Concurrent Physical Activity Protects Against C26 Adenocarcinoma Tumor-Mediated Cardiac and Skeletal Muscle Dysfunction and Wasting in Males
Abstract
1. Introduction
2. Materials & Methods
2.1. Experimental Design
2.2. Tumor Model
2.3. Running Wheels
2.4. Grip Strength
2.5. Echocardiography
2.6. Sacrifice and Harvest
2.7. Protein Expression
2.8. Statistical Analysis
3. Results
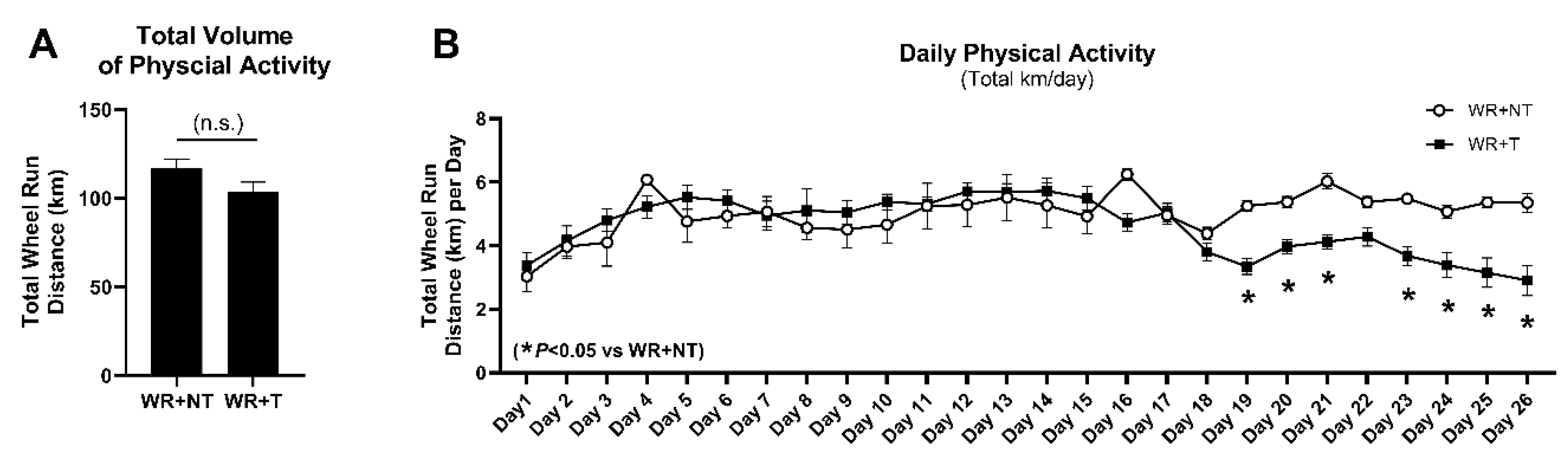
3.1. Voluntary Physical Activity Is Well-Tolerated by Tumor-Bearing Mice
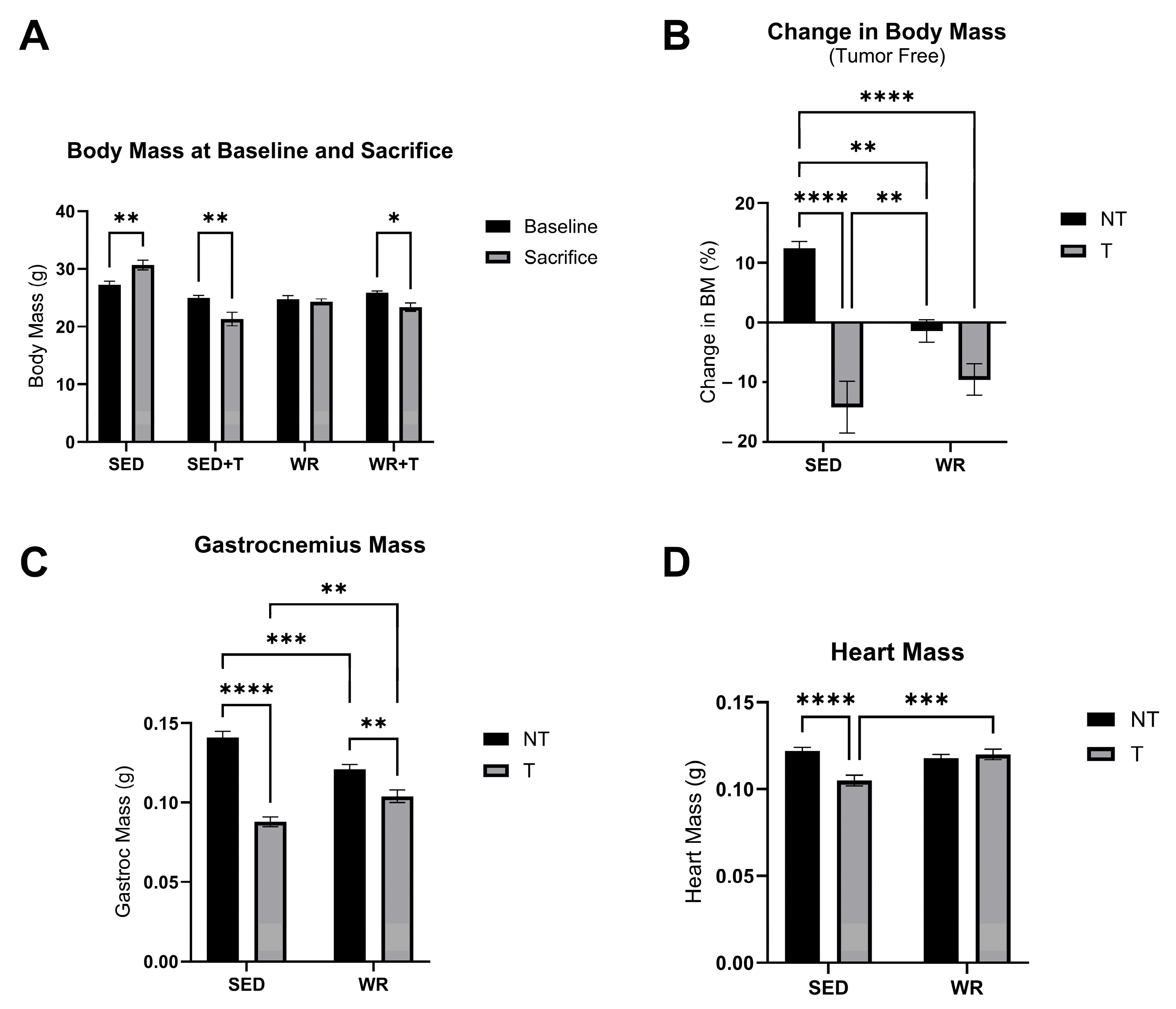
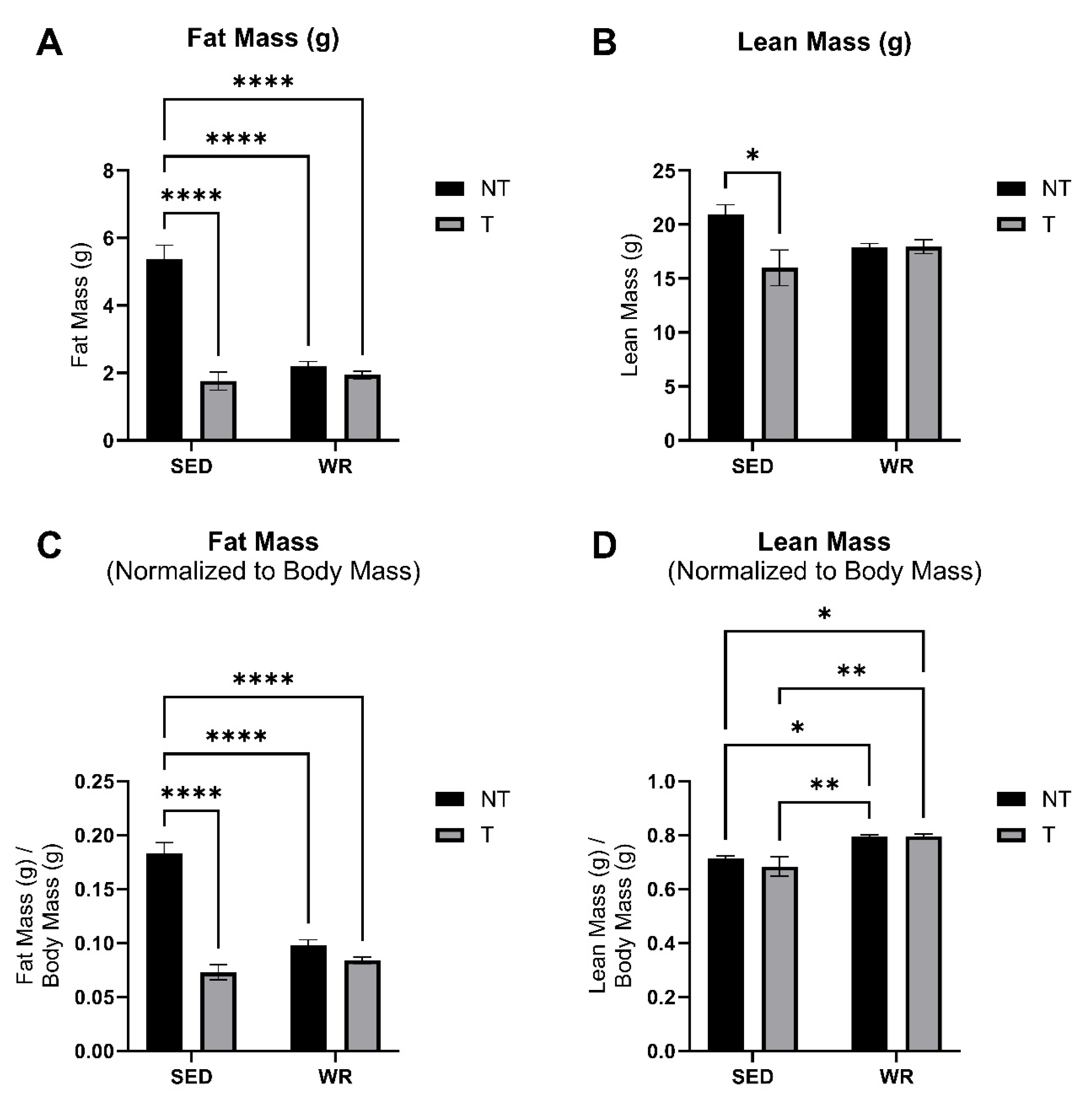
3.2. Physical Activity Protects Against C26 Tumor-Mediated Body Wasting
3.3. Voluntary Wheel Running Stunts Tumor Growth
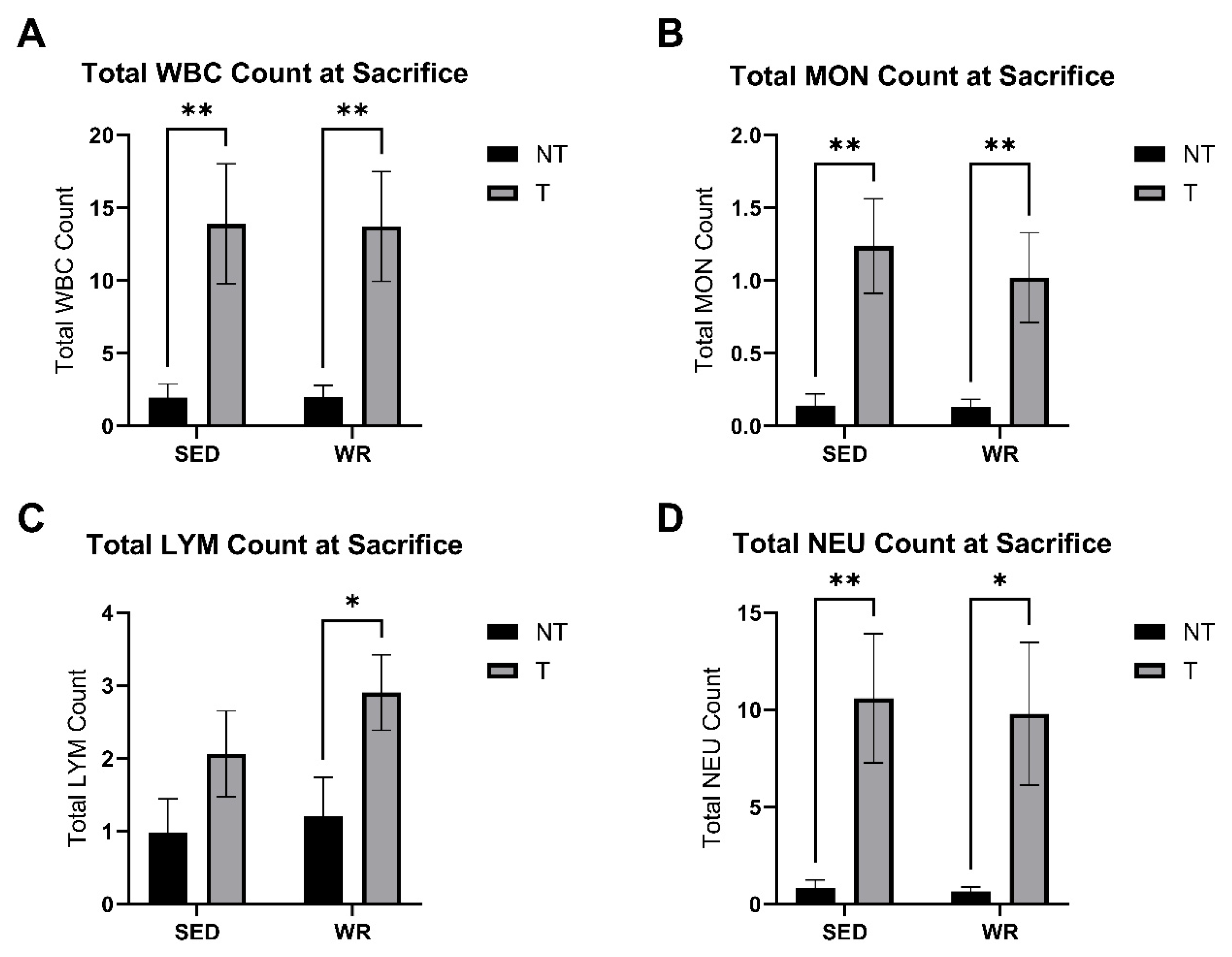
3.4. Tumor Bearing Influences White Blood Cell Profile
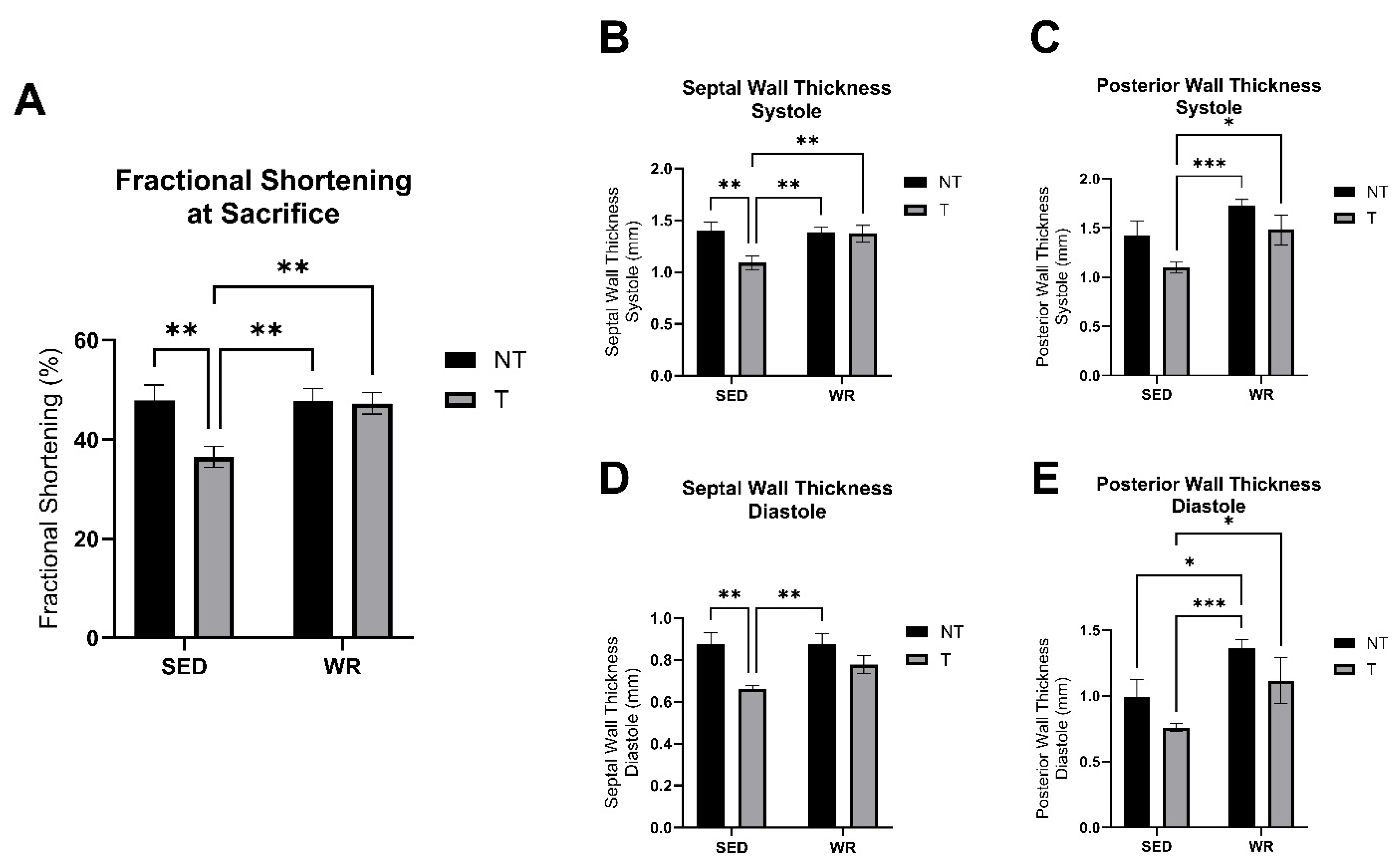
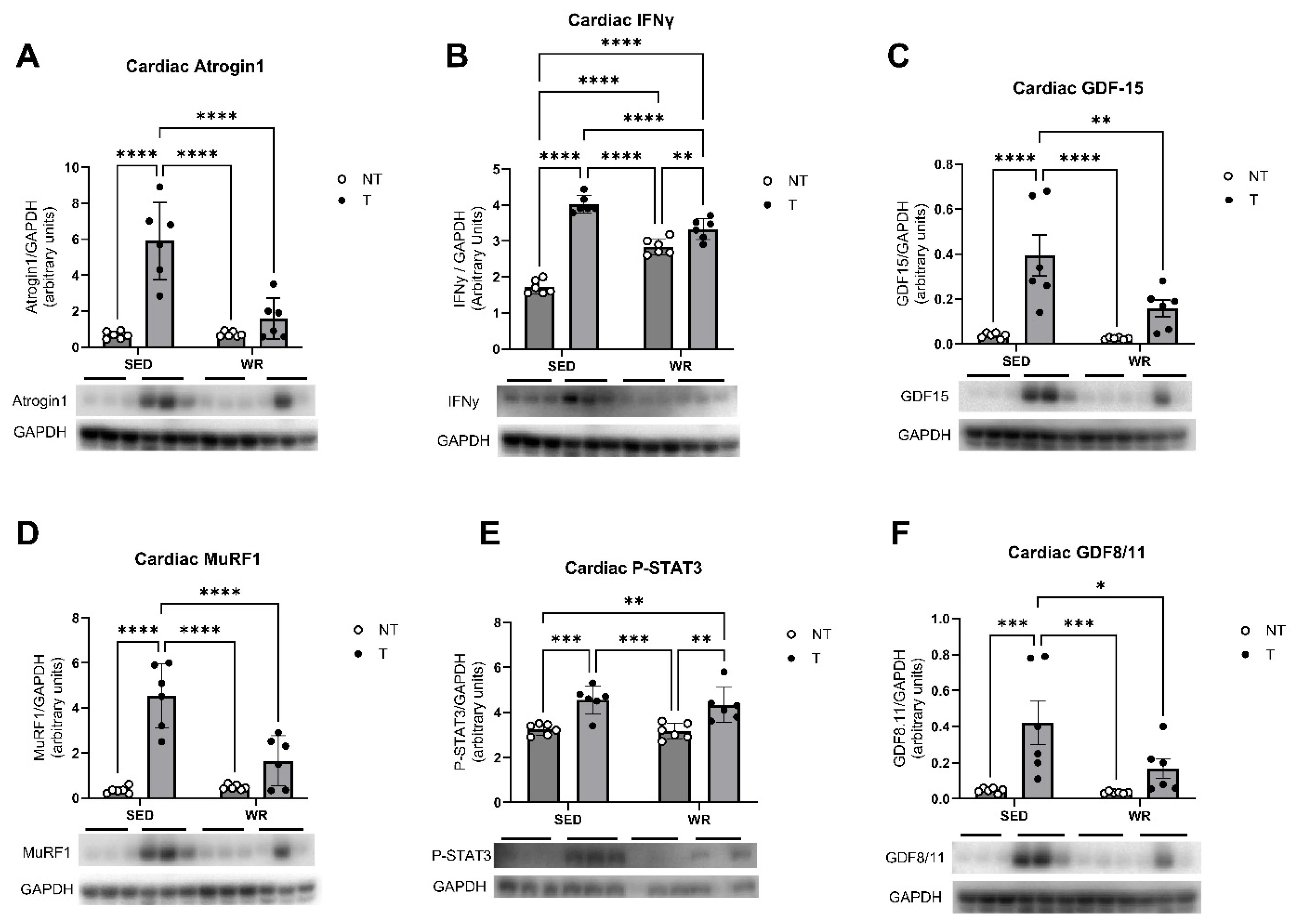
3.5. Physical Activity Protects Against C26 Tumor-Mediated Cardiac Dysfunction
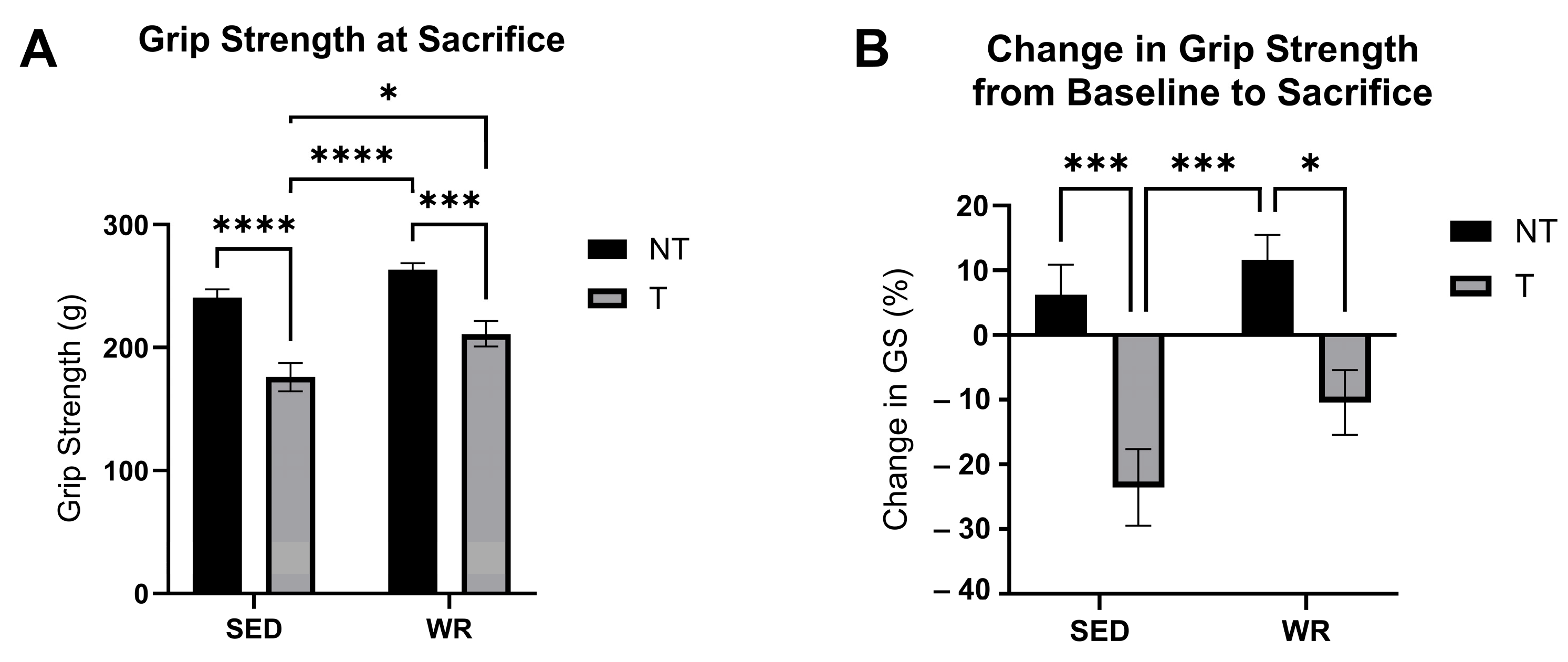
3.6. Physical Activity Protects Against C26 Tumor-Mediated Loss in Skeletal Muscle Strength
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Mariean, C.R.; Tiucă, O.M.; Mariean, A.; Cotoi, O.S. Cancer Cachexia: New Insights and Future Directions. Cancers 2023, 15, 5590. [Google Scholar] [CrossRef]
- Argillander, T.E.; Spek, D.; van der Zaag-Loonen, H.J.; van Raamt, A.F.; van Duijvendijk, P.; van Munster, B.C. Association between postoperative muscle wasting and survival in older patients undergoing surgery for non-metastatic colorectal cancer. J. Geriatr. Oncol. 2021, 12, 1052–1058. [Google Scholar] [CrossRef] [PubMed]
- Wiegert, E.V.M.; de Oliveira, L.C.; Calixto-Lima, L.; Chaves, G.V.; Silva Lopes, M.S.; Peres, W.A.F. New cancer cachexia staging system for use in clinical practice. Nutrition 2021, 90, 111271. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.; Muscaritoli, M.; Anker, S.; Audisio, R.; Barazzoni, R.; Bosnjak, S.; Bossi, P.; Bowman, J.; Gijssels, S.; Krznarić, Ž.; et al. Overcoming barriers to timely recognition and treatment of cancer cachexia: Sharing Progress in Cancer Care Task Force Position Paper and Call to Action. Crit. Rev. Oncol./Hematol. 2023, 185, 103965. [Google Scholar] [CrossRef]
- Cao, Z.; Zhao, K.; Jose, I.; Hoogenraad, N.J.; Osellame, L.D. Biomarkers for Cancer Cachexia: A Mini Review. Int. J. Mol. Sci. 2021, 22, 4501. [Google Scholar] [CrossRef]
- Robinson, T.P.; Hamidi, T.; Counts, B.; Guttridge, D.C.; Ostrowski, M.C.; Zimmers, T.A.; Koniaris, L.G. The impact of inflammation and acute phase activation in cancer cachexia. Front. Immunol. 2023, 14, 1207746. [Google Scholar] [CrossRef]
- Setiawan, T.; Sari, I.N.; Wijaya, Y.T.; Julianto, N.M.; Muhammad, J.A.; Lee, H.; Chae, J.H.; Kwon, H.Y. Cancer cachexia: Molecular mechanisms and treatment strategies. J. Hematol. Oncol. 2023, 16, 54. [Google Scholar] [CrossRef]
- Homa-Mlak, I.; Pigoń-Zając, D.; Wawrejko, P.; Małecka-Massalska, T.; Mlak, R. Three Pathways of Cancer Cachexia: Inflammation, Changes in Adipose Tissue and Loss of Muscle Mass—The Role of miRNAs. J. Pers. Med. 2022, 12, 1438. [Google Scholar] [CrossRef]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Solheim, T.S.; Laird, B.J.A.; Balstad, T.R.; Stene, G.B.; Bye, A.; Johns, N.; Pettersen, C.H.; Fallon, M.; Fayers, P.; Fearon, K.; et al. A randomized phase II feasibility trial of a multimodal intervention for the management of cachexia in lung and pancreatic cancer. J. Cachexia Sarcopenia Muscle 2017, 8, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Allan, J.; Buss, L.A.; Draper, N.; Currie, M.J. Exercise in People With Cancer: A Spotlight on Energy Regulation and Cachexia. Front. Physiol. 2022, 13, 836804. [Google Scholar] [CrossRef]
- Campbell, K.L.; Winters-Stone, K.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.; Matthews, C.; Ligibel, J.; Gerber, L.; et al. Exercise Guidelines for Cancer Survivors: Consensus statement from International Multidisciplinary Roundtable. Med. Sci. Sports Exerc. 2019, 51, 2375–2390. [Google Scholar] [CrossRef]
- Abo, S.; Ritchie, D.; Denehy, L.; Panek-Hudson, Y.; Irving, L.; Granger, C.L. A hospital and home-based exercise program to address functional decline in people following allogeneic stem cell transplantation. Support. Care Cancer 2018, 26, 1727–1736. [Google Scholar] [CrossRef]
- Alves, C.R.R.; das Neves, W.; Tobias, G.C.; de Almeida, N.R.; Barreto, R.F.; Melo, C.M.; Carneiro, C.d.G.; Garcez, A.T.; Faria, D.d.P.; Chammas, R.; et al. High-intensity interval training slows down tumor progression in mice bearing Lewis lung carcinoma. JCSM Rapid Commun. 2018, 1, 1–10. [Google Scholar] [CrossRef]
- Tsitkanou, S.; Murach, K.A.; Washington, T.A.; Greene, N.P. Exercise Counteracts the Deleterious Effects of Cancer Cachexia. Cancers 2022, 14, 2512. [Google Scholar] [CrossRef]
- Parry, T.L.; Tichy, L.; Brantley, J.T. Cardioprotective effects of preconditioning exercise in the female tumor bearing mouse. Front. Cell Dev. Biol. 2022, 10, 950479. [Google Scholar] [CrossRef]
- Ballarò, R.; Penna, F.; Pin, F.; Gómez-Cabrera, M.C.; Viña, J.; Costelli, P. Moderate Exercise Improves Experimental Cancer Cachexia by Modulating the Redox Homeostasis. Cancers 2019, 11, 285. [Google Scholar] [CrossRef] [PubMed]
- Fuller, K.N.Z.; Thyfault, J.P. Barriers in translating preclinical rodent exercise metabolism findings to human health. J. Appl. Physiol. 2021, 130, 182–192. [Google Scholar] [CrossRef]
- Bonetto, A.; Rupert, J.E.; Barreto, R.; Zimmers, T.A. The Colon-26 Carcinoma Tumor-bearing Mouse as a Model for the Study of Cancer Cachexia. J. Vis. Exp. 2016, 117, 54893. [Google Scholar] [CrossRef]
- Parry, T.L.; Hayward, R. Exercise Protects against Cancer-induced Cardiac Cachexia. Med. Sci. Sports Exerc. 2018, 50, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Owendoff, G.; Ray, A.; Bobbili, P.; Clark, L.; Baumann, C.W.; Clark, B.C.; Arnold, W.D. Optimization and construct validity of approaches to preclinical grip strength testing. J. Cachexia Sarcopenia Muscle 2023, 14, 2439–2445. [Google Scholar] [CrossRef] [PubMed]
- Tie, L.; Xiao, H.; Wu, D.; Yang, Y.; Wang, P. A brief guide to good practices in pharmacological experiments: Western blotting. Acta Pharmacol. Sin. 2021, 42, 1015–1017. [Google Scholar] [CrossRef]
- Montesi, L.; Moscatiello, S.; Malavolti, M.; Marzocchi, R.; Marchesini, G. Physical activity for the prevention and treatment of metabolic disorders. Intern. Emerg. Med. 2013, 8, 655–666. [Google Scholar] [CrossRef]
- Belanger, M.J.; Rao, P.; Robbins, J.M. Exercise, Physical Activity and Cardiometabolic Health: Pathophysiologic Insights. Cardiol. Rev. 2022, 30, 134–144. [Google Scholar] [CrossRef]
- Esteves, J.V.; Stanford, K.I. Exercise as a tool to mitigate metabolic disease. Am. J. Physiol.-Cell Physiol. 2024, 327, C587–C598. [Google Scholar] [CrossRef] [PubMed]
- Bayly, J.; Wilcock, A.; Higginson, I.J.; Maddocks, M. Early Engagement in Physical Activity and Exercise Is Key in Managing Cancer Cachexia. Oncology 2017, 31, 38–39. [Google Scholar]
- López, C.O.; Estéfane, J.L.; Rosales, M.R.; Ramirez, C.V.; Arriagada, C.M.; Argilés, J.M.; López-Soriano, F.J.; González, F.O.; Yañez, N.; Busquets, S. Prevalence of Cachexia in Cancer Patients. Eur. J. Cancer Care 2023, 5743872. [Google Scholar] [CrossRef]
- Baumfalk, D.R.; Opoku-Acheampong, A.B.; Caldwell, J.T.; Butenas, A.L.E.; Horn, A.G.; Kunkel, O.N.; Copp, S.W.; Ade, C.J.; Musch, T.I.; Behnke, B.J. Effects of high-intensity training on prostate cancer-induced cardiac atrophy. Am. J. Transl. Res. 2021, 13, 197–209. [Google Scholar]
- Nezamdoost, Z.; Saghebjoo, M.; Hoshyar, R.; Hedayati, M.; Keska, A. High-Intensity Training and Saffron: Effects on Breast Cancer-related Gene Expression. Med. Sci. Sports Exerc. 2020, 52, 1470–1476. [Google Scholar] [CrossRef]
- Goh, J.; Ladiges, W. Voluntary Wheel Running in Mice. Curr. Protoc. Mouse Biol. 2015, 5, 283–290. [Google Scholar] [CrossRef]
- Goldschmidt, S.; Schmidt, M.E.; Rosenberger, F.; Wiskemann, J.; Steindorf, K. Patterns and influencing factors of exercise attendance of breast cancer patients during neoadjuvant chemotherapy. Support. Care Cancer 2024, 32, 79. [Google Scholar] [CrossRef]
- Allen, D.L.; Harrison, B.C.; Maass, A.; Bell, M.L.; Byrnes, W.C.; Leinwand, L.A. Cardiac and skeletal muscle adaptations to voluntary wheel running in the mouse. J. Appl. Physiol. 2001, 90, 1900–1908. [Google Scholar] [CrossRef] [PubMed]
- Wood, N.R.; Garritson, J.; Mathias, A.; Haughian, J.M.; Hayward, R. Moderate Intensity Endurance and Resistance Exercise Attenuates Cachexia in Tumor-bearing Mice. Anticancer Res. 2022, 42, 397–405. [Google Scholar] [CrossRef]
- Hojman, P.; Stagaard, R.; Adachi-Fernandez, E.; Deshmukh, A.S.; Mund, A.; Olsen, C.H.; Keller, L.; Pedersen, B.K.; Gehl, J. Exercise suppresses tumor growth independent of high fat food intake and associated immune dysfunction. Sci. Rep. 2022, 12, 5476. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.; Idorn, M.; Olofsson, G.H.; Lauenborg, B.; Nookaew, I.; Hansen, R.H.; Johannesen, H.H.; Becker, J.C.; Pedersen, K.S.; Dethlefsen, C.; et al. Voluntary Running Suppresses Tumor Growth through Epinephrine- and IL-6-Dependent NK Cell Mobilization and Redistribution. Cell Metab. 2016, 23, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Jee, H.; Park, E.; Hur, K.; Kang, M.; Kim, Y. High-Intensity Aerobic Exercise Suppresses Cancer Growth by Regulating Skeletal Muscle-Derived Oncogenes and Tumor Suppressors. Front. Mol. Biosci. 2022, 9, 818470. [Google Scholar] [CrossRef]
- Daou, H.N. Exercise as an anti-inflammatory therapy for cancer cachexia: A focus on interleukin-6 regulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 318, R296–R310. [Google Scholar] [CrossRef]
- Hojman, P.; Gehl, J.; Christensen, J.F.; Pedersen, B.K. Molecular Mechanisms Linking Exercise to Cancer Prevention and Treatment. Cell Metab. 2018, 27, 10–21. [Google Scholar] [CrossRef]
- Peris-Moreno, D.; Taillandier, D.; Polge, C. MuRF1/TRIM63, Master Regulator of Muscle Mass. Int. J. Mol. Sci. 2020, 21, 6663. [Google Scholar] [CrossRef]
- Gumucio, J.P.; Mendias, C.L. Atrogin-1, MuRF-1, and sarcopenia. Endocrine 2013, 43, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Han, J.; Meng, Q.; Xi, Q.; Zhuang, Q.; Jiang, Y.; Han, Y.; Zhang, B.; Fang, J.; Wu, G. Muscle-specific E3 ubiquitin ligases are involved in muscle atrophy of cancer cachexia: An in vitro and in vivo study. Oncol. Rep. 2015, 33, 2261–2268. [Google Scholar] [CrossRef] [PubMed]
- Neyroud, D.; Laitano, O.; Dasgupta, A.; Lopez, C.; Schmitt, R.E.; Schneider, J.Z.; Hammers, D.W.; Sweeney, H.L.; Walter, G.A.; Doles, J.; et al. Blocking muscle wasting via deletion of the muscle-specific E3 ligase MuRF1 impedes pancreatic tumor growth. Commun. Biol. 2023, 6, 519. [Google Scholar] [CrossRef]
- Yang, L.; Morielli, A.R.; Heer, E.; Kirkham, A.A.; Cheung, W.Y.; Usmani, N.; Friedenreich, C.M.; Courneya, K.S. Effects of Exercise on Cancer Treatment Efficacy: A Systematic Review of Preclinical and Clinical Studies. Cancer Res. 2021, 81, 4889–4895. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.F.; Simonsen, C.; Hojman, P. Exercise Training in Cancer Control and Treatment. Compr. Physiol. 2018, 9, 165–205. [Google Scholar] [CrossRef]
- Fernandes, L.G.; Tobias, G.C.; Paixão, A.O.; Dourado, P.M.; Voltarelli, V.A.; Brum, P.C. Exercise training delays cardiac remodeling in a mouse model of cancer cachexia. Life Sci. 2020, 260, 118392. [Google Scholar] [CrossRef]
- Karekar, P.; Jensen, H.N.; Russart, K.L.G.; Ponnalagu, D.; Seeley, S.; Sanghvi, S.; Smith, S.A.; Pyter, L.M.; Singh, H.; Gururaja Rao, S. Tumor-Induced Cardiac Dysfunction: A Potential Role of ROS. Antioxidants 2021, 10, 1299. [Google Scholar] [CrossRef]
- Willis, M.S.; Rojas, M.; Li, L.; Selzman, C.H.; Tang, R.-H.; Stansfield, W.E.; Rodriguez, J.E.; Glass, D.J.; Patterson, C. Muscle ring finger 1 mediates cardiac atrophy in vivo. Am. J. Physiol.-Heart Circ. Physiol. 2009, 296, H997–H1006. [Google Scholar] [CrossRef]
- Li, H.-H.; Kedar, V.; Zhang, C.; McDonough, H.; Arya, R.; Wang, D.-Z.; Patterson, C. Atrogin-1/muscle atrophy F-box inhibits calcineurin-dependent cardiac hypertrophy by participating in an SCF ubiquitin ligase complex. J. Clin. Investig. 2004, 114, 1058–1071. [Google Scholar] [CrossRef]
- Zaglia, T.; Milan, G.; Ruhs, A.; Franzoso, M.; Bertaggia, E.; Pianca, N.; Carpi, A.; Carullo, P.; Pesce, P.; Sacerdoti, D.; et al. Atrogin-1 deficiency promotes cardiomyopathy and premature death via impaired autophagy. J. Clin. Investig. 2014, 124, 2410–2424. [Google Scholar] [CrossRef]
- Ahmed, D.S.; Isnard, S.; Lin, J.; Routy, B.; Routy, J.-P. GDF15/GFRAL Pathway as a Metabolic Signature for Cachexia in Patients with Cancer. J. Cancer 2021, 12, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, B.; Chen, X.; Hirenallur-Shanthappa, D.; Zhao, Y.; Stansfield, J.C.; Zhang, B.B.; Sheikh, A.; Wu, Z. Neutralization of GDF15 Prevents Anorexia and Weight Loss in the Monocrotaline-Induced Cardiac Cachexia Rat Model. Cells 2022, 11, 1073. [Google Scholar] [CrossRef]
- Kim-Muller, J.Y.; Song, L.; LaCarubba Paulhus, B.; Pashos, E.; Li, X.; Rinaldi, A.; Joaquim, S.; Stansfield, J.C.; Zhang, J.; Robertson, A.; et al. GDF15 neutralization restores muscle function and physical performance in a mouse model of cancer cachexia. Cell Rep. 2023, 42, 111947. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, W.; Gu, X.; Miao, C.; Feng, L.; Shen, Q.; Liu, X.; Zhang, X. GDF-15 in tumor-derived exosomes promotes muscle atrophy via Bcl-2/caspase-3 pathway. Cell Death Discov. 2022, 8, 162. [Google Scholar] [CrossRef]
- Crawford, J.; Lubaczewski, S.L.; Tarachandani, A.; Harrington, M.A.; Weng, Y.; Qiu, R.; Collins, S.M.; Rossulek, M.I.; Revkin, J.H. Phase 2 study to assess the efficacy, safety, and tolerability of the GDF-15 inhibitor ponsegromab in patients with cancer cachexia. J. Clin. Oncol. 2023, 41, TPS12147. [Google Scholar] [CrossRef]
- Winter, L.-M.; Reinhardt, D.; Schatter, A.; Tissen, V.; Wiora, H.; Gerlach, D.; Tontsch-Grunt, U.; Colbatzky, F.; Stierstorfer, B.; Yun, S.-W. Molecular basis of GDF15 induction and suppression by drugs in cardiomyocytes and cancer cells toward precision medicine. Sci. Rep. 2023, 13, 12061. [Google Scholar] [CrossRef] [PubMed]
- Ling, T.; Zhang, J.; Ding, F.; Ma, L. Role of growth differentiation factor 15 in cancer cachexia (Review). Oncol. Lett. 2023, 26, 462. [Google Scholar] [CrossRef] [PubMed]
- Lerner, L.; Tao, J.; Liu, Q.; Nicoletti, R.; Feng, B.; Krieger, B.; Mazsa, E.; Siddiquee, Z.; Wang, R.; Huang, L.; et al. MAP3K11/GDF15 axis is a critical driver of cancer cachexia. J. Cachexia Sarcopenia Muscle 2016, 7, 467–482. [Google Scholar] [CrossRef]
- Zimmers, T.A.; Jiang, Y.; Wang, M.; Liang, T.W.; Rupert, J.E.; Au, E.D.; Marino, F.E.; Couch, M.E.; Koniaris, L.G. Exogenous GDF11 Induces Cardiac and Skeletal Muscle Dysfunction and Wasting. Basic Res. Cardiol. 2017, 112, 48. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tichy, L.; Allred, K.F.; Rezeli, E.T.; Coleman, M.F.; Allred, C.D.; Hursting, S.D.; Parry, T.L. Concurrent Physical Activity Protects Against C26 Adenocarcinoma Tumor-Mediated Cardiac and Skeletal Muscle Dysfunction and Wasting in Males. Cells 2025, 14, 924. https://doi.org/10.3390/cells14120924
Tichy L, Allred KF, Rezeli ET, Coleman MF, Allred CD, Hursting SD, Parry TL. Concurrent Physical Activity Protects Against C26 Adenocarcinoma Tumor-Mediated Cardiac and Skeletal Muscle Dysfunction and Wasting in Males. Cells. 2025; 14(12):924. https://doi.org/10.3390/cells14120924
Chicago/Turabian StyleTichy, Louisa, Kimberly F. Allred, Erika T. Rezeli, Michael F. Coleman, Clinton D. Allred, Stephen D. Hursting, and Traci L. Parry. 2025. "Concurrent Physical Activity Protects Against C26 Adenocarcinoma Tumor-Mediated Cardiac and Skeletal Muscle Dysfunction and Wasting in Males" Cells 14, no. 12: 924. https://doi.org/10.3390/cells14120924
APA StyleTichy, L., Allred, K. F., Rezeli, E. T., Coleman, M. F., Allred, C. D., Hursting, S. D., & Parry, T. L. (2025). Concurrent Physical Activity Protects Against C26 Adenocarcinoma Tumor-Mediated Cardiac and Skeletal Muscle Dysfunction and Wasting in Males. Cells, 14(12), 924. https://doi.org/10.3390/cells14120924





