Mesenchymal Stem Cell Secretome Attenuates PrP106-126-Induced Neurotoxicity by Suppressing Neuroinflammation and Apoptosis and Enhances Cell Migration
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture of AdMSCs and Preparation of the Secretome
2.2. Preparation of PrP106−126 Peptide and Cell Culture
2.3. Release of Lactate Dehydrogenase (LDH)
2.4. Real-Time Polymerase Chain Reaction (RT-PCR)
2.5. Immunofluorescence of Cells
2.6. Western Blot Analysis
2.7. Scratch Assay
2.8. Statistical Analysis
3. Results
3.1. The AdMSC Secretome Preserves the Viability of SH-SY5Y Cells
3.2. AdMSC Secretome Suppresses Reactive Astrocytes
3.3. AdMSC Secretome Ameliorates Neuroinflammation
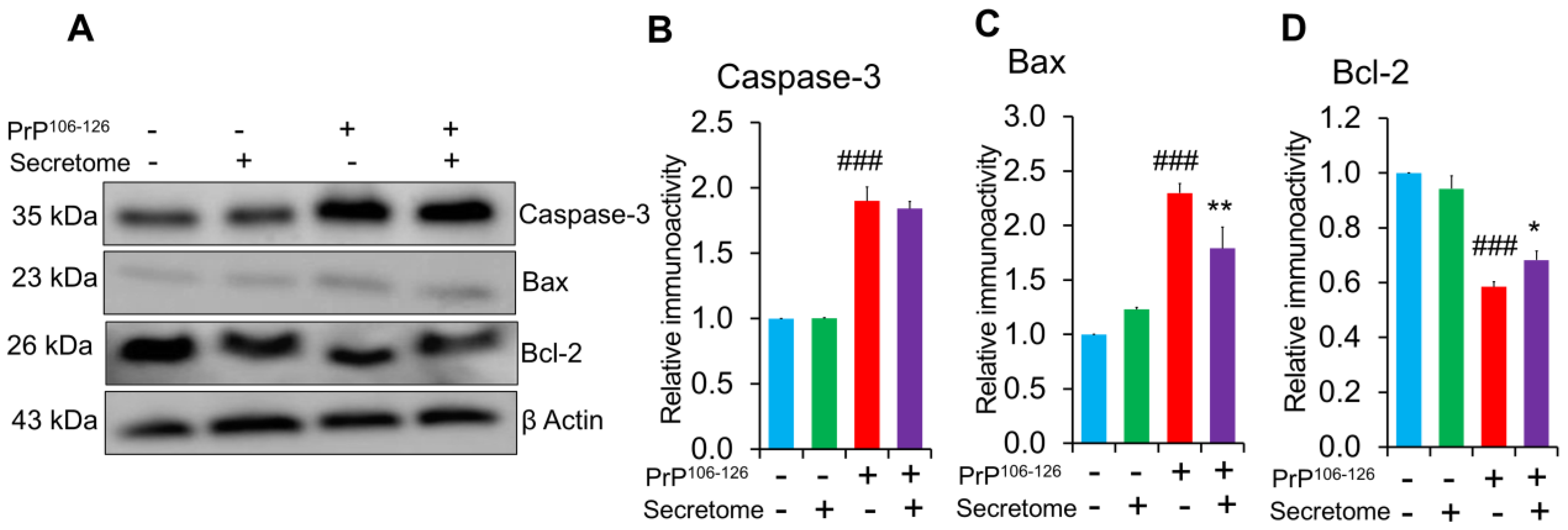
3.4. AdMSC Secretome Inhibits Apoptosis in SH-SY5Y Cells
3.5. AdMSC Secretome Enhances the Migratory Activity of SH-SY5Y Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pan, K.M.; Baldwin, M.; Nguyen, J.; Gasset, M.; Serban, A.; Groth, D.; Mehlhorn, I.; Huang, Z.; Fletterick, R.J.; Cohen, F.E.; et al. Conversion of alpha-helices into beta-sheets features in the formation of the scrapie prion proteins. Proc. Natl. Acad. Sci. USA 1993, 90, 10962–10966. [Google Scholar] [CrossRef] [PubMed]
- Prusiner, S.B. Prions. Proc. Natl. Acad. Sci. USA 1998, 95, 13363–13383. [Google Scholar] [CrossRef] [PubMed]
- du Plessis, D.G. Prion Protein Disease and Neuropathology of Prion Disease. Neuroimaging Clin. N. Am. 2008, 18, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, Y. Creutzfeldt-Jakob disease. Neuropathology 2017, 37, 174–188. [Google Scholar] [CrossRef]
- Wadsworth, J.D.F.; Collinge, J. Molecular pathology of human prion disease. Acta Neuropathol. 2011, 121, 69–77. [Google Scholar] [CrossRef]
- Li, B.; Chen, M.; Zhu, C. Neuroinflammation in Prion Disease. Int. J. Mol. Sci. 2021, 22, 2196. [Google Scholar] [CrossRef]
- Prasad, K.N.; Bondy, S.C. Oxidative and Inflammatory Events in Prion Diseases: Can They Be Therapeutic Targets? Curr. Aging Sci. 2019, 11, 216–225. [Google Scholar] [CrossRef]
- Srivastava, S.; Katorcha, E.; Makarava, N.; Barrett, J.P.; Loane, D.J.; Baskakov, I.V. Inflammatory response of microglia to prions is controlled by sialylation of PrP(Sc). Sci. Rep. 2018, 8, 11326. [Google Scholar] [CrossRef]
- Mühleisen, H.; Gehrmann, J.; Meyermann, R. Reactive microglia in Creutzfeldt-Jakob disease. Neuropathol. Appl. Neurobiol. 1995, 21, 505–517. [Google Scholar] [CrossRef]
- Williams, A.E.; Lawson, L.J.; Perry, V.H.; Fraser, H. Characterization of the microglial response in murine scrapie. Neuropathol. Appl. Neurobiol. 1994, 20, 47–55. [Google Scholar] [CrossRef]
- Giese, A.; Kretzschmar, H.A. Prion-induced neuronal damage—The mechanisms of neuronal destruction in the subacute spongiform encephalopathies. Curr. Top. Microbiol. Immunol. 2001, 253, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Na, Y.-J.; Jin, J.-K.; Kim, J.-I.; Choi, E.-K.; Carp, R.I.; Kim, Y.-S. JAK-STAT signaling pathway mediates astrogliosis in brains of scrapie-infected mice. J. Neurochem. 2007, 103, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Forloni, G.; Chiesa, R.; Bugiani, O.; Salmona, M.; Tagliavini, F. Review: PrP 106-126—25 years after. Neuropathol. Appl. Neurobiol. 2019, 45, 430–440. [Google Scholar] [CrossRef] [PubMed]
- O’Donovan, C.N.; Tobin, D.; Cotter, T.G. Prion protein fragment PrP-(106-126) induces apoptosis via mitochondrial disruption in human neuronal SH-SY5Y cells. J. Biol. Chem. 2001, 276, 43516–43523. [Google Scholar] [CrossRef]
- Singh, N.; Gu, Y.; Bose, S.; Kalepu, S.; Mishra, R.S.; Verghese, S. Prion peptide 106-126 as a model for prion replication and neurotoxicity. Front. Biosci. 2002, 7, a60–a71. [Google Scholar] [CrossRef]
- Brown, D.R.; Herms, J.W.; Schmidt, B.; Kretzschmar, H.A. PrP and beta-amyloid fragments activate different neurotoxic mechanisms in cultured mouse cells. Eur. J. Neurosci. 1997, 9, 1162–1169. [Google Scholar] [CrossRef]
- Wang, J.; Deng, G.; Wang, S.; Li, S.; Song, P.; Lin, K.; Xu, X.; He, Z. Enhancing regenerative medicine: The crucial role of stem cell therapy. Front. Neurosci. 2024, 18, 1269577. [Google Scholar] [CrossRef]
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 68. [Google Scholar] [CrossRef]
- Zayed, M.; Caniglia, C.; Misk, N.; Dhar, M.S. Donor-Matched Comparison of Chondrogenic Potential of Equine Bone Marrow- and Synovial Fluid-Derived Mesenchymal Stem Cells: Implications for Cartilage Tissue Regeneration. Front. Vet. Sci. 2016, 3, 121. [Google Scholar] [CrossRef]
- Zayed, M.; Iohara, K.; Watanabe, H.; Ishikawa, M.; Tominaga, M.; Nakashima, M. Characterization of stable hypoxia-preconditioned dental pulp stem cells compared with mobilized dental pulp stem cells for application for pulp regenerative therapy. Stem Cell Res. Ther. 2021, 12, 302. [Google Scholar] [CrossRef]
- Zayed, M.; Adair, S.; Dhar, M. Effects of Normal Synovial Fluid and Interferon Gamma on Chondrogenic Capability and Immunomodulatory Potential Respectively on Equine Mesenchymal Stem Cells. Int. J. Mol. Sci. 2021, 22, 6391. [Google Scholar] [CrossRef] [PubMed]
- Hay, A.J.D.; Latham, A.S.; Mumford, G.; Hines, A.D.; Risen, S.; Gordon, E.; Siebenaler, C.; Gilberto, V.S.; Zabel, M.D.; Moreno, J.A. Intranasally delivered mesenchymal stromal cells decrease glial inflammation early in prion disease. Front. Neurosci. 2023, 17, 1158408. [Google Scholar] [CrossRef] [PubMed]
- Zayed, M.; Kook, S.H.; Jeong, B.H. Potential Therapeutic Use of Stem Cells for Prion Diseases. Cells 2023, 12, 2413. [Google Scholar] [CrossRef] [PubMed]
- Relaño-Ginés, A.; Lehmann, S.; Bencsik, A.; Herva, M.E.; Torres, J.M.; Crozet, C.A. Stem Cell Therapy Extends Incubation and Survival Time in Prion-Infected Mice in a Time Window–Dependant Manner. J. Infect. Dis. 2011, 204, 1038–1045. [Google Scholar] [CrossRef]
- Blurton-Jones, M.; Kitazawa, M.; Martinez-Coria, H.; Castello, N.A.; Müller, F.J.; Loring, J.F.; Yamasaki, T.R.; Poon, W.W.; Green, K.N.; LaFerla, F.M. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc. Natl. Acad. Sci. USA 2009, 106, 13594–13599. [Google Scholar] [CrossRef]
- Yang, H.; Xie, Z.; Wei, L.; Yang, H.; Yang, S.; Zhu, Z.; Wang, P.; Zhao, C.; Bi, J. Human umbilical cord mesenchymal stem cell-derived neuron-like cells rescue memory deficits and reduce amyloid-beta deposition in an AβPP/PS1 transgenic mouse model. Stem Cell Res. Ther. 2013, 4, 76. [Google Scholar] [CrossRef]
- Ala, M. The beneficial effects of mesenchymal stem cells and their exosomes on myocardial infarction and critical considerations for enhancing their efficacy. Ageing Res. Rev. 2023, 89, 101980. [Google Scholar] [CrossRef]
- Ratajczak, M.Z.; Kucia, M.; Jadczyk, T.; Greco, N.J.; Wojakowski, W.; Tendera, M.; Ratajczak, J. Pivotal role of paracrine effects in stem cell therapies in regenerative medicine: Can we translate stem cell-secreted paracrine factors and microvesicles into better therapeutic strategies? Leukemia 2012, 26, 1166–1173. [Google Scholar] [CrossRef]
- Hofer, H.R.; Tuan, R.S. Secreted trophic factors of mesenchymal stem cells support neurovascular and musculoskeletal therapies. Stem Cell Res. Ther. 2016, 7, 131. [Google Scholar] [CrossRef]
- Cheng, L.; Zhao, W.; Hill, A.F. Exosomes and their role in the intercellular trafficking of normal and disease associated prion proteins. Mol. Asp. Med. 2018, 60, 62–68. [Google Scholar] [CrossRef]
- Han, D.; Zheng, X.; Wang, X.; Jin, T.; Cui, L.; Chen, Z. Mesenchymal Stem/Stromal Cell-Mediated Mitochondrial Transfer and the Therapeutic Potential in Treatment of Neurological Diseases. Stem Cells Int. 2020, 2020, 8838046. [Google Scholar] [CrossRef] [PubMed]
- Tseng, N.; Lambie, S.C.; Huynh, C.Q.; Sanford, B.; Patel, M.; Herson, P.S.; Ormond, D.R. Mitochondrial transfer from mesenchymal stem cells improves neuronal metabolism after oxidant injury in vitro: The role of Miro1. J. Cereb. Blood Flow. Metab. 2021, 41, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Angeloni, C.; Gatti, M.; Prata, C.; Hrelia, S.; Maraldi, T. Role of Mesenchymal Stem Cells in Counteracting Oxidative Stress-Related Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 3299. [Google Scholar] [CrossRef] [PubMed]
- Teli, P.; Kale, V.; Vaidya, A. Mesenchymal stromal cells-derived secretome protects Neuro-2a cells from oxidative stress-induced loss of neurogenesis. Exp. Neurol. 2022, 354, 114107. [Google Scholar] [CrossRef]
- Zayed, M.; Jeong, B.H. Adipose-Derived Mesenchymal Stem Cell Secretome Attenuates Prion Protein Peptide (106-126)-Induced Oxidative Stress via Nrf2 Activation. Stem Cell Rev. Rep. 2024, 21, 589–592. [Google Scholar] [CrossRef]
- Chen, Y.X.; Zeng, Z.C.; Sun, J.; Zeng, H.Y.; Huang, Y.; Zhang, Z.Y. Mesenchymal stem cell-conditioned medium prevents radiation-induced liver injury by inhibiting inflammation and protecting sinusoidal endothelial cells. J. Radiat. Res. 2015, 56, 700–708. [Google Scholar] [CrossRef]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef]
- Tang, J.; Kang, Y.; Zhou, Y.; Chen, Q.; Lan, J.; Liu, X.; Peng, Y. Umbilical cord mesenchymal stem cell-conditioned medium inhibits microglial activation to ameliorate neuroinflammation in amyotrophic lateral sclerosis mice and cell models. Brain Res. Bull. 2023, 202, 110760. [Google Scholar] [CrossRef]
- Zayed, M.; Kim, Y.-C.; Jeong, B.-H. Biological characteristics and transcriptomic profile of adipose-derived mesenchymal stem cells isolated from prion-infected murine model. Stem Cell Res. Ther. 2025, 16, 154. [Google Scholar] [CrossRef]
- Zayed, M.; Kim, Y.-C.; Jeong, B.-H. Assessment of the therapeutic potential of Hsp70 activator against prion diseases using in vitro and in vivo models. Front. Cell Dev. Biol. 2024, 12, 1411529. [Google Scholar] [CrossRef]
- Ghasemi, M.; Roshandel, E.; Mohammadian, M.; Farhadihosseinabadi, B.; Akbarzadehlaleh, P.; Shamsasenjan, K. Mesenchymal stromal cell-derived secretome-based therapy for neurodegenerative diseases: Overview of clinical trials. Stem Cell Res. Ther. 2023, 14, 122. [Google Scholar] [CrossRef] [PubMed]
- Makarava, N.; Chang, J.C.; Kushwaha, R.; Baskakov, I.V. Region-Specific Response of Astrocytes to Prion Infection. Front. Neurosci. 2019, 13, 1048. [Google Scholar] [CrossRef] [PubMed]
- Shephard, M.T.; Merkhan, M.M.; Forsyth, N.R. Human Mesenchymal Stem Cell Secretome Driven T Cell Immunomodulation Is IL-10 Dependent. Int. J. Mol. Sci. 2022, 23, 13596. [Google Scholar] [CrossRef] [PubMed]
- Kavaldzhieva, K.; Mladenov, N.; Markova, M.; Belemezova, K. Mesenchymal Stem Cell Secretome: Potential Applications in Human Infertility Caused by Hormonal Imbalance, External Damage, or Immune Factors. Biomedicines 2025, 13, 586. [Google Scholar] [CrossRef]
- Aguzzi, A.; Zhu, C. Microglia in prion diseases. J. Clin. Investig. 2017, 127, 3230–3239. [Google Scholar] [CrossRef]
- Wu, C.L.; Chou, Y.H.; Chang, Y.J.; Teng, N.Y.; Hsu, H.L.; Chen, L. Interplay between cell migration and neurite outgrowth determines SH2B1β-enhanced neurite regeneration of differentiated PC12 cells. PLoS ONE 2012, 7, e34999. [Google Scholar] [CrossRef]
- Han, Y.; Yang, J.; Fang, J.; Zhou, Y.; Candi, E.; Wang, J.; Hua, D.; Shao, C.; Shi, Y. The secretion profile of mesenchymal stem cells and potential applications in treating human diseases. Signal Transduct. Target. Ther. 2022, 7, 92. [Google Scholar] [CrossRef]
- Mattei, V.; Delle Monache, S. Mesenchymal Stem Cells and Their Role in Neurodegenerative Diseases. Cells 2024, 13, 779. [Google Scholar] [CrossRef]
- Mahmoud, E.E.; Mawas, A.S.; Mohamed, A.A.; Noby, M.A.; Abdel-Hady, A.A.; Zayed, M. Treatment strategies for meniscal lesions: From past to prospective therapeutics. Regen. Med. 2022, 17, 547–560. [Google Scholar] [CrossRef]
- Zayed, M.; Kim, Y.-C.; Jeong, B.-H. Therapeutic effects of adipose-derived mesenchymal stem cells combined with glymphatic system activation in prion disease. Mol. Neurodegener. 2025, 20, 42. [Google Scholar] [CrossRef]
- Lee, H.C.; An, S.G.; Lee, H.W.; Park, J.S.; Cha, K.S.; Hong, T.J.; Park, J.H.; Lee, S.Y.; Kim, S.P.; Kim, Y.D.; et al. Safety and effect of adipose tissue-derived stem cell implantation in patients with critical limb ischemia: A pilot study. Circ. J. 2012, 76, 1750–1760. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I.; Correa, D. The MSC: An Injury Drugstore. Cell Stem Cell 2011, 9, 11–15. [Google Scholar] [CrossRef]
- Giovannelli, L.; Bari, E.; Jommi, C.; Tartara, F.; Armocida, D.; Garbossa, D.; Cofano, F.; Torre, M.L.; Segale, L. Mesenchymal stem cell secretome and extracellular vesicles for neurodegenerative diseases: Risk-benefit profile and next steps for the market access. Bioact. Mater. 2023, 29, 16–35. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Ito, T.; Inden, M.; Kurita, H.; Yamamoto, A.; Hozumi, I. Stem Cells From Human Exfoliated Deciduous Teeth-Conditioned Medium (SHED-CM) is a Promising Treatment for Amyotrophic Lateral Sclerosis. Front. Pharmacol. 2022, 13, 805379. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, D.; Liu, C.; Ding, T.; Yang, L.; Yin, X.; Zhou, X. Prion protein participates in the protection of mice from lipopolysaccharide infection by regulating the inflammatory process. J. Mol. Neurosci. 2015, 55, 279–287. [Google Scholar] [CrossRef]
- Roberts, T.K.; Eugenin, E.A.; Morgello, S.; Clements, J.E.; Zink, M.C.; Berman, J.W. PrPC, the Cellular Isoform of the Human Prion Protein, Is a Novel Biomarker of HIV-Associated Neurocognitive Impairment and Mediates Neuroinflammation. Am. J. Pathol. 2010, 177, 1848–1860. [Google Scholar] [CrossRef]
- Bakkebø, M.K.; Mouillet-Richard, S.; Espenes, A.; Goldmann, W.; Tatzelt, J.; Tranulis, M.A. The Cellular Prion Protein: A Player in Immunological Quiescence. Front. Immunol. 2015, 6, 450. [Google Scholar] [CrossRef]
- Risen, S.J.; Boland, S.W.; Sharma, S.; Weisman, G.M.; Shirley, P.M.; Latham, A.S.; Hay, A.J.D.; Gilberto, V.S.; Hines, A.D.; Brindley, S.; et al. Targeting Neuroinflammation by Pharmacologic Downregulation of Inflammatory Pathways Is Neuroprotective in Protein Misfolding Disorders. ACS Chem. Neurosci. 2024, 15, 1533–1547. [Google Scholar] [CrossRef]
- Kordek, R.; Nerurkar, V.R.; Liberski, P.P.; Isaacson, S.; Yanagihara, R.; Gajdusek, D.C. Heightened expression of tumor necrosis factor alpha, interleukin 1 alpha, and glial fibrillary acidic protein in experimental Creutzfeldt-Jakob disease in mice. Proc. Natl. Acad. Sci. USA 1996, 93, 9754–9758. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Tang, Y.; Le, W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 1181–1194. [Google Scholar] [CrossRef] [PubMed]
- Kappy, N.S.; Chang, S.; Harris, W.M.; Plastini, M.; Ortiz, T.; Zhang, P.; Hazelton, J.P.; Carpenter, J.P.; Brown, S.A. Human adipose-derived stem cell treatment modulates cellular protection in both in vitro and in vivo traumatic brain injury models. J. Trauma. Acute Care Surg. 2018, 84, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Baez-Jurado, E.; Guio-Vega, G.; Hidalgo-Lanussa, O.; González, J.; Echeverria, V.; Ashraf, G.M.; Sahebkar, A.; Barreto, G.E. Mitochondrial Neuroglobin Is Necessary for Protection Induced by Conditioned Medium from Human Adipose-Derived Mesenchymal Stem Cells in Astrocytic Cells Subjected to Scratch and Metabolic Injury. Mol. Neurobiol. 2019, 56, 5167–5187. [Google Scholar] [CrossRef] [PubMed]
- Gray, F.; Chrétien, F.; Adle-Biassette, H.; Dorandeu, A.; Ereau, T.; Delisle, M.B.; Kopp, N.; Ironside, J.W.; Vital, C. Neuronal apoptosis in Creutzfeldt-Jakob disease. J. Neuropathol. Exp. Neurol. 1999, 58, 321–328. [Google Scholar] [CrossRef]
- Huang, Y.; Mei, X.; Jiang, W.; Zhao, H.; Yan, Z.; Zhang, H.; Liu, Y.; Hu, X.; Zhang, J.; Peng, W.; et al. Mesenchymal Stem Cell-Conditioned Medium Protects Hippocampal Neurons from Radiation Damage by Suppressing Oxidative Stress and Apoptosis. Dose Response 2021, 19, 1559325820984944. [Google Scholar] [CrossRef]
- Li, H.; Yahaya, B.H.; Ng, W.H.; Yusoff, N.M.; Lin, J. Conditioned Medium of Human Menstrual Blood-Derived Endometrial Stem Cells Protects Against MPP(+)-Induced Cytotoxicity in vitro. Front. Mol. Neurosci. 2019, 12, 80. [Google Scholar] [CrossRef]




| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| TNF-α | CCTCTCTCTAATCAGCCCTCTG | GAGGACCTGGGAGTAGATGAG |
| IL-1β | ATGATGGCTTATTACAGTGGCAA | GTCGGAGATTCGTAGCTGGA |
| IL-10 | ACCTGCCTAACATGCTTCGAG | CTGGGTCTTGGTTCTCAGCTT |
| IDO | CAAAGGTCATGGAGATGTCC | CCACCAATAGAGAGACCAGG |
| ACTB | TGGCACCCAGCACAATGAA | CTAAGTCATAGTCCGCCTAGAAGCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zayed, M.; Jeong, B.-H. Mesenchymal Stem Cell Secretome Attenuates PrP106-126-Induced Neurotoxicity by Suppressing Neuroinflammation and Apoptosis and Enhances Cell Migration. Cells 2025, 14, 851. https://doi.org/10.3390/cells14110851
Zayed M, Jeong B-H. Mesenchymal Stem Cell Secretome Attenuates PrP106-126-Induced Neurotoxicity by Suppressing Neuroinflammation and Apoptosis and Enhances Cell Migration. Cells. 2025; 14(11):851. https://doi.org/10.3390/cells14110851
Chicago/Turabian StyleZayed, Mohammed, and Byung-Hoon Jeong. 2025. "Mesenchymal Stem Cell Secretome Attenuates PrP106-126-Induced Neurotoxicity by Suppressing Neuroinflammation and Apoptosis and Enhances Cell Migration" Cells 14, no. 11: 851. https://doi.org/10.3390/cells14110851
APA StyleZayed, M., & Jeong, B.-H. (2025). Mesenchymal Stem Cell Secretome Attenuates PrP106-126-Induced Neurotoxicity by Suppressing Neuroinflammation and Apoptosis and Enhances Cell Migration. Cells, 14(11), 851. https://doi.org/10.3390/cells14110851









