PANoptosis as a Two-Edged Sword in Colorectal Cancer: A Pathogenic Mechanism and Therapeutic Opportunity
Abstract
1. Introduction
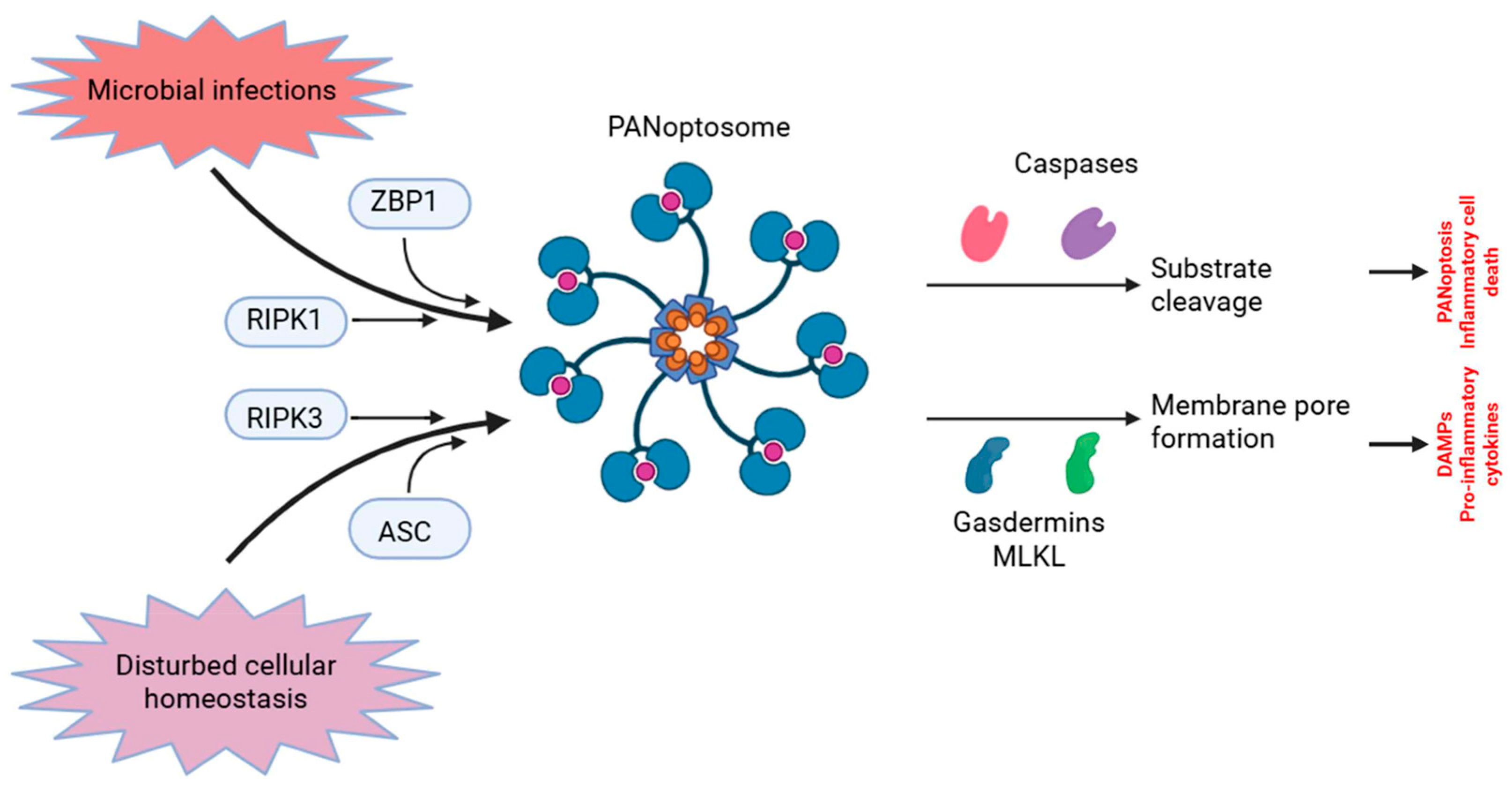
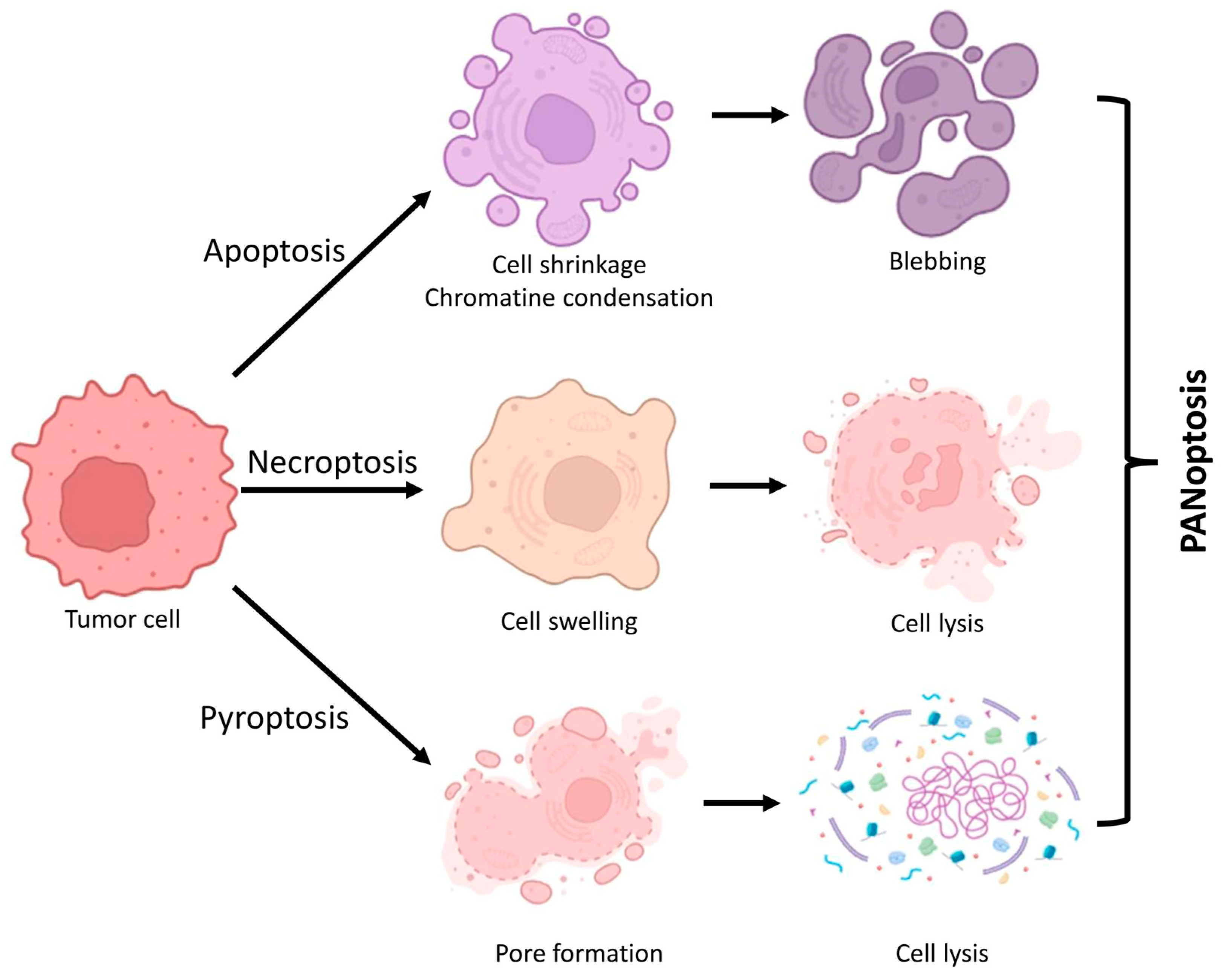
2. Fundamental Characteristics and Processes of PANoptosis
3. Synergistic and Evasive Functional Effects of the PANoptosome as a Central Integrative Hub
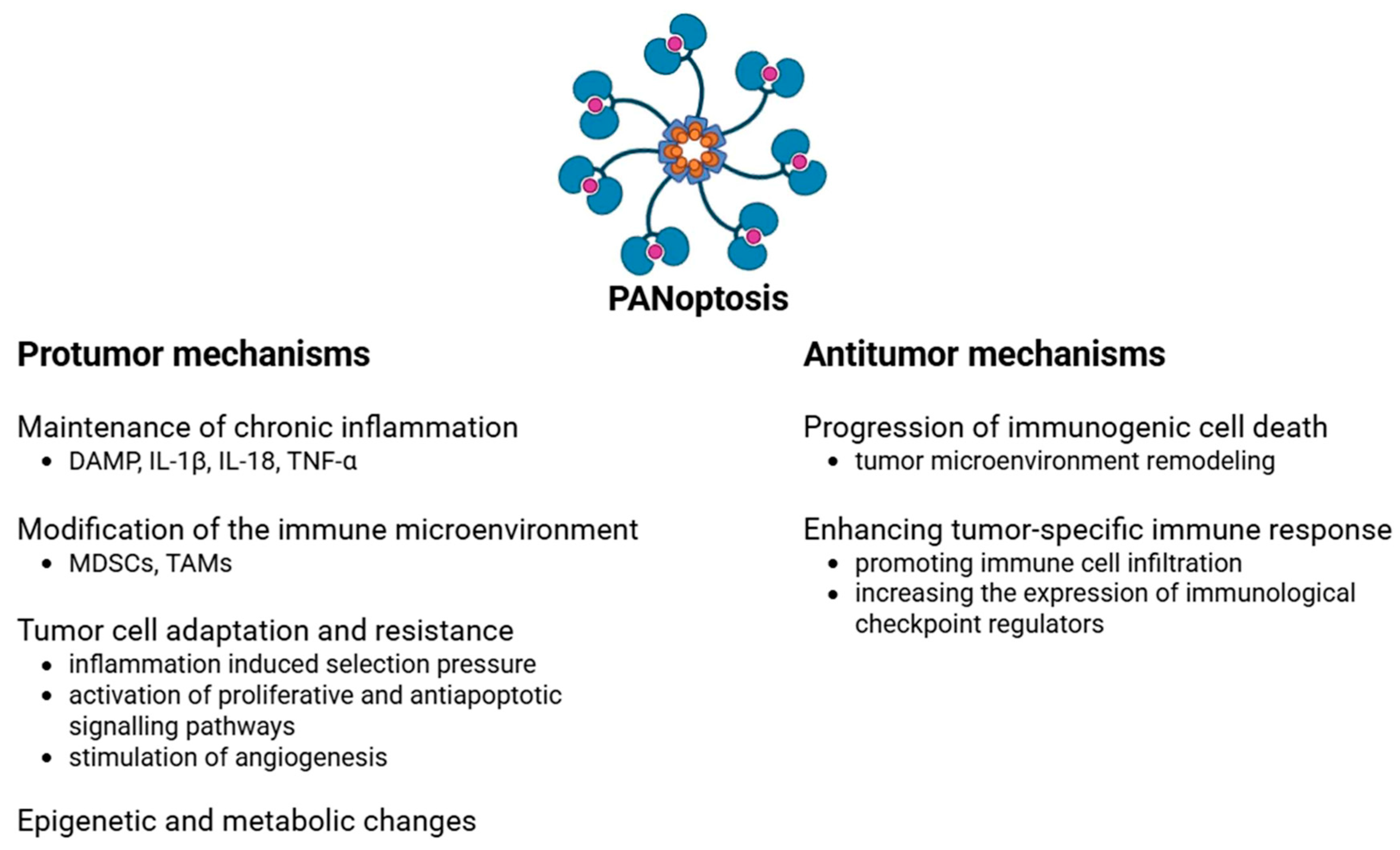
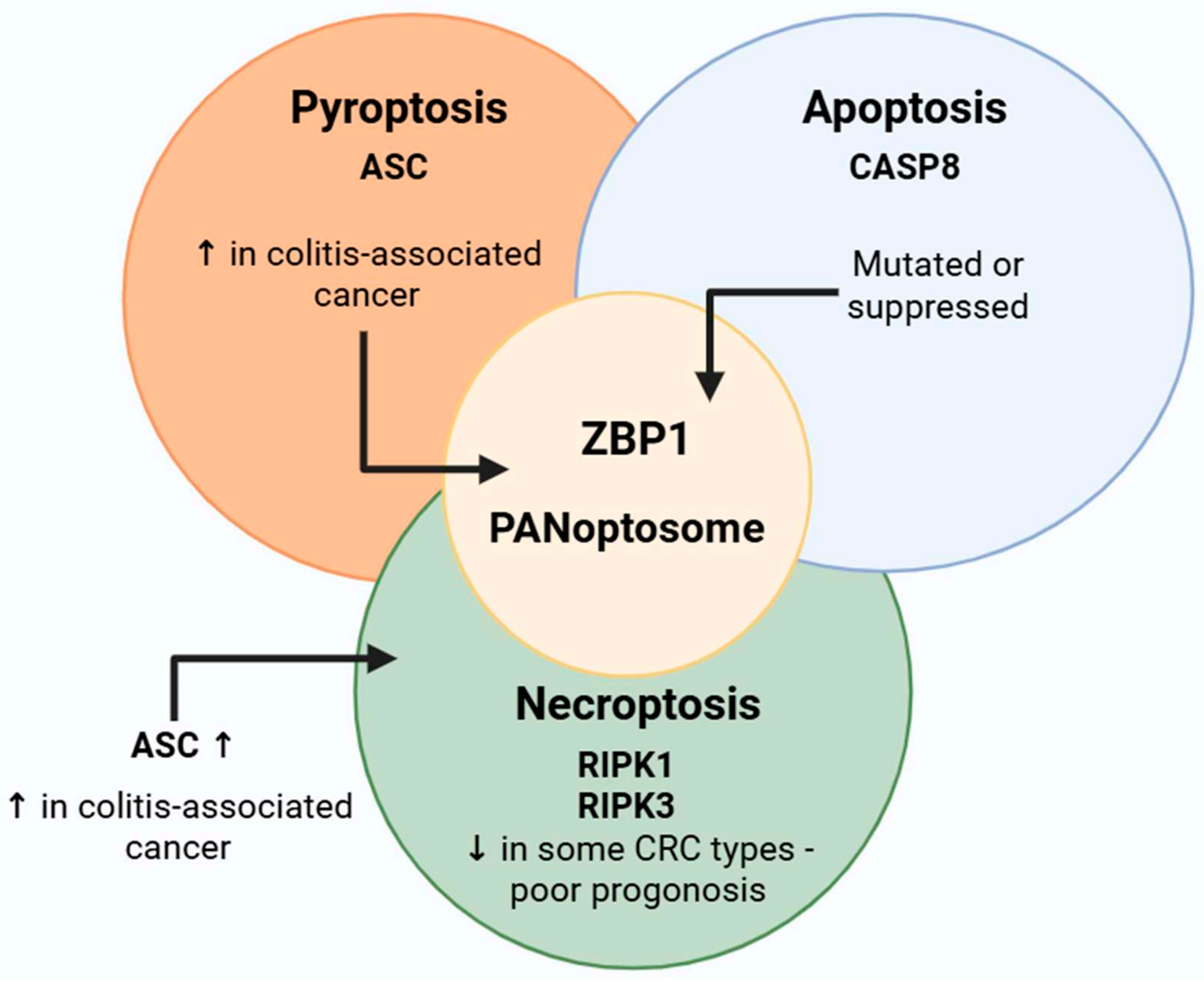
4. PANoptosis in Colorectal Tumor Development
4.1. Protumor Effects
4.2. Antitumor Effects
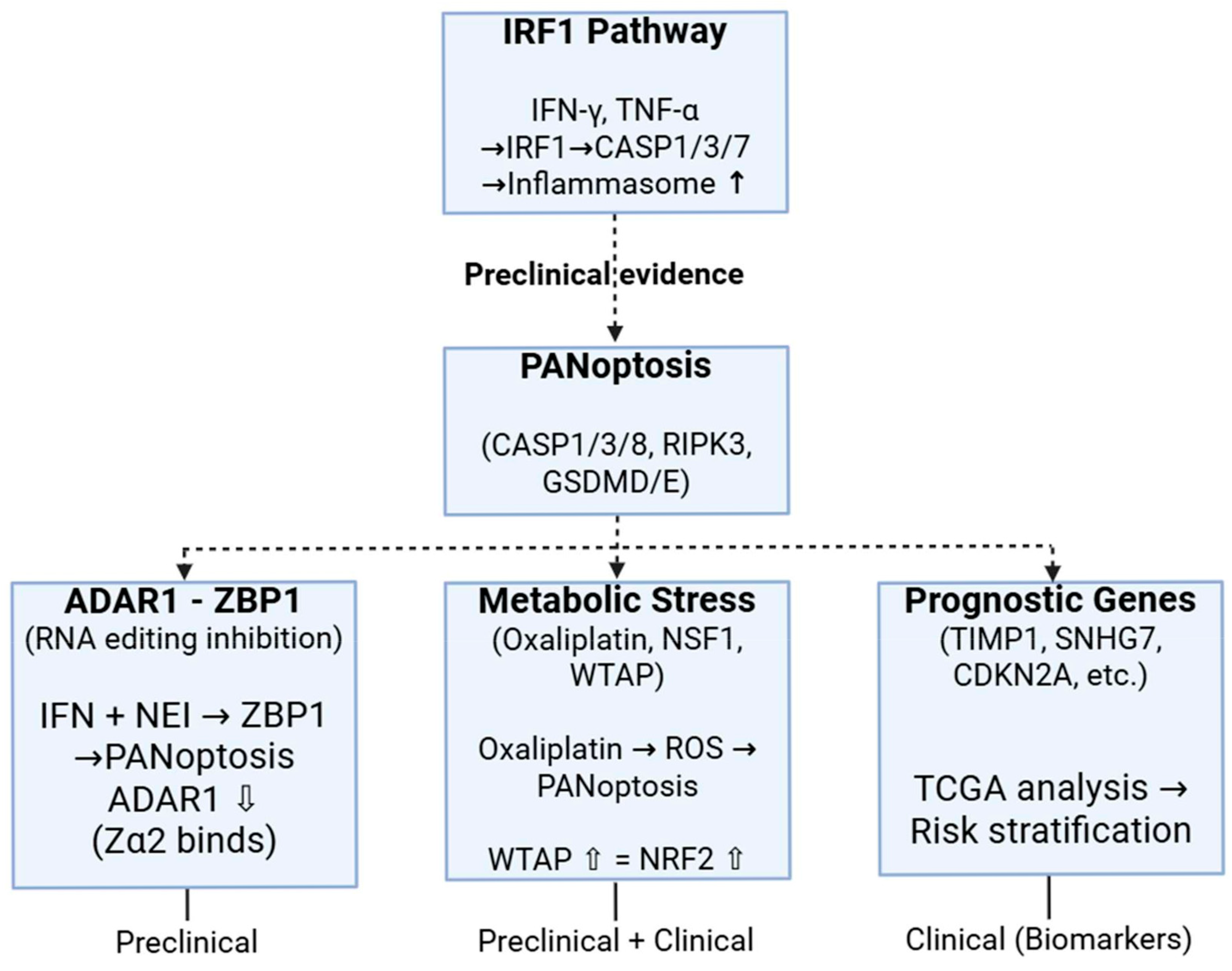
5. Preclinical and Clinical Dimensions of PANoptosis in Colorectal Cancer
Expression of Main Components of PANoptosis in Colorectal Cancer
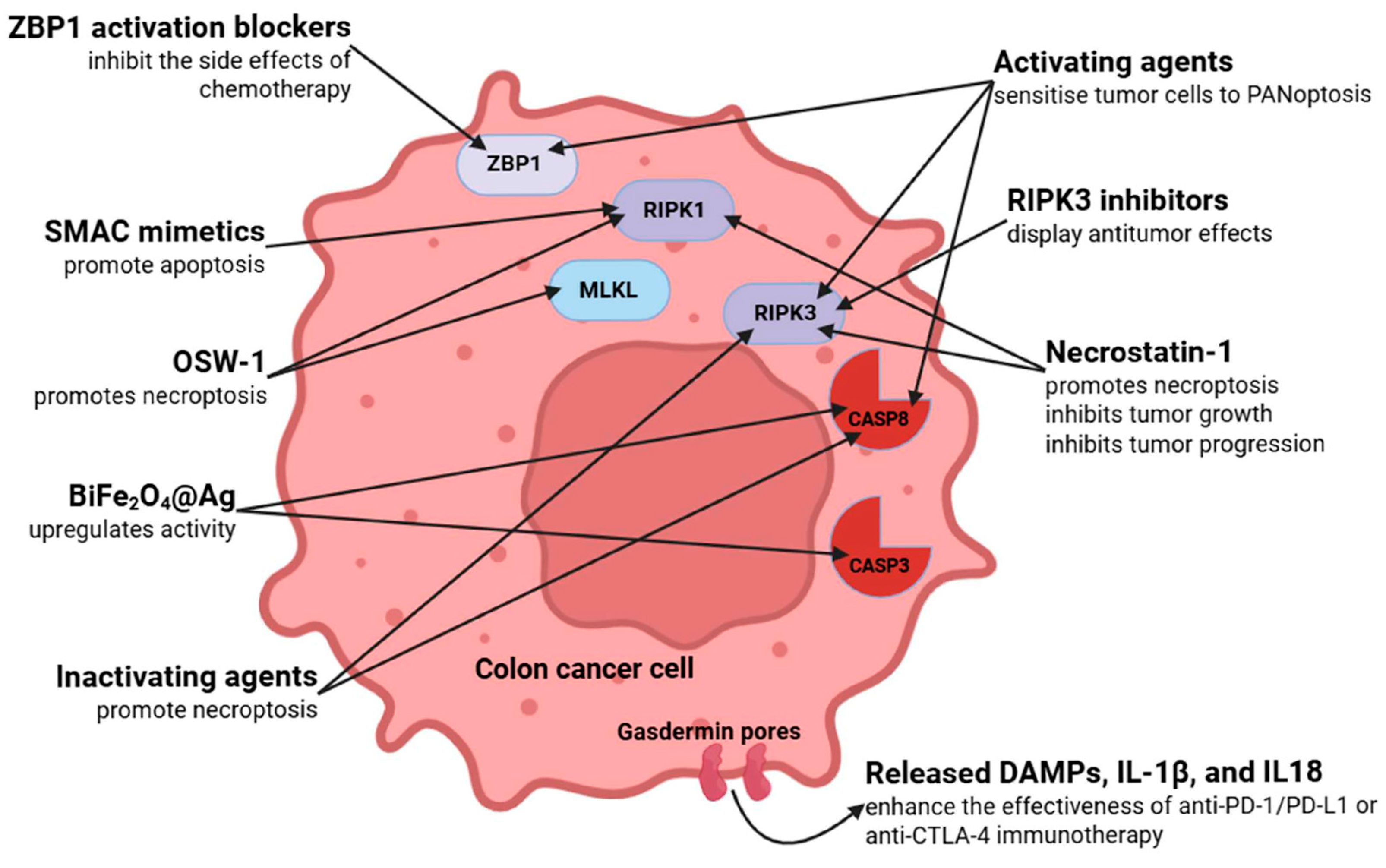
6. Therapeutic Strategies and Targets Based on PANoptosis in Colorectal Cancer
7. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Yan, L.; Shi, J.; Zhu, J. Cellular and molecular events in colorectal cancer: Biological mechanisms, cell death pathways, drug resistance and signalling network interactions. Discov. Oncol. 2024, 15, 294. [Google Scholar] [CrossRef] [PubMed]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef] [PubMed]
- Sagaert, X.; Vanstapel, A.; Verbeek, S. Tumor Heterogeneity in Colorectal Cancer: What Do We Know So Far? Pathobiology 2018, 85, 72–84. [Google Scholar] [CrossRef]
- Sasaki, N.; Clevers, H. Studying cellular heterogeneity and drug sensitivity in colorectal cancer using organoid technology. Curr. Opin. Genet. Dev. 2018, 52, 117–122. [Google Scholar] [CrossRef]
- Pinsky, P.F.; Doroudi, M. Colorectal Cancer Screening. JAMA 2016, 316, 1715. [Google Scholar] [CrossRef]
- Ma, J.-Y.; Wang, Y.-X.; Zhao, Z.-Y.; Xiong, Z.-Y.; Zhang, Z.-L.; Cai, J.; Guo, J.-W. Identification of key programmed cell death genes for predicting prognosis and treatment sensitivity in colorectal cancer. Front. Oncol. 2024, 14, 1483987. [Google Scholar] [CrossRef]
- Davidson, B.; Gurusamy, K.; Corrigan, N.; Croft, J.; Ruddock, S.; Pullan, A.; Brown, J.; Twiddy, M.; Birtwistle, J.; Morris, S.; et al. Liver resection surgery compared with thermal ablation in high surgical risk patients with colorectal liver metastases: The LAVA international RCT. Health Technol. Assess. 2020, 24, 1–38. [Google Scholar] [CrossRef]
- Hornbech, K.; Ravn, J.; Steinbrüchel, D.A. Outcome after pulmonary metastasectomy: Analysis of 5 years consecutive surgical resections 2002–2006. J. Thorac. Oncol. 2011, 6, 1733–1740. [Google Scholar] [CrossRef]
- Tang, D.; Kang, R.; Berghe, T.V.; Vandenabeele, P.; Kroemer, G. The molecular machinery of regulated cell death. Cell Res. 2019, 29, 347–364. [Google Scholar] [CrossRef]
- Malireddi, R.K.S.; Kesavardhana, S.; Kanneganti, T.D. ZBP1 and TAK1: Master Regulators of NLRP3 Inflammasome/Pyroptosis, Apoptosis, and Necroptosis (PAN-optosis). Front. Cell. Infect. Microbiol. 2019, 9, 406. [Google Scholar] [CrossRef]
- Shi, C.; Cao, P.; Wang, Y.; Zhang, Q.; Zhang, D.; Wang, Y.; Wang, L.; Gong, Z. PANoptosis: A Cell Death Characterized by Pyroptosis, Apoptosis, and Necroptosis. J. Inflamm. Res. 2023, 16, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Morana, O.; Wood, W.; Gregory, C.D. The Apoptosis Paradox in Cancer. Int. J. Mol. Sci. 2022, 23, 1328. [Google Scholar] [CrossRef]
- Gao, L.; Shay, C.; Teng, Y. Cell death shapes cancer immunity: Spotlighting PANoptosis. J. Exp. Clin. Cancer Res. 2024, 43, 168. [Google Scholar] [CrossRef]
- Pandey, A.; Li, Z.; Gautam, M.; Ghosh, A.; Man, S.M. Molecular mechanisms of emerging inflammasome complexes and their activation and signaling in inflammation and pyroptosis. Immunol. Rev. 2025, 329, e13406. [Google Scholar] [CrossRef]
- Wang, H.; Feng, X.; He, H.; Li, L.; Wetn, Y.; Liu, X.; He, B.; Hual, S.; Sun, S. Crosstalk between autophagy and other forms of programmed cell death. Eur. J. Pharmacol. 2025, 995, 177414. [Google Scholar] [CrossRef]
- Vogler, M.; Braun, Y.; Smith, V.M.; Westhoff, M.-A.; Pereira, R.S.; Pieper, N.M.; Anders, M.; Callens, M.; Vervliet, T.; Abbas, M.; et al. The BCL2 family: From apoptosis mechanisms to new advances in targeted therapy. Signal Transduct. Target. Ther. 2025, 10, 91. [Google Scholar] [CrossRef]
- Hao, M.-Y.; Li, H.-J.; Han, H.-S.; Chu, T.; Wang, Y.-W.; Si, W.-R.; Jiang, Q.-Y.; Wu, D.-D. Recent advances in the role of gasotransmitters in necroptosis. Apoptosis 2025, 30, 616–635. [Google Scholar] [CrossRef]
- Nadella, V.; Kanneganti, T.D. Inflammasomes and their role in PANoptosomes. Curr. Opin. Immunol. 2024, 91, 102489. [Google Scholar] [CrossRef]
- Liu, K.; Wang, M.; Li, D.; Duong, N.T.D.; Liu, Y.; Mal, J.; Xin, K.; Zhou, Z. PANoptosis in autoimmune diseases interplay between apoptosis, necrosis, and pyroptosis. Front. Immunol. 2024, 15, 1502855. [Google Scholar] [CrossRef]
- Sundaram, B.; Tweedell, R.E.; Prasanth Kumar, S.; Kanneganti, T.D. The NLR family of innate immune and cell death sensors. Immunity 2024, 57, 674–699. [Google Scholar] [CrossRef]
- Pandeya, A.; Kanneganti, T.D. Therapeutic potential of PANoptosis: Innate sensors, inflammasomes, and RIPKs in PANoptosomes. Trends Mol. Med. 2024, 30, 74–88. [Google Scholar] [CrossRef] [PubMed]
- You, Y.-P.; Yan, L.; Ke, H.-Y.; Li, Y.-P.; Shi, Z.-J.; Zhou, Z.-Y.; Yang, H.-Y.; Yuan, T.; Gan, Y.-Q.; Lu, N.; et al. Baicalin inhibits PANoptosis by blocking mitochondrial Z-DNA formation and ZBP1-PANoptosome assembly in macrophages. Acta Pharmacol. Sin. 2025, 46, 430–447. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Yang, H.-Y.; Li, Y.-P.; Shi, Z.-J.; Zhou, Z.-Y.; You, Y.-P.; Ke, H.-Y.; Yan, L.; Xu, L.-H.; Ouyang, D.-Y.; et al. Scutellarin inhibits inflammatory PANoptosis by diminishing mitochondrial ROS generation and blocking PANoptosome formation. Int. Immunopharmacol. 2024, 139, 112710. [Google Scholar] [CrossRef]
- Song, K.; Wu, Y.; Tan, S. Caspases in PANoptosis. Curr. Res. Transl. Med. 2025, 73, 103502. [Google Scholar] [CrossRef]
- Sahoo, G.; Samal, D.; Khandayataray, P.; Murthy, M.K. A Review on Caspases: Key Regulators of Biological Activities and Apoptosis. Mol. Neurobiol. 2023, 60, 5805–5837. [Google Scholar] [CrossRef]
- Zhou, Y.; Xiang, Y.; Liu, S.; Li, C.; Dong, J.; Kong, X.; Ji, X.; Cheng, X.; Zhang, L. RIPK3 signaling and its role in regulated cell death and diseases. Cell Death Discov. 2024, 10, 200. [Google Scholar] [CrossRef]
- Ye, K.; Chen, Z.; Xu, Y. The double-edged functions of necroptosis. Cell Death Dis. 2023, 14, 163. [Google Scholar] [CrossRef]
- Karki, R.; Kanneganti, T.D. PANoptosome signaling and therapeutic implications in infection: Central role for ZBP1 to activate the inflammasome and PANoptosis. Curr. Opin. Immunol. 2023, 83, 102348. [Google Scholar] [CrossRef]
- Dai, Z.; Liu, W.C.; Chen, X.Y.; Wang, X.; Li, J.L.; Zhang, X. Gasdermin D-mediated pyroptosis: Mechanisms, diseases, and inhibitors. Front. Immunol. 2023, 14, 1178662. [Google Scholar] [CrossRef]
- Oh, S.; Lee, S. Recent advances in ZBP1-derived PANoptosis against viral infections. Front. Immunol. 2023, 14, 1148727. [Google Scholar] [CrossRef]
- Orning, P.; Lien, E. Multiple roles of caspase-8 in cell death, inflammation, and innate immunity. J. Leukoc. Biol. 2021, 109, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Pandian, N.; Kanneganti, T.D. PANoptosis: A Unique Innate Immune Inflammatory Cell Death Modality. J. Immunol. 2022, 209, 1625–1633. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Ma, C.; Liang, S.; Chen, M.; He, Y.; Lei, W. PANoptosis: Novel insight into regulated cell death and its potential role in cardiovascular diseases (Review). Int. J. Mol. Med. 2024, 54, 74. [Google Scholar] [CrossRef]
- Zhu, L.; Qi, Z.; Zhang, H.; Wang, N. Nucleic Acid Sensor-Mediated PANoptosis in Viral Infection. Viruses 2024, 16, 966. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, Y.; Zhang, L.; Guo, L.; Wang, X.; Pan, Z.; Jiang, X.; Wu, F.; He, G. Mechanisms of PANoptosis and relevant small-molecule compounds for fighting diseases. Cell Death Dis. 2023, 14, 851. [Google Scholar] [CrossRef]
- Wang, Y.; Kanneganti, T.D. From pyroptosis, apoptosis and necroptosis to PANoptosis: A mechanistic compendium of programmed cell death pathways. Comput. Struct. Biotechnol. J. 2021, 19, 4641–4657. [Google Scholar] [CrossRef]
- Tian, R.; Song, H.; Li, J.; Yuan, T.; Liu, J.; Wang, Y.; Li, Y.; Song, X. PINCH-1 promotes tumor growth and metastasis by enhancing DRP1-mediated mitochondrial fission in head and neck squamous cell carcinoma. Cancer Biol. Ther. 2025, 26, 2477365. [Google Scholar] [CrossRef]
- Zhu, X.; Feng, Z.; Peng, X.; Di, T.; Li, Y.; Bai, J.; Ma, T.; Li, L.; Zhang, L. Threonine and tyrosine kinase promotes multiple myeloma progression by regulating regucalcin expression. Exp. Cell Res. 2025, 446, 114454. [Google Scholar] [CrossRef]
- Cui, Y.; Cao, X.; Zhang, Y.; Fu, C.; Li, D.; Sun, Y.; Zhang, Y.; Xu, T.; Tsukamoto, T.; Cao, D.; et al. Protein phosphatase 1 regulatory subunit 15 A (PPP1R15A) promoted the progression of gastric cancer by activating cell autophagy under energy stress. J. Exp. Clin. Cancer Res. 2025, 44, 52. [Google Scholar] [CrossRef]
- Yingsunthonwattana, W.; Sangsuriya, P.; Supungul, P.; Tassanakajon, A. Litopenaeus vannamei heat shock protein 90 (LvHSP90) interacts with white spot syndrome virus protein, WSSV322, to modulate hemocyte apoptosis during viral infection. Fish Shellfish. Immunol. 2024, 151, 109695. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, J.; Cheng, W.; Cheng, X.; Lu, L.; Gui, L.; Jiang, Y.; Zhang, Y.; Xu, D. miR-124 mediates the expression of ccBax to regulate Cyprinid herpesvirus 2 (CyHV-2)-induced apoptosis and viral replication. J. Fish Dis. 2023, 46, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Hu, D.; Xue, S.; Mao, F.; Obeng, E.; Quan, Y.; Yu, W. HN1L is essential for cell growth and survival during nucleopolyhedrovirus infection in silkworm, Bombyx mori. PLoS ONE 2019, 14, e0216719. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Shi, J.; Hou, R.; Huang, Y.; Wang, C.; Du, H. The necroptosis-related lncRNA ENSG00000253385.1 promotes the progression of esophageal squamous cell carcinoma by targeting the miR-16-2-3p/VDAC1 axis. Sci. Rep. 2025, 15, 2650. [Google Scholar] [CrossRef]
- Cong, L.; Liu, X.; Bai, Y.; Qin, Q.; Zhao, L.; Shi, Y.; Bai, Y.; Guo, Z. Melatonin alleviates pyroptosis by regulating the SIRT3/FOXO3α/ROS axis and interacting with apoptosis in Atherosclerosis progression. Biol. Res. 2023, 56, 62. [Google Scholar] [CrossRef]
- Yuan, S.; Wang, Y.; Li, Z.; Chen, X.; Song, P.; Chen, A.; Qu, Z.; Wen, S.; Liu, H.; Zhu, X. Gasdermin D is involved in switching from apoptosis to pyroptosis in TLR4-mediated renal tubular epithelial cells injury in diabetic kidney disease. Arch. Biochem. Biophys. 2022, 727, 109347. [Google Scholar] [CrossRef]
- Bock, F.J.; Riley, J.S. When cell death goes wrong: Inflammatory outcomes of failed apoptosis and mitotic cell death. Cell Death Differ. 2023, 30, 293–303. [Google Scholar] [CrossRef]
- Carty, M.; Guy, C.; Bowie, A.G. Detection of Viral Infections by Innate Immunity. Biochem. Pharmacol. 2021, 183, 114316. [Google Scholar] [CrossRef]
- Karki, R.; Kanneganti, T.D. The ‘cytokine storm’: Molecular mechanisms and therapeutic prospects. Trends Immunol. 2021, 42, 681–705. [Google Scholar] [CrossRef]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef]
- Karki, R.; Sharma, B.R.; Tuladhar, S.; Williams, E.P.; Zalduondo, L.; Samir, P.; Zheng, M.; Sundaram, B.; Banoth, B.; Malireddi, R.K.S.; et al. Synergism of TNF-α and IFN-γ Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes. Cell 2021, 184, 149–168.e17. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Kanneganti, T.D. PANoptosis in Viral Infection: The Missing Puzzle Piece in the Cell Death Field. J. Mol. Biol. 2022, 434, 167249. [Google Scholar] [CrossRef] [PubMed]
- Kuriakose, T.; Man, S.M.; Malireddi, R.K.S.; Karki, R.; Ketsavardhana, S.; Place, D.E.; Neale, G.; Vogel, P.; Kanneganti, T.-D. ZBP1/DAI is an innate sensor of influenza virus triggering the NLRP3 inflammasome and programmed cell death pathways. Sci. Immunol. 2016, 1, aag2045. [Google Scholar] [CrossRef]
- Zheng, M.; Williams, E.P.; Malireddi, R.K.S.; Karki, R.; Banoth, B.; Burton, A.; Webby, R.; Channappanavar, R.; Jonsson, C.B.; Kanneganti, T.-D. Impaired NLRP3 inflammasome activation/pyroptosis leads to robust inflammatory cell death via caspase-8/RIPK3 during coronavirus infection. J. Biol. Chem. 2020, 295, 14040–14052. [Google Scholar] [CrossRef]
- Christgen, S.; Zheng, M.; Kesavardhana, S.; Karki, R.; Malireddi, R.K.S.; Banoth, B.; Place, D.E.; Briard, B.; Sharma, B.R.; Tuladhar, S.; et al. Identification of the PANoptosome: A Molecular Platform Triggering Pyroptosis, Apoptosis, and Necroptosis (PANoptosis). Front. Cell. Infect. Microbiol. 2020, 10, 237. [Google Scholar] [CrossRef]
- Huang, Z.; Wu, S.-Q.; Liang, Y.; Zhou, X.; Chen, W.; Li, L.; Wu, J.; Zhuang, Q.; Chen, C.; Li, J.; et al. RIP1/RIP3 binding to HSV-1 ICP6 initiates necroptosis to restrict virus propagation in mice. Cell Host Microbe 2015, 17, 229–242. [Google Scholar] [CrossRef]
- Guo, H.; Omoto, S.; Harris, P.A.; Finger, J.N.; Bertin, J.; Gough, P.J.; Kaliser, W.J.; Mocarski, E.S. Herpes simplex virus suppresses necroptosis in human cells. Cell Host Microbe 2015, 17, 243–251. [Google Scholar] [CrossRef]
- Fletcher-Etherington, A.; Nobre, L.; Nightingale, K.; Antrobus, R.; Nichols, J.; Davison, A.J.; Stanton, R.J.; Weekes, M.P. Human cytomegalovirus protein pUL36: A dual cell death pathway inhibitor. Proc. Natl. Acad. Sci. USA. 2020, 117, 18771–18779. [Google Scholar] [CrossRef]
- He, X.; Jiang, X.; Guo, J.; Sun, H.; Yang, J. PANoptosis in Bacterial Infections: A Double-Edged Sword Balancing Host Immunity and Pathogenesis. Pathogens 2025, 14, 43. [Google Scholar] [CrossRef]
- Ashida, H.; Sasakawa, C.; Suzuki, T. A unique bacterial tactic to circumvent the cell death crosstalk induced by blockade of caspase-8. EMBO J. 2020, 39, e104469. [Google Scholar] [CrossRef]
- Kobayashi, T.; Ogawa, M.; Sanada, T.; Mimuro, H.; Kim, M.; Ashida, H.; Akakura, R.; Yoshida, M.; Kawalec, M.; Reichhart, J.-M.; et al. The Shigella OspC3 effector inhibits caspase-4, antagonizes inflammatory cell death, and promotes epithelial infection. Cell Host Microbe 2013, 13, 570–583. [Google Scholar] [CrossRef]
- Günther, S.D.; Fritsch, M.; Seeger, J.M.; Schiffmann, L.M.; Snipas, S.J.; Coutelle, M.; Kufer, T.A.; Higgins, P.G.; Hornung, V.; Bernardini, M.L.; et al. Cytosolic Gram-negative bacteria prevent apoptosis by inhibition of effector caspases through lipopolysaccharide. Nat. Microbiol. 2020, 5, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Bergounioux, J.; Elisee, R.; Prunier, A.-L.; Donnadieu, F.; Sperandio, B.; Sansonetti, P.; Arbibe, L. Calpain activation by the Shigella flexneri effector VirA regulates key steps in the formation and life of the bacterium’s epithelial niche. Cell Host Microbe 2012, 11, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Ramel, D.; Lagarrigue, F.; Pons, V.; Mounier, J.; Dupuis-Coronas, S.; Chicanne, G.; Sansonetti, P.J.; Gaits-Iacovoni, F.; Tronchère, H.; Payrastre, B. Shigella flexneri infection generates the lipid PI5P to alter endocytosis and prevent termination of EGFR signaling. Sci. Signal. 2011, 4, ra61. [Google Scholar] [CrossRef] [PubMed]
- Imani, S.; Farghadani, R.; Roozitalab, G.; Maghsoudloo, M.; Emadi, M.; Moradi, A.; Abedi, B.; Kaboli, P.J. Reprogramming the breast tumor immune microenvironment: Cold-to-hot transition for enhanced immunotherapy. J. Exp. Clin. Cancer Res. 2025, 44, 131. [Google Scholar] [CrossRef]
- Li, J.; Huang, L.; Zhao, H.; Yan, Y.; Lu, J. The Role of Interleukins in Colorectal Cancer. Int. J. Biol. Sci. 2020, 16, 2323–2339. [Google Scholar] [CrossRef]
- Kay, J.; Thadhani, E.; Samson, L.; Engelward, B. Inflammation-induced DNA damage, mutations and cancer. DNA Repair. 2019, 83, 102673. [Google Scholar] [CrossRef]
- Meira, L.B.; Bugni, J.M.; Green, S.L.; Lee, C.-W.; Pang, B.; Borenshtein, D.; Rickman, B.H.; Rogers, A.B.; Moroski-Erkul, C.A.; McFaline, J.L.; et al. DNA damage induced by chronic inflammation contributes to colon carcinogenesis in mice. J. Clin. Investig. 2008, 118, 2516–2525. [Google Scholar] [CrossRef]
- Mangerich, A.; Knutson, C.G.; Parry, N.M.; Muthupalani, S.; Ye, W.; Prestwich, E.; Cui, L.; McFaline, J.L.; Mobley, M.; Ge, Z.; et al. Infection-induced colitis in mice causes dynamic and tissue-specific changes in stress response and DNA damage leading to colon cancer. Proc. Natl. Acad. Sci. USA. 2012, 109, E1820–E1829. [Google Scholar] [CrossRef]
- Wang, K.; Wang, Y.; Yin, K. Role played by MDSC in colitis-associated colorectal cancer and potential therapeutic strategies. J. Cancer Res. Clin. Oncol. 2024, 150, 243. [Google Scholar] [CrossRef]
- Ke, Z.; Wang, C.; Wu, T.; Wang, W.; Yang, Y.; Dai, Y. PAR2 deficiency enhances myeloid cell-mediated immunosuppression and promotes colitis-associated tumorigenesis. Cancer Lett. 2020, 469, 437–446. [Google Scholar] [CrossRef]
- Wang, H.; Tian, T.; Zhang, J. Tumor-Associated Macrophages (TAMs) in Colorectal Cancer (CRC): From Mechanism to Therapy and Prognosis. Int. J. Mol. Sci. 2021, 22, 8470. [Google Scholar] [CrossRef]
- Traughber, C.A.; Deshpande, G.M.; Neupane, K.; Bhandari, N.; Khan, M.R.; McMullen, M.R.; Swaidani, S.; Opoku, E.; Muppala, S.; Smith, J.D.; et al. Myeloid-cell-specific role of Gasdermin D in promoting lung cancer progression in mice. iScience 2023, 26, 106076. [Google Scholar] [CrossRef]
- Yan, G.; Zhao, H.; Zhang, Q.; Zhou, Y.; Wu, L.; Lei, J.; Wang, X.; Zhang, J.; Zhang, X.; Zheng, L.; et al. A RIPK3-PGE2 Circuit Mediates Myeloid-Derived Suppressor Cell-Potentiated Colorectal Carcinogenesis. Cancer Res. 2018, 78, 5586–5599. [Google Scholar] [CrossRef]
- Jayakumar, A.; Bothwell, A.L.M. RIPK3-Induced Inflammation by I-MDSCs Promotes Intestinal Tumors. Cancer Res. 2019, 79, 1587–1599. [Google Scholar] [CrossRef]
- Zhang, N.; Gao, X.; Zhang, W.; Xiong, J.; Cao, X.; Fu, Z.F.; Cui, M. JEV Infection Induces M-MDSC Differentiation Into CD3+ Macrophages in the Brain. Front. Immunol. 2022, 13, 838990. [Google Scholar] [CrossRef]
- Herbert, A.; Balachandran, S. Z-DNA enhances immunotherapy by triggering death of inflammatory cancer-associated fibroblasts. J. Immunother. Cancer 2022, 10, e005704. [Google Scholar] [CrossRef]
- Ge, Y.; Jiang, L.; Yang, C.; Dong, Q.; Tang, C.; Xu, Y.; Zhong, X. Interactions between tumor-associated macrophages and regulated cell death: Therapeutic implications in immuno-oncology. Front. Oncol. 2024, 14, 1449696. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, B.; Luo, J.; Yang, Y.; Weng, Q.; Fang, S.; Zhao, Z.; Tu, J.; Chen, M.; Ji, J. Cuproptosis, ferroptosis and PANoptosis in tumor immune microenvironment remodeling and immunotherapy: Culprits or new hope. Mol. Cancer. 2024, 23, 255. [Google Scholar] [CrossRef]
- Seidel, J.A.; Otsuka, A.; Kabashima, K. Anti-PD-1 and Anti-CTLA-4 Therapies in Cancer: Mechanisms of Action, Efficacy, and Limitations. Front. Oncol. 2018, 8, 86. [Google Scholar] [CrossRef]
- Fang, L.; Liu, K.; Liu, C.; Wang, X.; Ma, W.; Xu, W.; Wu, J.; Sun, C. Tumor accomplice: T cell exhaustion induced by chronic inflammation. Front. Immunol. 2022, 13, 979116. [Google Scholar] [CrossRef]
- Koi, M.; Tseng-Rogenski, S.S.; Carethers, J.M. Inflammation-associated microsatellite alterations: Mechanisms and significance in the prognosis of patients with colorectal cancer. World J. Gastrointest. Oncol. 2018, 10, 1–14. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Y.; Zhu, B. T-cell exhaustion in the tumor microenvironment. Cell Death Dis. 2015, 6, e1792. [Google Scholar] [CrossRef]
- Cai, Y.; Xiao, H.; Zhou, Q.; Lin, J.; Liang, X.; Xu, W.; Cao, Y.; Zhang, X.; Wang, H. Comprehensive Analyses of PANoptosome with Potential Implications in Cancer Prognosis and Immunotherapy. Biochem. Genet. 2025, 63, 331–353. [Google Scholar] [CrossRef]
- Wen, Y.; Zhu, Y.; Zhang, C.; Yang, X.; Gao, Y.; Li, M.; Yang, H.; Liu, T.; Tang, H. Chronic inflammation, cancer development and immunotherapy. Front. Pharmacol. 2022, 13, 1040163. [Google Scholar] [CrossRef]
- Hu, F.; Song, D.; Yan, Y.; Huang, C.; Shen, C.; Lan, J.; Chen, Y.; Liu, A.; Wu, Q.; Sun, L.; et al. IL-6 regulates autophagy and chemotherapy resistance by promoting BECN1 phosphorylation. Nat. Commun. 2021, 12, 3651. [Google Scholar] [CrossRef]
- Han, J.; Soletti, R.C.; Sadarangani, A.; Sridevi, P.; Ramirez, M.E.; Eckmann, L.; Borges, H.L.; Wang, J.Y. Nuclear expression of β-catenin promotes RB stability and resistance to TNF-induced apoptosis in colon cancer cells. Mol. Cancer Res. 2013, 11, 207–218. [Google Scholar] [CrossRef]
- Wei, W.; Wang, J.; Huang, P.; Gou, S.; Yu, D.; Zong, L. Tumor necrosis factor-α induces proliferation and reduces apoptosis of colorectal cancer cells through STAT3 activation. Immunogenetics 2023, 75, 161–169. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Pappan, L.; Galliher-Beckley, A.; Shi, J. IL-1β promotes stemness and invasiveness of colon cancer cells through Zeb1 activation. Mol. Cancer. 2012, 11, 87. [Google Scholar] [CrossRef]
- Dong, Y.; Chen, J.; Chen, Y.; Liu, S. Targeting the STAT3 oncogenic pathway: Cancer immunotherapy and drug repurposing. Biomed. Pharmacother. 2023, 167, 115513. [Google Scholar] [CrossRef]
- Deng, Z.; Fan, T.; Xiao, C.; Tian, H.; Zheng, Y.; Li, C.; He, J. TGF-β signaling in health, disease, and therapeutics. Signal Transduct. Target. Ther. 2024, 9, 61. [Google Scholar] [CrossRef]
- Stefani, C.; Miricescu, D.; Stanescu-Spinu, I.-I.; Nica, R.I.; Greabu, M.; Totan, A.R.; Jinga, M. Growth Factors, PI3K/AKT/mTOR and MAPK Signaling Pathways in Colorectal Cancer Pathogenesis: Where Are We Now? Int. J. Mol. Sci. 2021, 22, 10260. [Google Scholar] [CrossRef]
- Wang, S.; He, H.; Qu, L.; Shen, Q.; Dai, Y. Dual roles of inflammatory programmed cell death in cancer: Insights into pyroptosis and necroptosis. Front. Pharmacol. 2024, 15, 1446486. [Google Scholar] [CrossRef]
- Wang, H.; Wang, T.; Yan, S.; Tang, J.; Zhang, Y.; Wang, L.; Xu, H.; Tu, C. Crosstalk of pyroptosis and cytokine in the tumor microenvironment: From mechanisms to clinical implication. Mol. Cancer. 2024, 23, 268. [Google Scholar] [CrossRef]
- Jin, B.; Miao, Z.; Pan, J.; Zhang, Z.; Yang, Y.; Zhou, Y.; Jin, Y.; Niu, Z.; Xu, Q. The emerging role of glycolysis and immune evasion in ovarian cancer. Cancer Cell Int. 2025, 25, 78. [Google Scholar] [CrossRef]
- Zhong, X.; He, X.; Wang, Y.; Hu, Z.; Huang, H.; Zhao, S.; Wei, P.; Li, D. Warburg effect in colorectal cancer: The emerging roles in tumor microenvironment and therapeutic implications. J. Hematol. Oncol. 2022, 15, 160. [Google Scholar] [CrossRef]
- Claycombe, K.J.; Brissette, C.A.; Ghribi, O. Epigenetics of inflammation, maternal infection, and nutrition. J. Nutr. 2015, 145, 1109S–1115S. [Google Scholar] [CrossRef]
- Da Silva, M.L.R.; De Albuquerque, B.H.D.R.; Allyrio, T.A.D.M.F.; De Almeida, V.D.; Cobucci, R.N.D.O.; Bezerra, F.L.; Andrade, V.S.; Lanza, D.C.F.; De Azevedo, J.C.V.; De Araújo, J.M.G.; et al. The role of HPV-induced epigenetic changes in cervical carcinogenesis (Review). Biomed. Rep. 2021, 15, 60. [Google Scholar] [CrossRef]
- Yang, Z.H.; Dang, Y.Q.; Ji, G. Role of epigenetics in transformation of inflammation into colorectal cancer. World J. Gastroenterol. 2019, 25, 2863–2877. [Google Scholar] [CrossRef]
- Cai, H.; Lv, M.; Wang, T. PANoptosis in cancer, the triangle of cell death. Cancer Med. 2023, 12, 22206–22223. [Google Scholar] [CrossRef]
- Zhu, P.; Ke, Z.R.; Chen, J.X.; Li, S.J.; Ma, T.L.; Fan, X.L. Advances in mechanism and regulation of PANoptosis: Prospects in disease treatment. Front. Immunol. 2023, 14, 1120034. [Google Scholar] [CrossRef]
- Meyiah, A.; Khan, F.I.; Alfaki, D.A.; Murshed, K.; Raza, A.; Elkord, E. The colorectal cancer microenvironment: Preclinical progress in identifying targets for cancer therapy. Transl. Oncol. 2025, 53, 102307. [Google Scholar] [CrossRef]
- Najafi-Fard, S.; Petruccioli, E.; Farroni, C.; Petrone, L.; Vanini, V.; Cuzzi, G.; Salmi, A.; Altera, A.M.G.; Navarra, A.; Alonzi, T.; et al. Evaluation of the immunomodulatory effects of interleukin-10 on peripheral blood immune cells of COVID-19 patients: Implication for COVID-19 therapy. Front. Immunol. 2022, 13, 984098. [Google Scholar] [CrossRef]
- Li, H.; Ni, H.; Li, Y.; Zhou, A.; Qin, X.; Li, Y.; Che, L.; Mo, H.; Qin, C.; Li, J. Tumors cells with mismatch repair deficiency induce hyperactivation of pyroptosis resistant to cell membrane damage but are more sensitive to co-treatment of IFN-γ and TNF-α to PANoptosis. Cell Death Discov. 2024, 10, 227. [Google Scholar] [CrossRef]
- Zhou, L.; Lyu, J.; Liu, F.; Su, Y.; Feng, L.; Zhang, X. Immunogenic PANoptosis-Initiated Cancer Sono-Immune Reediting Nanotherapy by Iteratively Boosting Cancer Immunity Cycle. Adv. Mater. 2024, 36, e2305361. [Google Scholar] [CrossRef]
- Yi, X.; Li, J.; Zheng, X.; Xu, H.; Liao, D.; Zhang, T.; Weti, Q.; Li, H.; Peng, J.; Ai, J. Construction of PANoptosis signature: Novel target discovery for prostate cancer immunotherapy. Mol. Ther. Nucleic Acids. 2023, 33, 376–390. [Google Scholar] [CrossRef]
- Xiong, Y. The emerging role of PANoptosis in cancer treatment. Biomed. Pharmacother. 2023, 168, 115696. [Google Scholar] [CrossRef]
- Kamijo, R.; Harada, H.; Matsuyama, T.; Bosland, M.; Gerecitano, J.; Shapiro, D.; Le, J.; Koh, S.I.; Kimura, T.; Green, S.J.; et al. Requirement for transcription factor IRF-1 in NO synthase induction in macrophages. Science 1994, 263, 1612–1615. [Google Scholar] [CrossRef]
- Bouker, K.B.; Skaar, T.C.; Riggins, R.B.; Harburger, D.S.; Fernandez, D.R.; Zwart, A.; Wang, A.; Clarke, R. Interferon regulatory factor-1 (IRF-1) exhibits tumor suppressor activities in breast cancer associated with caspase activation and induction of apoptosis. Carcinogenesis 2005, 26, 1527–1535. [Google Scholar] [CrossRef]
- Man, S.M.; Karki, R.; Malireddi, R.K.S.; Neale, G.; Vogel, P.; Yamamoto, M.; Lamkanfi, M.; Kanneganti, T.-D. The transcription factor IRF1 and guanylate-binding proteins target activation of the AIM2 inflammasome by Francisella infection. Nat. Immunol. 2015, 16, 467–475. [Google Scholar] [CrossRef]
- Briard, B.; Karki, R.; Malireddi, R.K.S.; Bhattacharya, A.; Place, D.E.; Mavuluri, J.; Peters, J.L.; Vogel, P.; Yamamoto, M.; Kanneganti, T.-D. Fungal ligands released by innate immune effectors promote inflammasome activation during Aspergillus fumigatus infection. Nat. Microbiol. 2019, 4, 316–327. [Google Scholar] [CrossRef]
- Zheng, M.; Kanneganti, T.D. The regulation of the ZBP1-NLRP3 inflammasome and its implications in pyroptosis, apoptosis, and necroptosis (PANoptosis). Immunol. rev. 2020, 297, 26–38. [Google Scholar] [CrossRef]
- Karki, R.; Kanneganti, T.D. Diverging inflammasome signals in tumorigenesis and potential targeting. Nat. Rev. Cancer. 2019, 19, 197–214. [Google Scholar] [CrossRef]
- Karki, R.; Man, S.M.; Kanneganti, T.D. Inflammasomes and Cancer. Cancer Immunol. Res. 2017, 5, 94–99. [Google Scholar] [CrossRef]
- Sharma, B.R.; Kanneganti, T.D. Inflammasome signaling in colorectal cancer. Transl. Res. 2023, 252, 45–52. [Google Scholar] [CrossRef]
- Karki, R.; Sharma, B.R.; Lee, E.; Banoth, B.; Malireddi, R.S.; Samir, P.; Tuladhar, S.; Mummareddy, H.; Burton, A.R.; Vogel, P.; et al. Interferon regulatory factor 1 regulates PANoptosis to prevent colorectal cancer. JCI Insight 2020, 5, e136720. [Google Scholar] [CrossRef]
- Karki, R.; Sundaram, B.; Sharma, B.R.; Lee, S.; Malireddi, R.S.; Nguyen, L.N.; Christgen, S.; Zheng, M.; Wang, Y.; Samir, P.; et al. ADAR1 restricts ZBP1-mediated immune response and PANoptosis to promote tumorigenesis. Cell Rep. 2021, 37, 109858. [Google Scholar] [CrossRef]
- Karki, R.; Kanneganti, T.D. ADAR1 and ZBP1 in innate immunity, cell death, and disease. Trends Immunol. 2023, 44, 201–216. [Google Scholar] [CrossRef]
- Hu, S.B.; Li, J.B. RNA editing and immune control: From mechanism to therapy. Curr. Opin. Genet. Dev. 2024, 86, 102195. [Google Scholar] [CrossRef]
- Faubert, B.; Solmonson, A.; DeBerardinis, R.J. Metabolic reprogramming and cancer progression. Science 2020, 368, eaaw5473. [Google Scholar] [CrossRef]
- Pavlova, N.N.; Zhu, J.; Thompson, C.B. The hallmarks of cancer metabolism: Still emerging. Cell Metab. 2022, 34, 355–377. [Google Scholar] [CrossRef]
- Ali, V.; Nozaki, T. Iron-sulphur clusters, their biosynthesis, and biological functions in protozoan parasites. Adv. Parasitol. 2013, 83, 1–92. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, Z.; Le, Y.; Gu, Z.; Zhao, H. Iron-Sulfur Clusters: A Key Factor of Regulated Cell Death in Cancer. Oxidative Med. Cell Longev. 2022, 2022, 7449941. [Google Scholar] [CrossRef]
- Lin, J.-F.; Hu, P.-S.; Wang, Y.-Y.; Tan, Y.-T.; Yu, K.; Liao, K.; Wu, Q.-N.; Li, T.; Meng, Q.; Lin, J.-Z.; et al. Phosphorylated NFS1 weakens oxaliplatin-based chemosensitivity of colorectal cancer by preventing PANoptosis. Signal Transduct. Target. Ther. 2022, 7, 54. [Google Scholar] [CrossRef]
- Majewska, J.; Ciesielski, S.J.; Schilke, B.; Kominek, J.; Blenska, A.; Delewski, W.; Song, J.-Y.; Marszalek, J.; Craig, E.A.; Dutkiewicz, R. Binding of the chaperone Jac1 protein and cysteine desulfurase Nfs1 to the iron-sulfur cluster scaffold Isu protein is mutually exclusive. J. Biol. Chem. 2013, 288, 29134–29142. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.-T.; Li, T.; Wang, R.-B.; Liu, Z.-K.; Ma, M.-Y.; Huang, R.-Z.; Mo, H.-Y.; Luo, S.-Y.; Lin, J.-F.; Xu, R.-H.; et al. WTAP weakens oxaliplatin chemosensitivity of colorectal cancer by preventing PANoptosis. Cancer Lett. 2024, 604, 217254. [Google Scholar] [CrossRef]
- Huang, J.; Jiang, S.; Liang, L.; He, H.; Liu, Y.; Cong, L.; Jiang, Y. Analysis of PANoptosis-Related LncRNA-miRNA-mRNA Network Reveals LncRNA SNHG7 Involved in Chemo-Resistance in Colon Adenocarcinoma. Front. Oncol. 2022, 12, 888105. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, X.; Liu, X.; Jiang, X.; Chen, S.; Li, H.; Ji, H.; Wang, S.; Liang, Q.; Ni, S.; et al. Characterizing PANoptosis gene signature in prognosis and chemosensitivity of colorectal cancer. J. Gastrointest. Oncol. 2024, 15, 2129–2144. [Google Scholar] [CrossRef]
- Yu, X.; Shao, Y.; Dong, H.; Zhang, X.; Ye, G. Biological function and potential application of PANoptosis-related genes in colorectal carcinogenesis. Sci. Rep. 2024, 14, 20672. [Google Scholar] [CrossRef]
- Zhang, M.; Li, W.; Zhao, Y.; Qi, L.; Xiao, Y.; Liu, D.; Peng, T. Molecular characterization analysis of PANoptosis-related genes in colorectal cancer based on bioinformatic analysis. PLoS ONE. 2024, 19, e0307651. [Google Scholar] [CrossRef]
- Wang, S.; Song, A.; Xie, J.; Wang, Y.-Y.; Wang, W.-D.; Zhang, M.-J.; Wu, Z.-Z.; Yang, Q.-C.; Li, H.; Zhang, J.; et al. Fn-OMV potentiates ZBP1-mediated PANoptosis triggered by oncolytic HSV-1 to fuel antitumor immunity. Nat. Commun. 2024, 15, 3669. [Google Scholar] [CrossRef]
- Wang, X.; Sun, R.; Chan, S.; Meng, L.; Xu, Y.; Zuo, X.; Wang, Z.; Hu, X.; Han, Q.; Dai, L.; et al. PANoptosis-based molecular clustering and prognostic signature predicts patient survival and immune landscape in colon cancer. Front. Genet. 2022, 13, 955355. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-M.; Yang, J.; Xia, W.-Y.; Wang, Y.-M.; Zhu, Y.-B.; Huang, Q.; Feng, T.; Xie, L.-S.; Li, S.-H.; Liu, S.-Q.; et al. Comprehensive Analysis of PANoptosis-Related Gene Signature of Ulcerative Colitis. Int. J. Mol. Sci. 2023, 25, 348. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Lin, C.; He, R.; Chen, H.; Teng, Z.; Yao, H.; Liu, S.; Hoffman, R.M.; Ye, J.; Zhu, G. TRAF6 regulates the abundance of RIPK1 and inhibits the RIPK1/RIPK3/MLKL necroptosis signaling pathway and affects the progression of colorectal cancer. Cell Death Dis. 2023, 14, 6. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Guo, J.; Cheng, X.; Liao, Y.; Bi, Y.; Gong, Y.; Zhang, X.; Guo, Y.; Wang, X.; Yu, W.; et al. RIPK3 Suppresses the Progression of Spontaneous Intestinal Tumorigenesis. Front. Oncol. 2021, 11, 664927. [Google Scholar] [CrossRef]
- Zhang, Z.; Ju, F.; Chen, F.; Wu, H.; Chen, J.; Zhong, J.; Shao, L.; Zheng, S.; Wang, L.; Xue, M. GDC-0326 Enhances the Effects of 5-Fu in Colorectal Cancer Cells by Inducing Necroptotic Death. Onco Targets Ther. 2021, 14, 2519–2530. [Google Scholar] [CrossRef]
- Conev, N.V.; Kashlov, Y.K.; Dimitrova, E.G.; Bogdanova, M.K.; Chaushev, B.G.; Radanova, M.A.; Petrov, D.P.; Georgiev, K.D.; Bachvarov, C.H.; Todorov, G.N.; et al. RIPK3 expression as a potential predictive and prognostic marker in metastatic colon cancer. Clin. Investig. Med. 2019, 42, E31–E38. [Google Scholar] [CrossRef]
- Kim, H.S.; Soung, Y.H.; Park, W.S.; Kim, S.Y.; Lee, J.H.; Park, J.Y.; Cho, Y.G.; Kim, C.J.; Jeong, S.W.; Nam, S.W.; et al. Inactivating mutations of caspase-8 gene in colorectal carcinomas. Gastroenterology 2003, 125, 708–715. [Google Scholar] [CrossRef]
- Cacina, C.; Turan Sürmen, S.; Arıkan, S.; Pençe, S.; Yaylım, İ. The influence of CASP8 D302H gene variant in colorectal cancer risk and prognosis. Turk. J. Biochem. 2023, 48, 234–238. [Google Scholar] [CrossRef]
- Sun, T.; Gao, Y.; Tan, W.; Ma, S.; Shi, Y.; Yao, J.; Guo, Y.; Yang, M.; Zhang, X.; Zhang, Q.; et al. A six-nucleotide insertion-deletion polymorphism in the CASP8 promoter is associated with susceptibility to multiple cancers. Nat. Genet. 2007, 39, 605–613. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, H.; Teraishi, F.; Davis, J.J.; Guo, W.; Fan, Z.; Fang, B. Accelerated degradation of caspase-8 protein correlates with TRAIL resistance in a DLD1 human colon cancer cell line. Neoplasia 2005, 7, 594–602. [Google Scholar] [CrossRef]
- Melo-Lima, S.; Celeste Lopes, M.; Mollinedo, F. Necroptosis is associated with low procaspase-8 and active RIPK1 and -3 in human glioma cells. Oncoscience 2014, 1, 649–664. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Qi, L.; Li, L.; Wu, Y.; Song, D.; Li, Y. Caspase-8: A key protein of cross-talk signal way in “PANoptosis” in cancer. Int. J. Cancer 2021, 149, 1408–1420. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, T.; Sagara, J.; Guan, X.; Masumoto, J.; Takeoka, M.; Komiyama, Y.; Miyata, K.; Higuchi, K.; Taniguchi, S. Methylation of ASC/TMS1, a proapoptotic gene responsible for activating procaspase-1, in human colorectal cancer. Cancer Lett. 2003, 202, 101–108. [Google Scholar] [CrossRef]
- Ohtsuka, T.; Liu, X.-F.; Koga, Y.; Kitajima, Y.; Nakafusa, Y.; Ha, C.-W.; Lee, S.W.; Miyazaki, K. Methylation-induced silencing of ASC and the effect of expressed ASC on p53-mediated chemosensitivity in colorectal cancer. Oncogene 2006, 25, 1807–1811. [Google Scholar] [CrossRef] [PubMed]
- Riojas, M.A.; Guo, M.; Glöckner, S.C.; Machida, E.O.; Baylin, S.B.; Ahuja, N. Methylation-induced silencing of ASC/TMS1, a pro-apoptotic gene, is a late-stage event in colorectal cancer. Cancer Biol. Ther. 2007, 6, 1710–1716. [Google Scholar] [CrossRef]
- Zhang, T.; Yin, C.; Fedorov, A.; Qiao, L.; Bao, H.; Beknazarov, N.; Wang, S.; Gautam, A.; Williams, R.M.; Crawford, J.C.; et al. ADAR1 masks the cancer immunotherapeutic promise of ZBP1-driven necroptosis. Nature 2022, 606, 594–602. [Google Scholar] [CrossRef]
- Gong, Z.; Jia, Q.; Guo, J.; Li, C.; Xu, S.; Jin, Z.; Chu, H.; Wan, Y.Y.; Zhu, B.; Zhou, Y. Caspase-8 contributes to an immuno-hot microenvironment by promoting phagocytosis via an ecto-calreticulin-dependent mechanism. Exp. Hematol. Oncol. 2023, 12, 7. [Google Scholar] [CrossRef]
- Chen, K.-S.; Manoury-Battais, S.; Kanaya, N.; Vogiatzi, I.; Borges, P.; Kruize, S.J.; Chen, Y.-C.; Lin, L.Y.; Rossignoli, F.; Mendonca, N.C.; et al. An inducible RIPK3-driven necroptotic system enhances cancer cell-based immunotherapy and ensures safety. J. Clin. Investig. 2024, 135, e181143. [Google Scholar] [CrossRef]
- Ocansey, D.K.W.; Qian, F.; Cai, P.; Ocansey, S.; Amoah, S.; Qian, Y.; Mao, F. Current evidence and therapeutic implication of PANoptosis in cancer. Theranostics 2024, 14, 640–661. [Google Scholar] [CrossRef]
- Liu, J.; Hong, M.; Li, Y.; Chen, D.; Wu, Y.; Hu, Y. Programmed Cell Death Tunes Tumor Immunity. Front. Immunol. 2022, 13, 847345. [Google Scholar] [CrossRef]
- Cai, Y.; Xiao, H.; Xue, S.; Li, P.; Zhan, Z.; Lin, J.; Song, Z.; Liu, J.; Xu, W.; Zhou, Q.; et al. Integrative analysis of immunogenic PANoptosis and experimental validation of cinobufagin-induced activation to enhance glioma immunotherapy. J. Exp. Clin. Cancer Res. 2025, 44, 35. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Yang, B.; Yang, J.; Shi, X.; Yang, X.; Zhang, D.; Zhao, D.; Yan, W.; Chen, L.; Zheng, H.; et al. ZBP1: A Powerful Innate Immune Sensor and Double-Edged Sword in Host Immunity. Int. J. Mol. Sci. 2022, 23, 10224. [Google Scholar] [CrossRef]
- Wang, R.; Li, H.; Wu, J.; Cai, Z.-Y.; Li, B.; Ni, H.; Qiu, X.; Chen, H.; Liu, W.; Yang, Z.-H.; et al. Gut stem cell necroptosis by genome instability triggers bowel inflammation. Nature 2020, 580, 386–390. [Google Scholar] [CrossRef]
- Wang, F.; Li, K.; Wang, W.; Hui, J.; He, J.; Cai, J.; Ren, W.; Zhao, Y.; Song, Q.; He, Y.; et al. Sensing of endogenous retroviruses-derived RNA by ZBP1 triggers PANoptosis in DNA damage and contributes to toxic side effects of chemotherapy. Cell Death Dis. 2024, 15, 779. [Google Scholar] [CrossRef]
- Zou, J.; Xia, H.; Zhang, C.; Xu, H.; Tang, Q.; Zhu, G.; Li, J.; Bi, F. Casp8 acts through A20 to inhibit PD-L1 expression: The mechanism and its implication in immunotherapy. Cancer sci. 2021, 112, 2664–2678. [Google Scholar] [CrossRef]
- Golrokh, F.J.; Tolami, H.F.; Ghanbarirad, M.; Mahmoudi, A.; Tabassi, N.R.; Alkinani, T.A.; Taramsari, S.M.; Aghajani, S.; Taati, H.; Akbari, F.; et al. Apoptosis induction in colon cancer cells (SW480) by BiFe2O4@Ag nanocomposite synthesized from Chlorella vulgaris extract and evaluation the expression of CASP8, BAX and BCL2 genes. J. Trace Elem. Med. Biol. 2024, 83, 127369. [Google Scholar] [CrossRef]
- Gyrd-Hansen, M.; Meier, P. IAPs: From caspase inhibitors to modulators of NF-kappaB, inflammation and cancer. Nat. Rev. Cancer 2010, 10, 561–574. [Google Scholar] [CrossRef]
- Fichtner, M.; Bozkurt, E.; Salvucci, M.; McCann, C.; McAllister, K.A.; Halang, L.; Düssmann, H.; Kinsella, S.; Crawford, N.; Sessler, T.; et al. Molecular subtype-specific responses of colon cancer cells to the SMAC mimetic Birinapant. Cell Death Dis. 2020, 11, 1020. [Google Scholar] [CrossRef]
- Xie, Y.; Zhao, Y.; Shi, L.; Li, W.; Chen, K.; Li, M.; Chen, X.; Zhang, H.; Li, T.; Matsuzawa-Ishimoto, Y.; et al. Gut epithelial TSC1/mTOR controls RIPK3-dependent necroptosis in intestinal inflammation and cancer. J. Clin. Investig. 2020, 130, 2111–2128. [Google Scholar] [CrossRef]
- Zhan, Z.; Liu, Z.; Lai, J.; Zhang, C.; Chen, Y.; Huang, H. Anticancer Effects and Mechanisms of OSW-1 Isolated From Ornithogalum saundersiae: A Review. Front. Oncol. 2021, 11, 747718. [Google Scholar] [CrossRef]
- Wang, N.; Li, C.Y.; Yao, T.F.; Kang, X.D.; Guo, H.S. OSW-1 triggers necroptosis in colorectal cancer cells through the RIPK1/RIPK3/MLKL signaling pathway facilitated by the RIPK1-p62/SQSTM1 complex. World J. Gastroenterol. 2024, 30, 2155–2174. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Mu, W. Necrostatin-1 and necroptosis inhibition: Pathophysiology and therapeutic implications. Pharmacol. Res. 2021, 163, 105297. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-Y.; Wu, B.; Guo, Y.-S.; Zhou, Y.-H.; Fu, Z.-G.; Xu, B.-Q.; Li, J.-H.; Jing, L.; Jiang, J.-L.; Tang, J.; et al. Necrostatin-1 reduces intestinal inflammation and colitis-associated tumorigenesis in mice. Am. J. Cancer Res. 2015, 5, 3174–3185. [Google Scholar]
- Cui, Z.; Li, Y.; Bi, Y.; Li, W.; Piao, J.; Ren, X. PANoptosis: A new era for anti-cancer strategies. Life Sci. 2024, 359, 123241. [Google Scholar] [CrossRef]
- Malireddi, R.K.S.; Karki, R.; Sundaram, B.; Kancharana, B.; Lee, S.; Samir, P.; Kanneganti, T.-D. Inflammatory Cell Death, PANoptosis, Mediated by Cytokines in Diverse Cancer Lineages Inhibits Tumor Growth. Immunohorizons 2021, 5, 568–580. [Google Scholar] [CrossRef]
- Zhuang, L.; Sun, Q.; Huang, S.; Hu, L.; Chen, Q. A comprehensive analysis of PANoptosome to prognosis and immunotherapy response in pan-cancer. Sci. Rep. 2023, 13, 3877. [Google Scholar] [CrossRef]
| Pathway | Key Molecules | Role in PANoptosis | Reference |
|---|---|---|---|
| Apoptosis | CASP3, CASP7, CASP8, and CASP9 | CASP3/7 execute apoptosis; CASP8 initiates apoptosis or inhibits necroptosis | [16,25] |
| Necroptosis | RIPK1, RIPK3, and MLKL | RIPK1/RIPK3 activate MLKL; MLKL disrupts membrane; inhibited by CASP8 | [17,26,27] |
| Pyroptosis | CASP1, CASP4, CASP5, CASP11, ASC, and GSDMD | CASP1 activates IL-1β/IL-18 and cleaves GSDMD; ASC scaffolds inflammasome formation | [14,24] |
| PANoptosome scaffold | ZBP1, RIPK3, and ASC | ZBP1 senses stress/DNA and recruits RIPK3, ASC, and CASP8 to form the PANoptosome | [21,22,28,30] |
| Executioners shared across pathways | CASP1, CASP3, CASP8, GSDMD, GSDME, and MLKL | Mediate final membrane disruption and cell death | [23,24,29] |
| lncRNA/miRNA or mRNA | Function/Role | Reference |
|---|---|---|
| lncRNA SNHG7 | Associated with CRC metastasis, chemoresistance, and prognosis; proposed as a predictive biomarker and therapeutic target. | [126] |
| miR-33ab; miR-34ac; miR-101; miR-187 | Affect PANoptosis in CRC through post-transcriptional regulation of the BCL10 gene. | [128] |
| miR-15abc; miR-31; miR-133abc; miR-191 | Influence PANoptosis in CRC via post-transcriptional modulation of the CDKN2A gene. | [128] |
| miR-23abc; miR-181abc; miR-217; miR-455-5p; | Modulate PANoptosis in CRC through post-transcriptional regulation of the DAPK1 gene. | [128] |
| miR-18ab; miR-19ab; miR-141; miR218 | Affect PANoptosis in CRC via post-transcriptional modulation of the TIMP1 gene. | [128] |
| miR-1ab; miR-145; miR-193; miR210 | Influence PANoptosis in CRC through post-transcriptional modulation of the PYGM gene. | [128] |
| TIMP1 (mRNA) | Part of a PANoptosis-related prognostic model; associated with poorer survival in CRC. | [127,128] |
| CDKN2A (mRNA) | Involved in CRC prognosis and progression; linked to immune microenvironment and drug sensitivity. | [127,128] |
| CAMK2B (mRNA) | Component of a PANoptosis-based prognostic model; contributes to CRC survival prediction. | [127] |
| TLR3 (mRNA) | Included in the prognostic model; high PANoptosis risk score correlates with worse CRC survival. | [127] |
| BCL10 (mRNA) | PANoptosis-related gene associated with CRC progression; involved in immune response and drug sensitivity. | [128] |
| DAPK1 (mRNA) | Plays a role in CRC and PANoptosis; potentially involved in signaling and therapeutic response. | [128] |
| PYGM (mRNA) | PANoptosis gene linked to CRC progression; shows prognostic value. | [128] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Műzes, G.; Sipos, F. PANoptosis as a Two-Edged Sword in Colorectal Cancer: A Pathogenic Mechanism and Therapeutic Opportunity. Cells 2025, 14, 730. https://doi.org/10.3390/cells14100730
Műzes G, Sipos F. PANoptosis as a Two-Edged Sword in Colorectal Cancer: A Pathogenic Mechanism and Therapeutic Opportunity. Cells. 2025; 14(10):730. https://doi.org/10.3390/cells14100730
Chicago/Turabian StyleMűzes, Györgyi, and Ferenc Sipos. 2025. "PANoptosis as a Two-Edged Sword in Colorectal Cancer: A Pathogenic Mechanism and Therapeutic Opportunity" Cells 14, no. 10: 730. https://doi.org/10.3390/cells14100730
APA StyleMűzes, G., & Sipos, F. (2025). PANoptosis as a Two-Edged Sword in Colorectal Cancer: A Pathogenic Mechanism and Therapeutic Opportunity. Cells, 14(10), 730. https://doi.org/10.3390/cells14100730







