NAA10 (N-Alpha-Acetyltransferase 10): A Multifunctional Regulator in Development, Disease, and Cancer
Abstract
1. Introduction
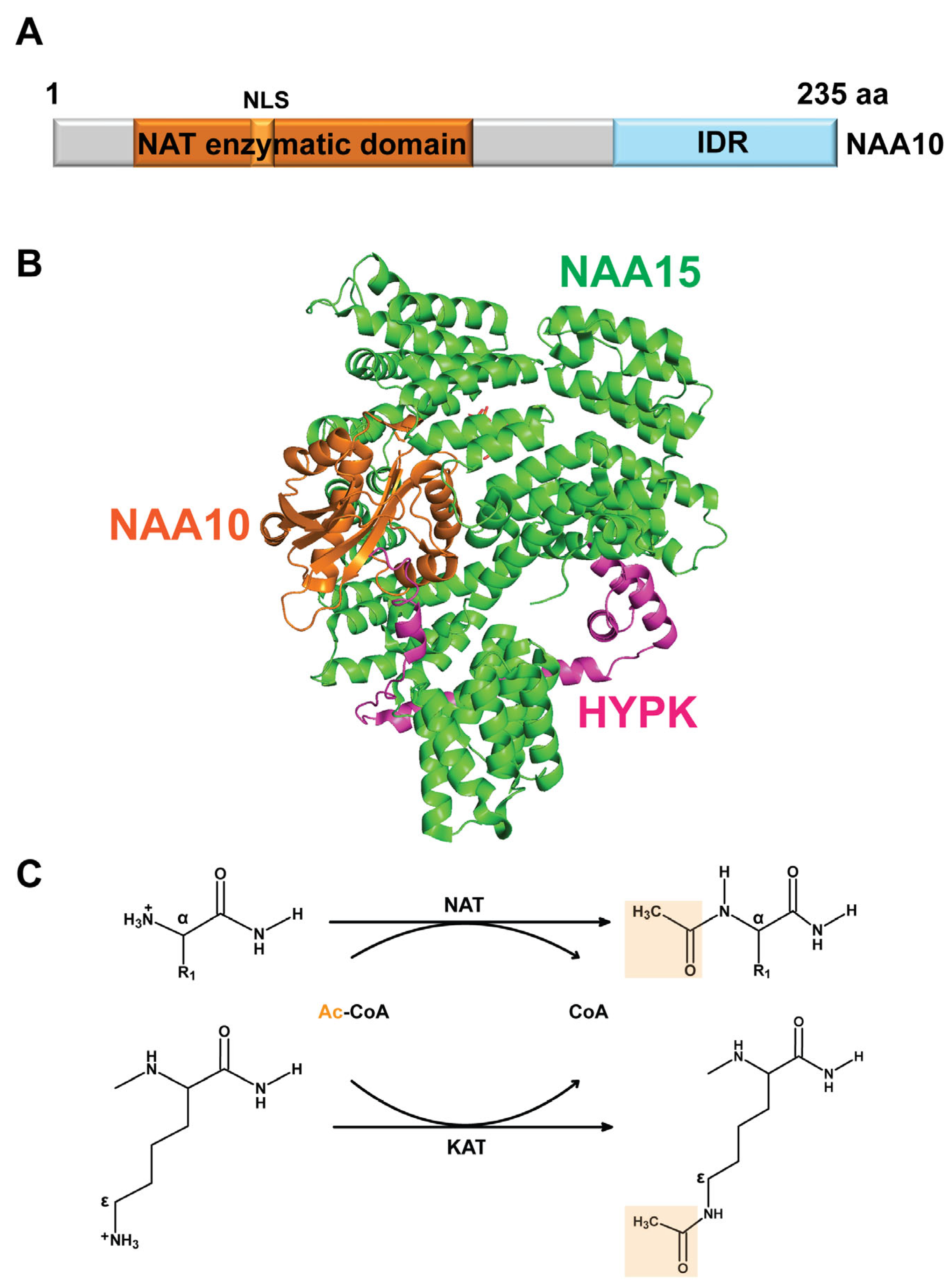
2. Structural Basis and Molecular Functions of NAA10
3. NAA10 in Developmental Disorders
3.1. Pathogenic Mutations and Developmental Roles of NAA10
3.2. Mechanistic Insights into NAA10-Related Developmental Syndromes
4. NAA10 in Cancer
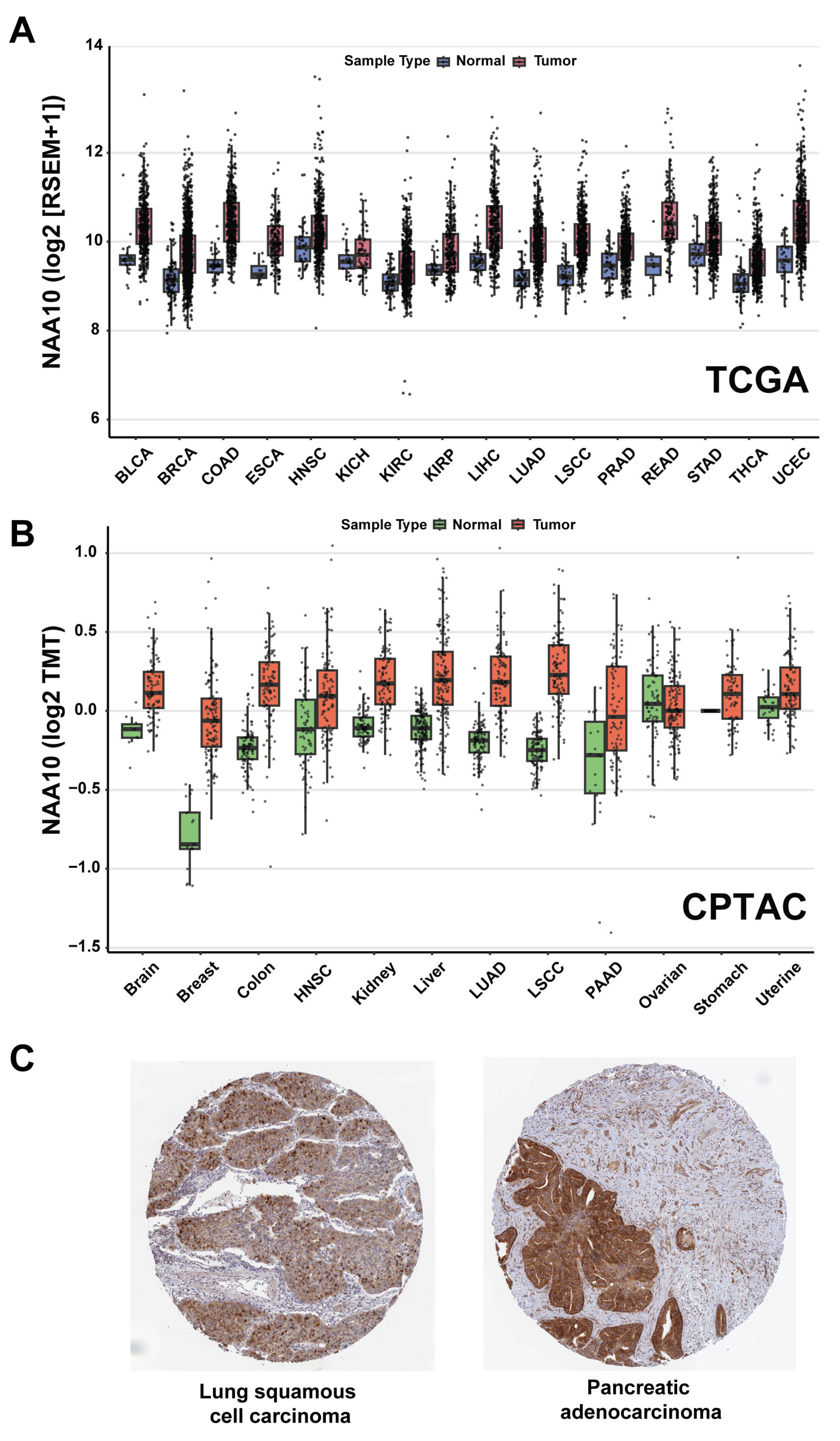
4.1. Alterations and Clinical Significance of NAA10 in Human Cancer
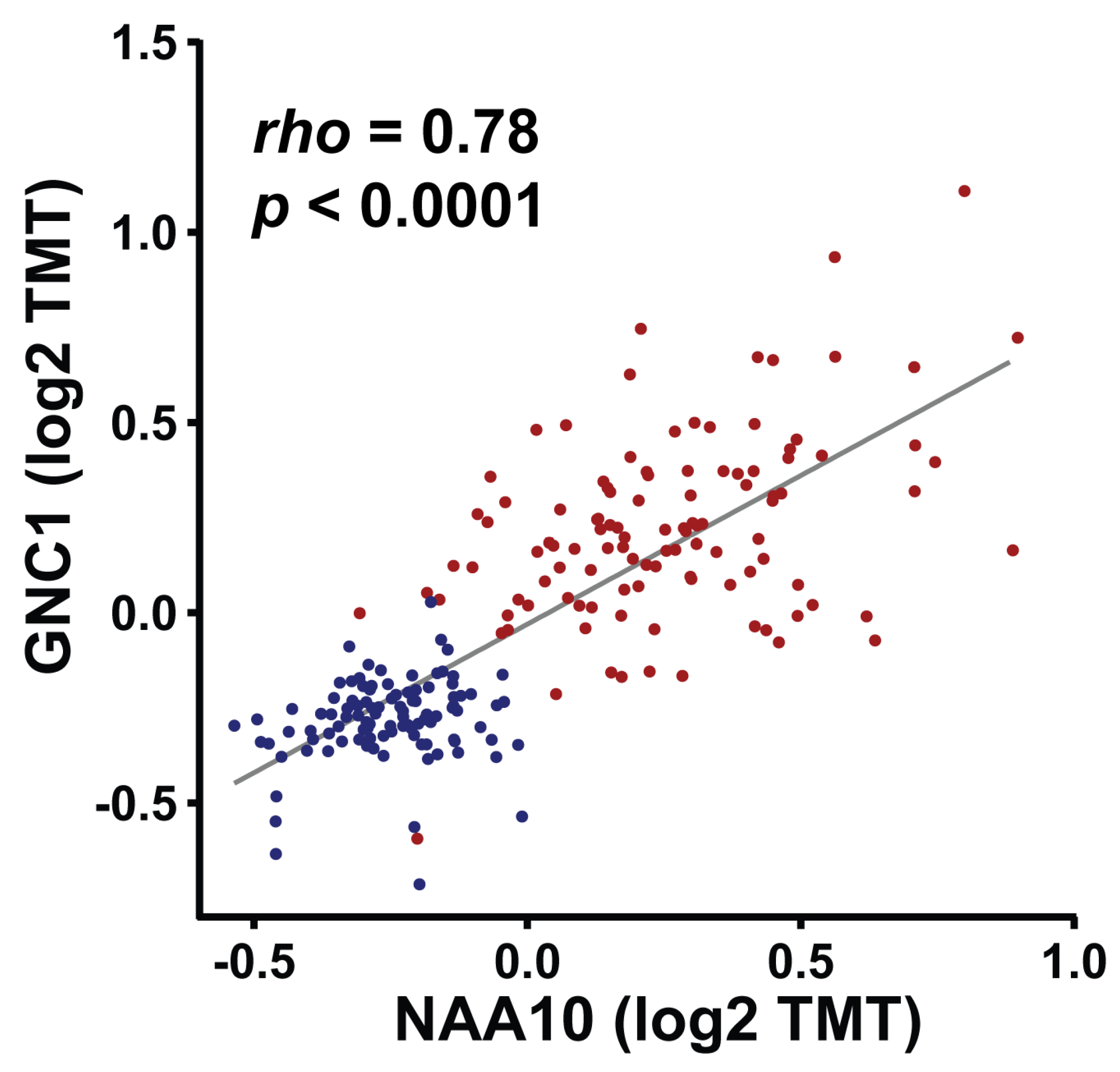
4.2. Multilayered Regulation of NAA10 in Cancer
4.3. Acetyltransferase-Dependent and -Independent Functions of NAA10 in Cancer
4.4. In Silico Identification of NAA10-Regulated Targets in Cancer
5. Therapeutic Potential of Targeting NAA10
6. Conclusions and Future Perspective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- McTiernan, N.; Kjosas, I.; Arnesen, T. Illuminating the impact of N-terminal acetylation: From protein to physiology. Nat. Commun. 2025, 16, 703. [Google Scholar] [CrossRef] [PubMed]
- Oye, H.; Lundekvam, M.; Caiella, A.; Hellesvik, M.; Arnesen, T. Protein N-terminal modifications: Molecular machineries and biological implications. Trends Biochem. Sci. 2025, 50, 290–310. [Google Scholar] [CrossRef]
- Deng, S.; Marmorstein, R. Protein N-Terminal Acetylation: Structural Basis, Mechanism, Versatility, and Regulation. Trends Biochem. Sci. 2021, 46, 15–27. [Google Scholar] [CrossRef]
- Aksnes, H.; Ree, R.; Arnesen, T. Co-translational, Post-translational, and Non-catalytic Roles of N-Terminal Acetyltransferases. Mol. Cell 2019, 73, 1097–1114. [Google Scholar] [CrossRef]
- Aksnes, H.; McTiernan, N.; Arnesen, T. NATs at a glance. J. Cell Sci. 2023, 136, jcs260766. [Google Scholar] [CrossRef]
- Calis, S.; Gevaert, K. The role of Nalpha-terminal acetylation in protein conformation. FEBS J. 2025, 292, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Ree, R.; Varland, S.; Arnesen, T. Spotlight on protein N-terminal acetylation. Exp. Mol. Med. 2018, 50, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Aksnes, H.; Drazic, A.; Marie, M.; Arnesen, T. First Things First: Vital Protein Marks by N-Terminal Acetyltransferases. Trends Biochem. Sci. 2016, 41, 746–760. [Google Scholar] [CrossRef]
- Constantinou, M.; Klavaris, A.; Koufaris, C.; Kirmizis, A. Cellular effects of NAT-mediated histone N-terminal acetylation. J. Cell Sci. 2023, 136, jcs260801. [Google Scholar] [CrossRef]
- Varland, S.; Osberg, C.; Arnesen, T. N-terminal modifications of cellular proteins: The enzymes involved, their substrate specificities and biological effects. Proteomics 2015, 15, 2385–2401. [Google Scholar] [CrossRef]
- Yamada, K.D.; Omori, S.; Nishi, H.; Miyagi, M. Identification of the sequence determinants of protein N-terminal acetylation through a decision tree approach. BMC Bioinform. 2017, 18, 289. [Google Scholar] [CrossRef] [PubMed]
- Polevoda, B.; Norbeck, J.; Takakura, H.; Blomberg, A.; Sherman, F. Identification and specificities of N-terminal acetyltransferases from Saccharomyces cerevisiae. EMBO J. 1999, 18, 6155–6168. [Google Scholar] [CrossRef] [PubMed]
- McTiernan, N.; Tranebjaerg, L.; Bjorheim, A.S.; Hogue, J.S.; Wilson, W.G.; Schmidt, B.; Boerrigter, M.M.; Nybo, M.L.; Smeland, M.F.; Tumer, Z.; et al. Biochemical analysis of novel NAA10 variants suggests distinct pathogenic mechanisms involving impaired protein N-terminal acetylation. Hum. Genet. 2022, 141, 1355–1369. [Google Scholar] [CrossRef]
- Lee, M.N.; Kweon, H.Y.; Oh, G.T. N-alpha-acetyltransferase 10 (NAA10) in development: The role of NAA10. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef]
- Mullen, J.R.; Kayne, P.S.; Moerschell, R.P.; Tsunasawa, S.; Gribskov, M.; Colavito-Shepanski, M.; Grunstein, M.; Sherman, F.; Sternglanz, R. Identification and characterization of genes and mutants for an N-terminal acetyltransferase from yeast. EMBO J. 1989, 8, 2067–2075. [Google Scholar] [CrossRef]
- Tribioli, C.; Mancini, M.; Plassart, E.; Bione, S.; Rivella, S.; Sala, C.; Torri, G.; Toniolo, D. Isolation of new genes in distal Xq28: Transcriptional map and identification of a human homologue of the ARD1 N-acetyl transferase of Saccharomyces cerevisiae. Hum. Mol. Genet. 1994, 3, 1061–1067. [Google Scholar] [CrossRef]
- Liszczak, G.; Goldberg, J.M.; Foyn, H.; Petersson, E.J.; Arnesen, T.; Marmorstein, R. Molecular basis for N-terminal acetylation by the heterodimeric NatA complex. Nat. Struct. Mol. Biol. 2013, 20, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- Pang, A.L.; Clark, J.; Chan, W.Y.; Rennert, O.M. Expression of human NAA11 (ARD1B) gene is tissue-specific and is regulated by DNA methylation. Epigenetics 2011, 6, 1391–1399. [Google Scholar] [CrossRef]
- Koufaris, C.; Demetriadou, C.; Nicolaidou, V.; Kirmizis, A. Bioinformatic Analysis Reveals the Association of Human N-Terminal Acetyltransferase Complexes with Distinct Transcriptional and Post-Transcriptional Processes. Biochem. Genet. 2024. [Google Scholar] [CrossRef]
- Vo, T.T.L.; Park, J.-H.; Lee, E.J.; Nguyen, Y.T.K.; Han, B.W.; Nguyen, H.T.T.; Mun, K.C.; Ha, E.; Kwon, T.K.; Kim, K.-W.; et al. Characterization of Lysine Acetyltransferase Activity of Recombinant Human ARD1/NAA10. Molecules 2020, 25, 588. [Google Scholar] [CrossRef]
- Vo, T.T.L.; Jeong, C.H.; Lee, S.; Kim, K.W.; Ha, E.; Seo, J.H. Versatility of ARD1/NAA10-mediated protein lysine acetylation. Exp. Mol. Med. 2018, 50, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Magin, R.S.; March, Z.M.; Marmorstein, R. The N-terminal Acetyltransferase Naa10/ARD1 Does Not Acetylate Lysine Residues. J. Biol. Chem. 2016, 291, 5270–5277. [Google Scholar] [CrossRef]
- Bienvenut, W.V.; Brunje, A.; Boyer, J.B.; Muhlenbeck, J.S.; Bernal, G.; Lassowskat, I.; Dian, C.; Linster, E.; Dinh, T.V.; Koskela, M.M.; et al. Dual lysine and N-terminal acetyltransferases reveal the complexity underpinning protein acetylation. Mol. Syst. Biol. 2020, 16, e9464. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Chun, Y.S.; Huh, J.; Park, J.W. FIH permits NAA10 to catalyze the oxygen-dependent lysyl-acetylation of HIF-1alpha. Redox Biol. 2018, 19, 364–374. [Google Scholar] [CrossRef]
- Ree, R.; Krogstad, K.; McTiernan, N.; Jakobsson, M.E.; Arnesen, T. Hydroxylation of the Acetyltransferase NAA10 Trp38 Is Not an Enzyme-Switch in Human Cells. Int. J. Mol. Sci. 2021, 22, 11805. [Google Scholar] [CrossRef]
- Wu, Y.; Lyon, G.J. NAA10-related syndrome. Exp. Mol. Med. 2018, 50, 1–10. [Google Scholar] [CrossRef]
- Gogoll, L.; Steindl, K.; Joset, P.; Zweier, M.; Baumer, A.; Gerth-Kahlert, C.; Tutschek, B.; Rauch, A. Confirmation of Ogden syndrome as an X-linked recessive fatal disorder due to a recurrent NAA10 variant and review of the literature. Am. J. Med. Genet. A 2021, 185, 2546–2560. [Google Scholar] [CrossRef]
- Lyon, G.J.; Vedaie, M.; Beisheim, T.; Park, A.; Marchi, E.; Gottlieb, L.; Hsieh, T.C.; Klinkhammer, H.; Sandomirsky, K.; Cheng, H.; et al. Expanding the phenotypic spectrum of NAA10-related neurodevelopmental syndrome and NAA15-related neurodevelopmental syndrome. Eur. J. Hum. Genet. 2023, 31, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.H.; Pan, K.F.; Cheng, T.Y.; Chien, M.H.; Hua, K.T. Multiple impacts of Naa10p on cancer progression: Molecular functions and clinical prospects. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188973. [Google Scholar] [CrossRef]
- Zhu, R.; Chen, M.; Luo, Y.; Cheng, H.; Zhao, Z.; Zhang, M. The role of N-acetyltransferases in cancers. Gene 2024, 892, 147866. [Google Scholar] [CrossRef]
- Jiang, Y.; Guo, X.; Liu, L.; Rode, S.; Wang, R.; Liu, H.; Yang, Z.Q. Metagenomic characterization of lysine acetyltransferases in human cancer and their association with clinicopathologic features. Cancer Sci. 2020, 111, 1829–1839. [Google Scholar] [CrossRef]
- Kim, S.M.; Ha, E.; Kim, J.; Cho, C.; Shin, S.-J.; Seo, J.H. NAA10 as a New Prognostic Marker for Cancer Progression. Int. J. Mol. Sci. 2020, 21, 8010. [Google Scholar] [CrossRef] [PubMed]
- Kuhns, K.J.; Zhang, G.; Wang, Z.; Liu, W. ARD1/NAA10 acetylation in prostate cancer. Exp. Mol. Med. 2018, 50, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Jang, M.K.; Seo, J.H.; Ryu, S.H.; Kim, J.A.; Chung, Y.H. ARD1/NAA10 in hepatocellular carcinoma: Pathways and clinical implications. Exp. Mol. Med. 2018, 50, 1–12. [Google Scholar] [CrossRef]
- Chaudhary, P.; Ha, E.; Vo, T.T.L.; Seo, J.H. Diverse roles of arrest defective 1 in cancer development. Arch. Pharm. Res. 2019, 42, 1040–1051. [Google Scholar] [CrossRef] [PubMed]
- Kalvik, T.V.; Arnesen, T. Protein N-terminal acetyltransferases in cancer. Oncogene 2013, 32, 269–276. [Google Scholar] [CrossRef]
- Arnesen, T.; Van Damme, P.; Polevoda, B.; Helsens, K.; Evjenth, R.; Colaert, N.; Varhaug, J.E.; Vandekerckhove, J.; Lillehaug, J.R.; Sherman, F.; et al. Proteomics analyses reveal the evolutionary conservation and divergence of N-terminal acetyltransferases from yeast and humans. Proc. Natl. Acad. Sci. USA 2009, 106, 8157–8162. [Google Scholar] [CrossRef]
- Consortium, G.T. The Genotype-Tissue Expression (GTEx) project. Nat. Genet. 2013, 45, 580–585. [Google Scholar] [CrossRef]
- Uhlen, M.; Fagerberg, L.; Hallstrom, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, A.; Kampf, C.; Sjostedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Arnesen, T.; Anderson, D.; Baldersheim, C.; Lanotte, M.; Varhaug, J.E.; Lillehaug, J.R. Identification and characterization of the human ARD1-NATH protein acetyltransferase complex. Biochem. J. 2005, 386, 433–443. [Google Scholar] [CrossRef]
- Gottlieb, L.; Marmorstein, R. Structure of Human NatA and Its Regulation by the Huntingtin Interacting Protein HYPK. Structure 2018, 26, 925–935.e8. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.; Wild, K.; Sinning, I. Multi-protein assemblies orchestrate co-translational enzymatic processing on the human ribosome. Nat. Commun. 2024, 15, 7681. [Google Scholar] [CrossRef]
- Lentzsch, A.M.; Yudin, D.; Gamerdinger, M.; Chandrasekar, S.; Rabl, L.; Scaiola, A.; Deuerling, E.; Ban, N.; Shan, S.O. NAC guides a ribosomal multienzyme complex for nascent protein processing. Nature 2024, 633, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Knorr, A.G.; Schmidt, C.; Tesina, P.; Berninghausen, O.; Becker, T.; Beatrix, B.; Beckmann, R. Ribosome-NatA architecture reveals that rRNA expansion segments coordinate N-terminal acetylation. Nat. Struct. Mol. Biol. 2019, 26, 35–39. [Google Scholar] [CrossRef]
- Weyer, F.A.; Gumiero, A.; Lapouge, K.; Bange, G.; Kopp, J.; Sinning, I. Structural basis of HypK regulating N-terminal acetylation by the NatA complex. Nat. Commun. 2017, 8, 15726. [Google Scholar] [CrossRef]
- Van Damme, P.; Hole, K.; Pimenta-Marques, A.; Helsens, K.; Vandekerckhove, J.; Martinho, R.G.; Gevaert, K.; Arnesen, T. NatF contributes to an evolutionary shift in protein N-terminal acetylation and is important for normal chromosome segregation. PLoS Genet. 2011, 7, e1002169. [Google Scholar] [CrossRef] [PubMed]
- Faber, P.W.; Barnes, G.T.; Srinidhi, J.; Chen, J.; Gusella, J.F.; MacDonald, M.E. Huntingtin interacts with a family of WW domain proteins. Hum. Mol. Genet. 1998, 7, 1463–1474. [Google Scholar] [CrossRef]
- Arnesen, T.; Starheim, K.K.; Van Damme, P.; Evjenth, R.; Dinh, H.; Betts, M.J.; Ryningen, A.; Vandekerckhove, J.; Gevaert, K.; Anderson, D. The chaperone-like protein HYPK acts together with NatA in cotranslational N-terminal acetylation and prevention of Huntingtin aggregation. Mol. Cell Biol. 2010, 30, 1898–1909. [Google Scholar] [CrossRef]
- Rabl, L.; Deuerling, E. The nascent polypeptide-associated complex (NAC) as regulatory hub on ribosomes. Biol. Chem. 2025. [Google Scholar] [CrossRef]
- Park, J.H.; Seo, J.H.; Wee, H.J.; Vo, T.T.; Lee, E.J.; Choi, H.; Cha, J.H.; Ahn, B.J.; Shin, M.W.; Bae, S.J.; et al. Nuclear translocation of hARD1 contributes to proper cell cycle progression. PLoS ONE 2014, 9, e105185. [Google Scholar] [CrossRef]
- Yoon, H.; Kim, H.L.; Chun, Y.S.; Shin, D.H.; Lee, K.H.; Shin, C.S.; Lee, D.Y.; Kim, H.H.; Lee, Z.H.; Ryoo, H.M.; et al. NAA10 controls osteoblast differentiation and bone formation as a feedback regulator of Runx2. Nat. Commun. 2014, 5, 5176. [Google Scholar] [CrossRef] [PubMed]
- Rojo de la Vega, M.; Chapman, E.; Zhang, D.D. NRF2 and the Hallmarks of Cancer. Cancer Cell 2018, 34, 21–43. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Lee, Y.H.; Jang, J.H.; Kim, S.J.; Kim, S.H.; Kim, D.H.; Na, H.K.; Kim, K.O.; Baek, J.H.; Surh, Y.J. ARD1 stabilizes NRF2 through direct interaction and promotes colon cancer progression. Life Sci. 2023, 313, 121217. [Google Scholar] [CrossRef]
- Zeng, Y.; Min, L.; Han, Y.; Meng, L.; Liu, C.; Xie, Y.; Dong, B.; Wang, L.; Jiang, B.; Xu, H.; et al. Inhibition of STAT5a by Naa10p contributes to decreased breast cancer metastasis. Carcinogenesis 2014, 35, 2244–2253. [Google Scholar] [CrossRef]
- Hua, K.T.; Tan, C.T.; Johansson, G.; Lee, J.M.; Yang, P.W.; Lu, H.Y.; Chen, C.K.; Su, J.L.; Chen, P.B.; Wu, Y.L.; et al. N-alpha-acetyltransferase 10 protein suppresses cancer cell metastasis by binding PIX proteins and inhibiting Cdc42/Rac1 activity. Cancer Cell 2011, 19, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.F.; Ou, D.S.; Lee, S.B.; Chang, L.H.; Lin, R.K.; Li, Y.S.; Upadhyay, A.K.; Cheng, X.; Wang, Y.C.; Hsu, H.S.; et al. hNaa10p contributes to tumorigenesis by facilitating DNMT1-mediated tumor suppressor gene silencing. J. Clin. Investig. 2010, 120, 2920–2930. [Google Scholar] [CrossRef]
- Lee, C.C.; Peng, S.H.; Shen, L.; Lee, C.F.; Du, T.H.; Kang, M.L.; Xu, G.L.; Upadhyay, A.K.; Cheng, X.; Yan, Y.T.; et al. The Role of N-alpha-acetyltransferase 10 Protein in DNA Methylation and Genomic Imprinting. Mol. Cell 2017, 68, 89–103.e7. [Google Scholar] [CrossRef]
- Lin, Y.W.; Wen, Y.C.; Chu, C.Y.; Tung, M.C.; Yang, Y.C.; Hua, K.T.; Pan, K.F.; Hsiao, M.; Lee, W.J.; Chien, M.H. Stabilization of ADAM9 by N-alpha-acetyltransferase 10 protein contributes to promoting progression of androgen-independent prostate cancer. Cell Death Dis. 2020, 11, 591. [Google Scholar] [CrossRef]
- Makwana, R.; Christ, C.; Marchi, E.; Harpell, R.; Lyon, G.J. Longitudinal adaptive behavioral outcomes in Ogden syndrome by seizure status and therapeutic intervention. Am. J. Med. Genet. A 2024, 194, e63651. [Google Scholar] [CrossRef]
- Rope, A.F.; Wang, K.; Evjenth, R.; Xing, J.; Johnston, J.J.; Swensen, J.J.; Johnson, W.E.; Moore, B.; Huff, C.D.; Bird, L.M.; et al. Using VAAST to identify an X-linked disorder resulting in lethality in male infants due to N-terminal acetyltransferase deficiency. Am. J. Hum. Genet. 2011, 89, 28–43. [Google Scholar] [CrossRef]
- Dorfel, M.J.; Lyon, G.J. The biological functions of Naa10—From amino-terminal acetylation to human disease. Gene 2015, 567, 103–131. [Google Scholar] [CrossRef] [PubMed]
- Myklebust, L.M.; Van Damme, P.; Stove, S.I.; Dorfel, M.J.; Abboud, A.; Kalvik, T.V.; Grauffel, C.; Jonckheere, V.; Wu, Y.; Swensen, J.; et al. Biochemical and cellular analysis of Ogden syndrome reveals downstream Nt-acetylation defects. Hum. Mol. Genet. 2015, 24, 1956–1976. [Google Scholar] [CrossRef]
- Cheng, H.; Gottlieb, L.; Marchi, E.; Kleyner, R.; Bhardwaj, P.; Rope, A.F.; Rosenheck, S.; Moutton, S.; Philippe, C.; Eyaid, W.; et al. Phenotypic and biochemical analysis of an international cohort of individuals with variants in NAA10 and NAA15. Hum. Mol. Genet. 2019, 28, 2900–2919. [Google Scholar] [CrossRef]
- Bottillo, I.; De Luca, C.; Cordella, A.; Passeri, M.; Salvatore, M.; Fortugno, P.; Leonardi, S.; Dofcaci, A.; Sciarra, L.; Romano, S.; et al. Cardiological Manifestations in Males and Females Affected by NAA10-Related Disease. Am. J. Med. Genet. A 2025, e64096. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, D.; Craven, I.; Feng, R.; Prondzynski, M.; Shani, K.; Tharani, Y.; Mayourian, J.; Joseph, M.; Walker, D.; Bortolin, R.H.; et al. Dysregulation of N-terminal acetylation causes cardiac arrhythmia and cardiomyopathy. Nat. Commun. 2025, 16, 3604. [Google Scholar] [CrossRef] [PubMed]
- Esmailpour, T.; Riazifar, H.; Liu, L.; Donkervoort, S.; Huang, V.H.; Madaan, S.; Shoucri, B.M.; Busch, A.; Wu, J.; Towbin, A.; et al. A splice donor mutation in NAA10 results in the dysregulation of the retinoic acid signalling pathway and causes Lenz microphthalmia syndrome. J. Med. Genet. 2014, 51, 185–196. [Google Scholar] [CrossRef]
- Wang, Y.; Mijares, M.; Gall, M.D.; Turan, T.; Javier, A.; Bornemann, D.J.; Manage, K.; Warrior, R. Drosophila variable nurse cells encodes arrest defective 1 (ARD1), the catalytic subunit of the major N-terminal acetyltransferase complex. Dev. Dyn. 2010, 239, 2813–2827. [Google Scholar] [CrossRef]
- Ingram, A.K.; Cross, G.A.; Horn, D. Genetic manipulation indicates that ARD1 is an essential N(alpha)-acetyltransferase in Trypanosoma brucei. Mol. Biochem. Parasitol. 2000, 111, 309–317. [Google Scholar] [CrossRef]
- Lyon, G.J.; Longo, J.; Garcia, A.; Inusa, F.; Marchi, E.; Shi, D.; Dorfel, M.; Arnesen, T.; Aldabe, R.; Lyons, S.; et al. Evaluating possible maternal effect lethality and genetic background effects in Naa10 knockout mice. PLoS ONE 2024, 19, e0301328. [Google Scholar] [CrossRef]
- Kweon, H.Y.; Lee, M.N.; Dorfel, M.; Seo, S.; Gottlieb, L.; PaPazyan, T.; McTiernan, N.; Ree, R.; Bolton, D.; Garcia, A.; et al. Naa12 compensates for Naa10 in mice in the amino-terminal acetylation pathway. eLife 2021, 10, e65952. [Google Scholar] [CrossRef]
- Sonnichsen, B.; Koski, L.B.; Walsh, A.; Marschall, P.; Neumann, B.; Brehm, M.; Alleaume, A.M.; Artelt, J.; Bettencourt, P.; Cassin, E.; et al. Full-genome RNAi profiling of early embryogenesis in Caenorhabditis elegans. Nature 2005, 434, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Dorfel, M.J.; Fang, H.; Crain, J.; Klingener, M.; Weiser, J.; Lyon, G.J. Proteomic and genomic characterization of a yeast model for Ogden syndrome. Yeast 2017, 34, 19–37. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, U.A.; Zedan, M.; Hessling, B.; Fenzl, K.; Gillet, L.; Barry, J.; Knop, M.; Kramer, G.; Bukau, B. N(alpha)-terminal acetylation of proteins by NatA and NatB serves distinct physiological roles in Saccharomyces cerevisiae. Cell Rep. 2021, 34, 108711. [Google Scholar] [CrossRef]
- Sugiura, N.; Adams, S.M.; Corriveau, R.A. An evolutionarily conserved N-terminal acetyltransferase complex associated with neuronal development. J. Biol. Chem. 2003, 278, 40113–40120. [Google Scholar] [CrossRef]
- Pang, A.; Rennert, O. Protein acetylation and spermatogenesis. Reprod. Syst. Sex. Disord. 2013, (Suppl. S1), 5. [Google Scholar] [CrossRef]
- Brown, S.D.; Moore, M.W. The International Mouse Phenotyping Consortium: Past and future perspectives on mouse phenotyping. Mamm. Genome 2012, 23, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Kajimura, S. Naa10P puts a brake on PGC1alpha and fat browning. Nat. Struct. Mol. Biol. 2019, 26, 849–851. [Google Scholar] [CrossRef]
- Handschin, C. The biology of PGC-1alpha and its therapeutic potential. Trends Pharmacol. Sci. 2009, 30, 322–329. [Google Scholar] [CrossRef]
- Lee, C.C.; Shih, Y.C.; Kang, M.L.; Chang, Y.C.; Chuang, L.M.; Devaraj, R.; Juan, L.J. Naa10p Inhibits Beige Adipocyte-Mediated Thermogenesis through N-alpha-acetylation of Pgc1alpha. Mol. Cell 2019, 76, 500–515.e8. [Google Scholar] [CrossRef]
- Makwana, R.; Christ, C.; Patel, R.; Marchi, E.; Harpell, R.; Lyon, G.J. Natural History of NAA15 -Related Neurodevelopmental Disorder Through Adolescence. Am. J. Med. Genet. A 2025, 197, e64009. [Google Scholar] [CrossRef]
- Ritter, A.; Berger, J.H.; Deardorff, M.; Izumi, K.; Lin, K.Y.; Medne, L.; Ahrens-Nicklas, R.C. Variants in NAA15 cause pediatric hypertrophic cardiomyopathy. Am. J. Med. Genet. A 2021, 185, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Ward, T.; Tai, W.; Morton, S.; Impens, F.; Van Damme, P.; Van Haver, D.; Timmerman, E.; Venturini, G.; Zhang, K.; Jang, M.Y.; et al. Mechanisms of Congenital Heart Disease Caused by NAA15 Haploinsufficiency. Circ. Res. 2021, 128, 1156–1169. [Google Scholar] [CrossRef]
- Pan, K.F.; Liu, Y.C.; Hsiao, M.; Cheng, T.Y.; Hua, K.T. Naa10p promotes cell invasiveness of esophageal cancer by coordinating the c-Myc and PAI1 regulatory axis. Cell Death Dis. 2022, 13, 995. [Google Scholar] [CrossRef]
- Chien, M.H.; Lee, W.J.; Yang, Y.C.; Tan, P.; Pan, K.F.; Liu, Y.C.; Tsai, H.C.; Hsu, C.H.; Wen, Y.C.; Hsiao, M.; et al. N-alpha-acetyltransferase 10 protein promotes metastasis by stabilizing matrix metalloproteinase-2 protein in human osteosarcomas. Cancer Lett. 2018, 433, 86–98. [Google Scholar] [CrossRef]
- Qian, X.; Li, X.; Lu, Z. Protein kinase activity of the glycolytic enzyme PGK1 regulates autophagy to promote tumorigenesis. Autophagy 2017, 13, 1246–1247. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Z.; Guo, J.; Li, Y.; Bavarva, J.H.; Qian, C.; Brahimi-Horn, M.C.; Tan, D.; Liu, W. Inactivation of androgen-induced regulator ARD1 inhibits androgen receptor acetylation and prostate tumorigenesis. Proc. Natl. Acad. Sci. USA 2012, 109, 3053–3058. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.H.; Cha, J.H.; Park, J.H.; Jeong, C.H.; Park, Z.Y.; Lee, H.S.; Oh, S.H.; Kang, J.H.; Suh, S.W.; Kim, K.H.; et al. Arrest defective 1 autoacetylation is a critical step in its ability to stimulate cancer cell proliferation. Cancer Res. 2010, 70, 4422–4432. [Google Scholar] [CrossRef]
- Kuo, H.P.; Lee, D.F.; Chen, C.T.; Liu, M.; Chou, C.K.; Lee, H.J.; Du, Y.; Xie, X.; Wei, Y.; Xia, W.; et al. ARD1 stabilization of TSC2 suppresses tumorigenesis through the mTOR signaling pathway. Sci. Signal. 2010, 3, ra9. [Google Scholar] [CrossRef]
- Lim, J.H.; Park, J.W.; Chun, Y.S. Human arrest defective 1 acetylates and activates beta-catenin, promoting lung cancer cell proliferation. Cancer Res. 2006, 66, 10677–10682. [Google Scholar] [CrossRef]
- Kuo, H.P.; Hung, M.C. Arrest-defective-1 protein (ARD1): Tumor suppressor or oncoprotein? Am. J. Transl. Res. 2010, 2, 56–64. [Google Scholar]
- Yu, M.; Gong, J.; Ma, M.; Yang, H.; Lai, J.; Wu, H.; Li, L.; Li, L.; Tan, D. Immunohistochemical analysis of human arrest-defective-1 expressed in cancers in vivo. Oncol. Rep. 2009, 21, 909–915. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Koufaris, C.; Kirmizis, A. N-Terminal Acetyltransferases Are Cancer-Essential Genes Prevalently Upregulated in Tumours. Cancers 2020, 12, 2631. [Google Scholar] [CrossRef]
- Liao, Y.; Savage, S.R.; Dou, Y.; Shi, Z.; Yi, X.; Jiang, W.; Lei, J.T.; Zhang, B. A proteogenomics data-driven knowledge base of human cancer. Cell Syst. 2023, 14, 777–787.e5. [Google Scholar] [CrossRef]
- Vasaikar, S.V.; Straub, P.; Wang, J.; Zhang, B. LinkedOmics: Analyzing multi-omics data within and across 32 cancer types. Nucleic Acids Res. 2018, 46, D956–D963. [Google Scholar] [CrossRef]
- Wang, D.; Qian, X.; Du, Y.N.; Sanchez-Solana, B.; Chen, K.; Kanigicherla, M.; Jenkins, L.M.; Luo, J.; Eng, S.; Park, B.; et al. cProSite: A web based interactive platform for online proteomics, phosphoproteomics, and genomics data analysis. J. Biotechnol. Biomed. 2023, 6, 573–578. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research, N.; Weinstein, J.N.; Collisson, E.A.; Mills, G.B.; Shaw, K.R.; Ozenberger, B.A.; Ellrott, K.; Shmulevich, I.; Sander, C.; Stuart, J.M. The Cancer Genome Atlas Pan-Cancer analysis project. Nat. Genet. 2013, 45, 1113–1120. [Google Scholar] [CrossRef]
- Li, Y.; Dou, Y.; Da Veiga Leprevost, F.; Geffen, Y.; Calinawan, A.P.; Aguet, F.; Akiyama, Y.; Anand, S.; Birger, C.; Cao, S.; et al. Proteogenomic data and resources for pan-cancer analysis. Cancer Cell 2023, 41, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Uhlen, M.; Zhang, C.; Lee, S.; Sjostedt, E.; Fagerberg, L.; Bidkhori, G.; Benfeitas, R.; Arif, M.; Liu, Z.; Edfors, F.; et al. A pathology atlas of the human cancer transcriptome. Science 2017, 357, 2507. [Google Scholar] [CrossRef]
- Dong, Q.; Shen, D.; Ye, J.; Chen, J.; Li, J. PhosCancer: A comprehensive database for investigating protein phosphorylation in human cancer. iScience 2024, 27, 111060. [Google Scholar] [CrossRef]
- Walsh, C.T.; Garneau-Tsodikova, S.; Gatto, G.J., Jr. Protein posttranslational modifications: The chemistry of proteome diversifications. Angew. Chem. Int. Ed. Engl. 2005, 44, 7342–7372. [Google Scholar] [CrossRef]
- Arafeh, R.; Shibue, T.; Dempster, J.M.; Hahn, W.C.; Vazquez, F. The present and future of the Cancer Dependency Map. Nat. Rev. Cancer 2025, 25, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Bachman, J.A.; Muhlich, J.L.; Mitchison, T.J. shinyDepMap, a tool to identify targetable cancer genes and their functional connections from Cancer Dependency Map data. eLife 2021, 10, e57116. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekaran, R.; Deutzmann, A.; Mahauad-Fernandez, W.D.; Hansen, A.S.; Gouw, A.M.; Felsher, D.W. The MYC oncogene—The grand orchestrator of cancer growth and immune evasion. Nat. Rev. Clin. Oncol. 2022, 19, 23–36. [Google Scholar] [CrossRef]
- Yang, H.; Li, Q.; Niu, J.; Li, B.; Jiang, D.; Wan, Z.; Yang, Q.; Jiang, F.; Wei, P.; Bai, S. microRNA-342-5p and miR-608 inhibit colon cancer tumorigenesis by targeting NAA10. Oncotarget 2016, 7, 2709–2720. [Google Scholar] [CrossRef]
- Qian, X.; Li, X.; Cai, Q.; Zhang, C.; Yu, Q.; Jiang, Y.; Lee, J.H.; Hawke, D.; Wang, Y.; Xia, Y.; et al. Phosphoglycerate Kinase 1 Phosphorylates Beclin1 to Induce Autophagy. Mol. Cell 2017, 65, 917–931.e6. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, H.; Tao, Y.; Liu, X.; Yuan, Z.; Nie, C. ARD1 contributes to IKKbeta-mediated breast cancer tumorigenesis. Cell Death Dis. 2018, 9, 860. [Google Scholar] [CrossRef]
- Kuo, H.P.; Lee, D.F.; Xia, W.; Lai, C.C.; Li, L.Y.; Hung, M.C. Phosphorylation of ARD1 by IKKbeta contributes to its destabilization and degradation. Biochem. Biophys. Res. Commun. 2009, 389, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Chun, K.H.; Cho, S.J.; Lee, J.W.; Seo, J.H.; Kim, K.W.; Lee, S.K. Protein kinase C-delta interacts with and phosphorylates ARD1. J. Cell Physiol. 2021, 236, 379–391. [Google Scholar] [CrossRef]
- Koufaris, C.; Nicolaidou, V.; Kirmizis, A. Letter to the Editor regarding The role of N-acetyltransferases in cancers: N-alpha acetyltransferase 10 (NAA10) and N-acetyltransferase 10 (NAT10) are distinct genes. Gene 2024, 898, 148087. [Google Scholar] [CrossRef]
- Dalhat, M.H.; Narayan, S.; Serio, H.; Arango, D. Dissecting the oncogenic properties of essential RNA-modifying enzymes: A focus on NAT10. Oncogene 2024, 43, 1077–1086. [Google Scholar] [CrossRef]
- Van Damme, P. Charting the N-Terminal Acetylome: A Comprehensive Map of Human NatA Substrates. Int. J. Mol. Sci. 2021, 22, 10692. [Google Scholar] [CrossRef] [PubMed]
- DePaolo, J.S.; Wang, Z.; Guo, J.; Zhang, G.; Qian, C.; Zhang, H.; Zabaleta, J.; Liu, W. Acetylation of androgen receptor by ARD1 promotes dissociation from HSP90 complex and prostate tumorigenesis. Oncotarget 2016, 7, 71417–71428. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.T.L.; Park, J.H.; Seo, J.H.; Lee, E.J.; Choi, H.; Bae, S.J.; Le, H.; An, S.; Lee, H.S.; Wee, H.J.; et al. ARD1-mediated aurora kinase A acetylation promotes cell proliferation and migration. Oncotarget 2017, 8, 57216–57230. [Google Scholar] [CrossRef]
- Seo, J.H.; Park, J.H.; Lee, E.J.; Vo, T.T.; Choi, H.; Kim, J.Y.; Jang, J.K.; Wee, H.J.; Lee, H.S.; Jang, S.H.; et al. ARD1-mediated Hsp70 acetylation balances stress-induced protein refolding and degradation. Nat. Commun. 2016, 7, 12882. [Google Scholar] [CrossRef]
- Xu, H.; Jiang, B.; Meng, L.; Ren, T.; Zeng, Y.; Wu, J.; Qu, L.; Shou, C. N-alpha-acetyltransferase 10 protein inhibits apoptosis through RelA/p65-regulated MCL1 expression. Carcinogenesis 2012, 33, 1193–1202. [Google Scholar] [CrossRef]
- Kang, R.; Zeh, H.J.; Lotze, M.T.; Tang, D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011, 18, 571–580. [Google Scholar] [CrossRef]
- Shi, Z.; Lei, J.T.; Elizarraras, J.M.; Zhang, B. Mapping the functional network of human cancer through machine learning and pan-cancer proteogenomics. Nat. Cancer 2025, 6, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Shin, D.H.; Chun, Y.S.; Lee, K.H.; Shin, H.W.; Park, J.W. Arrest defective-1 controls tumor cell behavior by acetylating myosin light chain kinase. PLoS ONE 2009, 4, e7451. [Google Scholar] [CrossRef]
- Foyn, H.; Jones, J.E.; Lewallen, D.; Narawane, R.; Varhaug, J.E.; Thompson, P.R.; Arnesen, T. Design, synthesis, and kinetic characterization of protein N-terminal acetyltransferase inhibitors. ACS Chem. Biol. 2013, 8, 1121–1127. [Google Scholar] [CrossRef]
- Albaugh, B.N.; Denu, J.M. Catalysis by protein acetyltransferase Gcn5. Biochim. Biophys. Acta Gene Regul. Mech. 2021, 1864, 194627. [Google Scholar] [CrossRef] [PubMed]
- Krtenic, B.; Drazic, A.; Arnesen, T.; Reuter, N. Classification and phylogeny for the annotation of novel eukaryotic GNAT acetyltransferases. PLoS Comput. Biol. 2020, 16, e1007988. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.M.; Cole, P.A. Targeting lysine acetylation readers and writers. Nat. Rev. Drug Discov. 2025, 24, 112–133. [Google Scholar] [CrossRef] [PubMed]
- Whedon, S.D.; Cole, P.A. KATs off: Biomedical insights from lysine acetyltransferase inhibitors. Curr. Opin. Chem. Biol. 2023, 72, 102255. [Google Scholar] [CrossRef]
- White, J.; Derheimer, F.A.; Jensen-Pergakes, K.; O’Connell, S.; Sharma, S.; Spiegel, N.; Paul, T.A. Histone lysine acetyltransferase inhibitors: An emerging class of drugs for cancer therapy. Trends Pharmacol. Sci. 2024, 45, 243–254. [Google Scholar] [CrossRef]
- Tao, H.; Wang, J.; Lu, W.; Zhang, R.; Xie, Y.; Liu, Y.C.; Liu, R.; Yue, L.; Chen, K.; Jiang, H.; et al. Discovery of trisubstituted nicotinonitrile derivatives as novel human GCN5 inhibitors through AlphaScreen-based high throughput screening. RSC Adv. 2019, 9, 4917–4924. [Google Scholar] [CrossRef]
- Haque, M.E.; Jakaria, M.; Akther, M.; Cho, D.Y.; Kim, I.S.; Choi, D.K. The GCN5: Its biological functions and therapeutic potentials. Clin. Sci. 2021, 135, 231–257. [Google Scholar] [CrossRef]
- Lasko, L.M.; Jakob, C.G.; Edalji, R.P.; Qiu, W.; Montgomery, D.; Digiammarino, E.L.; Hansen, T.M.; Risi, R.M.; Frey, R.; Manaves, V.; et al. Discovery of a selective catalytic p300/CBP inhibitor that targets lineage-specific tumours. Nature 2017, 550, 128–132. [Google Scholar] [CrossRef]
- Hogg, S.J.; Motorna, O.; Cluse, L.A.; Johanson, T.M.; Coughlan, H.D.; Raviram, R.; Myers, R.M.; Costacurta, M.; Todorovski, I.; Pijpers, L.; et al. Targeting histone acetylation dynamics and oncogenic transcription by catalytic P300/CBP inhibition. Mol. Cell 2021, 81, 2183–2200.e13. [Google Scholar] [CrossRef]
- Wei, W.; Song, Z.; Chiba, M.; Wu, W.; Jeong, S.; Zhang, J.P.; Kadin, M.E.; Nakagawa, M.; Yang, Y. Analysis and therapeutic targeting of the EP300 and CREBBP acetyltransferases in anaplastic large cell lymphoma and Hodgkin lymphoma. Leukemia 2023, 37, 396–407. [Google Scholar] [CrossRef]
- Sharma, S.; Chung, C.Y.; Uryu, S.; Petrovic, J.; Cao, J.; Rickard, A.; Nady, N.; Greasley, S.; Johnson, E.; Brodsky, O.; et al. Discovery of a highly potent, selective, orally bioavailable inhibitor of KAT6A/B histone acetyltransferases with efficacy against KAT6A-high ER+ breast cancer. Cell Chem. Biol. 2023, 30, 1191–1210.e20. [Google Scholar] [CrossRef]
- Baell, J.B.; Leaver, D.J.; Hermans, S.J.; Kelly, G.L.; Brennan, M.S.; Downer, N.L.; Nguyen, N.; Wichmann, J.; McRae, H.M.; Yang, Y.; et al. Inhibitors of histone acetyltransferases KAT6A/B induce senescence and arrest tumour growth. Nature 2018, 560, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Mukohara, T.; Park, Y.H.; Sommerhalder, D.; Yonemori, K.; Hamilton, E.; Kim, S.B.; Kim, J.H.; Iwata, H.; Yamashita, T.; Layman, R.M.; et al. Inhibition of lysine acetyltransferase KAT6 in ER(+)HER2(-) metastatic breast cancer: A phase 1 trial. Nat. Med. 2024, 30, 2242–2250. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, Y.; Aguilar, A.; Bernard, D.; Yang, C.Y. Targeting the MDM2-p53 Protein-Protein Interaction for New Cancer Therapy: Progress and Challenges. Cold Spring Harb. Perspect. Med. 2017, 7, a026245. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J.; Synnott, N.C.; O’Grady, S.; Crown, J. Targeting p53 for the treatment of cancer. Semin. Cancer Biol. 2022, 79, 58–67. [Google Scholar] [CrossRef]
- Uttarkar, S.; Dasse, E.; Coulibaly, A.; Steinmann, S.; Jakobs, A.; Schomburg, C.; Trentmann, A.; Jose, J.; Schlenke, P.; Berdel, W.E.; et al. Targeting acute myeloid leukemia with a small molecule inhibitor of the Myb/p300 interaction. Blood 2016, 127, 1173–1182. [Google Scholar] [CrossRef][Green Version]
- Uttarkar, S.; Piontek, T.; Dukare, S.; Schomburg, C.; Schlenke, P.; Berdel, W.E.; Muller-Tidow, C.; Schmidt, T.J.; Klempnauer, K.H. Small-Molecule Disruption of the Myb/p300 Cooperation Targets Acute Myeloid Leukemia Cells. Mol. Cancer Ther. 2016, 15, 2905–2915. [Google Scholar] [CrossRef] [PubMed]
- Nicosia, L.; Spencer, G.J.; Brooks, N.; Amaral, F.M.R.; Basma, N.J.; Chadwick, J.A.; Revell, B.; Wingelhofer, B.; Maiques-Diaz, A.; Sinclair, O.; et al. Therapeutic targeting of EP300/CBP by bromodomain inhibition in hematologic malignancies. Cancer Cell 2023, 41, 2136–2153.e13. [Google Scholar] [CrossRef]
- Caligiuri, M.; Williams, G.L.; Castro, J.; Battalagine, L.; Wilker, E.; Yao, L.; Schiller, S.; Toms, A.; Li, P.; Pardo, E.; et al. FT-6876, a Potent and Selective Inhibitor of CBP/p300, is Active in Preclinical Models of Androgen Receptor-Positive Breast Cancer. Target. Oncol. 2023, 18, 269–285. [Google Scholar] [CrossRef]
- Spriano, F.; Gaudio, E.; Cascione, L.; Tarantelli, C.; Melle, F.; Motta, G.; Priebe, V.; Rinaldi, A.; Golino, G.; Mensah, A.A.; et al. Antitumor activity of the dual BET and CBP/EP300 inhibitor NEO2734. Blood Adv. 2020, 4, 4124–4135. [Google Scholar] [CrossRef]
- Yu, J.; Xie, T.; Wang, Z.; Wang, X.; Zeng, S.; Kang, Y.; Hou, T. DNA methyltransferases: Emerging targets for the discovery of inhibitors as potent anticancer drugs. Drug Discov. Today 2019, 24, 2323–2331. [Google Scholar] [CrossRef] [PubMed]
- Mehdipour, P.; Chen, R.; De Carvalho, D.D. The next generation of DNMT inhibitors. Nat. Cancer 2021, 2, 1000–1001. [Google Scholar] [CrossRef] [PubMed]
- Chirnomas, D.; Hornberger, K.R.; Crews, C.M. Protein degraders enter the clinic—A new approach to cancer therapy. Nat. Rev. Clin. Oncol. 2023, 20, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.M.; Nowak, R.P.; Ebert, B.L.; Fischer, E.S. Targeted protein degradation: From mechanisms to clinic. Nat. Rev. Mol. Cell Biol. 2024, 25, 740–757. [Google Scholar] [CrossRef]


| Modification Type | Target Protein | Lysine Site | PubMed ID | Publication Year |
|---|---|---|---|---|
| N-terminal acetylation | 16 (ELOA, GCN1, PPIA, RPL13A, RPP30, SAE1) | 19420222 | 2009 | |
| TSC2 | 20145209 | 2010 | ||
| MMP2 | 29960050 | 2018 | ||
| PLIN2 | 30425097 | 2019 | ||
| PGC-1α | 31422874 | 2019 | ||
| 985 (MYH7, MYH6, TNNI3, DES) | 40234403 | 2025 | ||
| Internal lysine acetylation | HIF-1α | K532 | 12464182 | 2002 |
| β-catenin | N/A | 17108104 | 2006 | |
| MLCK | K608 | 19826488 | 2009 | |
| NAA10 | K136 | 20501853 | 2010 | |
| MSRA | K49 | 25341044 | 2014 | |
| RUNX2 | K225 | 25376646 | 2014 | |
| CDC25A | N/A | 26967250 | 2016 | |
| AR | K618 | 27659526 | 2016 | |
| HSP70 | K77 | 27708256 | 2016 | |
| PGK1 | K388 | 28486006 | 2017 | |
| AURKA | K75, K125 | 28915666 | 2017 | |
| SAMHD1 | K405 | 28978134 | 2017 | |
| NRF2 | K438 | 36442525 | 2023 | |
| Protein–protein interaction | DNMT1 | 20592467 | 2010 | |
| PIX | 21295525 | 2011 | ||
| RelA | 22496479 | 2012 | ||
| STAT5α | 24925029 | 2014 | ||
| ADAM9 | 32719332 | 2020 | ||
| IKKα | 34060226 | 2021 |
| Uniport ID | Protein Symbol | Description | NAA10 Meta P |
|---|---|---|---|
| Q9UQ80 | PA2G4 | proliferation-associated 2G4 | 30.1 |
| P62913 | RPL11 | ribosomal protein L11 | 23.5 |
| P26641 | EEF1G | eukaryotic translation elongation factor 1 gamma | 21.0 |
| Q7L2H7 | EIF3M | eukaryotic translation initiation factor 3 subunit M | 21.0 |
| Q14690 | PDCD11 | programmed cell death 11 | 21.0 |
| Q12904 | AIMP1 | aminoacyl tRNA synthetase complex interacting multifunctional protein 1 | 20.9 |
| Q92616 | GCN1 | GCN1 activator of EIF2AK4 | 20.5 |
| Q9UHD1 | CHORDC1 | cysteine and histidine rich domain containing 1 | 19.6 |
| P25205 | MCM3 | minichromosome maintenance complex component 3 | 17.4 |
| O43583 | DENR | density regulated re-initiation and release factor | 16.5 |
| Q15007 | WTAP | WT1 associated protein | 15.9 |
| Q9UL63 | MKLN1 | muskelin 1 | 14.9 |
| P62195 | PSMC5 | proteasome 26S subunit, ATPase 5 | 14.0 |
| P49790 | NUP153 | nucleoporin 153 | 13.5 |
| Q9NPD3 | EXOSC4 | exosome component 4 | 13.4 |
| Q9UHB9 | SRP68 | signal recognition particle 68 | 13.0 |
| Q9Y5J9 | TIMM8B | translocase of inner mitochondrial membrane 8 homolog B | 11.6 |
| Q9NR33 | POLE4 | DNA polymerase epsilon 4, accessory subunit | 10.5 |
| Q92990 | GLMN | glomulin, FKBP associated protein | 10.0 |
| P10412 | H1-4 | H1.4 linker histone | −10.1 |
| P07305 | H1-0 | H1.0 linker histone | −10.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.Q.; Campeanu, I.J.; Lopez, I.; Syed, M.; Jiang, Y.; Afisllari, H. NAA10 (N-Alpha-Acetyltransferase 10): A Multifunctional Regulator in Development, Disease, and Cancer. Cells 2025, 14, 863. https://doi.org/10.3390/cells14120863
Yang ZQ, Campeanu IJ, Lopez I, Syed M, Jiang Y, Afisllari H. NAA10 (N-Alpha-Acetyltransferase 10): A Multifunctional Regulator in Development, Disease, and Cancer. Cells. 2025; 14(12):863. https://doi.org/10.3390/cells14120863
Chicago/Turabian StyleYang, Zeng Quan, Ion John Campeanu, Ivan Lopez, Manaal Syed, Yuanyuan Jiang, and Hilda Afisllari. 2025. "NAA10 (N-Alpha-Acetyltransferase 10): A Multifunctional Regulator in Development, Disease, and Cancer" Cells 14, no. 12: 863. https://doi.org/10.3390/cells14120863
APA StyleYang, Z. Q., Campeanu, I. J., Lopez, I., Syed, M., Jiang, Y., & Afisllari, H. (2025). NAA10 (N-Alpha-Acetyltransferase 10): A Multifunctional Regulator in Development, Disease, and Cancer. Cells, 14(12), 863. https://doi.org/10.3390/cells14120863





