Neutrophils at the Crossroads of Inflammatory Bowel Disease and Atherosclerosis: A State-of-the-Art Review
Abstract
1. Inflammatory Bowel Disease (IBD) and Atherosclerosis: “Real-World” Data and Clinical Trial Results
2. The Role of Neutrophils in Atherogenesis: From Associations to Causality
3. The Role of Neutrophils in the Pathogenesis of IBD
4. Neutrophils as Key Drivers of Atherosclerosis in Patients with IBD
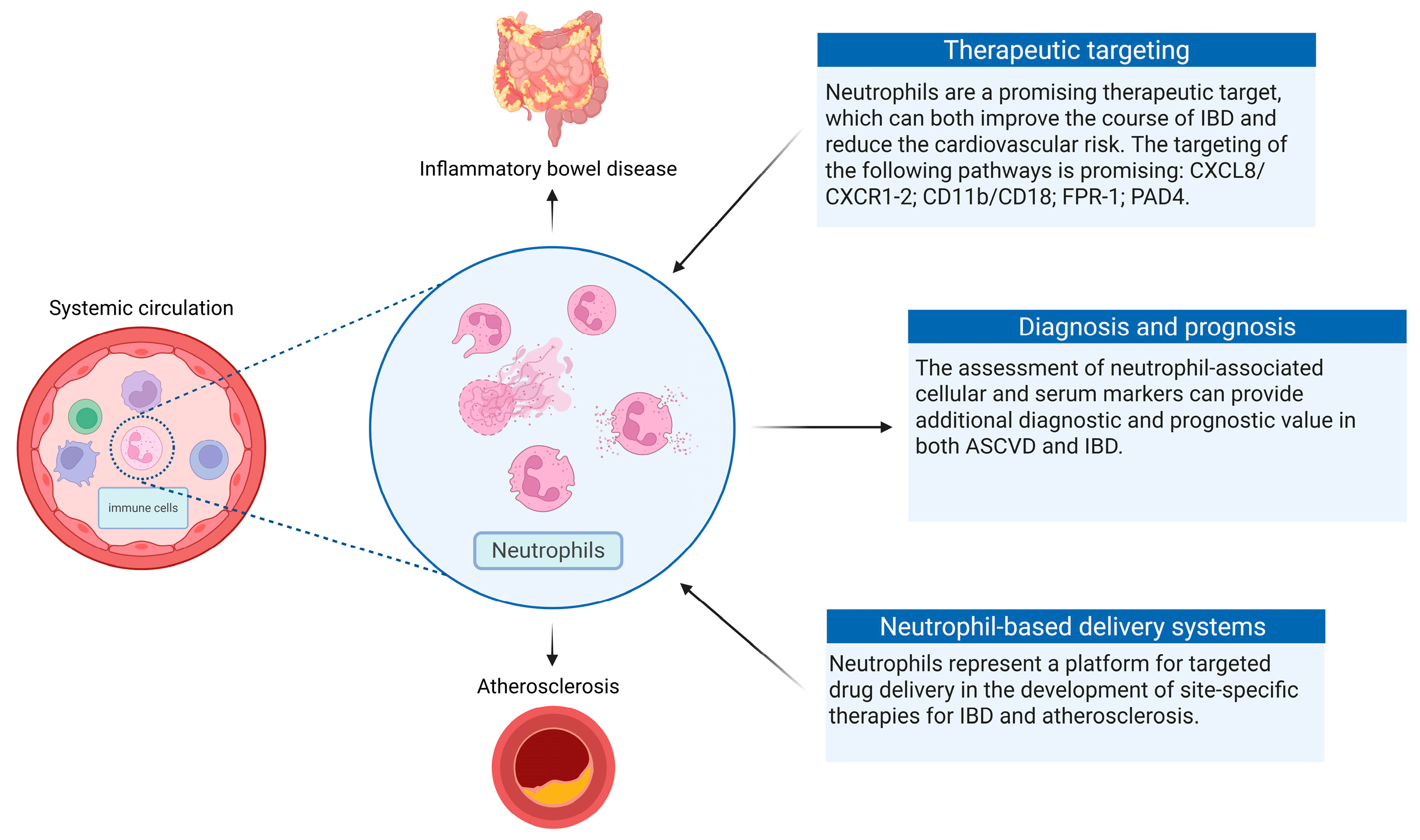
5. Clinical Implications
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, J.L.; Bao, J.C.; Liao, X.Y.; Chen, Y.J.; Wang, L.W.; Fan, Y.Y.; Xu, Q.Y.; Hao, L.X.; Li, K.J.; Liang, M.X.; et al. Trends and projections of inflammatory bowel disease at the global, regional and national levels, 1990–2050: A Bayesian age-period-cohort modeling study. BMC Public Health 2023, 23, 2507. [Google Scholar] [CrossRef] [PubMed]
- Forbes, A.J.; Frampton, C.M.A.; Day, A.S.; Kaplan, G.G.; Gearry, R.B. The epidemiology of inflammatory bowel disease in Oceania: A systematic review and meta-analysis of incidence and prevalence. Inflamm. Bowel. Dis. 2023, 30, izad295. [Google Scholar] [CrossRef]
- Wang, S.; Dong, Z.; Wan, X. Global, regional, and national burden of inflammatory bowel disease and its associated anemia, 1990 to 2019 and predictions to 2050: An analysis of the global burden of disease study 2019. Autoimmun. Rev. 2023, 23, 103498. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.M.; Lin, Z.L.; He, B.X.; Yan, W.T.; Zhang, X.Y.; Zhang, Z.H.; Wang, L.; Wang, J.Q.; Liu, D.M.; Zhang, W.; et al. Epidemiological analysis reveals a surge in inflammatory bowel disease among children and adolescents: A global, regional, and national perspective from 1990 to 2019–insights from the China study. J. Glob. Health 2023, 13, 04174. [Google Scholar] [CrossRef]
- Green, Z.; Ashton, J.J.; Rodrigues, A.; Spray, C.; Howarth, L.; Mallikarjuna, A.; Chanchlani, N.; Hart, J.; Bakewell, C.; Lee, K.Y.; et al. Sustained increase in pediatric inflammatory bowel disease incidence across the South West United Kingdom over the last 10 years. Inflamm. Bowel Dis. 2024, 19, izad302. [Google Scholar] [CrossRef]
- Cao, L.; Dayimu, A.; Guan, X.; Duan, M.; Zeng, S.; Wang, H.; Zong, J.; Sun, C.; Yang, X.; Yang, X. Global evolving patterns and cross-country inequalities of inflammatory bowel disease burden from 1990 to 2019: A worldwide report. Inflamm. Res. 2024, 73, 277–287. [Google Scholar] [CrossRef]
- Kichloo, A.; El-Amir, Z.; Dahiya, D.S.; Wani, F.; Shaka, H. Trends in hospitalizations and mortality for inflammatory bowel disease from a nationwide database study between 2008 and 2018. Bayl. Univ. Med Cent. Proc. 2021, 34, 550–554. [Google Scholar] [CrossRef]
- Chen, X.; Xiang, X.; Xia, W.; Li, X.; Wang, S.; Ye, S.; Tian, L.; Zhao, L.; Ai, F.; Shen, Z.; et al. Evolving trends and burden of inflammatory bowel disease in Asia, 1990–2019: A comprehensive analysis based on the Global Burden of Disease Study. J. Epidemiol. Glob. Health 2023, 13, 725–739. [Google Scholar] [CrossRef]
- Dupont-Lucas, C.; Leroyer, A.; Ley, D.; Spyckerelle, C.; Bertrand, V.; Turck, D.; Savoye, G.; Maunoury, V.; Guillon, N.; Fumery, M.; et al. Increased risk of cancer and mortality in a large French population-based paediatric-onset inflammatory bowel disease retrospective cohort. J. Crohns Colitis 2023, 17, 524–534. [Google Scholar] [CrossRef]
- Follin-Arbelet, B.; Cvancarova Småstuen, M.; Hovde, Ø.; Jelsness-Jørgensen, L.P.; Moum, B. Mortality in patients with inflammatory bowel disease: Results from 30 years of follow-up in a Norwegian inception cohort (the IBSEN study). J. Crohns Colitis 2023, 17, 497–503. [Google Scholar] [CrossRef]
- Shah, N.N.; Wass, S.; Hajjari, J.; Heisler, A.C.; Malakooti, S.; Janus, S.E.; Al-Kindi, S.G. Proportionate cardiovascular mortality in chronic inflammatory disease in adults in the United States from 1999 to 2019. J. Clin. Rheumatol. 2022, 28, 97–103. [Google Scholar] [CrossRef]
- Jaiswal, V.; Batra, N.; Dagar, M.; Butey, S.; Huang, H.; Chia, J.E.; Naz, S.; Endurance, E.O.; Raj, N.; Patel, S.; et al. Inflammatory bowel disease and associated cardiovascular disease outcomes: A systematic review. Medicine 2023, 102, e32775. [Google Scholar] [CrossRef]
- Groenendyk, J.W.; Rivera, A.S.; Sinha, A.; Lloyd-Jones, D.M.; Feinstein, M.J. Changes in proportionate cardiovascular mortality in patients with chronic infectious and inflammatory conditions in the United States, 1999–2018. Sci. Rep. 2021, 11, 23985. [Google Scholar] [CrossRef] [PubMed]
- Sleutjes, J.A.M.; van Lennep, J.E.R.; van der Woude, C.J.; de Vries, A.C. Thromboembolic and atherosclerotic cardiovascular events in inflammatory bowel disease: Epidemiology, pathogenesis, and clinical management. Ther. Adv. Gastroenterol. 2021, 14, 17562848211032126. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.H.; Tian, F. Inflammatory bowel disease and cardiovascular disease incidence and mortality: A meta-analysis. Eur. J. Prev. Cardiol. 2018, 25, 1623–1631. [Google Scholar] [CrossRef] [PubMed]
- Nasir, K.; Acquah, I.; Dey, A.K.; Agrawal, T.; Hassan, S.Z.; Glassner, K.; Abraham, B.; Quigley, E.M.M.; Blankstein, R.; Virani, S.S.; et al. Inflammatory bowel disease and atherosclerotic cardiovascular disease in U.S. adults—A population-level analysis in the National Health Interview Survey. Am. J. Prev. Cardiol. 2022, 9, 100316. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.T.; Mahtta, D.; Chen, L.; Hussain, A.; Al Rifai, M.; Sinh, P.; Khalid, U.; Nasir, K.; Ballantyne, C.M.; Petersen, L.A.; et al. Premature atherosclerotic cardiovascular disease risk among patients with inflammatory bowel disease. Am. J. Med. 2021, 134, 1047–1051.e2. [Google Scholar] [CrossRef]
- Alayo, Q.A.; Loftus, E.V., Jr.; Yarur, A.; Alvarado, D.; Ciorba, M.A.; de Las Fuentes, L.; Deepak, P. Inflammatory bowel disease is associated with an increased risk of incident acute arterial events: Analysis of the United Kingdom Biobank. Clin. Gastroenterol. Hepatol. 2023, 21, 761–770.e13. [Google Scholar] [CrossRef]
- Fang, L.; Gao, H.; Gao, X.; Wu, W.; Miao, Y.; Zhang, H.; Guleng, B.; Zhang, H.; Wang, Y.; Li, M.; et al. Risks of cardiovascular events in patients with inflammatory bowel disease in China: A retrospective multicenter cohort study. Inflamm. Bowel Dis. 2022, 28 (Suppl. 2), S52–S58. [Google Scholar] [CrossRef]
- Livzan, M.A.; Bikbavova, G.R.; Lisyutenko, N.S.; Romanyuk, A.E.; Drapkina, O.M. Cardiovascular Risk in Patients with Inflammatory Bowel Diseases-The Role of Endothelial Dysfunction. Diagnostics 2024, 14, 1722. [Google Scholar] [CrossRef]
- Sleutjes, J.A.M.; Roeters van Lennep, J.E.; Verploegh, P.J.P.; van Doorn, M.B.A.; Vis, M.; Kavousi, M.; van der Woude, C.J.; de Vries, A.C. Prevalence of ideal cardiovascular health and its correlates in patients with inflammatory bowel disease, psoriasis and spondyloarthropathy. Eur. J. Prev. Cardiol. 2022, 29, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, T.; Acquah, I.; Dey, A.K.; Glassner, K.; Abraham, B.; Blankstein, R.; Virani, S.S.; Blaha, M.J.; Valero-Elizondo, J.; Mehta, N.; et al. Prevalence of cardiovascular risk factors in a nationally representative adult population with inflammatory bowel disease without atherosclerotic cardiovascular disease. Am. J. Prev. Cardiol. 2021, 16, 100171. [Google Scholar] [CrossRef] [PubMed]
- Gravina, A.G.; Pellegrino, R.; Palladino, G.; Zanini, A.; Federico, A.; Zingone, F. Too Many Couch Potatoes Among Middle-Aged Inflammatory Bowel Disease Patients: Findings from the “BE-FIT-IBD-2” Study. Gastroenterol. Insights 2024, 15, 963–975. [Google Scholar] [CrossRef]
- Gravina, A.G.; Pellegrino, R.; Durante, T.; Palladino, G.; D’Onofrio, R.; Mammone, S.; Arboretto, G.; Auletta, S.; Imperio, G.; Ventura, A.; et al. Inflammatory bowel diseases patients suffer from significant low levels and barriers to physical activity: The “BE-FIT-IBD” study. World J. Gastroenterol. 2023, 29, 5668–5682. [Google Scholar] [CrossRef] [PubMed]
- Kaazan, P.; Seow, W.; Yong, S.; Heilbronn, L.K.; Segal, J.P. The Impact of Obesity on Inflammatory Bowel Disease. Biomedicines 2023, 11, 3256. [Google Scholar] [CrossRef]
- Khakoo, N.S.; Ioannou, S.; Khakoo, N.S.; Vedantam, S.; Pearlman, M. Impact of Obesity on Inflammatory Bowel Disease. Curr. Gastroenterol. Rep. 2022, 24, 26–36. [Google Scholar] [CrossRef]
- Gravina, A.G.; Panarese, I.; Trotta, M.C.; D’Amico, M.; Pellegrino, R.; Ferraraccio, F.; Galdiero, M.; Alfano, R.; Grieco, P.; Federico, A. Melanocortin 3,5 receptors immunohistochemical expression in colonic mucosa of inflammatory bowel disease patients: A matter of disease activity? World J. Gastroenterol. 2024, 30, 1132–1142. [Google Scholar] [CrossRef]
- Patel, T.P.; Jun, J.Y.; Seo, A.Y.; Levi, N.J.; Elizondo, D.M.; Chen, J.; Wong, A.M.; Tugarinov, N.; Altman, E.K.; Gehle, D.B.; et al. Melanocortin 3 receptor regulates hepatic autophagy and systemic adiposity. Nat. Commun. 2025, 16, 1690. [Google Scholar] [CrossRef]
- Nuutinen, S.; Ailanen, L.; Savontaus, E.; Rinne, P. Melanocortin overexpression limits diet-induced inflammation and atherosclerosis in LDLR-/- mice. J. Endocrinol. 2018, 236, 111–123. [Google Scholar] [CrossRef]
- Marín-Jiménez, I.; Carpio, D.; Hernández, V.; Muñoz, F.; Zatarain-Nicolás, E.; Zabana, Y.; Mañosa, M.; Rodríguez-Moranta, F.; Barreiro-de Acosta, M.; Gutiérrez Casbas, A. Spanish Working Group in Crohn’s Disease and Ulcerative Colitis (GETECCU) position paper on cardiovascular disease in patients with inflammatory bowel disease. Gastroenterol. Hepatol. 2025, 48, 502314, English, Spanish. [Google Scholar] [CrossRef]
- Hernández-Camba, A.; Carrillo-Palau, M.; Ramos, L.; Hernández Alvarez-Buylla, N.; Alonso-Abreu, I.; Hernández-Pérez, A.; Vela, M.; Arranz, L.; Hernández-Guerra, M.; González-Gay, M.Á.; et al. Carotid plaque assessment reclassifies patients with inflammatory bowel disease into very-high cardiovascular risk. J. Clin. Med. 2021, 10, 1671. [Google Scholar] [CrossRef]
- Naami, R.; Tashtish, N.; Neeland, I.J.; Katz, J.; Sinh, P.; Nasir, K.; Chittajallu, V.; Mansoor, E.; Rajagopalan, S.; Al-Kindi, S. Coronary artery calcium scoring for cardiovascular risk assessment in patients with inflammatory bowel disease. Am. Heart J. 2023, 266, 120–127. [Google Scholar] [CrossRef]
- Mantaka, A.; Galanakis, N.; Tsetis, D.; Koutroubakis, I.E. Abdominal aortic calcification in patients with inflammatory bowel disease: Does anti-tumor necrosis factor α use protect from chronic inflammation-induced atherosclerosis? Intestig. Res. 2022, 20, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Sleutjes, J.A.M.; van der Woude, C.J.; Verploegh, P.J.P.; Aribas, E.; Kavousi, M.; Roeters van Lennep, J.E.; de Vries, A.C. Cardiovascular risk profiles in patients with inflammatory bowel disease differ from matched controls from the general population. Eur. J. Prev. Cardiol. 2023, 30, 1615–1622. [Google Scholar] [CrossRef]
- Alayo, Q.A.; Famutimi, D.; Ayoub, M.; De Las Fuentes, L.; Deepak, P. Performance of ASCVD risk prediction models in individuals with inflammatory bowel disease: A UK Biobank study. Inflamm. Bowel Dis. 2024, 18, izae007. [Google Scholar] [CrossRef]
- Silvestre-Roig, C.; Braster, Q.; Ortega-Gomez, A.; Soehnlein, O. Neutrophils as regulators of cardiovascular inflammation. Nat. Rev. Cardiol. 2020, 17, 327–340. [Google Scholar] [CrossRef]
- Aroca-Crevillén, A.; Vicanolo, T.; Ovadia, S.; Hidalgo, A. Neutrophils in physiology and pathology. Annu. Rev. Pathol. 2024, 19, 227–259. [Google Scholar] [CrossRef]
- Garratt, L.W. Current understanding of the neutrophil transcriptome in health and disease. Cells 2021, 10, 2406. [Google Scholar] [CrossRef] [PubMed]
- Grieshaber-Bouyer, R.; Radtke, F.A.; Cunin, P.; Stifano, G.; Levescot, A.; Vijaykumar, B.; Nelson-Maney, N.; Blaustein, R.B.; Monach, P.A.; Nigrovic, P.A. The neutrotime transcriptional signature defines a single continuum of neutrophils across biological compartments. Nat. Commun. 2021, 12, 2856. [Google Scholar] [CrossRef]
- Xie, X.; Shi, Q.; Wu, P.; Zhang, X.; Kambara, H.; Su, J.; Yu, H.; Park, S.-Y.; Guo, R.; Ren, Q.; et al. Single-cell transcriptome profiling reveals neutrophil heterogeneity in homeostasis and infection. Nat. Immunol. 2020, 21, 1119–1133. [Google Scholar] [CrossRef]
- Shah, A.D.; Denaxas, S.; Nicholas, O.; Hingorani, A.D.; Hemingway, H. Neutrophil counts and initial presentation of 12 cardiovascular diseases: A CALIBER cohort study. J. Am. Coll. Cardiol. 2017, 69, 1160–1169, Erratum in: J. Am. Coll. Cardiol. 2017, 69, 3125–3126. https://doi.org/10.1016/j.jacc.2016.12.022. [Google Scholar] [CrossRef]
- Welsh, C.; Welsh, P.; Mark, P.B.; Celis-Morales, C.A.; Lewsey, J.; Gray, S.R.; Lyall, D.M.; Iliodromiti, S.; Gill, J.M.R.; Pell, J.; et al. Association of total and differential leukocyte counts with cardiovascular disease and mortality in the UK Biobank. Arter. Thromb. Vasc. Biol. 2018, 38, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Thomassen, J.Q.; Nordestgaard, B.G.; Tybjærg-Hansen, A.; Frikke-Schmidt, R. Neutrophil counts and cardiovascular disease. Eur. Heart J. 2023, 44, 4953–4964. [Google Scholar] [CrossRef]
- Soehnlein, O.; Döring, Y. Beyond association: High neutrophil counts are a causal risk factor for atherosclerotic cardiovascular disease. Eur. Heart J. 2023, 44, 4965–4967. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Kang, Z.; Yin, D.; Gao, J. Role of neutrophils in different stages of atherosclerosis. Innate Immun. 2023, 29, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Sreejit, G.; Johnson, J.; Jaggers, R.M.; Dahdah, A.; Murphy, A.J.; Hanssen, N.M.J.; Nagareddy, P.R. Neutrophils in cardiovascular disease: Warmongers, peacemakers, or both? Cardiovasc. Res. 2022, 118, 2596–2609. [Google Scholar] [CrossRef]
- Herrero-Cervera, A.; Soehnlein, O.; Kenne, E. Neutrophils in chronic inflammatory diseases. Cell Mol. Immunol. 2022, 19, 177–191. [Google Scholar] [CrossRef]
- Tamassia, N.; Bianchetto-Aguilera, F.; Arruda-Silva, F.; Gardiman, E.; Gasperini, S.; Calzetti, F.; Cassatella, M.A. Cytokine production by human neutrophils: Revisiting the “dark side of the moon”. Eur. J. Clin. Investig. 2018, 48 (Suppl. 2), e12952. [Google Scholar] [CrossRef]
- Hall, C.H.T.; Campbell, E.L.; Colgan, S.P. Neutrophils as components of mucosal homeostasis. Cell Mol. Gastroenterol. Hepatol. 2017, 4, 329–337. [Google Scholar] [CrossRef]
- Danne, C.; Skerniskyte, J.; Marteyn, B.; Sokol, H. Neutrophils: From IBD to the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 184–197. [Google Scholar] [CrossRef]
- Mortha, A.; Remark, R.; Del Valle, D.M.; Chuang, L.S.; Chai, Z.; Alves, I.; Azevedo, C.; Gaifem, J.; Martin, J.; Petralia, F.; et al. Neutralizing anti-granulocyte macrophage-colony stimulating factor autoantibodies recognize post-translational glycosylations on granulocyte macrophage-colony stimulating factor years before diagnosis and predict complicated Crohn’s disease. Gastroenterology 2022, 163, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Saez, A.; Herrero-Fernandez, B.; Gomez-Bris, R.; Sanchez-Martinez, H.; Gonzalez-Granado, J.M. Pathophysiology of inflammatory bowel disease: Innate immune system. Int. J. Mol. Sci. 2023, 24, 1526. [Google Scholar] [CrossRef] [PubMed]
- Segal, A.W. The role of neutrophils in the pathogenesis of Crohn’s disease. Eur. J. Clin. Investig. 2018, 48 (Suppl. 2), e12983. [Google Scholar] [CrossRef]
- Denson, L.A.; Jurickova, I.; Karns, R.; Shaw, K.A.; Cutler, D.J.; Okou, D.; Valencia, C.A.; Dodd, A.; Mondal, K.; Aronow, B.J.; et al. Genetic and transcriptomic variation linked to neutrophil granulocyte-macrophage colony-stimulating factor signaling in pediatric Crohn’s disease. Inflamm. Bowel Dis. 2019, 25, 547–560. [Google Scholar] [CrossRef]
- Bamias, G.; Zampeli, E.; Domènech, E. Targeting neutrophils in inflammatory bowel disease: Revisiting the role of adsorptive granulocyte and monocyte apheresis. Expert. Rev. Gastroenterol. Hepatol. 2022, 16, 721–735. [Google Scholar] [CrossRef]
- Parigi, T.L.; Cannatelli, R.; Nardone, O.M.; Zammarchi, I.; Shivaji, U.; Furfaro, F.; Zardo, D.; Spaggiari, P.; Del Sordo, R.; Setti, O.; et al. Neutrophil-only histological assessment of ulcerative colitis correlates with endoscopic activity and predicts long-term outcomes in a multicentre study. J. Crohn’s Colitis 2023, 17, 1931–1938. [Google Scholar] [CrossRef]
- Pai, R.K.; Hartman, D.J.; Rivers, C.R.; Regueiro, M.; Schwartz, M.; Binion, D.G.; Pai, R.K. Complete resolution of mucosal neutrophils associates with improved long-term clinical outcomes of patients with ulcerative colitis. Clin. Gastroenterol. Hepatol. 2020, 18, 2510–2517.e5. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, J.; Zhang, Y.; Song, Z.; Bian, J.; Yi, H.; Ma, Z. Identifying neutrophil-associated subtypes in ulcerative colitis and confirming neutrophils promote colitis-associated colorectal cancer. Front. Immunol. 2023, 14, 1095098. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, D.; Peyrin-Biroulet, L.; Estevinho, M.M.; Danese, S.; Magro, F. Pursuing neutrophils: Systematic scoping review on blood-based biomarkers as predictors of treatment outcomes in inflammatory bowel disease. Ther. Adv. Gastroenterol. 2023, 16, 17562848231155987. [Google Scholar] [CrossRef]
- Juzenas, S.; Hübenthal, M.; Lindqvist, C.M.; Kruse, R.; Steiert, T.A.; Degenhardt, F.; Schulte, D.; Nikolaus, S.; Zeissig, S.; Bergemalm, D.; et al. Detailed transcriptional landscape of peripheral blood points to increased neutrophil activation in treatment-naïve inflammatory bowel disease. J. Crohn’s Colitis 2022, 16, 1097–1109. [Google Scholar] [CrossRef]
- van Unen, V.; Ouboter, L.F.; Li, N.; Schreurs, M.; Abdelaal, T.; Kooy-Winkelaar, Y.; Beyrend, G.; Höllt, T.; Maljaars, P.W.J.; Mearin, M.L.; et al. Identification of a disease-associated network of intestinal immune cells in treatment-naïve inflammatory bowel disease. Front. Immunol. 2022, 13, 893803. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Liu, H.; Huang, G.; Dai, S.S. The emerging role of neutrophils in autoimmune-associated disorders: Effector, predictor, and therapeutic targets. Med. Commun. 2021, 2, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Wigerblad, G.; Kaplan, M.J. Neutrophil extracellular traps in systemic autoimmune and autoinflammatory diseases. Nat. Rev. Immunol. 2023, 23, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Bissenova, S.; Ellis, D.; Mathieu, C.; Gysemans, C. Neutrophils in autoimmunity: When the hero becomes the villain. Clin. Exp. Immunol. 2022, 210, 128–140. [Google Scholar] [CrossRef]
- Sadeghi, M.; Dehnavi, S.; Jamialahmadi, T.; Johnston, T.P.; Sahebkar, A. Neutrophil extracellular trap: A key player in the pathogenesis of autoimmune diseases. Int. Immunopharmacol. 2023, 116, 109843. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Zhang, W.; Yang, J.; Sun, N.; Qu, X. Identification of neutrophil extracellular traps and crosstalk genes linking inflammatory bowel disease and osteoporosis by integrated bioinformatics analysis and machine learning. Sci. Rep. 2023, 13, 23054. [Google Scholar] [CrossRef]
- Ding, Y.; Yang, Y.; Xue, L. Immune cells and their related genes provide a new perspective on the common pathogenesis of ankylosing spondylitis and inflammatory bowel diseases. Front. Immunol. 2023, 14, 1137523. [Google Scholar] [CrossRef]
- Jatana, S.; Ponti, A.K.; Johnson, E.E.; Rebert, N.A.; Smith, J.L.; Fulmer, C.G.; Maytin, E.V.; Achkar, J.P.; Fernandez, A.P.; McDonald, C. A novel murine model of pyoderma gangrenosum reveals that inflammatory skin-gut crosstalk is mediated by IL-1β-primed neutrophils. Front. Immunol. 2023, 14, 1148893. [Google Scholar] [CrossRef]
- He, R.; Zhao, S.; Cui, M.; Chen, Y.; Ma, J.; Li, J.; Wang, X. Cutaneous manifestations of inflammatory bowel disease: Basic characteristics, therapy, and potential pathophysiological associations. Front. Immunol. 2023, 14, 1234535. [Google Scholar] [CrossRef]
- Kaneko, R.; Matsui, A.; Watanabe, M.; Harada, Y.; Kanamori, M.; Awata, N.; Kawazoe, M.; Takao, T.; Kobayashi, Y.; Kikutake, C.; et al. Increased neutrophils in inflammatory bowel disease accelerate the accumulation of amyloid plaques in the mouse model of Alzheimer’s disease. Inflamm. Regen. 2023, 43, 20. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, Q.; Zhou, L.; Li, W.; Yuan, Y.; Lei, W.; Liu, K.; Xu, M.; Diao, T.; Gao, H.; et al. Associations of baseline and changes in leukocyte counts with incident cardiovascular events: The Dongfeng-Tongji Cohort Study. J. Atheroscler. Thromb. 2022, 29, 1040–1058. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wu, S.; Xu, F.; Shu, R.; Song, H.; Chen, S.; Shao, Z.; Cui, L. Distinct WBC trajectories are associated with the risks of incident CVD and all-cause mortality. J. Atheroscler. Thromb. 2023, 30, 1492–1506. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Liu, Y.; Zhu, L.; Xu, L.; Shen, H. Evaluation of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as potential markers for ulcerative colitis: A retrospective study. BMC Gastroenterol. 2022, 22, 485. [Google Scholar] [CrossRef] [PubMed]
- Kurimoto, N.; Nishida, Y.; Hosomi, S.; Itani, S.; Kobayashi, Y.; Nakata, R.; Ōminami, M.; Nadatani, Y.; Fukunaga, S.; Ōtani, K.; et al. Neutrophil-to-lymphocyte ratio may predict clinical relapse in ulcerative colitis patients with mucosal healing. PLoS ONE 2023, 18, e0280252. [Google Scholar] [CrossRef] [PubMed]
- Pang, W.; Zhang, B.; Jin, L.; Yao, Y.; Han, Q.; Zheng, X. Serological biomarker-based machine learning models for predicting the relapse of ulcerative colitis. J. Inflamm. Res. 2023, 16, 3531–3545. [Google Scholar] [CrossRef]
- Zhou, G.X.; Liu, Z.J. Potential roles of neutrophils in regulating intestinal mucosal inflammation of inflammatory bowel disease. J. Dig. Dis. 2017, 18, 495–503. [Google Scholar] [CrossRef]
- Luan, Y.; Hu, J.; Wang, Q.; Wang, X.; Li, W.; Qu, R.; Yang, C.; Rajendran, B.K.; Zhou, H.; Liu, P.; et al. Wnt5 controls splenic myelopoiesis and neutrophil functional ambivalency during DSS-induced colitis. Cell Rep. 2024, 43, 113934. [Google Scholar] [CrossRef]
- Bénard, A.; Mittelstädt, A.; Klösch, B.; Glanz, K.; Müller, J.; Schoen, J.; Nüse, B.; Brunner, M.; Naschberger, E.; Stürzl, M.; et al. IL-3 orchestrates ulcerative colitis pathogenesis by controlling the development and the recruitment of splenic reservoir neutrophils. Cell Rep. 2023, 42, 112637. [Google Scholar] [CrossRef]
- Arosa, L.; Camba-Gómez, M.; Conde-Aranda, J. Neutrophils in intestinal inflammation: What we know and what we could expect for the near future. Gastrointest. Disord. 2022, 4, 263–276. [Google Scholar] [CrossRef]
- Libby, P.; Hansson, G.K. From focal lipid storage to systemic inflammation: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 74, 1594–1607. [Google Scholar] [CrossRef]
- Ostendorf, Y.; Rolauer, L.; Pasch, N.; Schaefer, H.; Heitmann, S.; Petzsch, P.; Poschmann, G.; Hartwig, S.; Lehr, S.; Koehrer, K.; et al. Neutrophils as major drivers of increased atherosclerosis in a murine model of chronic colitis. Eur. Heart J. 2022, 43 (Suppl. 2), ehac544.3042. [Google Scholar] [CrossRef]
- Yao, Z.; Zhang, B.; Niu, G.; Yan, Z.; Tong, X.; Zou, Y.; Li, Y.; Yang, M. Neutrophil infiltration characterized by upregulation of S100A8, S100A9, S100A12, and CXCR2 is associated with the co-occurrence of Crohn’s disease and peripheral artery disease. Front. Immunol. 2022, 13, 896645. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zhou, Y.; Chen, Z.; Liu, C.; Wu, Z.; Zhou, Y.; Zhang, F.; Lu, X.; Tang, L. Identification of key biomarkers for predicting CAD progression in inflammatory bowel disease via machine-learning and bioinformatics strategies. J. Cell. Mol. Med. 2024, 28, e18175. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Wang, R.; Zhang, X.; Wen, X.; Deng, S.; Xie, W. Identification of CCL2, CXCR2, S100A9 as immune-related gene markers and immune infiltration characteristics of inflammatory bowel disease and heart failure via bioinformatics analysis and machine learning. Front. Cardiovasc. Med. 2023, 10, 1268675. [Google Scholar] [CrossRef]
- Fu, Q.; Shen, T.; Qiu, W.; Liao, Y.; Yu, M.; Zhou, Y. FOSB is a key factor in the genetic link between inflammatory bowel disease and acute myocardial infarction: Multiple bioinformatics analyses and validation. BMC Med. Genom. 2025, 18, 63. [Google Scholar] [CrossRef]
- Wu, M.; Liu, D.; Xiong, X.; Su, Q.; Xiang, Y.; Shen, L.; An, Z.; Yang, X. Analysis of the molecular mechanisms of ulcerative colitis and atherosclerosis by microarray data. Sci. Rep. 2025, 15, 10715. [Google Scholar] [CrossRef]
- Buso, G.; Faggin, E.; Bressan, A.; Galliazzo, S.; Cinetto, F.; Felice, C.; Fusaro, M.; Erdmann, A.; Pauletto, P.; Rattazzi, M.; et al. Biomarkers of neutrophil activation in patients with symptomatic chronic peripheral artery disease predict worse cardiovascular outcome. Biomedicines 2023, 11, 866. [Google Scholar] [CrossRef]
- Swaminathan, A.; Borichevsky, G.M.; Frampton, C.M.; Day, A.S.; Hampton, M.B.; Kettle, A.J.; Gearry, R.B. Comparison of fecal calprotectin and myeloperoxidase in predicting outcomes in inflammatory bowel disease. Inflamm. Bowel Dis. 2024, 28, izae032. [Google Scholar] [CrossRef]
- Ling Lundström, M.; Peterson, C.; Lampinen, M.; Hedin, C.R.H.; Keita, Å.V.; Kruse, R.; Magnusson, M.K.; Lindqvist, C.M.; Repsilber, D.; D’Amato, M.; et al. Fecal biomarkers of neutrophil and eosinophil origin reflect the response to biological therapy and corticosteroids in patients with inflammatory bowel disease. Clin. Transl. Gastroenterol. 2023, 14, e00605. [Google Scholar] [CrossRef]
- Pavlidis, P.; Tsakmaki, A.; Pantazi, E.; Li, K.; Cozzetto, D.; Bell, J.D.; Yang, F.; Lo, J.W.; Alberts, E.; Sa, A.C.C.; et al. Interleukin-22 regulates neutrophil recruitment in ulcerative colitis and is associated with resistance to ustekinumab therapy. Nat. Commun. 2022, 13, 5820. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, Y.; Yang, X.; Pan, Y.; Li, L.; Gao, C.; He, C. Clinical significance of novel neutrophil-based biomarkers in the diagnosis and prediction of response to infliximab therapy in Crohn’s disease. Front. Immunol. 2022, 13, 865968. [Google Scholar] [CrossRef]
- Friedrich, M.; Pohin, M.; Jackson, M.A.; Korsunsky, I.; Bullers, S.J.; Rue-Albrecht, K.; Christoforidou, Z.; Sathananthan, D.; Thomas, T.; Ravindran, R.; et al. IL-1-driven stromal-neutrophil interactions define a subset of patients with inflammatory bowel disease that does not respond to therapies. Nat. Med. 2021, 27, 1970–1981. [Google Scholar] [CrossRef]
- Everett, B.M.; MacFadyen, J.G.; Thuren, T.; Libby, P.; Glynn, R.J.; Ridker, P.M. Inhibition of interleukin-1β and reduction in atherothrombotic cardiovascular events in the CANTOS trial. J. Am. Coll. Cardiol. 2020, 76, 1660–1670. [Google Scholar] [CrossRef] [PubMed]
- Aggeletopoulou, I.; Kalafateli, M.; Tsounis, E.P.; Triantos, C. Exploring the role of IL-1β in inflammatory bowel disease pathogenesis. Front. Med. 2024, 11, 1307394. [Google Scholar] [CrossRef] [PubMed]
- McAllister, M.J.; Hall, R.; Whelan, R.J.; Fischer, L.J.; Chuah, C.S.; Cartlidge, P.D.; Drury, B.; Rutherford, D.G.; Duffin, R.M.; Cartwright, J.A.; et al. Formylated peptide receptor-1-mediated gut inflammation as a therapeutic target in inflammatory bowel disease. Crohns Colitis 360 2024, 6, otae003. [Google Scholar] [CrossRef]
- Dhayni, K.; Zibara, K.; Issa, H.; Kamel, S.; Bennis, Y. Targeting CXCR1 and CXCR2 receptors in cardiovascular diseases. Pharmacol. Ther. 2022, 237, 108257. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Kuang, W.; Wang, D.; Yuan, K.; Yang, P. Expanding role of CXCR2 and therapeutic potential of CXCR2 antagonists in inflammatory diseases and cancers. Eur. J. Med. Chem. 2023, 250, 115175. [Google Scholar] [CrossRef]
- Kelm, M.; Lehoux, S.; Azcutia, V.; Cummings, R.D.; Nusrat, A.; Parkos, C.A.; Brazil, J.C. Regulation of neutrophil function by selective targeting of glycan epitopes expressed on the integrin CD11b/CD18. FASEB J. 2020, 34, 2326–2343. [Google Scholar] [CrossRef]
- Zhou, G.; Zhu, F.; Zhang, H.; Wang, Y.; Yang, Y.; Jin, G.; Wang, Y.; Dong, G.; Xiong, H. PTK2B regulates immune responses of neutrophils and protects mucosal inflammation in ulcerative colitis. FASEB J. 2023, 37, e22967. [Google Scholar] [CrossRef]
- Gu, C.; Pang, B.; Sun, S.; An, C.; Wu, M.; Wang, N.; Yuan, Y.; Liu, G. Neutrophil extracellular traps contributing to atherosclerosis: From pathophysiology to clinical implications. Exp. Biol. Med. 2023, 248, 1302–1312. [Google Scholar] [CrossRef]
- Wang, C.J.; Ko, G.R.; Lee, Y.Y.; Park, J.; Park, W.; Park, T.E.; Jin, Y.; Kim, S.N.; Lee, J.S.; Park, C.G. Polymeric DNase-I nanozymes targeting neutrophil extracellular traps for the treatment of bowel inflammation. Nano Converg. 2024, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Hong, W.; Wan, M.; Zheng, L. Molecular mechanisms and therapeutic target of NETosis in diseases. Med. Commun. 2022, 3, e162. [Google Scholar] [CrossRef]
- Geng, S.; Zhang, Y.; Lee, C.; Li, L. Novel reprogramming of neutrophils modulates inflammation resolution during atherosclerosis. Sci. Adv. 2019, 5, eaav2309. [Google Scholar] [CrossRef]
- Lin, R.; Yi, Z.; Wang, J.; Geng, S.; Li, L. Generation of resolving memory neutrophils through pharmacological training with 4-PBA or genetic deletion of TRAM. Cell Death Dis. 2022, 13, 345. [Google Scholar] [CrossRef]
- Li, W.; Liu, C.; Wang, S.; Liu, N. Neutrophil membrane biomimetic delivery system (Ptdser-NM-Lipo/Fer-1) designed for targeting atherosclerosis therapy. IET Nanobiotechnology 2023, 17, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, M.; Yuan, Y.; Nie, C.; Wei, K.; Zhang, T.; Chen, T.; Chu, X. Neutrophil-membrane-coated biomineralized metal-organic framework nanoparticles for atherosclerosis treatment by targeting gene silencing. ACS Nano 2023, 17, 7721–7732. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Z.; ZhuGe, D.L.; Tong, M.Q.; Lin, M.T.; Zheng, Y.W.; Jiang, X.; Yang, W.G.; Yao, Q.; Xiang, Q.; Li, X.K.; et al. Ulcerative colitis-specific delivery of keratinocyte growth factor by neutrophils-simulated liposomes facilitates the morphologic and functional recovery of the damaged colon through alleviating the inflammation. J. Control. Release 2019, 299, 90–106. [Google Scholar] [CrossRef]
- Yennemadi, A.S.; Jordan, N.; Diong, S.; Keane, J.; Leisching, G. The link between dysregulated immunometabolism and vascular damage: Implications for the development of atherosclerosis in systemic lupus erythematosus and other rheumatic diseases. J. Rheumatol. 2024, 51, 234–241. [Google Scholar] [CrossRef]
- Legge, A.; Hanly, J.G. Managing premature atherosclerosis in patients with chronic inflammatory diseases. Can. Med. Assoc. J. 2018, 190, E430–E439. [Google Scholar] [CrossRef]
- Hosack, T.; Thomas, T.; Ravindran, R.; Uhlig, H.H.; Travis, S.P.L.; Buckley, C.D. Inflammation across tissues: Can shared cell biology help design smarter trials? Nat. Rev. Rheumatol. 2023, 19, 666–674. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genkel, V.; Zaripova, Y.; Kuznetsova, A.; Sluchanko, A.; Minasova, A.; Zotova, M.; Saenko, A.; Savochkina, A.; Dolgushina, A. Neutrophils at the Crossroads of Inflammatory Bowel Disease and Atherosclerosis: A State-of-the-Art Review. Cells 2025, 14, 738. https://doi.org/10.3390/cells14100738
Genkel V, Zaripova Y, Kuznetsova A, Sluchanko A, Minasova A, Zotova M, Saenko A, Savochkina A, Dolgushina A. Neutrophils at the Crossroads of Inflammatory Bowel Disease and Atherosclerosis: A State-of-the-Art Review. Cells. 2025; 14(10):738. https://doi.org/10.3390/cells14100738
Chicago/Turabian StyleGenkel, Vadim, Yana Zaripova, Alla Kuznetsova, Alena Sluchanko, Anna Minasova, Maria Zotova, Anna Saenko, Albina Savochkina, and Anastasiya Dolgushina. 2025. "Neutrophils at the Crossroads of Inflammatory Bowel Disease and Atherosclerosis: A State-of-the-Art Review" Cells 14, no. 10: 738. https://doi.org/10.3390/cells14100738
APA StyleGenkel, V., Zaripova, Y., Kuznetsova, A., Sluchanko, A., Minasova, A., Zotova, M., Saenko, A., Savochkina, A., & Dolgushina, A. (2025). Neutrophils at the Crossroads of Inflammatory Bowel Disease and Atherosclerosis: A State-of-the-Art Review. Cells, 14(10), 738. https://doi.org/10.3390/cells14100738







