Pharmacokinomic Profiling Using Patient-Derived Cell Lines Predicts Sensitivity to Imatinib in Dermatofibrosarcoma Protuberans
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Backgrounds
2.2. Cell Culture Procedure
2.3. Drug Screening for Tyrosine Kinase Inhibitors
2.4. Sample Preparation
2.5. Protein Extraction from Imatinib-Treated Cells
2.6. In Vitro Tyrosine Kinase Activity Assay
2.7. Kinase–Substrate Enrichment Analysis (KSEA)
2.8. Gene Ontology Analysis
2.9. Correlation Analysis to Predict Drug Sensitivity for Imatinib
2.10. Western Blotting
3. Results
3.1. Patient Characteristics
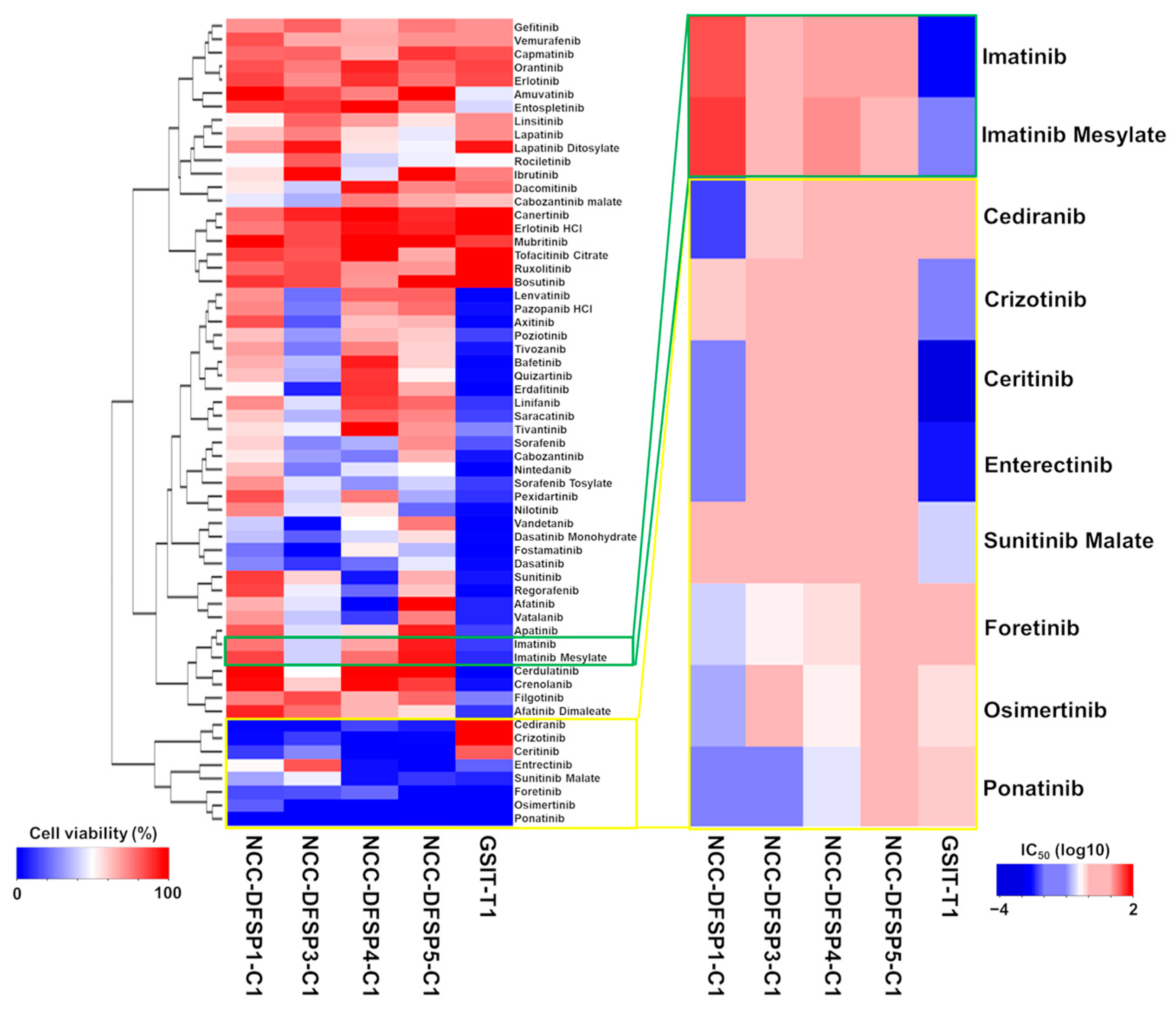
3.2. Sensitivity to Kinase Inhibitors
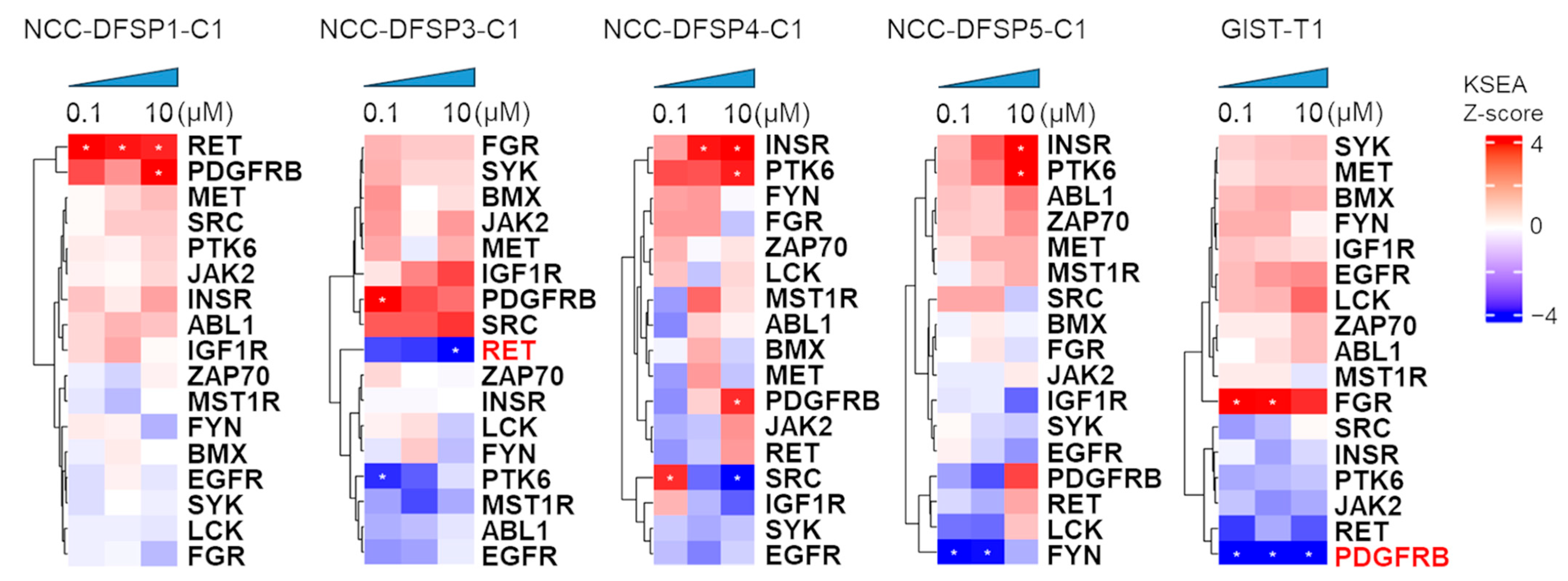
3.3. Effect of Imatinib on Kinase Activity in Four DFSP Cell Lines and GIST-T1 Cell
3.4. Dynamic Change in Kinases by Imatinib
3.5. Gene Ontology Analysis
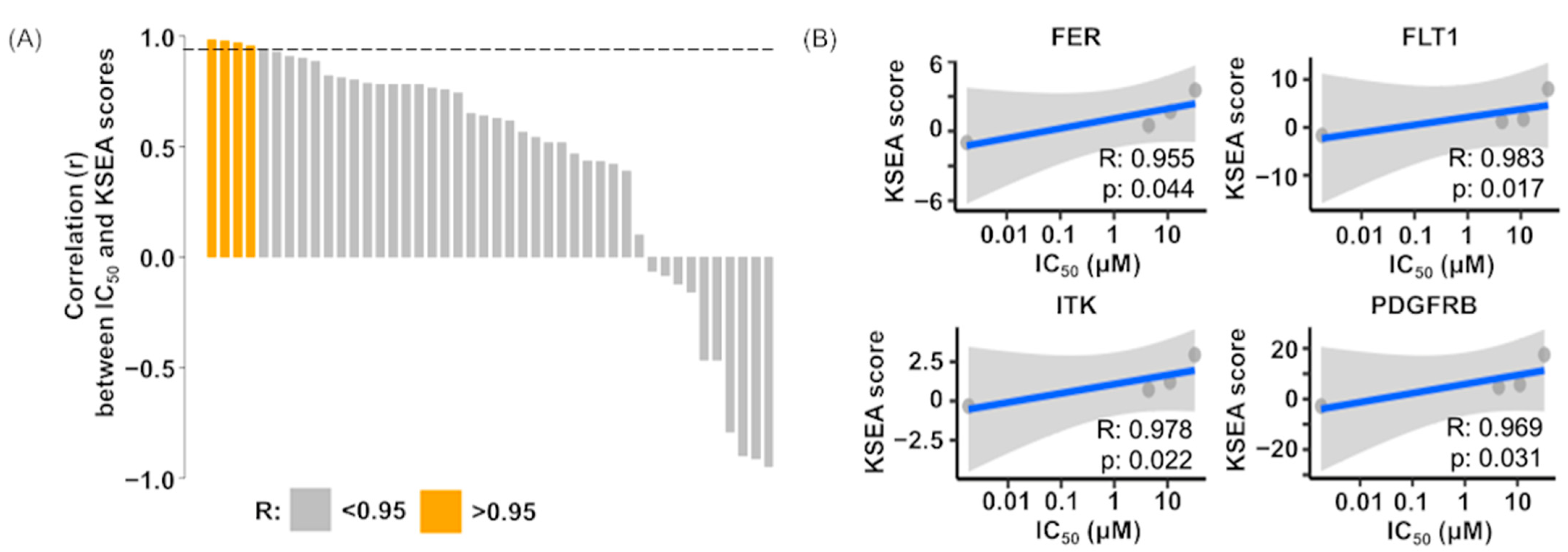
3.6. Correlation Analysis of Drug Sensitivity on Imatinib and Kinase Activity
4. Discussion
4.1. Patient-Derived DFSP Cell Lines
4.2. Kinase Activity Profiles Under Imatinib Treatment on DFSP Cell Lines and GIST-T1 Cells
4.3. Predictive Biomarkers of Imatinib Sensitivity
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kreicher, K.L.; Kurlander, D.E.; Gittleman, H.R.; Barnholtz-Sloan, J.S.; Bordeaux, J.S. Incidence and Survival of Primary Dermatofibrosarcoma Protuberans in the United States. Dermatol. Surg. 2016, 42 (Suppl. 1), S24–S31. [Google Scholar] [CrossRef] [PubMed]
- Rouhani, P.; Fletcher, C.D.; Devesa, S.S.; Toro, J.R. Cutaneous soft tissue sarcoma incidence patterns in the U.S.: An analysis of 12,114 cases. Cancer 2008, 113, 616–627. [Google Scholar] [CrossRef]
- Chang, C.K.; Jacobs, I.A.; Salti, G.I. Outcomes of surgery for dermatofibrosarcoma protuberans. Eur. J. Surg. Oncol. 2004, 30, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Bowne, W.B.; Antonescu, C.R.; Leung, D.H.; Katz, S.C.; Hawkins, W.G.; Woodruff, J.M.; Brennan, M.F.; Lewis, J.J. Dermatofibrosarcoma protuberans: A clinicopathologic analysis of patients treated and followed at a single institution. Cancer 2000, 88, 2711–2720. [Google Scholar] [CrossRef]
- McArthur, G. Dermatofibrosarcoma protuberans: Recent clinical progress. Ann. Surg. Oncol. 2007, 14, 2876–2886. [Google Scholar] [CrossRef]
- Simon, M.P.; Navarro, M.; Roux, D.; Pouyssegur, J. Structural and functional analysis of a chimeric protein COL1A1-PDGFB generated by the translocation t(17;22)(q22;q13.1) in Dermatofibrosarcoma protuberans (DP). Oncogene 2001, 20, 2965–2975. [Google Scholar] [CrossRef]
- Navarrete-Dechent, C.; Mori, S.; Barker, C.A.; Dickson, M.A.; Nehal, K.S. Imatinib Treatment for Locally Advanced or Metastatic Dermatofibrosarcoma Protuberans: A Systematic Review. JAMA Dermatol. 2019, 155, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, P.; Van Glabbeke, M.; Rankin, C.J.; Ruka, W.; Rubin, B.P.; Debiec-Rychter, M.; Lazar, A.; Gelderblom, H.; Sciot, R.; Lopez-Terrada, D.; et al. Imatinib mesylate in advanced dermatofibrosarcoma protuberans: Pooled analysis of two phase II clinical trials. J. Clin. Oncol. 2010, 28, 1772–1779. [Google Scholar] [CrossRef]
- Pawson, T.; Scott, J.D. Protein phosphorylation in signaling--50 years and counting. Trends Biochem. Sci. 2005, 30, 286–290. [Google Scholar] [CrossRef]
- Manning, G.; Plowman, G.D.; Hunter, T.; Sudarsanam, S. Evolution of protein kinase signaling from yeast to man. Trends Biochem. Sci. 2002, 27, 514–520. [Google Scholar] [CrossRef]
- Wilson, L.J.; Linley, A.; Hammond, D.E.; Hood, F.E.; Coulson, J.M.; MacEwan, D.J.; Ross, S.J.; Slupsky, J.R.; Smith, P.D.; Eyers, P.A.; et al. New Perspectives, Opportunities, and Challenges in Exploring the Human Protein Kinome. Cancer Res. 2018, 78, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Fleuren, E.D.; Zhang, L.; Wu, J.; Daly, R.J. The kinome ‘at large’ in cancer. Nat. Rev. Cancer 2016, 16, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.W.; Chien, C.H.; Sung, M.I.; Chen, C.W.; Chen, Y.T. dBMHCC: A comprehensive hepatocellular carcinoma (HCC) biomarker database provides a reliable prediction system for novel HCC phosphorylated biomarkers. PLoS ONE 2020, 15, e0234084. [Google Scholar] [CrossRef]
- Kolch, W.; Pitt, A. Functional proteomics to dissect tyrosine kinase signalling pathways in cancer. Nat. Rev. Cancer 2010, 10, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Oyama, R.; Kito, F.; Qiao, Z.; Sakumoto, M.; Shiozawa, K.; Toki, S.; Yoshida, A.; Kawai, A.; Kondo, T. Establishment of novel patient-derived models of dermatofibrosarcoma protuberans: Two cell lines, NCC-DFSP1-C1 and NCC-DFSP2-C1. In Vitro Cell. Dev. Biol. Anim. 2019, 55, 62–73. [Google Scholar] [CrossRef]
- Yoshimatsu, Y.; Noguchi, R.; Tsuchiya, R.; Sei, A.; Nakagawa, M.; Yoshida, A.; Kawai, A.; Kondo, T. Establishment and characterization of NCC-DFSP3-C1: A novel patient-derived dermatofibrosarcoma protuberans cell line. Hum. Cell 2020, 33, 894–903. [Google Scholar] [CrossRef]
- Akiyama, T.; Yoshimatsu, Y.; Noguchi, R.; Sin, Y.; Osaki, J.; Ono, T.; Adachi, Y.; Tsuchiya, R.; Toda, Y.; Ogura, K.; et al. Establishment and characterization of NCC-DFSP4-C1: A novel cell line from a patient with dermatofibrosarcoma protuberans having the fibrosarcomatous transformation. Hum. Cell 2023, 36, 2187–2194. [Google Scholar] [CrossRef]
- Ono, T.; Noguchi, R.; Osaki, J.; Akiyama, T.; Adachi, Y.; Kojima, N.; Toda, Y.; Fukushima, S.; Yoshimatsu, Y.; Yoshida, A.; et al. Establishment and characterization of NCC-DFSP5-C1: A novel patient-derived dermatofibrosarcoma protuberans cell line. Hum. Cell 2024, 37, 854–864. [Google Scholar] [CrossRef]
- Yoshimatsu, Y.; Noguchi, R.; Tsuchiya, R.; Kito, F.; Sei, A.; Sugaya, J.; Nakagawa, M.; Yoshida, A.; Iwata, S.; Kawai, A.; et al. Establishment and characterization of NCC-CDS2-C1: A novel patient-derived cell line of CIC-DUX4 sarcoma. Hum. Cell 2020, 33, 427–436. [Google Scholar] [CrossRef]
- Noguchi, R.; Yoshimura, A.; Uchino, J.; Takeda, T.; Chihara, Y.; Ota, T.; Hiranuma, O.; Gyotoku, H.; Takayama, K.; Kondo, T. Comprehensive Kinase Activity Profiling Revealed the Kinase Activity Patterns Associated with the Effects of EGFR Tyrosine Kinase Inhibitor Therapy in Advanced Non-Small-Cell Lung Cancer Patients with Sensitizing EGFR Mutations. Proteomes 2023, 11, 6. [Google Scholar] [CrossRef]
- Noguchi, R.; Osaki, J.; Ono, T.; Adachi, Y.; Iwata, S.; Yoshimatsu, Y.; Sasaki, K.; Kawai, A.; Kondo, T. Pharmacoproteogenomic approach identifies on-target kinase inhibitors for cancer drug repositioning. In Vitro Cell. Dev. Biol.-Anim. 2024, 60, 1200–1214. [Google Scholar] [CrossRef]
- Anderson, J.C.; Duarte, C.W.; Welaya, K.; Rohrbach, T.D.; Bredel, M.; Yang, E.S.; Choradia, N.V.; Thottassery, J.V.; Yancey Gillespie, G.; Bonner, J.A.; et al. Kinomic exploration of temozolomide and radiation resistance in Glioblastoma multiforme xenolines. Radiother. Oncol. 2014, 111, 468–474. [Google Scholar] [CrossRef]
- Wiredja, D.D.; Koyuturk, M.; Chance, M.R. The KSEA App: A web-based tool for kinase activity inference from quantitative phosphoproteomics. Bioinformatics 2017, 33, 3489–3491. [Google Scholar] [CrossRef]
- Casado, P.; Rodriguez-Prados, J.C.; Cosulich, S.C.; Guichard, S.; Vanhaesebroeck, B.; Joel, S.; Cutillas, P.R. Kinase-substrate enrichment analysis provides insights into the heterogeneity of signaling pathway activation in leukemia cells. Sci. Signal 2013, 6, rs6. [Google Scholar] [CrossRef]
- Bairoch, A. The Cellosaurus, a cell-Line knowledge resource. J. Biomol. Tech. 2018, 29, 25–38. [Google Scholar] [CrossRef]
- Gershwin, M.E.; Lentz, D.; Owens, R.B. Relationship between karyotype of tissue culture lines and tumorigenicity in nude mice. Exp. Cell Biol. 1984, 52, 361–370. [Google Scholar] [CrossRef]
- Shindo, Y.; Akiyama, J.; Yamaji, K.; Ishihara, Y.; Saida, T.; Takase, Y. Establishment of a human dermatofibrosarcoma protuberans cell line: Cytological characteristics. J. Dermatol. 1989, 16, 355–360. [Google Scholar] [CrossRef]
- Chan, J.Y.; Lee, E.C.Y.; Li, Z.; Lee, J.Y.; Lim, A.H.; Poon, E. Multi-omic profiling and real time ex vivo modelling of imatinib-resistant dermatofibrosarcoma protuberans with fibrosarcomatous transformation. Hum. Cell 2023, 36, 2228–2236. [Google Scholar] [CrossRef]
- Iqbal, N.; Iqbal, N. Imatinib: A breakthrough of targeted therapy in cancer. Chemother. Res. Pract. 2014, 2014, 357027. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.S.; Hussain, H.B.; Moley, J.F. Inhibition of medullary thyroid carcinoma cell proliferation and RET phosphorylation by tyrosine kinase inhibitors. Surgery 2002, 132, 960–966; discussion 966–967. [Google Scholar] [CrossRef] [PubMed]
- de Groot, J.W.; Plaza Menacho, I.; Schepers, H.; Drenth-Diephuis, L.J.; Osinga, J.; Plukker, J.T.; Links, T.P.; Eggen, B.J.; Hofstra, R.M. Cellular effects of imatinib on medullary thyroid cancer cells harboring multiple endocrine neoplasia Type 2A and 2B associated RET mutations. Surgery 2006, 139, 806–814. [Google Scholar] [CrossRef]
- Greco, A.; Roccato, E.; Miranda, C.; Cleris, L.; Formelli, F.; Pierotti, M.A. Growth-inhibitory effect of STI571 on cells transformed by the COL1A1/PDGFB rearrangement. Int. J. Cancer 2001, 92, 354–360. [Google Scholar] [CrossRef][Green Version]
- Sjoblom, T.; Shimizu, A.; O’Brien, K.P.; Pietras, K.; Dal Cin, P.; Buchdunger, E.; Dumanski, J.P.; Ostman, A.; Heldin, C.H. Growth inhibition of dermatofibrosarcoma protuberans tumors by the platelet-derived growth factor receptor antagonist STI571 through induction of apoptosis. Cancer Res. 2001, 61, 5778–5783. [Google Scholar]
- Ugurel, S.; Mentzel, T.; Utikal, J.; Helmbold, P.; Mohr, P.; Pfohler, C.; Schiller, M.; Hauschild, A.; Hein, R.; Kampgen, E.; et al. Neoadjuvant imatinib in advanced primary or locally recurrent dermatofibrosarcoma protuberans: A multicenter phase II DeCOG trial with long-term follow-up. Clin. Cancer Res. 2014, 20, 499–510. [Google Scholar] [CrossRef]
- McArthur, G.A.; Demetri, G.D.; van Oosterom, A.; Heinrich, M.C.; Debiec-Rychter, M.; Corless, C.L.; Nikolova, Z.; Dimitrijevic, S.; Fletcher, J.A. Molecular and clinical analysis of locally advanced dermatofibrosarcoma protuberans treated with imatinib: Imatinib Target Exploration Consortium Study B2225. J. Clin. Oncol. 2005, 23, 866–873. [Google Scholar] [CrossRef]
- Fischer, C.; Mazzone, M.; Jonckx, B.; Carmeliet, P. FLT1 and its ligands VEGFB and PlGF: Drug targets for anti-angiogenic therapy? Nat. Rev. Cancer 2008, 8, 942–956. [Google Scholar] [CrossRef]
- Mathew, P.; Wen, S.; Morita, S.; Thall, P.F. Placental growth factor and soluble c-kit receptor dynamics characterize the cytokine signature of imatinib in prostate cancer and bone metastases. J. Interferon Cytokine Res. 2011, 31, 539–544. [Google Scholar] [CrossRef]

| Cell Line Name | Diagnosis * | Age | Gender | Location | Site of Origin | Reference |
|---|---|---|---|---|---|---|
| NCC-DFSP1-C1 | FS-DFSP | 46 | M | Primary | Abdominal wall | [15] |
| NCC-DFSP3-C1 | FS-DFSP | 51 | F | Metastatic | Left thigh | [16] |
| NCC-DFSP4-C1 | FS-DFSP | 60 | M | Primary | Left lower back | [17] |
| NCC-DFSP5-C1 | DFSP | 52 | M | Primary | Left proximal tibia | [18] |
| Drug Name | NCC-DFSP1-C1 | NCC-DFSP3-C1 | NCC- DFSP4-C1 | NCC-DFSP5-C1 | GIST-T1 | |
|---|---|---|---|---|---|---|
| 10 μM cell viability (%) | Imatinib | 76.76 | 41.42 | 67.00 | 92.11 | 13.24 |
| Imatinib Mesylate | 85.85 | 41.11 | 77.40 | 93.28 | 10.14 | |
| Cediranib | 2.35 | 2.81 | 14.23 | 7.92 | 107.79 | |
| Crizotinib | 4.05 | 12.56 | 0.60 | 2.66 | 111.10 | |
| Ceritinib | 13.04 | 26.92 | 0.54 | 0.00 | 80.77 | |
| Entrectinib | 51.03 | 82.41 | 4.55 | 0.06 | 19.36 | |
| Sunitinib Malate | 32.96 | 46.95 | 5.68 | 11.92 | 8.53 | |
| Foretinib | 15.13 | 16.56 | 20.55 | 0.01 | −1.20 | |
| Osimertinib | 18.77 | 1.89 | 1.21 | 0.68 | −0.04 | |
| Ponatinib | 0.34 | 0.96 | 0.40 | 1.34 | −1.49 | |
| IC50 (μM) | Imatinib | 32.6900 | 4.4190 | 11.1700 | 11.7800 | 0.0018 |
| Imatinib Mesylate | 42.0700 | 2.3960 | 13.1900 | 4.5540 | 0.0136 | |
| Cediranib | 0.0050 | 0.8100 | 1.3500 | 4.6200 | 0.0035 | |
| Crizotinib | 0.0690 | 1.2300 | 2.1900 | 1.2500 | 0.2406 | |
| Ceritinib | 0.7900 | 3.9100 | 1.8700 | 2.2600 | 2.3630 | |
| Entrectinib | 0.0160 | 2.0600 | 1.5600 | 2.0300 | 1.3510 | |
| Sunitinib Malate | 4.6800 | 9.4100 | 6.5200 | 7.3000 | 0.0006 | |
| Foretinib | 0.2600 | 0.5300 | 0.6300 | 1.1800 | 0.6235 | |
| Osimertinib | 0.1500 | 2.6200 | 0.5700 | 2.0600 | 0.9135 | |
| Ponatinib | 0.0500 | 0.0800 | 0.3600 | 1.3700 | 0.0883 |
| Sample | Term Description | NES | Exact p-Value | False Discovery Rate |
|---|---|---|---|---|
| NCC-DFSP1-C1 | MAPK signaling pathway | 1.5 | 0.003 | 0.022 |
| NCC-DFSP3-C1 | EGFR tyrosine kinase inhibitor resistance | 1.811 | 0.001 | 0.005 |
| NCC-DFSP3-C1 | MAPK signaling pathway | −1.362 | 0.012 | 0.024 |
| NCC-DFSP4-C1 | NA | NA | NA | NA |
| NCC-DFSP5-C1 | NA | NA | NA | NA |
| GIST-T1 | MAPK signaling pathway | −1.747 | 0.001 | 0.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noguchi, R.; Ono, T.; Sasaki, K.; Masuda, M.; Kawai, A.; Yoshimatsu, Y.; Kondo, T. Pharmacokinomic Profiling Using Patient-Derived Cell Lines Predicts Sensitivity to Imatinib in Dermatofibrosarcoma Protuberans. Cells 2025, 14, 884. https://doi.org/10.3390/cells14120884
Noguchi R, Ono T, Sasaki K, Masuda M, Kawai A, Yoshimatsu Y, Kondo T. Pharmacokinomic Profiling Using Patient-Derived Cell Lines Predicts Sensitivity to Imatinib in Dermatofibrosarcoma Protuberans. Cells. 2025; 14(12):884. https://doi.org/10.3390/cells14120884
Chicago/Turabian StyleNoguchi, Rei, Takuya Ono, Kazuki Sasaki, Mari Masuda, Akira Kawai, Yuki Yoshimatsu, and Tadashi Kondo. 2025. "Pharmacokinomic Profiling Using Patient-Derived Cell Lines Predicts Sensitivity to Imatinib in Dermatofibrosarcoma Protuberans" Cells 14, no. 12: 884. https://doi.org/10.3390/cells14120884
APA StyleNoguchi, R., Ono, T., Sasaki, K., Masuda, M., Kawai, A., Yoshimatsu, Y., & Kondo, T. (2025). Pharmacokinomic Profiling Using Patient-Derived Cell Lines Predicts Sensitivity to Imatinib in Dermatofibrosarcoma Protuberans. Cells, 14(12), 884. https://doi.org/10.3390/cells14120884







