The Potential of Red Blood Cells in Regenerative Medicine: A Paradigm Shift in Cellular Therapy
Abstract
:1. Introduction
| Cell Type | Primary Function | Regenerative Properties | Potential Applications | References |
|---|---|---|---|---|
| Red Blood Cells (RBCs) | Oxygen Transport | Extracellular vesicles (EVs), immunomodulation, oxygenation | Emerging translational research for regenerative uses | [11] |
| Mesenchymal Stem Cells (MSCs) | Tissue Regeneration | Differentiation into various cell types, paracrine signaling | Widely used in orthopedic, cardiac, and neurological treatments | [12] |
| Platelets | Clot Formation & Healing | Growth factor release, immune modulation | PRP widely used in wound healing, orthopedics | [13] |
| Macrophages | Immune Regulation | Phagocytosis, cytokine secretion, tissue repair | Experimental therapies in chronic inflammation, tissue healing | [12] |
2. Biological Functions of Red Blood Cells in Regenerative Medicine
3. Current Use of Orthobiologic Formulations in Joint Disorders
3.1. PRP and RBC Exclusion
3.2. BMAC and RBCs
4. Potential Benefits of RBCs in Orthobiologic Therapies
4.1. Angiogenesis and Vascular Homeostasis
4.2. Oxidative Stress Regulation and Cytoprotective Pathways
4.3. RBC-Derived Extracellular Vesicles in Cartilage and Tendon Healing
5. Challenges, Controversies, and Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, S.; Rahaman, K.A.; Kim, Y.-C.; Jeon, H.; Han, H.-S. Fostering tissue engineering and regenerative medicine to treat musculoskeletal disorders in bone and muscle. Bioact. Mater. 2024, 40, 345–365. [Google Scholar] [CrossRef] [PubMed]
- Balusani, P.; Shrivastava, S.; Pundkar, A.; Kale, P. Navigating the Therapeutic Landscape: A Comprehensive Review of Platelet-Rich Plasma and Bone Marrow Aspirate Concentrate in Knee Osteoarthritis. Cureus 2024, 16, e54747. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.R.; Pires, L.; Martins, R.A.; Santos, M.; Santos, G.S.; Lana, J.V.; Costa, B.R.; Santos, N.; de Macedo, A.P.; Kruel, A.; et al. Orthobiologics Revisited: A Concise Perspective on Regenerative Orthopedics. Curr. Issues Mol. Biol. 2025, 47, 247. [Google Scholar] [CrossRef]
- Mohanty, J.; Nagababu, E.; Rifkind, J.M. Red blood cell oxidative stress impairs oxygen delivery and induces red blood cell aging. Front. Physiol. 2014, 5, 84. [Google Scholar] [CrossRef]
- Eigenschink, M.; Savran, D.; Zitterer, C.P.; Granitzer, S.; Fritz, M.; Baron, D.M.; Müllner, E.W.; Salzer, U. Redox Properties of Human Erythrocytes Are Adapted for Vitamin C Recycling. Front. Physiol. 2021, 12, 767439. [Google Scholar] [CrossRef]
- Weisel, J.W.; Litvinov, R.I. Red blood cells: The forgotten player in hemostasis and thrombosis. J. Thromb. Haemost. 2019, 17, 271–282. [Google Scholar] [CrossRef]
- Dobkin, J.; Mangalmurti, N.S. Immunomodulatory Roles of Red Blood Cells. Curr. Opin. Hematol. 2022, 29, 306–309. [Google Scholar] [CrossRef]
- Anderson, H.L.; Brodsky, I.E.; Mangalmurti, N.S. The evolving erythrocyte: RBCs as modulators of innate immunity. J. Immunol. 2018, 201, 1343–1351. [Google Scholar] [CrossRef]
- Braun, H.J.; Kim, H.J.; Chu, C.R.; Dragoo, J.L. The Effect of Platelet-Rich Plasma Formulations and Blood Products on Human Synoviocytes. Am. J. Sports Med. 2014, 42, 1204–1210. [Google Scholar] [CrossRef]
- Gupta, A.; Maffulli, N.; Jain, V.K. Red Blood Cells in Platelet-Rich Plasma: Avoid If at All Possible. Biomedicines 2023, 11, 2425. [Google Scholar] [CrossRef]
- Thangaraju, K.; Neerukonda, S.N.; Katneni, U.; Buehler, P.W. Extracellular Vesicles from Red Blood Cells and Their Evolving Roles in Health, Coagulopathy and Therapy. Int. J. Mol. Sci. 2020, 22, 153. [Google Scholar] [CrossRef] [PubMed]
- Hoang, D.M.; Pham, P.T.; Bach, T.Q.; Ngo, A.T.L.; Nguyen, Q.T.; Phan, T.T.K.; Nguyen, G.H.; Le, P.T.T.; Hoang, V.T.; Forsyth, N.R.; et al. Stem cell-based therapy for human diseases. Signal Transduct. Target. Ther. 2022, 7, 272. [Google Scholar] [CrossRef] [PubMed]
- Middleton, K.K.; Barro, V.; Muller, B.; Terada, S.; Fu, F.H. Evaluation of the effects of platelet-rich plasma (PRP) therapy involved in the healing of sports-related soft tissue injuries. Iowa Orthop. J. 2012, 32, 150. [Google Scholar]
- Rifkind, J.M.; Mohanty, J.G.; Nagababu, E. The pathophysiology of extracellular hemoglobin associated with enhanced oxidative reactions. Front. Physiol. 2015, 5, 500. [Google Scholar] [CrossRef]
- dos Santos, R.G.; Santos, G.S.; Alkass, N.; Chiesa, T.L.; Azzini, G.O.; da Fonseca, L.F.; dos Santos, A.F.; Rodrigues, B.L.; Mosaner, T.; Lana, J.F. The regenerative mechanisms of platelet-rich plasma: A review. Cytokine 2021, 144, 155560. [Google Scholar] [CrossRef]
- Ma, S.-R.; Xia, H.-F.; Gong, P.; Yu, Z.-L. Red Blood Cell-Derived Extracellular Vesicles: An Overview of Current Research Progress, Challenges, and Opportunities. Biomedicines 2023, 11, 2798. [Google Scholar] [CrossRef]
- Cortese-Krott, M.M.; Kelm, M. Endothelial nitric oxide synthase in red blood cells: Key to a new erythrocrine function? Redox Biol. 2014, 2, 251–258. [Google Scholar] [CrossRef]
- LoBue, A.; Heuser, S.K.; Lindemann, M.; Li, J.; Rahman, M.; Kelm, M.; Stegbauer, J.; Cortese-Krott, M.M. Red blood cell endothelial nitric oxide synthase: A major player in regulating cardiovascular health. Br. J. Pharmacol. 2023; Online ahead of print. [Google Scholar] [CrossRef]
- Chiangjong, W.; Netsirisawan, P.; Hongeng, S.; Chutipongtanate, S. Red Blood Cell Extracellular Vesicle-Based Drug Delivery: Challenges and Opportunities. Front. Med. 2021, 8, 761362. [Google Scholar] [CrossRef]
- Pham, T.T.; Le, A.H.; Dang, C.P.; Chong, S.Y.; Do, D.V.; Peng, B.; Jayasinghe, M.K.; Ong, H.B.; Hoang, D.V.; Louise, R.A.; et al. Endocytosis of red blood cell extracellular vesicles by macrophages leads to cytoplasmic heme release and prevents foam cell formation in atherosclerosis. J. Extracell. Vesicles 2023, 12, e12354. [Google Scholar] [CrossRef]
- Yang, L.; Huang, S.; Zhang, Z.; Liu, Z.; Zhang, L. Roles and Applications of Red Blood Cell-Derived Extracellular Vesicles in Health and Diseases. Int. J. Mol. Sci. 2022, 23, 5927. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.-J.; Xu, J.-M.; Wu, M.; Yue, K.; Cui, Y.-H.; Bai, Y.; You, L.-W.; Guo, J.-R. Exploration of the mechanism of reinfusion of fresh autologous blood in type 2 diabetes mice to induce macrophage polarization and inhibit erythrocyte damage. J. Diabetes Investig. 2024, 15, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Buttari, B.; Profumo, E.; Riganò, R. Crosstalk between Red Blood Cells and the Immune System and Its Impact on Atherosclerosis. Biomed. Res. Int. 2015, 2015, 616834. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Yang, M.; Zheng, H.; Cai, Y.; Luo, P.; Wang, X.; Xu, P. M2 Macrophage-Derived Extracellular Vesicles Encapsulated in Hyaluronic Acid Alleviate Osteoarthritis by Modulating Macrophage Polarization. ACS Biomater. Sci. Eng. 2024, 10, 3355–3377. [Google Scholar] [CrossRef]
- Wang, J.; Yu, C.; Zhuang, J.; Qi, W.; Jiang, J.; Liu, X.; Zhao, W.; Cao, Y.; Wu, H.; Qi, J.; et al. The role of phosphatidylserine on the membrane in immunity and blood coagulation. Biomark. Res. 2022, 10, 4. [Google Scholar] [CrossRef]
- Ryter, S.W. Heme Oxygenase-1: An Anti-Inflammatory Effector in Cardiovascular, Lung, and Related Metabolic Disorders. Antioxidants 2022, 11, 555. [Google Scholar] [CrossRef]
- Yamaguchi, F.S.M.; Shams, S.; Silva, E.A.; Stilhano, R.S. PRP and BMAC for musculoskeletal conditions via biomaterial carriers. Int. J. Mol. Sci. 2019, 20, 5328. [Google Scholar] [CrossRef]
- Lana, J.F.S.D.; da Fonseca, L.F.; Macedo, R.D.R.; Mosaner, T.; Murrell, W.; Kumar, A.; Purita, J.; de sAndrade, M.A.P. Platelet-rich plasma vs bone marrow aspirate concentrate: An overview of mechanisms of action and orthobiologic synergistic effects. World J. Stem Cells 2021, 13, 155–167. [Google Scholar] [CrossRef]
- Ren, Y.; Yan, C.; Yang, H. Erythrocytes: Member of the immune system that should not be ignored. Crit. Rev. Oncol./Hematol. 2023, 187, 104039. [Google Scholar] [CrossRef] [PubMed]
- Sunwoo, J.Y.; Eliasberg, C.D.; Carballo, C.B.; Rodeo, S.A. The role of the macrophage in tendinopathy and tendon healing. J. Orthop. Res. 2020, 38, 1666–1675. [Google Scholar] [CrossRef]
- Li, M.; Yin, H.; Yan, Z.; Li, H.; Wu, J.; Wang, Y.; Wei, F.; Tian, G.; Ning, C.; Li, H.; et al. The immune microenvironment in cartilage injury and repair. Acta Biomater. 2022, 140, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-Z.; Zhang, Z.-W.; Wang, H.; Chen, Y.-Q.; Zhou, X.-F.; Duan, L.-S.; Wang, X.-X.; Xu, F.; Guo, J.-R. Autologous transfusion of “old” red blood cells-induced M2 macrophage polarization through IL-10-Nrf2-HO-1 signaling complexes. Adv. Clin. Exp. Med. 2020, 29, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Naeini, M.B.; Bianconi, V.; Pirro, M.; Sahebkar, A. The role of phosphatidylserine recognition receptors in multiple biological functions. Cell. Mol. Biol. Lett. 2020, 25, 23. [Google Scholar] [CrossRef]
- Horuk, R. The Duffy Antigen Receptor for Chemokines DARC/ACKR1. Front. Immunol. 2015, 6, 279. [Google Scholar] [CrossRef]
- Novitzky-Basso, I.; Rot, A. Duffy antigen receptor for chemokines and its involvement in patterning and control of inflammatory chemokines. Front. Immunol. 2012, 3, 266. [Google Scholar] [CrossRef]
- Peng, B.; Yang, Y.; Wu, Z.; Tan, R.; Pham, T.T.; Yeo, E.Y.M.; Pirisinu, M.; Jayasinghe, M.K.; Pham, T.C.; Liang, K.; et al. Red blood cell extracellular vesicles deliver therapeutic siRNAs to skeletal muscles for treatment of cancer cachexia. Mol. Ther. 2023, 31, 1418–1436. [Google Scholar] [CrossRef]
- Xu, L.; Xu, X.; Liang, Y.; Wen, C.; Ouyang, K.; Huang, J.; Xiao, Y.; Deng, X.; Xia, J.; Duan, L. Osteoclast-targeted delivery of anti-miRNA oligonucleotides by red blood cell extracellular vesicles. J. Control. Release 2023, 358, 259–272. [Google Scholar] [CrossRef]
- Ateeq, M.; Broadwin, M.; Sellke, F.W.; Abid, M.R. Extracellular Vesicles’ Role in Angiogenesis and Altering Angiogenic Signaling. Med. Sci. 2024, 12, 4. [Google Scholar] [CrossRef]
- Impieri, L.; Pezzi, A.; Hadad, H.; Peretti, G.M.; Mangiavini, L.; Rossi, N. Orthobiologics in delayed union and non-union of adult long bones fractures: A systematic review. Bone Rep. 2024, 21, 101760. [Google Scholar] [CrossRef]
- Lana, J.F.S.D.; Lana, A.V.S.D.; da Fonseca, L.F.; Coelho, M.A.; Marques, G.G.; Mosaner, T.; Ribeiro, L.L.; Azzini, G.O.M.; Santos, G.S.; Fonseca, E.; et al. Stromal Vascular Fraction for Knee Osteoarthritis—An Update. J. Stem Cells Regen. Med. 2022, 18, 11–20. [Google Scholar] [CrossRef]
- Lana, J.F.; Macedo, A.; Ingrao, I.L.G.; Huber, S.C.; Santos, G.S.; Santana, M.H.A. Leukocyte-rich PRP for knee osteoarthritis: Current concepts. J. Clin. Orthop. Trauma. 2019, 10, S179–S182. [Google Scholar] [CrossRef] [PubMed]
- Lana, J.F.; da Fonseca, L.F.; Azzini, G.; Santos, G.; Braga, M.; Cardoso Junior, A.M.; Murrell, W.D.; Gobbi, A.; Purita, J.; de Andrade, M.A.P. Bone marrow aspirate matrix: A convenient ally in regenerative medicine. Int. J. Mol. Sci. 2021, 22, 2762. [Google Scholar] [CrossRef] [PubMed]
- Lana, J.F.; Purita, J.; Everts, P.A.; De Mendonça Neto, P.A.T.; de Moraes Ferreira Jorge, D.; Mosaner, T.; Huber, S.C.; Azzini, G.O.M.; da Fonseca, L.F.; Jeyaraman, M.; et al. Platelet-Rich Plasma Power-Mix Gel (ppm)-An Orthobiologic Optimization Protocol Rich in Growth Factors and Fibrin. Gels 2023, 9, 553. [Google Scholar] [CrossRef]
- Domingues, R.B.; von Rautenfeld, M.; Kavalco, C.M.; Caliari, C.; Dellagiustina, C.; da Fonseca, L.F.; Costa, F.R.; da Cruz Silva Reis, A.; Santos, G.S.; Azzini, G.; et al. The role of orthobiologics in chronic wound healing. Int. Wound J. 2024, 21, e14854. [Google Scholar] [CrossRef]
- Lana, J.F.; Huber, S.C.; Purita, J.; Tambeli, C.H.; Santos, G.S.; Paulus, C.; Annichino-Bizzacchi, J.M. Leukocyte-rich PRP versus leukocyte-poor PRP—The role of monocyte/macrophage function in the healing cascade. J. Clin. Orthop. Trauma. 2019, 10, S7–S12. [Google Scholar] [CrossRef]
- Lana, J.F.S.D.; Purita, J.; Paulus, C.; Huber, S.C.; Rodrigues, B.L.; Rodrigues, A.A.; Santana, M.H.; Madureira, J.L.; Luzo, Â.C.M.; Belangero, W.D.; et al. Contributions for Classification of Platelet Rich Plasma—Proposal of a New Classification: MARSPILL. Regen. Med. 2017, 12, 565–574. [Google Scholar] [CrossRef]
- Obeagu, E.I.; Igwe, M.C.; Obeagu, G.U. Oxidative stress’s impact on red blood cells: Unveiling implications for health and disease. Medicine 2024, 103, e37360. [Google Scholar] [CrossRef]
- Kim, G.B.; Seo, M.-S.; Park, W.T.; Lee, G.W. Bone Marrow Aspirate Concentrate: Its Uses in Osteoarthritis. Int. J. Mol. Sci. 2020, 21, 3224. [Google Scholar] [CrossRef]
- Gianakos, A.L.; Sun, L.; Patel, J.N.; Adams, D.M.; Liporace, F.A. Clinical application of concentrated bone marrow aspirate in orthopaedics: A systematic review. World J. Orthop. 2017, 8, 491–506. [Google Scholar] [CrossRef]
- Purita, J.; Lana, J.F.S.D.; Kolber, M.; Rodrigues, B.L.; Mosaner, T.; Santos, G.S.; Caliari-Oliveira, C.; Huber, S.C. Bone marrow-derived products: A classification proposal—Bone marrow aspirate, bone marrow aspirate concentrate or hybrid? World J. Stem Cells 2020, 12, 241–250. [Google Scholar] [CrossRef]
- Lee, A.J.; Gangi, L.R.; Zandkarimi, F.; Stockwell, B.R.; Hung, C.T. Red blood cell exposure increases chondrocyte susceptibility to oxidative stress following hemarthrosis. Osteoarthr. Cartil. 2023, 31, 1365–1376. [Google Scholar] [CrossRef] [PubMed]
- Dregalla, R.C.; Herrera, J.A.; Donner, E.J. Red blood cells and their releasates compromise bone marrow-derived human mesenchymal stem/stromal cell survival in vitro. Stem Cell Res. Ther. 2021, 12, 547. [Google Scholar] [CrossRef] [PubMed]
- Kou, M.; Huang, L.; Yang, J.; Chiang, Z.; Chen, S.; Liu, J.; Guo, L.; Zhang, X.; Zhou, X.; Xu, X.; et al. Mesenchymal stem cell-derived extracellular vesicles for immunomodulation and regeneration: A next generation therapeutic tool? Cell Death Dis. 2022, 13, 580. [Google Scholar] [CrossRef]
- Chen, B.; Hong, H.; Sun, Y.; Chen, C.; Wu, C.; Xu, G.; Bao, G.; Cui, Z. Role of macrophage polarization in osteoarthritis (Review). Exp. Ther. Med. 2022, 24, 757. [Google Scholar] [CrossRef]
- Karsten, E.; Breen, E.; Herbert, B.R. Red blood cells are dynamic reservoirs of cytokines. Sci. Rep. 2018, 8, 3101. [Google Scholar] [CrossRef]
- Terkawi, M.A.; Ebata, T.; Yokota, S.; Takahashi, D.; Endo, T.; Matsumae, G.; Shimizu, T.; Kadoya, K.; Iwasaki, N. Low-Grade Inflammation in the Pathogenesis of Osteoarthritis: Cellular and Molecular Mechanisms and Strategies for Future Therapeutic Intervention. Biomedicines 2022, 10, 1109. [Google Scholar] [CrossRef]
- Yeom, M.; Hahm, D.-H.; Sur, B.-J.; Han, J.-J.; Lee, H.-J.; Yang, H.-I.; Kim, K.S. Phosphatidylserine inhibits inflammatory responses in interleukin-1β-stimulated fibroblast-like synoviocytes and alleviates carrageenan-induced arthritis in rat. Nutr. Res. 2013, 33, 242–250. [Google Scholar] [CrossRef]
- Loi, F.; Córdova, L.A.; Pajarinen, J.; Lin, T.; Yao, Z.; Goodman, S.B. Inflammation, Fracture and Bone Repair. Bone 2016, 86, 119–130. [Google Scholar] [CrossRef]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef]
- Jang, H.-J.; Yoon, J.-K. The Role of Vasculature and Angiogenic Strategies in Bone Regeneration. Biomimetics 2024, 9, 75. [Google Scholar] [CrossRef]
- Ramires, L.C.; Jeyaraman, M.; Muthu, S.; Shankar, A.N.; Santos, G.S.; da Fonseca, L.F.; Lana, J.F.; Rajendran, R.L.; Gangadaran, P.; Jogalekar, M.P.; et al. Application of Orthobiologics in Achilles Tendinopathy: A Review. Life 2022, 12, 399. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-R.; Lin, C.-N.; Lee, C.-C.; Chen, Y.-C.; Chen, Y.-J.; Chen, M.-H.; Lin, Y.-C.; Chang, S.-H. Effects of Intra-Articular Stromal Vascular Fraction Injection on Clinical Symptoms and Cartilage Health in Osteoarthritic Knees: A Single-Center Pilot Study. Life 2024, 14, 1468. [Google Scholar] [CrossRef]
- Kuhn, V.; Diederich, L.; Keller, T.C.S.; Kramer, C.M.; Lückstädt, W.; Panknin, C.; Suvorava, T.; Isakson, B.E.; Kelm, M.; Cortese-Krott, M.M. Red Blood Cell Function and Dysfunction: Redox Regulation, Nitric Oxide Metabolism, Anemia. Antioxid. Redox Signal 2017, 26, 718–742. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, Y.; Xin, J.; Zhang, Y.; Yang, K.; Luo, Y.; Wang, B. Red blood cells in biology and translational medicine: Natural vehicle inspires new biomedical applications. Theranostics 2024, 14, 220–248. [Google Scholar] [CrossRef]
- Todorova, D.; Simoncini, S.; Lacroix, R.; Sabatier, F.; Dignat-George, F. Extracellular Vesicles in Angiogenesis. Circ. Res. 2017, 120, 1658–1673. [Google Scholar] [CrossRef]
- Dennery, P.A. Signaling Function of Heme Oxygenase Proteins. Antioxid. Redox Signal 2014, 20, 1743–1753. [Google Scholar] [CrossRef]
- Daraghmeh, D.N.; Karaman, R. The Redox Process in Red Blood Cells: Balancing Oxidants and Antioxidants. Antioxidants 2025, 14, 36. [Google Scholar] [CrossRef]
- Setti, T.; Arab, M.G.L.; Santos, G.S.; Alkass, N.; Andrade, M.A.P.; Lana, J.F.S.D. The protective role of glutathione in osteoarthritis. J. Clin. Orthop. Trauma. 2020, 15, 145–151. [Google Scholar] [CrossRef]
- Jung, H.; Jung, Y.; Seo, J.; Bae, Y.; Kim, H.-S.; Jeong, W. Roles of extracellular vesicles from mesenchymal stem cells in regeneration. Mol. Cells 2024, 47, 100151. [Google Scholar] [CrossRef]
- Amorim, C.S.; Moraes, J.A.; Magdalena, I.J.; López, S.G.; Carneiro, A.C.D.; da Nunes, I.K.C.; Pizzatti, L.; Sardela, V.F.; Aquino Neto, F.R.; Mirotti, L.C.; et al. Extracellular Vesicles from Stored Red Blood Cells Convey Heme and Induce Spic Expression on Human Monocytes. Front. Immunol. 2022, 13, 833286. [Google Scholar] [CrossRef]
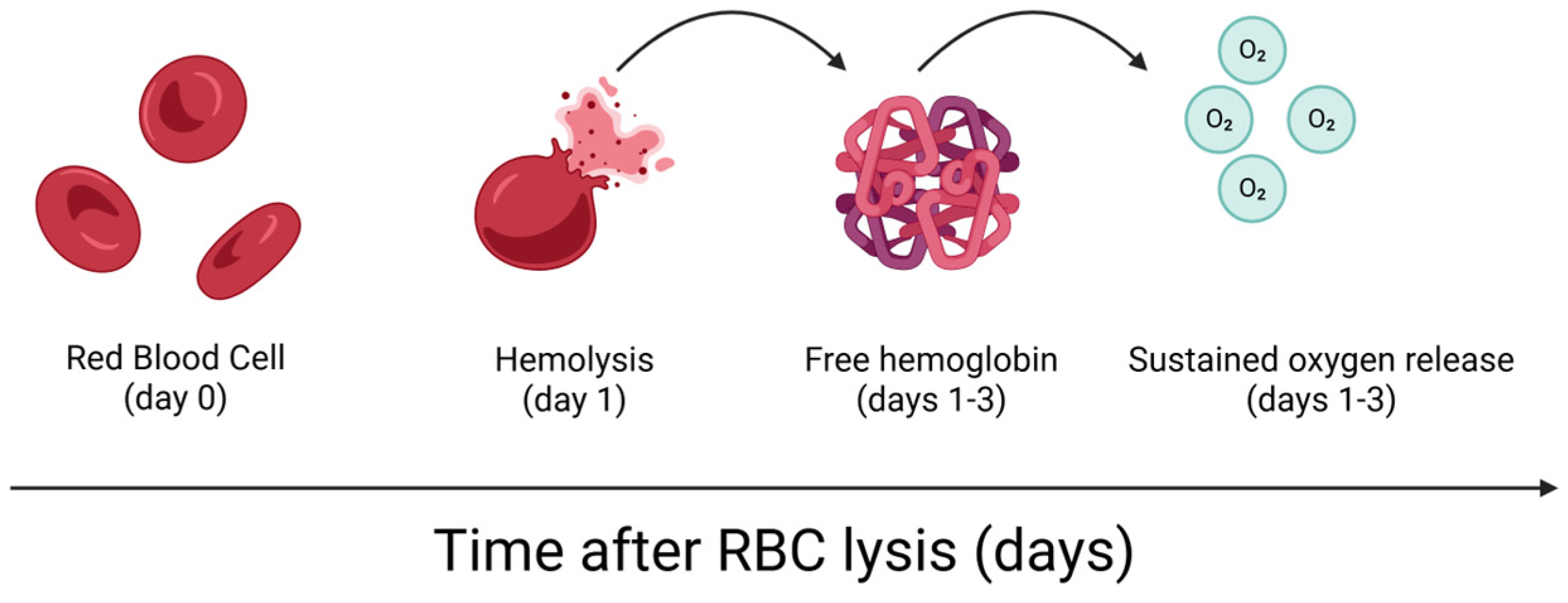
- Everts, P.A.; Malanga, G.A.; Paul, R.V.; Rothenberg, J.B.; Stephens, N.; Mautner, K.R. Assessing clinical implications and perspectives of the pathophysiological effects of erythrocytes and plasma free hemoglobin in autologous biologics for use in musculoskeletal regenerative medicine therapies. A review. Regen. Ther. 2019, 11, 56–64. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, F.R.; Purita, J.; Mahmood, A.; Martins, R.; Costa, B.; Rodrigues, B.L.; Huber, S.C.; Santos, G.S.; Pires, L.; Azzini, G.; et al. The Potential of Red Blood Cells in Regenerative Medicine: A Paradigm Shift in Cellular Therapy. Cells 2025, 14, 797. https://doi.org/10.3390/cells14110797
Costa FR, Purita J, Mahmood A, Martins R, Costa B, Rodrigues BL, Huber SC, Santos GS, Pires L, Azzini G, et al. The Potential of Red Blood Cells in Regenerative Medicine: A Paradigm Shift in Cellular Therapy. Cells. 2025; 14(11):797. https://doi.org/10.3390/cells14110797
Chicago/Turabian StyleCosta, Fábio Ramos, Joseph Purita, Ansar Mahmood, Rubens Martins, Bruno Costa, Bruno Lima Rodrigues, Stephany Cares Huber, Gabriel Silva Santos, Luyddy Pires, Gabriel Azzini, and et al. 2025. "The Potential of Red Blood Cells in Regenerative Medicine: A Paradigm Shift in Cellular Therapy" Cells 14, no. 11: 797. https://doi.org/10.3390/cells14110797
APA StyleCosta, F. R., Purita, J., Mahmood, A., Martins, R., Costa, B., Rodrigues, B. L., Huber, S. C., Santos, G. S., Pires, L., Azzini, G., Kruel, A., & Lana, J. F. (2025). The Potential of Red Blood Cells in Regenerative Medicine: A Paradigm Shift in Cellular Therapy. Cells, 14(11), 797. https://doi.org/10.3390/cells14110797








