Mitogen-Activated Protein Kinase Kinase Kinase 1 Overexpression Disrupts Development of the Ocular Surface Epithelium
Abstract
1. Introduction
2. Materials and Methods
2.1. Mouse Strains and Genotyping
2.2. Phenotype Evaluation, Histology, and Immunohistochemistry
2.3. Keratinocyte Culture
2.4. Proliferation and Apoptosis
2.5. Wound Healing Assay
2.6. Western Blot Analyses
2.7. Statistical Analysis
3. Results
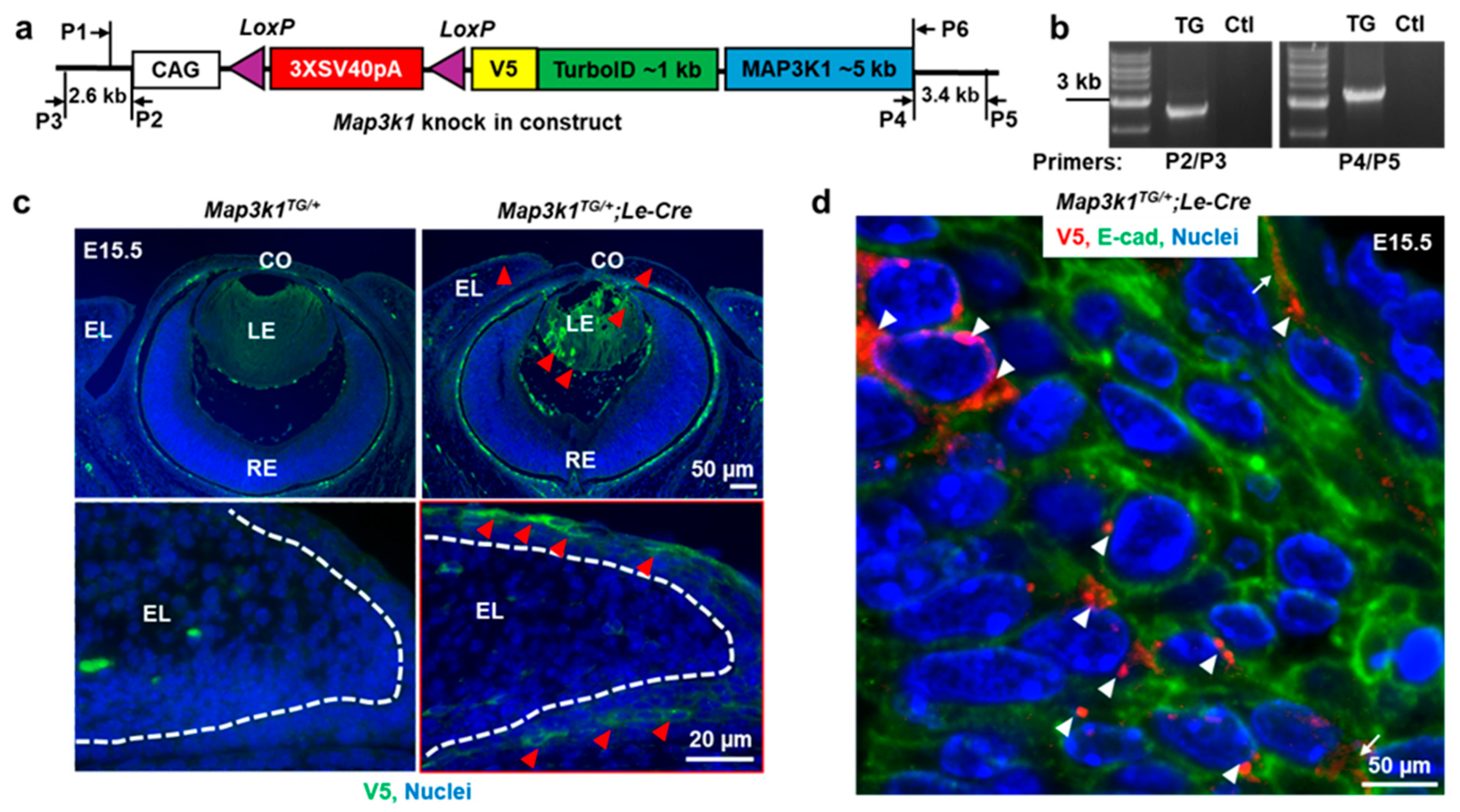
3.1. MAP3K1 Overexpression in OSE: Transgenic Mouse Model Characterization
3.2. MAP3K1 Overexpression Alters Corneal, Lens, and Eyelid Development
3.3. MAP3K1 Expression Level Correlates with Severity of Ocular Defects
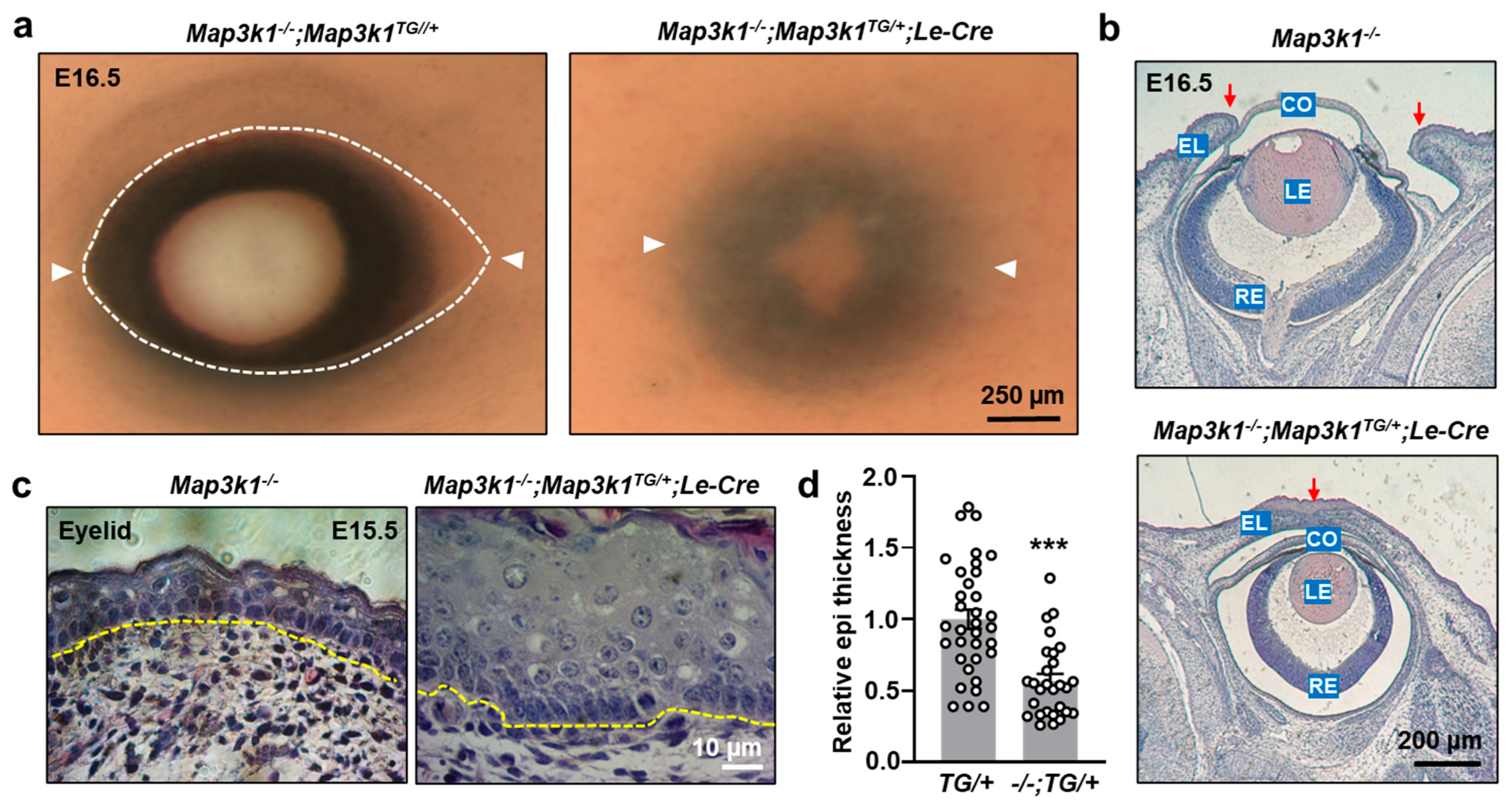
3.4. MAP3K1 Overexpression Rescues the Eye-Open at Birth Phenotype in Knockout Mice
3.5. MAP3K1 Overexpression Activates JNK, WNT, TGFβ, and Notch Signaling Pathways
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karin, M. Mitogen-activated protein kinase cascades as regulators of stress responses. Ann. N. Y. Acad. Sci. 1998, 851, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Craig, E.A.; Stevens, M.V.; Vaillancourt, R.R.; Camenisch, T.D. MAP3Ks as central regulators of cell fate during development. Dev. Dyn. 2008, 237, 3102–3114. [Google Scholar] [CrossRef]
- Uhlik, M.T.; Abell, A.N.; Cuevas, B.D.; Nakamura, K.; Johnson, G.L. Wiring diagrams of MAPK regulation by MEKK1, 2, and 3. Biochem. Cell Biol. 2004, 82, 658–663. [Google Scholar] [CrossRef]
- Suddason, T.; Gallagher, E. A RING to rule them all? Insights into the Map3k1 PHD motif provide a new mechanistic understanding into the diverse roles of Map3k1. Cell Death Differ. 2015, 22, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kimura, E.; Mongan, M.; Xia, Y. Genetic Control of MAP3K1 in Eye Development and Sex Differentiation. Cells 2021, 11, 34. [Google Scholar] [CrossRef]
- Yujiri, T.; Fanger, G.R.; Garrington, T.P.; Schlesinger, T.K.; Gibson, S.; Johnson, G.L. MEK kinase 1 (MEKK1) transduces c-Jun NH2-terminal kinase activation in response to changes in the microtubule cytoskeleton. J. Biol. Chem. 1999, 274, 12605–12610. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, W.; Hayashi, Y.; Jester, J.V.; Birk, D.E.; Gao, M.; Liu, C.Y.; Kao, W.W.; Karin, M.; Xia, Y. A role for MEK kinase 1 in TGF-beta/activin-induced epithelium movement and embryonic eyelid closure. EMBO J. 2003, 22, 4443–4454. [Google Scholar] [CrossRef]
- Juriloff, D.M.; Harris, M.J.; Mah, D.G. The open-eyelid mutation, lidgap-Gates, is an eight-exon deletion in the mouse Map3k1 gene. Genomics 2005, 85, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.; Cross, S.H.; Jackson, I.J.; Hardisty-Hughes, R.; Morse, S.; Nicholson, G.; Coghill, E.; Bowl, M.R.; Brown, S.D. The goya mouse mutant reveals distinct newly identified roles for MAP3K1 in the development and survival of cochlear sensory hair cells. Dis. Model. Mech. 2015, 8, 1555–1568. [Google Scholar]
- Mohamed, Y.H.; Gong, H.; Amemiya, T. Role of apoptosis in eyelid development. Exp. Eye Res. 2003, 76, 115–123. [Google Scholar] [CrossRef]
- Harris, M.J.; Juriloff, D.M. Eyelid development and fusion induced by cortisone treatment in mutant, lidgap-Miller, foetal mice. A scanning electron microscope study. J. Embryol. Exp. Morphol. 1986, 91, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Findlater, G.S.; McDougall, R.D.; Kaufman, M.H. Eyelid development, fusion and subsequent reopening in the mouse. J. Anat. 1993, 183, 121–129. [Google Scholar] [PubMed]
- Teramoto, S.; Fujii, S.; Yoshida, A.; Shirasu, Y. Morphological and genetic characteristics of the open-eyelid mutant spontaneously occurring in NC-strain mice. Jikken Dobutsu 1988, 37, 455–462. [Google Scholar]
- Meng, Q.; Mongan, M.; Carreira, V.; Kurita, H.; Liu, C.Y.; Kao, W.W.; Xia, Y. Eyelid closure in embryogenesis is required for ocular adnexa development. Invest. Ophthalmol. Vis. Sci. 2014, 55, 7652–7661. [Google Scholar] [CrossRef]
- Wang, J.; Call, M.; Mongan, M.; Kao, W.W.; Xia, Y. Meibomian gland morphogenesis requires developmental eyelid closure and lid fusion. Ocul. Surf. 2017, 15, 704–712. [Google Scholar] [CrossRef]
- Yujiri, T.; Ware, M.; Widmann, C.; Oyer, R.; Russell, D.; Chan, E.; Zaitsu, Y.; Clarke, P.; Tyler, K.; Oka, Y.; et al. MEK kinase 1 gene disruption alters cell migration and c-Jun NH2-terminal kinase regulation but does not cause a measurable defect in NF-kappa B activation. Proc. Natl. Acad. Sci. USA 2000, 97, 7272–7277. [Google Scholar] [CrossRef]
- Wang, J.; Xiao, B.; Kimura, E.; Mongan, M.; Xia, Y. The combined effects of Map3k1 mutation and dioxin on differentiation of keratinocytes derived from mouse embryonic stem cells. Sci. Rep. 2022, 12, 11482. [Google Scholar] [CrossRef] [PubMed]
- Heller, E.; Kumar, K.V.; Grill, S.W.; Fuchs, E. Forces generated by cell intercalation tow epidermal sheets in mammalian tissue morphogenesis. Dev. Cell 2014, 28, 617–632. [Google Scholar] [CrossRef]
- Pham, T.T.; Angus, S.P.; Johnson, G.L. MAP3K1: Genomic Alterations in Cancer and Function in Promoting Cell Survival or Apoptosis. Genes Cancer 2013, 4, 419–426. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013, 6, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Glubb, D.M.; Maranian, M.J.; Michailidou, K.; Pooley, K.A.; Meyer, K.B.; Kar, S.; Carlebur, S.; O’Reilly, M.; Betts, J.A.; Hillman, K.M.; et al. Fine-scale mapping of the 5q11.2 breast cancer locus reveals at least three independent risk variants regulating MAP3K1. Am. J. Hum. Genet. 2015, 96, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Kan, Z.; Jaiswal, B.S.; Stinson, J.; Janakiraman, V.; Bhatt, D.; Stern, H.M.; Yue, P.; Haverty, P.M.; Bourgon, R.; Zheng, J.; et al. Diverse somatic mutation patterns and pathway alterations in human cancers. Nature 2010, 466, 869–873. [Google Scholar] [CrossRef]
- Fatemi, N.; Tu, S.J.; Chung, C.C.; Moghadam, P.K.; Mojarad, E.N.; Sadeghi, A.; Totonchi, M.; Aghdaei, H.A.; Chang, J.G. Whole exome sequencing identifies MAP3K1, MSH2, and MLH1 as potential cancer-predisposing genes in familial early-onset colorectal cancer. Kaohsiung J. Med. Sci. 2023, 39, 896–903. [Google Scholar] [CrossRef]
- Xie, N.; Yao, Y.; Wan, L.; Zhu, T.; Liu, L.; Yuan, J. Next-generation sequencing reveals lymph node metastasis associated genetic markers in colorectal cancer. Exp. Ther. Med. 2017, 14, 338–343. [Google Scholar] [CrossRef][Green Version]
- Pearlman, A.; Loke, J.; Le Caignec, C.; White, S.; Chin, L.; Friedman, A.; Warr, N.; Willan, J.; Brauer, D.; Farmer, C.; et al. Mutations in MAP3K1 cause 46,XY disorders of sex development and implicate a common signal transduction pathway in human testis determination. Am. J. Hum. Genet. 2010, 87, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Mongan, M.; Zhang, X.; Xia, Y. Isolation and long-term expansion of murine epidermal stem-like cells. PLoS ONE 2021, 16, e0254731. [Google Scholar] [CrossRef]
- Wang, W.L.; Lai, Y.H.; Huang, C.H.; Lai, J.Y.; Yao, C.H. Lumbrokinase-containing gelatin nanofibers with multiple bioactivities for effective skin wound healing. Mater. Today Bio 2025, 32, 101713. [Google Scholar] [CrossRef]
- Hochedlinger, K.; Yamada, Y.; Beard, C.; Jaenisch, R. Ectopic expression of Oct-4 blocks progenitor-cell differentiation and causes dysplasia in epithelial tissues. Cell 2005, 121, 465–477. [Google Scholar] [CrossRef]
- Fuchs, E.; Green, H. Changes in keratin gene expression during terminal differentiation of the keratinocyte. Cell 1980, 19, 1033–1042. [Google Scholar] [CrossRef]
- Park, S. Building vs. Rebuilding Epidermis: Comparison Embryonic Development and Adult Wound Repair. Front. Cell Dev. Biol. 2021, 9, 796080. [Google Scholar] [CrossRef] [PubMed]
- Ota, T.; Takekoshi, S.; Takagi, T.; Kitatani, K.; Toriumi, K.; Kojima, T.; Kato, M.; Ikoma, N.; Mabuchi, T.; Ozawa, A. Notch signaling may be involved in the abnormal differentiation of epidermal keratinocytes in psoriasis. Acta Histochem. Cytochem. 2014, 47, 175–183. [Google Scholar] [CrossRef]
- Fuchs, E.; Horsley, V. More than one way to skin. Genes Dev. 2008, 22, 976–985. [Google Scholar] [CrossRef]
- Paramio, J.M.; Casanova, M.L.; Segrelles, C.; Mittnacht, S.; Lane, E.B.; Jorcano, J.L. Modulation of cell proliferation by cytokeratins K10 and K16. Mol. Cell Biol. 1999, 19, 3086–3094. [Google Scholar] [CrossRef]
- Santos, M.; Paramio, J.M.; Bravo, A.; Ramirez, A.; Jorcano, J.L. The expression of keratin k10 in the basal layer of the epidermis inhibits cell proliferation and prevents skin tumorigenesis. J. Biol. Chem. 2002, 277, 19122–19130. [Google Scholar] [CrossRef]
- Rossi, A.; Jang, S.I.; Ceci, R.; Steinert, P.M.; Markova, N.G. Effect of AP1 transcription factors on the regulation of transcription in normal human epidermal keratinocytes. J. Investig. Dermatol. 1998, 110, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, F.E.; Dusold, J.E.; Shaik, J.A.; Walsh, H.A.; Glick, A.B. Targeted deletion of TGFbeta1 in basal keratinocytes causes profound defects in stratified squamous epithelia and aberrant melanocyte migration. Dev. Biol. 2022, 485, 9–23. [Google Scholar] [CrossRef]
- Leng, L.; Ma, J.; Lv, L.; Wang, W.; Gao, D.; Zhu, Y.; Wu, Z. Both Wnt signaling and epidermal stem cell-derived extracellular vesicles are involved in epidermal cell growth. Stem Cell Res. Ther. 2020, 11, 415. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.S.; Zhang, Y.; Xu, M.; Yang, Y.; Ito, M.; Peng, T.; Cui, Z.; Nagy, A.; Hadjantonakis, A.K.; Lang, R.A.; et al. Distinct functions for Wnt/beta-catenin in hair follicle stem cell proliferation and survival and interfollicular epidermal homeostasis. Cell Stem Cell 2013, 13, 720–733. [Google Scholar] [CrossRef]
- Mumm, J.S.; Kopan, R. Notch signaling: From the outside in. Dev. Biol. 2000, 228, 151–165. [Google Scholar] [CrossRef]
- Blanpain, C.; Lowry, W.E.; Pasolli, H.A.; Fuchs, E. Canonical notch signaling functions as a commitment switch in the epidermal lineage. Genes Dev. 2006, 20, 3022–3035. [Google Scholar] [CrossRef] [PubMed]
- Zenz, R.; Wagner, E.F. Jun signalling in the epidermis: From developmental defects to psoriasis and skin tumors. Int. J. Biochem. Cell Biol. 2006, 38, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, T.K.; Bonvin, C.; Jarpe, M.B.; Fanger, G.R.; Cardinaux, J.R.; Johnson, G.L.; Widmann, C. Apoptosis stimulated by the 91-kDa caspase cleavage MEKK1 fragment requires translocation to soluble cellular compartments. J. Biol. Chem. 2002, 277, 10283–10291. [Google Scholar] [CrossRef] [PubMed]
- Gibson, E.M.; Henson, E.S.; Villanueva, J.; Gibson, S.B. MEK kinase 1 induces mitochondrial permeability transition leading to apoptosis independent of cytochrome c release. J. Biol. Chem. 2002, 277, 10573–10580. [Google Scholar] [CrossRef]
- Bild, A.H.; Mendoza, F.J.; Gibson, E.M.; Huang, M.; Villanueva, J.; Garrington, T.P.; Jove, R.; Johnson, G.L.; Gibson, S.B. MEKK1-induced apoptosis requires TRAIL death receptor activation and is inhibited by AKT/PKB through inhibition of MEKK1 cleavage. Oncogene 2002, 21, 6649–6656. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mongan, M.; Xiao, B.; Christianto, A.; Hu, Y.-C.; Xia, Y. Mitogen-Activated Protein Kinase Kinase Kinase 1 Overexpression Disrupts Development of the Ocular Surface Epithelium. Cells 2025, 14, 894. https://doi.org/10.3390/cells14120894
Mongan M, Xiao B, Christianto A, Hu Y-C, Xia Y. Mitogen-Activated Protein Kinase Kinase Kinase 1 Overexpression Disrupts Development of the Ocular Surface Epithelium. Cells. 2025; 14(12):894. https://doi.org/10.3390/cells14120894
Chicago/Turabian StyleMongan, Maureen, Bo Xiao, Antonius Christianto, Yueh-Chiang Hu, and Ying Xia. 2025. "Mitogen-Activated Protein Kinase Kinase Kinase 1 Overexpression Disrupts Development of the Ocular Surface Epithelium" Cells 14, no. 12: 894. https://doi.org/10.3390/cells14120894
APA StyleMongan, M., Xiao, B., Christianto, A., Hu, Y.-C., & Xia, Y. (2025). Mitogen-Activated Protein Kinase Kinase Kinase 1 Overexpression Disrupts Development of the Ocular Surface Epithelium. Cells, 14(12), 894. https://doi.org/10.3390/cells14120894






