SHROOM3 Deficiency Aggravates Adriamycin-Induced Nephropathy Accompanied by Focal Adhesion Disassembly and Stress Fiber Disorganization
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Analysis Methods
2.2. Animal Models of ADR-Induced Nephropathy
2.3. Podocyte-Specific Shroom3 Knockout Mice
2.4. Proteinuria and Renal Function Assessment
2.5. Histology and Immunostaining
2.6. Isolation of Glomeruli and Tubules of Kidney Cortex
2.7. Cell Culture and Treatment
2.8. Quantification of Podocyte Size, Adhesion and Apoptosis
2.9. Calpain Activity Assay
2.10. RhoA Activity Assay
2.11. Real-Time Quantitative PCR (RT-qPCR)
2.12. Immunofluorescence Analysis
2.13. Western Blot Analysis
2.14. RNA Sequencing and Analysis
2.15. Statistical Analyses
3. Results
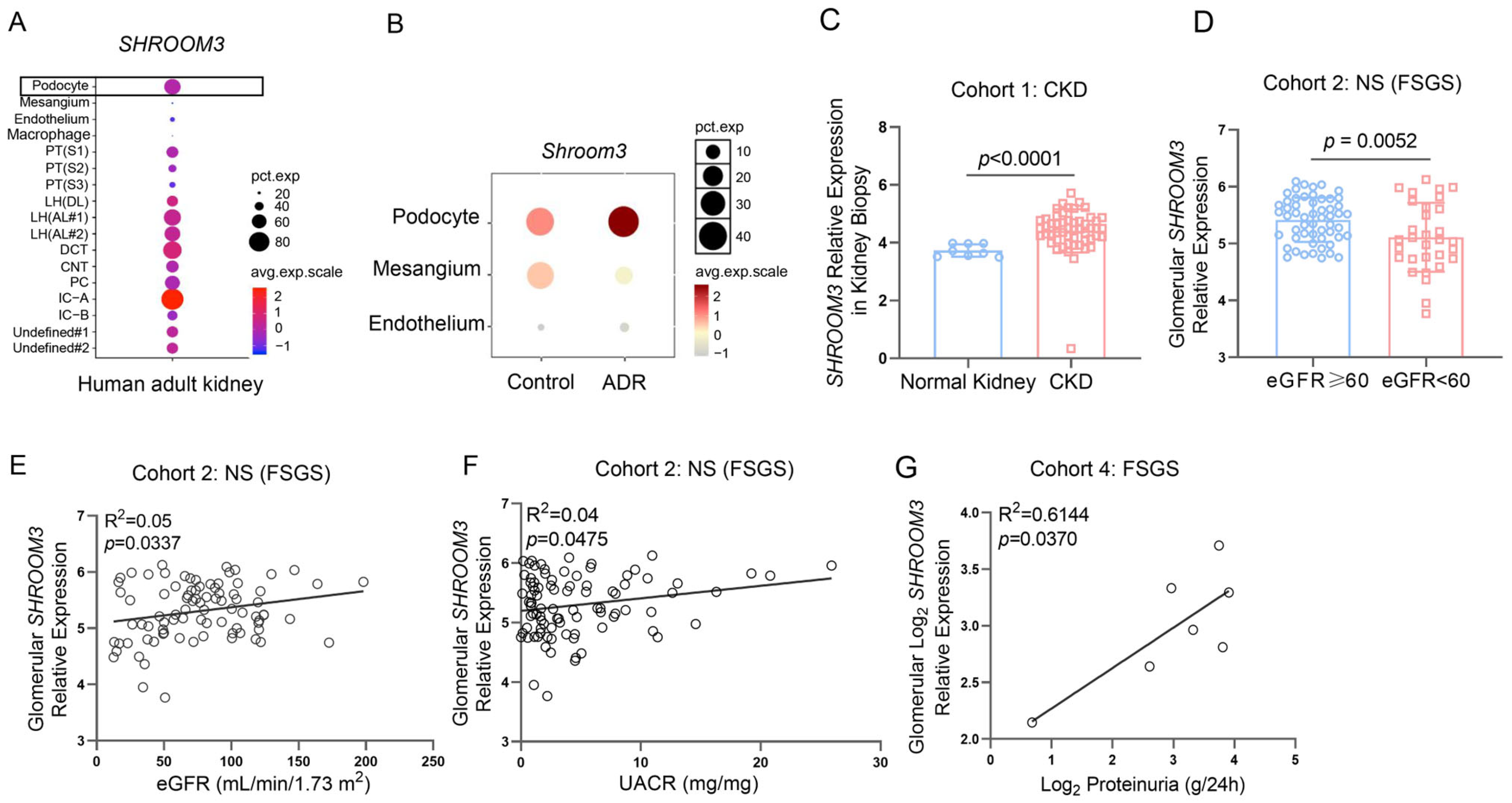
3.1. Dynamic Regulation and Clinical Relevance of SHROOM3 Expression in Proteinuric Kidney Diseases
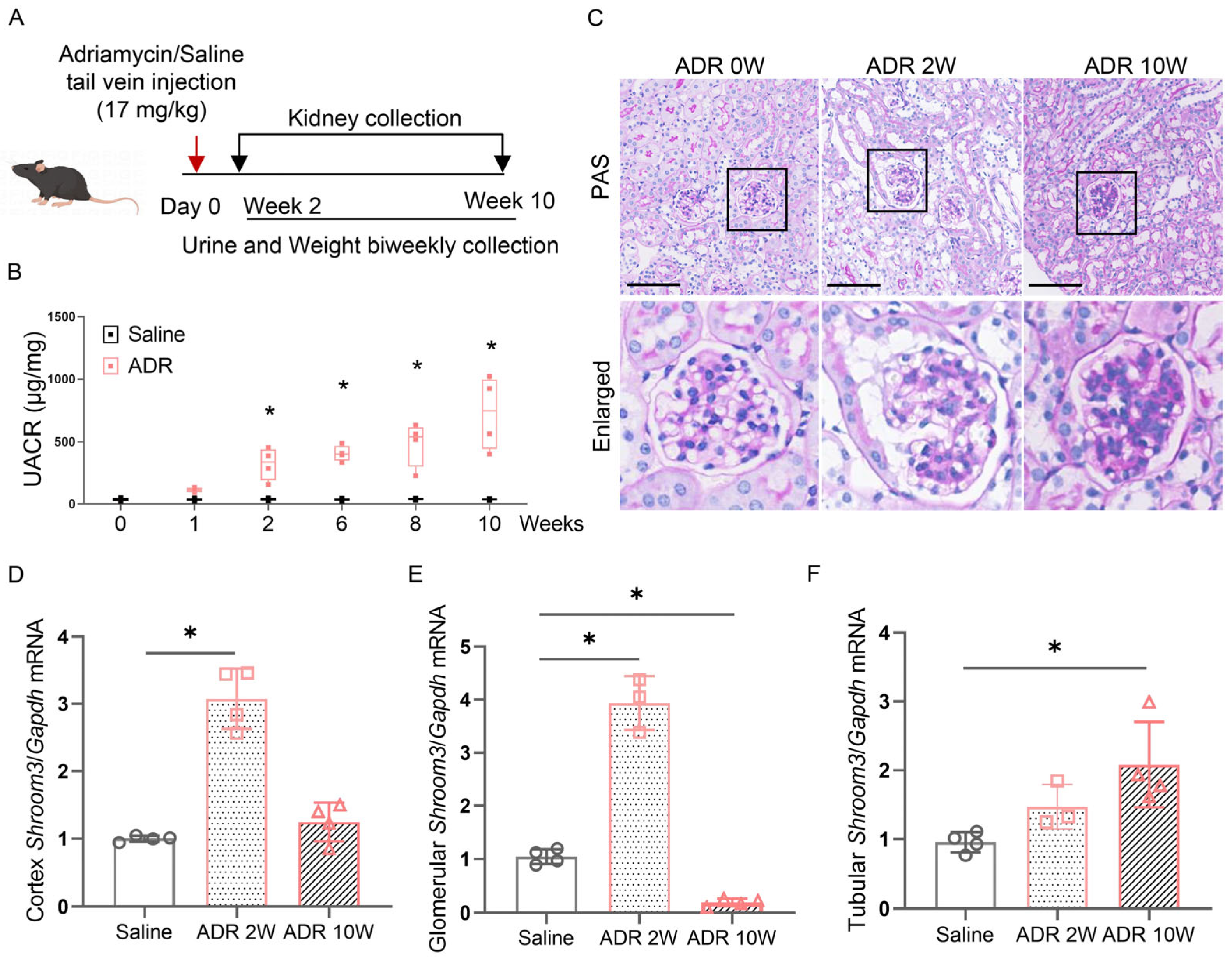
3.2. Induction of Shroom3 Expression in Podocytes Following Glomerular Injury
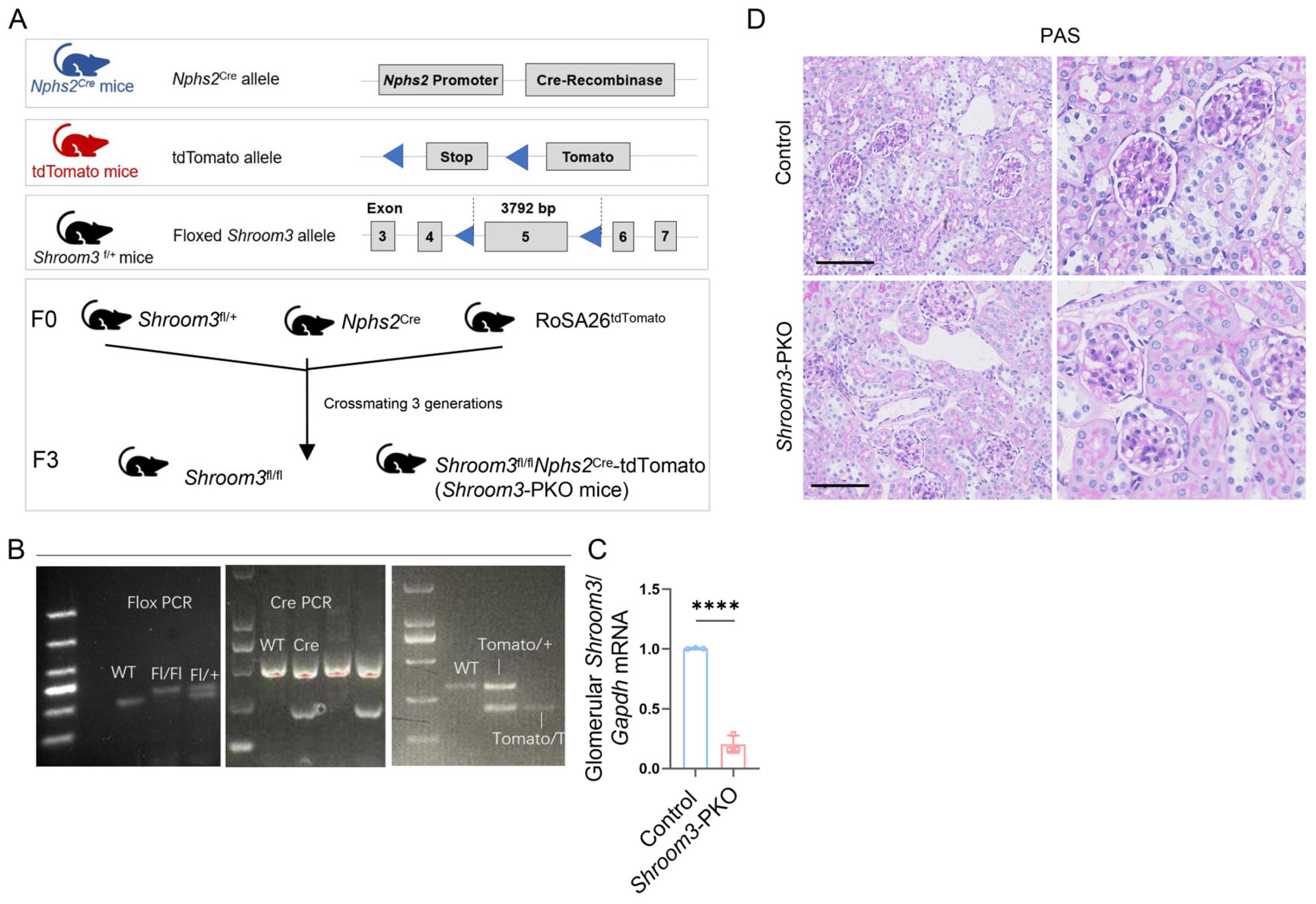
3.3. Establishment of Podocyte-Specific Ablation of Shroom3 Mouse Model
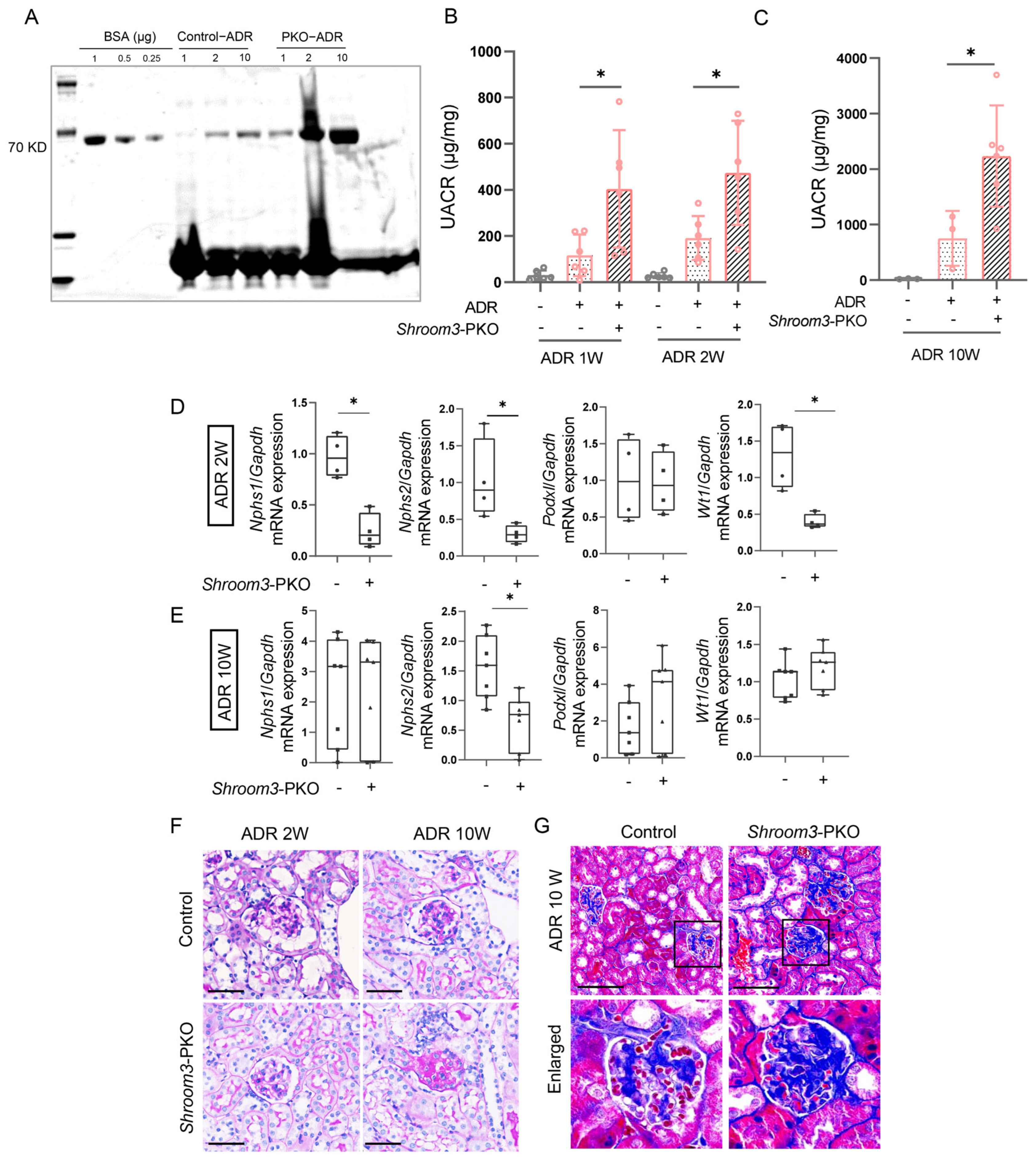
3.4. Podocyte-Specific Ablation of Shroom3 Aggravates ADR-Induced Nephropathy
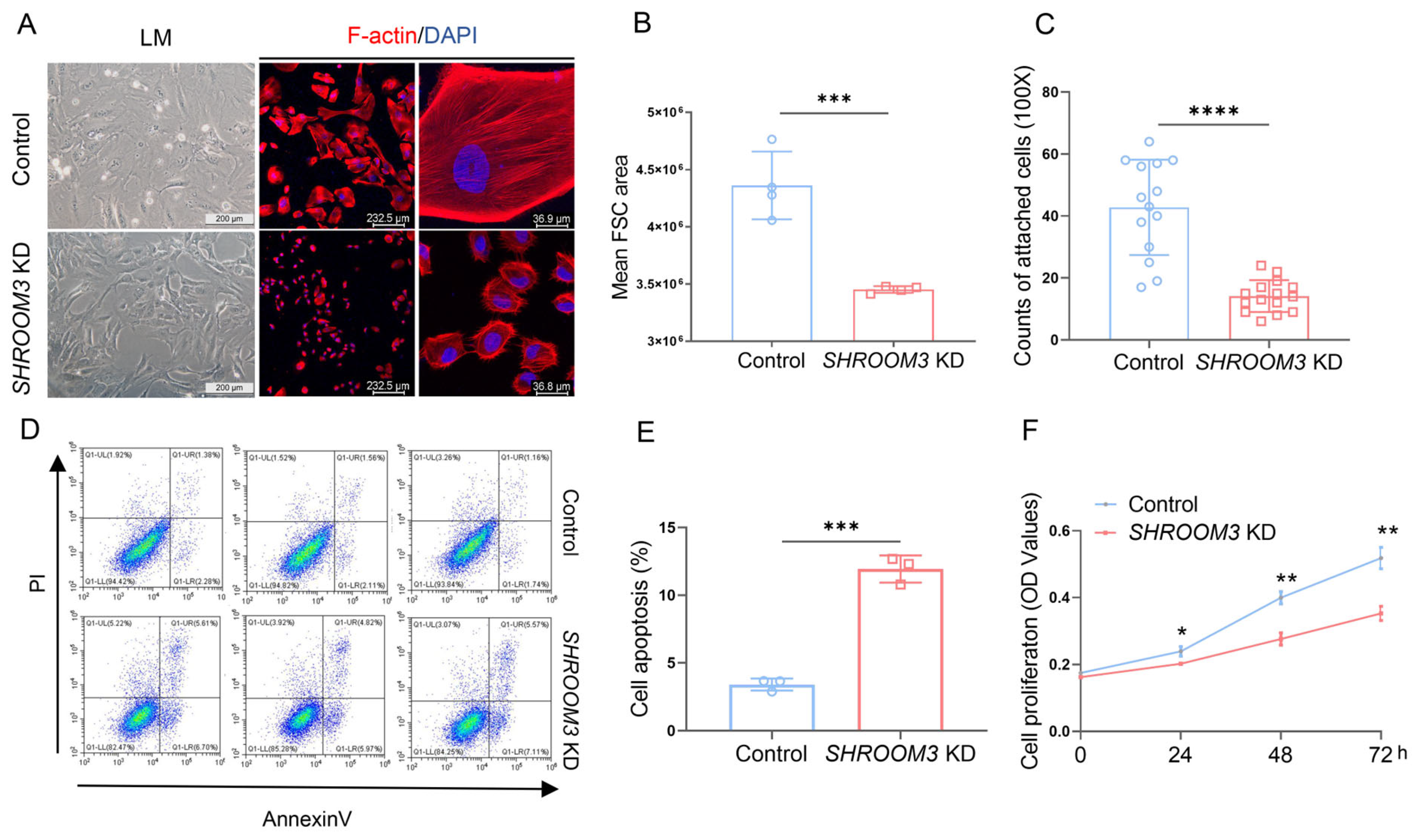
3.5. SHROOM3 Deficiency Impairs Podocyte Morphology and Function In Vitro
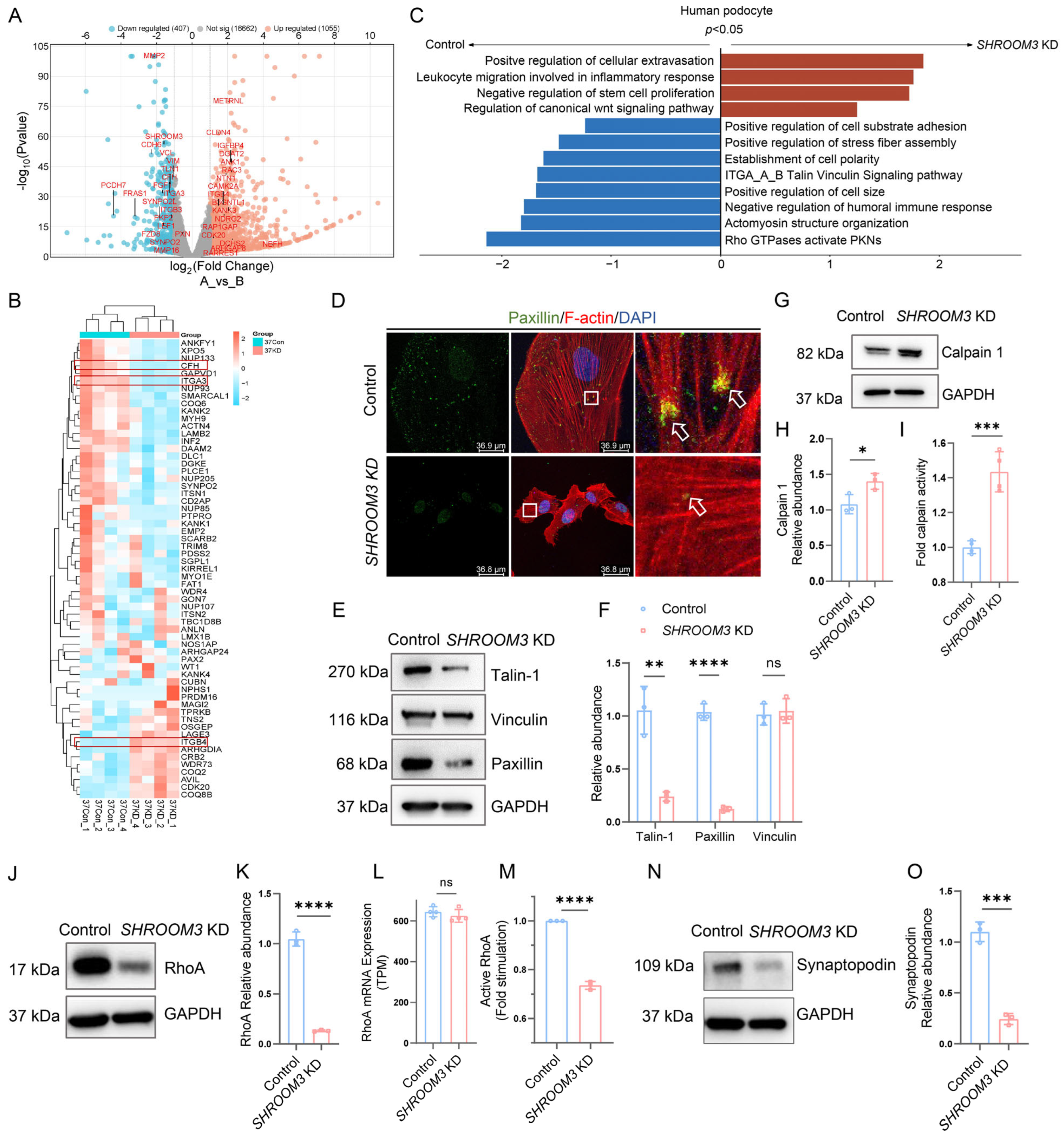
3.6. mRNA Profiling Analysis Reveals Calpain Activation in SHROOM3-Deficient Podocytes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADR | adriamycin |
| CKD | chronic kidney disease |
| eGFR | estimated glomerular filtration rate |
| FSGS | focal segmental glomerulosclerosis |
| KD | knockdown |
| KIT | kidney interactive transcript |
| MCD | minimal change disease |
| NS | nephrotic syndrome |
| PAS | periodic acid–Schiff |
| PKO | podocyte-specific knockout |
| UACR | urine-albumin-to-creatinine ratio |
References
- Noone, D.G.; Iijima, K.; Parekh, R. Idiopathic nephrotic syndrome in children. Lancet 2018, 392, 61–74. [Google Scholar] [CrossRef]
- Perico, L.; Conti, S.; Benigni, A.; Remuzzi, G. Podocyte-actin dynamics in health and disease. Nat. Rev. Nephrol. 2016, 12, 692–710. [Google Scholar] [CrossRef]
- Hildebrand, J.D.; Soriano, P. Shroom, a PDZ domain-containing actin-binding protein, is required for neural tube morphogenesis in mice. Cell 1999, 99, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, J.D. Shroom regulates epithelial cell shape via the apical positioning of an actomyosin network. J. Cell Sci. 2005, 118, 5191–5203. [Google Scholar] [CrossRef]
- Nishimura, T.; Takeichi, M. Shroom3-mediated recruitment of Rho kinases to the apical cell junctions regulates epithelial and neuroepithelial planar remodeling. Development 2008, 135, 1493–1502. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Scherr, H.M.; Wallingford, J.B. Shroom family proteins regulate gamma-tubulin distribution and microtubule architecture during epithelial cell shape change. Development 2007, 134, 1431–1441. [Google Scholar] [CrossRef] [PubMed]
- Khalili, H.; Sull, A.; Sarin, S.; Boivin, F.J.; Halabi, R.; Svajger, B.; Li, A.; Cui, V.W.; Drysdale, T.; Bridgewater, D. Developmental Origins for Kidney Disease Due to Shroom3 Deficiency. J. Am. Soc. Nephrol. 2016, 27, 2965–2973. [Google Scholar] [CrossRef]
- Ellis, J.W.; Chen, M.H.; Foster, M.C.; Liu, C.T.; Larson, M.G.; de Boer, I.; Kottgen, A.; Parsa, A.; Bochud, M.; Boger, C.A.; et al. Validated SNPs for eGFR and their associations with albuminuria. Hum. Mol. Genet. 2012, 21, 3293–3298. [Google Scholar] [CrossRef]
- Gorski, M.; Tin, A.; Garnaas, M.; McMahon, G.M.; Chu, A.Y.; Tayo, B.O.; Pattaro, C.; Teumer, A.; Chasman, D.I.; Chalmers, J.; et al. Genome-wide association study of kidney function decline in individuals of European descent. Kidney Int. 2015, 87, 1017–1029. [Google Scholar] [CrossRef]
- Menon, M.C.; Chuang, P.Y.; Li, Z.; Wei, C.; Zhang, W.; Luan, Y.; Yi, Z.; Xiong, H.; Woytovich, C.; Greene, I.; et al. Intronic locus determines SHROOM3 expression and potentiates renal allograft fibrosis. J. Clin. Investig. 2015, 125, 208–221. [Google Scholar] [CrossRef]
- Paul, A.; Lawlor, A.; Cunanan, K.; Gaheer, P.S.; Kalra, A.; Napoleone, M.; Lanktree, M.B.; Bridgewater, D. The Good and the Bad of SHROOM3 in Kidney Development and Disease: A Narrative Review. Can. J. Kidney Health Dis. 2023, 10, 20543581231212038. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Uchimura, K.; Donnelly, E.L.; Kirita, Y.; Morris, S.A.; Humphreys, B.D. Comparative Analysis and Refinement of Human PSC-Derived Kidney Organoid Differentiation with Single-Cell Transcriptomics. Cell Stem Cell 2018, 23, 869–881.e8. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Kirita, Y.; Donnelly, E.L.; Humphreys, B.D. Advantages of Single-Nucleus over Single-Cell RNA Sequencing of Adult Kidney: Rare Cell Types and Novel Cell States Revealed in Fibrosis. J. Am. Soc. Nephrol. 2019, 30, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, S.; Nishihara, K.; Miyata, H.; Shinke, H.; Tomita, E.; Kajiwara, M.; Matsubara, T.; Iehara, N.; Igarashi, Y.; Yamada, H.; et al. Molecular Markers of Tubulointerstitial Fibrosis and Tubular Cell Damage in Patients with Chronic Kidney Disease. PLoS ONE 2015, 10, e0136994. [Google Scholar] [CrossRef]
- Mariani, L.H.; Eddy, S.; AlAkwaa, F.M.; McCown, P.J.; Harder, J.L.; Nair, V.; Eichinger, F.; Martini, S.; Ademola, A.D.; Boima, V.; et al. Precision nephrology identified tumor necrosis factor activation variability in minimal change disease and focal segmental glomerulosclerosis. Kidney Int. 2023, 103, 565–579. [Google Scholar] [CrossRef]
- Hodgin, J.B.; Nair, V.; Zhang, H.; Randolph, A.; Harris, R.C.; Nelson, R.G.; Weil, E.J.; Cavalcoli, J.D.; Patel, J.M.; Brosius, F.C., 3rd; et al. Identification of cross-species shared transcriptional networks of diabetic nephropathy in human and mouse glomeruli. Diabetes 2013, 62, 299–308. [Google Scholar] [CrossRef]
- Chung, J.J.; Goldstein, L.; Chen, Y.J.; Lee, J.; Webster, J.D.; Roose-Girma, M.; Paudyal, S.C.; Modrusan, Z.; Dey, A.; Shaw, A.S. Single-Cell Transcriptome Profiling of the Kidney Glomerulus Identifies Key Cell Types and Reactions to Injury. J. Am. Soc. Nephrol. 2020, 31, 2341–2354. [Google Scholar] [CrossRef]
- Li, F.; Fang, Y.; Zhuang, Q.; Cheng, M.; Moronge, D.; Jue, H.; Meyuhas, O.; Ding, X.; Zhang, Z.; Chen, J.K.; et al. Blocking ribosomal protein S6 phosphorylation inhibits podocyte hypertrophy and focal segmental glomerulosclerosis. Kidney Int. 2022, 102, 121–135. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Lykke-Andersen, S.; Jensen, T.H. Nonsense-mediated mRNA decay: An intricate machinery that shapes transcriptomes. Nat. Rev. Mol. Cell Biol. 2015, 16, 665–677. [Google Scholar] [CrossRef]
- Wang, H.; Sheng, J.; He, H.; Chen, X.; Li, J.; Tan, R.; Wang, L.; Lan, H.Y. A simple and highly purified method for isolation of glomeruli from the mouse kidney. Am. J. Physiol.-Ren. Physiol. 2019, 317, F1217–F1223. [Google Scholar] [CrossRef] [PubMed]
- Frank, C.N.; Hou, X.; Petrosyan, A.; Villani, V.; Zhao, R.; Hansen, J.R.; Clair, G.; Salem, F.; De Filippo, R.E.; Cravedi, P.; et al. Effect of disease progression on the podocyte cell cycle in Alport Syndrome. Kidney Int. 2022, 101, 106–118. [Google Scholar] [CrossRef]
- Ding, W.Y.; Kuzmuk, V.; Hunter, S.; Lay, A.; Hayes, B.; Beesley, M.; Rollason, R.; Hurcombe, J.A.; Barrington, F.; Masson, C.; et al. Adeno-associated virus gene therapy prevents progression of kidney disease in genetic models of nephrotic syndrome. Sci. Transl. Med. 2023, 15, eabc8226. [Google Scholar] [CrossRef]
- Chen, A.; Feng, Y.; Lai, H.; Ju, W.; Li, Z.; Li, Y.; Wang, A.; Hong, Q.; Zhong, F.; Wei, C.; et al. Soluble RARRES1 induces podocyte apoptosis to promote glomerular disease progression. J. Clin. Investig. 2020, 130, 5523–5535. [Google Scholar] [CrossRef]
- Zipfel, P.F.; Skerka, C. Complement regulators and inhibitory proteins. Nat. Rev. Immunol. 2009, 9, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Asanuma, K.; Yanagida-Asanuma, E.; Faul, C.; Tomino, Y.; Kim, K.; Mundel, P. Synaptopodin orchestrates actin organization and cell motility via regulation of RhoA signalling. Nat. Cell Biol. 2006, 8, 485–491. [Google Scholar] [CrossRef]
- Li, A.; Cunanan, J.; Khalili, H.; Plageman, T.; Ask, K.; Khan, A.; Hunjan, A.; Drysdale, T.; Bridgewater, D. Shroom3, a Gene Associated with CKD, Modulates Epithelial Recovery after AKI. Kidney360 2022, 3, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Moeller, M.J.; Sanden, S.K.; Soofi, A.; Wiggins, R.C.; Holzman, L.B. Podocyte-specific expression of cre recombinase in transgenic mice. Genesis 2003, 35, 39–42. [Google Scholar] [CrossRef]
- Liu, X.; Schnellmann, R.G. Calpain mediates progressive plasma membrane permeability and proteolysis of cytoskeleton-associated paxillin, talin, and vinculin during renal cell death. J. Pharmacol. Exp. Ther. 2003, 304, 63–70. [Google Scholar] [CrossRef]
- Tian, X.; Inoue, K.; Zhang, Y.; Wang, Y.; Sperati, C.J.; Pedigo, C.E.; Zhao, T.; Yan, M.; Groener, M.; Moledina, D.G.; et al. Inhibiting calpain 1 and 2 in cyclin G associated kinase-knockout mice mitigates podocyte injury. JCI Insight 2020, 5, e142740. [Google Scholar] [CrossRef]
- Buvall, L.; Wallentin, H.; Sieber, J.; Andreeva, S.; Choi, H.Y.; Mundel, P.; Greka, A. Synaptopodin Is a Coincidence Detector of Tyrosine versus Serine/Threonine Phosphorylation for the Modulation of Rho Protein Crosstalk in Podocytes. J. Am. Soc. Nephrol. 2017, 28, 837–851. [Google Scholar] [CrossRef] [PubMed]
- Prokop, J.W.; Yeo, N.C.; Ottmann, C.; Chhetri, S.B.; Florus, K.L.; Ross, E.J.; Sosonkina, N.; Link, B.A.; Freedman, B.I.; Coppola, C.J.; et al. Characterization of Coding/Noncoding Variants for SHROOM3 in Patients with CKD. J. Am. Soc. Nephrol. 2018, 29, 1525–1535. [Google Scholar] [CrossRef] [PubMed]
- Kambham, N.; Tanji, N.; Seigle, R.L.; Markowitz, G.S.; Pulkkinen, L.; Uitto, J.; D’Agati, V.D. Congenital focal segmental glomerulosclerosis associated with β4 integrin mutation and epidermolysis bullosa. Am. J. Kidney Dis. 2000, 36, 190–196. [Google Scholar] [CrossRef]
- Nicolaou, N.; Margadant, C.; Kevelam, S.H.; Lilien, M.R.; Oosterveld, M.J.; Kreft, M.; van Eerde, A.M.; Pfundt, R.; Terhal, P.A.; van der Zwaag, B.; et al. Gain of glycosylation in integrin alpha3 causes lung disease and nephrotic syndrome. J. Clin. Investig. 2012, 122, 4375–4387. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.-N.; Sun, Y.-Y.; Tan, Y.-F.; Zhou, X.-Y.; Xiang, T.-C.; Fang, Y.; Li, F.; Shen, Q.; Xu, H.; Rao, J. SHROOM3 Deficiency Aggravates Adriamycin-Induced Nephropathy Accompanied by Focal Adhesion Disassembly and Stress Fiber Disorganization. Cells 2025, 14, 895. https://doi.org/10.3390/cells14120895
Xu L-N, Sun Y-Y, Tan Y-F, Zhou X-Y, Xiang T-C, Fang Y, Li F, Shen Q, Xu H, Rao J. SHROOM3 Deficiency Aggravates Adriamycin-Induced Nephropathy Accompanied by Focal Adhesion Disassembly and Stress Fiber Disorganization. Cells. 2025; 14(12):895. https://doi.org/10.3390/cells14120895
Chicago/Turabian StyleXu, Li-Nan, Ying-Ying Sun, Yan-Feng Tan, Xin-Yue Zhou, Tian-Chao Xiang, Ye Fang, Fei Li, Qian Shen, Hong Xu, and Jia Rao. 2025. "SHROOM3 Deficiency Aggravates Adriamycin-Induced Nephropathy Accompanied by Focal Adhesion Disassembly and Stress Fiber Disorganization" Cells 14, no. 12: 895. https://doi.org/10.3390/cells14120895
APA StyleXu, L.-N., Sun, Y.-Y., Tan, Y.-F., Zhou, X.-Y., Xiang, T.-C., Fang, Y., Li, F., Shen, Q., Xu, H., & Rao, J. (2025). SHROOM3 Deficiency Aggravates Adriamycin-Induced Nephropathy Accompanied by Focal Adhesion Disassembly and Stress Fiber Disorganization. Cells, 14(12), 895. https://doi.org/10.3390/cells14120895







