ARID4B: An Orchestrator from Stem Cell Fate to Carcinogenesis
Abstract
1. Introduction
1.1. Epigenetic Regulation of Cell Fate/Cellular Phenotype
1.2. AT Rich Interaction Domains-Containing Protein Family
2. ARID4 Subfamily: ARID4A and ARID4B
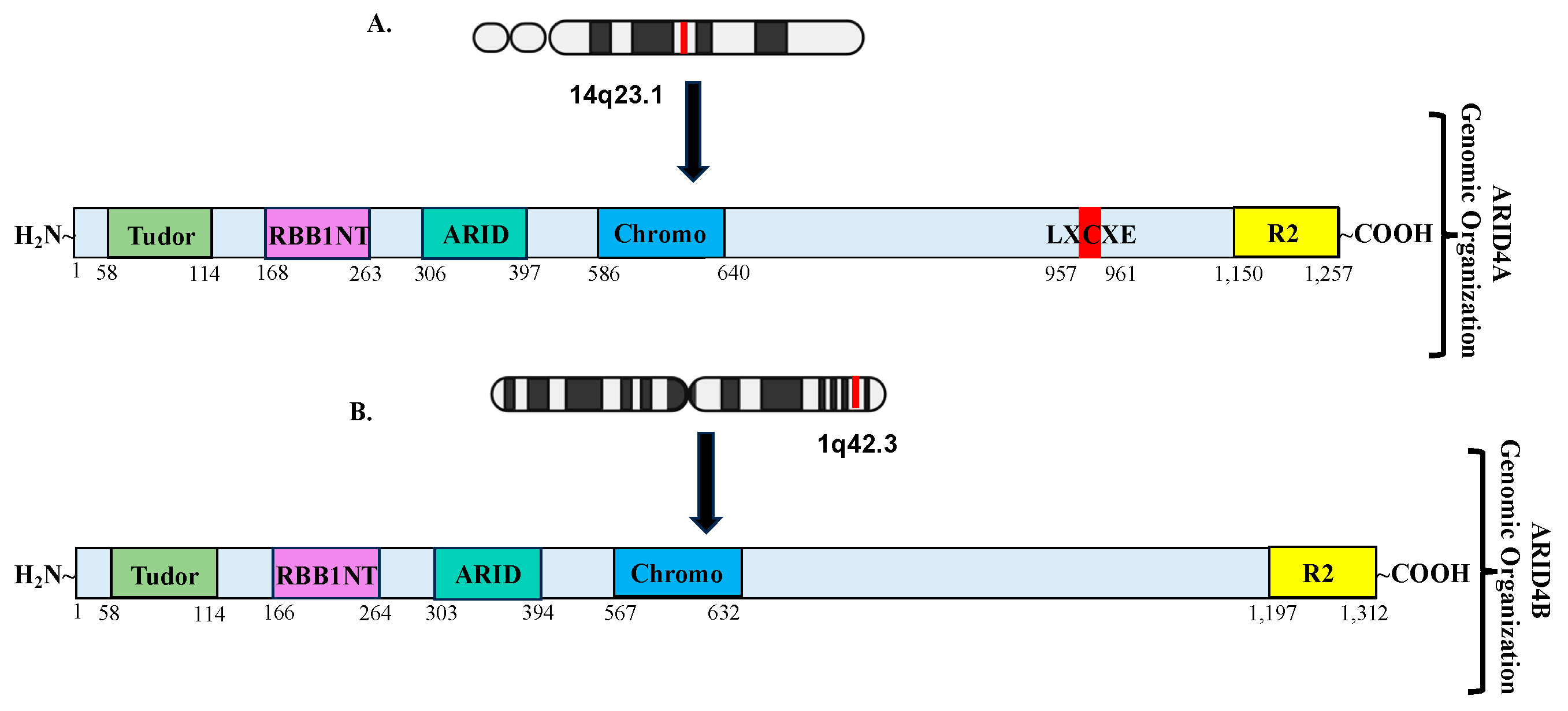
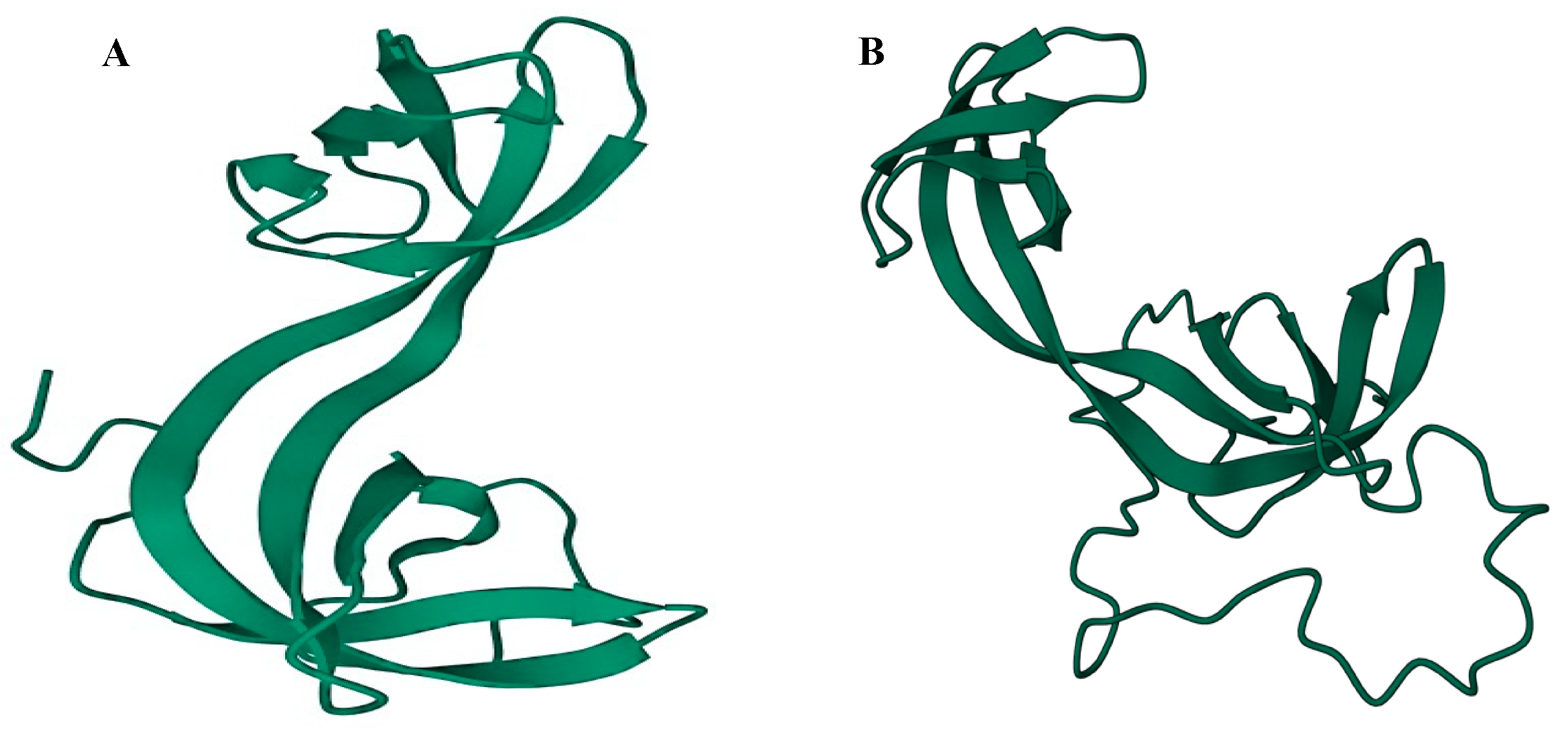
2.1. Structural/Functional Domains of ARID4A and ARID4B
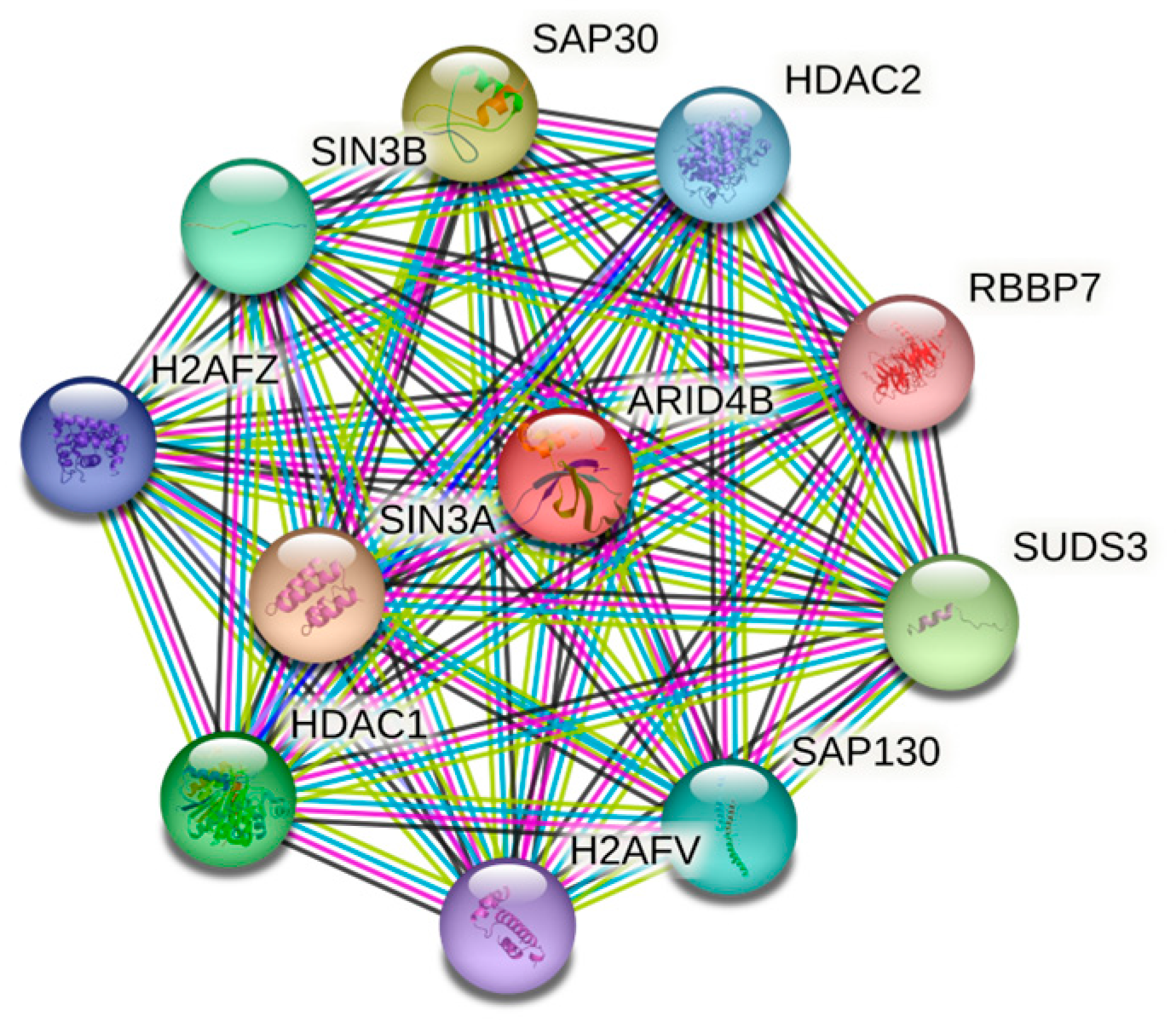
2.2. Functional Redundancy of Arid4a and Arid4b
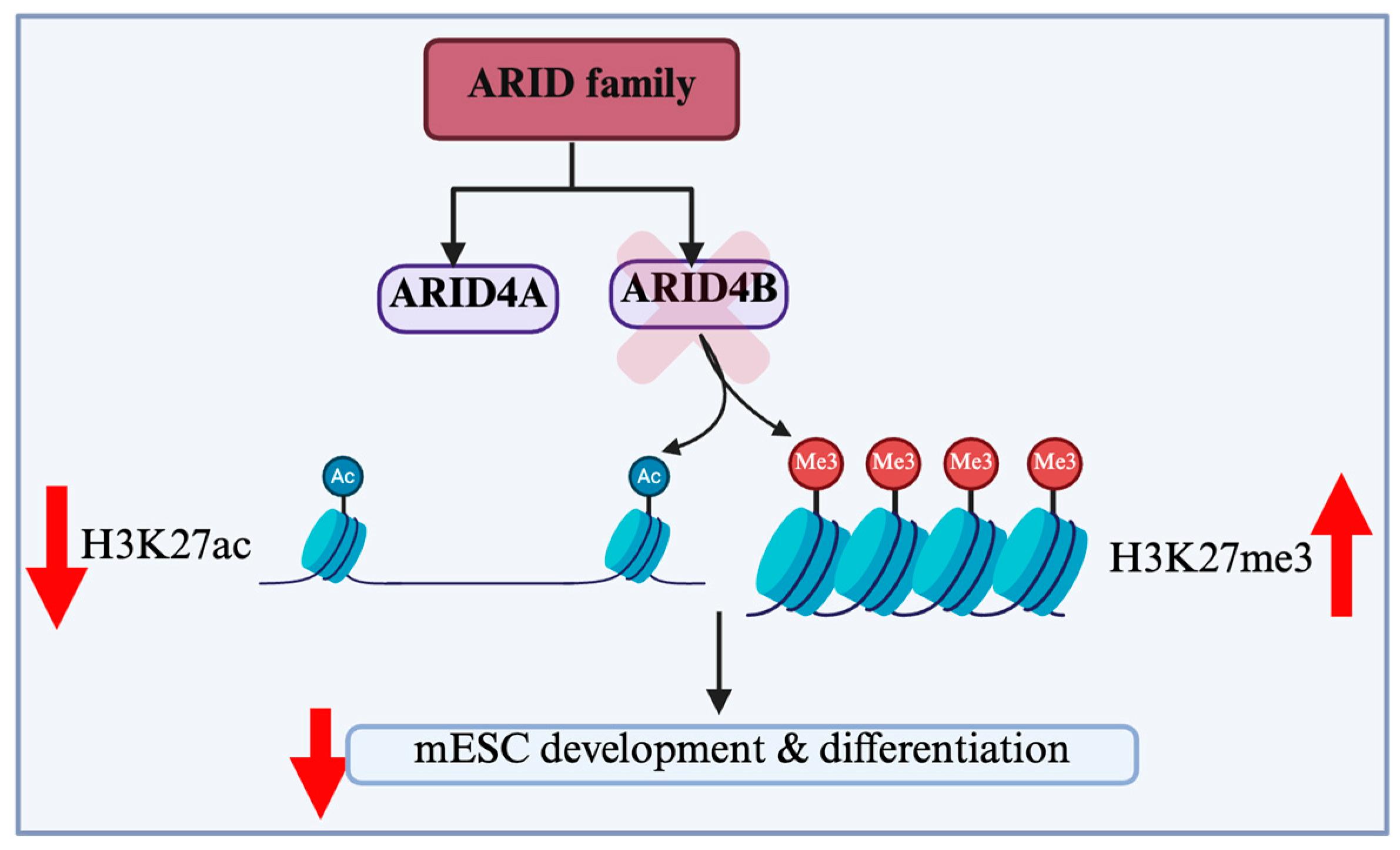
2.3. ARID4B: An Orchestrator of Stem Cell Fate
2.4. Spermatogenesis
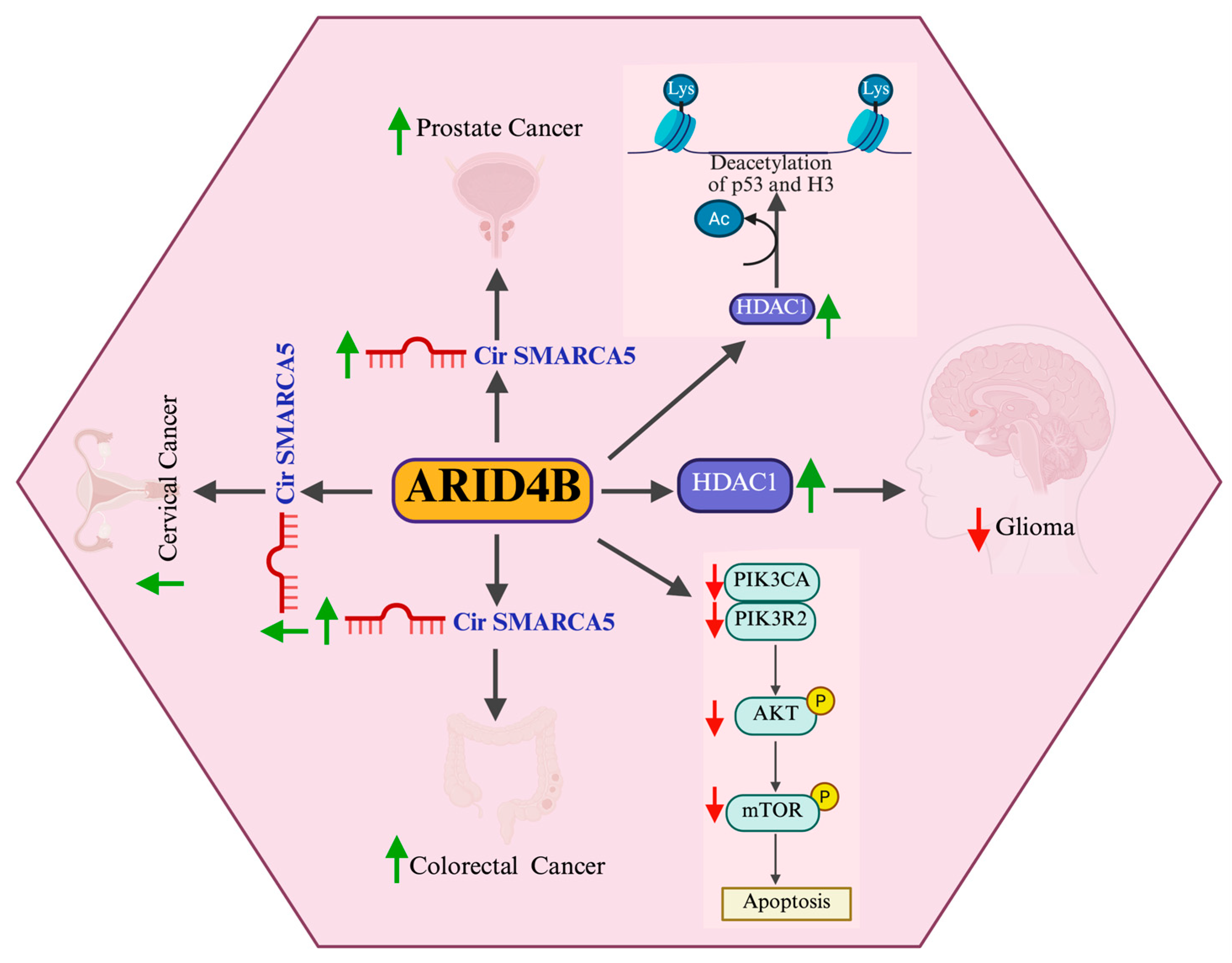
3. The Role of ARID4B in Cancer
3.1. Prostate Cancer
3.2. Breast Cancer
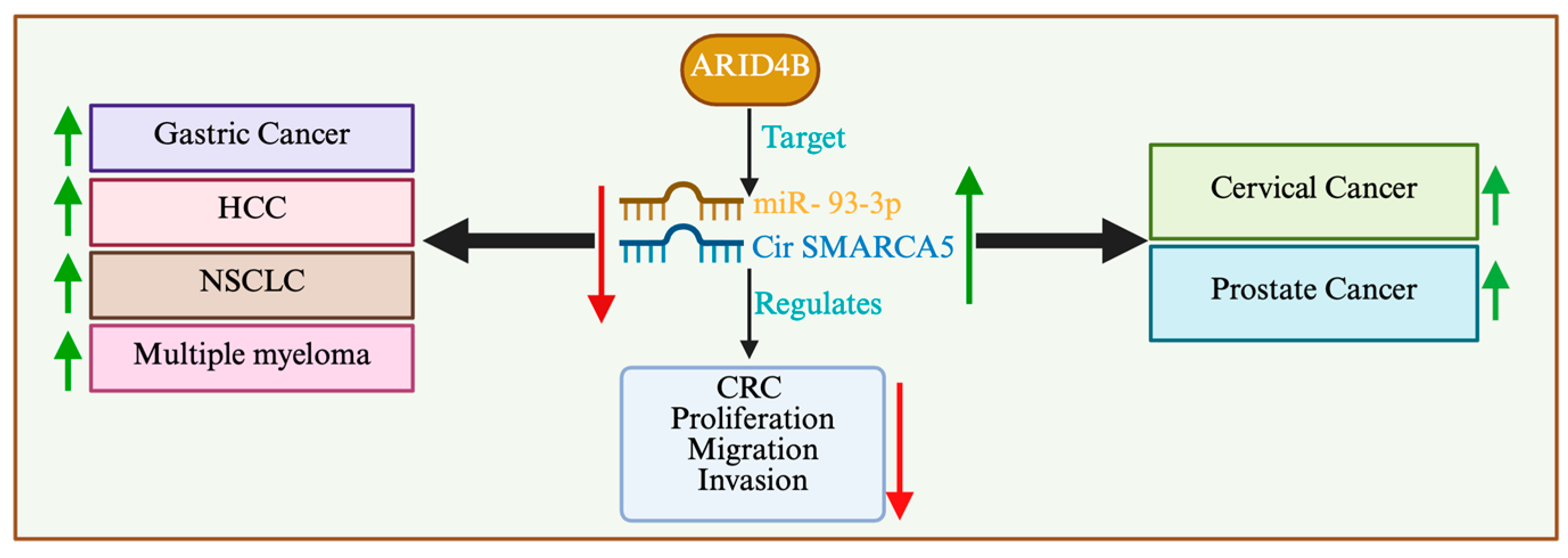
3.3. Colorectal Carcinoma
3.4. Hepatocellular Carcinoma
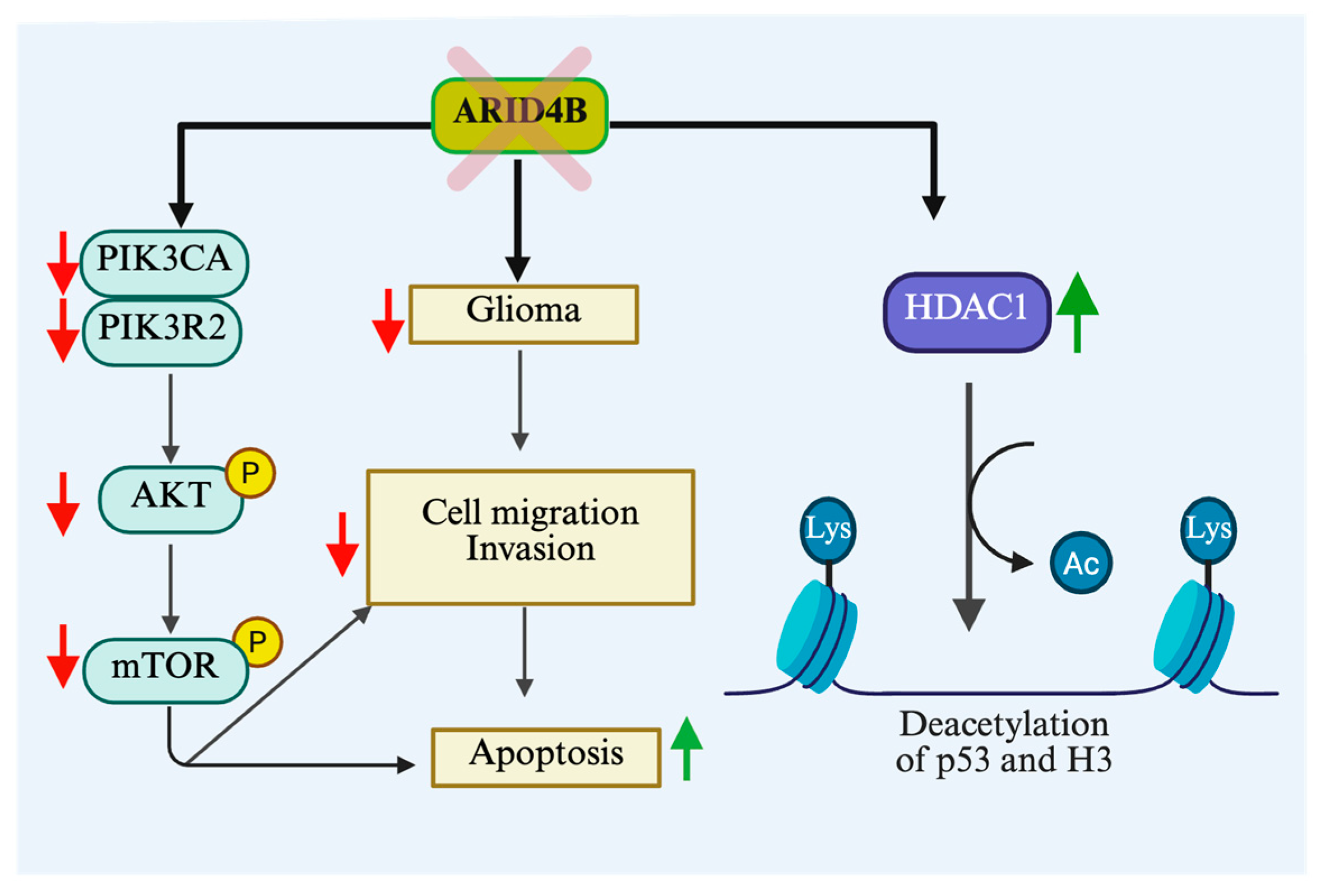
3.5. Glioblastoma
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AKT | Protein Kinase B |
| ARID | AT rich interaction domains |
| circ-SMARCA5 | Circular RNA SMARCA5 |
| H3K27me3 | Trimethylation of Histone H3 at Lysine 27 |
| HDAC1 | Histone Deacetylase 1 |
| Lys | Lysine |
| mTOR | Mammalian target of rapamycin |
| NSCLC | Non-small cell lung cancer |
| p53 | Tumor Protein p53 |
| PI3K | Phosphoinositide 3-Kinase |
| PIK3CA | Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha |
| PIK3R2 | Phosphoinositide-3-Kinase Regulatory Subunit 2 |
| PIP2 | Phosphatidylinositol 4,5-bisphosphate |
| PIP3 | Phosphatidyl-inositol 3,4,5-trisphosphate |
References
- Waddington, C.H. The epigenotype. 1942. Int. J. Epidemiol. 2012, 41, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Dawson, M.A.; Kouzarides, T. Cancer epigenetics: From mechanism to therapy. Cell 2012, 150, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Egger, G.; Liang, G.; Aparicio, A.; Jones, P.A. Epigenetics in human disease and prospects for epigenetic therapy. Nature 2004, 429, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.A.; Baylin, S.B. The fundamental role of epigenetic events in cancer. Nat. Rev. Genet. 2002, 3, 415–428. [Google Scholar] [CrossRef]
- Wilsker, D.; Probst, L.; Wain, H.M.; Maltais, L.; Tucker, P.W.; Moran, E. Nomenclature of the ARID family of DNA-binding proteins. Genomics 2005, 86, 242–251. [Google Scholar] [CrossRef]
- Herrscher, R.F.; Kaplan, M.H.; Lelsz, D.L.; Das, C.; Scheuermann, R.; Tucker, P.W. The immunoglobulin heavy-chain matrix-associating regions are bound by Bright: A B cell-specific trans-activator that describes a new DNA-binding protein family. Genes. Dev. 1995, 9, 3067–3082. [Google Scholar] [CrossRef]
- Gregory, S.L.; Kortschak, R.D.; Kalionis, B.; Saint, R. Characterization of the dead ringer gene identifies a novel, highly conserved family of sequence-specific DNA-binding proteins. Mol. Cell Biol. 1996, 16, 792–799. [Google Scholar] [CrossRef]
- D’Elia, A.V.; Tell, G.; Paron, I.; Pellizzari, L.; Lonigro, R.; Damante, G. Missense mutations of human homeoboxes: A review. Hum. Mutat. 2001, 18, 361–374. [Google Scholar] [CrossRef]
- Kortschak, R.D.; Tucker, P.W.; Saint, R. ARID proteins come in from the desert. Trends Biochem. Sci. 2000, 25, 294–299. [Google Scholar] [CrossRef]
- Wilsker, D.; Patsialou, A.; Dallas, P.B.; Moran, E. ARID proteins: A diverse family of DNA binding proteins implicated in the control of cell growth, differentiation, and development. Cell Growth Differ. 2002, 13, 95–106. [Google Scholar]
- Deak, G.; Cook, A.G. Missense Variants Reveal Functional Insights into the Human ARID Family of Gene Regulators. J. Mol. Biol. 2022, 434, 167529. [Google Scholar] [CrossRef] [PubMed]
- Shandala, T.; Kortschak, R.D.; Saint, R. The Drosophila retained/dead ringer gene and ARID gene family function during development. Int. J. Dev. Biol. 2002, 46, 423–430. [Google Scholar] [PubMed]
- Baba, A.; Ohtake, F.; Okuno, Y.; Yokota, K.; Okada, M.; Imai, Y.; Ni, M.; Meyer, C.A.; Igarashi, K.; Kanno, J.; et al. PKA-dependent regulation of the histone lysine demethylase complex PHF2-ARID5B. Nat. Cell Biol. 2011, 13, 668–675. [Google Scholar] [CrossRef]
- Gong, W.; Zhou, T.; Mo, J.; Perrett, S.; Wang, J.; Feng, Y. Structural insight into recognition of methylated histone tails by retinoblastoma-binding protein 1. J. Biol. Chem. 2012, 287, 8531–8540. [Google Scholar] [CrossRef]
- Lai, A.; Kennedy, B.K.; Barbie, D.A.; Bertos, N.R.; Yang, X.J.; Theberge, M.C.; Tsai, S.C.; Seto, E.; Zhang, Y.; Kuzmichev, A.; et al. RBP1 recruits the mSIN3-histone deacetylase complex to the pocket of retinoblastoma tumor suppressor family proteins found in limited discrete regions of the nucleus at growth arrest. Mol. Cell Biol. 2001, 21, 2918–2932. [Google Scholar] [CrossRef]
- Plch, J.; Hrabeta, J.; Eckschlager, T. KDM5 demethylases and their role in cancer cell chemoresistance. Int. J. Cancer 2019, 144, 221–231. [Google Scholar] [CrossRef]
- Kasinath, V.; Beck, C.; Sauer, P.; Poepsel, S.; Kosmatka, J.; Faini, M.; Toso, D.; Aebersold, R.; Nogales, E. JARID2 and AEBP2 regulate PRC2 in the presence of H2AK119ub1 and other histone modifications. Science 2021, 371, eabc3393. [Google Scholar] [CrossRef]
- Mashtalir, N.; D’Avino, A.R.; Michel, B.C.; Luo, J.; Pan, J.; Otto, J.E.; Zullow, H.J.; McKenzie, Z.M.; Kubiak, R.L.; St Pierre, R.; et al. Modular Organization and Assembly of SWI/SNF Family Chromatin Remodeling Complexes. Cell 2018, 175, 1272–1288. [Google Scholar] [CrossRef]
- Defeo-Jones, D.; Huang, P.S.; Jones, R.E.; Haskell, K.M.; Vuocolo, G.A.; Hanobik, M.G.; Huber, H.E.; Oliff, A. Cloning of cDNAs for cellular proteins that bind to the retinoblastoma gene product. Nature 1991, 352, 251–254. [Google Scholar] [CrossRef]
- Fattaey, A.R.; Helin, K.; Dembski, M.S.; Dyson, N.; Harlow, E.; Vuocolo, G.A.; Hanobik, M.G.; Haskell, K.M.; Oliff, A.; Defeo-Jones, D.; et al. Characterization of the retinoblastoma binding proteins RBP1 and RBP2. Oncogene 1993, 8, 3149–3156. [Google Scholar]
- Lai, A.; Marcellus, R.C.; Corbeil, H.B.; Branton, P.E. RBP1 induces growth arrest by repression of E2F-dependent transcription. Oncogene 1999, 18, 2091–2100. [Google Scholar] [CrossRef] [PubMed]
- Lai, A.; Lee, J.M.; Yang, W.M.; DeCaprio, J.A.; Kaelin, W.G., Jr.; Seto, E.; Branton, P.E. RBP1 recruits both histone deacetylase-dependent and -independent repression activities to retinoblastoma family proteins. Mol. Cell Biol. 1999, 19, 6632–6641. [Google Scholar] [CrossRef] [PubMed]
- Patsialou, A.; Wilsker, D.; Moran, E. DNA-binding properties of ARID family proteins. Nucleic Acids Res. 2005, 33, 66–80. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Yao, H.; Hu, W.; Perrett, S.; Gong, W.; Feng, Y. Correction: Structural basis for the DNA-binding activity of human ARID4B Tudor domain. J. Biol. Chem. 2022, 298, 102492. [Google Scholar] [CrossRef]
- Fleischer, T.C.; Yun, U.J.; Ayer, D.E. Identification and characterization of three new components of the mSin3A corepressor complex. Mol. Cell. Biol. 2003, 23, 3456–3467. [Google Scholar] [CrossRef]
- Cao, J.; Gao, T.; Stanbridge, E.J.; Irie, R. RBP1L1, a retinoblastoma-binding protein-related gene encoding an antigenic epitope abundantly expressed in human carcinomas and normal testis. J. Natl. Cancer Inst. 2001, 93, 1159–1165. [Google Scholar] [CrossRef]
- Brookfield, J.F. Genetic redundancy. Adv. Genet. 1997, 36, 137–155. [Google Scholar] [CrossRef]
- Tautz, D. Redundancies, development and the flow of information. Bioessays 1992, 14, 263–266. [Google Scholar] [CrossRef]
- O’Brien, S.J. On estimating functional gene number in eukaryotes. Nat. New Biol. 1973, 242, 52–54. [Google Scholar] [CrossRef]
- Wu, M.Y.; Eldin, K.W.; Beaudet, A.L. Identification of chromatin remodeling genes Arid4a and Arid4b as leukemia suppressor genes. J. Natl. Cancer Inst. 2008, 100, 1247–1259. [Google Scholar] [CrossRef]
- Wu, R.C.; Jiang, M.; Beaudet, A.L.; Wu, M.Y. ARID4A and ARID4B regulate male fertility, a functional link to the AR and RB pathways. Proc. Natl. Acad. Sci. USA 2013, 110, 4616–4621. [Google Scholar] [CrossRef] [PubMed]
- Blanpain, C.; Simons, B.D. Unravelling stem cell dynamics by lineage tracing. Nat. Rev. Mol. Cell Biol. 2013, 14, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Goodell, M.A.; Rando, T.A. Stem cells and healthy aging. Science 2015, 350, 1199–1204. [Google Scholar] [CrossRef]
- Evans, M.J.; Kaufman, M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981, 292, 154–156. [Google Scholar] [CrossRef]
- Yao, S.; Chen, S.; Clark, J.; Hao, E.; Beattie, G.M.; Hayek, A.; Ding, S. Long-term self-renewal and directed differentiation of human embryonic stem cells in chemically defined conditions. Proc. Natl. Acad. Sci. USA 2006, 103, 6907–6912. [Google Scholar] [CrossRef]
- Orkin, S.H.; Hochedlinger, K. Chromatin connections to pluripotency and cellular reprogramming. Cell 2011, 145, 835–850. [Google Scholar] [CrossRef]
- Morey, L.; Santanach, A.; Di Croce, L. Pluripotency and Epigenetic Factors in Mouse Embryonic Stem Cell Fate Regulation. Mol. Cell. Biol. 2015, 35, 2716–2728. [Google Scholar] [CrossRef]
- Seruggia, D.; Oti, M.; Tripathi, P.; Canver, M.C.; LeBlanc, L.; Di Giammartino, D.C.; Bullen, M.J.; Nefzger, C.M.; Sun, Y.B.Y.; Farouni, R.; et al. TAF5L and TAF6L Maintain Self-Renewal of Embryonic Stem Cells via the MYC Regulatory Network. Mol. Cell 2019, 74, 1148–1163.e1147. [Google Scholar] [CrossRef]
- Fraser, J.; Ferrai, C.; Chiariello, A.M.; Schueler, M.; Rito, T.; Laudanno, G.; Barbieri, M.; Moore, B.L.; Kraemer, D.C.; Aitken, S.; et al. Hierarchical folding and reorganization of chromosomes are linked to transcriptional changes in cellular differentiation. Mol. Syst. Biol. 2015, 11, 852. [Google Scholar] [CrossRef]
- Bonev, B.; Mendelson Cohen, N.; Szabo, Q.; Fritsch, L.; Papadopoulos, G.L.; Lubling, Y.; Xu, X.; Lv, X.; Hugnot, J.P.; Tanay, A.; et al. Multiscale 3D Genome Rewiring during Mouse Neural Development. Cell 2017, 171, 557–572.e524. [Google Scholar] [CrossRef]
- Dixon, J.R.; Jung, I.; Selvaraj, S.; Shen, Y.; Antosiewicz-Bourget, J.E.; Lee, A.Y.; Ye, Z.; Kim, A.; Rajagopal, N.; Xie, W.; et al. Chromatin architecture reorganization during stem cell differentiation. Nature 2015, 518, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Schultz, M.D.; Lister, R.; Hou, Z.; Rajagopal, N.; Ray, P.; Whitaker, J.W.; Tian, S.; Hawkins, R.D.; Leung, D.; et al. Epigenomic analysis of multilineage differentiation of human embryonic stem cells. Cell 2013, 153, 1134–1148. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Adli, M.; Zou, J.Y.; Verstappen, G.; Coyne, M.; Zhang, X.; Durham, T.; Miri, M.; Deshpande, V.; De Jager, P.L.; et al. Genome-wide chromatin state transitions associated with developmental and environmental cues. Cell 2013, 152, 642–654. [Google Scholar] [CrossRef] [PubMed]
- Fouse, S.D.; Shen, Y.; Pellegrini, M.; Cole, S.; Meissner, A.; Van Neste, L.; Jaenisch, R.; Fan, G. Promoter CpG methylation contributes to ES cell gene regulation in parallel with Oct4/Nanog, PcG complex, and histone H3 K4/K27 trimethylation. Cell Stem Cell 2008, 2, 160–169. [Google Scholar] [CrossRef]
- Mas, G.; Blanco, E.; Ballare, C.; Sanso, M.; Spill, Y.G.; Hu, D.; Aoi, Y.; Le Dily, F.; Shilatifard, A.; Marti-Renom, M.A.; et al. Promoter bivalency favors an open chromatin architecture in embryonic stem cells. Nat. Genet. 2018, 50, 1452–1462. [Google Scholar] [CrossRef]
- Denissov, S.; Hofemeister, H.; Marks, H.; Kranz, A.; Ciotta, G.; Singh, S.; Anastassiadis, K.; Stunnenberg, H.G.; Stewart, A.F. Mll2 is required for H3K4 trimethylation on bivalent promoters in embryonic stem cells, whereas Mll1 is redundant. Development 2014, 141, 526–537. [Google Scholar] [CrossRef]
- Terzi Cizmecioglu, N.; Huang, J.; Keskin, E.G.; Wang, X.; Esen, I.; Chen, F.; Orkin, S.H. ARID4B is critical for mouse embryonic stem cell differentiation towards mesoderm and endoderm, linking epigenetics to pluripotency exit. J. Biol. Chem. 2020, 295, 17738–17751. [Google Scholar] [CrossRef]
- Keskin, E.G.; Huang, J.; Terzi Cizmecioglu, N. Arid4b physically interacts with Tfap2c in mouse embryonic stem cells. Turk. J. Biol. 2021, 45, 162–170. [Google Scholar] [CrossRef]
- GÜven, G.; ÇİzmecİoĞlu, N.T. Arid4b alters cell cycle and cell death dynamics during mouse embryonic stem cell differentiation. Turk. J. Biol. 2021, 45, 56–64. [Google Scholar] [CrossRef]
- Wu, M.Y.; Tsai, T.F.; Beaudet, A.L. Deficiency of Rbbp1/Arid4a and Rbbp1l1/Arid4b alters epigenetic modifications and suppresses an imprinting defect in the PWS/AS domain. Genes. Dev. 2006, 20, 2859–2870. [Google Scholar] [CrossRef]
- Marone, M.; De Ritis, D.; Bonanno, G.; Mozzetti, S.; Rutella, S.; Scambia, G.; Pierelli, L. Cell cycle regulation in human hematopoietic stem cells: From isolation to activation. Leuk. Lymphoma 2002, 43, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.A.; Lemischka, I.R. Stem cells and their niches. Science 2006, 311, 1880–1885. [Google Scholar] [CrossRef]
- Wilson, A.; Trumpp, A. Bone-marrow haematopoietic-stem-cell niches. Nat. Rev. Immunol. 2006, 6, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Laurenti, E.; Gottgens, B. From haematopoietic stem cells to complex differentiation landscapes. Nature 2018, 553, 418–426. [Google Scholar] [CrossRef]
- Oguro, H.; Ding, L.; Morrison, S.J. SLAM family markers resolve functionally distinct subpopulations of hematopoietic stem cells and multipotent progenitors. Cell Stem Cell 2013, 13, 102–116. [Google Scholar] [CrossRef]
- Yamamoto, R.; Morita, Y.; Ooehara, J.; Hamanaka, S.; Onodera, M.; Rudolph, K.L.; Ema, H.; Nakauchi, H. Clonal analysis unveils self-renewing lineage-restricted progenitors generated directly from hematopoietic stem cells. Cell 2013, 154, 1112–1126. [Google Scholar] [CrossRef]
- Cabezas-Wallscheid, N.; Klimmeck, D.; Hansson, J.; Lipka, D.B.; Reyes, A.; Wang, Q.; Weichenhan, D.; Lier, A.; von Paleske, L.; Renders, S.; et al. Identification of regulatory networks in HSCs and their immediate progeny via integrated proteome, transcriptome, and DNA methylome analysis. Cell Stem Cell 2014, 15, 507–522. [Google Scholar] [CrossRef]
- Chabot, B.; Stephenson, D.A.; Chapman, V.M.; Besmer, P.; Bernstein, A. The proto-oncogene c-kit encoding a transmembrane tyrosine kinase receptor maps to the mouse W locus. Nature 1988, 335, 88–89. [Google Scholar] [CrossRef]
- Geissler, E.N.; Ryan, M.A.; Housman, D.E. The dominant-white spotting (W) locus of the mouse encodes the c-kit proto-oncogene. Cell 1988, 55, 185–192. [Google Scholar] [CrossRef]
- Russell, E.S. Hereditary anemias of the mouse: A review for geneticists. Adv. Genet. 1979, 20, 357–459. [Google Scholar]
- Geissler, E.N.; Russell, E.S. Analysis of the hematopoietic effects of new dominant spotting (W) mutations of the mouse. II. Effects on mast cell development. Exp. Hematol. 1983, 11, 461–466. [Google Scholar] [PubMed]
- Sharma, Y.; Astle, C.M.; Harrison, D.E. Heterozygous kit mutants with little or no apparent anemia exhibit large defects in overall hematopoietic stem cell function. Exp. Hematol. 2007, 35, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, S.; Bosbach, B.; Yozgat, Y.; Park, C.Y.; Moore, M.A.; Besmer, P. KIT receptor gain-of-function in hematopoiesis enhances stem cell self-renewal and promotes progenitor cell expansion. Stem Cells 2013, 31, 1683–1695. [Google Scholar] [CrossRef] [PubMed]
- Young, I.C.; Wu, B.; Andricovich, J.; Chuang, S.T.; Li, R.; Tzatsos, A.; Wu, R.C.; Wu, M.Y. Differentiation of fetal hematopoietic stem cells requires ARID4B to restrict autocrine KITLG/KIT-Src signaling. Cell Rep. 2021, 37, 110036. [Google Scholar] [CrossRef]
- Newton, S.C.; Blaschuk, O.W.; Millette, C.F. N-cadherin mediates Sertoli cell-spermatogenic cell adhesion. Dev. Dyn. 1993, 197, 1–13. [Google Scholar] [CrossRef]
- Pratt, S.A.; Scully, N.F.; Shur, B.D. Cell surface beta 1,4 galactosyltransferase on primary spermatocytes facilitates their initial adhesion to Sertoli cells in vitro. Biol. Reprod. 1993, 49, 470–482. [Google Scholar] [CrossRef]
- Wu, R.C.; Zeng, Y.; Pan, I.W.; Wu, M.Y. Androgen Receptor Coactivator ARID4B Is Required for the Function of Sertoli Cells in Spermatogenesis. Mol. Endocrinol. 2015, 29, 1334–1346. [Google Scholar] [CrossRef][Green Version]
- Jan, S.Z.; Hamer, G.; Repping, S.; de Rooij, D.G.; van Pelt, A.M.; Vormer, T.L. Molecular control of rodent spermatogenesis. Biochim. Biophys. Acta 2012, 1822, 1838–1850. [Google Scholar] [CrossRef]
- Wu, R.C.; Zeng, Y.; Chen, Y.F.; Lanz, R.B.; Wu, M.Y. Temporal-Spatial Establishment of Initial Niche for the Primary Spermatogonial Stem Cell Formation Is Determined by an ARID4B Regulatory Network. Stem Cells 2017, 35, 1554–1565. [Google Scholar] [CrossRef]
- Godia, M.; Casellas, J.; Ruiz-Herrera, A.; Rodriguez-Gil, J.E.; Castello, A.; Sanchez, A.; Clop, A. Whole genome sequencing identifies allelic ratio distortion in sperm involving genes related to spermatogenesis in a swine model. DNA Res. 2020, 27, dsaa019. [Google Scholar] [CrossRef]
- Pernar, C.H.; Ebot, E.M.; Wilson, K.M.; Mucci, L.A. The Epidemiology of Prostate Cancer. Cold Spring Harb. Perspect. Med. 2018, 8, a030361. [Google Scholar] [CrossRef] [PubMed]
- Taitt, H.E. Global Trends and Prostate Cancer: A Review of Incidence, Detection, and Mortality as Influenced by Race, Ethnicity, and Geographic Location. Am. J. Mens. Health 2018, 12, 1807–1823. [Google Scholar] [CrossRef] [PubMed]
- Brandao, A.; Paulo, P.; Teixeira, M.R. Hereditary Predisposition to Prostate Cancer: From Genetics to Clinical Implications. Int. J. Mol. Sci. 2020, 21, 5036. [Google Scholar] [CrossRef] [PubMed]
- Hjelmborg, J.B.; Scheike, T.; Holst, K.; Skytthe, A.; Penney, K.L.; Graff, R.E.; Pukkala, E.; Christensen, K.; Adami, H.O.; Holm, N.V.; et al. The heritability of prostate cancer in the Nordic Twin Study of Cancer. Cancer Epidemiol. Biomark. Prev. 2014, 23, 2303–2310. [Google Scholar] [CrossRef]
- Wen, S.; Chang, H.C.; Tian, J.; Shang, Z.; Niu, Y.; Chang, C. Stromal androgen receptor roles in the development of normal prostate, benign prostate hyperplasia, and prostate cancer. Am. J. Pathol. 2015, 185, 293–301. [Google Scholar] [CrossRef]
- Cittadini, A.; Isidori, A.M.; Salzano, A. Testosterone therapy and cardiovascular diseases. Cardiovasc. Res. 2022, 118, 2039–2057. [Google Scholar] [CrossRef]
- Benafif, S.; Kote-Jarai, Z.; Eeles, R.A.; Consortium, P. A Review of Prostate Cancer Genome-Wide Association Studies (GWAS). Cancer Epidemiol. Biomark. Prev. 2018, 27, 845–857. [Google Scholar] [CrossRef]
- Agalliu, I.; Karlins, E.; Kwon, E.M.; Iwasaki, L.M.; Diamond, A.; Ostrander, E.A.; Stanford, J.L. Rare germline mutations in the BRCA2 gene are associated with early-onset prostate cancer. Br. J. Cancer 2007, 97, 826–831. [Google Scholar] [CrossRef]
- Ford, D.; Easton, D.F.; Bishop, D.T.; Narod, S.A.; Goldgar, D.E. Risks of cancer in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Lancet 1994, 343, 692–695. [Google Scholar] [CrossRef]
- Rebello, R.J.; Oing, C.; Knudsen, K.E.; Loeb, S.; Johnson, D.C.; Reiter, R.E.; Gillessen, S.; Van der Kwast, T.; Bristow, R.G. Prostate cancer. Nat. Rev. Dis. Primers 2021, 7, 9. [Google Scholar] [CrossRef]
- Edwards, S.M.; Kote-Jarai, Z.; Meitz, J.; Hamoudi, R.; Hope, Q.; Osin, P.; Jackson, R.; Southgate, C.; Singh, R.; Falconer, A.; et al. Two percent of men with early-onset prostate cancer harbor germline mutations in the BRCA2 gene. Am. J. Hum. Genet. 2003, 72, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yu, J.; Mani, R.S.; Cao, Q.; Brenner, C.J.; Cao, X.; Wang, X.; Wu, L.; Li, J.; Hu, M.; et al. An integrated network of androgen receptor, polycomb, and TMPRSS2-ERG gene fusions in prostate cancer progression. Cancer Cell 2010, 17, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Kissick, H.T.; On, S.T.; Dunn, L.K.; Sanda, M.G.; Asara, J.M.; Pellegrini, K.L.; Noel, J.K.; Arredouani, M.S. The transcription factor ERG increases expression of neurotransmitter receptors on prostate cancer cells. BMC Cancer 2015, 15, 604. [Google Scholar] [CrossRef]
- Chen, H.; Liu, W.; Roberts, W.; Hooker, S.; Fedor, H.; DeMarzo, A.; Isaacs, W.; Kittles, R.A. 8q24 allelic imbalance and MYC gene copy number in primary prostate cancer. Prostate Cancer Prostatic Dis. 2010, 13, 238–243. [Google Scholar] [CrossRef]
- Fromont, G.; Godet, J.; Peyret, A.; Irani, J.; Celhay, O.; Rozet, F.; Cathelineau, X.; Cussenot, O. 8q24 amplification is associated with Myc expression and prostate cancer progression and is an independent predictor of recurrence after radical prostatectomy. Hum. Pathol. 2013, 44, 1617–1623. [Google Scholar] [CrossRef]
- Liu, J.; Lichtenberg, T.; Hoadley, K.A.; Poisson, L.M.; Lazar, A.J.; Cherniack, A.D.; Kovatich, A.J.; Benz, C.C.; Levine, D.A.; Lee, A.V.; et al. An Integrated TCGA Pan-Cancer Clinical Data Resource to Drive High-Quality Survival Outcome Analytics. Cell 2018, 173, 400–416.e411. [Google Scholar] [CrossRef]
- Wu, R.C.; Young, I.C.; Chen, Y.F.; Chuang, S.T.; Toubaji, A.; Wu, M.Y. Identification of the PTEN-ARID4B-PI3K pathway reveals the dependency on ARID4B by PTEN-deficient prostate cancer. Nat. Commun. 2019, 10, 4332. [Google Scholar] [CrossRef]
- Liang, Y.K.; Han, Z.D.; Lu, J.M.; Liu, Z.Z.; Zhuo, Y.J.; Zhu, X.J.; Chen, J.X.; Ye, J.H.; Liang, Y.X.; He, H.C.; et al. Downregulation of ARID4A and ARID4B promote tumor progression and directly regulated by microRNA-30d in patient with prostate cancer. J. Cell Biochem. 2018, 119, 7245–7255. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Ferrini, K.; Ghelfi, F.; Mannucci, R.; Titta, L. Lifestyle, nutrition and breast cancer: Facts and presumptions for consideration. Ecancermedicalscience 2015, 9, 557. [Google Scholar] [CrossRef]
- Castello, A.; Martin, M.; Ruiz, A.; Casas, A.M.; Baena-Canada, J.M.; Lope, V.; Antolin, S.; Sanchez, P.; Ramos, M.; Anton, A.; et al. Lower Breast Cancer Risk among Women following the World Cancer Research Fund and American Institute for Cancer Research Lifestyle Recommendations: EpiGEICAM Case-Control Study. PLoS ONE 2015, 10, e0126096. [Google Scholar] [CrossRef] [PubMed]
- Winter, S.F.; Lukes, L.; Walker, R.C.; Welch, D.R.; Hunter, K.W. Allelic variation and differential expression of the mSIN3A histone deacetylase complex gene Arid4b promote mammary tumor growth and metastasis. PLoS Genet. 2012, 8, e1002735. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hou, S.; You, Z.; Li, G.; Xu, S.; Li, X.; Zhang, X.; Lei, B.; Pang, D. Expression and prognostic values of ARID family members in breast cancer. Aging 2021, 13, 5621–5637. [Google Scholar] [CrossRef] [PubMed]
- Goldberger, N.; Walker, R.C.; Kim, C.H.; Winter, S.; Hunter, K.W. Inherited variation in miR-290 expression suppresses breast cancer progression by targeting the metastasis susceptibility gene Arid4b. Cancer Res. 2013, 73, 2671–2681. [Google Scholar] [CrossRef]
- Rabeneck, L.; Chiu, H.M.; Senore, C. International Perspective on the Burden of Colorectal Cancer and Public Health Effects. Gastroenterology 2020, 158, 447–452. [Google Scholar] [CrossRef]
- Hofseth, L.J.; Hebert, J.R.; Chanda, A.; Chen, H.; Love, B.L.; Pena, M.M.; Murphy, E.A.; Sajish, M.; Sheth, A.; Buckhaults, P.J.; et al. Early-onset colorectal cancer: Initial clues and current views. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 352–364. [Google Scholar] [CrossRef]
- Yu, J.; Xu, Q.G.; Wang, Z.G.; Yang, Y.; Zhang, L.; Ma, J.Z.; Sun, S.H.; Yang, F.; Zhou, W.P. Circular RNA cSMARCA5 inhibits growth and metastasis in hepatocellular carcinoma. J. Hepatol. 2018, 68, 1214–1227. [Google Scholar] [CrossRef]
- Lu, Q.; Fang, T. Circular RNA SMARCA5 correlates with favorable clinical tumor features and prognosis, and increases chemotherapy sensitivity in intrahepatic cholangiocarcinoma. J. Clin. Lab. Anal. 2020, 34, e23138. [Google Scholar] [CrossRef]
- Liu, H.; Wu, Y.; Wang, S.; Jiang, J.; Zhang, C.; Jiang, Y.; Wang, X.; Hong, L.; Huang, H. Circ-SMARCA5 suppresses progression of multiple myeloma by targeting miR-767-5p. BMC Cancer 2019, 19, 937. [Google Scholar] [CrossRef]
- Kong, Z.; Wan, X.; Zhang, Y.; Zhang, P.; Zhang, Y.; Zhang, X.; Qi, X.; Wu, H.; Huang, J.; Li, Y. Androgen-responsive circular RNA circSMARCA5 is up-regulated and promotes cell proliferation in prostate cancer. Biochem. Biophys. Res. Commun. 2017, 493, 1217–1223. [Google Scholar] [CrossRef]
- Miao, X.; Xi, Z.; Zhang, Y.; Li, Z.; Huang, L.; Xin, T.; Shen, R.; Wang, T. Circ-SMARCA5 suppresses colorectal cancer progression via downregulating miR-39-3p and upregulating ARID4B. Dig. Liver Dis. 2020, 52, 1494–1502. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Liu, L.; Zhou, N.; Shen, J.; Sun, Q.; Zhu, Y.; Chen, M. miR-519b-3p promotes responsiveness to preoperative chemoradiotherapy in rectal cancer patients by targeting ARID4B. Gene 2018, 655, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Singal, A.G.; Lampertico, P.; Nahon, P. Epidemiology and surveillance for hepatocellular carcinoma: New trends. J. Hepatol. 2020, 72, 250–261. [Google Scholar] [CrossRef]
- Sun, J.; Cheng, N.S. Comprehensive Landscape of ARID Family Members and Their Association with Prognosis and Tumor Microenvironment in Hepatocellular Carcinoma. J. Immunol. Res. 2022, 2022, 1688460. [Google Scholar] [CrossRef]
- Wang, R.; Yu, Z.; Chen, F.; Liao, C.; Wang, Q.; Huang, X. Overexpression of ARID4B predicts poor survival in patients with hepatocellular carcinoma. Hum. Pathol. 2018, 73, 114–121. [Google Scholar] [CrossRef]
- Clarke, J.; Penas, C.; Pastori, C.; Komotar, R.J.; Bregy, A.; Shah, A.H.; Wahlestedt, C.; Ayad, N.G. Epigenetic pathways and glioblastoma treatment. Epigenetics 2013, 8, 785–795. [Google Scholar] [CrossRef]
- Tsai, W.C.; Hueng, D.Y.; Nieh, S.; Gao, H.W. ARID4B is a good biomarker to predict tumour behaviour and decide WHO grades in gliomas and meningiomas. J. Clin. Pathol. 2017, 70, 162–167. [Google Scholar] [CrossRef]
- Luo, S.M.; Tsai, W.C.; Tsai, C.K.; Chen, Y.; Hueng, D.Y. ARID4B Knockdown Suppresses PI3K/AKT Signaling and Induces Apoptosis in Human Glioma Cells. Onco Targets Ther. 2021, 14, 1843–1855. [Google Scholar] [CrossRef]
| Human | Mouse | ||
|---|---|---|---|
| ARID1A | Chromosome 1p36.11 | Arid1a | Chromosome 4qD2.3 |
| ARID1B | Chromosome 6q25.3 | Arid1b | Chromosome 17qA1 |
| ARID2 | Chromosome 12q12 | Arid2 | Chromosome 15qF1 |
| ARID3A | Chromosome 19p13.3 | Arid3a | Chromosome 10qC1 |
| ARID3B | Chromosome 15q24.1 | Arid3b | Chromosome 9qB |
| ARID3C | Chromosome 9p13.3 | Arid3c | Chromosome 10qC1 |
| ARID4A | Chromosome 14q23.1 | Arid4a | Chromosome 12qC2 |
| ARID4B | Chromosome 1q42.3 | Arid4b | Chromosome 13qA1 |
| ARID5A | Chromosome 2q11.2 | Arid5a | Chromosome 1qB |
| ARID5B | Chromosome 10q21.2 | Arid5b | Chromosome 10qB5.2 |
| JARID1A | Chromosome 12p13.33 | Jarid1a | Chromosome 6qF1 |
| JARID1B | Chromosome 1q32.1 | Jarid1b | Chromosome 1qE4 |
| JARID2 | Chromosome 6p22.3 | Jarid2 | Chromosome 13qA5 |
| JARID1C | Chromosome Xp11.22 | Jarid1c | Chromosome XqF3 |
| JARID1D | Chromosome Yq11.223 | Jarid1d | Chromosome YqA1 |
| Physiological State | Model System | Associated Cellular Mechanisms | Reference |
|---|---|---|---|
| ESC renewal | In vitro | Arid4b regulates transcriptional networks critical for maintaining pluripotency and self-renewal of ESCs | [30,31,51] |
| Impaired hematopoietic stem cell function | In vivo, In vitro, In silico | Arid4b maintains HSPC homeostasis by regulating self-renewal, differentiation, and lineage commitment via the KITLG/KIT-Src signaling axis | [64] |
| Embryonic germ layer differentiation | In vitro | Arid4b binds to the transcription factor Tfap2c, regulating the differentiation of endoderm and mesoderm | [48] |
| Cell cycle regulation and apoptosis during embryonic development | In vitro | Arid4b altered cell cycle and cell death in a Caspase 3-dependent manner | [49] |
| Hormonal regulation of male germ cell development | In vivo, In vitro | Arid4b coactivates the androgen receptor (AR), which modulates AR-dependent transcription essential for the development and maturation of male germ cells | [67] |
| Stem cell niche establishment during spermatogenesis | In vivo | Arid4b regulates the signaling network required to establish a niche in the gonocyte–spermatogonial stem cell transition | [69] |
| Cancer Type | Model System | Oncogene/Tumor Suppressor | Associated Cellular Functions | Reference |
|---|---|---|---|---|
| Prostate cancer | In vivo, In vitro | Oncogene | Arid4b regulates PIK3CA and PIK3R2, leading to PTEN downregulation and AKT pathway activation, thereby promoting prostate cancer cell survival and proliferation. | [87] |
| Prostate cancer | In vivo, In vitro | Tumor suppressor | Arid4b expression is negatively regulated by miRNA-30d, leading to reduced tumor suppressor activity | [88] |
| Breast cancer | In vivo, In vitro | Oncogene | Arid4b mediates estrogen receptor signaling through miRNA-290 regulation, promoting cancer cell proliferation and survival | [94] |
| Breast cancer | In vivo, In vitro | Oncogene | Arid4b inhibits pulmonary metastasis by interacting with BRMS1, indicating its complex role in regulating metastatic potential | [92] |
| Hepatocellular carcinoma | In vivo | Oncogene | Arid4b overexpression suggests a potential role in liver tumor development or progression | [106] |
| Glioblastoma | In vitro, In silico | Oncogene | Knockdown of Arid4b favors apoptosis through PI3K/AKT pathway | [102] |
| Glioblastoma | In vivo, In vitro | Oncogene | Enhanced expression of Arid4b in primary brain tumor highly correlated with WHO grades | [108] |
| Acute myeloid leukemia | In vivo | Tumor suppressor | Haplo-deficiency of Arid4b in adult mice enhanced onset of acute myeloid leukemia in Arid4a null mice | [30] |
| Colorectal cancer | In vivo, In vitro | Tumor suppressor | miRNA-mediated activation of cSMARCA5/miR-39a-3p/ARID4B pathway enhances Arid4b expression and inhibits tumorigenesis | [101] |
| Colorectal cancer | In vivo, In vitro | Oncogene | Enhanced cellular responsiveness to chemoradiation through binding of miR-519b-3p to Arid4b | [102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kandy, R.R.K.; Arumugam, M.K.; Yadav, M.P.; Mishra, B.B.; Sharma, J. ARID4B: An Orchestrator from Stem Cell Fate to Carcinogenesis. Cells 2025, 14, 872. https://doi.org/10.3390/cells14120872
Kandy RRK, Arumugam MK, Yadav MP, Mishra BB, Sharma J. ARID4B: An Orchestrator from Stem Cell Fate to Carcinogenesis. Cells. 2025; 14(12):872. https://doi.org/10.3390/cells14120872
Chicago/Turabian StyleKandy, Rakhee Rathnam Kalari, Madan Kumar Arumugam, Mukesh Pratap Yadav, Bibhuti Bhusan Mishra, and Jyotika Sharma. 2025. "ARID4B: An Orchestrator from Stem Cell Fate to Carcinogenesis" Cells 14, no. 12: 872. https://doi.org/10.3390/cells14120872
APA StyleKandy, R. R. K., Arumugam, M. K., Yadav, M. P., Mishra, B. B., & Sharma, J. (2025). ARID4B: An Orchestrator from Stem Cell Fate to Carcinogenesis. Cells, 14(12), 872. https://doi.org/10.3390/cells14120872








