Mesenchymal Stem Cell Secretome Attenuates PrP106-126-Induced Neurotoxicity by Suppressing Neuroinflammation and Apoptosis and Enhances Cell Migration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture of AdMSCs and Preparation of the Secretome
2.2. Preparation of PrP106−126 Peptide and Cell Culture
2.3. Release of Lactate Dehydrogenase (LDH)
2.4. Real-Time Polymerase Chain Reaction (RT-PCR)
2.5. Immunofluorescence of Cells
2.6. Western Blot Analysis
2.7. Scratch Assay
2.8. Statistical Analysis
3. Results
3.1. The AdMSC Secretome Preserves the Viability of SH-SY5Y Cells
3.2. AdMSC Secretome Suppresses Reactive Astrocytes
3.3. AdMSC Secretome Ameliorates Neuroinflammation
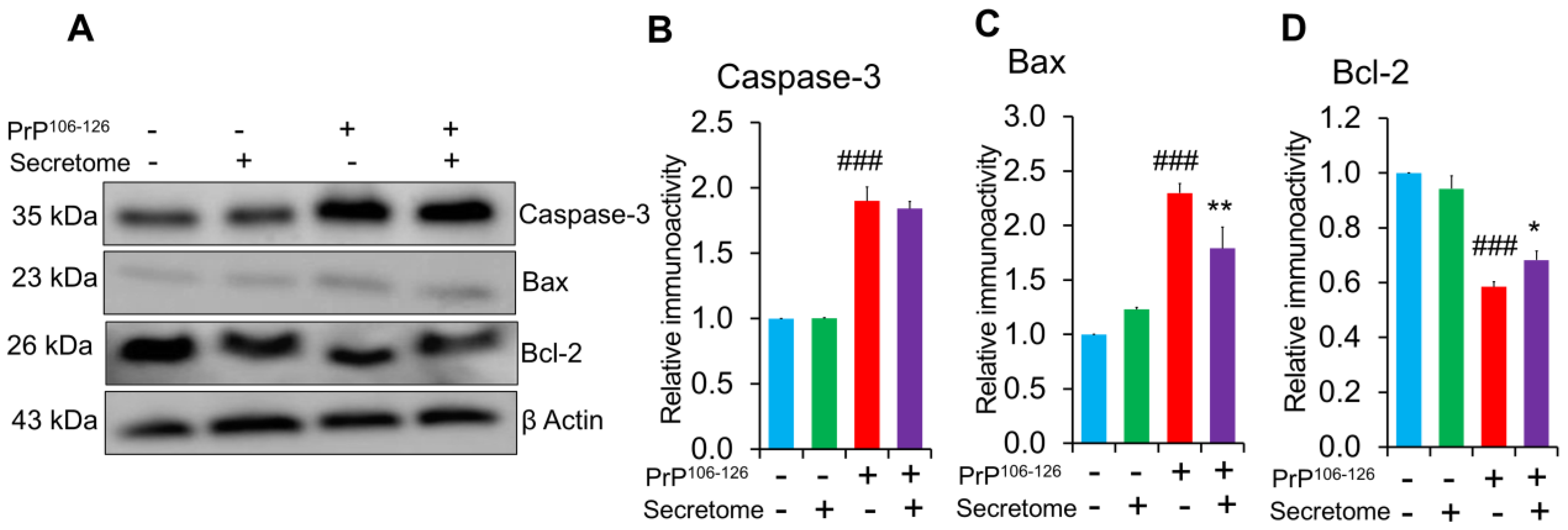
3.4. AdMSC Secretome Inhibits Apoptosis in SH-SY5Y Cells
3.5. AdMSC Secretome Enhances the Migratory Activity of SH-SY5Y Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pan, K.M.; Baldwin, M.; Nguyen, J.; Gasset, M.; Serban, A.; Groth, D.; Mehlhorn, I.; Huang, Z.; Fletterick, R.J.; Cohen, F.E.; et al. Conversion of alpha-helices into beta-sheets features in the formation of the scrapie prion proteins. Proc. Natl. Acad. Sci. USA 1993, 90, 10962–10966. [Google Scholar] [CrossRef] [PubMed]
- Prusiner, S.B. Prions. Proc. Natl. Acad. Sci. USA 1998, 95, 13363–13383. [Google Scholar] [CrossRef] [PubMed]
- du Plessis, D.G. Prion Protein Disease and Neuropathology of Prion Disease. Neuroimaging Clin. N. Am. 2008, 18, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, Y. Creutzfeldt-Jakob disease. Neuropathology 2017, 37, 174–188. [Google Scholar] [CrossRef]
- Wadsworth, J.D.F.; Collinge, J. Molecular pathology of human prion disease. Acta Neuropathol. 2011, 121, 69–77. [Google Scholar] [CrossRef]
- Li, B.; Chen, M.; Zhu, C. Neuroinflammation in Prion Disease. Int. J. Mol. Sci. 2021, 22, 2196. [Google Scholar] [CrossRef]
- Prasad, K.N.; Bondy, S.C. Oxidative and Inflammatory Events in Prion Diseases: Can They Be Therapeutic Targets? Curr. Aging Sci. 2019, 11, 216–225. [Google Scholar] [CrossRef]
- Srivastava, S.; Katorcha, E.; Makarava, N.; Barrett, J.P.; Loane, D.J.; Baskakov, I.V. Inflammatory response of microglia to prions is controlled by sialylation of PrP(Sc). Sci. Rep. 2018, 8, 11326. [Google Scholar] [CrossRef]
- Mühleisen, H.; Gehrmann, J.; Meyermann, R. Reactive microglia in Creutzfeldt-Jakob disease. Neuropathol. Appl. Neurobiol. 1995, 21, 505–517. [Google Scholar] [CrossRef]
- Williams, A.E.; Lawson, L.J.; Perry, V.H.; Fraser, H. Characterization of the microglial response in murine scrapie. Neuropathol. Appl. Neurobiol. 1994, 20, 47–55. [Google Scholar] [CrossRef]
- Giese, A.; Kretzschmar, H.A. Prion-induced neuronal damage—The mechanisms of neuronal destruction in the subacute spongiform encephalopathies. Curr. Top. Microbiol. Immunol. 2001, 253, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Na, Y.-J.; Jin, J.-K.; Kim, J.-I.; Choi, E.-K.; Carp, R.I.; Kim, Y.-S. JAK-STAT signaling pathway mediates astrogliosis in brains of scrapie-infected mice. J. Neurochem. 2007, 103, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Forloni, G.; Chiesa, R.; Bugiani, O.; Salmona, M.; Tagliavini, F. Review: PrP 106-126—25 years after. Neuropathol. Appl. Neurobiol. 2019, 45, 430–440. [Google Scholar] [CrossRef] [PubMed]
- O’Donovan, C.N.; Tobin, D.; Cotter, T.G. Prion protein fragment PrP-(106-126) induces apoptosis via mitochondrial disruption in human neuronal SH-SY5Y cells. J. Biol. Chem. 2001, 276, 43516–43523. [Google Scholar] [CrossRef]
- Singh, N.; Gu, Y.; Bose, S.; Kalepu, S.; Mishra, R.S.; Verghese, S. Prion peptide 106-126 as a model for prion replication and neurotoxicity. Front. Biosci. 2002, 7, a60–a71. [Google Scholar] [CrossRef]
- Brown, D.R.; Herms, J.W.; Schmidt, B.; Kretzschmar, H.A. PrP and beta-amyloid fragments activate different neurotoxic mechanisms in cultured mouse cells. Eur. J. Neurosci. 1997, 9, 1162–1169. [Google Scholar] [CrossRef]
- Wang, J.; Deng, G.; Wang, S.; Li, S.; Song, P.; Lin, K.; Xu, X.; He, Z. Enhancing regenerative medicine: The crucial role of stem cell therapy. Front. Neurosci. 2024, 18, 1269577. [Google Scholar] [CrossRef]
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 68. [Google Scholar] [CrossRef]
- Zayed, M.; Caniglia, C.; Misk, N.; Dhar, M.S. Donor-Matched Comparison of Chondrogenic Potential of Equine Bone Marrow- and Synovial Fluid-Derived Mesenchymal Stem Cells: Implications for Cartilage Tissue Regeneration. Front. Vet. Sci. 2016, 3, 121. [Google Scholar] [CrossRef]
- Zayed, M.; Iohara, K.; Watanabe, H.; Ishikawa, M.; Tominaga, M.; Nakashima, M. Characterization of stable hypoxia-preconditioned dental pulp stem cells compared with mobilized dental pulp stem cells for application for pulp regenerative therapy. Stem Cell Res. Ther. 2021, 12, 302. [Google Scholar] [CrossRef]
- Zayed, M.; Adair, S.; Dhar, M. Effects of Normal Synovial Fluid and Interferon Gamma on Chondrogenic Capability and Immunomodulatory Potential Respectively on Equine Mesenchymal Stem Cells. Int. J. Mol. Sci. 2021, 22, 6391. [Google Scholar] [CrossRef] [PubMed]
- Hay, A.J.D.; Latham, A.S.; Mumford, G.; Hines, A.D.; Risen, S.; Gordon, E.; Siebenaler, C.; Gilberto, V.S.; Zabel, M.D.; Moreno, J.A. Intranasally delivered mesenchymal stromal cells decrease glial inflammation early in prion disease. Front. Neurosci. 2023, 17, 1158408. [Google Scholar] [CrossRef] [PubMed]
- Zayed, M.; Kook, S.H.; Jeong, B.H. Potential Therapeutic Use of Stem Cells for Prion Diseases. Cells 2023, 12, 2413. [Google Scholar] [CrossRef] [PubMed]
- Relaño-Ginés, A.; Lehmann, S.; Bencsik, A.; Herva, M.E.; Torres, J.M.; Crozet, C.A. Stem Cell Therapy Extends Incubation and Survival Time in Prion-Infected Mice in a Time Window–Dependant Manner. J. Infect. Dis. 2011, 204, 1038–1045. [Google Scholar] [CrossRef]
- Blurton-Jones, M.; Kitazawa, M.; Martinez-Coria, H.; Castello, N.A.; Müller, F.J.; Loring, J.F.; Yamasaki, T.R.; Poon, W.W.; Green, K.N.; LaFerla, F.M. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc. Natl. Acad. Sci. USA 2009, 106, 13594–13599. [Google Scholar] [CrossRef]
- Yang, H.; Xie, Z.; Wei, L.; Yang, H.; Yang, S.; Zhu, Z.; Wang, P.; Zhao, C.; Bi, J. Human umbilical cord mesenchymal stem cell-derived neuron-like cells rescue memory deficits and reduce amyloid-beta deposition in an AβPP/PS1 transgenic mouse model. Stem Cell Res. Ther. 2013, 4, 76. [Google Scholar] [CrossRef]
- Ala, M. The beneficial effects of mesenchymal stem cells and their exosomes on myocardial infarction and critical considerations for enhancing their efficacy. Ageing Res. Rev. 2023, 89, 101980. [Google Scholar] [CrossRef]
- Ratajczak, M.Z.; Kucia, M.; Jadczyk, T.; Greco, N.J.; Wojakowski, W.; Tendera, M.; Ratajczak, J. Pivotal role of paracrine effects in stem cell therapies in regenerative medicine: Can we translate stem cell-secreted paracrine factors and microvesicles into better therapeutic strategies? Leukemia 2012, 26, 1166–1173. [Google Scholar] [CrossRef]
- Hofer, H.R.; Tuan, R.S. Secreted trophic factors of mesenchymal stem cells support neurovascular and musculoskeletal therapies. Stem Cell Res. Ther. 2016, 7, 131. [Google Scholar] [CrossRef]
- Cheng, L.; Zhao, W.; Hill, A.F. Exosomes and their role in the intercellular trafficking of normal and disease associated prion proteins. Mol. Asp. Med. 2018, 60, 62–68. [Google Scholar] [CrossRef]
- Han, D.; Zheng, X.; Wang, X.; Jin, T.; Cui, L.; Chen, Z. Mesenchymal Stem/Stromal Cell-Mediated Mitochondrial Transfer and the Therapeutic Potential in Treatment of Neurological Diseases. Stem Cells Int. 2020, 2020, 8838046. [Google Scholar] [CrossRef] [PubMed]
- Tseng, N.; Lambie, S.C.; Huynh, C.Q.; Sanford, B.; Patel, M.; Herson, P.S.; Ormond, D.R. Mitochondrial transfer from mesenchymal stem cells improves neuronal metabolism after oxidant injury in vitro: The role of Miro1. J. Cereb. Blood Flow. Metab. 2021, 41, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Angeloni, C.; Gatti, M.; Prata, C.; Hrelia, S.; Maraldi, T. Role of Mesenchymal Stem Cells in Counteracting Oxidative Stress-Related Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 3299. [Google Scholar] [CrossRef] [PubMed]
- Teli, P.; Kale, V.; Vaidya, A. Mesenchymal stromal cells-derived secretome protects Neuro-2a cells from oxidative stress-induced loss of neurogenesis. Exp. Neurol. 2022, 354, 114107. [Google Scholar] [CrossRef]
- Zayed, M.; Jeong, B.H. Adipose-Derived Mesenchymal Stem Cell Secretome Attenuates Prion Protein Peptide (106-126)-Induced Oxidative Stress via Nrf2 Activation. Stem Cell Rev. Rep. 2024, 21, 589–592. [Google Scholar] [CrossRef]
- Chen, Y.X.; Zeng, Z.C.; Sun, J.; Zeng, H.Y.; Huang, Y.; Zhang, Z.Y. Mesenchymal stem cell-conditioned medium prevents radiation-induced liver injury by inhibiting inflammation and protecting sinusoidal endothelial cells. J. Radiat. Res. 2015, 56, 700–708. [Google Scholar] [CrossRef]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef]
- Tang, J.; Kang, Y.; Zhou, Y.; Chen, Q.; Lan, J.; Liu, X.; Peng, Y. Umbilical cord mesenchymal stem cell-conditioned medium inhibits microglial activation to ameliorate neuroinflammation in amyotrophic lateral sclerosis mice and cell models. Brain Res. Bull. 2023, 202, 110760. [Google Scholar] [CrossRef]
- Zayed, M.; Kim, Y.-C.; Jeong, B.-H. Biological characteristics and transcriptomic profile of adipose-derived mesenchymal stem cells isolated from prion-infected murine model. Stem Cell Res. Ther. 2025, 16, 154. [Google Scholar] [CrossRef]
- Zayed, M.; Kim, Y.-C.; Jeong, B.-H. Assessment of the therapeutic potential of Hsp70 activator against prion diseases using in vitro and in vivo models. Front. Cell Dev. Biol. 2024, 12, 1411529. [Google Scholar] [CrossRef]
- Ghasemi, M.; Roshandel, E.; Mohammadian, M.; Farhadihosseinabadi, B.; Akbarzadehlaleh, P.; Shamsasenjan, K. Mesenchymal stromal cell-derived secretome-based therapy for neurodegenerative diseases: Overview of clinical trials. Stem Cell Res. Ther. 2023, 14, 122. [Google Scholar] [CrossRef] [PubMed]
- Makarava, N.; Chang, J.C.; Kushwaha, R.; Baskakov, I.V. Region-Specific Response of Astrocytes to Prion Infection. Front. Neurosci. 2019, 13, 1048. [Google Scholar] [CrossRef] [PubMed]
- Shephard, M.T.; Merkhan, M.M.; Forsyth, N.R. Human Mesenchymal Stem Cell Secretome Driven T Cell Immunomodulation Is IL-10 Dependent. Int. J. Mol. Sci. 2022, 23, 13596. [Google Scholar] [CrossRef] [PubMed]
- Kavaldzhieva, K.; Mladenov, N.; Markova, M.; Belemezova, K. Mesenchymal Stem Cell Secretome: Potential Applications in Human Infertility Caused by Hormonal Imbalance, External Damage, or Immune Factors. Biomedicines 2025, 13, 586. [Google Scholar] [CrossRef]
- Aguzzi, A.; Zhu, C. Microglia in prion diseases. J. Clin. Investig. 2017, 127, 3230–3239. [Google Scholar] [CrossRef]
- Wu, C.L.; Chou, Y.H.; Chang, Y.J.; Teng, N.Y.; Hsu, H.L.; Chen, L. Interplay between cell migration and neurite outgrowth determines SH2B1β-enhanced neurite regeneration of differentiated PC12 cells. PLoS ONE 2012, 7, e34999. [Google Scholar] [CrossRef]
- Han, Y.; Yang, J.; Fang, J.; Zhou, Y.; Candi, E.; Wang, J.; Hua, D.; Shao, C.; Shi, Y. The secretion profile of mesenchymal stem cells and potential applications in treating human diseases. Signal Transduct. Target. Ther. 2022, 7, 92. [Google Scholar] [CrossRef]
- Mattei, V.; Delle Monache, S. Mesenchymal Stem Cells and Their Role in Neurodegenerative Diseases. Cells 2024, 13, 779. [Google Scholar] [CrossRef]
- Mahmoud, E.E.; Mawas, A.S.; Mohamed, A.A.; Noby, M.A.; Abdel-Hady, A.A.; Zayed, M. Treatment strategies for meniscal lesions: From past to prospective therapeutics. Regen. Med. 2022, 17, 547–560. [Google Scholar] [CrossRef]
- Zayed, M.; Kim, Y.-C.; Jeong, B.-H. Therapeutic effects of adipose-derived mesenchymal stem cells combined with glymphatic system activation in prion disease. Mol. Neurodegener. 2025, 20, 42. [Google Scholar] [CrossRef]
- Lee, H.C.; An, S.G.; Lee, H.W.; Park, J.S.; Cha, K.S.; Hong, T.J.; Park, J.H.; Lee, S.Y.; Kim, S.P.; Kim, Y.D.; et al. Safety and effect of adipose tissue-derived stem cell implantation in patients with critical limb ischemia: A pilot study. Circ. J. 2012, 76, 1750–1760. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I.; Correa, D. The MSC: An Injury Drugstore. Cell Stem Cell 2011, 9, 11–15. [Google Scholar] [CrossRef]
- Giovannelli, L.; Bari, E.; Jommi, C.; Tartara, F.; Armocida, D.; Garbossa, D.; Cofano, F.; Torre, M.L.; Segale, L. Mesenchymal stem cell secretome and extracellular vesicles for neurodegenerative diseases: Risk-benefit profile and next steps for the market access. Bioact. Mater. 2023, 29, 16–35. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Ito, T.; Inden, M.; Kurita, H.; Yamamoto, A.; Hozumi, I. Stem Cells From Human Exfoliated Deciduous Teeth-Conditioned Medium (SHED-CM) is a Promising Treatment for Amyotrophic Lateral Sclerosis. Front. Pharmacol. 2022, 13, 805379. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, D.; Liu, C.; Ding, T.; Yang, L.; Yin, X.; Zhou, X. Prion protein participates in the protection of mice from lipopolysaccharide infection by regulating the inflammatory process. J. Mol. Neurosci. 2015, 55, 279–287. [Google Scholar] [CrossRef]
- Roberts, T.K.; Eugenin, E.A.; Morgello, S.; Clements, J.E.; Zink, M.C.; Berman, J.W. PrPC, the Cellular Isoform of the Human Prion Protein, Is a Novel Biomarker of HIV-Associated Neurocognitive Impairment and Mediates Neuroinflammation. Am. J. Pathol. 2010, 177, 1848–1860. [Google Scholar] [CrossRef]
- Bakkebø, M.K.; Mouillet-Richard, S.; Espenes, A.; Goldmann, W.; Tatzelt, J.; Tranulis, M.A. The Cellular Prion Protein: A Player in Immunological Quiescence. Front. Immunol. 2015, 6, 450. [Google Scholar] [CrossRef]
- Risen, S.J.; Boland, S.W.; Sharma, S.; Weisman, G.M.; Shirley, P.M.; Latham, A.S.; Hay, A.J.D.; Gilberto, V.S.; Hines, A.D.; Brindley, S.; et al. Targeting Neuroinflammation by Pharmacologic Downregulation of Inflammatory Pathways Is Neuroprotective in Protein Misfolding Disorders. ACS Chem. Neurosci. 2024, 15, 1533–1547. [Google Scholar] [CrossRef]
- Kordek, R.; Nerurkar, V.R.; Liberski, P.P.; Isaacson, S.; Yanagihara, R.; Gajdusek, D.C. Heightened expression of tumor necrosis factor alpha, interleukin 1 alpha, and glial fibrillary acidic protein in experimental Creutzfeldt-Jakob disease in mice. Proc. Natl. Acad. Sci. USA 1996, 93, 9754–9758. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Tang, Y.; Le, W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 1181–1194. [Google Scholar] [CrossRef] [PubMed]
- Kappy, N.S.; Chang, S.; Harris, W.M.; Plastini, M.; Ortiz, T.; Zhang, P.; Hazelton, J.P.; Carpenter, J.P.; Brown, S.A. Human adipose-derived stem cell treatment modulates cellular protection in both in vitro and in vivo traumatic brain injury models. J. Trauma. Acute Care Surg. 2018, 84, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Baez-Jurado, E.; Guio-Vega, G.; Hidalgo-Lanussa, O.; González, J.; Echeverria, V.; Ashraf, G.M.; Sahebkar, A.; Barreto, G.E. Mitochondrial Neuroglobin Is Necessary for Protection Induced by Conditioned Medium from Human Adipose-Derived Mesenchymal Stem Cells in Astrocytic Cells Subjected to Scratch and Metabolic Injury. Mol. Neurobiol. 2019, 56, 5167–5187. [Google Scholar] [CrossRef] [PubMed]
- Gray, F.; Chrétien, F.; Adle-Biassette, H.; Dorandeu, A.; Ereau, T.; Delisle, M.B.; Kopp, N.; Ironside, J.W.; Vital, C. Neuronal apoptosis in Creutzfeldt-Jakob disease. J. Neuropathol. Exp. Neurol. 1999, 58, 321–328. [Google Scholar] [CrossRef]
- Huang, Y.; Mei, X.; Jiang, W.; Zhao, H.; Yan, Z.; Zhang, H.; Liu, Y.; Hu, X.; Zhang, J.; Peng, W.; et al. Mesenchymal Stem Cell-Conditioned Medium Protects Hippocampal Neurons from Radiation Damage by Suppressing Oxidative Stress and Apoptosis. Dose Response 2021, 19, 1559325820984944. [Google Scholar] [CrossRef]
- Li, H.; Yahaya, B.H.; Ng, W.H.; Yusoff, N.M.; Lin, J. Conditioned Medium of Human Menstrual Blood-Derived Endometrial Stem Cells Protects Against MPP(+)-Induced Cytotoxicity in vitro. Front. Mol. Neurosci. 2019, 12, 80. [Google Scholar] [CrossRef]




| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| TNF-α | CCTCTCTCTAATCAGCCCTCTG | GAGGACCTGGGAGTAGATGAG |
| IL-1β | ATGATGGCTTATTACAGTGGCAA | GTCGGAGATTCGTAGCTGGA |
| IL-10 | ACCTGCCTAACATGCTTCGAG | CTGGGTCTTGGTTCTCAGCTT |
| IDO | CAAAGGTCATGGAGATGTCC | CCACCAATAGAGAGACCAGG |
| ACTB | TGGCACCCAGCACAATGAA | CTAAGTCATAGTCCGCCTAGAAGCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zayed, M.; Jeong, B.-H. Mesenchymal Stem Cell Secretome Attenuates PrP106-126-Induced Neurotoxicity by Suppressing Neuroinflammation and Apoptosis and Enhances Cell Migration. Cells 2025, 14, 851. https://doi.org/10.3390/cells14110851
Zayed M, Jeong B-H. Mesenchymal Stem Cell Secretome Attenuates PrP106-126-Induced Neurotoxicity by Suppressing Neuroinflammation and Apoptosis and Enhances Cell Migration. Cells. 2025; 14(11):851. https://doi.org/10.3390/cells14110851
Chicago/Turabian StyleZayed, Mohammed, and Byung-Hoon Jeong. 2025. "Mesenchymal Stem Cell Secretome Attenuates PrP106-126-Induced Neurotoxicity by Suppressing Neuroinflammation and Apoptosis and Enhances Cell Migration" Cells 14, no. 11: 851. https://doi.org/10.3390/cells14110851
APA StyleZayed, M., & Jeong, B.-H. (2025). Mesenchymal Stem Cell Secretome Attenuates PrP106-126-Induced Neurotoxicity by Suppressing Neuroinflammation and Apoptosis and Enhances Cell Migration. Cells, 14(11), 851. https://doi.org/10.3390/cells14110851









