Taurine Prevents Impairments in Skin Barrier Function and Dermal Collagen Synthesis Triggered by Sleep Deprivation-Induced Estrogen Circadian Rhythm Disruption
Abstract
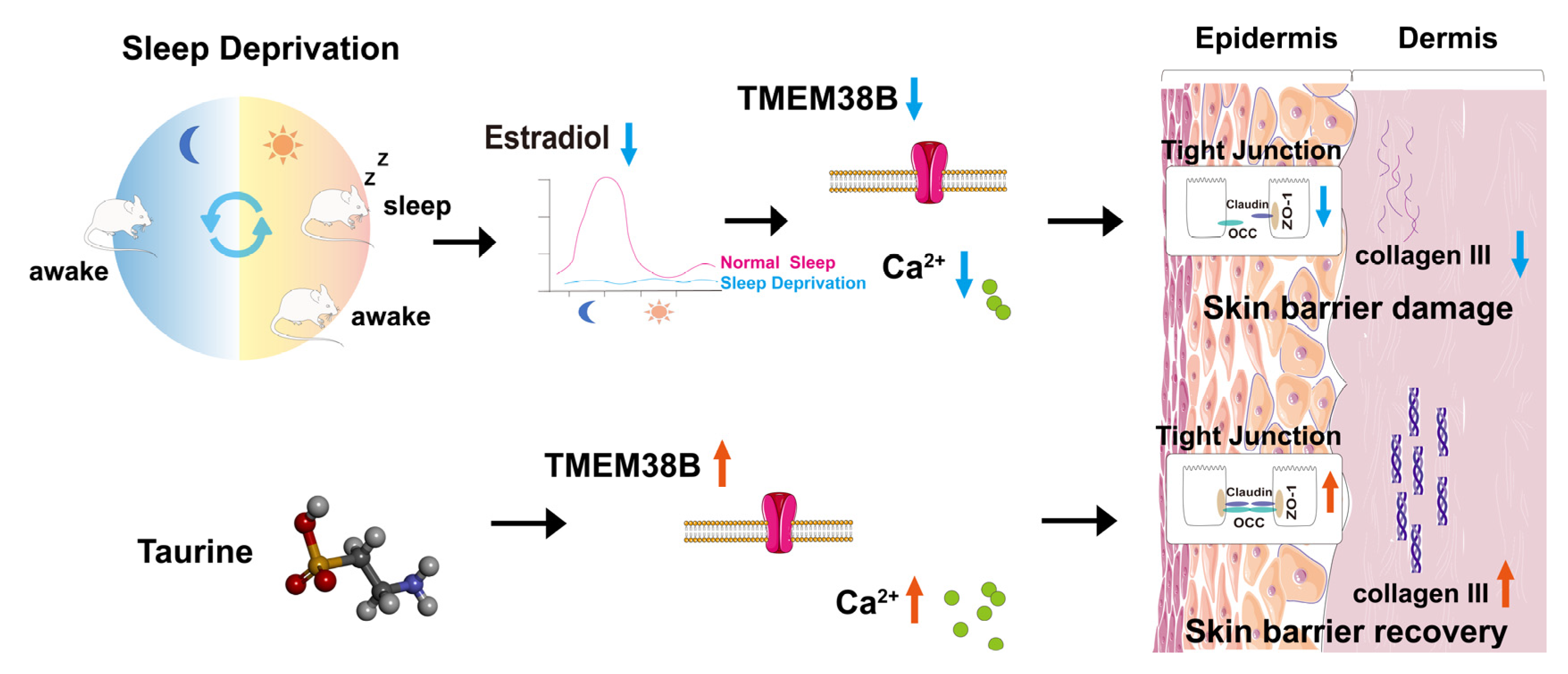
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Drug Delivery Treatments
2.2. Quantitative Real-Time PCR (qRT-PCR)
2.3. Western Blot Assay
2.4. Transepithelial Electrical Resistance (TEER) Measurements
2.5. Knockdown and Overexpression
2.6. Calcium Ion Content Detection Assay
2.7. Immunofluorescence Assay
2.8. Animal Experiment
2.9. FITC Penetration Assessment of the Skin Barrier
2.10. Hematoxylin-Eosin Staining
2.11. Masson Staining
2.12. Sirus Red Stain
2.13. Serum Estradiol Assay
2.14. Statistical Analysis
3. Results
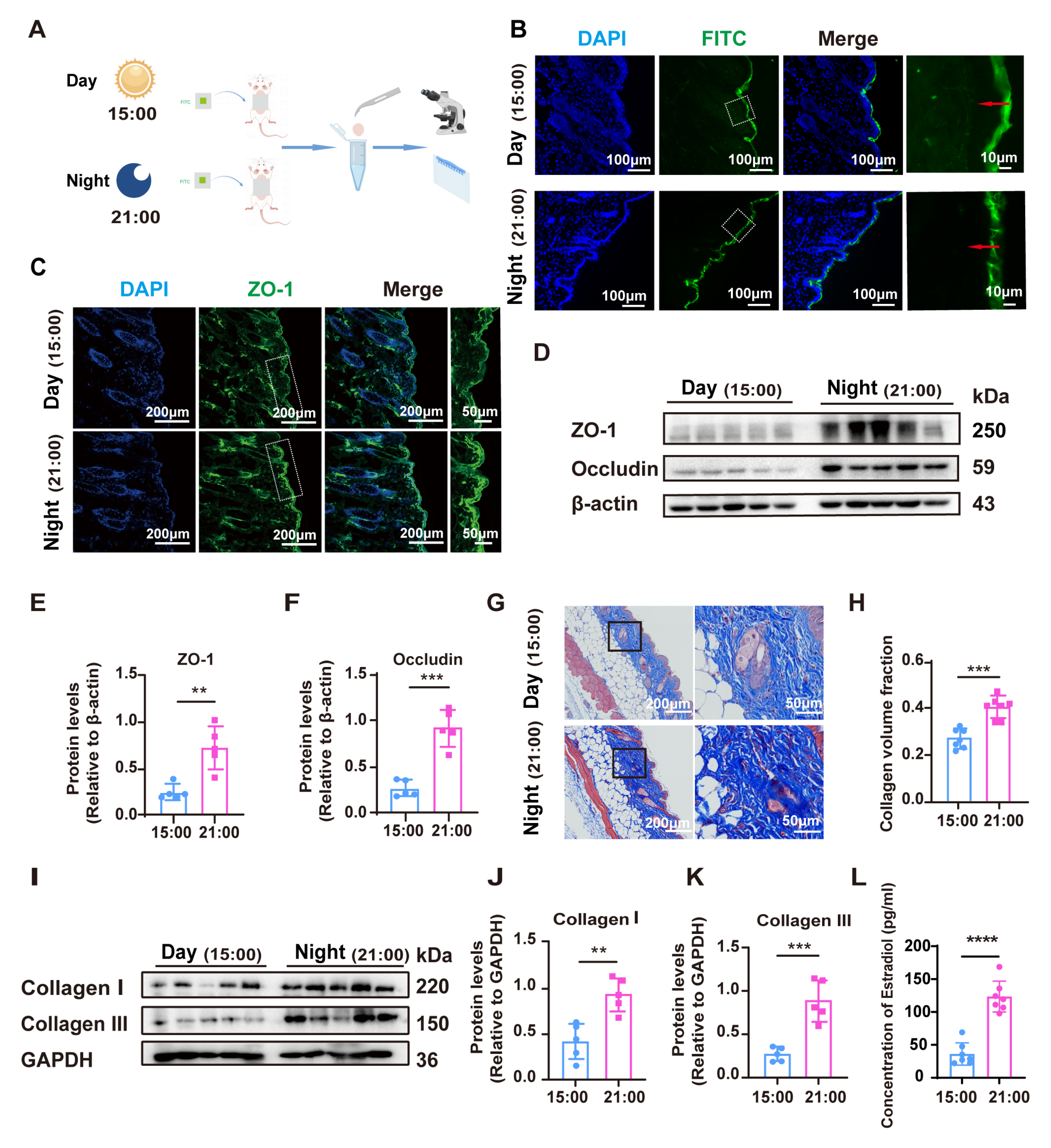
3.1. The Alterations of Skin Barrier and Collagen Production Under the Influence of Circadian Rhythm
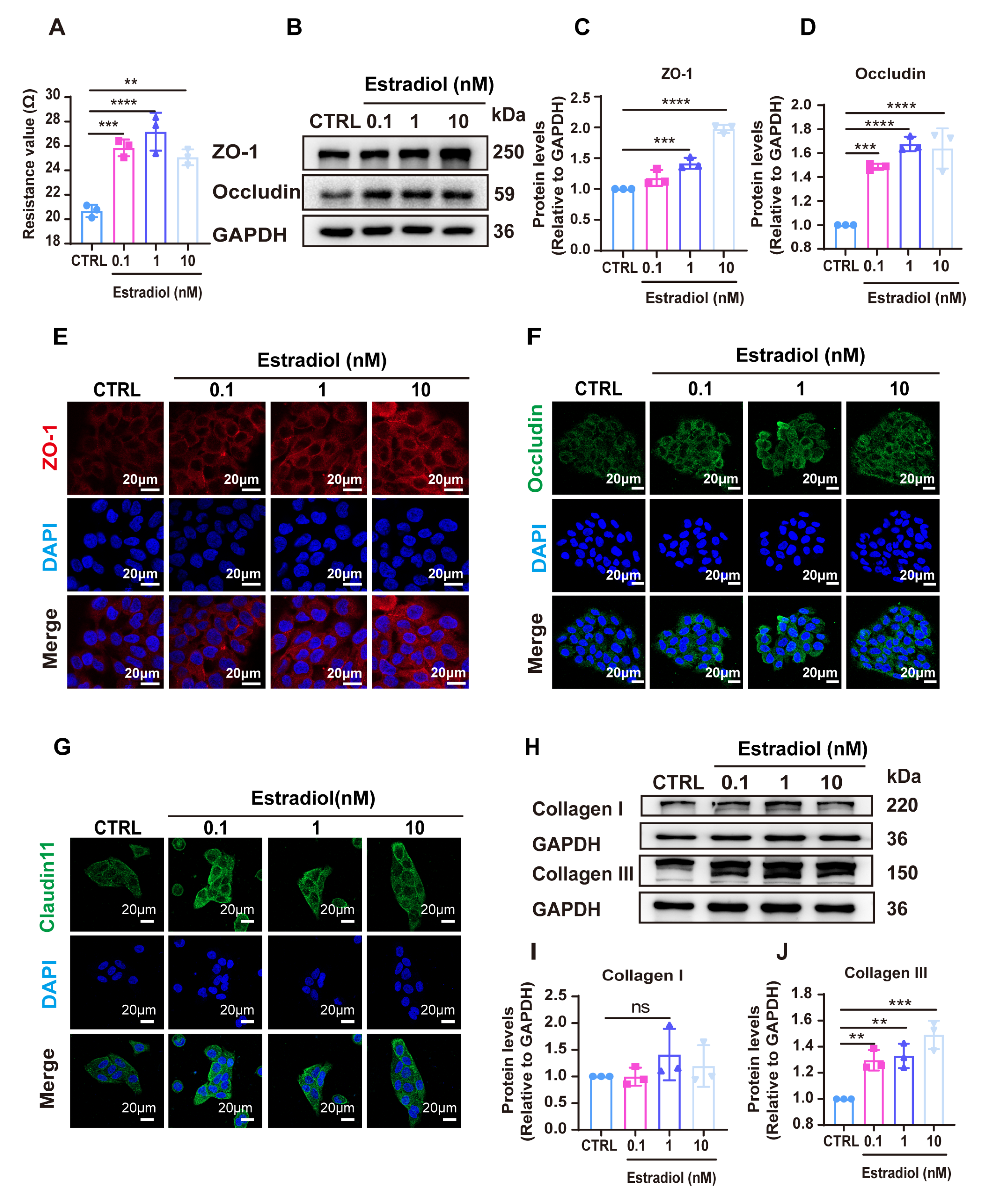
3.2. Estradiol Promoted the Expression of Tight Junction (TJ)-Related Proteins and the Production of Collagen
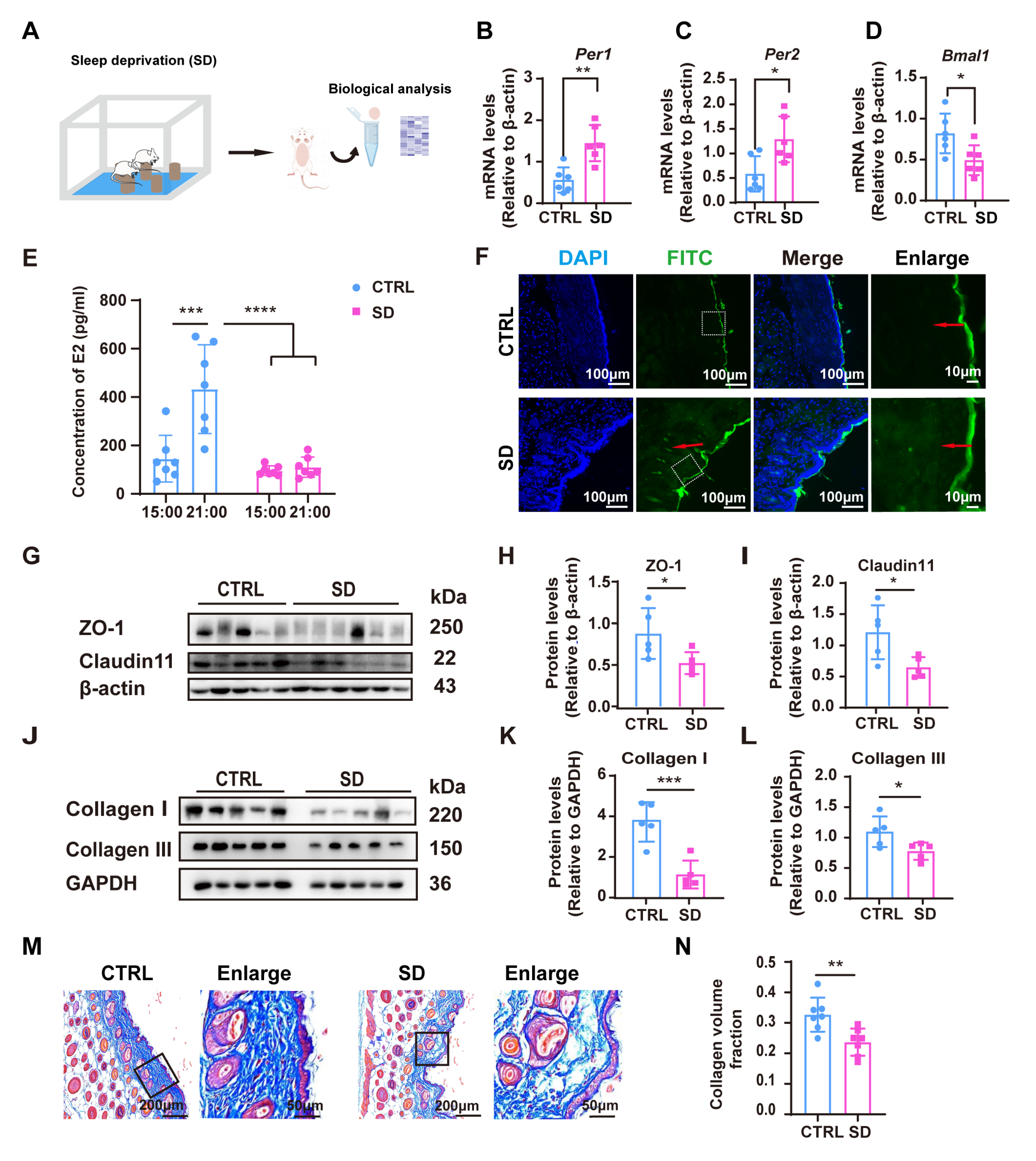
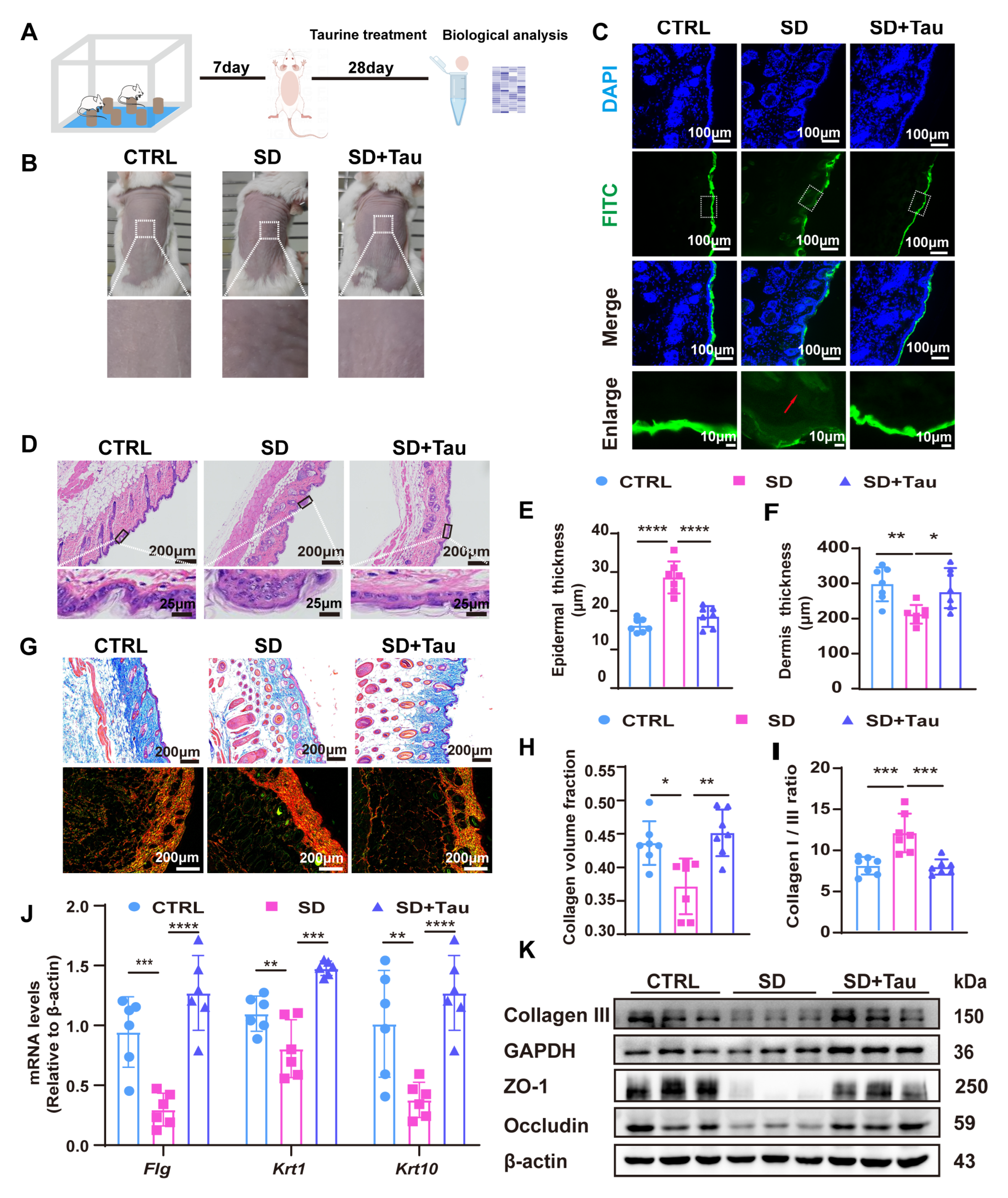
3.3. Effects of Circadian Rhythm Disruption on Skin Barrier Function and Collagen Synthesis in Sleep-Deprived Mice
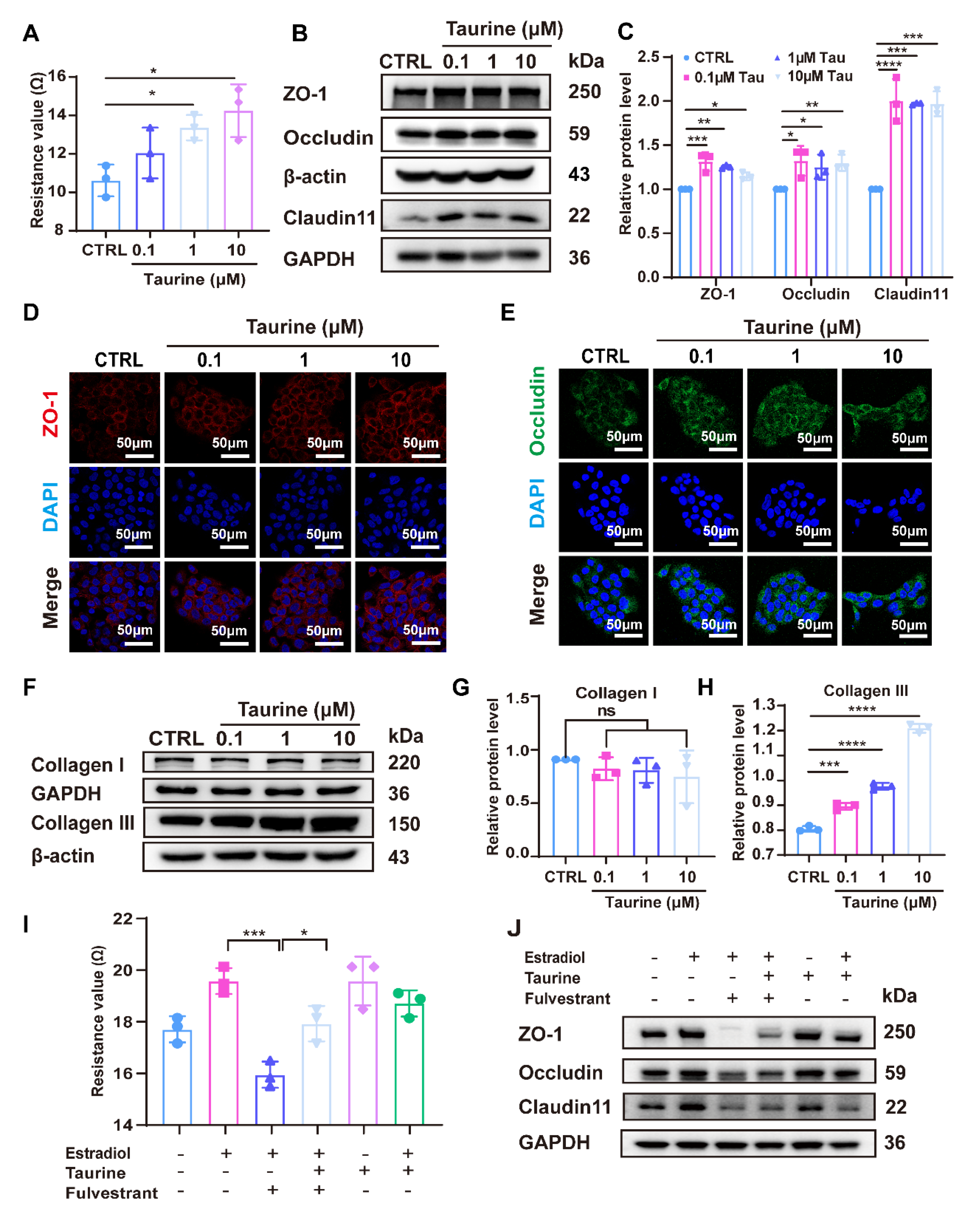
3.4. Taurine Restored the Expression of Tight Junction (TJ) Proteins and Collagen Production Reduced by Estradiol Deficiency
3.5. Taurine Restored Skin Barrier Disruption in Sleep-Deprived (SD) Mice
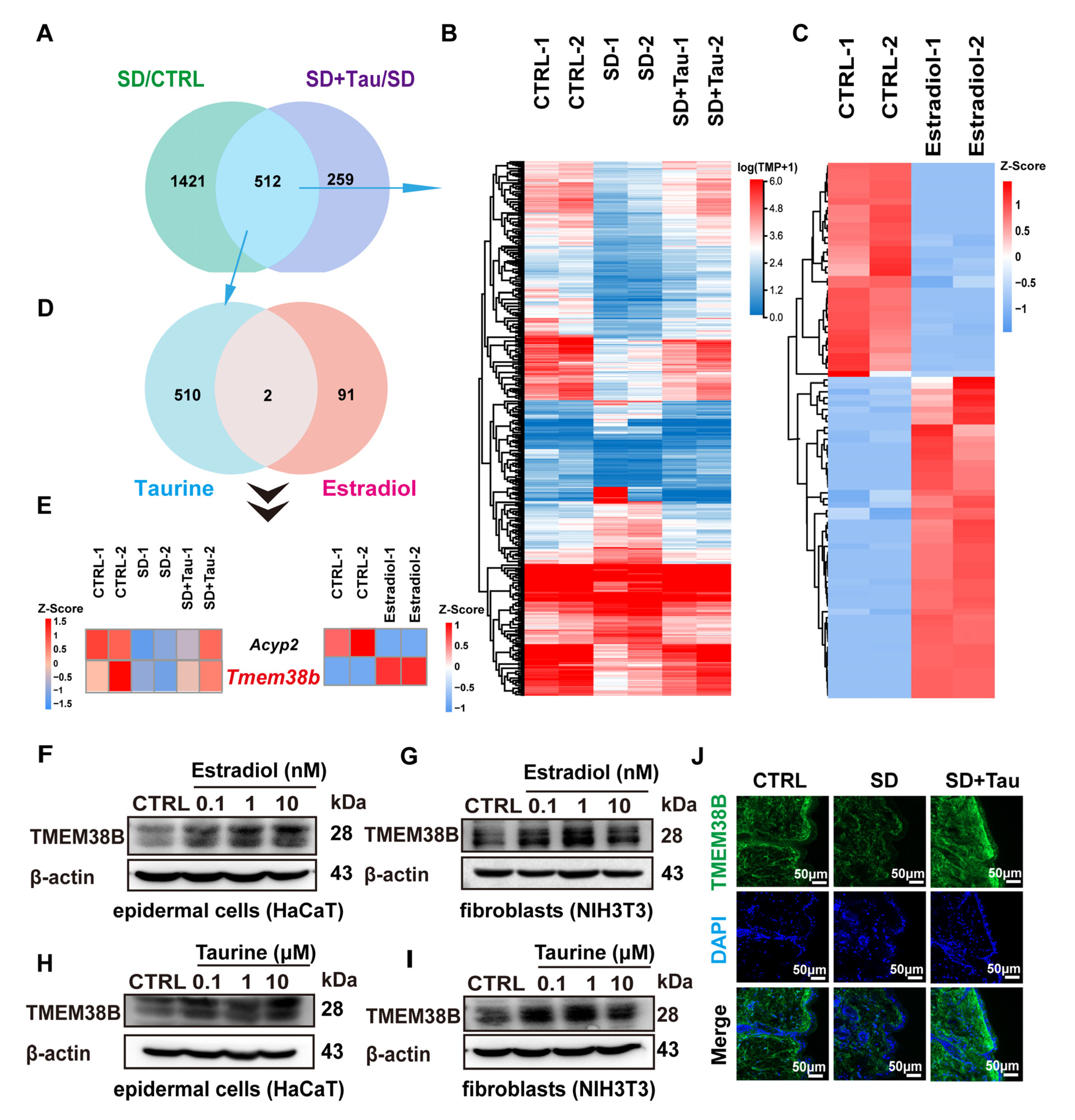
3.6. Estradiol and Taurine Regulated the Expression of TJ Proteins and Collagen Production by Up-Regulating TMEM38B
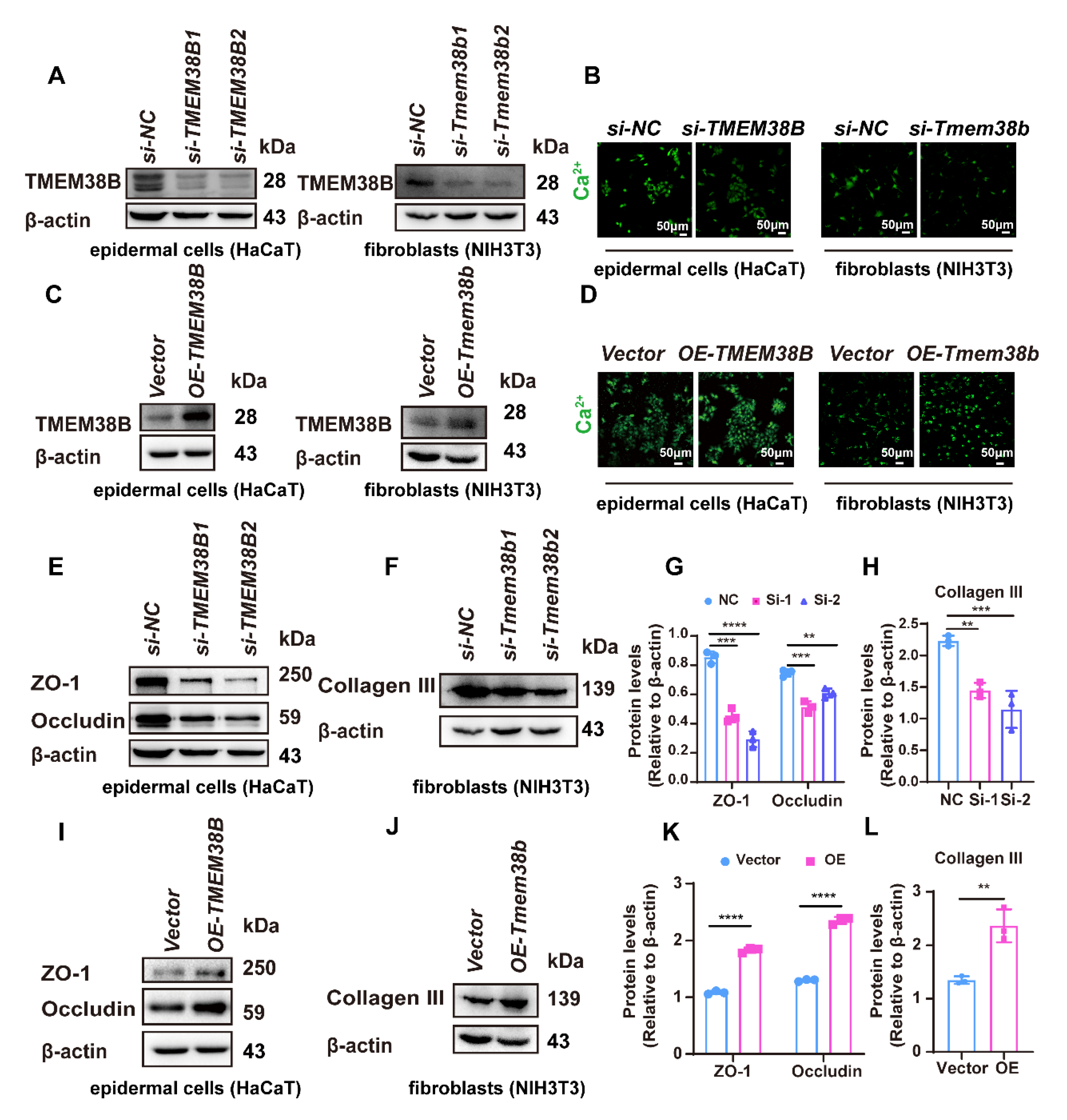
3.7. TMEM38B Affects Tight Junction-Related Proteins and Collagen Expression by Regulating Intracellular Calcium Levels
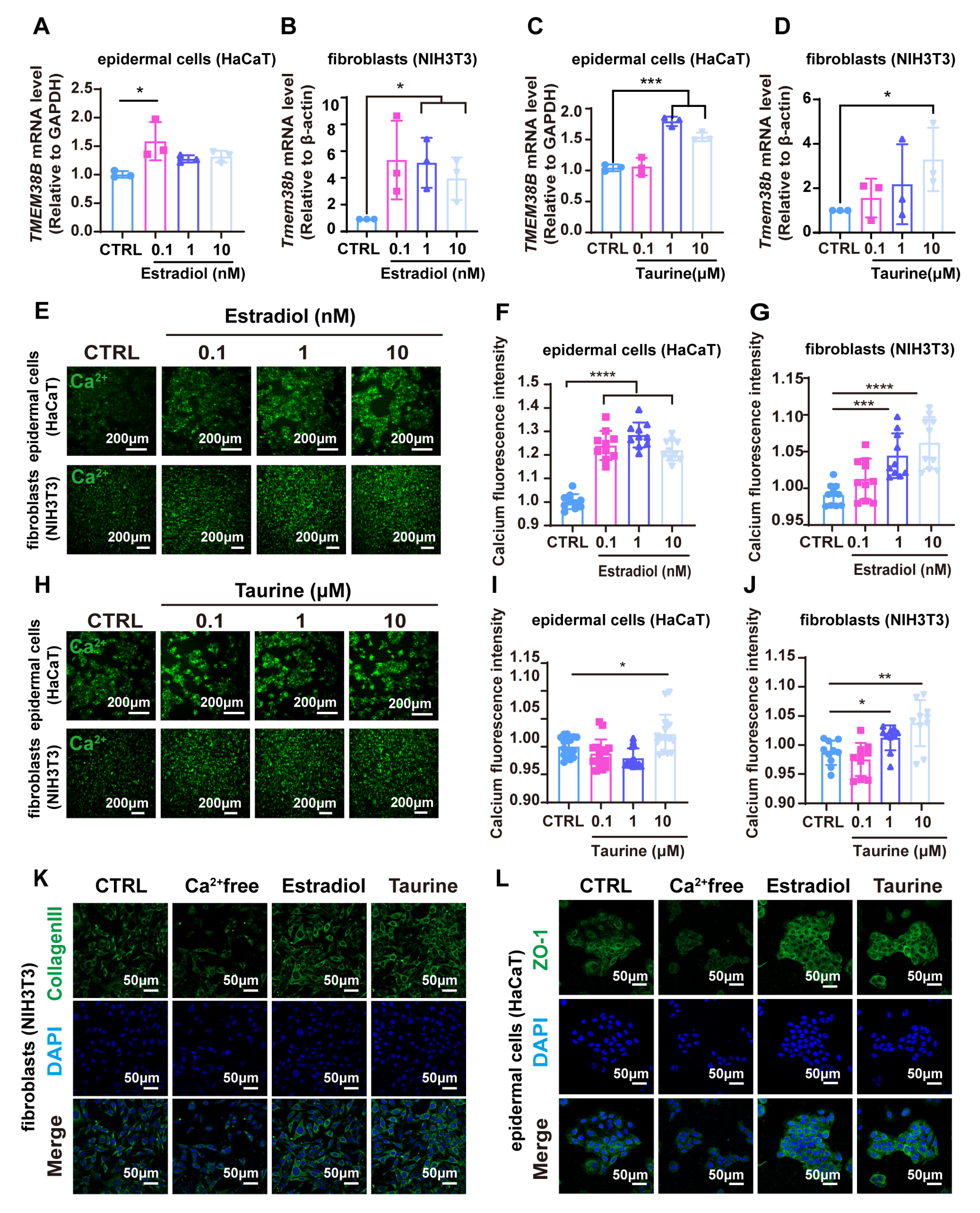
3.8. Estradiol and Taurine Augment Tight Junction-Related Proteins and Collagen Protein Expression via Elevating Intracellular Calcium Levels by Targeting TMEM38B
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jensen, J.M.; Proksch, E. The skin’s barrier. G Ital. Dermatol. Venereol. 2009, 144, 689–700. [Google Scholar] [PubMed]
- Wikramanayake, T.C.; Stojadinovic, O.; Tomic-Canic, M. Epidermal Differentiation in Barrier Maintenance and Wound Healing. Adv. Wound Care 2014, 3, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Lefevre-Utile, A.; Braun, C.; Haftek, M.; Aubin, F. Five Functional Aspects of the Epidermal Barrier. Int. J. Mol. Sci. 2021, 22, 11676. [Google Scholar] [CrossRef] [PubMed]
- Porter, R.M.; Lunny, D.P.; Ogden, P.H.; Morley, S.M.; McLean, W.H.; Evans, A.; Harrison, D.L.; Rugg, E.L.; Lane, E.B. K15 expression implies lateral differentiation within stratified epithelial basal cells. Lab. Investig. 2000, 80, 1701–1710. [Google Scholar] [CrossRef]
- Taylor, D.K.; Bubier, J.A.; Silva, K.A.; Sundberg, J.P. Development, structure, and keratin expression in C57BL/6J mouse eccrine glands. Vet. Pathol. 2012, 49, 146–154. [Google Scholar] [CrossRef]
- Otani, T.; Furuse, M. Tight Junction Structure and Function Revisited. Trends Cell Biol. 2020, 30, 805–817, Erratum in Trends Cell Biol. 2020, 30, 1014. [Google Scholar] [CrossRef]
- Vitellaro-Zuccarello, L.; Garbelli, R.; Rossi, V.D. Immunocytochemical localization of collagen types I, III, IV, and fibronectin in the human dermis. Modifications with ageing. Cell Tissue Res. 1992, 268, 505–511. [Google Scholar] [CrossRef]
- Chaput, J.P.; McHill, A.W.; Cox, R.C.; Broussard, J.L.; Dutil, C.; da Costa, B.G.G.; Sampasa-Kanyinga, H.; Wright, K.P., Jr. The role of insufficient sleep and circadian misalignment in obesity. Nat. Rev. Endocrinol. 2023, 19, 82–97. [Google Scholar] [CrossRef]
- Lindberg, E.; Janson, C.; Gislason, T.; Björnsson, E.; Hetta, J.; Boman, G. Sleep disturbances in a young adult population: Can gender differences be explained by differences in psychological status? Sleep 1997, 20, 381–387. [Google Scholar] [CrossRef]
- Sandu, C.; Liu, T.; Malan, A.; Challet, E.; Pevet, P.; Felder-Schmittbuhl, M.P. Circadian clocks in rat skin and dermal fibroblasts: Differential effects of aging, temperature and melatonin. Cell Mol. Life Sci. 2015, 72, 2237–2248. [Google Scholar] [CrossRef]
- Sandu, C.; Dumas, M.; Malan, A.; Sambakhe, D.; Marteau, C.; Nizard, C.; Schnebert, S.; Perrier, E.; Challet, E.; Pevet, P.; et al. Human skin keratinocytes, melanocytes, and fibroblasts contain distinct circadian clock machineries. Cell Mol. Life Sci. 2012, 69, 3329–3339. [Google Scholar] [CrossRef] [PubMed]
- Buhr, E.D.; Vemaraju, S.; Diaz, N.; Lang, R.A.; Van Gelder, R.N. Neuropsin (OPN5) Mediates Local Light-Dependent Induction of Circadian Clock Genes and Circadian Photoentrainment in Exposed Murine Skin. Curr. Biol. 2019, 29, 3478–3487.e4. [Google Scholar] [CrossRef]
- Yeung, C.C.; Kadler, K.E. Importance of the circadian clock in tendon development. Curr. Top. Dev. Biol. 2019, 133, 309–342. [Google Scholar] [CrossRef]
- Hoyle, N.P.; Seinkmane, E.; Putker, M.; Feeney, K.A.; Krogager, T.P.; Chesham, J.E.; Bray, L.K.; Thomas, J.M.; Dunn, K.; Blaikley, J.; et al. Circadian actin dynamics drive rhythmic fibroblast mobilization during wound healing. Sci. Transl. Med. 2017, 9, eaal2774. [Google Scholar] [CrossRef]
- Kim, P.; Oster, H.; Lehnert, H.; Schmid, S.M.; Salamat, N.; Barclay, J.L.; Maronde, E.; Inder, W.; Rawashdeh, O. Coupling the Circadian Clock to Homeostasis: The Role of Period in Timing Physiology. Endocr. Rev. 2019, 40, 66–95. [Google Scholar] [CrossRef]
- Thornton, M.J. Estrogens and aging skin. Dermato-Endocrinology 2013, 5, 264–270. [Google Scholar] [CrossRef]
- Shao, S.; Zhao, H.; Lu, Z.; Lei, X.; Zhang, Y. Circadian Rhythms Within the Female HPG Axis: From Physiology to Etiology. Endocrinology 2021, 162, bqab117. [Google Scholar] [CrossRef]
- Shah, M.G.; Maibach, H.I. Estrogen and skin. An overview. Am. J. Clin. Dermatol. 2001, 2, 143–150. [Google Scholar] [CrossRef]
- Affinito, P.; Palomba, S.; Sorrentino, C.; Di Carlo, C.; Bifulco, G.; Arienzo, M.P.; Nappi, C. Effects of postmenopausal hypoestrogenism on skin collagen. Maturitas 1999, 33, 239–247. [Google Scholar] [CrossRef]
- Shu, Y.Y.; Maibach, H.I. Estrogen and skin: Therapeutic options. Am. J. Clin. Dermatol. 2011, 12, 297–311. [Google Scholar] [CrossRef]
- Castelo-Branco, C.; Duran, M.; González-Merlo, J. Skin collagen changes related to age and hormone replacement therapy. Maturitas 1992, 15, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Varila, E.; Rantala, I.; Oikarinen, A.; Risteli, J.; Reunala, T.; Oksanen, H.; Punnonen, R. The effect of topical oestradiol on skin collagen of postmenopausal women. Br. J. Obstet. Gynaecol. 1995, 102, 985–989. [Google Scholar] [CrossRef] [PubMed]
- Archer, D.F. Postmenopausal skin and estrogen. Gynecol. Endocrinol. 2012, 28 (Suppl. S2), 2–6. [Google Scholar] [CrossRef] [PubMed]
- Poggio, F.; Del Mastro, L.; Bruzzone, M.; Ceppi, M.; Razeti, M.G.; Fregatti, P.; Ruelle, T.; Pronzato, P.; Massarotti, C.; Franzoi, M.A.; et al. Safety of systemic hormone replacement therapy in breast cancer survivors: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2022, 191, 269–275. [Google Scholar] [CrossRef]
- Collaborative Group on Hormonal Factors in Breast Cancer. Type and timing of menopausal hormone therapy and breast cancer risk: Individual participant meta-analysis of the worldwide epidemiological evidence. Lancet 2019, 394, 1159–1168. [Google Scholar] [CrossRef]
- Deli, T.; Orosz, M.; Jakab, A. Hormone Replacement Therapy in Cancer Survivors—Review of the Literature. Pathol. Oncol. Res. 2020, 26, 63–78. [Google Scholar] [CrossRef]
- Schaffer, S.; Azuma, J.; Takahashi, K.; Mozaffari, M. Why is taurine cytoprotective? Adv. Exp. Med. Biol. 2003, 526, 307–321. [Google Scholar] [CrossRef]
- Janeke, G.; Siefken, W.; Carstensen, S.; Springmann, G.; Bleck, O.; Steinhart, H.; Höger, P.; Wittern, K.P.; Wenck, H.; Stäb, F.; et al. Role of taurine accumulation in keratinocyte hydration. J. Investig. Dermatol. 2003, 121, 354–361. [Google Scholar] [CrossRef]
- El-Chami, C.; Foster, A.R.; Johnson, C.; Clausen, R.P.; Cornwell, P.; Haslam, I.S.; Steward, M.C.; Watson, R.E.B.; Young, H.S.; O’Neill, C.A. Organic osmolytes increase expression of specific tight junction proteins in skin and alter barrier function in keratinocytes. Br. J. Dermatol. 2021, 184, 482–494. [Google Scholar] [CrossRef]
- Yoshimura, T.; Manabe, C.; Inokuchi, Y.; Mutou, C.; Nagahama, T.; Murakami, S. Protective effect of taurine on UVB-induced skin aging in hairless mice. Biomed. Pharmacother. 2021, 141, 111898. [Google Scholar] [CrossRef]
- Yoshimura, T.; Manabe, C.; Nagumo, J.I.; Nagahama, T.; Sato, T.; Murakami, S. Taurine accelerates the synthesis of ceramides and hyaluronic acid in cultured epidermis and dermal fibroblasts. Exp. Ther. Med. 2023, 26, 512. [Google Scholar] [CrossRef] [PubMed]
- Nandhini, T.A.; Thirunavukkarasu, V.; Ravichandran, M.K.; Anuradha, C.V. Taurine prevents fructose-diet induced collagen abnormalities in rat skin. J. Diabetes Its Complicat. 2005, 19, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Grafe, F.; Wohlrab, W.; Neubert, R.H.; Brandsch, M. Functional characterization of sodium- and chloride-dependent taurine transport in human keratinocytes. Eur. J. Pharm. Biopharm. 2004, 57, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.; Wu, J.L.; Huang, W.C. Effects of prebiotics on intestinal physiology, neuropsychological function, and exercise capacity of mice with sleep deprivation. Food Res. Int. 2023, 165, 112568. [Google Scholar] [CrossRef]
- Ma, C.; Wu, G.; Wang, Z.; Wang, P.; Wu, L.; Zhu, G.; Zhao, H. Effects of chronic sleep deprivation on the extracellular signal-regulated kinase pathway in the temporomandibular joint of rats. PLoS ONE 2014, 9, e107544. [Google Scholar] [CrossRef]
- Gachon, F.; Firsov, D. The role of circadian timing system on drug metabolism and detoxification. Expert Opin. Drug Metab. Toxicol. 2011, 7, 147–158. [Google Scholar] [CrossRef]
- Lu, D.; Zhao, M.; Chen, M.; Wu, B. Circadian Clock-Controlled Drug Metabolism: Implications for Chronotherapeutics. Drug Metab. Dispos. 2020, 48, 395–406. [Google Scholar] [CrossRef]
- Alvord, V.M.; Kantra, E.J.; Pendergast, J.S. Estrogens and the circadian system. Semin. Cell Dev. Biol. 2022, 126, 56–65. [Google Scholar] [CrossRef]
- Kim, T.W.; Jeong, J.H.; Hong, S.C. The impact of sleep and circadian disturbance on hormones and metabolism. Int. J. Endocrinol. 2015, 2015, 591729. [Google Scholar] [CrossRef]
- Sciarra, F.; Franceschini, E.; Campolo, F.; Gianfrilli, D.; Pallotti, F.; Paoli, D.; Isidori, A.M.; Venneri, M.A. Disruption of Circadian Rhythms: A Crucial Factor in the Etiology of Infertility. Int. J. Mol. Sci. 2020, 21, 3943. [Google Scholar] [CrossRef]
- Ayyar, V.S.; Sukumaran, S. Circadian rhythms: Influence on physiology, pharmacology, and therapeutic interventions. J. Pharmacokinet. Pharmacodyn. 2021, 48, 321–338. [Google Scholar] [CrossRef] [PubMed]
- Mukai, K.; Nakajima, Y.; Urai, T.; Komatsu, E.; Nasruddin; Sugama, J.; Nakatani, T. 17beta-Estradiol administration promotes delayed cutaneous wound healing in 40-week ovariectomised female mice. Int. Wound J. 2016, 13, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Ehrhardt, C.; Wessels, J.T.; Wuttke, W.; Seidlova-Wuttke, D. The effects of 20-hydroxyecdysone and 17beta-estradiol on the skin of ovariectomized rats. Menopause 2011, 18, 323–327. [Google Scholar] [CrossRef]
- Ichimasu, N.; Chen, Y.; Kobayashi, K.; Suzuki, S.; Chikazawa, S.; Shimura, S.; Katagiri, K. Possible involvement of type 2 cytokines in alloknesis in mouse models of menopause and dry skin. Exp. Dermatol. 2021, 30, 1745–1753. [Google Scholar] [CrossRef]
- Saville, C.R.; Holmes, D.F.; Swift, J.; Derby, B.; Sherratt, M.J. Estrogen mediates acute elastic fibre homeostasis in skin. bioRxiv 2019. preprint. [Google Scholar] [CrossRef]
- Li, W.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Melatonin improves skin barrier damage caused by sleep restriction through gut microbiota. J. Pineal Res. 2023, 75, e12874. [Google Scholar] [CrossRef]
- Pingel, J.; Langberg, H.; Skovgård, D.; Koskinen, S.; Flyvbjerg, A.; Frystyk, J.; Kjær, M.; Hansen, M. Effects of transdermal estrogen on collagen turnover at rest and in response to exercise in postmenopausal women. J. Appl. Physiol 2012, 113, 1040–1047. [Google Scholar] [CrossRef]
- Brincat, M.P. Oestrogens and the skin. J. Cosmet. Dermatol. 2004, 3, 41–49. [Google Scholar] [CrossRef]
- Wolff, E.F.; Narayan, D.; Taylor, H.S. Long-term effects of hormone therapy on skin rigidity and wrinkles. Fertil. Steril. 2005, 84, 285–288. [Google Scholar] [CrossRef]
- Liu, T.; Li, N.; Yan, Y.Q.; Liu, Y.; Xiong, K.; Liu, Y.; Xia, Q.M.; Zhang, H.; Liu, Z.D. Recent advances in the anti-aging effects of phytoestrogens on collagen, water content, and oxidative stress. Phytother. Res. 2020, 34, 435–447. [Google Scholar] [CrossRef]
- Jakaria, M.; Azam, S.; Haque, M.E.; Jo, S.H.; Uddin, M.S.; Kim, I.S.; Choi, D.K. Taurine and its analogs in neurological disorders: Focus on therapeutic potential and molecular mechanisms. Redox Biol. 2019, 24, 101223. [Google Scholar] [CrossRef]
- Sirdah, M.M.; El-Agouza, I.M.; Abu Shahla, A.N. Possible ameliorative effect of taurine in the treatment of iron-deficiency anaemia in female university students of Gaza, Palestine. Eur. J. Haematol. 2002, 69, 236–242. [Google Scholar] [CrossRef]
- Kim, Y.; Lim, K.M. Skin barrier dysfunction and filaggrin. Arch. Pharm. Res. 2021, 44, 36–48. [Google Scholar] [CrossRef]
- Anderheggen, B.; Jassoy, C.; Waldmann-Laue, M.; Förster, T.; Wadle, A.; Doering, T. Taurine improves epidermal barrier properties stressed by surfactants-a role for osmolytes in barrier homeostasis. J. Cosmet. Sci. 2006, 57, 1–10. [Google Scholar]
- Bukhari, S.N.A.; Roswandi, N.L.; Waqas, M.; Habib, H.; Hussain, F.; Khan, S.; Sohail, M.; Ramli, N.A.; Thu, H.E.; Hussain, Z. Hyaluronic acid, a promising skin rejuvenating biomedicine: A review of recent updates and pre-clinical and clinical investigations on cosmetic and nutricosmetic effects. Int. J. Biol. Macromol. 2018, 120, 1682–1695. [Google Scholar] [CrossRef]
- Meckfessel, M.H.; Brandt, S. The structure, function, and importance of ceramides in skin and their use as therapeutic agents in skin-care products. J. Am. Acad. Dermatol. 2014, 71, 177–184. [Google Scholar] [CrossRef]
- Madaan, P.; Sikka, P.; Malik, D.S. Cosmeceutical Aptitudes of Niacinamide: A Review. Recent Adv. Anti-Infect. Drug Discov. 2021, 16, 196–208. [Google Scholar] [CrossRef]
- Yazawa, M.; Ferrante, C.; Feng, J.; Mio, K.; Ogura, T.; Zhang, M.; Lin, P.H.; Pan, Z.; Komazaki, S.; Kato, K.; et al. TRIC channels are essential for Ca2+ handling in intracellular stores. Nature 2007, 448, 78–82. [Google Scholar] [CrossRef]
- Cabral, W.A.; Ishikawa, M.; Garten, M.; Makareeva, E.N.; Sargent, B.M.; Weis, M.; Barnes, A.M.; Webb, E.A.; Shaw, N.J.; Ala-Kokko, L.; et al. Absence of the ER Cation Channel TMEM38B/TRIC-B Disrupts Intracellular Calcium Homeostasis and Dysregulates Collagen Synthesis in Recessive Osteogenesis Imperfecta. PLoS Genet. 2016, 12, e1006156. [Google Scholar] [CrossRef]
- Celli, A.; Sanchez, S.; Behne, M.; Hazlett, T.; Gratton, E.; Mauro, T. The epidermal Ca2+ gradient: Measurement using the phasor representation of fluorescent lifetime imaging. Biophys. J. 2010, 98, 911–921. [Google Scholar] [CrossRef]
- Murata, T.; Honda, T.; Egawa, G.; Yamamoto, Y.; Ichijo, R.; Toyoshima, F.; Dainichi, T.; Kabashima, K. Transient elevation of cytoplasmic calcium ion concentration at a single cell level precedes morphological changes of epidermal keratinocytes during cornification. Sci. Rep. 2018, 8, 6610. [Google Scholar] [CrossRef]
- Pondeljak, N.; Lugovic-Mihic, L.; Tomic, L.; Parac, E.; Pedic, L.; Lazic-Mosler, E. Key Factors in the Complex and Coordinated Network of Skin Keratinization: Their Significance and Involvement in Common Skin Conditions. Int. J. Mol. Sci. 2023, 25, 236. [Google Scholar] [CrossRef]
- Byun, K.A.; Lee, J.H.; Lee, S.Y.; Oh, S.; Batsukh, S.; Cheon, G.W.; Lee, D.; Hong, J.H.; Son, K.H.; Byun, K. Piezo1 Activation Drives Enhanced Collagen Synthesis in Aged Animal Skin Induced by Poly L-Lactic Acid Fillers. Int. J. Mol. Sci. 2024, 25, 7232. [Google Scholar] [CrossRef]
| Name | Sense Primers (5′–3′) | Anti-Sense Primers |
|---|---|---|
| Per1 | CAACGTGATGGGTCGCTTTC | GTAAAGGGGTCGTGTTCGCT |
| Per2 | GCGCACCAAGTGACGGG | ACTTGGGGAGAAGTCCACGTA |
| Bmal1 | GGCCCAAAGAGGACTCATCC | GCGATGACCCTCTTATCCTGT |
| Flg | TCCTGGAAAGCATCACTAGCA | TGTCTTGGTCATCTGGATTCTTCA |
| Krt1 | GTTGCAAGAAGCAGATCTCCCA | AGTGCTTTCTCACCACGCTG |
| Krt10 | AGTGGTACGAGAAGCATGGC | GCACGTTGGCATTGTCAGTT |
| TMEM38B | GTGCCCTCTCCTACTCCTCA | GCTGCTCCCGGCTGA |
| GAPDH | CACCATCTTCCAGGAGCGAG | AGAGGGGGCAGAGATGATGA |
| Tmem38b | GCCCTCGTGACCTAGTTTCC | CCGCCTACTATTTTCCACGTC |
| β-actin | GAGCGCAAGTACTCTGTGTG | AACGCAGCTCAGTAACAGTC |
| Antibody | Source | Item Number | Working Dilution |
|---|---|---|---|
| ZO-1 | Abcam | Ab221547 | 1:100 (IF)–1:1000 (WB) |
| Occludin | Abcam | Ab216327 | 1:1000 (WB) |
| Claudin-11 | Invitrogen | 364500 | 1:100 (IF)–1:1000 (WB) |
| Collagen III | Abcam | EPR17673 | 1:1000 (WB) |
| Collagen I | Abcam | Ab21286 | 1:1000 (WB) |
| Collagen I | Abcam | Ab260043 | 1:1000 (WB) |
| TMEM38B | Proteintech | 19919-1-AP | 1:100 (IF)–1:500 (WB) |
| GAPDH | Abcam | ab245355 | 1:5000 (WB) |
| β-actin | Abcam | ab8226 | 1:5000 (WB) |
| Name | Sense Primers (5′–3′) | Anti-Sense Primers (5′–3′) |
|---|---|---|
| si-TMEM38B-1 | CCAUUGAAGUUUCUUGCAATT | UUGCAAGAAACUUCAAUGGTT |
| si-TMEM38B-2 | GGAUAGUCAUGAUAGCUAUUGTT | CAAUAGCUAUCAUGACUAUCCTT |
| si-Tmem38b-1 | CCAGGGUUAUUCAUAUCAATT | UUGAUAUGAAUAACCCUGGTT |
| si-Tmem38b-2 | CCUGGAUAGUCAUGAUAGUTT | ACUAUCAUGACUAUCCAGGTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, Q.; Wang, Z.; Li, Y.; Tang, X.; Li, Z.; Xia, H.; Wu, Q.; Chang, R.; Wu, C.; Meng, T.; et al. Taurine Prevents Impairments in Skin Barrier Function and Dermal Collagen Synthesis Triggered by Sleep Deprivation-Induced Estrogen Circadian Rhythm Disruption. Cells 2025, 14, 727. https://doi.org/10.3390/cells14100727
Shao Q, Wang Z, Li Y, Tang X, Li Z, Xia H, Wu Q, Chang R, Wu C, Meng T, et al. Taurine Prevents Impairments in Skin Barrier Function and Dermal Collagen Synthesis Triggered by Sleep Deprivation-Induced Estrogen Circadian Rhythm Disruption. Cells. 2025; 14(10):727. https://doi.org/10.3390/cells14100727
Chicago/Turabian StyleShao, Qi, Zhaoyang Wang, Yifang Li, Xun Tang, Ziyi Li, Huan Xia, Qihong Wu, Ruxue Chang, Chunna Wu, Tao Meng, and et al. 2025. "Taurine Prevents Impairments in Skin Barrier Function and Dermal Collagen Synthesis Triggered by Sleep Deprivation-Induced Estrogen Circadian Rhythm Disruption" Cells 14, no. 10: 727. https://doi.org/10.3390/cells14100727
APA StyleShao, Q., Wang, Z., Li, Y., Tang, X., Li, Z., Xia, H., Wu, Q., Chang, R., Wu, C., Meng, T., Fan, Y., Huang, Y., & Yang, Y. (2025). Taurine Prevents Impairments in Skin Barrier Function and Dermal Collagen Synthesis Triggered by Sleep Deprivation-Induced Estrogen Circadian Rhythm Disruption. Cells, 14(10), 727. https://doi.org/10.3390/cells14100727







