progression. Oncogene 2010, 29, 6485–6498. [Google Scholar] [CrossRef] [PubMed]
- Sossey-Alaoui, K.; Pluskota, E.; Szpak, D.; Schiemann, W.P.; Plow, E.F. The Kindlin-2 regulation of epithelial-to-mesenchymal transition in breast cancer metastasis is mediated through miR-200b. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sossey-Alaoui, K.; Pluskota, E.; Bialkowska, K.; Szpak, D.; Parker, Y.; Morrison, C.D.; Lindner, D.J.; Schiemann, W.P.; Plow, E.F. Kindlin-2 Regulates the Growth of Breast Cancer Tumors by Activating CSF-1–Mediated Macrophage Infiltration. Cancer Res. 2017, 77, 5129–5141. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yousafzai, N.A.; Santos, M.F.; Kim, Y.; Avihen Schahaf, N.; Zielke, K.; Languino, L.; Sossey-Alaoui, K.; Lorico, A. Role of Kindlin-2-Expressing Extracellular Vesicles in the Invasiveness of Triple Negative Breast Cancer Tumor Cells. Cells 2025, 14, 1034. https://doi.org/10.3390/cells14131034
Yousafzai NA, Santos MF, Kim Y, Avihen Schahaf N, Zielke K, Languino L, Sossey-Alaoui K, Lorico A. Role of Kindlin-2-Expressing Extracellular Vesicles in the Invasiveness of Triple Negative Breast Cancer Tumor Cells. Cells. 2025; 14(13):1034. https://doi.org/10.3390/cells14131034
Chicago/Turabian StyleYousafzai, Neelum Aziz, Mark F. Santos, Yeaji Kim, Nofar Avihen Schahaf, Kim Zielke, Lucia Languino, Khalid Sossey-Alaoui, and Aurelio Lorico. 2025. "Role of Kindlin-2-Expressing Extracellular Vesicles in the Invasiveness of Triple Negative Breast Cancer Tumor Cells" Cells 14, no. 13: 1034. https://doi.org/10.3390/cells14131034
APA StyleYousafzai, N. A., Santos, M. F., Kim, Y., Avihen Schahaf, N., Zielke, K., Languino, L., Sossey-Alaoui, K., & Lorico, A. (2025). Role of Kindlin-2-Expressing Extracellular Vesicles in the Invasiveness of Triple Negative Breast Cancer Tumor Cells. Cells, 14(13), 1034. https://doi.org/10.3390/cells14131034







