Investigation of the Roles of the Adenosine A(2A) and Metabotropic Glutamate Receptor Type 5 (mGlu5) Receptors in Prepulse Inhibition and CREB Signaling in a Heritable Rodent Model of Psychosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment 1: Subjects
2.2. Experiment 1: Drugs
2.3. Experiments 1 and 2: Research Design
2.4. Experiments 1 and 2: Sensorimotor Gating Apparatus
2.5. Experiments 1 and 2: Prepulse Inhibition (PPI) Methods
2.6. Experiments 1 and 2: Cyclic AMP Response Element Binding Protein (CREB) ELISA
2.7. Experiment 2: Subjects
2.8. Experiment 2: Drugs
2.9. Experiments 1 and 2: Statistical Analysis
3. Results
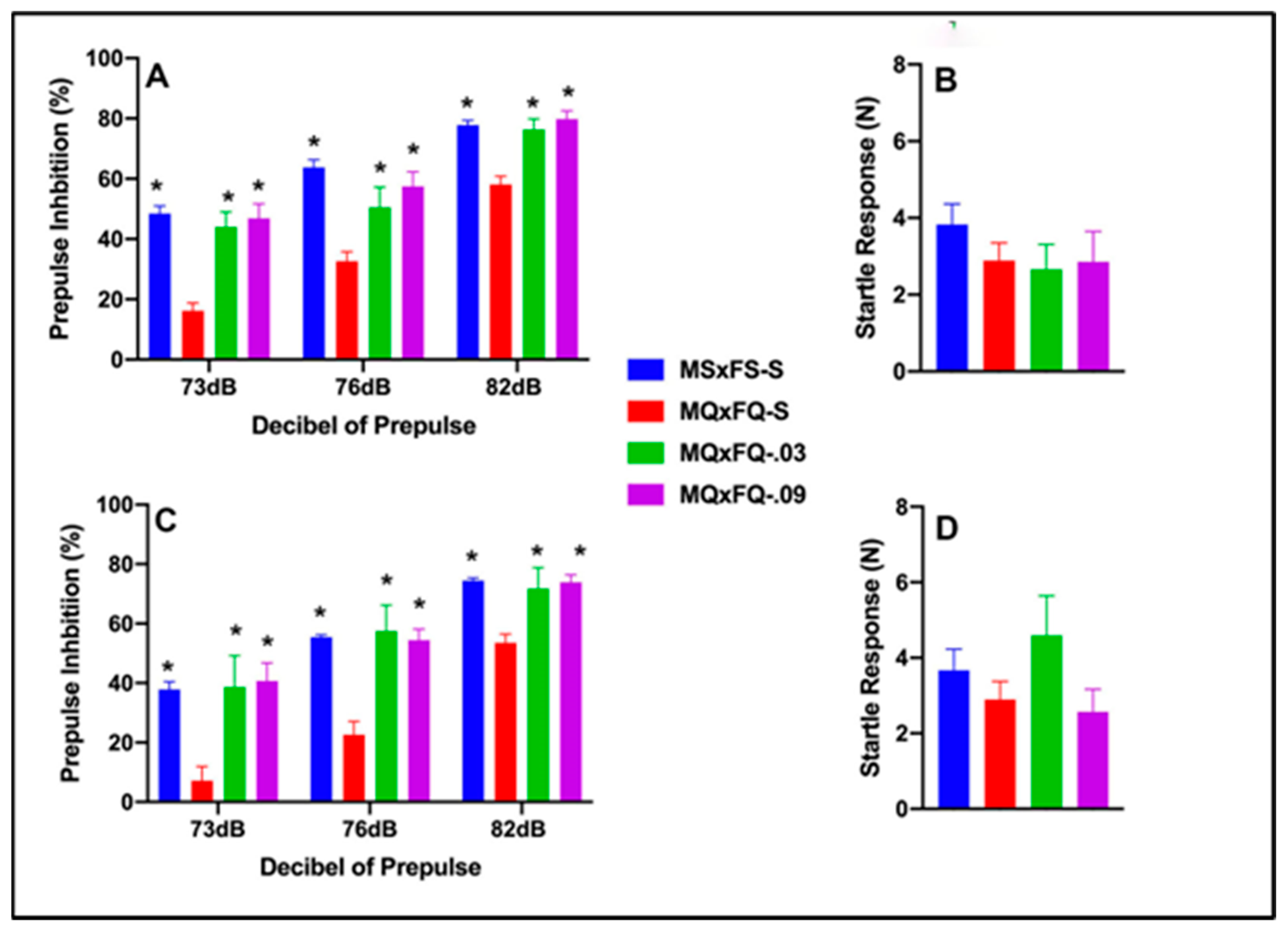
3.1. CGS21680–MQxFQ PPI and Acoustic Startle
3.2. CGS21680–MQxFS PPI and Acoustic Startle
3.3. CGS21680–MSxFQ PPI and Acoustic Startle
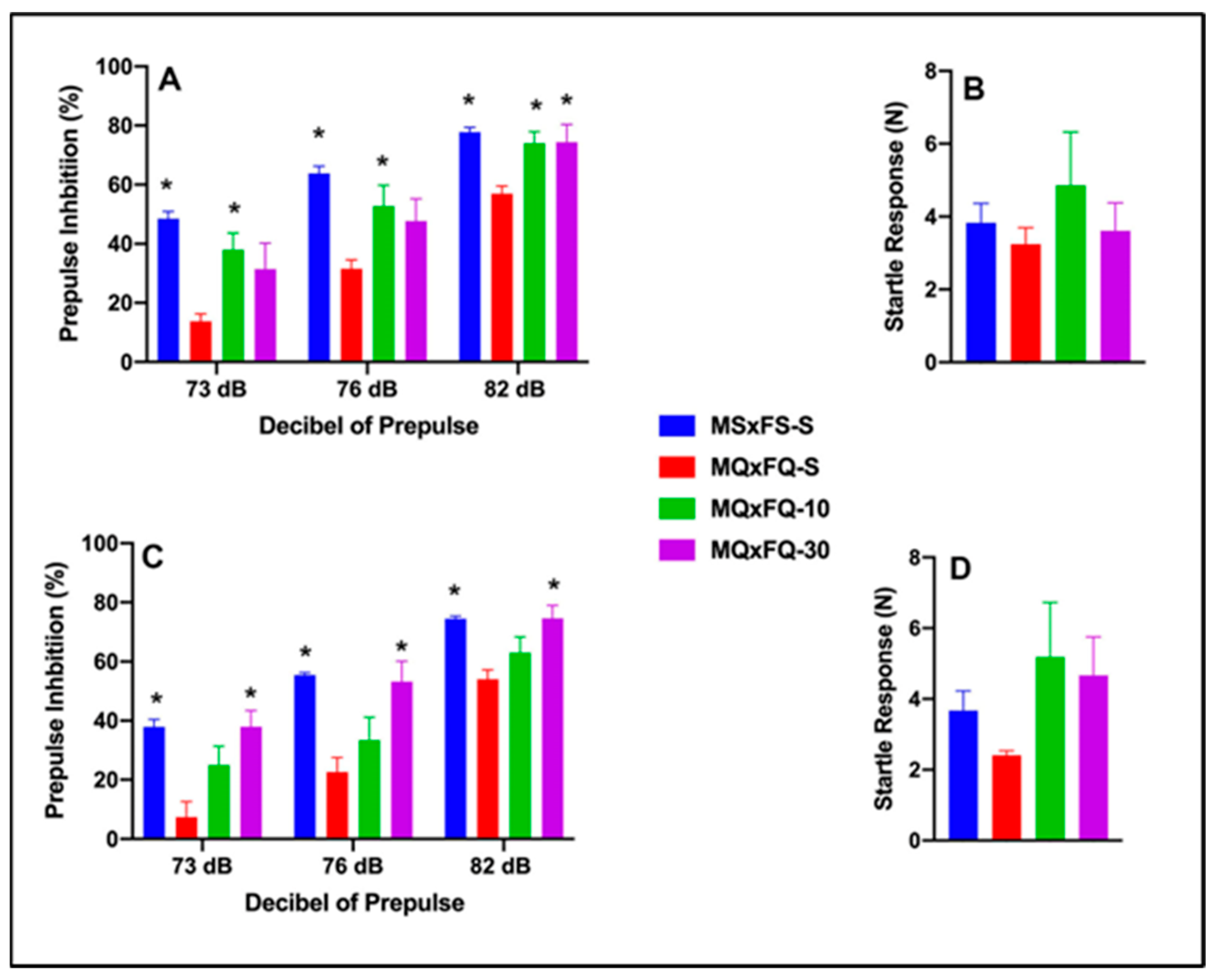
3.4. CDPPB–MQxFQ PPI and Acoustic Startle
3.5. CDPPB–MQxFS PPI and Acoustic Startle
3.6. CDPPB–MSxFQ PPI and Acoustic Startle
3.7. A(2A) Agonist: CGS21680–CREB
3.7.1. Numbers of Animals for CREB Analyses
3.7.2. MQxFQ Group
3.7.3. MQxFS Group
3.7.4. MSxFQ Group
3.8. mGlu5 PAM: CDPPB–CREB
3.8.1. MQxFQ Group
3.8.2. MQxFS Group
3.8.3. MSxFQ Group
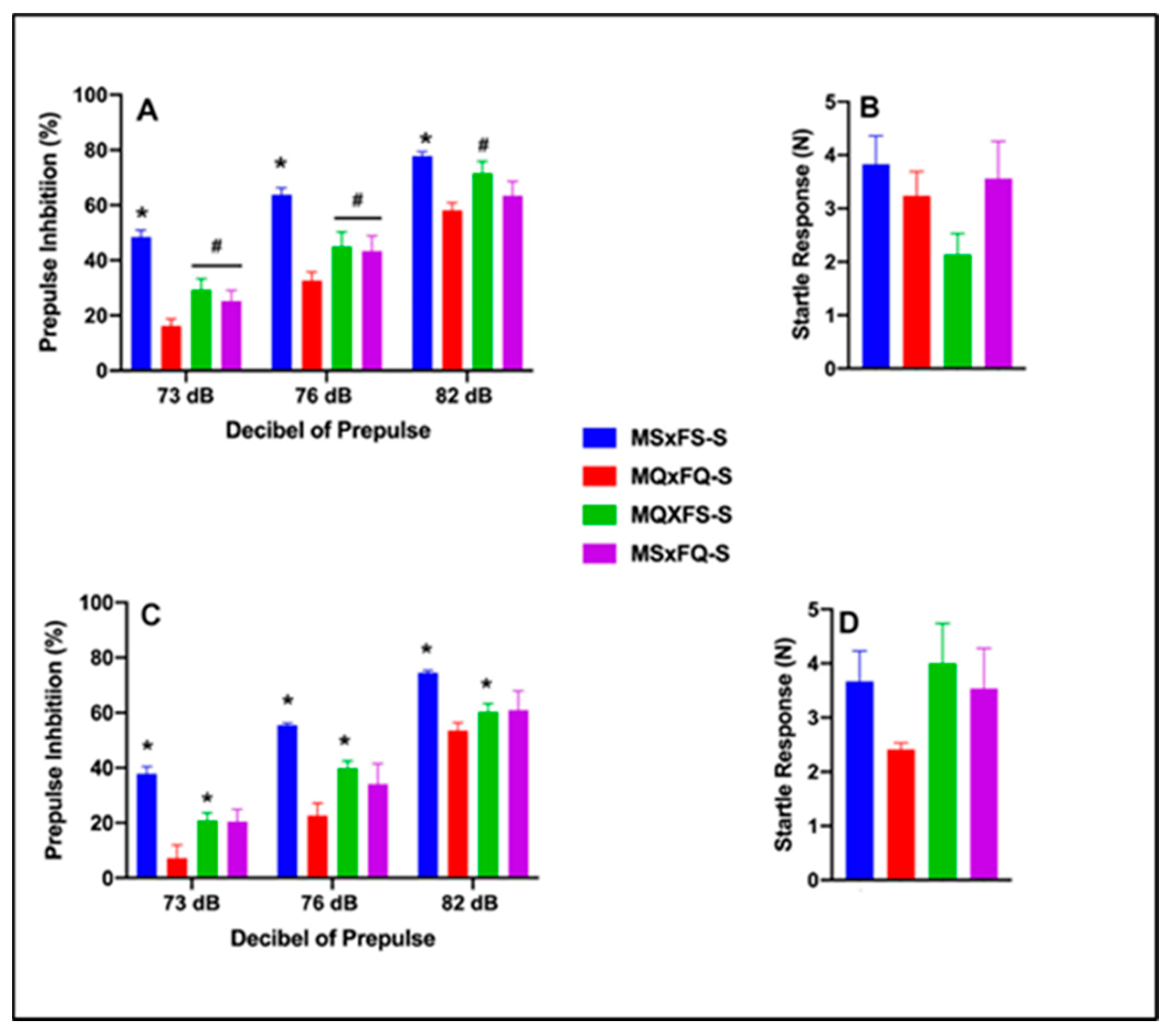
3.9. F1-Generation Comparison of Offspring of NQ-Treated Rats in PPI and Acoustic Startle
3.10. F1-Generation Comparison of Offspring of NQ-Treated Rats in NAc CREB
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dunwiddie, T.V.; Masino, S.A. The Role and Regulation of Adenosine in the Central Nervous System. Annu. Rev. Neurosci. 2001, 24, 31–55. [Google Scholar] [CrossRef] [PubMed]
- Cunha, R.A. How Does Adenosine Control Neuronal Dysfunction and Neurodegeneration? J. Neurochem. 2016, 139, 1019–1055. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.-Y.; Chen, J.-F. Adenosine A2A Receptors in Psychopharmacology: Modulators of Behavior, Mood and Cognition. Curr. Neuropharmacol. 2009, 7, 195. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.W.; Bhide, P.G.; Gill, W.D.; Peeters, L.D. The Adenosine A(2A) Receptor Agonist CGS 21680 Alleviates Auditory Sensorimotor Gating Deficits and Increases in Accumbal CREB in Rats Neonatally Treated with Quinpirole. Psychopharmacology 2020, 237, 3519. [Google Scholar] [CrossRef]
- Mena, A.; Ruiz-Salas, J.C.; Puentes, A.; Dorado, I.; Ruiz-Veguilla, M.; De la Casa, L.G. Reduced Prepulse Inhibition as a Biomarker of Schizophrenia. Front. Behav. Neurosci. 2016, 10, 202. [Google Scholar] [CrossRef] [PubMed]
- Brisch, R.; Saniotis, A.; Wolf, R.; Bielau, H.; Bernstein, H.-G.; Steiner, J.; Bogerts, B.; Braun, K.; Jankowski, Z.; Kumaratilake, J.; et al. The Role of Dopamine in Schizophrenia from a Neurobiological and Evolutionary Perspective: Old Fashioned, but Still in Vogue. Front. Psychiatry 2014, 5, 47. [Google Scholar] [CrossRef]
- Kesby, J.P.; Eyles, D.W.; McGrath, J.J.; Scott, J.G. Dopamine, Psychosis and Schizophrenia: The Widening Gap between Basic and Clinical Neuroscience. Transl. Psychiatry 2018, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Singer, P.; Yee, B.K. The Adenosine Hypothesis of Schizophrenia into Its Third Decade: From Neurochemical Imbalance to Early Life Etiological Risks. Front. Cell. Neurosci. 2023, 17, 1120532. [Google Scholar] [CrossRef] [PubMed]
- Boison, D.; Singer, P.; Shen, H.-Y.; Feldon, J.; Yee, B.K. Adenosine Hypothesis of Schizophrenia –Opportunities for Pharmacotherapy. Neuropharmacology 2011, 62, 1527. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine, Board on Health Sciences Policy, Forum on Neuroscience and Nervous System Disorders. Overview of the Glutamatergic System. In Glutamate-Related Biomarkers in Drug Development for Disorders of the Nervous System: Workshop Summary; National Academies Press (US): Cambridge, MA, USA, 2011. [Google Scholar]
- Budgett, R.F.; Bakker, G.; Sergeev, E.; Bennett, K.A.; Bradley, S.J. Targeting the Type 5 Metabotropic Glutamate Receptor: A Potential Therapeutic Strategy for Neurodegenerative Diseases? Front. Pharmacol. 2022, 13, 893422. [Google Scholar] [CrossRef] [PubMed]
- Matosin, N.; Newell, K.A. Metabotropic Glutamate Receptor 5 in the Pathology and Treatment of Schizophrenia. Neurosci. Biobehav. Rev. 2013, 37, 256–268. [Google Scholar] [CrossRef]
- Brown, R.W.; Varnum, C.G.; Wills, L.J.; Peeters, L.D.; Gass, J.T. Modulation of mGlu5 Improves Sensorimotor Gating Deficits in Rats Neonatally Treated with Quinpirole through Changes in Dopamine D2 Signaling. Pharmacol. Biochem. Behav. 2021, 211, 173292. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.-Y.; Wu, D.C.; Zhou, N. Astrocytic Regulation of Glutamate Transmission in Schizophrenia. Front. Psychiatry 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-Y.; MacDonald, M.L.; Borgmann-Winter, K.E.; Banerjee, A.; Sleiman, P.; Tom, A.; Khan, A.; Lee, K.-C.; Roussos, P.; Siegel, S.J.; et al. mGluR5 Hypofunction Is Integral to Glutamatergic Dysregulation in Schizophrenia. Mol. Psychiatry 2018, 25, 750. [Google Scholar] [CrossRef] [PubMed]
- Janus, A.; Lustyk, K.; Pytka, K. MK-801 and Cognitive Functions: Investigating the Behavioral Effects of a Non-Competitive NMDA Receptor Antagonist. Psychopharmacology 2023, 240, 2435–2457. [Google Scholar] [CrossRef]
- Azargoonjahromi, A. Current Findings and Potential Mechanisms of KarXT (Xanomeline-Trospium) in Schizophrenia Treatment. Clin. Drug Investig. 2024, 44, 471–493. [Google Scholar] [CrossRef]
- Ferré, S.; Karcz-Kubicha, M.; Hope, B.T.; Popoli, P.; Burgueño, J.; Gutiérrez, M.A.; Casadó, V.; Fuxe, K.; Goldberg, S.R.; Lluis, C.; et al. Synergistic Interaction between Adenosine A2A and Glutamate mGlu5 Receptors: Implications for Striatal Neuronal Function. Proc. Natl. Acad. Sci. USA 2002, 99, 11940–11945. [Google Scholar] [CrossRef] [PubMed]
- Beggiato, S.; Tomasini, M.C.; Borelli, A.C.; Borroto-Escuela, D.O.; Fuxe, K.; Antonelli, T.; Tanganelli, S.; Ferraro, L. Functional Role of Striatal A2A, D2, and mGlu5 Receptor Interactions in Regulating Striatopallidal GABA Neuronal Transmission. J. Neurochem. 2016, 138, 254–264. [Google Scholar] [CrossRef]
- Kostrzewa, R.M.; Wydra, K.; Filip, M.; Crawford, C.A.; McDougall, S.A.; Brown, R.W.; Borroto-Escuela, D.O.; Fuxe, K.; Gainetdinov, R.R. Dopamine D2 Receptor Supersensitivity as a Spectrum of Neurotoxicity and Status in Psychiatric Disorders. J. Pharmacol. Exp. Ther. 2018, 366, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Gill, W.D.; Burgess, K.C.; Vied, C.; Brown, R.W. Transgenerational Evidence of Increases in Dopamine D2 Receptor Sensitivity in Rodents: Impact on Sensorimotor Gating, the Behavioral Response to Nicotine and BDNF. J. Psychopharmacol. 2021, 35, 1188–1203. [Google Scholar] [CrossRef]
- Brown, R.W.; Maple, A.M.; Perna, M.K.; Sheppard, A.B.; Cope, Z.A.; Kostrzewa, R.M. Schizophrenia and Substance Abuse Comorbidity: Nicotine Addiction and the Neonatal Quinpirole Model. Dev. Neurosci. 2012, 34, 140–151. [Google Scholar] [CrossRef]
- Kostrzewa, R.M.; Nowak, P.; Brus, R.; Brown, R.W. Perinatal Treatments with the Dopamine D2-Receptor Agonist Quinpirole Produces Permanent D2-Receptor Supersensitization: A Model of Schizophrenia. Neurochem. Res. 2016, 41, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.B.; Hu, K. Cigarette Smoking and Schizophrenia: Etiology, Clinical, Pharmacological, and Treatment Implications. Schizophr. Res. Treat. 2021, 2021, 7698030. [Google Scholar] [CrossRef] [PubMed]
- Peeters, L.D.; Wills, L.J.; Cuozzo, A.M.; Ahmed, C.D.; Massey, S.R.; Chen, W.; Chen, Z.; Wang, C.; Gass, J.T.; Brown, R.W. Effects of Positive mGlu5 Modulation on D2 Signaling and Nicotine-Conditioned Place Preference: Mechanisms of Epigenetic Inheritance in a Transgenerational Model of Drug Abuse Vulnerability in Psychosis. J. Psychopharmacol. 2024. [Google Scholar] [CrossRef]
- Halberstadt, A.L. Recent Advances in the Neuropsychopharmacology of Serotonergic Hallucinogens. Behav. Brain Res. 2015, 277, 99–120. [Google Scholar] [CrossRef] [PubMed]
- Wan, F.J.; Taaid, N.; Swerdlow, N.R. Do D1/D2 Interactions Regulate Prepulse Inhibition in Rats? Neuropsychopharmacology 1996, 14, 265–274. [Google Scholar] [CrossRef]
- Braff, D.L. Prepulse Inhibition of the Startle Reflex: A Window on the Brain in Schizophrenia. Curr. Top. Behav. Neurosci. 2010, 4, 349–371. [Google Scholar] [CrossRef] [PubMed]
- Powell, S.B.; Zhou, X.; Geyer, M.A. Prepulse Inhibition and Genetic Mouse Models of Schizophrenia. Behav. Brain Res. 2009, 204, 282–294. [Google Scholar] [CrossRef]
- Yamashima, T. ‘PUFA–GPR40–CREB Signaling’ Hypothesis for the Adult Primate Neurogenesis. Prog. Lipid Res. 2012, 51, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Rizavi, H.S.; Khan, M.A.; Bhaumik, R.; Dwivedi, Y.; Pandey, G.N. Alteration of Cyclic-AMP Response Element Binding Protein in the Postmortem Brain of Subjects with Bipolar Disorder and Schizophrenia. J. Affect. Disord. 2014, 152–154, 326–333. [Google Scholar] [CrossRef]
- Barrot, M.; Olivier, J.D.A.; Perrotti, L.I.; DiLeone, R.J.; Berton, O.; Eisch, A.J.; Impey, S.; Storm, D.R.; Neve, R.L.; Yin, J.C.; et al. CREB Activity in the Nucleus Accumbens Shell Controls Gating of Behavioral Responses to Emotional Stimuli. Proc. Natl. Acad. Sci. USA 2002, 99, 11435–11440. [Google Scholar] [CrossRef] [PubMed]
- Bahlinger, K.; Lincoln, T.M.; Clamor, A. Do Deficits in Subjective Stress Recovery Predict Subsequent Stress Sensitivity and Symptoms in Schizophrenia Spectrum Disorders? Schizophr. Res. 2024, 264, 170–177. [Google Scholar] [CrossRef]
- Li, L.; Du, Y.; Li, N.; Wu, X.; Wu, Y. Top–down Modulation of Prepulse Inhibition of the Startle Reflex in Humans and Rats. Neurosci. Biobehav. Rev. 2009, 33, 1157–1167. [Google Scholar] [CrossRef]
- Kostrzewa, R.M. Dopamine receptor supersensitivity. Neurosci. Biobehav. Rev. 1995, 19, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Maple, A.M.; Smith, K.J.; Perna, M.K.; Brown, R.W. Neonatal Quinpirole Treatment Produces Prepulse Inhibition Deficits in Adult Male and Female Rats. Pharmacol. Biochem. Behav. 2015, 137, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Uslaner, J.M.; Parmentier-Batteur, S.; Flick, R.B.; Surles, N.O.; Lam, J.S.H.; McNaughton, C.H.; Jacobson, M.A.; Hutson, P.H. Dose-Dependent Effect of CDPPB, the mGluR5 Positive Allosteric Modulator, on Recognition Memory Is Associated with GluR1 and CREB Phosphorylation in the Prefrontal Cortex and Hippocampus. Neuropharmacology 2009, 57, 531–538. [Google Scholar] [CrossRef]
- Kahn, R.S.; Sommer, I.E.; Murray, R.M.; Meyer-Lindenberg, A.; Weinberger, D.R.; Cannon, T.D.; O’Donovan, M.; Correll, C.U.; Kane, J.M.; van Os, J.; et al. Schizophrenia. Nat. Rev. Dis. Primers 2015, 1, 15067. [Google Scholar] [CrossRef] [PubMed]
- Monte, A.S.; Mello, B.S.F.; Borella, V.C.M.; da Silva Araujo, T.D.S.; Da Silva, F.E.R.; De Sousa, F.C.F.; De Oliveira, A.C.P.; Gama, C.S.; Seeman, M.V.; Vasconcelos, S.M.M.; et al. Two-Hit Model of Schizophrenia Induced by Neonatal Immune Activation and Peripubertal Stress in Rats: Study of Sex Differences and Brain Oxidative Alterations. Behav. Brain Res. 2017, 331, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Blendy, J.A. The Role of CREB in Depression and Antidepressant Treatment. Biol. Psychiatry 2006, 59, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Ferre, S.; von Euler, G.; Johansson, B.; Fredholm, B.B.; Fuxe, K. Stimulation of High-Affinity Adenosine A2 Receptors Decreases the Affinity of Dopamine D2 Receptors in Rat Striatal Membranes. Proc. Natl. Acad. Sci. USA 1991, 88, 7238–7241. [Google Scholar] [CrossRef]
- Muschamp, J.W.; Veer, A.V.; Parsegian, A.; Gallo, M.S.; Chen, M.; Neve, R.L.; Meloni, E.G.; Carlezon, W.A. Activation of CREB in the Nucleus Accumbens Shell Produces Anhedonia and Resistance to Extinction of Fear in Rats. J. Neurosci. 2011, 31, 3095–3103. [Google Scholar] [CrossRef] [PubMed]
- Lassen, M.L.; Byrne, C.; Sheykhzade, M.; Wissenberg, M.; Hurry, P.K.; Schmedes, A.V.; Kjaer, A.; Hasbak, P. Sex Differences and Caffeine Impact in Adenosine-Induced Hyperemia. J. Nucl. Med. 2022, 63, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Janse van Rensburg, H.D.; Terre’Blanche, G.; Van der Walt, M.M. On the Basis of Sex: Male vs. Female Rat Adenosine A1/A2A Receptor Affinity. BMC Res. Notes 2023, 16, 165. [Google Scholar] [CrossRef]
- Auger, A.P.; Hexter, D.P.; McCarthy, M.M. Sex Difference in the Phosphorylation of cAMP Response Element Binding Protein (CREB) in Neonatal Rat Brain. Brain Res. 2001, 890, 110–117. [Google Scholar] [CrossRef]
- Taura, J.; Valle-León, M.; Sahlholm, K.; Watanabe, M.; Van Craenenbroeck, K.; Fernández-Dueñas, V.; Ferré, S.; Ciruela, F. Behavioral Control by Striatal Adenosine A2A-Dopamine D2 Receptor Heteromers. Genes Brain Behav. 2018, 17, e12432. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Roy, K.; Ioka, S.; Otani, R.; Amezawa, M.; Ishikawa, Y.; Cherasse, Y.; Kaushik, M.K.; Klewe-Nebenius, D.; Zhou, L.; et al. Positive Allosteric Adenosine A2A Receptor Modulation Suppresses Insomnia Associated with Mania- and Schizophrenia-like Behaviors in Mice. Front. Pharmacol. 2023, 14, 1138666. [Google Scholar] [CrossRef]
- Valle-León, M.; Callado, L.F.; Aso, E.; Cajiao-Manrique, M.M.; Sahlholm, K.; López-Cano, M.; Soler, C.; Altafaj, X.; Watanabe, M.; Ferré, S.; et al. Decreased striatal adenosine A2A-dopamine D2 receptor heteromerization in schizophrenia. Neuropsychopharmacology 2021, 46, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Wickens, M.M.; Bangasser, D.A.; Briand, L.A. Sex Differences in Psychiatric Disease: A Focus on the Glutamate System. Front. Mol. Neurosci. 2018, 11, 197. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, Y.; Hu, J.; Cheng, W.; Jiang, H.; Zhang, X.; Li, M.; Ren, J.; Li, X. Prenatal Chronic Mild Stress Induces Depression-like Behavior and Sex-Specific Changes in Regional Glutamate Receptor Expression Patterns in Adult Rats. Neuroscience 2015, 301, 363–374. [Google Scholar] [CrossRef]
- Knouse, M.C.; Deutschmann, A.U.; Nenov, M.N.; Wimmer, M.E.; Briand, L.A. Sex Differences in Pre- and Post-Synaptic Glutamate Signaling in the Nucleus Accumbens Core. Biol. Sex Differ. 2023, 14, 52. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Feng, J.; Fienberg, A.A.; Greengard, P. D2 Dopamine Receptors Induce Mitogen-Activated Protein Kinase and cAMP Response Element-Binding Protein Phosphorylation in Neurons. Proc. Natl. Acad. Sci. USA 1999, 96, 11607–11612. [Google Scholar] [CrossRef]
- Nijholt, I.; Blank, T.; Ahi, J.; Spiess, J. In Vivo CREB Phosphorylation Mediated by Dopamine and NMDA Receptor Activation in Mouse Hippocampus and Caudate Nucleus. Gene Expr. Patterns 2002, 1, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, L.; Håkansson, K.; Usiello, A.; Borgkvist, A.; Lindskog, M.; Greengard, P.; Fisone, G. Opposite Regulation by Typical and Atypical Anti-Psychotics of ERK1/2, CREB and Elk-1 Phosphorylation in Mouse Dorsal Striatum. J. Neurochem. 2003, 86, 451–459. [Google Scholar] [CrossRef]
- Yang, S.N.; Dasgupta, S.; Lledo, P.M.; Vincent, J.D.; Fuxe, K. Reduction of Dopamine D2 Receptor Transduction by Activation of Adenosine A2a Receptors in Stably A2a/D2 (Long-Form) Receptor Co-Transfected Mouse Fibroblast Cell Lines: Studies on Intracellular Calcium Levels. Neuroscience 1995, 68, 729–736. [Google Scholar] [CrossRef]
- Lo, L.E.; Kaur, R.; Meiser, B.G.; Green, M.J. Risk of Schizophrenia in Relatives of Individuals Affected by Schizophrenia: A Meta-Analysis. Psychiatry Res. 2020, 286, 112852. [Google Scholar] [CrossRef]
- Malaspina, D.; Gilman, C.; Kranz, T.M. Paternal Age and Mental Health of Offspring. Fertil. Steril. 2015, 103, 1392–1396. [Google Scholar] [CrossRef] [PubMed]
- Hauber, W.; Koch, M. Adenosine A2a Receptors in the Nucleus Accumbens Modulate Prepulse Inhibition of the Startle Response. Neuroreport 1997, 8, 1515–1518. [Google Scholar] [CrossRef]
- Bugarski-Kirola, D.; Blaettler, T.; Arango, C.; Fleischhacker, W.W.; Garibaldi, G.; Wang, A.; Dixon, M.; Bressan, R.A.; Nasrallah, H.; Lawrie, S.; et al. Bitopertin in Negative Symptoms of Schizophrenia—Results From the Phase III FlashLyte and DayLyte Studies. Biol. Psychiatry 2017, 82, 8–16. [Google Scholar] [CrossRef]
- Weiser, M.; Gershon, A.A.; Rubinstein, K.; Petcu, C.; Ladea, M.; Sima, D.; Podea, D.; Keefe, R.S.; Davis, J.M. A randomized controlled trial of allopurinol vs. placebo added on to antipsychotics in patients with schizophrenia or schizoaffective disorder. Schizophr. Res. 2012, 138, 35–38. [Google Scholar] [CrossRef] [PubMed]
- LaCrosse, A.L.; May, C.E.; Griffin, W.C.; Olive, M.F. mGluR5 positive allosteric modulation prevents MK-801 induced increases in extracellular glutamate in the rat medial prefrontal cortex. Neuroscience 2024, 555, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, K.; Asaoka, N.; Nagayasu, K.; Shirakawa, H.; Kaneko, S. Enhancement of adenosine A2A signaling improves dopamine D2 receptor antagonist-induced dyskinesia via β-arrestin signaling. Front. Neurosci. 2023, 16, 1082375. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Matsumoto, M.; Iijima, K.; Sumiyoshi, T. Specificity and Continuity of Schizophrenia and Bipolar Disorder: Relation to Biomarkers. Curr. Pharm. Des. 2020, 26, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Stentebjerg-Olesen, M.; Pagsberg, A.K.; Fink-Jensen, A.; Correll, C.U.; Jeppesen, P. Clinical Characteristics and Predictors of Outcome of Schizophrenia-Spectrum Psychosis in Children and Adolescents: A Systematic Review. J. Child. Adolesc. Psychopharmacol. 2016, 26, 410–427. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuozzo, A.M.; Peeters, L.D.; Ahmed, C.D.; Wills, L.J.; Gass, J.T.; Brown, R.W. Investigation of the Roles of the Adenosine A(2A) and Metabotropic Glutamate Receptor Type 5 (mGlu5) Receptors in Prepulse Inhibition and CREB Signaling in a Heritable Rodent Model of Psychosis. Cells 2025, 14, 182. https://doi.org/10.3390/cells14030182
Cuozzo AM, Peeters LD, Ahmed CD, Wills LJ, Gass JT, Brown RW. Investigation of the Roles of the Adenosine A(2A) and Metabotropic Glutamate Receptor Type 5 (mGlu5) Receptors in Prepulse Inhibition and CREB Signaling in a Heritable Rodent Model of Psychosis. Cells. 2025; 14(3):182. https://doi.org/10.3390/cells14030182
Chicago/Turabian StyleCuozzo, Anthony M., Loren D. Peeters, Cristal D. Ahmed, Liza J. Wills, Justin T. Gass, and Russell W. Brown. 2025. "Investigation of the Roles of the Adenosine A(2A) and Metabotropic Glutamate Receptor Type 5 (mGlu5) Receptors in Prepulse Inhibition and CREB Signaling in a Heritable Rodent Model of Psychosis" Cells 14, no. 3: 182. https://doi.org/10.3390/cells14030182
APA StyleCuozzo, A. M., Peeters, L. D., Ahmed, C. D., Wills, L. J., Gass, J. T., & Brown, R. W. (2025). Investigation of the Roles of the Adenosine A(2A) and Metabotropic Glutamate Receptor Type 5 (mGlu5) Receptors in Prepulse Inhibition and CREB Signaling in a Heritable Rodent Model of Psychosis. Cells, 14(3), 182. https://doi.org/10.3390/cells14030182





