Revisiting Pathogen Exploitation of Clathrin-Independent Endocytosis: Mechanisms and Implications
Abstract
1. Introduction
2. Dynamin-Dependent CIE Pathways
2.1. Caveolae-Mediated Endocytosis
2.2. Small GTPases-Regulated Endocytosis
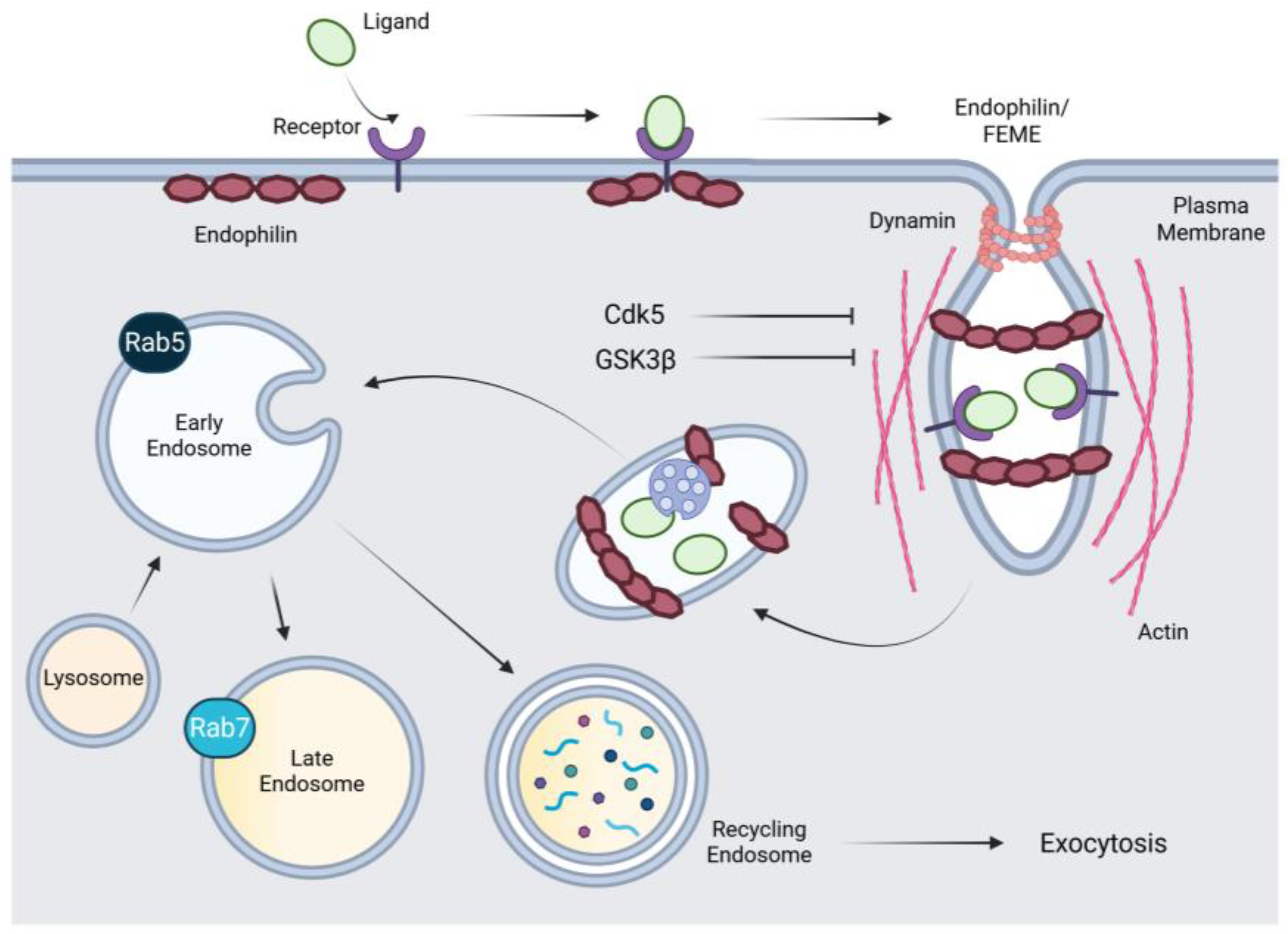
2.3. Fast Endophilin-Mediated Endocytosis (FEME)
2.4. Clathrin-Independent Internalization of the Epidermal Growth Factor Receptor (EGFR)
3. Dynamin-Independent CIE Pathways
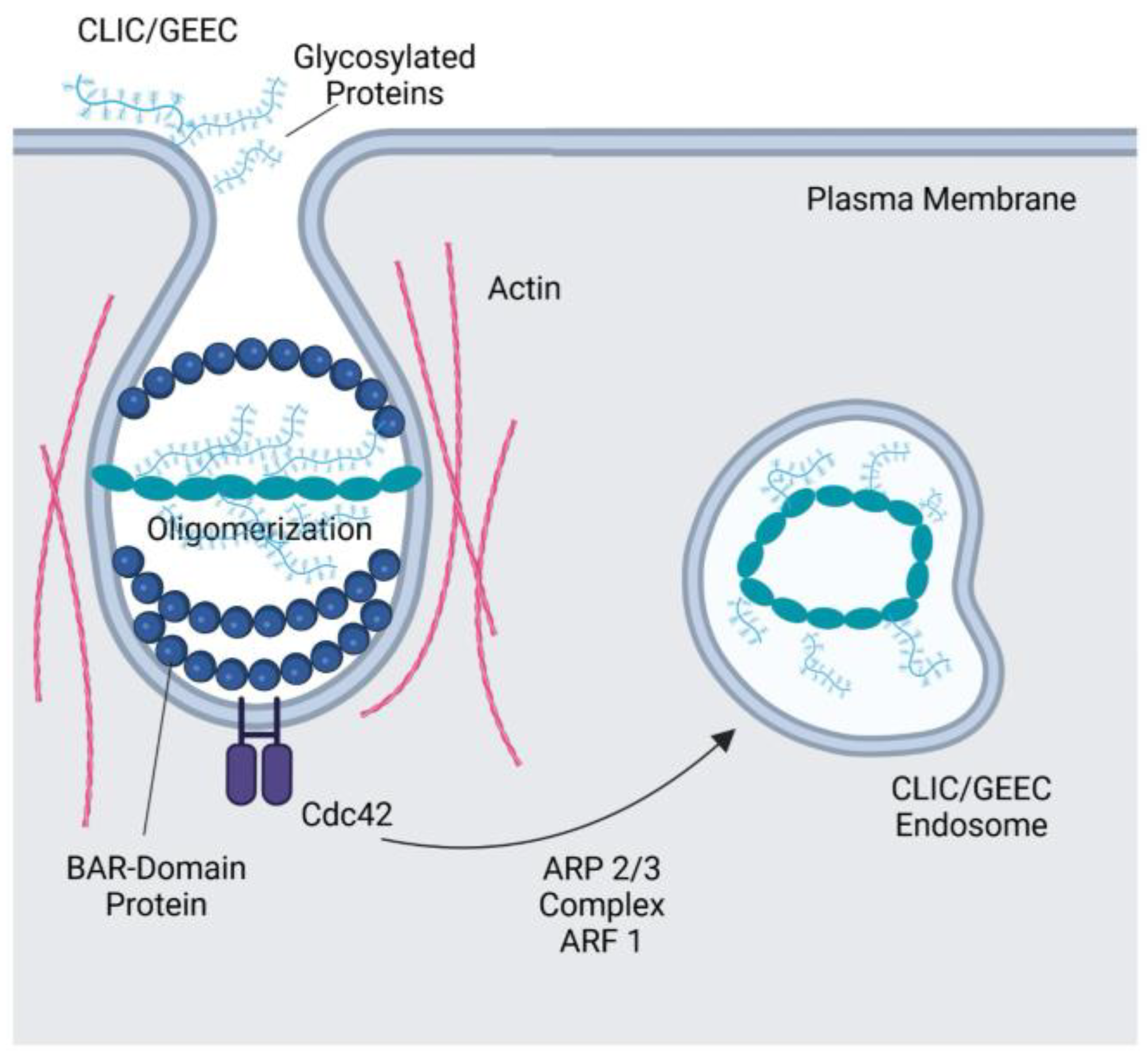
3.1. CLIC/GEEC Pathway
3.2. Arf6-Dependent Endocytosis
3.3. Flotillin-Mediated Endocytosis (FME)
3.4. Macropinocytosis
3.5. Convergence and Crosstalk Between CIE Pathways
4. Exploitation of CIE Pathways by Pathogens for Host Cell Entry and Infection
4.1. Exploitation of CIE by Bacterial Pathogens
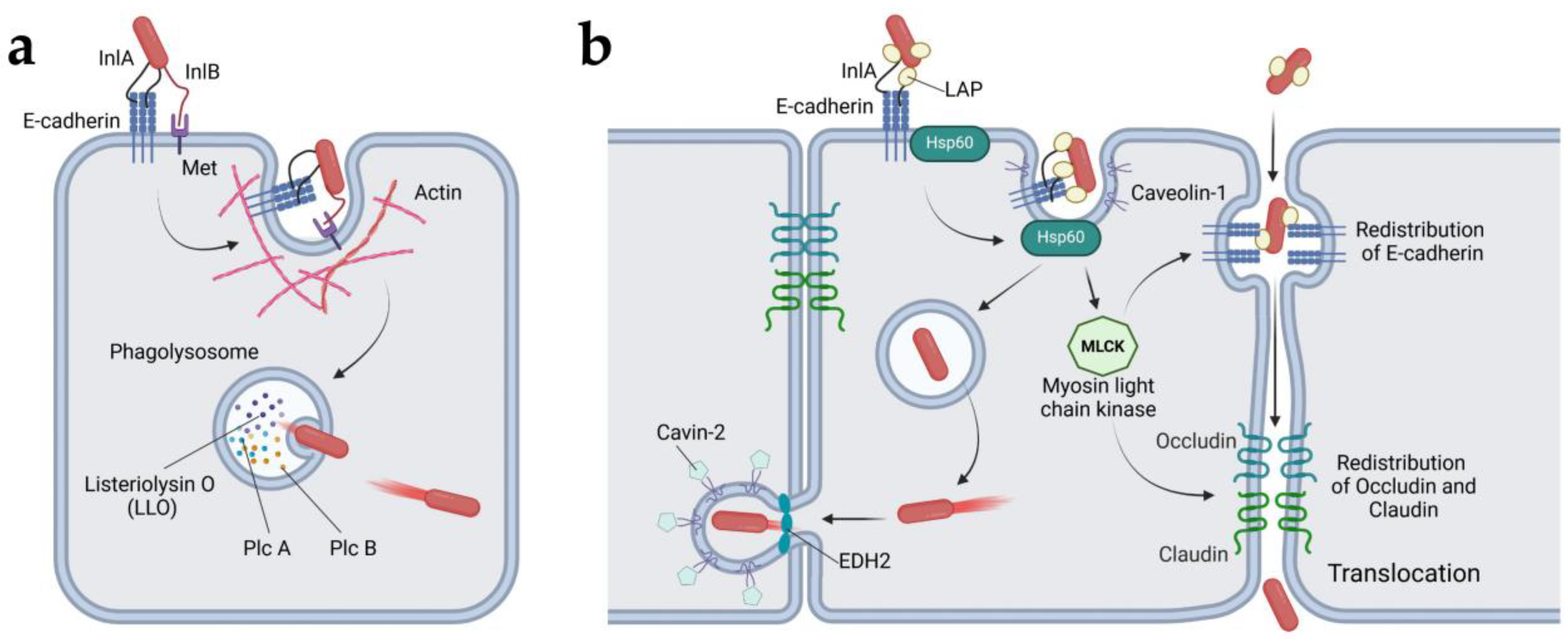
4.1.1. Listeria monocytogenes
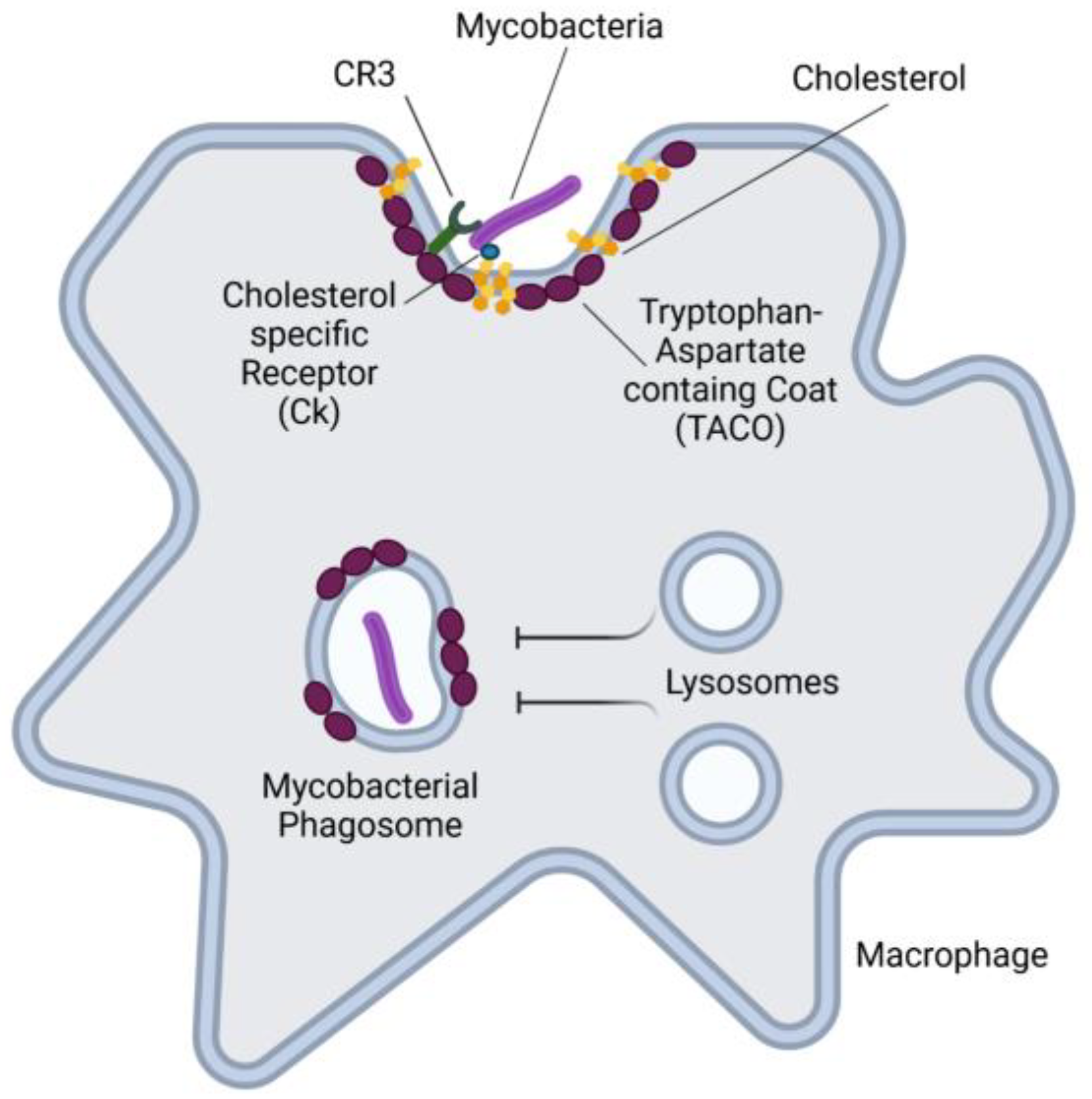
4.1.2. Mycobacterium tuberculosis
4.1.3. Streptococcus pyogenes
4.1.4. Staphylococcus aureus
4.1.5. Escherichia coli
4.1.6. Salmonella typhimurium
4.1.7. Chlamydia
4.1.8. Other Bacterial Pathogens
4.2. Exploitation of CIE by Viral Pathogens
4.2.1. Simian Virus 40 (SV40)
4.2.2. Echoviruses
4.2.3. Coronavirus
4.2.4. Human Immunodeficiency Virus 1 (HIV-1)
4.2.5. Japanese Encephalitis Virus (JEV)
5. Conclusions and Future Research Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CME | Clathrin-mediated endocytosis |
| CIE | Clathrin-independent endocytosis |
| EGF | Epidermal growth factor |
| EGFR | Epidermal growth factor receptor |
| Arf6 | ADP-ribosylation factor 6 |
| ER | Endoplasmic reticulum |
| RhoA | Ras homolog family member A |
| Rac1 | Rac Family Small GTPase 1 |
| Cdc42 | Cell division control protein 42 |
| RhoG | Ras homolog family member G |
| IL-2R-β | Interleukin-2 receptor subunit beta |
| GPI-Aps | Glycosylphosphatidylinositol-anchored proteins |
| FEME | Fast endophilin-mediated endocytosis |
| SH3 | Src homology 3 |
| BAR | Bin-Amphiphysin-Rvs domain |
| CIP4 | Cdc42-interacting protein 4 |
| FBP17 | formin-binding protein 17 GTPase-activating proteins |
| SHP2 | SH2-containing inositol phosphatase 2 |
| Cdk5 | Cyclin-dependent kinase 5 |
| GSK3β | Glycogen synthase kinase-3 beta |
| RTN3 | Reticulon-3 |
| IP3R | Inositol triphosphate receptor |
| IRSp53 | Insulin receptor substrate p53 |
| CLIC/GEEC | Clathrin-independent carrier (CLIC)/GPI-anchored protein-enriched early endocytic compartments (GEEC) |
| GPI-Aps | GPI-anchored proteins |
| PI3K | Phosphoinositide 3-kinases |
| GTP | Guanosine triphosphate |
| GDP | Guanosine diphosphate |
| GBF1 | Golgi Brefeldin A Resistant Guanine Nucleotide Exchange Factor 1 |
| GEF | Guanine nucleotide exchange factors |
| Arf1 | ADP-ribosylation factor 1 |
| ARHGAP10 | Rho GTPase Activating Protein 10 |
| GRAF1 | GTPase Regulator Associated with Focal Adhesion Kinase 1 |
| GAPs | GTPase-activating proteins |
| PIP2 | phosphatidylinositol 4,5-bisphosphate |
| FME | Flotillin-mediated endocytosis |
| SPFH | stomatin/prohibitin/flotillin/HflK/C |
| InlA | Internalin A |
| InlB | Internalin B |
| PlcA | Phosphatidylinositol-Specific Phospholipase C |
| PlcB | Broad-Range Phospholipase C |
| LIPI-1 | Listeria pathogenicity island 1 |
| EHD2 | EH Domain Containing 2 |
| LAP | Listeria adhesion protein |
| Hsp60 | Heat shock protein 60 |
| CR3 | Complement receptor 3 |
| TACO | Tryptophan-aspartate-containing coat |
| BCG | Mycobacterium bovis Bacillus Calmette-Guérin |
| SfbI | Streptococcal fibronectin-binding protein I |
| OCRL | Oculocerebrorenal Syndrome of Lowe |
| EPEC | Enteropathogenic E. coli |
| EHEC | Enterohaemorrhagic E. coli |
| UPEC | Uropathogenic E. coli |
| UTIs | Urinary tract infections |
| CNF1 | Cytotoxic necrotizing factor 1 |
| UP1a | Glycosylated uroplakin Ia |
| CEACAM | Carcinoembryonic antigen-related cell adhesion molecule |
| IBCs | Intracellular bacterial communities |
| DAEC | Afa/Dr diffusely adhering E. coli |
| PKCa | Protein kinase C alpha |
| T3SS | Type III secretion system |
| SPI-1 | Salmonella Pathogenicity Island 1 |
| SCVs | Salmonella-containing vacuoles |
| SPI-2 | Salmonella Pathogenicity Island 2 |
| Ebs | Elementary bodies |
| SV40 | Simian virus 40 |
| SARS | Severe acute respiratory syndrome |
| ACE2 | Angiotensin-converting enzyme 2 |
| HIV-1 | Human immunodeficiency virus 1 |
| JEV | Japanese Encephalitis Virus |
| CRISPR | Clustered regularly interspaced short palindromic repeats |
References
- Doherty, G.J.; McMahon, H.T. Mechanisms of endocytosis. Annu. Rev. Biochem. 2009, 78, 857–902. [Google Scholar] [CrossRef] [PubMed]
- Cossart, P.; Helenius, A. Endocytosis of viruses and bacteria. Cold Spring Harb. Perspect. Biol. 2014, 6, a016972. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kumari, S.; Mg, S.; Mayor, S. Endocytosis unplugged: Multiple ways to enter the cell. Cell Res. 2010, 20, 256–275. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McMahon, H.T.; Boucrot, E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2011, 12, 517–533. [Google Scholar] [CrossRef] [PubMed]
- Kaksonen, M.; Roux, A. Mechanisms of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2018, 19, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Mettlen, M.; Chen, P.H.; Srinivasan, S.; Danuser, G.; Schmid, S.L. Regulation of Clathrin-Mediated Endocytosis. Annu. Rev. Biochem. 2018, 87, 871–896. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reider, A.; Wendland, B. Endocytic adaptors–social networking at the plasma membrane. J. Cell Sci. 2011, 124, 1613–1622. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hansen, C.G.; Nichols, B.J. Molecular mechanisms of clathrin-independent endocytosis. J. Cell Sci. 2009, 122, 1713–1721. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sandvig, K.; Kavaliauskiene, S.; Skotland, T. Clathrin-independent endocytosis: An increasing degree of complexity. Histochem. Cell Biol. 2018, 150, 107–118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sandvig, K.; Pust, S.; Skotland, T.; van Deurs, B. Clathrin-independent endocytosis: Mechanisms and function. Curr. Opin. Cell Biol. 2011, 23, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Howes, M.T.; Mayor, S.; Parton, R.G. Molecules, mechanisms, and cellular roles of clathrin-independent endocytosis. Curr. Opin. Cell Biol. 2010, 22, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Mayor, S.; Pagano, R.E. Pathways of clathrin-independent endocytosis. Nat. Rev. Mol. Cell Biol. 2007, 8, 603–612. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lajoie, P.; Nabi, I.R. Lipid rafts, caveolae, and their endocytosis. Int. Rev. Cell Mol. Biol. 2010, 282, 135–163. [Google Scholar] [CrossRef] [PubMed]
- Nabi, I.R.; Le, P.U. Caveolae/raft-dependent endocytosis. J. Cell Biol. 2003, 161, 673–677. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Simons, K.; Ikonen, E. Functional rafts in cell membranes. Nature 1997, 387, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Pike, L.J. Lipid rafts: Bringing order to chaos. J. Lipid Res. 2003, 44, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Sezgin, E.; Levental, I.; Mayor, S.; Eggeling, C. The mystery of membrane organization: Composition, regulation and roles of lipid rafts. Nat. Rev. Mol. Cell Biol. 2017, 18, 361–374. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rennick, J.J.; Johnston, A.P.R.; Parton, R.G. Key principles and methods for studying the endocytosis of biological and nanoparticle therapeutics. Nat. Nanotechnol. 2021, 16, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Hinshaw, J.E. Dynamin and its role in membrane fission. Annu. Rev. Cell Dev. Biol. 2000, 16, 483–519. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pelkmans, L.; Helenius, A. Endocytosis via caveolae. Traffic 2002, 3, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.M.; Smart, E.J. Caveolae structure and function. J. Cell. Mol. Med. 2008, 12, 796–809. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Parton, R.G. Caveolae—From ultrastructure to molecular mechanisms. Nat. Rev. Mol. Cell Biol. 2003, 4, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Parton, R.G.; McMahon, K.A.; Wu, Y. Caveolae: Formation, dynamics, and function. Curr. Opin. Cell Biol. 2020, 65, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Pilch, P.F.; Liu, L. Fat caves: Caveolae, lipid trafficking and lipid metabolism in adipocytes. Trends Endocrinol. Metab. 2011, 22, 318–324. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chow, B.W.; Nunez, V.; Kaplan, L.; Granger, A.J.; Bistrong, K.; Zucker, H.L.; Kumar, P.; Sabatini, B.L.; Gu, C. Caveolae in CNS arterioles mediate neurovascular coupling. Nature 2020, 579, 106–110. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Frank, P.G.; Pavlides, S.; Lisanti, M.P. Caveolae and transcytosis in endothelial cells: Role in atherosclerosis. Cell Tissue Res. 2009, 335, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Parton, R.G.; del Pozo, M.A. Caveolae as plasma membrane sensors, protectors and organizers. Nat. Rev. Mol. Cell Biol. 2013, 14, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Sinha, B.; Koster, D.; Ruez, R.; Gonnord, P.; Bastiani, M.; Abankwa, D.; Stan, R.V.; Butler-Browne, G.; Vedie, B.; Johannes, L.; et al. Cells respond to mechanical stress by rapid disassembly of caveolae. Cell 2011, 144, 402–413. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nassoy, P.; Lamaze, C. Stressing caveolae new role in cell mechanics. Trends Cell Biol. 2012, 22, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Lamaze, C.; Tardif, N.; Dewulf, M.; Vassilopoulos, S.; Blouin, C.M. The caveolae dress code: Structure and signaling. Curr. Opin. Cell Biol. 2017, 47, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Kovtun, O.; Tillu, V.A.; Ariotti, N.; Parton, R.G.; Collins, B.M. Cavin family proteins and the assembly of caveolae. J. Cell Sci. 2015, 128, 1269–1278. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ludwig, A.; Nichols, B.J.; Sandin, S. Architecture of the caveolar coat complex. J. Cell Sci. 2016, 129, 3077–3083. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stoeber, M.; Stoeck, I.K.; Hanni, C.; Bleck, C.K.E.; Balistreri, G.; Helenius, A. Oligomers of the ATPase EHD2 confine caveolae to the plasma membrane through association with actin. EMBO J. 2012, 31, 2350–2364. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stoeber, M.; Schellenberger, P.; Siebert, C.A.; Leyrat, C.; Helenius, A.; Grunewald, K. Model for the architecture of caveolae based on a flexible, net-like assembly of Cavin1 and Caveolin discs. Proc. Natl. Acad. Sci. USA 2016, 113, E8069–E8078. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Drab, M.; Verkade, P.; Elger, M.; Kasper, M.; Lohn, M.; Lauterbach, B.; Menne, J.; Lindschau, C.; Mende, F.; Luft, F.C.; et al. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science 2001, 293, 2449–2452. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.M.; Bastiani, M.; Luetterforst, R.; Kirkham, M.; Kirkham, A.; Nixon, S.J.; Walser, P.; Abankwa, D.; Oorschot, V.M.; Martin, S.; et al. PTRF-Cavin, a conserved cytoplasmic protein required for caveola formation and function. Cell 2008, 132, 113–124. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tillu, V.A.; Lim, Y.W.; Kovtun, O.; Mureev, S.; Ferguson, C.; Bastiani, M.; McMahon, K.A.; Lo, H.P.; Hall, T.E.; Alexandrov, K.; et al. A variable undecad repeat domain in cavin1 regulates caveola formation and stability. EMBO Rep. 2018, 19, e45775. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tillu, V.A.; Rae, J.; Gao, Y.; Ariotti, N.; Floetenmeyer, M.; Kovtun, O.; McMahon, K.A.; Chaudhary, N.; Parton, R.G.; Collins, B.M. Cavin1 intrinsically disordered domains are essential for fuzzy electrostatic interactions and caveola formation. Nat. Commun. 2021, 12, 931. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hansen, C.G.; Bright, N.A.; Howard, G.; Nichols, B.J. SDPR induces membrane curvature and functions in the formation of caveolae. Nat. Cell Biol. 2009, 11, 807–814. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McMahon, K.A.; Zajicek, H.; Li, W.P.; Peyton, M.J.; Minna, J.D.; Hernandez, V.J.; Luby-Phelps, K.; Anderson, R.G.W. SRBC/cavin-3 is a caveolin adapter protein that regulates caveolae function. EMBO J. 2009, 28, 1001–1015. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bastiani, M.; Liu, L.; Hill, M.M.; Jedrychowski, M.P.; Nixon, S.J.; Lo, H.P.; Abankwa, D.; Luetterforst, R.; Fernandez-Rojo, M.; Breen, M.R.; et al. MURC/Cavin-4 and cavin family members form tissue-specific caveolar complexes. J. Cell Biol. 2009, 185, 1259–1273. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hansen, C.G.; Howard, G.; Nichols, B.J. Pacsin 2 is recruited to caveolae and functions in caveolar biogenesis. J. Cell Sci. 2011, 124, 2777–2785. [Google Scholar] [CrossRef] [PubMed]
- Senju, Y.; Itoh, Y.; Takano, K.; Hamada, S.; Suetsugu, S. Essential role of PACSIN2/syndapin-II in caveolae membrane sculpting. J. Cell Sci. 2011, 124, 2032–2040. [Google Scholar] [CrossRef] [PubMed]
- Moren, B.; Shah, C.; Howes, M.T.; Schieber, N.L.; McMahon, H.T.; Parton, R.G.; Daumke, O.; Lundmark, R. EHD2 regulates caveolar dynamics via ATP-driven targeting and oligomerization. Mol. Biol. Cell 2012, 23, 1316–1329. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hoernke, M.; Mohan, J.; Larsson, E.; Blomberg, J.; Kahra, D.; Westenhoff, S.; Schwieger, C.; Lundmark, R. EHD2 restrains dynamics of caveolae by an ATP-dependent, membrane-bound, open conformation. Proc. Natl. Acad. Sci. USA 2017, 114, E4360–E4369. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matthaeus, C.; Lahmann, I.; Kunz, S.; Jonas, W.; Melo, A.A.; Lehmann, M.; Larsson, E.; Lundmark, R.; Kern, M.; Bluher, M.; et al. EHD2-mediated restriction of caveolar dynamics regulates cellular fatty acid uptake. Proc. Natl. Acad. Sci. USA 2020, 117, 7471–7481. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sonnino, S.; Prinetti, A. Sphingolipids and membrane environments for caveolin. FEBS Lett. 2009, 583, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Stan, R.V. Structure of caveolae. Biochim. Biophys. Acta Mol. Cell Res. 2005, 1746, 334–348. [Google Scholar] [CrossRef] [PubMed]
- Fielding, C.J.; Fielding, P.E. Cholesterol and caveolae: Structural and functional relationships. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2000, 1529, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Ikonen, E.; Heino, S.; Lusa, S. Caveolins and membrane cholesterol. Biochem. Soc. Trans. 2004, 32, 121–123. [Google Scholar] [CrossRef] [PubMed]
- Schubert, W.; Frank, P.G.; Razani, B.; Park, D.S.; Chow, C.W.; Lisanti, M.P. Caveolae-deficient endothelial cells show defects in the uptake and transport of albumin in vivo. J. Biol. Chem. 2001, 276, 48619–48622. [Google Scholar] [CrossRef] [PubMed]
- Shogomori, H.; Futerman, A.H. Cholera toxin is found in detergent-insoluble rafts/domains at the cell surface of hippocampal neurons but is internalized via a raft-independent mechanism. J. Biol. Chem. 2001, 276, 9182–9188. [Google Scholar] [CrossRef] [PubMed]
- Zimnicka, A.M.; Husain, Y.S.; Shajahan, A.N.; Sverdlov, M.; Chaga, O.; Chen, Z.; Toth, P.T.; Klomp, J.; Karginov, A.V.; Tiruppathi, C.; et al. Src-dependent phosphorylation of caveolin-1 Tyr-14 promotes swelling and release of caveolae. Mol. Biol. Cell 2016, 27, 2090–2106. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sverdlov, M.; Shajahan, A.N.; Minshall, R.D. Tyrosine phosphorylation-dependence of caveolae-mediated endocytosis. J. Cell. Mol. Med. 2007, 11, 1239–1250. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cao, H.; Courchesne, W.E.; Mastick, C.C. A phosphotyrosine-dependent protein interaction screen reveals a role for phosphorylation of caveolin-1 on tyrosine 14: Recruitment of C-terminal Src kinase. J. Biol. Chem. 2002, 277, 8771–8774. [Google Scholar] [CrossRef] [PubMed]
- Oh, P.; McIntosh, D.P.; Schnitzer, J.E. Dynamin at the neck of caveolae mediates their budding to form transport vesicles by GTP-driven fission from the plasma membrane of endothelium. J. Cell Biol. 1998, 141, 101–114. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Henley, J.R.; Krueger, E.W.; Oswald, B.J.; McNiven, M.A. Dynamin-mediated internalization of caveolae. J. Cell Biol. 1998, 141, 85–99. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Larsson, E.; Moren, B.; McMahon, K.A.; Parton, R.G.; Lundmark, R. Dynamin2 functions as an accessory protein to reduce the rate of caveola internalization. J. Cell Biol. 2023, 222, e202205122. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matthaeus, C.; Sochacki, K.A.; Dickey, A.M.; Puchkov, D.; Haucke, V.; Lehmann, M.; Taraska, J.W. The molecular organization of differentially curved caveolae indicates bendable structural units at the plasma membrane. Nat. Commun. 2022, 13, 7234. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Preta, G.; Cronin, J.G.; Sheldon, I.M. Dynasore-not just a dynamin inhibitor. Cell Commun. Signal. 2015, 13, 24. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mundy, D.I.; Machleidt, T.; Ying, Y.S.; Anderson, R.G.W.; Bloom, G.S. Dual control of caveolar membrane traffic by microtubules and the actin cytoskeleton. J. Cell Sci. 2002, 115, 4327–4339. [Google Scholar] [CrossRef] [PubMed]
- Pelkmans, L.; Puntener, D.; Helenius, A. Local actin polymerization and dynamin recruitment in SV40-induced internalization of caveolae. Science 2002, 296, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Matthaeus, C.; Taraska, J.W. Energy and Dynamics of Caveolae Trafficking. Front. Cell Dev. Biol. 2021, 8, 614472. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hayer, A.; Stoeber, M.; Ritz, D.; Engel, S.; Meyer, H.H.; Helenius, A. Caveolin-1 is ubiquitinated and targeted to intralumenal vesicles in endolysosomes for degradation. J. Cell Biol. 2010, 191, 615–629. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pelkmans, L.; Burli, T.; Zerial, M.; Helenius, A. Caveolin-stabilized membrane domains as multifunctional transport and sorting devices in endocytic membrane traffic. Cell 2004, 118, 767–780. [Google Scholar] [CrossRef] [PubMed]
- Pelkmans, L.; Kartenbeck, J.; Helenius, A. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat. Cell Biol. 2001, 3, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Le, P.U.; Nabi, I.R. Distinct caveolae-mediated endocytic pathways target the Golgi apparatus and the endoplasmic reticulum. J. Cell Sci. 2003, 116, 1059–1071. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Nino, W.R.; Correa, F.; Rodriguez-Barrena, J.I.; Leon-Contreras, J.C.; Buelna-Chontal, M.; Soria-Castro, E.; Hernandez-Pando, R.; Pedraza-Chaverri, J.; Zazueta, C. Cardioprotective kinase signaling to subsarcolemmal and interfibrillar mitochondria is mediated by caveolar structures. Basic Res. Cardiol. 2017, 112, 15. [Google Scholar] [CrossRef] [PubMed]
- Correa, F.; Enriquez-Cortina, C.; Silva-Palacios, A.; Roman-Anguiano, N.; Gil-Hernandez, A.; Ostolga-Chavarria, M.; Soria-Castro, E.; Hernandez-Rizo, S.; de Los Heros, P.; Chavez-Canales, M.; et al. Actin-Cytoskeleton Drives Caveolae Signaling to Mitochondria during Postconditioning. Cells 2023, 12, 492. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pelkmans, L.; Zerial, M. Kinase-regulated quantal assemblies and kiss-and-run recycling of caveolae. Nature 2005, 436, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.S.; Abraham, S.N. Caveolae as portals of entry for microbes. Microbes Infect. 2001, 3, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Barman, D.; Drolia, R. Caveolin-Mediated Endocytosis: Bacterial Pathogen Exploitation and Host–Pathogen Interaction. Cells 2024, 14, 2. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xing, Y.; Wen, Z.; Gao, W.; Lin, Z.; Zhong, J.; Jiu, Y. Multifaceted Functions of Host Cell Caveolae/Caveolin-1 in Virus Infections. Viruses 2020, 12, 487. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sabharanjak, S.; Sharma, P.; Parton, R.G.; Mayor, S. GPI-anchored proteins are delivered to recycling endosomes via a distinct cdc42-regulated, clathrin-independent pinocytic pathway. Dev. Cell 2002, 2, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Chadda, R.; Howes, M.T.; Plowman, S.J.; Hancock, J.F.; Parton, R.G.; Mayor, S. Cholesterol-sensitive Cdc42 activation regulates actin polymerization for endocytosis via the GEEC pathway. Traffic 2007, 8, 702–717. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kumari, S.; Mayor, S. ARF1 is directly involved in dynamin-independent endocytosis. Nat. Cell Biol. 2008, 10, 30–41. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Prieto-Sanchez, R.M.; Berenjeno, I.M.; Bustelo, X.R. Involvement of the Rho/Rac family member RhoG in caveolar endocytosis. Oncogene 2006, 25, 2961–2973. [Google Scholar] [CrossRef] [PubMed]
- Lamaze, C.; Dujeancourt, A.; Baba, T.; Lo, C.G.; Benmerah, A.; Dautry-Varsat, A. Interleukin 2 receptors and detergent-resistant membrane domains define a clathrin-independent endocytic pathway. Mol. Cell 2001, 7, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Grassart, A.; Dujeancourt, A.; Lazarow, P.B.; Dautry-Varsat, A.; Sauvonnet, N. Clathrin-independent endocytosis used by the IL-2 receptor is regulated by Rac1, Pak1 and Pak2. EMBO Rep. 2008, 9, 356–362. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Casamento, A.; Boucrot, E. Molecular mechanism of Fast Endophilin-Mediated Endocytosis. Biochem. J. 2020, 477, 2327–2345. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boucrot, E.; Ferreira, A.P.A.; Almeida-Souza, L.; Debard, S.; Vallis, Y.; Howard, G.; Bertot, L.; Sauvonnet, N.; McMahon, H.T. Endophilin marks and controls a clathrin-independent endocytic pathway. Nature 2015, 517, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.Q.; Huang, A.F.; Xu, W.D. Biology of endophilin and it’s role in disease. Front. Immunol. 2023, 14, 1297506. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kjaerulff, O.; Brodin, L.; Jung, A. The structure and function of endophilin proteins. Cell Biochem. Biophys. 2011, 60, 137–154. [Google Scholar] [CrossRef] [PubMed]
- Renard, H.F.; Simunovic, M.; Lemiere, J.; Boucrot, E.; Garcia-Castillo, M.D.; Arumugam, S.; Chambon, V.; Lamaze, C.; Wunder, C.; Kenworthy, A.K.; et al. Endophilin-A2 functions in membrane scission in clathrin-independent endocytosis. Nature 2015, 517, 493–496. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Simunovic, M.; Manneville, J.B.; Renard, H.F.; Evergren, E.; Raghunathan, K.; Bhatia, D.; Kenworthy, A.K.; Voth, G.A.; Prost, J.; McMahon, H.T.; et al. Friction Mediates Scission of Tubular Membranes Scaffolded by BAR Proteins. Cell 2017, 170, 172–184.e11. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chan Wah Hak, L.; Khan, S.; Di Meglio, I.; Law, A.L.; Lucken-Ardjomande Hasler, S.; Quintaneiro, L.M.; Ferreira, A.P.A.; Krause, M.; McMahon, H.T.; Boucrot, E. FBP17 and CIP4 recruit SHIP2 and lamellipodin to prime the plasma membrane for fast endophilin-mediated endocytosis. Nat. Cell Biol. 2018, 20, 1023–1031. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferreira, A.P.A.; Casamento, A.; Carrillo Roas, S.; Halff, E.F.; Panambalana, J.; Subramaniam, S.; Schutzenhofer, K.; Chan Wah Hak, L.; McGourty, K.; Thalassinos, K.; et al. Cdk5 and GSK3β inhibit fast endophilin-mediated endocytosis. Nat. Commun. 2021, 12, 2424. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Murphrey, M.B.; Quaim, L.; Rahimi, N.; Varacallo, M.A. Biochemistry, Epidermal Growth Factor Receptor; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Tito, C.; Masciarelli, S.; Colotti, G.; Fazi, F. EGF receptor in organ development, tissue homeostasis and regeneration. J. Biomed. Sci. 2025, 32, 24. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sigismund, S.; Algisi, V.; Nappo, G.; Conte, A.; Pascolutti, R.; Cuomo, A.; Bonaldi, T.; Argenzio, E.; Verhoef, L.G.G.C.; Maspero, E.; et al. Threshold-controlled ubiquitination of the EGFR directs receptor fate. EMBO J. 2013, 32, 2140–2157. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Caldieri, G.; Barbieri, E.; Nappo, G.; Raimondi, A.; Bonora, M.; Conte, A.; Verhoef, L.G.G.C.; Confalonieri, S.; Malabarba, M.G.; Bianchi, F.; et al. Reticulon 3–dependent ER-PM contact sites control EGFR nonclathrin endocytosis. Science 2017, 356, 617–624. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alwan, H.A.; van Zoelen, E.J.; van Leeuwen, J.E. Ligand-induced lysosomal epidermal growth factor receptor (EGFR) degradation is preceded by proteasome-dependent EGFR de-ubiquitination. J. Biol. Chem. 2003, 278, 35781–35790. [Google Scholar] [CrossRef] [PubMed]
- Gundu, C.; Arruri, V.K.; Yadav, P.; Navik, U.; Kumar, A.; Amalkar, V.S.; Vikram, A.; Gaddam, R.R. Dynamin-Independent Mechanisms of Endocytosis and Receptor Trafficking. Cells 2022, 11, 2557. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ojha, R.; Jiang, A.; Mantyla, E.; Quirin, T.; Modhira, N.; Witte, R.; Gaudin, A.; De Zanetti, L.; Gormal, R.S.; Vihinen-Ranta, M.; et al. Dynamin independent endocytosis is an alternative cell entry mechanism for multiple animal viruses. PLoS Pathog. 2024, 20, e1012690. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sathe, M.; Muthukrishnan, G.; Rae, J.; Disanza, A.; Thattai, M.; Scita, G.; Parton, R.G.; Mayor, S. Small GTPases and BAR domain proteins regulate branched actin polymerisation for clathrin and dynamin-independent endocytosis. Nat. Commun. 2018, 9, 1835. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kalia, M.; Kumari, S.; Chadda, R.; Hill, M.M.; Parton, R.G.; Mayor, S. Arf6-independent GPI-anchored protein-enriched early endosomal compartments fuse with sorting endosomes via a Rab5/phosphatidylinositol-3′-kinase–dependent machinery. Mol. Biol. Cell 2006, 17, 3689–3704. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Howes, M.T.; Kirkham, M.; Riches, J.; Cortese, K.; Walser, P.J.; Simpson, F.; Hill, M.M.; Jones, A.; Lundmark, R.; Lindsay, M.R.; et al. Clathrin-independent carriers form a high capacity endocytic sorting system at the leading edge of migrating cells. J. Cell Biol. 2010, 190, 675–691. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Banfer, S.; Schneider, D.; Dewes, J.; Strauss, M.T.; Freibert, S.A.; Heimerl, T.; Maier, U.G.; Elsasser, H.P.; Jungmann, R.; Jacob, R. Molecular mechanism to recruit galectin-3 into multivesicular bodies for polarized exosomal secretion. Proc. Natl. Acad. Sci. USA 2018, 115, E4396–E4405. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lakshminarayan, R.; Wunder, C.; Becken, U.; Howes, M.T.; Benzing, C.; Arumugam, S.; Sales, S.; Ariotti, N.; Chambon, V.; Lamaze, C.; et al. Galectin-3 drives glycosphingolipid-dependent biogenesis of clathrin-independent carriers. Nat. Cell Biol. 2014, 16, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Goswami, D.; Gowrishankar, K.; Bilgrami, S.; Ghosh, S.; Raghupathy, R.; Chadda, R.; Vishwakarma, R.; Rao, M.; Mayor, S. Nanoclusters of GPI-anchored proteins are formed by cortical actin-driven activity. Cell 2008, 135, 1085–1097. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lundmark, R.; Doherty, G.J.; Howes, M.T.; Cortese, K.; Vallis, Y.; Parton, R.G.; McMahon, H.T. The GTPase-activating protein GRAF1 regulates the CLIC/GEEC endocytic pathway. Curr. Biol. 2008, 18, 1802–1808. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- D’Souza-Schorey, C.; Chavrier, P. ARF proteins: Roles in membrane traffic and beyond. Nat. Rev. Mol. Cell Biol. 2006, 7, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, J.K.; Sedgwick, A.E.; D’Souza-Schorey, C. ARF6-mediated endocytic recycling impacts cell movement, cell division and lipid homeostasis. Semin. Cell Dev. Biol. 2011, 22, 39–47. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Van Acker, T.; Tavernier, J.; Peelman, F. The Small GTPase Arf6: An Overview of Its Mechanisms of Action and of Its Role in Host–Pathogen Interactions and Innate Immunity. Int. J. Mol. Sci. 2019, 20, 2209. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grant, B.D.; Donaldson, J.G. Pathways and mechanisms of endocytic recycling. Nat. Rev. Mol. Cell Biol. 2009, 10, 597–608. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Naslavsky, N.; Weigert, R.; Donaldson, J.G. Characterization of a nonclathrin endocytic pathway: Membrane cargo and lipid requirements. Mol. Biol. Cell 2004, 15, 3542–3552. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schafer, D.A.; D’Souza-Schorey, C.; Cooper, J.A. Actin assembly at membranes controlled by ARF6. Traffic 2000, 1, 892–903. [Google Scholar] [CrossRef] [PubMed]
- Hongu, T.; Kanaho, Y. Activation machinery of the small GTPase Arf6. Adv. Biol. Regul. 2014, 54, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Naslavsky, N.; Weigert, R.; Donaldson, J.G. Convergence of non-clathrin- and clathrin-derived endosomes involves Arf6 inactivation and changes in phosphoinositides. Mol. Biol. Cell 2003, 14, 417–431. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chesneau, L.; Dambournet, D.; Machicoane, M.; Kouranti, I.; Fukuda, M.; Goud, B.; Echard, A. An ARF6/Rab35 GTPase cascade for endocytic recycling and successful cytokinesis. Curr. Biol. 2012, 22, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Brown, F.D.; Rozelle, A.L.; Yin, H.L.; Balla, T.; Donaldson, J.G. Phosphatidylinositol 4,5-bisphosphate and Arf6-regulated membrane traffic. J. Cell Biol. 2001, 154, 1007–1017. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cauvin, C.; Rosendale, M.; Gupta-Rossi, N.; Rocancourt, M.; Larraufie, P.; Salomon, R.; Perrais, D.; Echard, A. Rab35 GTPase Triggers Switch-like Recruitment of the Lowe Syndrome Lipid Phosphatase OCRL on Newborn Endosomes. Curr. Biol. 2016, 26, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Donaldson, J.G. Sorting of Clathrin-Independent Cargo Proteins Depends on Rab35 Delivered by Clathrin-Mediated Endocytosis. Traffic 2015, 16, 994–1009. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Glebov, O.O.; Bright, N.A.; Nichols, B.J. Flotillin-1 defines a clathrin-independent endocytic pathway in mammalian cells. Nat. Cell Biol. 2006, 8, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Otto, G.P.; Nichols, B.J. The roles of flotillin microdomains–endocytosis and beyond. J. Cell Sci. 2011, 124, 3933–3940. [Google Scholar] [CrossRef] [PubMed]
- Riento, K.; Frick, M.; Schafer, I.; Nichols, B.J. Endocytosis of flotillin-1 and flotillin-2 is regulated by Fyn kinase. J. Cell Sci. 2009, 122, 912–918. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Langhorst, M.F.; Reuter, A.; Stuermer, C.A.O. Scaffolding microdomains and beyond: The function of reggie/flotillin proteins. Cell. Mol. Life Sci. 2005, 62, 2228–2240. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rivera-Milla, E.; Stuermer, C.A.O.; Malaga-Trillo, E. Ancient origin of reggie (flotillin), reggie-like, and other lipid-raft proteins: Convergent evolution of the SPFH domain. Cell. Mol. Life Sci. 2006, 63, 343–357. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Babuke, T.; Ruonala, M.; Meister, M.; Amaddii, M.; Genzler, C.; Esposito, A.; Tikkanen, R. Hetero-oligomerization of reggie-1/flotillin-2 and reggie-2/flotillin-1 is required for their endocytosis. Cell. Signal. 2009, 21, 1287–1297. [Google Scholar] [CrossRef] [PubMed]
- Solis, G.P.; Hoegg, M.; Munderloh, C.; Schrock, Y.; Malaga-Trillo, E.; Rivera-Milla, E.; Stuermer, C.A.O. Reggie/flotillin proteins are organized into stable tetramers in membrane microdomains. Biochem. J. 2007, 403, 313–322. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Frick, M.; Bright, N.A.; Riento, K.; Bray, A.; Merrified, C.; Nichols, B.J. Coassembly of flotillins induces formation of membrane microdomains, membrane curvature, and vesicle budding. Curr. Biol. 2007, 17, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Babuke, T.; Tikkanen, R. Dissecting the molecular function of reggie/flotillin proteins. Eur. J. Cell Biol. 2007, 86, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Ait-Slimane, T.; Galmes, R.; Trugnan, G.; Maurice, M. Basolateral internalization of GPI-anchored proteins occurs via a clathrin-independent flotillin-dependent pathway in polarized hepatic cells. Mol. Biol. Cell 2009, 20, 3792–3800. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schneider, A.; Rajendran, L.; Honsho, M.; Gralle, M.; Donnert, G.; Wouters, F.; Hell, S.W.; Simons, M. Flotillin-dependent clustering of the amyloid precursor protein regulates its endocytosis and amyloidogenic processing in neurons. J. Neurosci. 2008, 28, 2874–2882. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, J.; Deyoung, S.M.; Zhang, M.; Dold, L.H.; Saltiel, A.R. The stomatin/prohibitin/flotillin/HflK/C domain of flotillin-1 contains distinct sequences that direct plasma membrane localization and protein interactions in 3T3-L1 adipocytes. J. Biol. Chem. 2005, 280, 16125–16134. [Google Scholar] [CrossRef] [PubMed]
- Neumann-Giesen, C.; Fernow, I.; Amaddii, M.; Tikkanen, R. Role of EGF-induced tyrosine phosphorylation of reggie-1/flotillin-2 in cell spreading and signaling to the actin cytoskeleton. J. Cell Sci. 2007, 120, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Stow, J.L.; Hung, Y.; Wall, A.A. Macropinocytosis: Insights from immunology and cancer. Curr. Opin. Cell Biol. 2020, 65, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Kay, R.R. Macropinocytosis: Biology and mechanisms. Cells Dev. 2021, 168, 203713. [Google Scholar] [CrossRef] [PubMed]
- Salloum, G.; Bresnick, A.R.; Backer, J.M. Macropinocytosis: Mechanisms and regulation. Biochem. J. 2023, 480, 335–362. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Hu, X.; Wei, Z.; Lin, Q. Cellular Regulation of Macropinocytosis. Int. J. Mol. Sci. 2024, 25, 6963. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Araki, N.; Johnson, M.T.; Swanson, J.A. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J. Cell Biol. 1996, 135, 1249–1260. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Amyere, M.; Payrastre, B.; Krause, U.; Van Der Smissen, P.; Veithen, A.; Courtoy, P.J. Constitutive macropinocytosis in oncogene-transformed fibroblasts depends on sequential permanent activation of phosphoinositide 3-kinase and phospholipase C. Mol. Biol. Cell 2000, 11, 3453–3467. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Quinn, S.E.; Huang, L.; Kerkvliet, J.G.; Swanson, J.A.; Smith, S.; Hoppe, A.D.; Anderson, R.B.; Thiex, N.W.; Scott, B.L. The structural dynamics of macropinosome formation and PI3-kinase-mediated sealing revealed by lattice light sheet microscopy. Nat. Commun. 2021, 12, 4838. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lim, J.P.; Gleeson, P.A. Macropinocytosis: An endocytic pathway for internalising large gulps. Immunol. Cell Biol. 2011, 89, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Buckley, C.M.; King, J.S. Drinking problems: Mechanisms of macropinosome formation and maturation. FEBS J. 2017, 284, 3778–3790. [Google Scholar] [CrossRef] [PubMed]
- Garcia-del Portillo, F.; Finlay, B.B. Salmonella invasion of nonphagocytic cells induces formation of macropinosomes in the host cell. Infect. Immun. 1994, 62, 4641–4645. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Weiner, A.; Mellouk, N.; Lopez-Montero, N.; Chang, Y.Y.; Souque, C.; Schmitt, C.; Enninga, J. Macropinosomes are Key Players in Early Shigella Invasion and Vacuolar Escape in Epithelial Cells. PLoS Pathog. 2016, 12, e1005602. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ford, C.; Nans, A.; Boucrot, E.; Hayward, R.D. Chlamydia exploits filopodial capture and a macropinocytosis-like pathway for host cell entry. PLoS Pathog. 2018, 14, e1007051. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Watarai, M.; Makino, S.; Fujii, Y.; Okamoto, K.; Shirahata, T. Modulation of Brucella-induced macropinocytosis by lipid rafts mediates intracellular replication. Cell. Microbiol. 2002, 4, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Perez, B.E.; Mondragon-Flores, R.; Luna-Herrera, J. Internalization of Mycobacterium tuberculosis by macropinocytosis in non-phagocytic cells. Microb. Pathog. 2003, 35, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Perez, B.E.; Hernandez-Gonzalez, J.C.; Garcia-Nieto, S.; Luna-Herrera, J. Internalization of a non-pathogenic mycobacteria by macropinocytosis in human alveolar epithelial A549 cells. Microb. Pathog. 2008, 45, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Watarai, M.; Derre, I.; Kirby, J.; Growney, J.D.; Dietrich, W.F.; Isberg, R.R. Legionella pneumophila is internalized by a macropinocytotic uptake pathway controlled by the Dot/Icm system and the mouse Lgn1 locus. J. Exp. Med. 2001, 194, 1081–1096. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Loh, L.N.; McCarthy, E.M.C.; Narang, P.; Khan, N.A.; Ward, T.H. Escherichia coli K1 utilizes host macropinocytic pathways for invasion of brain microvascular endothelial cells. Traffic 2017, 18, 733–746. [Google Scholar] [CrossRef] [PubMed]
- Mercer, J.; Helenius, A. Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science 2008, 320, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Marechal, V.; Prevost, M.C.; Petit, C.; Perret, E.; Heard, J.M.; Schwartz, O. Human immunodeficiency virus type 1 entry into macrophages mediated by macropinocytosis. J. Virol. 2001, 75, 11166–11177. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Parton, R.G.; Simons, K. The multiple faces of caveolae. Nat. Rev. Mol. Cell Biol. 2007, 8, 185–194. [Google Scholar] [CrossRef]
- Carrasco, G.A.; Van de Kar, L.D.; Jia, C.; Xu, H.; Chen, Z.; Chadda, R.; Garcia, F.; Muma, N.A.; Battaglia, G. Single exposure to a serotonin 1A receptor agonist, (+)8-hydroxy-2-(di-n-propylamino)-tetralin, produces a prolonged heterologous desensitization of serotonin 2A receptors in neuroendocrine neurons in vivo. J. Pharmacol. Exp. Ther. 2007, 320, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, N.; Gomez, G.A.; Howes, M.T.; Lo, H.P.; McMahon, K.A.; Rae, J.A.; Schieber, N.L.; Hill, M.M.; Gaus, K.; Yap, A.S.; et al. Endocytic crosstalk: Cavins, caveolins, and caveolae regulate clathrin-independent endocytosis. PLoS Biol. 2014, 12, e1001832. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Damke, H.; Baba, T.; van der Bliek, A.M.; Schmid, S.L. Clathrin-independent pinocytosis is induced in cells overexpressing a temperature-sensitive mutant of dynamin. J. Cell Biol. 1995, 131, 69–80. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kirkham, M.; Fujita, A.; Chadda, R.; Nixon, S.J.; Kurzchalia, T.V.; Sharma, D.K.; Pagano, R.E.; Hancock, J.F.; Mayor, S.; Parton, R.G. Ultrastructural identification of uncoated caveolin-independent early endocytic vehicles. J. Cell Biol. 2005, 168, 465–476. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cossart, P.; Sansonetti, P.J. Bacterial invasion: The paradigms of enteroinvasive pathogens. Science 2004, 304, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Kiss, A.L.; Botos, E. Endocytosis via caveolae: Alternative pathway with distinct cellular compartments to avoid lysosomal degradation? J. Cell. Mol. Med. 2009, 13, 1228–1237. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dhanda, A.S.; Yu, C.; Lulic, K.T.; Vogl, A.W.; Rausch, V.; Yang, D.; Nichols, B.J.; Kim, S.H.; Polo, S.; Hansen, C.G.; et al. Listeria monocytogenes Exploits Host Caveolin for Cell-to-Cell Spreading. mBio 2020, 11, 10–1128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Drolia, R.; Bryant, D.B.; Tenguria, S.; Jules-Culver, Z.A.; Thind, J.; Amelunke, B.; Liu, D.; Gallina, N.L.F.; Mishra, K.K.; Samaddar, M.; et al. Listeria adhesion protein orchestrates caveolae-mediated apical junctional remodeling of epithelial barrier for Listeria monocytogenes translocation. mBio 2024, 15, e0282123. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gatfield, J.; Pieters, J. Essential role for cholesterol in entry of mycobacteria into macrophages. Science 2000, 288, 1647–1650. [Google Scholar] [CrossRef] [PubMed]
- Kaul, D.; Anand, P.K.; Verma, I. Cholesterol-sensor initiates M. tuberculosis entry into human macrophages. Mol. Cell. Biochem. 2004, 258, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Munoz, S.; Rivas-Santiago, B.; Enciso, J.A. Mycobacterium tuberculosis entry into mast cells through cholesterol-rich membrane microdomains. Scand. J. Immunol. 2009, 70, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Riehle, A.; Pollmeier, B.; Kadow, S.; Schumacher, F.; Drab, M.; Kleuser, B.; Gulbins, E.; Grassme, H. Caveolin-1 affects early mycobacterial infection and apoptosis in macrophages and mice. Tuberculosis 2024, 147, 102493. [Google Scholar] [CrossRef] [PubMed]
- Rohde, M.; Muller, E.; Chhatwal, G.S.; Talay, S.R. Host cell caveolae act as an entry-port for group A streptococci. Cell. Microbiol. 2003, 5, 323–342. [Google Scholar] [CrossRef] [PubMed]
- Goldmann, O.; Lang, J.C.; Rohde, M.; May, T.; Molinari, G.; Medina, E. Alpha-hemolysin promotes internalization of Staphylococcus aureus into human lung epithelial cells via caveolin-1- and cholesterol-rich lipid rafts. Cell. Mol. Life Sci. 2024, 81, 435. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martinez, J.J.; Hultgren, S.J. Requirement of Rho-family GTPases in the invasion of Type 1-piliated uropathogenic Escherichia coli. Cell. Microbiol. 2002, 4, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Eto, D.S.; Jones, T.A.; Sundsbak, J.L.; Mulvey, M.A. Integrin-mediated host cell invasion by type 1–piliated uropathogenic Escherichia coli. PLoS Pathog. 2007, 3, e100. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Duncan, M.J.; Li, G.; Shin, J.S.; Carson, J.L.; Abraham, S.N. Bacterial penetration of bladder epithelium through lipid rafts. J. Biol. Chem. 2004, 279, 18944–18951. [Google Scholar] [CrossRef] [PubMed]
- Servin, A.L. Pathogenesis of human diffusely adhering Escherichia coli expressing Afa/Dr adhesins (Afa/Dr DAEC): Current insights and future challenges. Clin. Microbiol. Rev. 2014, 27, 823–869. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guignot, J.; Bernet-Camard, M.F.; Pous, C.; Plancon, L.; Le Bouguenec, C.; Servin, A.L. Polarized entry of uropathogenic Afa/Dr diffusely adhering Escherichia coli strain IH11128 into human epithelial cells: Evidence for α5β1 integrin recognition and subsequent internalization through a pathway involving caveolae and dynamic unstable microtubules. Infect. Immun. 2001, 69, 1856–1868. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Baorto, D.M.; Gao, Z.; Malaviya, R.; Dustin, M.L.; van der Merwe, A.; Lublin, D.M.; Abraham, S.N. Survival of FimH-expressing enterobacteria in macrophages relies on glycolipid traffic. Nature 1997, 389, 636–639. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.S.; Gao, Z.; Abraham, S.N. Involvement of cellular caveolae in bacterial entry into mast cells. Science 2000, 289, 785–788. [Google Scholar] [CrossRef] [PubMed]
- Sukumaran, S.K.; Quon, M.J.; Prasadarao, N.V. Escherichia coli K1 internalization via caveolae requires caveolin-1 and protein kinase Cα interaction in human brain microvascular endothelial cells. J. Biol. Chem. 2002, 277, 50716–50724. [Google Scholar] [CrossRef] [PubMed]
- Francis, C.L.; Ryan, T.A.; Jones, B.D.; Smith, S.J.; Falkow, S. Ruffles induced by Salmonella and other stimuli direct macropinocytosis of bacteria. Nature 1993, 364, 639–642. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.S.; Shin, M.; Kim, H.J.; Kim, K.S.; Choy, H.E.; Cho, K.A. Caveolin-1 mediates Salmonella invasion via the regulation of SopE-dependent Rac1 activation and actin reorganization. J. Infect. Dis. 2014, 210, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.S.; Choy, H.E.; Park, S.C.; Han, J.M.; Jang, I.S.; Cho, K.A. Caveolae-mediated entry of Salmonella typhimurium into senescent nonphagocytotic host cells. Aging Cell 2010, 9, 243–251. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lim, J.S.; Na, H.S.; Lee, H.C.; Choy, H.E.; Park, S.C.; Han, J.M.; Cho, K.A. Caveolae-mediated entry of Salmonella typhimurium in a human M-cell model. Biochem. Biophys. Res. Commun. 2009, 390, 1322–1327. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, J.T.; Puolakkainen, M.; Haveri, A.; Tammiruusu, A.; Sarvas, M.; Lahesmaa, R. Chlamydia pneumoniae entry into epithelial cells by clathrin-independent endocytosis. Microb. Pathog. 2012, 52, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Norkin, L.C.; Wolfrom, S.A.; Stuart, E.S. Association of caveolin with Chlamydia trachomatis inclusions at early and late stages of infection. Exp. Cell Res. 2001, 266, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Jutras, I.; Abrami, L.; Dautry-Varsat, A. Entry of the lymphogranuloma venereum strain of Chlamydia trachomatis into host cells involves cholesterol-rich membrane domains. Infect. Immun. 2003, 71, 260–266. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Olofsson, A.; Nygard Skalman, L.; Obi, I.; Lundmark, R.; Arnqvist, A. Uptake of Helicobacter pylori vesicles is facilitated by clathrin-dependent and clathrin-independent endocytic pathways. mBio 2014, 5, e00979-14. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Naroeni, A.; Porte, F. Role of cholesterol and the ganglioside GM1 in entry and short-term survival of Brucella suis in murine macrophages. Infect. Immun. 2002, 70, 1640–1644. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Watson, R.O.; Galan, J.E. Campylobacter jejuni survives within epithelial cells by avoiding delivery to lysosomes. PLoS Pathog. 2008, 4, e14. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tamilselvam, B.; Daefler, S. Francisella targets cholesterol-rich host cell membrane domains for entry into macrophages. J. Immunol. 2008, 180, 8262–8271. [Google Scholar] [CrossRef] [PubMed]
- Farber, J.M.; Peterkin, P.I. Listeria monocytogenes, a food-borne pathogen. Microbiol. Rev. 1991, 55, 476–511. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Melton-Witt, J.A.; McKay, S.L.; Portnoy, D.A. Development of a single-gene, signature-tag-based approach in combination with alanine mutagenesis to identify listeriolysin O residues critical for the in vivo survival of Listeria monocytogenes. Infect. Immun. 2012, 80, 2221–2230. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, T.; Abel, S.; Abel Zur Wiesch, P.; Sasabe, J.; Davis, B.M.; Higgins, D.E.; Waldor, M.K. Deciphering the landscape of host barriers to Listeria monocytogenes infection. Proc. Natl. Acad. Sci. USA 2017, 114, 6334–6339. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pizarro-Cerda, J.; Cossart, P. Listeria monocytogenes: Cell biology of invasion and intracellular growth. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Radoshevich, L.; Cossart, P. Listeria monocytogenes: Towards a complete picture of its physiology and pathogenesis. Nat. Rev. Microbiol. 2018, 16, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, J.L.; Berche, P.; Frehel, C.; Gouin, E.; Cossart, P. Entry of L. monocytogenes into cells is mediated by internalin, a repeat protein reminiscent of surface antigens from gram-positive cocci. Cell 1991, 65, 1127–1141. [Google Scholar] [CrossRef] [PubMed]
- Lecuit, M.; Dramsi, S.; Gottardi, C.; Fedor-Chaiken, M.; Gumbiner, B.; Cossart, P. A single amino acid in E-cadherin responsible for host specificity towards the human pathogen Listeria monocytogenes. EMBO J. 1999, 18, 3956–3963. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shen, Y.; Naujokas, M.; Park, M.; Ireton, K. InIB-dependent internalization of Listeria is mediated by the Met receptor tyrosine kinase. Cell 2000, 103, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Rezelj, S.; Bedina Zavec, A.; Anderluh, G.; Scheuring, S. Listeriolysin O Membrane Damaging Activity Involves Arc Formation and Lineaction—Implication for Listeria monocytogenes Escape from Phagocytic Vacuole. PLoS Pathog. 2016, 12, e1005597. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Petrisic, N.; Adamek, M.; Kezar, A.; Hocevar, S.B.; Zagar, E.; Anderluh, G.; Podobnik, M. Structural basis for the unique molecular properties of broad-range phospholipase C from Listeria monocytogenes. Nat. Commun. 2023, 14, 6474. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lambrechts, A.; Gevaert, K.; Cossart, P.; Vandekerckhove, J.; Van Troys, M. Listeria comet tails: The actin-based motility machinery at work. Trends Cell Biol. 2008, 18, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Bonazzi, M.; Vasudevan, L.; Mallet, A.; Sachse, M.; Sartori, A.; Prevost, M.C.; Roberts, A.; Taner, S.B.; Wilbur, J.D.; Brodsky, F.M.; et al. Clathrin phosphorylation is required for actin recruitment at sites of bacterial adhesion and internalization. J. Cell Biol. 2011, 195, 525–536. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Veiga, E.; Cossart, P. Listeria hijacks the clathrin-dependent endocytic machinery to invade mammalian cells. Nat. Cell Biol. 2005, 7, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Drolia, R.; Tenguria, S.; Durkes, A.C.; Turner, J.R.; Bhunia, A.K. Listeria Adhesion Protein Induces Intestinal Epithelial Barrier Dysfunction for Bacterial Translocation. Cell Host Microbe 2018, 23, 470–484.e7. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Houben, R.M.G.J.; Dodd, P.J. The Global Burden of Latent Tuberculosis Infection: A Re-estimation Using Mathematical Modelling. PLoS Med. 2016, 13, e1002152. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gagneux, S. Ecology and evolution of Mycobacterium tuberculosis. Nat. Rev. Microbiol. 2018, 16, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Guirado, E.; Schlesinger, L.S.; Kaplan, G. Macrophages in tuberculosis: Friend or foe. Semin. Immunopathol. 2013, 35, 563–583. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pieters, J.; Gatfield, J. Hijacking the host: Survival of pathogenic mycobacteria inside macrophages. Trends Microbiol. 2002, 10, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Hmama, Z.; Pena-Diaz, S.; Joseph, S.; Av-Gay, Y. Immunoevasion and immunosuppression of the macrophage by Mycobacterium tuberculosis. Immunol. Rev. 2015, 264, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Chai, Q.; Wang, L.; Liu, C.H.; Ge, B. New insights into the evasion of host innate immunity by Mycobacterium tuberculosis. Cell. Mol. Immunol. 2020, 17, 901–913. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hasan, Z.; Schlax, C.; Kuhn, L.; Lefkovits, I.; Young, D.; Thole, J.; Pieters, J. Isolation and characterization of the mycobacterial phagosome: Segregation from the endosomal/lysosomal pathway. Mol. Microbiol. 1997, 25, 427. [Google Scholar] [CrossRef] [PubMed]
- Houben, E.N.; Nguyen, L.; Pieters, J. Interaction of pathogenic mycobacteria with the host immune system. Curr. Opin. Microbiol. 2006, 9, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Schorey, J.S.; Carroll, M.C.; Brown, E.J. A macrophage invasion mechanism of pathogenic mycobacteria. Science 1997, 277, 1091–1093. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, G.; Langen, H.; Naito, M.; Pieters, J. A coat protein on phagosomes involved in the intracellular survival of mycobacteria. Cell 1999, 97, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.Y.; Claus, R.A. Keep Your Friends Close, but Your Enemies Closer: Role of Acid Sphingomyelinase During Infection and Host Response. Front. Med. 2020, 7, 616500. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Munoz, S.; Hernandez-Pando, R.; Abraham, S.N.; Enciso, J.A. Mast cell activation by Mycobacterium tuberculosis: Mediator release and role of CD48. J. Immunol. 2003, 170, 5590–5596. [Google Scholar] [CrossRef] [PubMed]
- Dudeck, A.; Koberle, M.; Goldmann, O.; Meyer, N.; Dudeck, J.; Lemmens, S.; Rohde, M.; Roldan, N.G.; Dietze-Schwonberg, K.; Orinska, Z.; et al. Mast cells as protectors of health. J. Allergy Clin. Immunol. 2019, 144, S4–S18. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.J.; Barnett, T.C.; McArthur, J.D.; Cole, J.N.; Gillen, C.M.; Henningham, A.; Sriprakash, K.S.; Sanderson-Smith, M.L.; Nizet, V. Disease manifestations and pathogenic mechanisms of Group A Streptococcus. Clin. Microbiol. Rev. 2014, 27, 264–301. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Amelung, S.; Nerlich, A.; Rohde, M.; Spellerberg, B.; Cole, J.N.; Nizet, V.; Chhatwal, G.S.; Talay, S.R. The FbaB-type fibronectin-binding protein of Streptococcus pyogenes promotes specific invasion into endothelial cells. Cell Microbiol. 2011, 13, 1200–1211. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Molinari, G.; Rohde, M.; Guzman, C.A.; Chhatwal, G.S. Two distinct pathways for the invasion of Streptococcus pyogenes in non-phagocytic cells. Cell. Microbiol. 2000, 2, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Siemens, N.; Patenge, N.; Otto, J.; Fiedler, T.; Kreikemeyer, B. Streptococcus pyogenes M49 plasminogen/plasmin binding facilitates keratinocyte invasion via integrin-integrin-linked kinase (ILK) pathways and protects from macrophage killing. J. Biol. Chem. 2011, 286, 21612–21622. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- LaPenta, D.; Rubens, C.; Chi, E.; Cleary, P.P. Group A streptococci efficiently invade human respiratory epithelial cells. Proc. Natl. Acad. Sci. USA 1994, 91, 12115–12119. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kaplan, E.L.; Chhatwal, G.S.; Rohde, M. Reduced ability of penicillin to eradicate ingested group A streptococci from epithelial cells: Clinical and pathogenetic implications. Clin. Infect. Dis. 2006, 43, 1398–1406. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Cleary, P.P. Intracellular Invasion by Streptococcus pyogenes: Invasins, Host Receptors, and Relevance to Human Disease. Microbiol. Spectr. 2019, 7, 35–44. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Medina, E.; Goldmann, O.; Toppel, A.W.; Chhatwal, G.S. Survival of Streptococcus pyogenes within host phagocytic cells: A pathogenic mechanism for persistence and systemic invasion. J. Infect. Dis. 2003, 187, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Medina, E.; Rohde, M.; Chhatwal, G.S. Intracellular survival of Streptococcus pyogenes in polymorphonuclear cells results in increased bacterial virulence. Infect. Immun. 2003, 71, 5376–5380. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Talay, S.R.; Zock, A.; Rohde, M.; Molinari, G.; Oggioni, M.; Pozzi, G.; Guzman, C.A.; Chhatwal, G.S. Co-operative binding of human fibronectin to Sfbl protein triggers streptococcal invasion into respiratory epithelial cells. Cell. Microbiol. 2000, 2, 521–535. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.; Barnett, T.C.; Bastiani, M.; McMahon, K.A.; Ferguson, C.; Webb, R.I.; Parton, R.G.; Walker, M.J. Caveolin 1 restricts Group A Streptococcus invasion of nonphagocytic host cells. Cell. Microbiol. 2017, 19, e12772. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chambers, H.F.; Deleo, F.R. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 2009, 7, 629–641. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thammavongsa, V.; Kim, H.K.; Missiakas, D.; Schneewind, O. Staphylococcal manipulation of host immune responses. Nat. Rev. Microbiol. 2015, 13, 529–543. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gresham, H.D.; Lowrance, J.H.; Caver, T.E.; Wilson, B.S.; Cheung, A.L.; Lindberg, F.P. Survival of Staphylococcus aureus inside neutrophils contributes to infection. J. Immunol. 2000, 164, 3713–3722. [Google Scholar] [CrossRef] [PubMed]
- Horn, J.; Stelzner, K.; Rudel, T.; Fraunholz, M. Inside job: Staphylococcus aureus host-pathogen interactions. Int. J. Med. Microbiol. 2018, 308, 607–624. [Google Scholar] [CrossRef] [PubMed]
- Hommes, J.W.; Surewaard, B.G.J. Intracellular Habitation of Staphylococcus aureus: Molecular Mechanisms and Prospects for Antimicrobial Therapy. Biomedicines 2022, 10, 1804. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Berube, B.J.; Bubeck Wardenburg, J. Staphylococcus aureus α-toxin: Nearly a century of intrigue. Toxins 2013, 5, 1140–1166. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vijayvargia, R.; Kaur, S.; Krishnasastry, M.V. α-Hemolysin-induced dephosphorylation of EGF receptor of A431 cells is carried out by rPTPσ. Biochem. Biophys. Res. Commun. 2004, 325, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Vijayvargia, R.; Suresh, C.G.; Krishnasastry, M.V. Functional form of Caveolin-1 is necessary for the assembly of α-hemolysin. Biochem. Biophys. Res. Commun. 2004, 324, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Vijayvargia, R.; Kaur, S.; Sangha, N.; Sahasrabuddhe, A.A.; Surolia, I.; Shouche, Y.; Krishnasastry, M.V. Assembly of α-hemolysin on A431 cells leads to clustering of Caveolin-1. Biochem. Biophys. Res. Commun. 2004, 324, 1124–1129. [Google Scholar] [CrossRef] [PubMed]
- Pany, S.; Vijayvargia, R.; Krishnasastry, M.V. Caveolin-1 binding motif of α-hemolysin: Its role in stability and pore formation. Biochem. Biophys. Res. Commun. 2004, 322, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Pany, S.; Krishnasastry, M.V. Aromatic residues of Caveolin-1 binding motif of α-hemolysin are essential for membrane penetration. Biochem. Biophys. Res. Commun. 2007, 363, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.; Berking, A.; Agerer, F.; Buntru, A.; Neske, F.; Chhatwal, G.S.; Ohlsen, K.; Hauck, C.R. Caveolin limits membrane microdomain mobility and integrin-mediated uptake of fibronectin-binding pathogens. J. Cell Sci. 2010, 123, 4280–4291. [Google Scholar] [CrossRef] [PubMed]
- Croxen, M.A.; Finlay, B.B. Molecular mechanisms of Escherichia coli pathogenicity. Nat. Rev. Microbiol. 2010, 8, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Whelan, S.; Lucey, B.; Finn, K. Uropathogenic Escherichia coli (UPEC)-Associated Urinary Tract Infections: The Molecular Basis for Challenges to Effective Treatment. Microorganisms 2023, 11, 2196. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, X.; Chen, H.; Zheng, Y.; Qu, S.; Wang, H.; Yi, F. Disease burden and long-term trends of urinary tract infections: A worldwide report. Front. Public Health 2022, 10, 888205. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sihra, N.; Goodman, A.; Zakri, R.; Sahai, A.; Malde, S. Nonantibiotic prevention and management of recurrent urinary tract infection. Nat. Rev. Urol. 2018, 15, 750–776. [Google Scholar] [CrossRef] [PubMed]
- Servin, A.L. Pathogenesis of Afa/Dr diffusely adhering Escherichia coli. Clin. Microbiol. Rev. 2005, 18, 264–292. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martinez, J.J.; Mulvey, M.A.; Schilling, J.D.; Pinkner, J.S.; Hultgren, S.J. Type 1 pilus-mediated bacterial invasion of bladder epithelial cells. EMBO J. 2000, 19, 2803–2812. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mulvey, M.A.; Lopez-Boado, Y.S.; Wilson, C.L.; Roth, R.; Parks, W.C.; Heuser, J.; Hultgren, S.J. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 1998, 282, 1494–1497. [Google Scholar] [CrossRef] [PubMed]
- Mulvey, M.A. Adhesion and entry of uropathogenic Escherichia coli. Cell. Microbiol. 2002, 4, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Mulvey, M.A.; Schilling, J.D.; Hultgren, S.J. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect. Immun. 2001, 69, 4572–4579. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Doye, A.; Mettouchi, A.; Bossis, G.; Clement, R.; Buisson-Touati, C.; Flatau, G.; Gagnoux, L.; Piechaczyk, M.; Boquet, P.; Lemichez, E. CNF1 exploits the ubiquitin-proteasome machinery to restrict Rho GTPase activation for bacterial host cell invasion. Cell 2002, 111, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Springall, T.; Sheerin, N.S.; Abe, K.; Holers, V.M.; Wan, H.; Sacks, S.H. Epithelial secretion of C3 promotes colonization of the upper urinary tract by Escherichia coli. Nat. Med. 2001, 7, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Poole, N.M.; Green, S.I.; Rajan, A.; Vela, L.E.; Zeng, X.L.; Estes, M.K.; Maresso, A.W. Role for FimH in Extraintestinal Pathogenic Escherichia coli Invasion and Translocation through the Intestinal Epithelium. Infect. Immun. 2017, 85, 10–1128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, G.; Mo, W.J.; Sebbel, P.; Min, G.; Neubert, T.A.; Glockshuber, R.; Wu, X.R.; Sun, T.T.; Kong, X.P. Uroplakin Ia is the urothelial receptor for uropathogenic Escherichia coli: Evidence from in vitro FimH binding. J. Cell Sci. 2001, 114, 4095–4103. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, A.; Fleckenstein, J.M. Interactions of pathogenic Escherichia coli with CEACAMs. Front. Immunol. 2023, 14, 1120331. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anderson, G.G.; Palermo, J.J.; Schilling, J.D.; Roth, R.; Heuser, J.; Hultgren, S.J. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 2003, 301, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Ouchenir, L.; Renaud, C.; Khan, S.; Bitnun, A.; Boisvert, A.A.; McDonald, J.; Bowes, J.; Brophy, J.; Barton, M.; Ting, J.; et al. The Epidemiology, Management, and Outcomes of Bacterial Meningitis in Infants. Pediatrics 2017, 140, e20170476. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S. Pathogenesis of bacterial meningitis: From bacteraemia to neuronal injury. Nat. Rev. Neurosci. 2003, 4, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Fabrega, A.; Vila, J. Salmonella enterica serovar Typhimurium skills to succeed in the host: Virulence and regulation. Clin. Microbiol. Rev. 2013, 26, 308–341. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Jong, H.K.; Parry, C.M.; van der Poll, T.; Wiersinga, W.J. Host–pathogen interaction in invasive Salmonellosis. PLoS Pathog. 2012, 8, e1002933. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jones, B.D.; Ghori, N.; Falkow, S. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer’s patches. J. Exp. Med. 1994, 180, 15–23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lou, L.; Zhang, P.; Piao, R.; Wang, Y. Salmonella Pathogenicity Island 1 (SPI-1) and Its Complex Regulatory Network. Front. Cell. Infect. Microbiol. 2019, 9, 270. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ly, K.T.; Casanova, J.E. Mechanisms of Salmonella entry into host cells. Cell. Microbiol. 2007, 9, 2103–2111. [Google Scholar] [CrossRef] [PubMed]
- Bakowski, M.A.; Braun, V.; Brumell, J.H. Salmonella-containing vacuoles: Directing traffic and nesting to grow. Traffic 2008, 9, 2022–2031. [Google Scholar] [CrossRef] [PubMed]
- Cheong, H.C.; Lee, C.Y.Q.; Cheok, Y.Y.; Tan, G.M.Y.; Looi, C.Y.; Wong, W.F. Chlamydiaceae: Diseases in Primary Hosts and Zoonosis. Microorganisms 2019, 7, 146. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Elwell, C.; Mirrashidi, K.; Engel, J. Chlamydia cell biology and pathogenesis. Nat. Rev. Microbiol. 2016, 14, 385–400. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cocchiaro, J.L.; Valdivia, R.H. New insights into Chlamydia intracellular survival mechanisms. Cell. Microbiol. 2009, 11, 1571–1578. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Caven, L.; Carabeo, R.A. Pathogenic Puppetry: Manipulation of the Host Actin Cytoskeleton by Chlamydia trachomatis. Int. J. Mol. Sci. 2019, 21, 90. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhong, G. Chlamydia Spreading from the Genital Tract to the Gastrointestinal Tract—A Two-Hit Hypothesis. Trends Microbiol. 2018, 26, 611–623. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bebear, C.; de Barbeyrac, B. Genital Chlamydia trachomatis infections. Clin. Microbiol. Infect. 2009, 15, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.R.; Burton, M.J.; Haddad, D.; West, S.; Wright, H. Trachoma. Lancet 2014, 384, 2142–2152. [Google Scholar] [CrossRef] [PubMed]
- Carabeo, R.A.; Grieshaber, S.S.; Fischer, E.; Hackstadt, T. Chlamydia trachomatis induces remodeling of the actin cytoskeleton during attachment and entry into HeLa cells. Infect. Immun. 2002, 70, 3793–3803. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boleti, H.; Benmerah, A.; Ojcius, D.M.; Cerf-Bensussan, N.; Dautry-Varsat, A. Chlamydia infection of epithelial cells expressing dynamin and Eps15 mutants: Clathrin-independent entry into cells and dynamin-dependent productive growth. J. Cell Sci. 1999, 112, 1487–1496. [Google Scholar] [CrossRef] [PubMed]
- Stuart, E.S.; Webley, W.C.; Norkin, L.C. Lipid rafts, caveolae, caveolin-1, and entry by Chlamydiae into host cells. Exp. Cell Res. 2003, 287, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Watarai, M.; Makino, S.; Michikawa, M.; Yanagisawa, K.; Murakami, S.; Shirahata, T. Macrophage plasma membrane cholesterol contributes to Brucella abortus infection of mice. Infect. Immun. 2002, 70, 4818–4825. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pietiainen, V.M.; Marjomaki, V.; Heino, J.; Hyypia, T. Viral entry, lipid rafts and caveosomes. Ann. Med. 2005, 37, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Pelkmans, L. Secrets of caveolae- and lipid raft-mediated endocytosis revealed by mammalian viruses. Biochim. Biophys. Acta Mol. Cell Res. 2005, 1746, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Anderson, H.A.; Chen, Y.; Norkin, L.C. Bound simian virus 40 translocates to caveolin-enriched membrane domains, and its entry is inhibited by drugs that selectively disrupt caveolae. Mol. Biol. Cell 1996, 7, 1825–1834. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marjomaki, V.; Pietiainen, V.; Matilainen, H.; Upla, P.; Ivaska, J.; Nissinen, L.; Reunanen, H.; Huttunen, P.; Hyypia, T.; Heino, J. Internalization of echovirus 1 in caveolae. J. Virol. 2002, 76, 1856–1865. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pietiainen, V.; Marjomaki, V.; Upla, P.; Pelkmans, L.; Helenius, A.; Hyypia, T. Echovirus 1 endocytosis into caveosomes requires lipid rafts, dynamin II, and signaling events. Mol. Biol. Cell 2004, 15, 4911–4925. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, H.; Yang, P.; Liu, K.; Guo, F.; Zhang, Y.; Zhang, G.; Jiang, C. SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res. 2008, 18, 290–301. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, G.M.; Li, Y.G.; Yamate, M.; Li, S.M.; Ikuta, K. Lipid rafts play an important role in the early stage of severe acute respiratory syndrome-coronavirus life cycle. Microbes Infect. 2007, 9, 96–102. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lu, Y.; Liu, D.X.; Tam, J.P. Lipid rafts are involved in SARS-CoV entry into Vero E6 cells. Biochem. Biophys. Res. Commun. 2008, 369, 344–349. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nomura, R.; Kiyota, A.; Suzaki, E.; Kataoka, K.; Ohe, Y.; Miyamoto, K.; Senda, T.; Fujimoto, T. Human coronavirus 229E binds to CD13 in rafts and enters the cell through caveolae. J. Virol. 2004, 78, 8701–8708. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Owczarek, K.; Szczepanski, A.; Milewska, A.; Baster, Z.; Rajfur, Z.; Sarna, M.; Pyrc, K. Early events during human coronavirus OC43 entry to the cell. Sci. Rep. 2018, 8, 7124. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Manes, S.; del Real, G.; Lacalle, R.A.; Lucas, P.; Gomez-Mouton, C.; Sanchez-Palomino, S.; Delgado, R.; Alcami, J.; Mira, E.; Martinez, A.C. Membrane raft microdomains mediate lateral assemblies required for HIV-1 infection. EMBO Rep. 2000, 1, 190–196. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nguyen, D.H.; Hildreth, J.E. Evidence for budding of human immunodeficiency virus type 1 selectively from glycolipid-enriched membrane lipid rafts. J. Virol. 2000, 74, 3264–3272. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, Q.; Cao, M.; Song, H.; Chen, S.; Qian, X.; Zhao, P.; Ren, H.; Tang, H.; Wang, Y.; Wei, Y.; et al. Caveolin-1-mediated Japanese encephalitis virus entry requires a two-step regulation of actin reorganization. Futur. Microbiol. 2016, 11, 1227–1248. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.Z.; Xu, Q.Q.; Wu, D.G.; Ren, H.; Zhao, P.; Lao, W.G.; Wang, Y.; Tao, Q.Y.; Qian, X.J.; Wei, Y.H.; et al. Japanese encephalitis virus enters rat neuroblastoma cells via a pH-dependent, dynamin and caveola-mediated endocytosis pathway. J. Virol. 2012, 86, 13407–13422. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Campanero-Rhodes, M.A.; Smith, A.; Chai, W.; Sonnino, S.; Mauri, L.; Childs, R.A.; Zhang, Y.; Ewers, H.; Helenius, A.; Imberty, A.; et al. N-glycolyl GM1 ganglioside as a receptor for simian virus 40. J. Virol. 2007, 81, 12846–12858. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tsai, B.; Gilbert, J.M.; Stehle, T.; Lencer, W.; Benjamin, T.L.; Rapoport, T.A. Gangliosides are receptors for murine polyoma virus and SV40. EMBO J. 2003, 22, 4346–4355. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schelhaas, M.; Malmstrom, J.; Pelkmans, L.; Haugstetter, J.; Ellgaard, L.; Grunewald, K.; Helenius, A. Simian Virus 40 depends on ER protein folding and quality control factors for entry into host cells. Cell 2007, 131, 516–529. [Google Scholar] [CrossRef] [PubMed]
- Milavetz, B.I.; Balakrishnan, L. Simian virus 40 (SV40)—Fresh perspectives on a historic virus. Virology 2025, 604, 110427. [Google Scholar] [CrossRef] [PubMed]
- Breau, W.C.; Atwood, W.J.; Norkin, L.C. Class I major histocompatibility proteins are an essential component of the simian virus 40 receptor. J. Virol. 1992, 66, 2037–2045. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peters, A.H.; O’Grady, J.E.; Milanovich, R.A. Aseptic meningitis associated with Echovirus type 3 in very young children. Am. J. Dis. Child. 1972, 123, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Mercer, J.; Schelhaas, M.; Helenius, A. Virus entry by endocytosis. Annu. Rev. Biochem. 2010, 79, 803–833. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Liu, Z.; Chen, D. Human coronaviruses: Origin, host and receptor. J. Clin. Virol. 2022, 155, 105246. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- V’Kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus biology and replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021, 19, 155–170. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020, 367, 1444–1448. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tortorici, M.A.; Walls, A.C.; Lang, Y.; Wang, C.; Li, Z.; Koerhuis, D.; Boons, G.J.; Bosch, B.J.; Rey, F.A.; de Groot, R.J.; et al. Structural basis for human coronavirus attachment to sialic acid receptors. Nat. Struct. Mol. Biol. 2019, 26, 481–489. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C.; et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003, 426, 450–454. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Harrison, C.M.; Doster, J.M.; Landwehr, E.H.; Kumar, N.P.; White, E.J.; Beachboard, D.C.; Stobart, C.C. Evaluating the Virology and Evolution of Seasonal Human Coronaviruses Associated with the Common Cold in the COVID-19 Era. Microorganisms 2023, 11, 445. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yeager, C.L.; Ashmun, R.A.; Williams, R.K.; Cardellichio, C.B.; Shapiro, L.H.; Look, A.T.; Holmes, K.V. Human aminopeptidase N is a receptor for human coronavirus 229E. Nature 1992, 357, 420–422. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Murray, R.S.; Brown, B.; Brain, D.; Cabirac, G.F. Detection of coronavirus RNA and antigen in multiple sclerosis brain. Ann. Neurol. 1992, 31, 525–533. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lau, S.K.P.; Li, K.S.M.; Li, X.; Tsang, K.Y.; Sridhar, S.; Woo, P.C.Y. Fatal Pneumonia Associated with a Novel Genotype of Human Coronavirus OC43. Front. Microbiol. 2022, 12, 795449. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Collins, A.R. HLA class I antigen serves as a receptor for human coronavirus OC43. Immunol. Investig. 1993, 22, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Ono, A.; Freed, E.O. Plasma membrane rafts play a critical role in HIV-1 assembly and release. Proc. Natl. Acad. Sci. USA 2001, 98, 13925–13930. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Turtle, L.; Solomon, T. Japanese encephalitis—The prospects for new treatments. Nat. Rev. Neurol. 2018, 14, 298–313. [Google Scholar] [CrossRef] [PubMed]

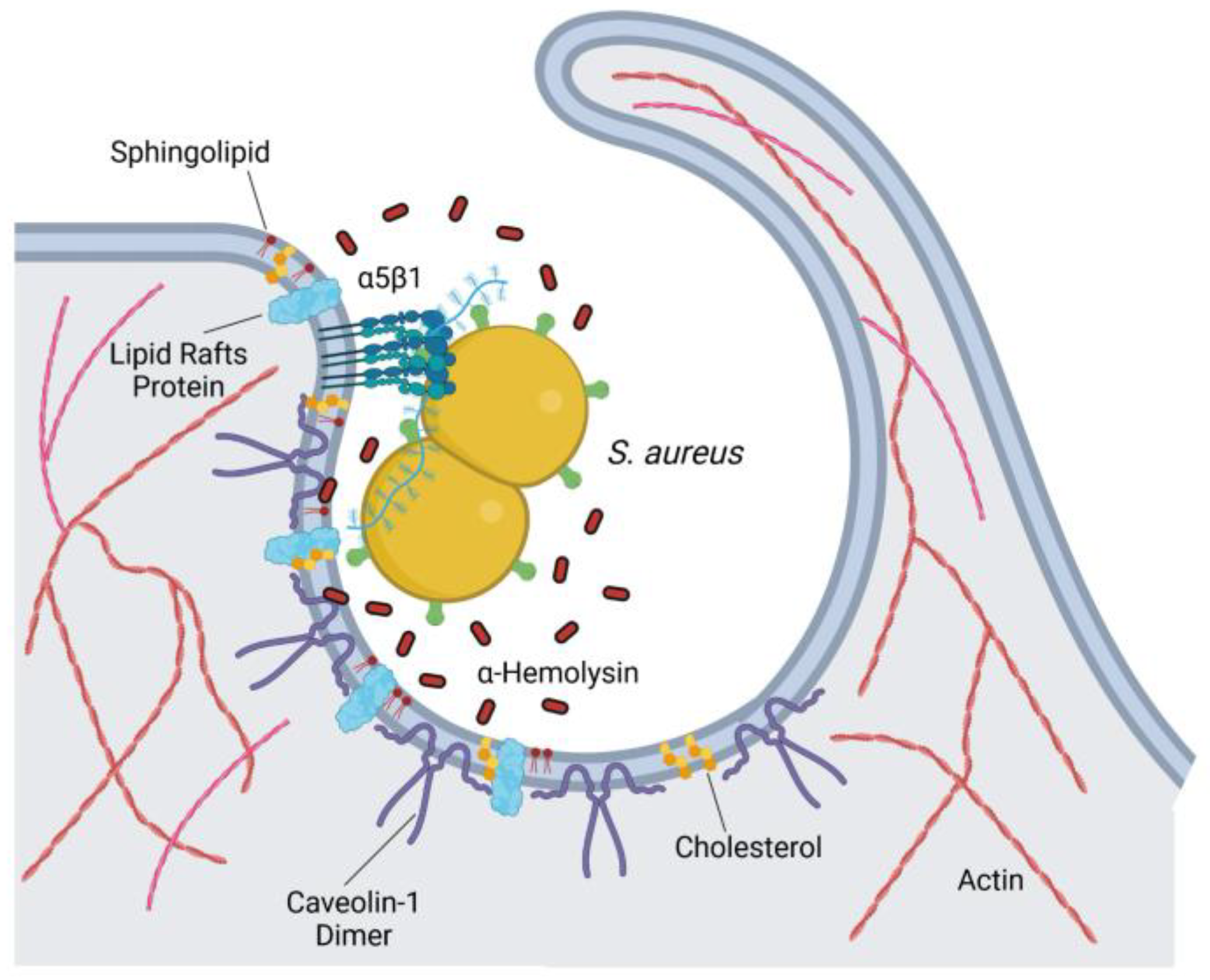
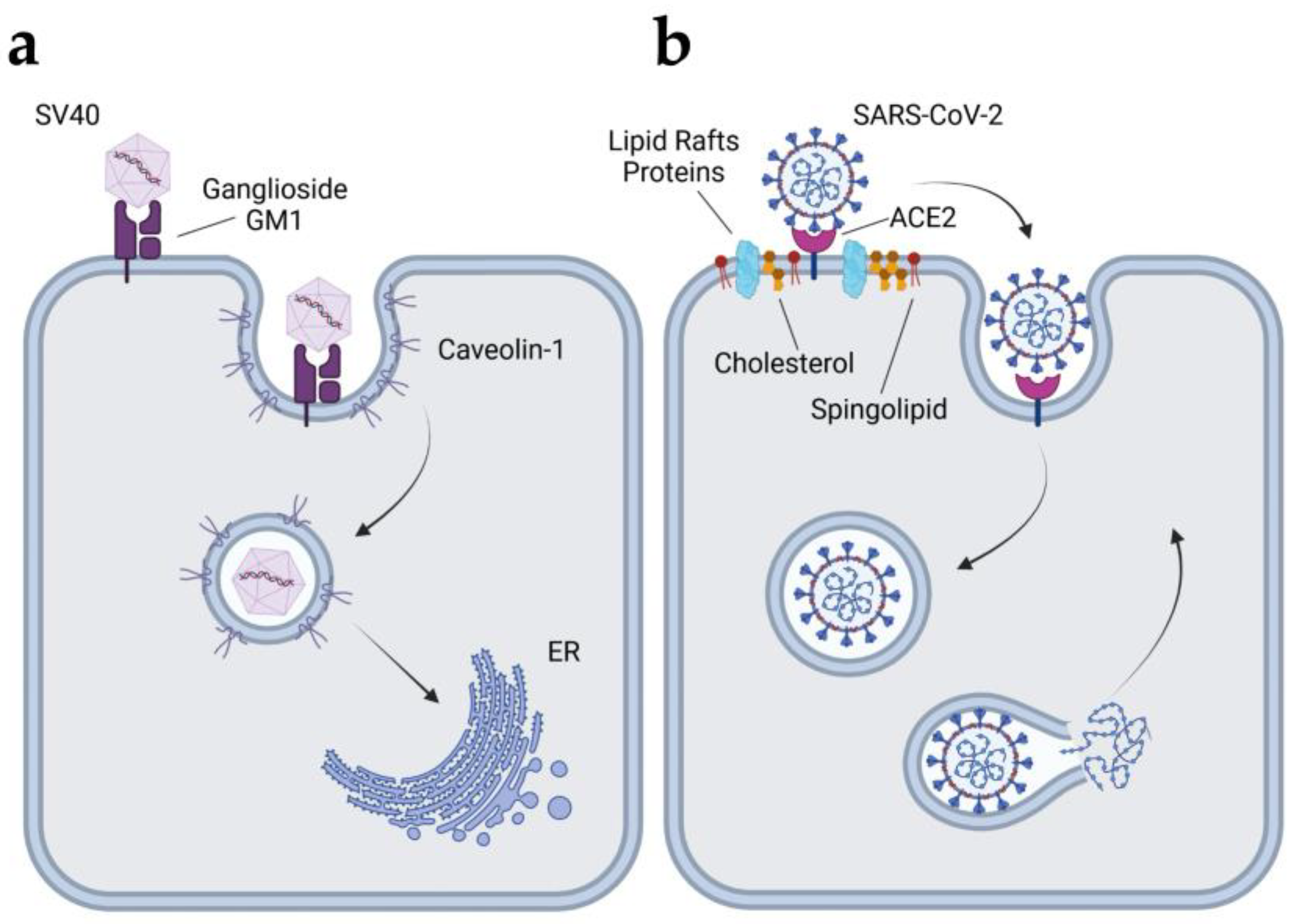
| Bacteria | Cell Type | CIE Pathway | References |
|---|---|---|---|
| Listeria monocytogenes | Epithelial cells | Caveolin-1-mediated | [153,154] |
| Mycobacterium tuberculosis | Macrophages | Cholesterol-rich domains | [155,156] |
| Mast cells | Lipid rafts | [157] | |
| BCG | Macrophages | Caveolin-1-mediated | [158] |
| Streptococcus pyogenes | Epithelial cells (HEP-2) | Caveolae-mediated | [159] |
| Staphylococcus aureus | Human respiratory epithelial cells | caveolin-1- and cholesterol-rich lipid rafts | [160] |
| Escherichia coli | Human bladder epithelial cells | Rho-family GTPases-mediated | [161] |
| Focal adhesion and Src family kinases | [162] | ||
| Caveolae/lipid raft | [163,164,165] | ||
| Macrophages | Lipid-rich microdomains | [166] | |
| Mast cells | Caveolae | [167] | |
| Human brain microvascular endothelial cells | Caveolae | [168] | |
| Salmonella typhimurium | Epithelial cells (HEP-2) | Macropinocytosis | [169] |
| Senescent human diploid fibroblasts | Caveolae | [170,171] | |
| Human M cells | Caveolae | [170,172] | |
| Chlamydia | Epithelial cells | Cholesterol- and sphingomyelin-rich plasma membrane microdomain | [173,174,175] |
| Macrophages | Cholesterol- and sphingomyelin-rich plasma membrane microdomain | [174] | |
| Helicobacter pylori | Gastric epithelial cells | Undefined CIE | [176] |
| Brucella | Macrophages | Lipid rafts | [139,177] |
| Campylobacter jejuni | Intestinal epithelial cells | Caveolae-mediated | [178] |
| Francisella tularensis | Macrophages | Lipid rafts | [179] |
| Virus | Cell Type | CIE Pathway | References |
|---|---|---|---|
| Simian virus 40 (SV40) | African green monkey fibroblast cells (CV-1) | Caveolae-mediated | [62,267] |
| Echoviruses | Primary osteosarcoma cells (Saos cells) | Caveolae-mediated | [268,269] |
| African green monkey fibroblast cells (CV-1) | Caveolae-mediated | [269] | |
| Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) | 293E-ACE2-Myc cells | Cholesterol- and sphingolipid-rich lipid raft microdomains | [270] |
| African green monkey fibroblast cells (Vero E6 cells) | Lipid rafts | [271,272] | |
| Human coronavirus 229E (HCoV-229E) | Fibroblasts | Caveolae-mediated | [273] |
| Human coronavirus OC43 | Human ileocecal colorectal adenocarcinoma (HCT-8 cells) | Caveolin-1-mediated | [274] |
| Human immunodeficiency virus 1 (HIV-1) | MT-2 cells | Membrane raft microdomains | [275] |
| Jurkat cells | Lipid rafts | [276] | |
| Japanese Encephalitis Virus (JEV) | Human neuronal cells | Caveolin-1-mediated | [277] |
| B104 rat neuroblastoma cells | Caveolae-mediated | [278] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goldmann, O.; Medina, E. Revisiting Pathogen Exploitation of Clathrin-Independent Endocytosis: Mechanisms and Implications. Cells 2025, 14, 731. https://doi.org/10.3390/cells14100731
Goldmann O, Medina E. Revisiting Pathogen Exploitation of Clathrin-Independent Endocytosis: Mechanisms and Implications. Cells. 2025; 14(10):731. https://doi.org/10.3390/cells14100731
Chicago/Turabian StyleGoldmann, Oliver, and Eva Medina. 2025. "Revisiting Pathogen Exploitation of Clathrin-Independent Endocytosis: Mechanisms and Implications" Cells 14, no. 10: 731. https://doi.org/10.3390/cells14100731
APA StyleGoldmann, O., & Medina, E. (2025). Revisiting Pathogen Exploitation of Clathrin-Independent Endocytosis: Mechanisms and Implications. Cells, 14(10), 731. https://doi.org/10.3390/cells14100731






