Physiological Oxygen Levels in the Microenvironment Program Ex Vivo-Generated Conventional Dendritic Cells Toward a Tolerogenic Phenotype
Abstract
1. Introduction
2. Materials and Methods
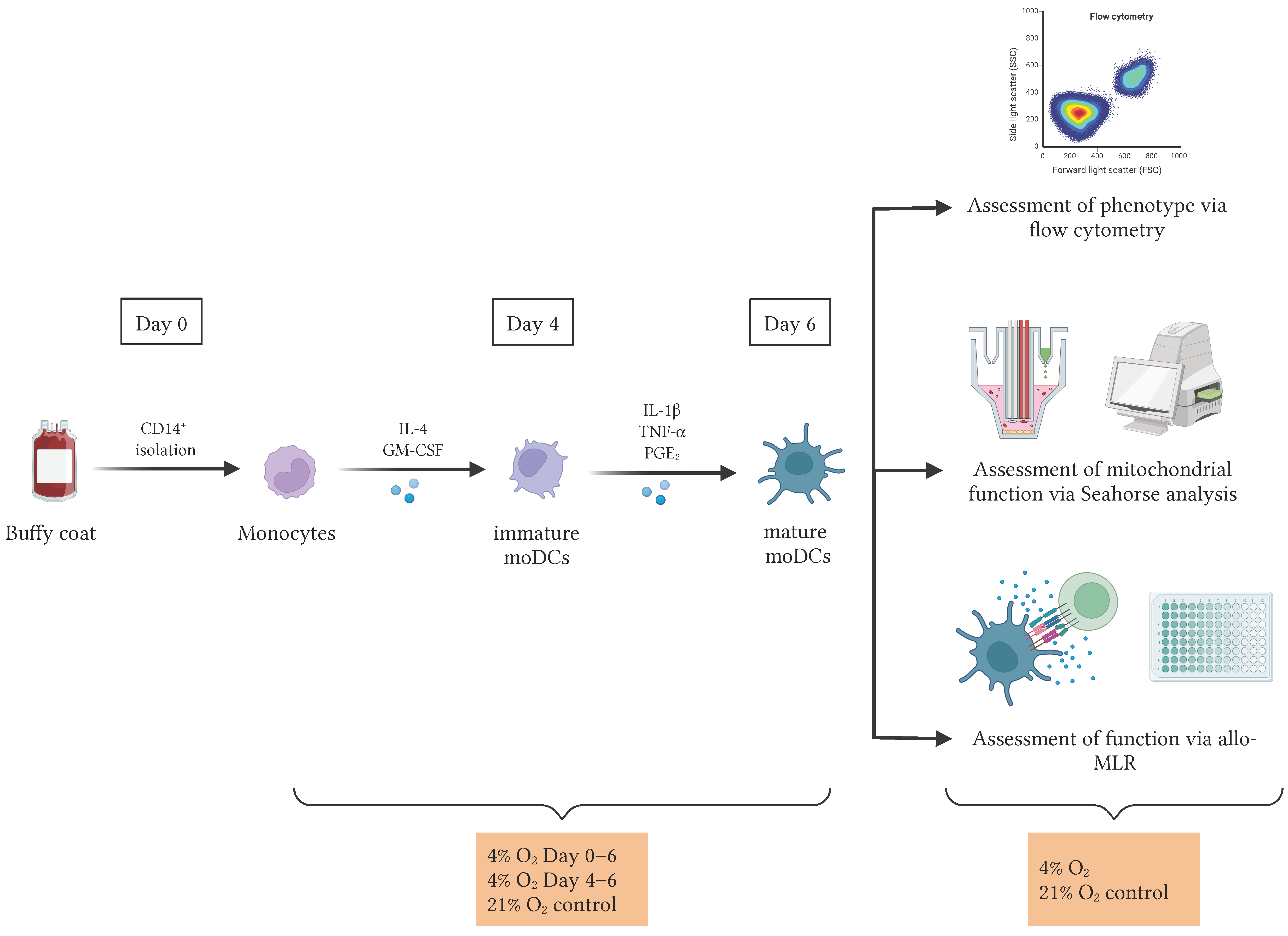
2.1. Cells and Culture Conditions
2.2. Immunophenotyping of DCs
2.3. Lactate Measurement
2.4. Seahorse Assay for Mitochondrial Function
2.5. Allogeneic Mixed Lymphocyte Reaction (Allo-MLR)
2.6. Immunophenotyping of PBLs
2.7. Statistical Analysis
3. Results
3.1. Physiological O2 Levels During the Generation Period Resulted in a Decreased Transformation of Monocytes into DCs
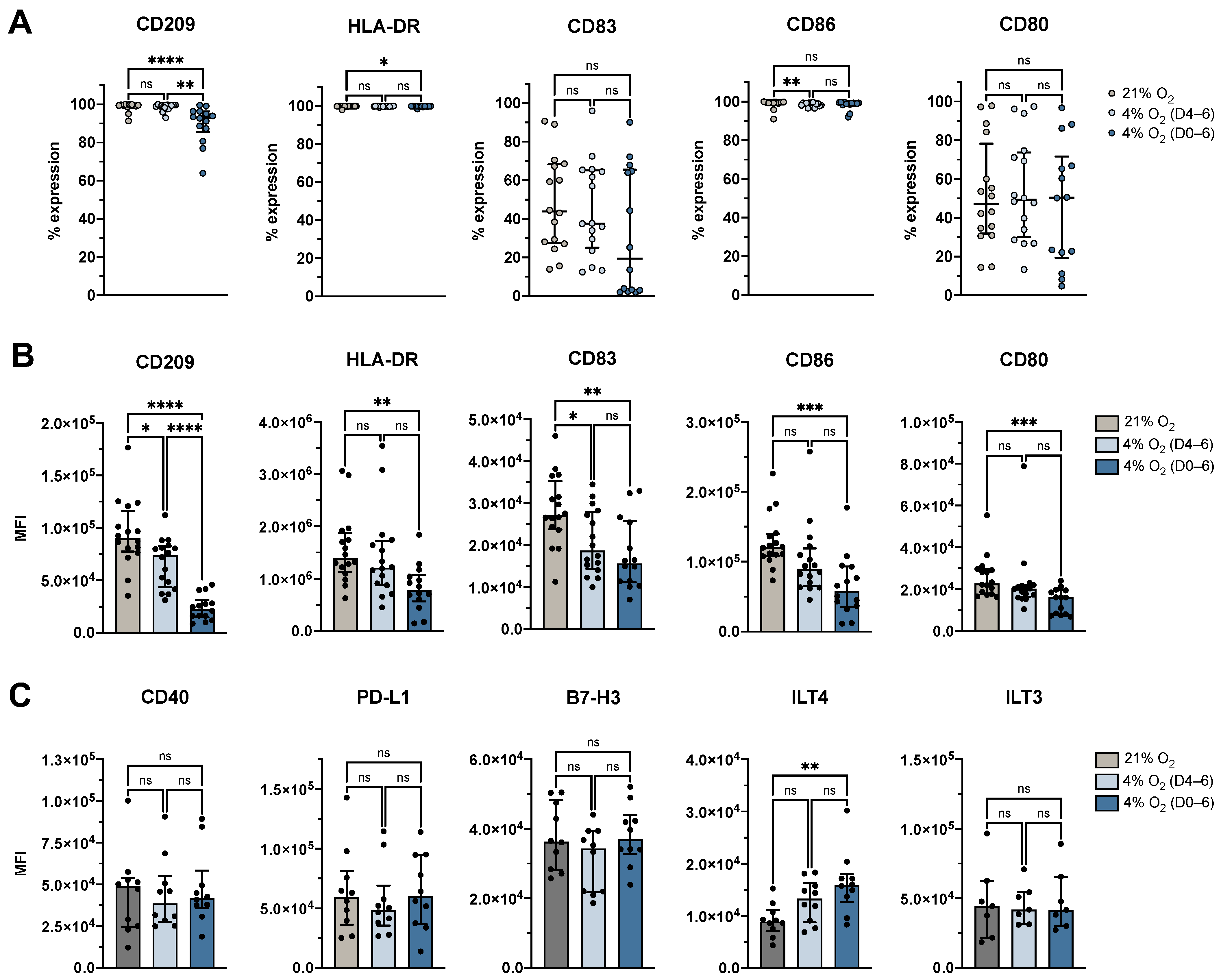
3.2. Physiological O2 Levels During the Generation Period Resulted in Decreased Surface Expression of Identity and Maturation Markers, and Increased Expression of Tolerance-Associated Marker ILT4
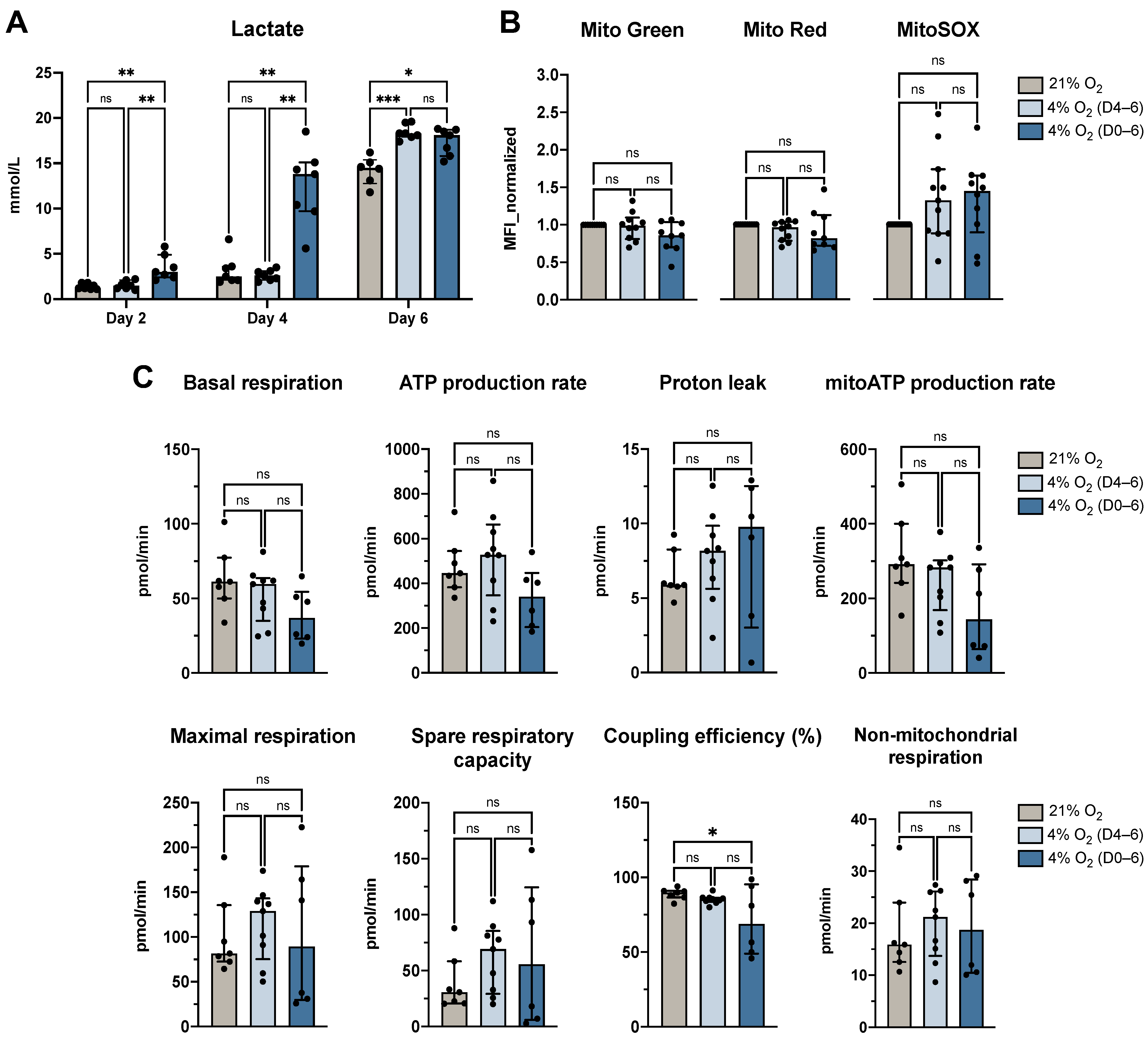
3.3. Physiological O2 Levels During the Generation Period Increased Lactate Secretion but Largely Preserved Mitochondrial Function
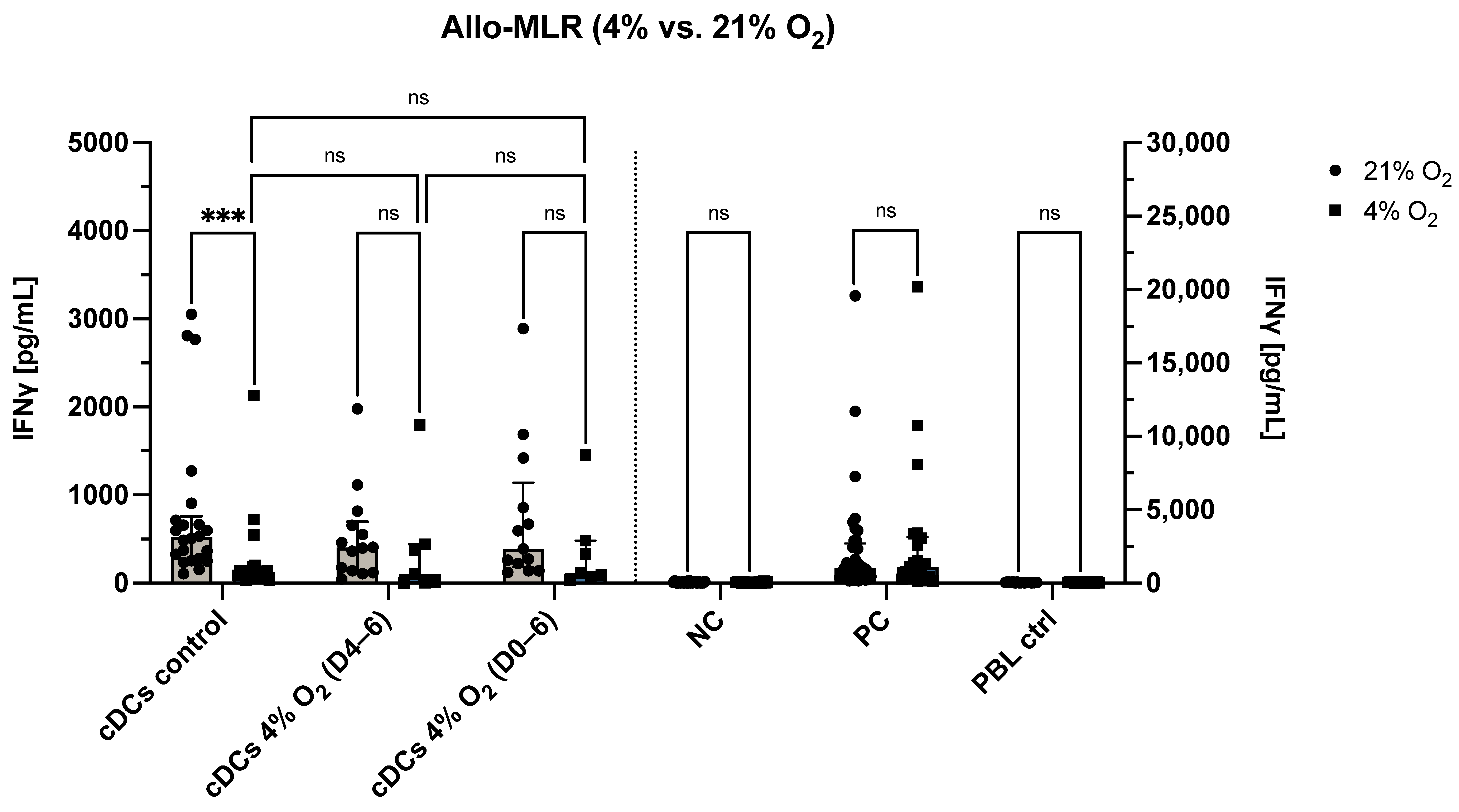
3.4. O2 Levels During the Allo-MLR Modulate T Cell Responses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
| allo-MLR | Allogeneic mixed lymphocyte reaction |
| ATP | Adenosine triphosphate |
| BSA | Bovine serum albumin |
| CCR7 | C-C chemokine receptor 7 |
| CD | Cluster of differentiation |
| moDCs | Monocyte-derived dendritic cells |
| CO2 | Carbon dioxide |
| CTLA-4 | Cytotoxic T-lymphocyte-associated protein 4 |
| DAMPs | Damage-associated molecular patterns |
| DCs | Dendritic cells |
| DMSO | Dimethyl sulfoxide |
| FBS | Fetal bovine serum |
| FCCP | Carbonyl cyanide-p-trifluoromethoxyphenylhydrazone |
| FMO | Fluorescence-minus-one |
| FSC | Forward scatter |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| hAB | Human AB serum |
| HLA-DR | Human leukocyte antigen-DR |
| iDCs | Immature dendritic cells |
| IL | Interleukin |
| ILT | Immunoglobulin-like transcript |
| IFN-γ | Interferon-gamma |
| IQR | Interquartile range |
| LAG-3 | Lymphocyte activation gene 3 |
| mDCs | Mature dendritic cells |
| MFI | Mean fluorescence intensity |
| moDCs | Monocyte-derived dendritic cells |
| ns | Not significant |
| O2 | Oxygen |
| OCR | Oxygen consumption rate |
| PAMPs | Pathogen-associated molecular patterns |
| PBLs | Peripheral blood lymphocytes |
| PBS | Phosphate-buffered saline |
| PD-1 | Programmed death-1 |
| PD-L1/L2 | Programmed death-ligand 1/2 |
| PDL | Poly-D-lysine |
| PGE2 | Prostaglandin E2 |
| PI | Propidium iodide |
| SSC | Side scatter |
| Th | T helper cell subset |
| TIGIT | T cell immunoreceptor with immunoglobulin and ITIM domain |
| TIGIT | T cell immunoreceptor with immunoglobulin and ITIM domain |
| TNF | Tumor necrosis factor |
| Tregs | Regulatory T cells |
References
- Caux, C.; Massacrier, C.; Vanbervliet, B.; Dubois, B.; Durand, I.; Cella, M.; Lanzavecchia, A.; Banchereau, J. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to granulocyte-macrophage colony-stimulating factor plus tumor necrosis factor alpha: II. Functional analysis. Blood 1997, 90, 1458–1470. [Google Scholar] [CrossRef] [PubMed]
- Cravens, P.D.; Melkus, M.W.; Padgett-Thomas, A.; Islas-Ohlmayer, M.; Del P. Martin, M.; Garcia, J.V. Development and activation of human dendritic cells in vivo in a xenograft model of human hematopoiesis. Stem Cells 2005, 23, 264–278. [Google Scholar] [CrossRef]
- Patente, T.A.; Pinho, M.P.; Oliveira, A.A.; Evangelista, G.C.M.; Bergami-Santos, P.C.; Barbuto, J.A.M. Human dendritic cells: Their heterogeneity and clinical application potential in cancer immunotherapy. Front. Immunol. 2018, 9, 3176. [Google Scholar] [CrossRef]
- Wilson, N.S.; El-Sukkari, D.; Villadangos, J.A. Dendritic cells constitutively present self antigens in their immature state in vivo and regulate antigen presentation by controlling the rates of MHC class II synthesis and endocytosis. Blood 2004, 103, 2187–2195. [Google Scholar] [CrossRef] [PubMed]
- Steinman, R.M.; Swanson, J. The endocytic activity of dendritic cells. J. Exp. Med. 1995, 182, 283–288. [Google Scholar] [CrossRef]
- Skelton, L.; Cooper, M.; Murphy, M.; Platt, A. Human immature monocyte-derived dendritic cells express the G protein-coupled receptor GPR105 (KIAA0001, P2Y14) and increase intracellular calcium in response to its agonist, uridine diphosphoglucose. J. Immunol. 2003, 171, 1941–1949. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, E.S.; Mellman, I. Cell biology of antigen processing in vitro and in vivo. Annu. Rev. Immunol. 2005, 23, 975–1028. [Google Scholar] [CrossRef] [PubMed]
- Gouwy, M.; Struyf, S.; Leutenez, L.; Pörtner, N.; Sozzani, S.; Van Damme, J. Chemokines and other GPCR ligands synergize in receptor-mediated migration of monocyte-derived immature and mature dendritic cells. Immunobiology 2014, 219, 218–229. [Google Scholar] [CrossRef]
- Hemmi, H.; Akira, S. TLR signalling and the function of dendritic cells. Chem. Immunol. Allergy 2005, 86, 120–135. [Google Scholar] [CrossRef]
- Tang, D.; Kang, R.; Coyne, C.B.; Zeh, H.J.; Lotze, M.T. PAMPs and DAMPs: Signal 0s that spur autophagy and immunity. Immunol. Rev. 2012, 249, 158–175. [Google Scholar] [CrossRef]
- Cerboni, S.; Gentili, M.; Manel, N. Diversity of pathogen sensors in dendritic cells. Adv. Immunol. 2013, 120, 211–237. [Google Scholar] [CrossRef]
- López, C.B.; García-Sastre, A.; Williams, B.R.G.; Moran, T.M. Type I interferon induction pathway, but not released interferon, participates in the maturation of dendritic cells induced by negative-strand RNA viruses. J. Infect. Dis. 2003, 187, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- McIlroy, D.; Tanguy-Royer, S.; Le Meur, N.; Guisle, I.; Royer, P.-J.; Léger, J.; Meflah, K.; Grégoire, M. Profiling dendritic cell maturation with dedicated microarrays. J. Leukoc. Biol. 2005, 78, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Dalod, M.; Chelbi, R.; Malissen, B.; Lawrence, T. Dendritic cell maturation: Functional specialization through signaling specificity and transcriptional programming. EMBO J. 2014, 33, 1104–1116. [Google Scholar] [CrossRef] [PubMed]
- Allenspach, E.J.; Lemos, M.P.; Porrett, P.M.; Turka, L.A.; Laufer, T.M. Migratory and lymphoid-resident dendritic cells cooperate to efficiently prime naive CD4 T cells. Immunity 2008, 29, 795–806. [Google Scholar] [CrossRef]
- Bourque, J.; Hawiger, D. Immunomodulatory Bonds of the Partnership between Dendritic Cells and T Cells. Crit. Rev. Immunol. 2018, 38, 379–401. [Google Scholar] [CrossRef]
- Steinman, R.M.; Hawiger, D.; Liu, K.; Bonifaz, L.; Bonnyay, D.; Mahnke, K.; Iyoda, T.; Ravetch, J.; Dhodapkar, M.; Inaba, K.; et al. Dendritic cell function in vivo during the steady state: A role in peripheral tolerance. Ann. N. Y. Acad. Sci. 2003, 987, 15–25. [Google Scholar] [CrossRef]
- Cools, N.; Ponsaerts, P.; Van Tendeloo, V.F.I.; Berneman, Z.N. Balancing between immunity and tolerance: An interplay between dendritic cells, regulatory T cells, and effector T cells. J. Leukoc. Biol. 2007, 82, 1365–1374. [Google Scholar] [CrossRef]
- Novak, N.; Bieber, T. 2. Dendritic cells as regulators of immunity and tolerance. J. Allergy Clin. Immunol. 2008, 121, S370–S374. [Google Scholar] [CrossRef]
- Nam, J.-H.; Lee, J.-H.; Choi, S.-Y.; Jung, N.-C.; Song, J.-Y.; Seo, H.-G.; Lim, D.-S. Functional ambivalence of dendritic cells: Tolerogenicity and immunogenicity. Int. J. Mol. Sci. 2021, 22, 4430. [Google Scholar] [CrossRef]
- Lozano, E.; Dominguez-Villar, M.; Kuchroo, V.; Hafler, D.A. The TIGIT/CD226 axis regulates human T cell function. J. Immunol. 2012, 188, 3869–3875. [Google Scholar] [CrossRef] [PubMed]
- Machicote, A.; Belén, S.; Baz, P.; Billordo, L.A.; Fainboim, L. Human CD8+HLA-DR+ Regulatory T Cells, Similarly to Classical CD4+Foxp3+ Cells, Suppress Immune Responses via PD-1/PD-L1 Axis. Front. Immunol. 2018, 9, 2788. [Google Scholar] [CrossRef]
- Dilek, N.; Poirier, N.; Hulin, P.; Coulon, F.; Mary, C.; Ville, S.; Vie, H.; Clémenceau, B.; Blancho, G.; Vanhove, B. Targeting CD28, CTLA-4 and PD-L1 costimulation differentially controls immune synapses and function of human regulatory and conventional T-cells. PLoS ONE 2013, 8, e83139. [Google Scholar] [CrossRef]
- Tekguc, M.; Wing, J.B.; Osaki, M.; Long, J.; Sakaguchi, S. Treg-expressed CTLA-4 depletes CD80/CD86 by trogocytosis, releasing free PD-L1 on antigen-presenting cells. Proc. Natl. Acad. Sci. USA 2021, 118, e2023739118. [Google Scholar] [CrossRef]
- Fuse, S.; Obar, J.J.; Bellfy, S.; Leung, E.K.; Zhang, W.; Usherwood, E.J. CD80 and CD86 control antiviral CD8+ T-cell function and immune surveillance of murine gammaherpesvirus 68. J. Virol. 2006, 80, 9159–9170. [Google Scholar] [CrossRef] [PubMed]
- Koorella, C.; Nair, J.R.; Murray, M.E.; Carlson, L.M.; Watkins, S.K.; Lee, K.P. Novel regulation of CD80/CD86-induced phosphatidylinositol 3-kinase signaling by NOTCH1 protein in interleukin-6 and indoleamine 2,3-dioxygenase production by dendritic cells. J. Biol. Chem. 2014, 289, 7747–7762. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M. Regulatory T cells and immune tolerance. Cell 2008, 133, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Medzhitov, R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015, 16, 343–353. [Google Scholar] [CrossRef]
- Collin, M.; Bigley, V. Human dendritic cell subsets: An update. Immunology 2018, 154, 3–20. [Google Scholar] [CrossRef]
- Buck, M.D.; Sowell, R.T.; Kaech, S.M.; Pearce, E.L. Metabolic instruction of immunity. Cell 2017, 169, 570–586. [Google Scholar] [CrossRef]
- Kim, J. Regulation of immune cell functions by metabolic reprogramming. J. Immunol. Res. 2018, 2018, 8605471. [Google Scholar] [CrossRef] [PubMed]
- Kedia-Mehta, N.; Finlay, D.K. Competition for nutrients and its role in controlling immune responses. Nat. Commun. 2019, 10, 2123. [Google Scholar] [CrossRef]
- Gottfried, E.; Kunz-Schughart, L.A.; Ebner, S.; Mueller-Klieser, W.; Hoves, S.; Andreesen, R.; Mackensen, A.; Kreutz, M. Tumor-derived lactic acid modulates dendritic cell activation and antigen expression. Blood 2006, 107, 2013–2021. [Google Scholar] [CrossRef] [PubMed]
- Okajima, F. Regulation of inflammation by extracellular acidification and proton-sensing GPCRs. Cell. Signal. 2013, 25, 2263–2271. [Google Scholar] [CrossRef]
- Cao, L.; Shi, X.; Chang, H.; Zhang, Q.; He, Y. pH-Dependent recognition of apoptotic and necrotic cells by the human dendritic cell receptor DEC205. Proc. Natl. Acad. Sci. USA 2015, 112, 7237–7242. [Google Scholar] [CrossRef]
- Hosonuma, M.; Yoshimura, K. Association between pH regulation of the tumor microenvironment and immunological state. Front. Oncol. 2023, 13, 1175563. [Google Scholar] [CrossRef]
- Steurer, J.; Hoffmann, U.; Dür, P.; Russi, E.; Vetter, W. Changes in arterial and transcutaneous oxygen and carbon dioxide tensions during and after voluntary hyperventilation. Respiration 1997, 64, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, C.C.; Kojima, H.; Lukashev, D.; Armstrong, J.; Farber, M.; Apasov, S.G.; Sitkovsky, M.V. Differential effects of physiologically relevant hypoxic conditions on T lymphocyte development and effector functions. J. Immunol. 2001, 167, 6140–6149. [Google Scholar] [CrossRef]
- Chow, D.C.; Wenning, L.A.; Miller, W.M.; Papoutsakis, E.T. Modeling pO2 distributions in the bone marrow hematopoietic compartment. II. Modified Kroghian models. Biophys. J. 2001, 81, 685–696. [Google Scholar] [CrossRef]
- Carreau, A.; El Hafny-Rahbi, B.; Matejuk, A.; Grillon, C.; Kieda, C. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J. Cell. Mol. Med. 2011, 15, 1239–1253. [Google Scholar] [CrossRef]
- Keeley, T.P.; Mann, G.E. Defining physiological normoxia for improved translation of cell physiology to animal models and humans. Physiol. Rev. 2019, 99, 161–234. [Google Scholar] [CrossRef] [PubMed]
- Chapple, S.J.; Keeley, T.P.; Mastronicola, D.; Arno, M.; Vizcay-Barrena, G.; Fleck, R.; Siow, R.C.M.; Mann, G.E. Bach1 differentially regulates distinct Nrf2-dependent genes in human venous and coronary artery endothelial cells adapted to physiological oxygen levels. Free Radic. Biol. Med. 2016, 92, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, D.C.J.; Smerdon, G.R.; Harries, L.W.; Dodd, N.J.F.; Murphy, M.P.; Curnow, A.; Winyard, P.G. Altered cellular redox homeostasis and redox responses under standard oxygen cell culture conditions versus physioxia. Free Radic. Biol. Med. 2018, 126, 322–333. [Google Scholar] [CrossRef]
- Stuart, J.A.; Fonseca, J.; Moradi, F.; Cunningham, C.; Seliman, B.; Worsfold, C.R.; Dolan, S.; Abando, J.; Maddalena, L.A. How Supraphysiological Oxygen Levels in Standard Cell Culture Affect Oxygen-Consuming Reactions. Oxid. Med. Cell. Longev. 2018, 2018, 8238459. [Google Scholar] [CrossRef] [PubMed]
- Timpano, S.; Guild, B.D.; Specker, E.J.; Melanson, G.; Medeiros, P.J.; Sproul, S.L.J.; Uniacke, J. Physioxic human cell culture improves viability, metabolism, and mitochondrial morphology while reducing DNA damage. FASEB J. 2019, 33, 5716–5728. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.-Y.; Huang, X.; Gil, C.-H.; Aljoufi, A.; Ropa, J.; Broxmeyer, H.E. Physioxia enhances T-cell development ex vivo from human hematopoietic stem and progenitor cells. Stem Cells 2020, 38, 1454–1466. [Google Scholar] [CrossRef]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef]
- Igarashi, K.J.; Kucinski, I.; Chan, Y.Y.; Tan, T.-K.; Khoo, H.M.; Kealy, D.; Bhadury, J.; Hsu, I.; Ho, P.Y.; Niizuma, K.; et al. Physioxia improves the selectivity of hematopoietic stem cell expansion cultures. Blood Adv. 2023, 7, 3366–3377. [Google Scholar] [CrossRef]
- Peter, A.; Berneman, Z.N.; Cools, N. Cellular respiration in dendritic cells: Exploring oxygen-dependent pathways for potential therapeutic interventions. Free Radic. Biol. Med. 2025, 227, 536–556. [Google Scholar] [CrossRef]
- Van Delen, M.; Janssens, I.; Dams, A.; Roosens, L.; Ogunjimi, B.; Berneman, Z.N.; Derdelinckx, J.; Cools, N. Tolerogenic Dendritic Cells Induce Apoptosis-Independent T Cell Hyporesponsiveness of SARS-CoV-2-Specific T Cells in an Antigen-Specific Manner. Int. J. Mol. Sci. 2022, 23, 15201. [Google Scholar] [CrossRef]
- Vaupel, P.; Multhoff, G. Revisiting the Warburg effect: Historical dogma versus current understanding. J. Physiol. 2021, 599, 1745–1757. [Google Scholar] [CrossRef] [PubMed]
- Caronni, N.; Simoncello, F.; Stafetta, F.; Guarnaccia, C.; Ruiz-Moreno, J.S.; Opitz, B.; Galli, T.; Proux-Gillardeaux, V.; Benvenuti, F. Downregulation of membrane trafficking proteins and lactate conditioning determine loss of dendritic cell function in lung cancer. Cancer Res. 2018, 78, 1685–1699. [Google Scholar] [CrossRef] [PubMed]
- Marin, E.; Bouchet-Delbos, L.; Renoult, O.; Louvet, C.; Nerriere-Daguin, V.; Managh, A.J.; Even, A.; Giraud, M.; Vu Manh, T.P.; Aguesse, A.; et al. Human Tolerogenic Dendritic Cells Regulate Immune Responses through Lactate Synthesis. Cell Metab. 2019, 30, 1075–1090.e8. [Google Scholar] [CrossRef]
- Raychaudhuri, D.; Bhattacharya, R.; Sinha, B.P.; Liu, C.S.C.; Ghosh, A.R.; Rahaman, O.; Bandopadhyay, P.; Sarif, J.; D’Rozario, R.; Paul, S.; et al. Lactate Induces Pro-tumor Reprogramming in Intratumoral Plasmacytoid Dendritic Cells. Front. Immunol. 2019, 10, 1878. [Google Scholar] [CrossRef] [PubMed]
- Kanemaru, H.; Mizukami, Y.; Kaneko, A.; Tagawa, H.; Kimura, T.; Kuriyama, H.; Sawamura, S.; Kajihara, I.; Makino, K.; Miyashita, A.; et al. A mechanism of cooling hot tumors: Lactate attenuates inflammation in dendritic cells. iScience 2021, 24, 103067. [Google Scholar] [CrossRef]
- Sanmarco, L.M.; Rone, J.M.; Polonio, C.M.; Fernandez Lahore, G.; Giovannoni, F.; Ferrara, K.; Gutierrez-Vazquez, C.; Li, N.; Sokolovska, A.; Plasencia, A.; et al. Lactate limits CNS autoimmunity by stabilizing HIF-1α in dendritic cells. Nature 2023, 620, 881–889. [Google Scholar] [CrossRef]
- Futalan, D.; Huang, C.-T.; Schmidt-Wolf, I.G.H.; Larsson, M.; Messmer, D. Effect of oxygen levels on the physiology of dendritic cells: Implications for adoptive cell therapy. Mol. Med. 2011, 17, 910–916. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peter, A.; Vermeulen, M.; Van Delen, M.; Dams, A.; Peeters, S.; De Reu, H.; Marei, W.F.A.; Berneman, Z.N.; Cools, N. Physiological Oxygen Levels in the Microenvironment Program Ex Vivo-Generated Conventional Dendritic Cells Toward a Tolerogenic Phenotype. Cells 2025, 14, 736. https://doi.org/10.3390/cells14100736
Peter A, Vermeulen M, Van Delen M, Dams A, Peeters S, De Reu H, Marei WFA, Berneman ZN, Cools N. Physiological Oxygen Levels in the Microenvironment Program Ex Vivo-Generated Conventional Dendritic Cells Toward a Tolerogenic Phenotype. Cells. 2025; 14(10):736. https://doi.org/10.3390/cells14100736
Chicago/Turabian StylePeter, Antonia, Morgane Vermeulen, Mats Van Delen, Amber Dams, Stefanie Peeters, Hans De Reu, Waleed F. A. Marei, Zwi N. Berneman, and Nathalie Cools. 2025. "Physiological Oxygen Levels in the Microenvironment Program Ex Vivo-Generated Conventional Dendritic Cells Toward a Tolerogenic Phenotype" Cells 14, no. 10: 736. https://doi.org/10.3390/cells14100736
APA StylePeter, A., Vermeulen, M., Van Delen, M., Dams, A., Peeters, S., De Reu, H., Marei, W. F. A., Berneman, Z. N., & Cools, N. (2025). Physiological Oxygen Levels in the Microenvironment Program Ex Vivo-Generated Conventional Dendritic Cells Toward a Tolerogenic Phenotype. Cells, 14(10), 736. https://doi.org/10.3390/cells14100736





