Modulating Cognition-Linked Histone Acetyltransferases (HATs) as a Therapeutic Strategy for Neurodegenerative Diseases: Recent Advances and Future Trends
Abstract
1. Introduction
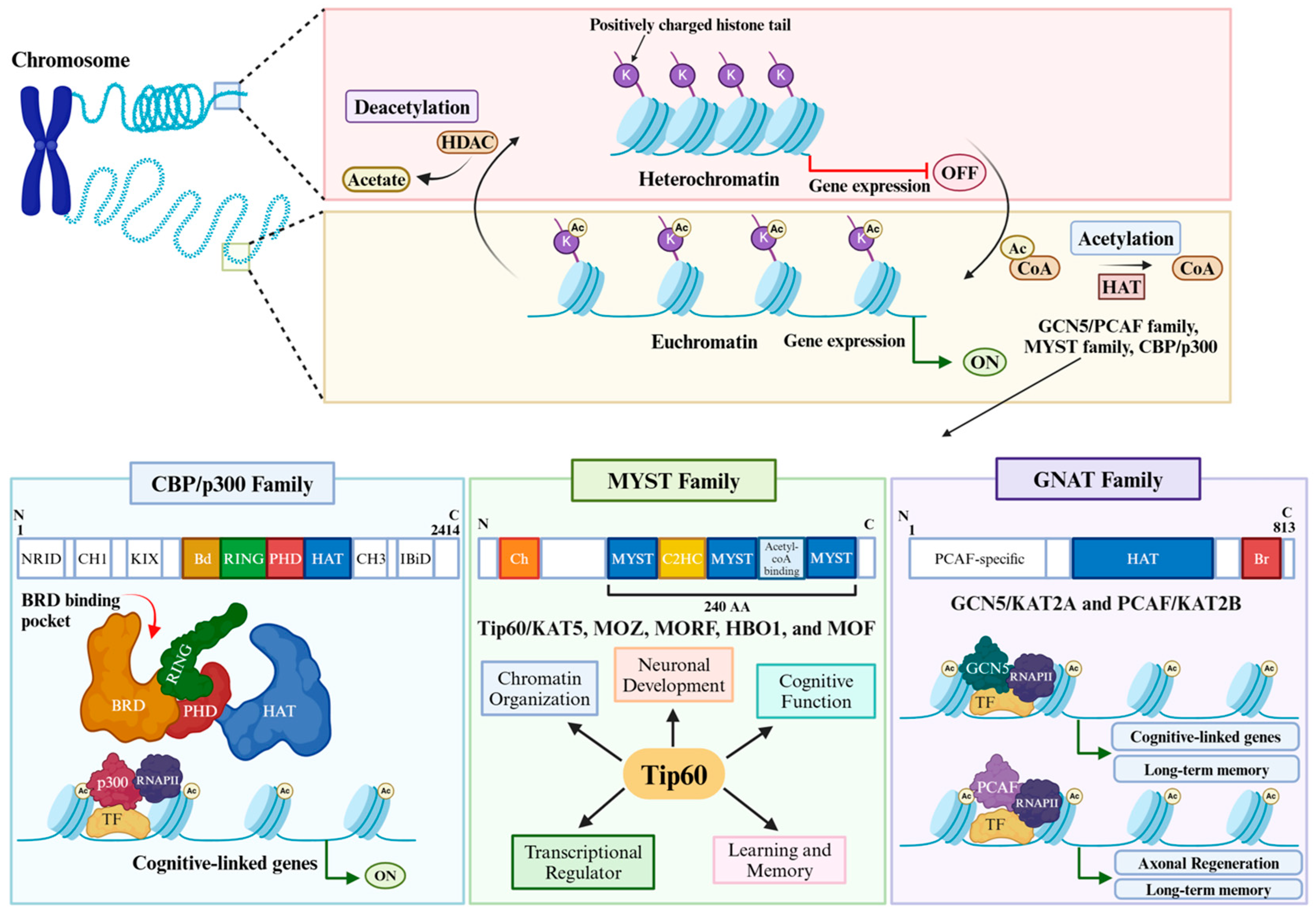
2. HAT Families in Brain Function and Cognition
3. HAT: HDAC Interplay in Activity-Dependent Cognition-Linked Gene Control
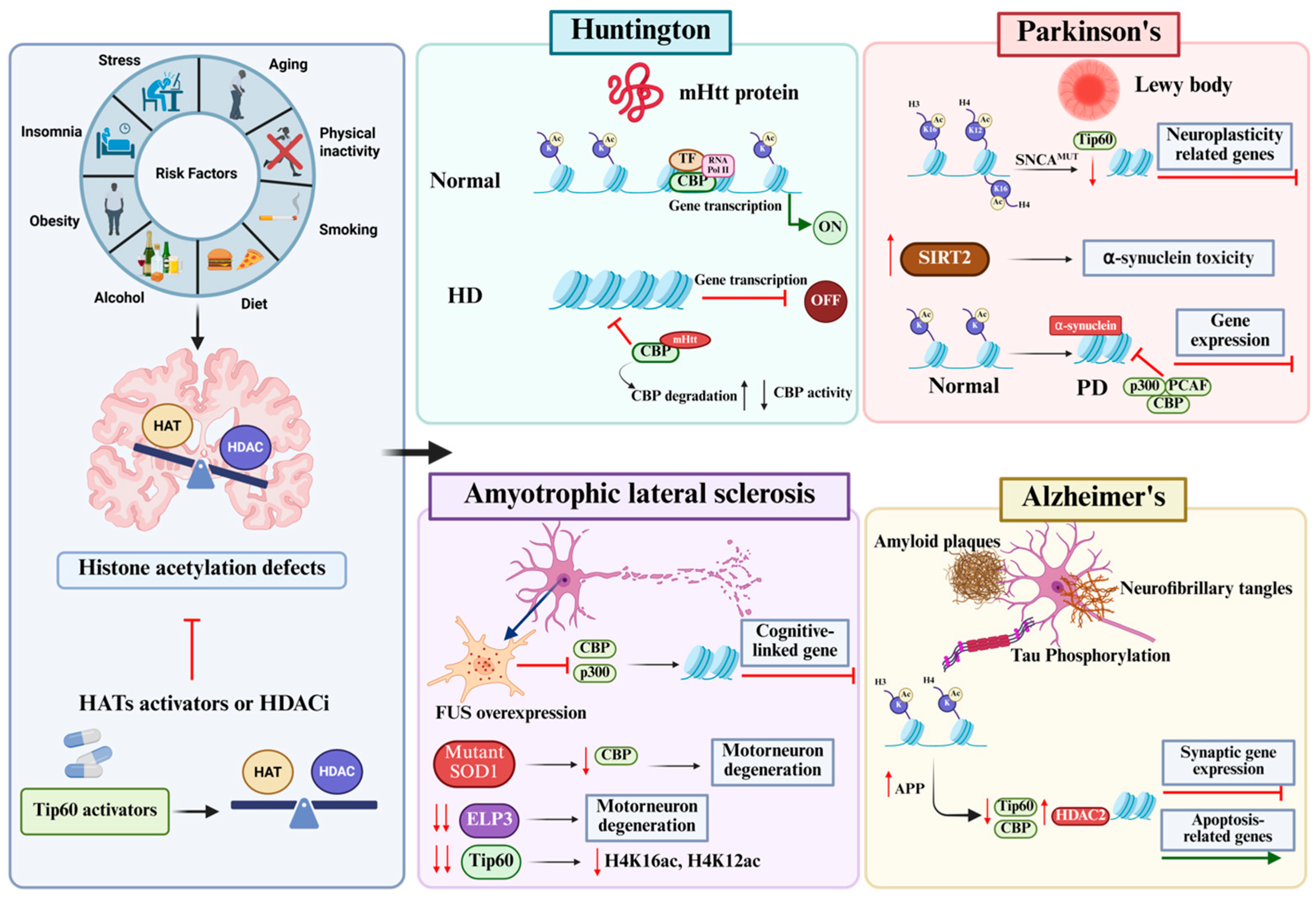
4. Neuroepigenetic Alterations Drive Multiple Age-Related Neurodegenerative Diseases
4.1. Huntington’s Disease
4.2. Parkinson’s Disease
4.3. Amyotrophic Lateral Sclerosis (ALS)
4.4. Alzheimer’s Disease
5. HAT Activation as a Promising Therapeutic Strategy for Age-Related Neurodegenerative Disease
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviations | Meaning |
| PTM | Post-translational modification |
| ND | Neurodegenerative disease |
| AD | Alzheimer’s disease |
| PD | Parkinson’s disease |
| ALS | Amyotrophic lateral sclerosis |
| HD | Huntington’s disease |
| HAT | Histone acetyltransferase |
| TAFII | TBP-associated Factor |
| TFIID | Transcription Factor IID |
| TBP | TATA-binding protein |
| RNA PolII | RNA Polymerase II |
| GCN5 | General Control non-depressible 5 (HAT2) |
| PCAF | p300/CREB-binding protein-associated factor |
| MYST | MOF,Ybf2/Sas3,Sas2,Tip60 |
| MOF | Monocytic leukemia zinc finger protein |
| Ybf2 | Yeast Binding Factor two (HAT) |
| Tip60 | Tat-interacting protein 60 |
| CBP | CREB Binding Protein |
| CREB | cAMP Response Element-Binding protein |
| RID | Receptor interaction domain |
| KIX | Kinase-inducible domain |
| PHD | Plant homeodomain |
| RTS | Rubinstein–Taybi Syndrome |
| LTM | Long-term memory |
| MOZ | Monocytic leukemia zinc finger protein (KAT6A) |
| MORF | MOZ-related factor |
| HBO1 | Histone acetyl transferase |
| sLNv | Small lateral neurons ventral |
| KAT | Lysine acetyl transferase |
| ChIP-seq | Chromatin immunoprecipitation sequencing |
| GNAT | Gcn5-related N-acetyltransferase |
| ERK | Extra-cellular signal related kinase |
| KO | Knockout |
| HDAC | Histone deacetylase |
| TSA | Trichostatin A |
| KCL | Potassium Chloride |
| NMDA | N-methyl D-aspartate receptor (glutamate receptor) |
| LTP | Long-term potentiation |
| LTD | Long-term depression |
| EE | Environmental enrichment |
| HDACi | Histone Deacetylase inhibitor |
| SIRT2 | Sirtuin 2(NAD+-dependent deacetylase) |
| SNCA | Gene that encodes alpha-synuclein |
| APP | Amyloid precursor protein |
| HTT | Huntington protein |
| mHTT | Mutant Huntington protein |
| polyQ | Polyglutamine |
| SAHA | Suberoylanilide hydroxamic acid |
| Sirtuin 2 | Sirtuin 2 |
| a-syn | Alpha synuclein |
| PFFs | a-syn folded fibrils |
| CNS | Central nervous system |
| FUS | Fused in Sarcoma |
| PARK7 | Gene name for Parkinsonism associate deglycase |
| MAPT | Microtubule-associated Protein tau |
| ELP3 | Elongator Protein 3 |
| HeLa cells | Cell line derived of cancerous tissues |
| SOD1 | Superoxide dismutase 1 |
| VPA | Valproic acid |
| mRNA | Messenger RNA |
| LOAD | Late-onset Alzheimer’s disease |
| ApoE4 | Apolioprotein E4 |
| PSEN | Presenilin |
| AB | Amyloid Beta |
| NTF | Neurofibrillary Tangle |
| MCI | Mild cognitive impairment |
| PS1/2 | Presenilin 1/2 |
| BDNF | Brain-derived neurotrophic factor |
| ZBGs | Zinc-binding groups |
| NaB | Sodium burytate |
| Httex1p | Polyglutamine-containing domain of Htt-htt exon 1 protein |
| TH | Tyrosine hydroxylase |
References
- Boros, I.M. Histone modification in Drosophila. Brief. Funct. Genom. 2012, 11, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.L.; Laniel, M.A. Histones and histone modifications. Curr. Biol. 2004, 14, R546–R551. [Google Scholar] [CrossRef] [PubMed]
- Levenson, J.M.; Sweatt, J.D. Epigenetic mechanisms in memory formation. Nat. Rev. Neurosci. 2005, 6, 108–118. [Google Scholar] [CrossRef]
- Schneider, A.; Chatterjee, S.; Bousiges, O.; Selvi, B.R.; Swaminathan, A.; Cassel, R.; Blanc, F.; Kundu, T.K.; Boutillier, A.L. Acetyltransferases (HATs) as targets for neurological therapeutics. Neurotherapeutics 2013, 10, 568–588. [Google Scholar] [CrossRef]
- Wood, M.A.; Hawk, J.D.; Abel, T. Combinatorial chromatin modifications and memory storage: A code for memory? Learn. Mem. 2006, 13, 241–244. [Google Scholar] [CrossRef]
- Kuo, M.H.; Allis, C.D. Roles of histone acetyltransferases and deacetylases in gene regulation. Bioessays 1998, 20, 615–626. [Google Scholar] [CrossRef]
- Scharf, A.N.; Imhof, A. Every methyl counts--epigenetic calculus. FEBS Lett. 2011, 585, 2001–2007. [Google Scholar] [CrossRef]
- Day, J.J.; Sweatt, J.D. Epigenetic treatments for cognitive impairments. Neuropsychopharmacology 2012, 37, 247–260. [Google Scholar] [CrossRef]
- Lardenoije, R.; Iatrou, A.; Kenis, G.; Kompotis, K.; Steinbusch, H.W.; Mastroeni, D.; Coleman, P.; Lemere, C.A.; Hof, P.R.; van den Hove, D.L.; et al. The epigenetics of aging and neurodegeneration. Prog. Neurobiol. 2015, 131, 21–64. [Google Scholar] [CrossRef]
- Borrelli, E.; Nestler, E.J.; Allis, C.D.; Sassone-Corsi, P. Decoding the epigenetic language of neuronal plasticity. Neuron 2008, 60, 961–974. [Google Scholar] [CrossRef]
- Graff, J.; Rei, D.; Guan, J.S.; Wang, W.Y.; Seo, J.; Hennig, K.M.; Nieland, T.J.; Fass, D.M.; Kao, P.F.; Kahn, M.; et al. An epigenetic blockade of cognitive functions in the neurodegenerating brain. Nature 2012, 483, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Mizar, P.; Cassel, R.; Neidl, R.; Selvi, B.R.; Mohankrishna, D.V.; Vedamurthy, B.M.; Schneider, A.; Bousiges, O.; Mathis, C.; et al. A novel activator of CBP/p300 acetyltransferases promotes neurogenesis and extends memory duration in adult mice. J. Neurosci. 2013, 33, 10698–10712. [Google Scholar] [CrossRef] [PubMed]
- Hegarty, S.V.; O’Leary, E.; Solger, F.; Stanicka, J.; Sullivan, A.M.; O’Keeffe, G.W. A Small Molecule Activator of p300/CBP Histone Acetyltransferase Promotes Survival and Neurite Growth in a Cellular Model of Parkinson’s Disease. Neurotox. Res. 2016, 30, 510–520. [Google Scholar] [CrossRef]
- Jakovcevski, M.; Akbarian, S. Epigenetic mechanisms in neurological disease. Nat. Med. 2012, 18, 1194–1204. [Google Scholar] [CrossRef]
- Kabir, F.; Atkinson, R.; Cook, A.L.; Phipps, A.J.; King, A.E. The role of altered protein acetylation in neurodegenerative disease. Front. Aging Neurosci. 2022, 14, 1025473. [Google Scholar] [CrossRef]
- Johnson, A.A.; Sarthi, J.; Pirooznia, S.K.; Reube, W.; Elefant, F. Increasing Tip60 HAT levels rescues axonal transport defects and associated behavioral phenotypes in a Drosophila Alzheimer’s disease model. J. Neurosci. 2013, 33, 7535–7547. [Google Scholar] [CrossRef]
- Pirooznia, S.K.; Elefant, F. Targeting specific HATs for neurodegenerative disease treatment: Translating basic biology to therapeutic possibilities. Front. Cell Neurosci. 2013, 7, 30. [Google Scholar] [CrossRef]
- Meaney, M.J.; Ferguson-Smith, A.C. Epigenetic regulation of the neural transcriptome: The meaning of the marks. Nat. Neurosci. 2010, 13, 1313–1318. [Google Scholar] [CrossRef]
- Nelson, E.D.; Monteggia, L.M. Epigenetics in the mature mammalian brain: Effects on behavior and synaptic transmission. Neurobiol. Learn. Mem. 2011, 96, 53–60. [Google Scholar] [CrossRef]
- Riccio, A. Dynamic epigenetic regulation in neurons: Enzymes, stimuli and signaling pathways. Nat. Neurosci. 2010, 13, 1330–1337. [Google Scholar] [CrossRef]
- Sweatt, J.D. Neuroscience. Epigenetics and cognitive aging. Science 2010, 328, 701–702. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wilf, R.; Menon, T.; Panikker, P.; Sarthi, J.; Elefant, F. Epigenetic control of learning and memory in Drosophila by Tip60 HAT action. Genetics 2014, 198, 1571–1586. [Google Scholar] [CrossRef] [PubMed]
- Grunstein, M. Histone acetylation in chromatin structure and transcription. Nature 1997, 389, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.F.; Lin, Y.T.; Gallegos, D.A.; Hazlett, M.F.; Gomez-Schiavon, M.; Yang, M.G.; Kalmeta, B.; Zhou, A.S.; Holtzman, L.; Gersbach, C.A.; et al. Enhancer Histone Acetylation Modulates Transcriptional Bursting Dynamics of Neuronal Activity-Inducible Genes. Cell Rep. 2019, 26, 1174–1188. [Google Scholar] [CrossRef]
- Peixoto, L.; Abel, T. The role of histone acetylation in memory formation and cognitive impairments. Neuropsychopharmacology 2013, 38, 62–76. [Google Scholar] [CrossRef]
- Krajewski, W.A.; Becker, P.B. Reconstitution of hyperacetylated, DNase I-sensitive chromatin characterized by high conformational flexibility of nucleosomal DNA. Proc. Natl. Acad. Sci. USA 1998, 95, 1540–1545. [Google Scholar] [CrossRef]
- Ikura, T.; Ogryzko, V.V.; Grigoriev, M.; Groisman, R.; Wang, J.; Horikoshi, M.; Scully, R.; Qin, J.; Nakatani, Y. Involvement of the TIP60 histone acetylase complex in DNA repair and apoptosis. Cell 2000, 102, 463–473. [Google Scholar] [CrossRef]
- Mizzen, C.A.; Yang, X.J.; Kokubo, T.; Brownell, J.E.; Bannister, A.J.; Owen-Hughes, T.; Workman, J.; Wang, L.; Berger, S.L.; Kouzarides, T.; et al. The TAF(II)250 subunit of TFIID has histone acetyltransferase activity. Cell 1996, 87, 1261–1270. [Google Scholar] [CrossRef]
- Liu, X.; Yang, S.; Zhao, M.; Luo, M.; Yu, C.W.; Chen, C.Y.; Tai, R.; Wu, K. Transcriptional repression by histone deacetylases in plants. Mol. Plant 2014, 7, 764–772. [Google Scholar] [CrossRef]
- Roth, S.Y.; Denu, J.M.; Allis, C.D. Histone acetyltransferases. Annu. Rev. Biochem. 2001, 70, 81–120. [Google Scholar] [CrossRef]
- Bedford, D.C.; Brindle, P.K. Is histone acetylation the most important physiological function for CBP and p300? Aging 2012, 4, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Dyson, H.J.; Wright, P.E. Role of Intrinsic Protein Disorder in the Function and Interactions of the Transcriptional Coactivators CREB-binding Protein (CBP) and p300. J. Biol. Chem. 2016, 291, 6714–6722. [Google Scholar] [CrossRef] [PubMed]
- Petrij, F.; Giles, R.H.; Dauwerse, H.G.; Saris, J.J.; Hennekam, R.C.; Masuno, M.; Tommerup, N.; van Ommen, G.J.; Goodman, R.H.; Peters, D.J.; et al. Rubinstein-Taybi syndrome caused by mutations in the transcriptional co-activator CBP. Nature 1995, 376, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Alarcon, J.M.; Malleret, G.; Touzani, K.; Vronskaya, S.; Ishii, S.; Kandel, E.R.; Barco, A. Chromatin acetylation, memory, and LTP are impaired in CBP+/- mice: A model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron 2004, 42, 947–959. [Google Scholar] [CrossRef]
- Korzus, E.; Rosenfeld, M.G.; Mayford, M. CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron 2004, 42, 961–972. [Google Scholar] [CrossRef]
- Maurice, T.; Duclot, F.; Meunier, J.; Naert, G.; Givalois, L.; Meffre, J.; Celerier, A.; Jacquet, C.; Copois, V.; Mechti, N.; et al. Altered memory capacities and response to stress in p300/CBP-associated factor (PCAF) histone acetylase knockout mice. Neuropsychopharmacology 2008, 33, 1584–1602. [Google Scholar] [CrossRef]
- Oliveira, A.M.; Wood, M.A.; McDonough, C.B.; Abel, T. Transgenic mice expressing an inhibitory truncated form of p300 exhibit long-term memory deficits. Learn. Mem. 2007, 14, 564–572. [Google Scholar] [CrossRef][Green Version]
- Oliveira, A.M.; Abel, T.; Brindle, P.K.; Wood, M.A. Differential role for CBP and p300 CREB-binding domain in motor skill learning. Behav. Neurosci. 2006, 120, 724–729. [Google Scholar] [CrossRef]
- Chatterjee, S.; Angelakos, C.C.; Bahl, E.; Hawk, J.D.; Gaine, M.E.; Poplawski, S.G.; Schneider-Anthony, A.; Yadav, M.; Porcari, G.S.; Cassel, J.C.; et al. The CBP KIX domain regulates long-term memory and circadian activity. BMC Biol. 2020, 18, 155. [Google Scholar] [CrossRef]
- Sapountzi, V.; Cote, J. MYST-family histone acetyltransferases: Beyond chromatin. Cell Mol. Life Sci. 2011, 68, 1147–1156. [Google Scholar] [CrossRef]
- Shukla, S.; Levine, C.; Sripathi, R.P.; Elson, G.; Lutz, C.S.; Leibovich, S.J. The Kat in the HAT: The Histone Acetyl Transferase Kat6b (MYST4) Is Downregulated in Murine Macrophages in Response to LPS. Mediat. Inflamm. 2018, 2018, 7852742. [Google Scholar] [CrossRef] [PubMed]
- Stilling, R.M.; Ronicke, R.; Benito, E.; Urbanke, H.; Capece, V.; Burkhardt, S.; Bahari-Javan, S.; Barth, J.; Sananbenesi, F.; Schutz, A.L.; et al. K-Lysine acetyltransferase 2a regulates a hippocampal gene expression network linked to memory formation. EMBO J. 2014, 33, 1912–1927. [Google Scholar] [CrossRef] [PubMed]
- Pirooznia, S.K.; Sarthi, J.; Johnson, A.A.; Toth, M.S.; Chiu, K.; Koduri, S.; Elefant, F. Tip60 HAT activity mediates APP induced lethality and apoptotic cell death in the CNS of a Drosophila Alzheimer’s disease model. PLoS ONE 2012, 7, e41776. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Elefant, F. Tip off the HAT- Epigenetic control of learning and memory by Drosophila Tip60. Fly 2015, 9, 22–28. [Google Scholar] [CrossRef]
- Hofmann, A.; Brunner, M.; Korge, G. The winged-helix transcription factor JUMU is a haplo-suppressor/triplo-enhancer of PEV in various tissues but exhibits reverse PEV effects in the brain of Drosophila melanogaster. Chromosome Res. 2009, 17, 347–358. [Google Scholar] [CrossRef]
- Lorbeck, M.; Pirooznia, K.; Sarthi, J.; Zhu, X.; Elefant, F. Microarray analysis uncovers a role for Tip60 in nervous system function and general metabolism. PLoS ONE 2011, 6, e18412. [Google Scholar] [CrossRef]
- Panikker, P.; Xu, S.J.; Zhang, H.; Sarthi, J.; Beaver, M.; Sheth, A.; Akhter, S.; Elefant, F. Restoring Tip60 HAT/HDAC2 Balance in the Neurodegenerative Brain Relieves Epigenetic Transcriptional Repression and Reinstates Cognition. J. Neurosci. 2018, 38, 4569–4583. [Google Scholar] [CrossRef]
- Urban, I.; Kerimoglu, C.; Sakib, M.S.; Wang, H.; Benito, E.; Thaller, C.; Zhou, X.; Yan, J.; Fischer, A.; Eichele, G. TIP60/KAT5 is required for neuronal viability in hippocampal CA1. Sci. Rep. 2019, 9, 16173. [Google Scholar] [CrossRef]
- Uchida, S.; Teubner, B.J.W.; Hevi, C.; Hara, K.; Kobayashi, A.; Dave, R.M.; Shintaku, T.; Jaikhan, P.; Yamagata, H.; Suzuki, T.; et al. CRTC1 Nuclear Translocation Following Learning Modulates Memory Strength via Exchange of Chromatin Remodeling Complexes on the Fgf1 Gene. Cell Rep. 2017, 18, 352–366. [Google Scholar] [CrossRef]
- Wiley, J.C.; Young, J.E.; Jayadev, S.; Darvas, M.; Swerdlow, R.H.; Wolfe, M.S.; Heath, L.M.; Gockley, J.; Cary, G.A.; Poehlman, W.L.; et al. Alternative Theory of AD pathogenesis: Membrane Delimitation of the histone Acetyltransferase Tip60/Kat5. Alzheimers Dement. 2022, S4, 068190. [Google Scholar] [CrossRef]
- Kueh, A.J.; Dixon, M.P.; Voss, A.K.; Thomas, T. HBO1 is required for H3K14 acetylation and normal transcriptional activity during embryonic development. Mol. Cell Biol. 2011, 31, 845–860. [Google Scholar] [CrossRef]
- Bergamasco, M.I.; Abeysekera, W.; Garnham, A.L.; Hu, Y.; Li-Wai-Suen, C.S.; Sheikh, B.N.; Smyth, G.K.; Thomas, T.; Voss, A.K. KAT6B is required for histone 3 lysine 9 acetylation and SOX gene expression in the developing brain. Life Sci. Alliance 2025, 8, e202402969. [Google Scholar] [CrossRef] [PubMed]
- Bergamasco, M.I.; Ozturk, E.; Casillas-Espinosa, P.M.; Garnham, A.L.; Abeysekera, W.; Wimmer, V.C.; Rajasekhar, P.; Vanyai, H.K.; Whitehead, L.; Blewitt, M.E.; et al. KAT6B overexpression in mice causes aggression, anxiety, and epilepsy. iScience 2025, 28, 111953. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, B.N.; Guhathakurta, S.; Tsang, T.H.; Schwabenland, M.; Renschler, G.; Herquel, B.; Bhardwaj, V.; Holz, H.; Stehle, T.; Bondareva, O.; et al. Neural metabolic imbalance induced by MOF dysfunction triggers pericyte activation and breakdown of vasculature. Nat. Cell Biol. 2020, 22, 828–841. [Google Scholar] [CrossRef]
- Hu, Y.; Ma, Y.; Liu, L.; Hong, Y.; Wang, G.; Tang, B.; Guo, J.; Yang, P.; Cao, Y.; Ren, H. Loss of NgBR causes neuronal damage through decreasing KAT7-mediated RFX1 acetylation and FGF1 expression. Cell Mol. Life Sci. 2025, 82, 140. [Google Scholar] [CrossRef]
- Grant, P.A.; Duggan, L.; Cote, J.; Roberts, S.M.; Brownell, J.E.; Candau, R.; Ohba, R.; Owen-Hughes, T.; Allis, C.D.; Winston, F.; et al. Yeast Gcn5 functions in two multisubunit complexes to acetylate nucleosomal histones: Characterization of an Ada complex and the SAGA (Spt/Ada) complex. Genes. Dev. 1997, 11, 1640–1650. [Google Scholar] [CrossRef]
- Sterner, D.E.; Berger, S.L. Acetylation of histones and transcription-related factors. Microbiol. Mol. Biol. Rev. 2000, 64, 435–459. [Google Scholar] [CrossRef]
- Peleg, S.; Sananbenesi, F.; Zovoilis, A.; Burkhardt, S.; Bahari-Javan, S.; Agis-Balboa, R.C.; Cota, P.; Wittnam, J.L.; Gogol-Doering, A.; Opitz, L.; et al. Altered histone acetylation is associated with age-dependent memory impairment in mice. Science 2010, 328, 753–756. [Google Scholar] [CrossRef]
- Puttagunta, R.; Tedeschi, A.; Soria, M.G.; Hervera, A.; Lindner, R.; Rathore, K.I.; Gaub, P.; Joshi, Y.; Nguyen, T.; Schmandke, A.; et al. PCAF-dependent epigenetic changes promote axonal regeneration in the central nervous system. Nat. Commun. 2014, 5, 3527. [Google Scholar] [CrossRef]
- Das, C.; Lucia, M.S.; Hansen, K.C.; Tyler, J.K. CBP/p300-mediated acetylation of histone H3 on lysine 56. Nature 2009, 459, 113–117. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Liu, L.; Wang, X. HDAC inhibitor trichostatin A-inhibited survival of dopaminergic neuronal cells. Neurosci. Lett. 2009, 467, 212–216. [Google Scholar] [CrossRef]
- Contreras, P.S.; Gonzalez-Zuniga, M.; Gonzalez-Hodar, L.; Yanez, M.J.; Dulcey, A.; Marugan, J.; Seto, E.; Alvarez, A.R.; Zanlungo, S. Neuronal gene repression in Niemann-Pick type C models is mediated by the c-Abl/HDAC2 signaling pathway. Biochim. Biophys. Acta 2016, 1859, 269–279. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rouaux, C.; Jokic, N.; Mbebi, C.; Boutillier, S.; Loeffler, J.P.; Boutillier, A.L. Critical loss of CBP/p300 histone acetylase activity by caspase-6 during neurodegeneration. EMBO J. 2003, 22, 6537–6549. [Google Scholar] [CrossRef] [PubMed]
- Fitzsimons, H.L.; Scott, M.J. Genetic modulation of Rpd3 expression impairs long-term courtship memory in Drosophila. PLoS ONE 2011, 6, e29171. [Google Scholar] [CrossRef][Green Version]
- Fitzsimons, H.L.; Schwartz, S.; Given, F.M.; Scott, M.J. The histone deacetylase HDAC4 regulates long-term memory in Drosophila. PLoS ONE 2013, 8, e83903. [Google Scholar] [CrossRef]
- Monday, H.R.; Younts, T.J.; Castillo, P.E. Long-Term Plasticity of Neurotransmitter Release: Emerging Mechanisms and Contributions to Brain Function and Disease. Annu. Rev. Neurosci. 2018, 41, 299–322. [Google Scholar] [CrossRef]
- Kim, T.K.; Hemberg, M.; Gray, J.M.; Costa, A.M.; Bear, D.M.; Wu, J.; Harmin, D.A.; Laptewicz, M.; Barbara-Haley, K.; Kuersten, S.; et al. Widespread transcription at neuronal activity-regulated enhancers. Nature 2010, 465, 182–187. [Google Scholar] [CrossRef]
- Karnay, A.; Karisetty, B.C.; Beaver, M.; Elefant, F. Hippocampal stimulation promotes intracellular Tip60 dynamics with concomitant genome reorganization and synaptic gene activation. Mol. Cell. Neurosci. 2019, 101, 103412. [Google Scholar] [CrossRef]
- Schlumm, F.; Mauceri, D.; Freitag, H.E.; Bading, H. Nuclear calcium signaling regulates nuclear export of a subset of class IIa histone deacetylases following synaptic activity. J. Biol. Chem. 2013, 288, 8074–8084. [Google Scholar] [CrossRef]
- Guan, Z.; Giustetto, M.; Lomvardas, S.; Kim, J.H.; Miniaci, M.C.; Schwartz, J.H.; Thanos, D.; Kandel, E.R. Integration of long-term-memory-related synaptic plasticity involves bidirectional regulation of gene expression and chromatin structure. Cell 2002, 111, 483–493. [Google Scholar] [CrossRef]
- Tessier, C.R.; Broadie, K. Activity-dependent modulation of neural circuit synaptic connectivity. Front. Mol. Neurosci. 2009, 2, 8. [Google Scholar] [CrossRef]
- Mayford, M.; Siegelbaum, S.A.; Kandel, E.R. Synapses and memory storage. Cold Spring Harb. Perspect. Biol. 2012, 4, a005751. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yuan, M.; Guo, Y.S.; Shen, X.Y.; Gao, Z.K.; Bi, X. The role of enriched environment in neural development and repair. Front. Cell Neurosci. 2022, 16, 890666. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Sananbenesi, F.; Wang, X.; Dobbin, M.; Tsai, L.H. Recovery of learning and memory is associated with chromatin remodelling. Nature 2007, 447, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Panikker, P.; Iqbal, S.; Elefant, F. Tip60 HAT Action Mediates Environmental Enrichment Induced Cognitive Restoration. PLoS ONE 2016, 11, e0159623. [Google Scholar] [CrossRef]
- Sarantis, K.; Antoniou, K.; Matsokis, N.; Angelatou, F. Exposure to novel environment is characterized by an interaction of D1/NMDA receptors underlined by phosphorylation of the NMDA and AMPA receptor subunits and activation of ERK1/2 signaling, leading to epigenetic changes and gene expression in rat hippocampus. Neurochem. Int. 2012, 60, 55–67. [Google Scholar] [CrossRef]
- Fischer, A.; Sananbenesi, F.; Mungenast, A.; Tsai, L.H. Targeting the correct HDAC(s) to treat cognitive disorders. Trends Pharmacol. Sci. 2010, 31, 605–617. [Google Scholar] [CrossRef]
- Francis, Y.I.; Fa, M.; Ashraf, H.; Zhang, H.; Staniszewski, A.; Latchman, D.S.; Arancio, O. Dysregulation of histone acetylation in the APP/PS1 mouse model of Alzheimer’s disease. J. Alzheimers Dis. 2009, 18, 131–139. [Google Scholar] [CrossRef]
- Graff, J.; Kim, D.; Dobbin, M.M.; Tsai, L.H. Epigenetic regulation of gene expression in physiological and pathological brain processes. Physiol. Rev. 2011, 91, 603–649. [Google Scholar] [CrossRef]
- Graff, J.; Mansuy, I.M. Epigenetic dysregulation in cognitive disorders. Eur. J. Neurosci. 2009, 30, 1–8. [Google Scholar] [CrossRef]
- Stilling, R.M.; Fischer, A. The role of histone acetylation in age-associated memory impairment and Alzheimer’s disease. Neurobiol. Learn. Mem. 2011, 96, 19–26. [Google Scholar] [CrossRef]
- Langley, B.; Gensert, J.M.; Beal, M.F.; Ratan, R.R. Remodeling chromatin and stress resistance in the central nervous system: Histone deacetylase inhibitors as novel and broadly effective neuroprotective agents. Curr. Drug Targets CNS Neurol. Disord. 2005, 4, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Yang, T.; Xu, S.; Li, X.; Liu, L.; Zhou, G.; Yang, S.; Yin, S.; Li, X.J.; Li, S. Huntington’s Disease: Complex Pathogenesis and Therapeutic Strategies. Int. J. Mol. Sci. 2024, 25, 3845. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Vaish, M.; Ratan, R.R. Transcriptional dysregulation in Huntington’s disease: A failure of adaptive transcriptional homeostasis. Drug Discov. Today 2014, 19, 956–962. [Google Scholar] [CrossRef] [PubMed]
- Francelle, L.; Lotz, C.; Outeiro, T.; Brouillet, E.; Merienne, K. Contribution of Neuroepigenetics to Huntington’s Disease. Front. Hum. Neurosci. 2017, 11, 17. [Google Scholar] [CrossRef]
- McCampbell, A.; Fischbeck, K.H. Polyglutamine and CBP: Fatal attraction? Nat. Med. 2001, 7, 528–530. [Google Scholar] [CrossRef]
- Gao, R.; Chakraborty, A.; Geater, C.; Pradhan, S.; Gordon, K.L.; Snowden, J.; Yuan, S.; Dickey, A.S.; Choudhary, S.; Ashizawa, T.; et al. Mutant huntingtin impairs PNKP and ATXN3, disrupting DNA repair and transcription. Elife 2019, 8, 42988. [Google Scholar] [CrossRef]
- Cornett, J.; Friedman, M.; Li, S.H.; Li, X.J. Transcriptional Dysregulation: A therapeutic Target for Polyglutamine Diseases. Curr. Med. Chem. Cenetral Nerv. Syst. Agents 2005, 5, 119–127. [Google Scholar] [CrossRef]
- Beaver, M.; Bhatnagar, A.; Panikker, P.; Zhang, H.; Snook, R.; Parmar, V.; Vijayakumar, G.; Betini, N.; Akhter, S.; Elefant, F. Disruption of Tip60 HAT mediated neural histone acetylation homeostasis is an early common event in neurodegenerative diseases. Sci. Rep. 2020, 10, 18265. [Google Scholar] [CrossRef]
- Steffan, J.S.; Bodai, L.; Pallos, J.; Poelman, M.; McCampbell, A.; Apostol, B.L.; Kazantsev, A.; Schmidt, E.; Zhu, Y.Z.; Greenwald, M.; et al. Histone deacetylase inhibitors arrest polyglutamine-dependent neurodegeneration in Drosophila. Nature 2001, 413, 739–743. [Google Scholar] [CrossRef]
- Cong, S.Y.; Pepers, B.A.; Evert, B.O.; Rubinsztein, D.C.; Roos, R.A.; van Ommen, G.J.; Dorsman, J.C. Mutant huntingtin represses CBP, but not p300, by binding and protein degradation. Mol. Cell. Neurosci. 2005, 30, 12–23. [Google Scholar] [CrossRef]
- Jiang, H.; Nucifora, F.C., Jr.; Ross, C.A.; DeFranco, D.B. Cell death triggered by polyglutamine-expanded huntingtin in a neuronal cell line is associated with degradation of CREB-binding protein. Hum. Mol. Genet. 2003, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pallos, J.; Bodai, L.; Lukacsovich, T.; Purcell, J.M.; Steffan, J.S.; Thompson, L.M.; Marsh, J.L. Inhibition of specific HDACs and sirtuins suppresses pathogenesis in a Drosophila model of Huntington’s disease. Hum. Mol. Genet. 2008, 17, 3767–3775. [Google Scholar] [CrossRef] [PubMed]
- Mielcarek, M.; Benn, C.L.; Franklin, S.A.; Smith, D.L.; Woodman, B.; Marks, P.A.; Bates, G.P. SAHA decreases HDAC 2 and 4 levels in vivo and improves molecular phenotypes in the R6/2 mouse model of Huntington’s disease. PLoS ONE 2011, 6, e27746. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Pallos, J.; Jacques, V.; Lau, A.; Tang, B.; Cooper, A.; Syed, A.; Purcell, J.; Chen, Y.; Sharma, S.; et al. Histone deacetylase (HDAC) inhibitors targeting HDAC3 and HDAC1 ameliorate polyglutamine-elicited phenotypes in model systems of Huntington’s disease. Neurobiol. Dis. 2012, 46, 351–361. [Google Scholar] [CrossRef]
- Suelves, N.; Kirkham-McCarthy, L.; Lahue, R.S.; Gines, S. A selective inhibitor of histone deacetylase 3 prevents cognitive deficits and suppresses striatal CAG repeat expansions in Huntington’s disease mice. Sci. Rep. 2017, 7, 6082. [Google Scholar] [CrossRef]
- Rathore, A.S.; Birla, H.; Singh, S.S.; Zahra, W.; Dilnashin, H.; Singh, R.; Keshri, P.K.; Singh, S.P. Epigenetic Modulation in Parkinson’s Disease and Potential Treatment Therapies. Neurochem. Res. 2021, 46, 1618–1626. [Google Scholar] [CrossRef]
- Srinivasan, E.; Chandrasekhar, G.; Chandrasekar, P.; Anbarasu, K.; Vickram, A.S.; Karunakaran, R.; Rajasekaran, R.; Srikumar, P.S. Alpha-Synuclein Aggregation in Parkinson’s Disease. Front. Med. 2021, 8, 736978. [Google Scholar] [CrossRef]
- Maiti, P.; Manna, J.; Dunbar, G.L. Current understanding of the molecular mechanisms in Parkinson’s disease: Targets for potential treatments. Transl. Neurodegener. 2017, 6, 28. [Google Scholar] [CrossRef]
- Kouli, A.; Torsney, K.M.; Kuan, W.L. Parkinson’s Disease: Etiology, Neuropathology, and Pathogenesis. In Parkinson’s Disease: Pathogenesis and Clinical Aspects; Stoker, T.B., Greenland, J.C., Eds.; Codon Publications: Brisbane, CA, USA, 2018. [Google Scholar] [CrossRef]
- Song, H.; Chen, J.; Huang, J.; Sun, P.; Liu, Y.; Xu, L.; Wei, C.; Mu, X.; Lu, X.; Wang, W.; et al. Epigenetic modification in Parkinson’s disease. Front. Cell Dev. Biol. 2023, 11, 1123621. [Google Scholar] [CrossRef]
- Kontopoulos, E.; Parvin, J.D.; Feany, M.B. Alpha-synuclein acts in the nucleus to inhibit histone acetylation and promote neurotoxicity. Hum. Mol. Genet. 2006, 15, 3012–3023. [Google Scholar] [CrossRef]
- Jin, H.; Kanthasamy, A.; Ghosh, A.; Yang, Y.; Anantharam, V.; Kanthasamy, A.G. alpha-Synuclein negatively regulates protein kinase Cdelta expression to suppress apoptosis in dopaminergic neurons by reducing p300 histone acetyltransferase activity. J. Neurosci. 2011, 31, 2035–2051. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, H.; Jo, A.; Khang, R.; Park, C.H.; Park, S.J.; Kwag, E.; Shin, J.H. alpha-Synuclein A53T Binds to Transcriptional Adapter 2-Alpha and Blocks Histone H3 Acetylation. Int. J. Mol. Sci. 2021, 22, 5392. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, Y. Cross-talk between the H3K36me3 and H4K16ac histone epigenetic marks in DNA double-strand break repair. J. Biol. Chem. 2017, 292, 11951–11959. [Google Scholar] [CrossRef]
- Pfister, J.A.; Ma, C.; Morrison, B.E.; D’Mello, S.R. Opposing effects of sirtuins on neuronal survival: SIRT1-mediated neuroprotection is independent of its deacetylase activity. PLoS ONE 2008, 3, e4090. [Google Scholar] [CrossRef]
- Suzuki, K.; Koike, T. Resveratrol abolishes resistance to axonal degeneration in slow Wallerian degeneration (WldS) mice: Activation of SIRT2, an NAD-dependent tubulin deacetylase. Biochem. Biophys. Res. Commun. 2007, 359, 665–671. [Google Scholar] [CrossRef]
- Outeiro, T.F.; Kontopoulos, E.; Altmann, S.M.; Kufareva, I.; Strathearn, K.E.; Amore, A.M.; Volk, C.B.; Maxwell, M.M.; Rochet, J.C.; McLean, P.J.; et al. Sirtuin 2 inhibitors rescue alpha-synuclein-mediated toxicity in models of Parkinson’s disease. Science 2007, 317, 516–519. [Google Scholar] [CrossRef]
- Park, G.; Tan, J.; Garcia, G.; Kang, Y.; Salvesen, G.; Zhang, Z. Regulation of Histone Acetylation by Autophagy in Parkinson Disease. J. Biol. Chem. 2016, 291, 3531–3540. [Google Scholar] [CrossRef]
- Toker, L.; Tran, G.T.; Sundaresan, J.; Tysnes, O.B.; Alves, G.; Haugarvoll, K.; Nido, G.S.; Dolle, C.; Tzoulis, C. Genome-wide histone acetylation analysis reveals altered transcriptional regulation in the Parkinson’s disease brain. Mol. Neurodegener. 2021, 16, 31. [Google Scholar] [CrossRef]
- Kim, T.; Song, S.; Park, Y.; Kang, S.; Seo, H. HDAC Inhibition by Valproic Acid Induces Neuroprotection and Improvement of PD-like Behaviors in LRRK2 R1441G Transgenic Mice. Exp. Neurobiol. 2019, 28, 504–515. [Google Scholar] [CrossRef]
- Guo, T.T.; Zhang, Z.; Sun, Y.; Zhu, R.Y.; Wang, F.X.; Ma, L.J.; Jiang, L.; Liu, H.D. Neuroprotective Effects of Sodium Butyrate by Restoring Gut Microbiota and Inhibiting TLR4 Signaling in Mice with MPTP-Induced Parkinson’s Disease. Nutrients 2023, 15, 930. [Google Scholar] [CrossRef]
- Baddour, E.; Tewksbury, A.; Stauner, N. Valproic acid-induced hyperammonemia: Incidence, clinical significance, and treatment management. Ment. Health Clin. 2018, 8, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Buoli, M.; Serati, M.; Botturi, A.; Altamura, A.C. The Risk of Thrombocytopenia During Valproic Acid Therapy: A Critical Summary of Available Clinical Data. Drugs R D 2018, 18, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, F.; Tampi, R.R. Valproic acid-induced parkinsonism in the elderly: A comprehensive review of the literature. Am. J. Geriatr. Pharmacother. 2011, 9, 405–412. [Google Scholar] [CrossRef]
- Silver, M.; Factor, S.A. Valproic acid-induced parkinsonism: Levodopa responsiveness with dyskinesia. Park. Relat. Disord. 2013, 19, 758–760. [Google Scholar] [CrossRef]
- Muralidharan, A.; Rahman, J.; Banerjee, D.; Hakim Mohammed, A.R.; Malik, B.H. Parkinsonism: A Rare Adverse Effect of Valproic Acid. Cureus 2020, 12, e8782. [Google Scholar] [CrossRef]
- Ranieri, F.; Mariotto, S.; Dubbioso, R.; Di Lazzaro, V. Brain Stimulation as a Therapeutic Tool in Amyotrophic Lateral Sclerosis: Current Status and Interaction With Mechanisms of Altered Cortical Excitability. Front. Neurol. 2021, 11, 605335. [Google Scholar] [CrossRef]
- Xu, R.S.; Yuan, M. Considerations on the concept, definition, and diagnosis of amyotrophic lateral sclerosis. Neural Regen. Res. 2021, 16, 1723–1729. [Google Scholar] [CrossRef]
- Al-Chalabi, A.; Hardiman, O. The epidemiology of ALS: A conspiracy of genes, environment and time. Nat. Rev. Neurol. 2013, 9, 617–628. [Google Scholar] [CrossRef]
- Kaur, S.J.; McKeown, S.R.; Rashid, S. Mutant SOD1 mediated pathogenesis of Amyotrophic Lateral Sclerosis. Gene 2016, 577, 109–118. [Google Scholar] [CrossRef]
- Wang, Z.; Zang, C.; Rosenfeld, J.A.; Schones, D.E.; Barski, A.; Cuddapah, S.; Cui, K.; Roh, T.Y.; Peng, W.; Zhang, M.Q.; et al. Combinatorial patterns of histone acetylations and methylations in the human genome. Nat. Genet. 2008, 40, 897–903. [Google Scholar] [CrossRef]
- Bennett, S.A.; Tanaz, R.; Cobos, S.N.; Torrente, M.P. Epigenetics in amyotrophic lateral sclerosis: A role for histone post-translational modifications in neurodegenerative disease. Transl. Res. 2019, 204, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Bennett, S.A.; Rana, N.; Yousuf, H.; Said, M.; Taaseen, S.; Mendo, N.; Meltser, S.M.; Torrente, M.P. Neurodegenerative Disease Proteinopathies Are Connected to Distinct Histone Post-translational Modification Landscapes. ACS Chem. Neurosci. 2018, 9, 838–848. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Lorbeck, M.T.; Zervos, A.; Zimmerman, J.; Elefant, F. The histone acetyltransferase Elp3 plays in active role in the control of synaptic bouton expansion and sleep in Drosophila. J. Neurochem. 2010, 115, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Simpson, C.L.; Lemmens, R.; Miskiewicz, K.; Broom, W.J.; Hansen, V.K.; van Vught, P.W.; Landers, J.E.; Sapp, P.; Van Den Bosch, L.; Knight, J.; et al. Variants of the elongator protein 3 (ELP3) gene are associated with motor neuron degeneration. Hum. Mol. Genet. 2009, 18, 472–481. [Google Scholar] [CrossRef]
- Bento-Abreu, A.; Jager, G.; Swinnen, B.; Rue, L.; Hendrickx, S.; Jones, A.; Staats, K.A.; Taes, I.; Eykens, C.; Nonneman, A.; et al. Elongator subunit 3 (ELP3) modifies ALS through tRNA modification. Hum. Mol. Genet. 2018, 27, 1276–1289. [Google Scholar] [CrossRef]
- Figueroa-Romero, C.; Hur, J.; Bender, D.E.; Delaney, C.E.; Cataldo, M.D.; Smith, A.L.; Yung, R.; Ruden, D.M.; Callaghan, B.C.; Feldman, E.L. Identification of epigenetically altered genes in sporadic amyotrophic lateral sclerosis. PLoS ONE 2012, 7, e52672. [Google Scholar] [CrossRef]
- Oates, N.; Pamphlett, R. An epigenetic analysis of SOD1 and VEGF in ALS. Amyotroph. Lateral Scler. 2007, 8, 83–86. [Google Scholar] [CrossRef]
- Joyce, P.I.; McGoldrick, P.; Saccon, R.A.; Weber, W.; Fratta, P.; West, S.J.; Zhu, N.; Carter, S.; Phatak, V.; Stewart, M.; et al. A novel SOD1-ALS mutation separates central and peripheral effects of mutant SOD1 toxicity. Hum. Mol. Genet. 2015, 24, 1883–1897. [Google Scholar] [CrossRef]
- Yoo, Y.E.; Ko, C.P. Treatment with trichostatin A initiated after disease onset delays disease progression and increases survival in a mouse model of amyotrophic lateral sclerosis. Exp. Neurol. 2011, 231, 147–159. [Google Scholar] [CrossRef]
- Guo, W.; Naujock, M.; Fumagalli, L.; Vandoorne, T.; Baatsen, P.; Boon, R.; Ordovas, L.; Patel, A.; Welters, M.; Vanwelden, T.; et al. HDAC6 inhibition reverses axonal transport defects in motor neurons derived from FUS-ALS patients. Nat. Commun. 2017, 8, 861. [Google Scholar] [CrossRef]
- Janssen, C.; Schmalbach, S.; Boeselt, S.; Sarlette, A.; Dengler, R.; Petri, S. Differential histone deacetylase mRNA expression patterns in amyotrophic lateral sclerosis. J. Neuropathol. Exp. Neurol. 2010, 69, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, W.; Ye, Q.; Fu, Y.; Li, X.; Yang, K.; Gao, F.; Zhou, A.; Wei, Y.; Tian, S.; et al. Histone deacetylase inhibitors VPA and WT161 ameliorate the pathological features and cognitive impairments of the APP/PS1 Alzheimer’s disease mouse model by regulating the expression of APP secretases. Alzheimers Res. Ther. 2024, 16, 15. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jiao, B.; Shen, L. The Epigenetics of Alzheimer’s Disease: Factors and Therapeutic Implications. Front. Genet. 2018, 9, 579. [Google Scholar] [CrossRef]
- Rajmohan, R.; Reddy, P.H. Amyloid-Beta and Phosphorylated Tau Accumulations Cause Abnormalities at Synapses of Alzheimer’s disease Neurons. J. Alzheimers Dis. 2017, 57, 975–999. [Google Scholar] [CrossRef]
- Mastroeni, D.; Grover, A.; Delvaux, E.; Whiteside, C.; Coleman, P.D.; Rogers, J. Epigenetic mechanisms in Alzheimer’s disease. Neurobiol. Aging 2011, 32, 1161–1180. [Google Scholar] [CrossRef]
- Karisetty, B.C.; Bhatnagar, A.; Armour, E.M.; Beaver, M.; Zhang, H.; Elefant, F. Amyloid-beta Peptide Impact on Synaptic Function and Neuroepigenetic Gene Control Reveal New Therapeutic Strategies for Alzheimer’s Disease. Front. Mol. Neurosci. 2020, 13, 577622. [Google Scholar] [CrossRef]
- Zhang, H.; Karisetty, B.C.; Bhatnagar, A.; Armour, E.M.; Beaver, M.; Roach, T.V.; Mortazavi, S.; Mandloi, S.; Elefant, F. Tip60 protects against amyloid-beta-induced transcriptomic alterations via different modes of action in early versus late stages of neurodegeneration. Mol. Cell. Neurosci. 2020, 109, 103570. [Google Scholar] [CrossRef]
- Currais, A.; Huang, L.; Goldberg, J.; Petrascheck, M.; Ates, G.; Pinto-Duarte, A.; Shokhirev, M.N.; Schubert, D.; Maher, P. Elevating acetyl-CoA levels reduces aspects of brain aging. Elife 2019, 8, 47866. [Google Scholar] [CrossRef]
- Ricobaraza, A.; Cuadrado-Tejedor, M.; Perez-Mediavilla, A.; Frechilla, D.; Del Rio, J.; Garcia-Osta, A. Phenylbutyrate ameliorates cognitive deficit and reduces tau pathology in an Alzheimer’s disease mouse model. Neuropsychopharmacology 2009, 34, 1721–1732. [Google Scholar] [CrossRef]
- Marzi, S.J.; Leung, S.K.; Ribarska, T.; Hannon, E.; Smith, A.R.; Pishva, E.; Poschmann, J.; Moore, K.; Troakes, C.; Al-Sarraj, S.; et al. A histone acetylome-wide association study of Alzheimer’s disease identifies disease-associated H3K27ac differences in the entorhinal cortex. Nat Neurosci. 2018, 21, 1618–1627. [Google Scholar] [CrossRef] [PubMed]
- Nativio, R.; Donahue, G.; Berson, A.; Lan, Y.; Amlie-Wolf, A.; Tuzer, F.; Toledo, J.B.; Gosai, S.J.; Gregory, B.D.; Torres, C.; et al. Dysregulation of the epigenetic landscape of normal aging in Alzheimer’s disease. Nat. Neurosci. 2018, 21, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Hendrickx, A.; Peirrot, N.; Tasiaux, B.; Schakman, O.; Kienlen-Campard, P.; De Smet, C.; Octabe, J.-N. Epigenetic regulationsof immediate early genes experssion involved in memory formation by the amyloid precursor protein of Alzheimer Disease. PLoS ONE 2014, 9, e99467. [Google Scholar] [CrossRef] [PubMed]
- Ettcheto, M.; Abad, S.; Petrov, D.; Pedros, I.; Busquets, O.; Sanchez-Lopez, E.; Casadesus, G.; Beas-Zarate, C.; Carro, E.; Auladell, C.; et al. Early Preclinical Changes in Hippocampal CREB-Binding Protein Expression in a Mouse Model of Familial Alzheimer’s Disease. Mol. Neurobiol. 2018, 55, 4885–4895. [Google Scholar] [CrossRef]
- Saura, C.A.; Choi, S.Y.; Beglopoulos, V.; Malkani, S.; Zhang, D.; Shankaranarayana Rao, B.S.; Chattarji, S.; Kelleher, R.J., 3rd; Kandel, E.R.; Duff, K.; et al. Loss of presenilin function causes impairments of memory and synaptic plasticity followed by age-dependent neurodegeneration. Neuron 2004, 42, 23–36. [Google Scholar] [CrossRef]
- Kilgore, M.; Miller, C.A.; Fass, D.M.; Hennig, K.M.; Haggarty, S.J.; Sweatt, J.D.; Rumbaugh, G. Inhibitors of class 1 histone deacetylases reverse contextual memory deficits in a mouse model of Alzheimer’s disease. Neuropsychopharmacology 2010, 35, 870–880. [Google Scholar] [CrossRef]
- Ricobaraza, A.; Cuadrado-Tejedor, M.; Garcia-Osta, A. Long-term phenylbutyrate administration prevents memory deficits in Tg2576 mice by decreasing Abeta. Front Biosci (Elite Ed) 2011, 3, 1375–1384. [Google Scholar] [CrossRef]
- Selvi, B.R.; Cassel, J.C.; Kundu, T.K.; Boutillier, A.L. Tuning acetylation levels with HAT activators: Therapeutic strategy in neurodegenerative diseases. Biochim. Biophys. Acta 2010, 1799, 840–853. [Google Scholar] [CrossRef]
- NCT03061474. Nicotinamide as an Early Alzheimer’s Disease Treatment. Available online: https://clinicaltrials.gov/study/NCT03061474 (accessed on 5 March 2025).
- NTC03056495. Clinical Trial to Determine Tolerable Dosis of Vorinostat in Patients with Mild Alzheimer’s Disease. Available online: https://clinicaltrials.gov/study/NCT03056495 (accessed on 5 March 2025).
- Hanson, J.E.; La, H.; Plise, E.; Chen, Y.H.; Ding, X.; Hanania, T.; Sabath, E.V.; Alexandrov, V.; Brunner, D.; Leahy, E.; et al. SAHA enhances synaptic function and plasticity in vitro but has limited brain availability in vivo and does not impact cognition. PLoS ONE 2013, 8, e69964. [Google Scholar] [CrossRef]
- Didonna, A.; Opal, P. The promise and perils of HDAC inhibitors in neurodegeneration. Ann. Clin. Transl. Neurol. 2015, 2, 79–101. [Google Scholar] [CrossRef]
- Jeong, H.; Shin, S.; Lee, J.S.; Lee, S.H.; Baik, J.H.; Lim, S.; Kim, Y.K. Pan-HDAC Inhibitors Promote Tau Aggregation by Increasing the Level of Acetylated Tau. Int. J. Mol. Sci. 2019, 20, 4283. [Google Scholar] [CrossRef]
- Phelan, M.; Mulnard, R.; Gillen, D.; Schreiber, S. Phase II Clinical Trial of Nicotinamide for the Treatment of Mild to Moderate Alzheimer’s Disease. Available online: https://clinmedjournals.org/articles/jgmg/journal-of-geriatric-medicine-and-gerontology-jgmg-3-021.php?jid=jgmg (accessed on 5 March 2025).
- Harrison, I.F.; Powell, N.M.; Dexter, D.T. The histone deacetylase inhibitor nicotinamide exacerbates neurodegeneration in the lactacystin rat model of Parkinson’s disease. J. Neurochem. 2019, 148, 136–156. [Google Scholar] [CrossRef] [PubMed]
- Beaver, M.; Karisetty, B.C.; Zhang, H.; Bhatnagar, A.; Armour, E.; Parmar, V.; Brown, R.; Xiang, M.; Elefant, F. Chromatin and transcriptomic profiling uncover dysregulation of the Tip60 HAT/HDAC2 epigenomic landscape in the neurodegenerative brain. Epigenetics 2022, 17, 786–807. [Google Scholar] [CrossRef] [PubMed]
- Sarthi, J.; Elefant, F. dTip60 HAT activity controls synaptic bouton expansion at the Drosophila neuromuscular junction. PLoS ONE 2011, 6, e26202. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhu, X.; Singh, N.; Donnelly, C.; Boimel, P.; Elefant, F. The cloning and characterization of the histone acetyltransferase human homolog Dmel\TIP60 in Drosophila melanogaster: Dmel\TIP60 is essential for multicellular development. Genetics 2007, 175, 1229–1240. [Google Scholar] [CrossRef]
- Gaub, P.; Joshi, Y.; Wuttke, A.; Naumann, U.; Schnichels, S.; Heiduschka, P.; Di Giovanni, S. The histone acetyltransferase p300 promotes intrinsic axonal regeneration. Brain 2011, 134, 2134–2148. [Google Scholar] [CrossRef]
- Vecsey, C.G.; Hawk, J.D.; Lattal, K.M.; Stein, J.M.; Fabian, S.A.; Attner, M.A.; Cabrera, S.M.; McDonough, C.B.; Brindle, P.K.; Abel, T.; et al. Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB:CBP-dependent transcriptional activation. J. Neurosci. 2007, 27, 6128–6140. [Google Scholar] [CrossRef]
- Nguyen, L.; Humbert, S.; Saudou, F.; Chariot, A. Elongator—An emerging role in neurological disorders. Trends Mol. Med. 2010, 16, 1–6. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Krick, K.; Karisetty, B.C.; Armour, E.M.; Heller, E.A.; Elefant, F. Tip60’s Novel RNA-Binding Function Modulates Alternative Splicing of Pre-mRNA Targets Implicated in Alzheimer’s Disease. J. Neurosci. 2023, 43, 2398–2423. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Thomas, C.M.; Nge, G.G.; Zaya, A.; Dasari, R.; Chongtham, N.; Manandhar, B.; Kortagere, S.; Elefant, F. Tip60 HAT activators as therapeutic modulators for Alzheimer’s disease. Nat. Commun. 2025, 16, 3347. [Google Scholar] [CrossRef]
| HAT/HDAC | ND | Modulation | Reference |
|---|---|---|---|
| CBP (HAT) | Huntington’s | Redistributed in cytoplasm vs nuclear | [86] |
 ubiquitylation and degradation ubiquitylation and degradation | [87,91,92] | ||
 Inhibition caused by inappropriate binding of first exon of HTT Inhibition caused by inappropriate binding of first exon of HTT | [88] | ||
| Parkinson’s | Inactivation caused by alpha-syn protein | [102,103] | |
| Unaltered levels | |||
| Amyotrophic Lateral Sclerosis |  Inhibition caused by overexpression of FUS gene Inhibition caused by overexpression of FUS gene | [63,123,130] | |
| Alzheimer’s |  levels by loss of function mutations in PS1 and PS2 levels by loss of function mutations in PS1 and PS2 | [146] | |
| PCAF (HAT) | Huntington’s disease |  Inhibition caused by inappropriate binding of first exon of HTT Inhibition caused by inappropriate binding of first exon of HTT | [87,88] |
| Parkinson’s | Inactivation caused by alpha-syn protein | [102,103] | |
| P300 (HAT) | Parkinson’s | Inactivation caused by alpha-syn protein | [102,103] |
| Amyotrophic Lateral Sclerosis | Inhibition caused by overexpression of FUS gene | [122,123] | |
| SIRT2 (HDAC) | Parkinson’s |  levels and accumulation levels and accumulation | [108] |
| Huntington’s | Suppression leads to neuroprotection | [93] | |
| ELP3 (HAT) | Amyotrophic Lateral Sclerosis | 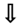 levels levels | [127] |
| Tip60 (HAT) | Huntington’s |  levels levels | [157] |
| Parkingson’s |  levels levels | [157] | |
| Amyotrophic Lateral Sclerosis |  levels levels | [157] | |
| Alzheimer’s | 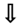 protein levels protein levels | [47] | |
| RPD3 (HDAC) | Huntington’s | Suppression leads to neuroprotection | [93] |
| Therapeutic | Modulation | ND Applicable | Clinical Application | Reference |
|---|---|---|---|---|
| TSA | Small molecule HDAC Inhibitor | Huntington | Pre-clinical/experimental | [90] |
| ALS | Pre-clinical/ experimental | [131] | ||
| Vorinostat (SAHA) | Small molecule pan-HDAC inhibitor | Huntington | Pre-clinical/experimental | [90] |
| Parkinson’s | Pre-clinical/ experimental | [102] | ||
| Alzheimer’s | Pre-clinical/experimental | [147] | ||
| Sodium Butyrate | Short fatty acid HDAC inhibitor | Alzheimer’s | Pre-clinical/experimental | [147] |
| Parkinson’s | Pre-clinical/experimental | [112] | ||
| Huntington | Pre-clinical/experimental | [102] | ||
| Sodium Valproate (VPA) | Small molecule HDAC inhibitor Negatively impacts Acetyl-CoA action | Parkinson’s | Pre-clinical/experimental | [111] |
| ALS | Pre-clinical/experimental | [63] | ||
| Alzheimer’s | Pre-clinical/experimental | [134,147] | ||
| 4B | Pimelic diphenylamide HDAC inhibitor | Huntington’s | Pre-clinical/experimental | [95] |
| RGFP966 | Selective HDAC 3 inhibitor | Huntington’s | Pre-clinical/ experimental | [96] |
| CSP-TTK21 | P300/CBP Hat activator | Alzheimer’s | Pre-clinical/experimental | [12,13] |
| CTPB | HAT Activator | Alzheimer’s | Pre-clinical/experimental | [13] |
| P-compounds (P10, P13) | Specific Tip60 HAT Activator | Alzheimer’s | Pre-clinical/experimental | [163] |
| WT161 | Selective HDAC6 inhibitor | Alzheimer’s | Preclinical/ experimental | [134] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mai, H.A.; Thomas, C.M.; Nge, G.G.; Elefant, F. Modulating Cognition-Linked Histone Acetyltransferases (HATs) as a Therapeutic Strategy for Neurodegenerative Diseases: Recent Advances and Future Trends. Cells 2025, 14, 873. https://doi.org/10.3390/cells14120873
Mai HA, Thomas CM, Nge GG, Elefant F. Modulating Cognition-Linked Histone Acetyltransferases (HATs) as a Therapeutic Strategy for Neurodegenerative Diseases: Recent Advances and Future Trends. Cells. 2025; 14(12):873. https://doi.org/10.3390/cells14120873
Chicago/Turabian StyleMai, Huong Anh, Christina M. Thomas, Gu Gu Nge, and Felice Elefant. 2025. "Modulating Cognition-Linked Histone Acetyltransferases (HATs) as a Therapeutic Strategy for Neurodegenerative Diseases: Recent Advances and Future Trends" Cells 14, no. 12: 873. https://doi.org/10.3390/cells14120873
APA StyleMai, H. A., Thomas, C. M., Nge, G. G., & Elefant, F. (2025). Modulating Cognition-Linked Histone Acetyltransferases (HATs) as a Therapeutic Strategy for Neurodegenerative Diseases: Recent Advances and Future Trends. Cells, 14(12), 873. https://doi.org/10.3390/cells14120873






