Gut Microbiota in a Viral Model of Multiple Sclerosis: Modulation and Pitfalls by Oral Antibiotic Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. TMEV Infection, Antibiotic Treatment, and Sample Collection
2.3. DNA Extraction and Real-Time PCR
2.4. Neuropathology
2.5. Anti-TMEV Antibody Enzyme-Linked Immunosorbent Assays (ELISAs) and Lymphoproliferative Assay
2.6. Statistical Analysis
3. Results
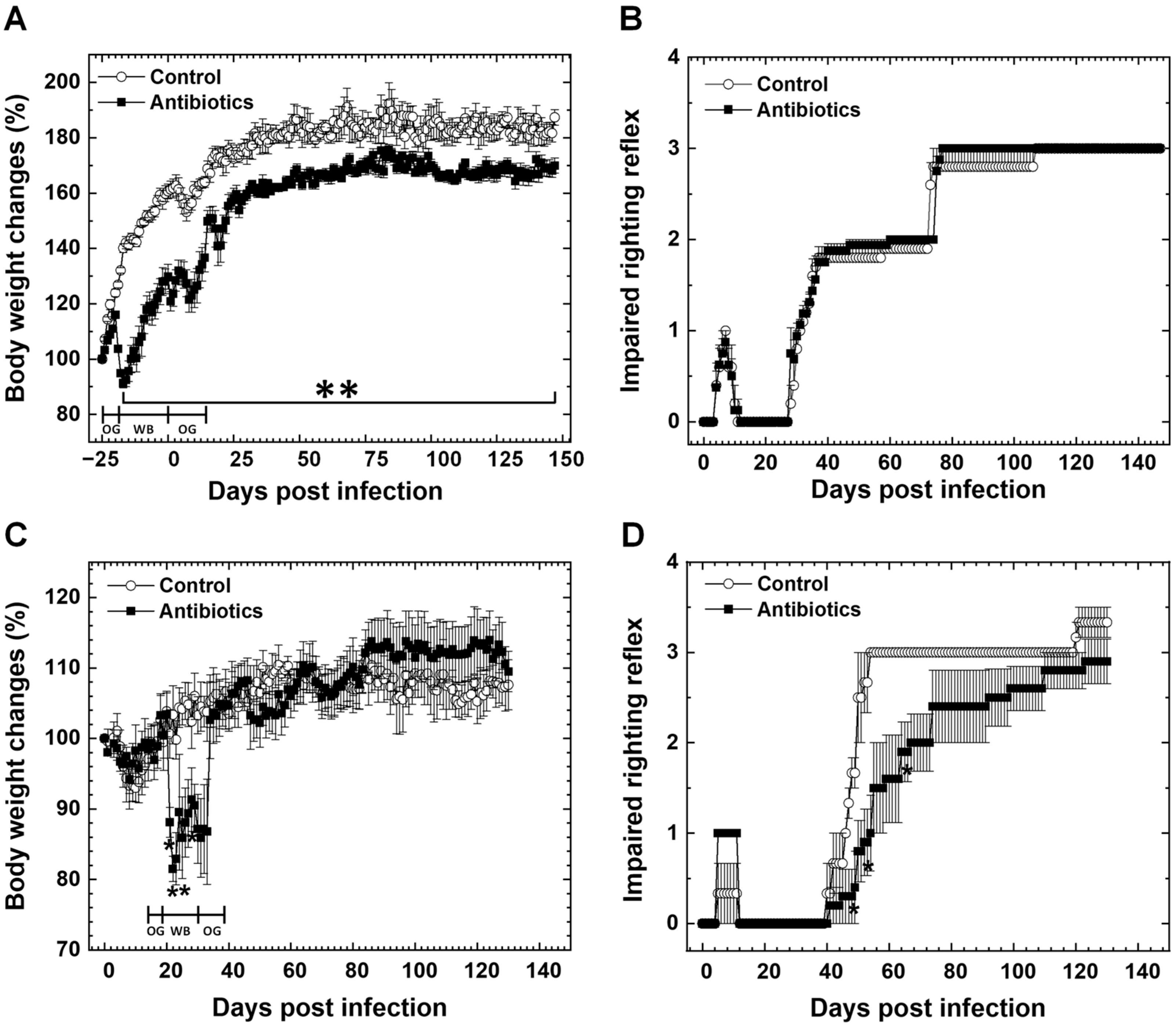
3.1. Effects of Antibiotic Treatment on Body Weights and Clinical Signs in TMEV Infection
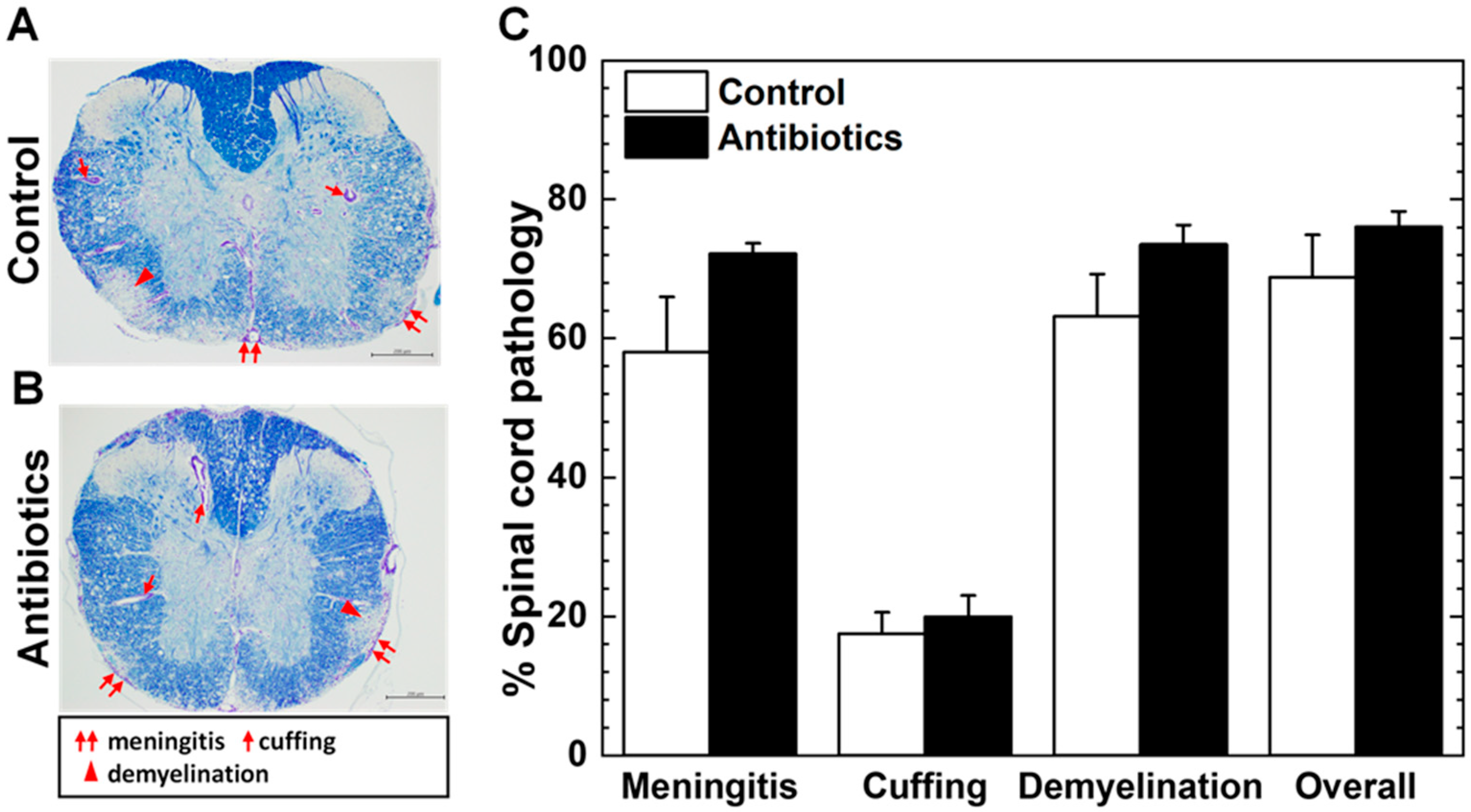
3.2. Antibiotic Treatment and Neuropathology of TMEV Infection
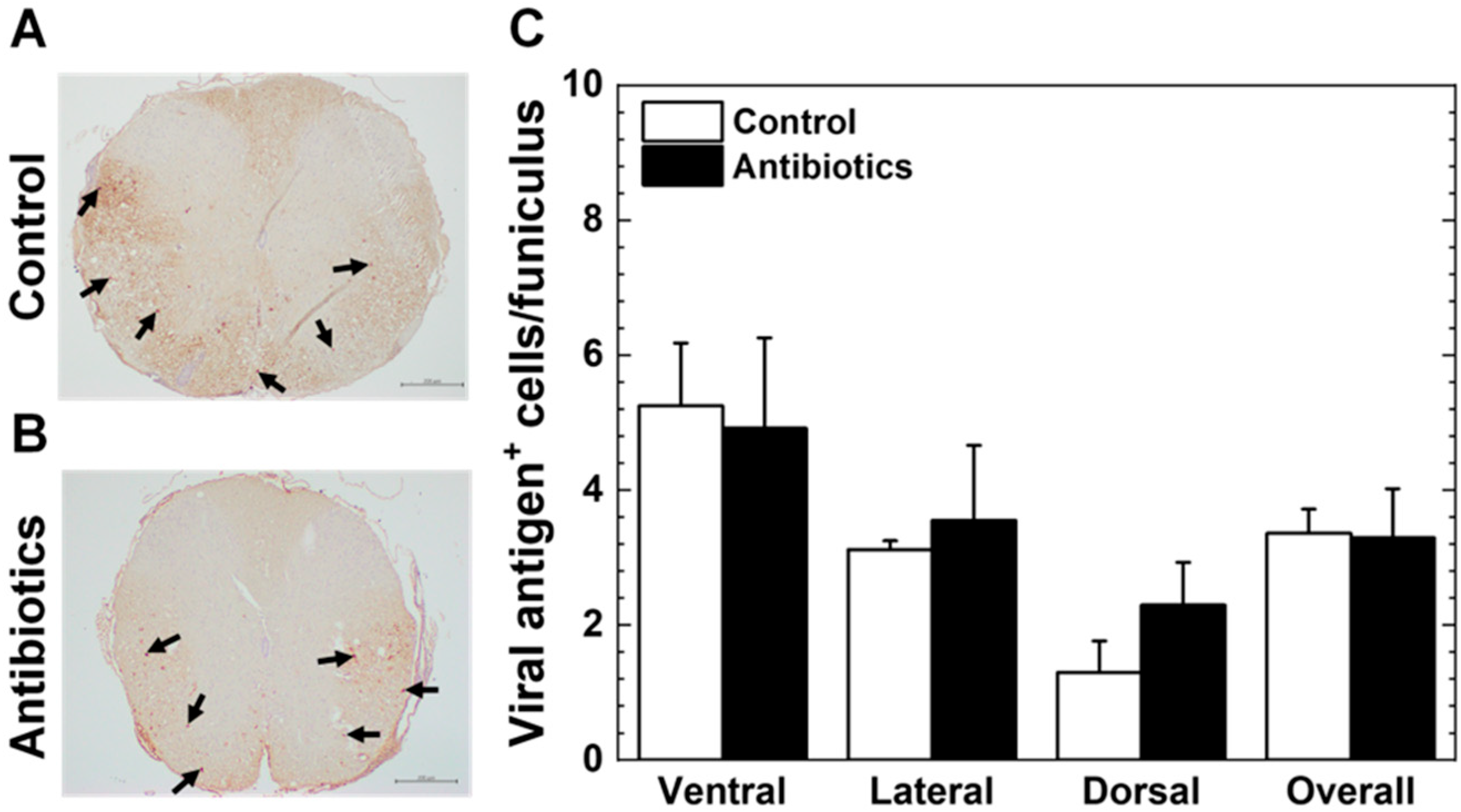
3.3. Antibiotic Treatment and Viral Persistence
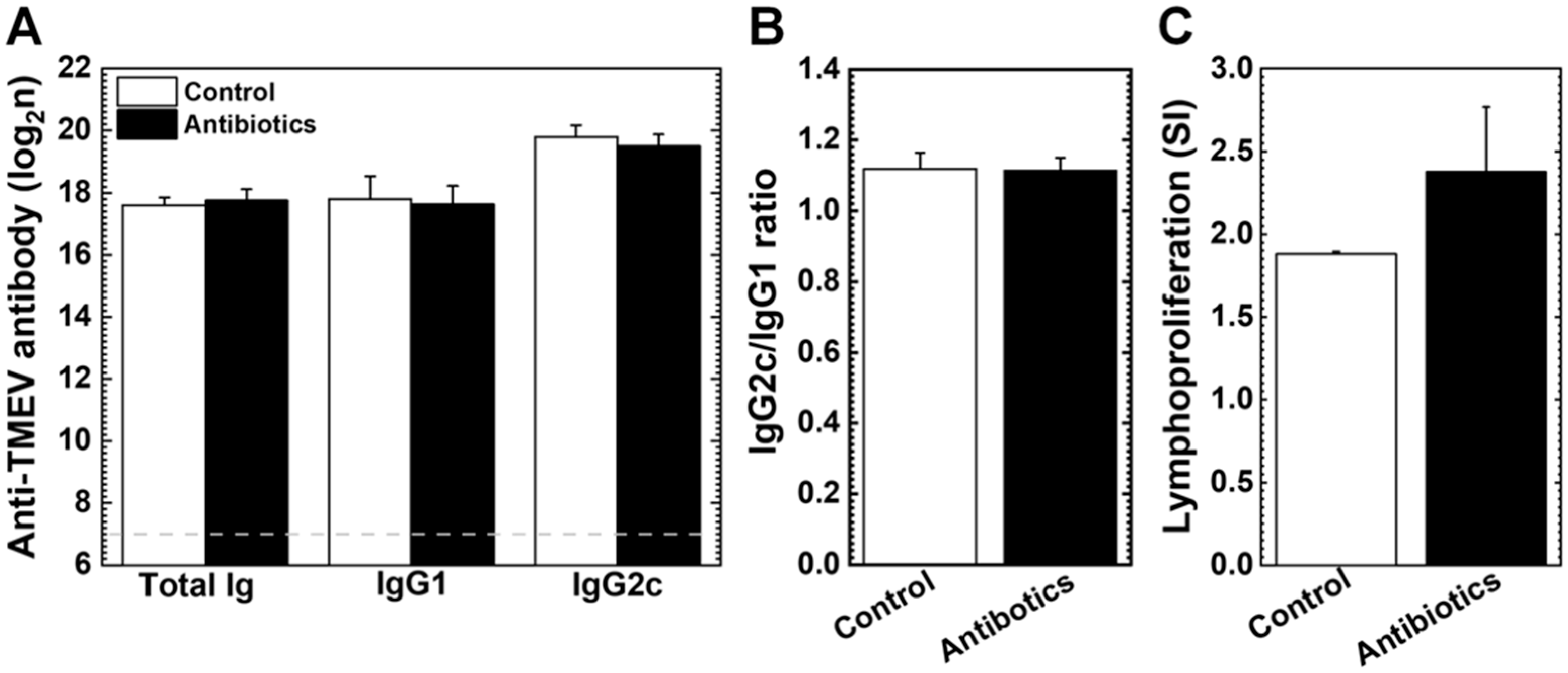
3.4. Anti-Viral Antibody Isotypes and Lymphoproliferative Responses
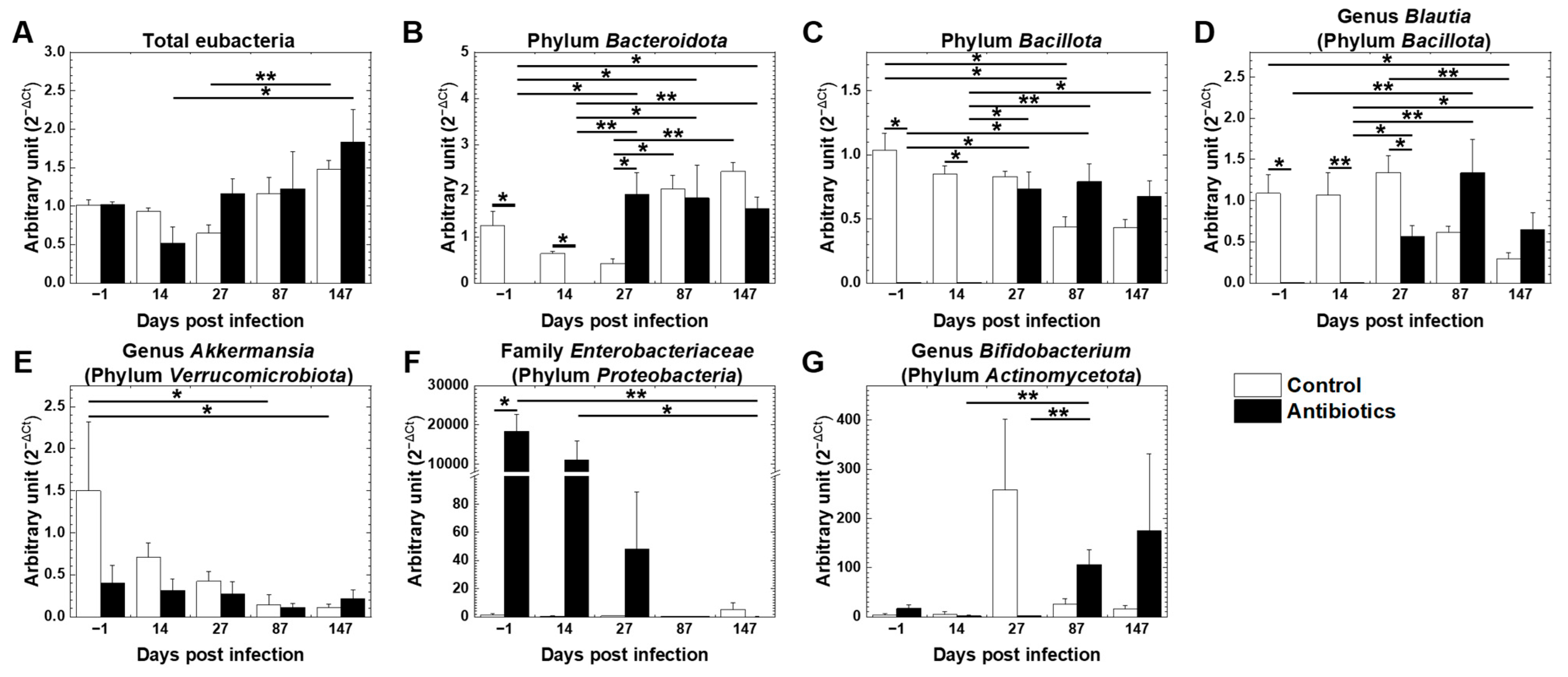
3.5. Effects of Antibiotic Treatment on Gut Microbiota in TMEV Infection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, M.; Chu, J.; Feng, S.; Guo, C.; Xue, B.; He, K.; Li, L. Immunological mechanisms of inflammatory diseases caused by gut microbiota dysbiosis: A review. Biomed. Pharmacother. 2023, 164, 114985. [Google Scholar] [CrossRef] [PubMed]
- Shao, T.; Hsu, R.; Rafizadeh, D.L.; Wang, L.; Bowlus, C.L.; Kumar, N.; Mishra, J.; Timilsina, S.; Ridgway, W.M.; Gershwin, M.E.; et al. The gut ecosystem and immune tolerance. J. Autoimmun. 2023, 141, 103114. [Google Scholar] [CrossRef]
- Socała, K.; Doboszewska, U.; Szopa, A.; Serefko, A.; Włodarczyk, M.; Zielińska, A.; Poleszak, E.; Fichna, J.; Wlaź, P. The role of microbiota-gut-brain axis in neuropsychiatric and neurological disorders. Pharmacol. Res. 2021, 172, 105840. [Google Scholar] [CrossRef]
- Hillman, E.T.; Lu, H.; Yao, T.; Nakatsu, C.H. Microbial ecology along the gastrointestinal tract. Microbes Environ. 2017, 32, 300–313. [Google Scholar] [CrossRef]
- Pang, I.K.; Iwasaki, A. Control of antiviral immunity by pattern recognition and the microbiome. Immunol. Rev. 2012, 245, 209–226. [Google Scholar] [CrossRef]
- Kane, M.; Case, L.K.; Kopaskie, K.; Kozlova, A.; MacDearmid, C.; Chervonsky, A.V.; Golovkina, T.V. Successful transmission of a retrovirus depends on the commensal microbiota. Science 2011, 334, 245–249. [Google Scholar] [CrossRef]
- Kuss, S.K.; Best, G.T.; Etheredge, C.A.; Pruijssers, A.J.; Frierson, J.M.; Hooper, L.V.; Dermody, T.S.; Pfeiffer, J.K. Intestinal microbiota promote enteric virus replication and systemic pathogenesis. Science 2011, 334, 249–252. [Google Scholar] [CrossRef]
- Kirby, T.O.; Ochoa-Repáraz, J. The gut microbiome in multiple sclerosis: A potential therapeutic avenue. Med. Sci. 2018, 6, 69. [Google Scholar] [CrossRef]
- Park, A.-M.; Omura, S.; Fujita, M.; Sato, F.; Tsunoda, I. Helicobacter pylori and gut microbiota in multiple sclerosis versus Alzheimer’s disease: 10 pitfalls of microbiome studies. Clin. Exp. Neuroimmunol. 2017, 8, 215–232. [Google Scholar] [CrossRef]
- Santana, P.T.; Rosas, S.L.B.; Ribeiro, B.E.; Marinho, Y.; de Souza, H.S.P. Dysbiosis in inflammatory bowel disease: Pathogenic role and potential therapeutic targets. Int. J. Mol. Sci. 2022, 23, 3464. [Google Scholar] [CrossRef]
- Kujawa, D.; Laczmanski, L.; Budrewicz, S.; Pokryszko-Dragan, A.; Podbielska, M. Targeting gut microbiota: New therapeutic opportunities in multiple sclerosis. Gut Microbes 2023, 15, 2274126. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Z.; Xu, Y.; Zhang, C.-J. The gut microbiota and associated metabolites in multiple sclerosis. Adv. Neurol. 2023, 2, 413. [Google Scholar] [CrossRef]
- Tsunoda, I.; Fujinami, R.S. Two models for multiple sclerosis: Experimental allergic encephalomyelitis and Theiler’s murine encephalomyelitis virus. J. Neuropathol. Exp. Neurol. 1996, 55, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Trapp, B.D.; Nave, K.-A. Multiple sclerosis: An immune or neurodegenerative disorder? Annu. Rev. Neurosci. 2008, 31, 247–269. [Google Scholar] [CrossRef]
- Park, E.; Barclay, W.E.; Barrera, A.; Liao, T.-C.; Salzler, H.R.; Reddy, T.E.; Shinohara, M.L.; Ciofani, M. Integrin α3 promotes TH17 cell polarization and extravasation during autoimmune neuroinflammation. Sci. Immunol. 2023, 8, eadg7597. [Google Scholar] [CrossRef]
- Sato, F.; Omura, S.; Martinez, N.E.; Tsunoda, I. Animal models of multiple sclerosis. In Neuroinflammation, 2nd ed.; Minagar, A., Ed.; Elsevier: Burlington, MA, USA, 2018; pp. 37–72. [Google Scholar] [CrossRef]
- Hafler, D.A.; Weiner, H.L. In vivo labeling of blood T cells: Rapid traffic into cerebrospinal fluid in multiple sclerosis. Ann. Neurol. 1987, 22, 89–93. [Google Scholar] [CrossRef]
- Burns, J.; Bartholomew, B.; Lobo, S. Isolation of myelin basic protein-specific T cells predominantly from the memory T-cell compartment in multiple sclerosis. Ann. Neurol. 1999, 45, 33–39. [Google Scholar] [CrossRef]
- Sato, F.; Nakamura, Y.; Katsuki, A.; Khadka, S.; Ahmad, I.; Omura, S.; Martinez, N.E.; Tsunoda, I. Curdlan, a microbial β-glucan, has contrasting effects on autoimmune and viral models of multiple sclerosis. Front. Cell Infect. Microbiol. 2022, 12, 805302. [Google Scholar] [CrossRef]
- Miyamoto, K.; Miyake, S.; Mizuno, M.; Oka, N.; Kusunoki, S.; Yamamura, T. Selective COX-2 inhibitor celecoxib prevents experimental autoimmune encephalomyelitis through COX-2-independent pathway. Brain 2006, 129, 1984–1992. [Google Scholar] [CrossRef]
- Mechelli, R.; Romano, C.; Reniè, R.; Manfrè, G.; Buscarinu, M.C.; Romano, S.; Marrone, A.; Bigi, R.; Bellucci, G.; Ballerini, C.; et al. Viruses and neuroinflammation in multiple sclerosis. Neurosciences 2021, 8, 269–283. [Google Scholar] [CrossRef]
- Virtanen, J.O.; Jacobson, S. Viruses and multiple sclerosis. CNS Neurol. Disord. Drug Targets 2012, 11, 528–544. [Google Scholar] [CrossRef] [PubMed]
- Omura, S.; Sato, F.; Martinez, N.E.; Range, T.; Ekshyyan, L.; Minagar, A.; Alexander, J.S.; Tsunoda, I. Immunoregulation of Theiler’s virus-induced demyelinating disease by glatiramer acetate without suppression of antiviral immune responses. Arch. Virol. 2018, 163, 1279–1284. [Google Scholar] [CrossRef] [PubMed]
- Lanz, T.V.; Brewer, R.C.; Ho, P.P.; Moon, J.-S.; Jude, K.M.; Fernandez, D.; Fernandes, R.A.; Gomez, A.M.; Nadj, G.-S.; Bartley, C.M.; et al. Clonally expanded B cells in multiple sclerosis bind EBV EBNA1 and GlialCAM. Nature 2022, 603, 321–327. [Google Scholar] [CrossRef]
- Rani, A.; Ergün, S.; Karnati, S.; Jha, H.C. Understanding the link between neurotropic viruses, BBB permeability, and MS pathogenesis. J. Neurovirol 2024, 30, 22–38. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, W.; Zheng, M.; Zhang, N. Contribution of microglia/macrophage to the pathogenesis of TMEV infection in the central nervous system. Front. Microbiol. 2024, 15, 1452390. [Google Scholar] [CrossRef]
- Tsunoda, I.; Sato, F.; Omura, S.; Fujita, M.; Sakiyama, N.; Park, A.-M. Three immune-mediated disease models induced by Theiler’s virus: Multiple sclerosis, seizures and myocarditis. Clin. Exp. Neuroimmunol. 2016, 7, 330–345. [Google Scholar] [CrossRef]
- Li, L.; Zhou, R.; Sun, L. Application of Theiler’s murine encephalomyelitis virus in treatment of multiple sclerosis. Front. Microbiol. 2024, 15, 1415365. [Google Scholar] [CrossRef]
- DePaula-Silva, A.B. The contribution of microglia and brain-infiltrating macrophages to the pathogenesis of neuroinflammatory and neurodegenerative diseases during TMEV infection of the central nervous system. Viruses 2024, 16, 119. [Google Scholar] [CrossRef]
- Tsunoda, I.; Kurtz, C.I.B.; Fujinami, R.S. Apoptosis in acute and chronic central nervous system disease induced by Theiler’s murine encephalomyelitis virus. Virology 1997, 228, 388–393. [Google Scholar] [CrossRef]
- Lassmann, H.; Bradl, M. Multiple sclerosis: Experimental models and reality. Acta Neuropathol. 2017, 133, 223–244. [Google Scholar] [CrossRef]
- Chitrala, K.N.; Guan, H.; Singh, N.P.; Busbee, B.; Gandy, A.; Mehrpouya-Bahrami, P.; Ganewatta, M.S.; Tang, C.; Chatterjee, S.; Nagarkatti, P.; et al. CD44 deletion leading to attenuation of experimental autoimmune encephalomyelitis results from alterations in gut microbiome in mice. Eur. J. Immunol. 2017, 47, 1188–1199. [Google Scholar] [CrossRef] [PubMed]
- Chu, F.; Shi, M.; Lang, Y.; Shen, D.; Jin, T.; Zhu, J.; Cui, L. Gut microbiota in multiple sclerosis and experimental autoimmune encephalomyelitis: Current applications and future perspectives. Mediat. Inflamm. 2018, 2018, 8168717. [Google Scholar] [CrossRef] [PubMed]
- Gandy, K.A.O.; Zhang, J.; Nagarkatti, P.; Nagarkatti, M. The role of gut microbiota in shaping the relapse-remitting and chronic-progressive forms of multiple sclerosis in mouse models. Sci. Rep. 2019, 9, 6923. [Google Scholar] [CrossRef]
- Mielcarz, D.W.; Kasper, L.H. The gut microbiome in multiple sclerosis. Curr. Treat. Options Neurol. 2015, 17, 344. [Google Scholar] [CrossRef]
- Miyake, S.; Kim, S.; Suda, W.; Oshima, K.; Nakamura, M.; Matsuoka, T.; Chihara, N.; Tomita, A.; Sato, W.; Kim, S.-W.; et al. Dysbiosis in the gut microbiota of patients with multiple sclerosis, with a striking depletion of species belonging to Clostridia XIVa and IV clusters. PLoS ONE 2015, 10, e0137429. [Google Scholar] [CrossRef]
- Chen, J.; Chia, N.; Kalari, K.R.; Yao, J.Z.; Novotna, M.; Paz Soldan, M.M.; Luckey, D.H.; Marietta, E.V.; Jeraldo, P.R.; Chen, X.; et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci. Rep. 2016, 6, 28484. [Google Scholar] [CrossRef]
- Jangi, S.; Gandhi, R.; Cox, L.M.; Li, N.; von Glehn, F.; Yan, R.; Patel, B.; Mazzola, M.A.; Liu, S.; Glanz, B.L.; et al. Alterations of the human gut microbiome in multiple sclerosis. Nat. Commun. 2016, 7, 12015. [Google Scholar] [CrossRef]
- Lee, Y.K.; Menezes, J.S.; Umesaki, Y.; Mazmanian, S.K. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4615–4622. [Google Scholar] [CrossRef]
- Omura, S.; Sato, F.; Park, A.-M.; Fujita, M.; Khadka, S.; Nakamura, Y.; Katsuki, A.; Nishio, K.; Gavins, F.N.E.; Tsunoda, I. Bioinformatics analysis of gut microbiota and CNS transcriptome in virus-induced acute myelitis and chronic inflammatory demyelination; potential association of distinct bacteria with CNS IgA upregulation. Front. Immunol. 2020, 11, 1138. [Google Scholar] [CrossRef]
- Zierath, D.K.; Davidson, S.; Manoukian, J.; Knox, K.M.; White, H.S.; Meeker, S.; Ericsson, A.; Barker-Haliski, M. Diet composition and sterilization modifies intestinal microbiome diversity and burden of Theiler’s virus infection-induced acute seizures. Epilepsia 2024, 65, 1777–1790. [Google Scholar] [CrossRef]
- Khadka, S.; Omura, S.; Sato, F.; Tsunoda, I. Adjuvant injections altered the ileal and fecal microbiota differently with changes in immunoglobulin isotypes and antimycobacterial antibody responses. Int. J. Mol. Sci. 2023, 24, 2818. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Jick, S.S.; Jick, H.; Hernán, M.A. Antibiotic use and risk of multiple sclerosis. Am. J. Epidemiol. 2006, 163, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Nørgaard, M.; Nielsen, R.B.; Jacobsen, J.B.; Gradus, J.L.; Stenager, E.; Koch-Henriksen, N.; Lash, T.L.; Sørensen, H.T. Use of penicillin and other antibiotics and risk of multiple sclerosis: A population-based case-control study. Am. J. Epidemiol. 2011, 174, 945–948. [Google Scholar] [CrossRef] [PubMed]
- Baldin, E.; Zenesini, C.; Antonazzo, I.C.; Bartolomei, I.; Caniatti, L.; Costa, M.; Curti, E.; Ferraro, D.; Foschi, M.; Granella, F.; et al. Antibiotic use and risk of multiple sclerosis: A nested case-control study in Emilia-Romagna region, Italy. Neuroepidemiology 2021, 55, 224–231. [Google Scholar] [CrossRef]
- Seifert, H.A.; Benedek, G.; Nguyen, H.; Gerstner, G.; Zhang, Y.; Kent, G.; Vandenbark, A.A.; Bernhagen, J.; Offner, H. Antibiotics protect against EAE by increasing regulatory and anti-inflammatory cells. Metab. Brain Dis. 2018, 33, 1599–1607. [Google Scholar] [CrossRef]
- Yokote, H.; Miyake, S.; Croxford, J.L.; Oki, S.; Mizusawa, H.; Yamamura, T. NKT cell-dependent amelioration of a mouse model of multiple sclerosis by altering gut flora. Am. J. Pathol. 2008, 173, 1714–1723. [Google Scholar] [CrossRef]
- Stanisavljević, S.; Čepić, A.; Bojić, S.; Veljović, K.; Mihajlović, S.; Đedović, N.; Jevtić, B.; Momčilović, M.; Lazarević, M.; Mostarica Stojković, M.; et al. Oral neonatal antibiotic treatment perturbs gut microbiota and aggravates central nervous system autoimmunity in dark agouti rats. Sci. Rep. 2019, 9, 918. [Google Scholar] [CrossRef]
- Ochoa-Repáraz, J.; Mielcarz, D.W.; Ditrio, L.E.; Burroughs, A.R.; Foureau, D.M.; Haque-Begum, S.; Kasper, L.H. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J. Immunol. 2009, 183, 6041–6050. [Google Scholar] [CrossRef]
- Colpitts, S.L.; Kasper, E.J.; Keever, A.; Liljenberg, C.; Kirby, T.; Magori, K.; Kasper, L.H.; Ochoa-Repáraz, J. A bidirectional association between the gut microbiota and CNS disease in a biphasic murine model of multiple sclerosis. Gut Microbes 2017, 8, 561–573. [Google Scholar] [CrossRef]
- Ceylani, T.; Jakubowska-Doğru, E.; Gurbanov, R.; Teker, H.T.; Gozen, A.G. The effects of repeated antibiotic administration to juvenile BALB/c mice on the microbiota status and animal behavior at the adult age. Heliyon 2018, 4, e00644. [Google Scholar] [CrossRef]
- National Research Council of The National Academies (US). Committee for the Update of the Guide for the Care and Use of Laboratory Animals. In Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Daniels, J.B.; Pappenheimer, A.M.; Richardson, S. Observations on encephalomyelitis of mice (DA strain). J. Exp. Med. 1952, 96, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Salinas, F.J.; Mestre, L.; Mecha, M.; Feliú, A.; Del Campo, R.; Villarrubia, N.; Espejo, C.; Montalbán, X.; Álvarez-Cermeño, J.C.; Villar, L.M.; et al. Gut dysbiosis and neuroimmune responses to brain infection with Theiler’s murine encephalomyelitis virus. Sci. Rep. 2017, 7, 44377. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, I.; Tanaka, T.; Fujinami, R.S. Regulatory role of CD1d in neurotropic virus infection. J. Virol. 2008, 82, 10279–10289. [Google Scholar] [CrossRef]
- Guo, X.; Xia, X.; Tang, R.; Zhou, J.; Zhao, H.; Wang, K. Development of a real-time PCR method for Firmicutes and Bacteroidetes in faeces and its application to quantify intestinal population of obese and lean pigs. Lett. Appl. Microbiol. 2008, 47, 367–373. [Google Scholar] [CrossRef]
- Lee, D.-H.; Zo, Y.-G.; Kim, S.-J. Nonradioactive method to study genetic profiles of natural bacterial communities by PCR-single-strand-conformation polymorphism. Appl. Environ. Microbiol. 1996, 62, 3112–3120. [Google Scholar] [CrossRef]
- Yang, Y.-W.; Chen, M.-K.; Yang, B.-Y.; Huang, X.-J.; Zhang, X.-R.; He, L.-Q.; Zhang, J.; Hua, Z.-C. Use of 16S rRNA gene-targeted group-specific primers for real-time PCR analysis of predominant bacteria in mouse feces. Appl. Environ. Microbiol. 2015, 81, 6749–6756. [Google Scholar] [CrossRef]
- Bartosch, S.; Fite, A.; Macfarlane, G.T.; McMurdo, M.E.T. Characterization of bacterial communities in feces from healthy elderly volunteers and hospitalized elderly patients by using real-time PCR and effects of antibiotic treatment on the fecal microbiota. Appl. Environ. Microbiol. 2004, 70, 3575–3581. [Google Scholar] [CrossRef]
- Rinttilä, T.; Kassinen, A.; Malinen, E.; Krogius, L.; Palva, A. Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J. Appl. Microbiol. 2004, 97, 1166–1177. [Google Scholar] [CrossRef]
- Miyamoto, J.; Shimizu, H.; Hisa, K.; Matsuzaki, C.; Inuki, S.; Ando, Y.; Nishida, A.; Izumi, A.; Yamano, M.; Ushiroda, C.; et al. Host metabolic benefits of prebiotic exopolysaccharides produced by Leuconostoc mesenteroides. Gut Microbes 2023, 15, 2161271. [Google Scholar] [CrossRef]
- Ahmad, I.; Omura, S.; Sato, F.; Park, A.-M.; Khadka, S.; Gavins, F.N.E.; Tanaka, H.; Kimura, M.Y.; Tsunoda, I. Exploring the role of platelets in virus-induced inflammatory demyelinating disease and myocarditis. Int. J. Mol. Sci. 2024, 25, 3460. [Google Scholar] [CrossRef]
- Sato, F.; Martinez, N.E.; Shahid, M.; Rose, J.W.; Carlson, N.G.; Tsunoda, I. Resveratrol exacerbates both autoimmune and viral models of multiple sclerosis. Am. J. Pathol. 2013, 183, 1390–1396. [Google Scholar] [CrossRef] [PubMed]
- Martinez, N.E.; Karlsson, F.; Sato, F.; Kawai, E.; Omura, S.; Minagar, A.; Grisham, M.B.; Tsunoda, I. Protective and detrimental roles for regulatory T cells in a viral model for multiple sclerosis. Brain Pathol. 2014, 24, 436–451. [Google Scholar] [CrossRef] [PubMed]
- Nitayaphan, S.; Toth, M.M.; Roos, R.P. Neutralizing monoclonal antibodies to Theiler’s murine encephalomyelitis viruses. J. Virol. 1985, 53, 651–657. [Google Scholar] [CrossRef]
- Mestre, L.; Carrillo-Salinas, F.J.; Mecha, M.; Feliú, A.; Espejo, C.; Álvarez-Cermeño, J.C.; Villar, L.M.; Guaza, C. Manipulation of gut microbiota influences immune responses, axon preservation, and motor disability in a model of progressive multiple sclerosis. Front. Immunol. 2019, 10, 1374. [Google Scholar] [CrossRef]
- Goulding, D.R.; Myers, P.H.; Dickerson, A.B.; Comins, M.M.; Wiltshire, R.A.; Blankenship-Paris, T.L. Comparative efficacy of two types of antibiotic mixtures in gut flora depletion in female C57BL/6 mice. Comp. Med. 2021, 71, 203–209. [Google Scholar] [CrossRef]
- Hill, D.A.; Hoffmann, C.; Abt, M.C.; Du, Y.; Kobuley, D.; Kirn, T.J.; Bushman, F.D.; Artis, D. Metagenomic analyses reveal antibiotic-induced temporal and spatial changes in intestinal microbiota with associated alterations in immune cell homeostasis. Mucosal Immunol. 2010, 3, 148–158. [Google Scholar] [CrossRef]
- Tsai, Y.-C.; Tai, W.-C.; Liang, C.-M.; Wu, C.-K.; Tsai, M.-C.; Hu, W.-H.; Huang, P.-Y.; Chen, C.-H.; Kuo, Y.-H.; Yao, C.-C.; et al. Alternations of the gut microbiota and the Firmicutes/Bacteroidetes ratio after biologic treatment in inflammatory bowel disease. J. Microbiol. Immunol. Infect. 2025, 58, 62–69. [Google Scholar] [CrossRef]
- Hassan, N.E.; El Shebini, S.M.; El-Masry, S.A.; Ahmed, N.H.; Kamal, A.N.; Ismail, A.S.; Alian, K.M.; Mostafa, M.I.; Selim, M.; Afify, M.A.S. Brief overview of dietary intake, some types of gut microbiota, metabolic markers and research opportunities in sample of Egyptian women. Sci. Rep. 2022, 12, 17291. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The influence of probiotics on the firmicutes/bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef]
- Rumah, K.R.; Linden, J.; Fischetti, V.A.; Vartanian, T. Isolation of Clostridium perfringens type B in an individual at first clinical presentation of multiple sclerosis provides clues for environmental triggers of the disease. PLoS ONE 2013, 8, e76359. [Google Scholar] [CrossRef]
- Cantarel, B.L.; Waubant, E.; Chehoud, C.; Kuczynski, J.; DeSantis, T.Z.; Warrington, J.; Venkatesan, A.; Fraser, C.M.; Mowry, E.M. Gut microbiota in multiple sclerosis: Possible influence of immunomodulators. J. Investig. Med. 2015, 63, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Baumann, R.; Gao, X.; Mendoza, M.; Singh, S.; Sand, I.K.; Xia, Z.; Cox, L.M.; Chitnis, T.; Yoon, H.; et al. Gut microbiome of multiple sclerosis patients and paired household healthy controls reveal associations with disease risk and course. Cell 2022, 185, 3467–3486.e16. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.M.; Maghzi, A.H.; Liu, S.; Tankou, S.K.; Dhang, F.H.; Willocq, V.; Song, A.; Wasén, C.; Tauhid, S.; Chu, R.; et al. The gut microbiome in progressive multiple sclerosis. Ann. Neurol. 2021, 89, 1195–1211. [Google Scholar] [CrossRef]
- Thirion, F.; Sellebjerg, F.; Fan, Y.; Lyu, L.; Hansen, T.H.; Pons, N.; Levenez, F.; Quinquis, B.; Stankevic, E.; Søndergaard, H.B.; et al. The gut microbiota in multiple sclerosis varies with disease activity. Genome Med. 2023, 15, 1. [Google Scholar] [CrossRef]
- Ghimire, S.; Lehman, P.C.; Aguilar Meza, L.S.; Shahi, S.K.; Hoang, J.; Olalde, H.; Paullus, M.; Cherwin, C.; Wang, K.; Gill, C.; et al. Specific microbial ratio in the gut microbiome is associated with multiple sclerosis. Proc. Natl. Acad. Sci. USA 2025, 122, e2413953122. [Google Scholar] [CrossRef]
- Yang, M.; Yang, Y.; He, Q.; Zhu, P.; Liu, M.; Xu, J.; Zhao, M. Intestinal microbiota-a promising target for antiviral therapy? Front. Immunol. 2021, 12, 676232. [Google Scholar] [CrossRef]
- Abt, M.C.; Osborne, L.C.; Monticelli, L.A.; Doering, T.A.; Alenghat, T.; Sonnenberg, G.F.; Paley, M.A.; Antenus, M.; Williams, K.L.; Erikson, J.; et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity 2012, 37, 158–170. [Google Scholar] [CrossRef]
- Ichinohe, T.; Pang, I.K.; Kumamoto, Y.; Peaper, D.R.; Ho, J.H.; Murray, T.S.; Iwasaki, A. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc. Natl. Acad. Sci. USA 2011, 108, 5354–5359. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, L.; Guo, B.; Zhu, B. Regulation of adaptive immune responses by guiding cell movements in the spleen. Front. Microbiol. 2015, 6, 645. [Google Scholar] [CrossRef]
- Miao, Z.; Cheng, R.; Zhang, Y.; Liang, H.; Jiang, F.; Shen, X.; Chen, G.; Zhang, Q.; He, F.; Li, M. Antibiotics can cause weight loss by impairing gut microbiota in mice and the potent benefits of lactobacilli. Biosci. Biotechnol. Biochem. 2020, 84, 411–420. [Google Scholar] [CrossRef]
- Fröhlich, E.E.; Farzi, A.; Mayerhofer, R.; Reichmann, F.; Jačan, A.; Wagner, B.; Zinser, E.; Bordag, N.; Magnes, C.; Fröhlich, E.; et al. Cognitive impairment by antibiotic-induced gut dysbiosis: Analysis of gut microbiota-brain communication. Brain Behav. Immun. 2016, 56, 140–155. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, R.; Rolinson, G.N. Activity of ampicillin in vitro compared with other antibiotics. J. Clin. Pathol. 1964, 17, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Samuelson, J. Why metronidazole is active against both bacteria and parasites. Antimicrob. Agents Chemother. 1999, 43, 1533–1541. [Google Scholar] [CrossRef]
- Löfmark, S.; Edlund, C.; Nord, C.E. Metronidazole is still the drug of choice for treatment of anaerobic infections. Clin. Infect. Dis. 2010, 50 (Suppl. S1), S16–S23. [Google Scholar] [CrossRef]
- The LiverTox Team. Neomycin. In LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012. Available online: http://www.ncbi.nlm.nih.gov/books/NBK547874/ (accessed on 15 May 2025).
- Block, M.; Blanchard, D.L. Aminoglycosides. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK541105/ (accessed on 15 May 2025).
- The LiverTox Team. Vancomycin. In LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012. Available online: http://www.ncbi.nlm.nih.gov/books/NBK548881/ (accessed on 31 March 2025).
- Josefsdottir, K.S.; Baldridge, M.T.; Kadmon, C.S.; King, K.Y. Antibiotics impair murine hematopoiesis by depleting the intestinal microbiota. Blood 2017, 129, 729–739. [Google Scholar] [CrossRef]
- Gödel, C.; Kunkel, B.; Kashani, A.; Lassmann, H.; Arumugam, M.; Krishnamoorthy, G. Perturbation of gut microbiota decreases susceptibility but does not modulate ongoing autoimmune neurological disease. J. Neuroinflamm. 2020, 17, 79. [Google Scholar] [CrossRef]
- Reikvam, D.H.; Erofeev, A.; Sandvik, A.; Grcic, V.; Jahnsen, F.L.; Gaustad, P.; McCoy, K.D.; Macpherson, A.J.; Meza-Zepeda, L.A.; Johansen, F.-E. Depletion of murine intestinal microbiota: Effects on gut mucosa and epithelial gene expression. PLoS ONE 2011, 6, e17996. [Google Scholar] [CrossRef]
- Kipnis, J. Multifaceted interactions between adaptive immunity and the central nervous system. Science 2016, 353, 766–771. [Google Scholar] [CrossRef]
- Mittal, R.; Debs, L.H.; Patel, A.P.; Nguyen, D.; Patel, K.; O’Connor, G.; Grati, M.; Mittal, J.; Yan, D.; Eshraghi, A.A.; et al. Neurotransmitters: The critical modulators regulating gut-brain axis. J. Cell Physiol. 2017, 232, 2359–2372. [Google Scholar] [CrossRef]
- Lee, B.; Lee, S.M.; Song, J.W.; Choi, J.W. Gut microbiota metabolite messengers in brain function and pathology at a view of cell type-based receptor and enzyme reaction. Biomol. Ther. 2024, 32, 403–423. [Google Scholar] [CrossRef]
- Mans, C.; Fink, D. Effects of commercial metronidazole and metronidazole benzoate suspensions on food intake in chinchillas. J. Small Anim. Pract. 2021, 62, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Arai, K.; Watanabe, K.; Kusu, F. Effects of bitter antibiotics on electrical potential oscillation across a water/octanol/water liquid membrane. IEEJ 1999, 119, 544–548. [Google Scholar] [CrossRef]
- Rakoff-Nahoum, S.; Paglino, J.; Eslami-Varzaneh, F.; Edberg, S.; Medzhitov, R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 2004, 118, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Battson, M.L.; Lee, D.M.; Jarrell, D.K.; Hou, S.; Ecton, K.E.; Weir, T.L.; Gentile, C.L. Suppression of gut dysbiosis reverses western diet-induced vascular dysfunction. Am. J. Physiol. Endocrinol. Metab. 2018, 314, E468–E477. [Google Scholar] [CrossRef]
- Emal, D.; Rampanelli, E.; Stroo, I.; Butter, L.M.; Teske, G.J.; Claessen, N.; Stokman, G.; Florquin, S.; Leemans, J.C.; Dessing, M.C. Depletion of gut microbiota protects against renal ischemia-reperfusion injury. J. Am. Soc. Nephrol. 2017, 28, 1450–1461. [Google Scholar] [CrossRef]
- Yan, J.; Herzog, J.W.; Tsang, K.; Brennan, C.A.; Bower, M.A.; Garrett, W.S.; Sartor, B.R.; Aliprantis, A.O.; Charles, J.F. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc. Natl. Acad. Sci. USA 2016, 113, E7554–E7563. [Google Scholar] [CrossRef]
- Burkholder, T.; Foltz, C.; Karlsson, E.; Linton, C.G.; Smith, J.M. Health evaluation of experimental laboratory mice. Curr. Protoc. Mouse Biol. 2012, 2, 145–165. [Google Scholar] [CrossRef]
- Fan, S.; Cai, X.; Cui, W.; Ma, P.; Wu, M.; Guo, J.; Zhang, Y.; Xuan, K.; Li, Z. Restoration of gut integrity by Bacteroides acidifaciens in water-deprived conditions. Biochem. Biophys. Res. Commun. 2025, 767, 151917. [Google Scholar] [CrossRef]
- Remus, J.L.; Stewart, L.T.; Camp, R.M.; Novak, C.M.; Johnson, J.D. Interaction of metabolic stress with chronic mild stress in altering brain cytokines and sucrose preference. Behav. Neurosci. 2015, 129, 321–330. [Google Scholar] [CrossRef]
- Kim, C.-S.; Shin, D.-M. Gut microbiota and cognitive development in infant mice: Quantity and source of potable water. PLoS ONE 2023, 18, e0286951. [Google Scholar] [CrossRef]
- Cignarella, F.; Cantoni, C.; Ghezzi, L.; Salter, A.; Dorsett, Y.; Chen, L.; Phillips, D.; Weinstock, G.M.; Fontana, L.; Cross, A.H.; et al. Intermittent fasting confers protection in CNS autoimmunity by altering the gut microbiota. Cell Metab. 2018, 27, 1222–1235.e6. [Google Scholar] [CrossRef] [PubMed]
- Balcombe, J.P.; Barnard, N.D.; Sandusky, C. Laboratory routines cause animal stress. Contemp. Top. Lab. Anim. Sci. 2004, 43, 42–51. [Google Scholar] [PubMed]
- Hoggatt, A.F.; Hoggatt, J.; Honerlaw, M.; Pelus, L.M. A spoonful of sugar helps the medicine go down: A novel technique to improve oral gavage in mice. J. Am. Assoc. Lab. Anim. Sci. 2010, 49, 329–334. [Google Scholar] [PubMed]
- Germann, P.G.; Ockert, D. Granulomatous inflammation of the oropharyngeal cavity as a possible cause for unexpected high mortality in a fischer 344 rat carcinogenicity study. Lab. Anim. Sci. 1994, 44, 338–343. [Google Scholar]
- Murphy, S.J.; Smith, P.; Shaivitz, A.B.; Rossberg, M.I.; Hurn, P.D. The effect of brief halothane anesthesia during daily gavage on complications and body weight in rats. Contemp. Top. Lab. Anim. Sci. 2001, 40, 9–12. [Google Scholar]
- Geng, S.; Yang, L.; Cheng, F.; Zhang, Z.; Li, J.; Liu, W.; Li, Y.; Chen, Y.; Bao, Y.; Chen, L.; et al. Gut microbiota are associated with psychological stress-induced defections in intestinal and blood–brain barriers. Front. Microbiol. 2020, 10, 3067. [Google Scholar] [CrossRef]
- Stilling, R.M.; van de Wouw, M.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis? Neurochem. Int. 2016, 99, 110–132. [Google Scholar] [CrossRef]
- Fung, T.C.; Olson, C.A.; Hsiao, E.Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 2017, 20, 145–155. [Google Scholar] [CrossRef]
- Padgett, D.A.; Glaser, R. How stress influences the immune response. Trends Immunol. 2003, 24, 444–448. [Google Scholar] [CrossRef]
- Song, H.; Fang, F.; Tomasson, G.; Arnberg, F.K.; Mataix-Cols, D.; Fernández de la Cruz, L.; Almqvist, C.; Fall, K.; Valdimarsdóttir, U.A. Association of stress-related disorders with subsequent autoimmune disease. JAMA 2018, 319, 2388–2400. [Google Scholar] [CrossRef]
- Weygandt, M.; Meyer-Arndt, L.; Behrens, J.R.; Wakonig, K.; Bellmann-Strobl, J.; Ritter, K.; Scheel, M.; Brandt, A.U.; Labadie, C.; Hetzer, S.; et al. Stress-induced brain activity, brain atrophy, and clinical disability in multiple sclerosis. Proc. Natl. Acad. Sci. USA 2016, 113, 13444–13449. [Google Scholar] [CrossRef] [PubMed]
- Bukilica, M.; Djordjević, S.; Marić, I.; Dimitrijević, M.; Marković, B.M.; Janković, B.D. Stress-induced suppression of experimental allergic encephalomyelitis in the rat. Int. J. Neurosci. 1991, 59, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Mohr, D.C.; Hart, S.L.; Julian, L.; Cox, D.; Pelletier, D. Association between stressful life events and exacerbation in multiple sclerosis: A meta-analysis. BMJ 2004, 328, 731. [Google Scholar] [CrossRef] [PubMed]
- Welsh, C.J.; Steelman, A.J.; Mi, W.; Young, C.R.; Dean, D.D.; Storts, R.; Welsh, T.H.; Meagher, M.W. Effects of stress on the immune response to Theiler’s virus—Implications for virus-induced autoimmunity. Neuroimmunomodulation 2010, 17, 169–172. [Google Scholar] [CrossRef]
- Johnson, R.R.; Prentice, T.W.; Bridegam, P.; Young, C.R.; Steelman, A.J.; Welsh, T.H.; Welsh, C.J.R.; Meagher, M.W. Social stress alters the severity and onset of the chronic phase of Theiler’s virus infection. J. Neuroimmunol. 2006, 175, 39–51. [Google Scholar] [CrossRef]
- Mi, W.; Young, C.R.; Storts, R.W.; Steelman, A.J.; Meagher, M.W.; Welsh, C.J.R. Restraint stress facilitates systemic dissemination of Theiler’s virus and alters its pathogenecity. Microb. Pathog. 2006, 41, 133–143. [Google Scholar] [CrossRef]
| Exp. No. | Groups | TMEV Infection | Mouse No. | Antibiotic Treatment | ||
|---|---|---|---|---|---|---|
| Oral Gavage | Water Bottle | Oral Gavage | ||||
| Exp. 1 | prophylactic | day 0 | 8 | days −25 to −21 | days −20 to 0 | days 1 to 14 |
| control | day 0 | 5 | (−) | (−) | (−) | |
| Exp. 2 | therapeutic | day 0 | 5 | days 14 to 18 | days 19 to 31 | days 32 to 38 |
| control | day 0 | 3 | (−) | (−) | (−) | |
| Target Bacterial Taxa | Tm (°C) | Primer Sequence | References |
|---|---|---|---|
| Total eubacteria | 68 69 | F: CGGYCCAGACTCCTACGGG R: TTACCGAGGCTGCTGGCAC | [56] |
| Phylum Bacillota | 65 66 | F: GGAGYATGTGGTTTAATTCGAAGCA R: AGCTGACGACAACCATGCAC | [57] |
| Phylum Bacteroidota | 62 61 | F: GTTTAATTCGATGATACGCGAG R: TTAASCCGACACCTCACGG | [58] |
| Family Enterobacteriaceae | 72 61 | F: CATTGACGTTACCCGCAGAAGAAGC R: CTCTACGAGACTCAAGCTTGC | [59] |
| Genus Bifidobacterium | 59 57 | F: TCGCGTCYGGTGTGAAAG R: CCACATCCAGCRTCCAC | [60] |
| Genus Akkermansia | 70 68 | F: CAGCACGTGAAGGTGGGGAC R: CCTTGCGGTTGGCTTCAGAT | [61] |
| Genus Blautia | 71 69 | F: TCTGATGTGAAAGGCTGGGGCTTA R: GGCTTAGCCACCCGACACCTA | [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, I.; Omura, S.; Khadka, S.; Sato, F.; Park, A.-M.; Rimal, S.; Tsunoda, I. Gut Microbiota in a Viral Model of Multiple Sclerosis: Modulation and Pitfalls by Oral Antibiotic Treatment. Cells 2025, 14, 871. https://doi.org/10.3390/cells14120871
Ahmad I, Omura S, Khadka S, Sato F, Park A-M, Rimal S, Tsunoda I. Gut Microbiota in a Viral Model of Multiple Sclerosis: Modulation and Pitfalls by Oral Antibiotic Treatment. Cells. 2025; 14(12):871. https://doi.org/10.3390/cells14120871
Chicago/Turabian StyleAhmad, Ijaz, Seiichi Omura, Sundar Khadka, Fumitaka Sato, Ah-Mee Park, Sandesh Rimal, and Ikuo Tsunoda. 2025. "Gut Microbiota in a Viral Model of Multiple Sclerosis: Modulation and Pitfalls by Oral Antibiotic Treatment" Cells 14, no. 12: 871. https://doi.org/10.3390/cells14120871
APA StyleAhmad, I., Omura, S., Khadka, S., Sato, F., Park, A.-M., Rimal, S., & Tsunoda, I. (2025). Gut Microbiota in a Viral Model of Multiple Sclerosis: Modulation and Pitfalls by Oral Antibiotic Treatment. Cells, 14(12), 871. https://doi.org/10.3390/cells14120871











