Investigation of Biodegradation and Biocompatibility of Chitosan–Bacterial Cellulose Composite Scaffold for Bone Tissue Engineering Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of CS–BC Composite Scaffold
2.2. Scanning Electron Microscopy
2.3. In Vitro Biodegradable Study
2.4. In Vitro Biocompatible Study
2.4.1. Cell Culturing and Seeding Procedure
2.4.2. Cell Attachment
2.4.3. Metabolic Activity of Cells
2.5. Cell Differentiation
2.5.1. Alkaline Phosphatase (ALP) Enzyme Activity Assay
2.5.2. Gene Expression Analysis by RT-qPCR
2.6. Statistical Analysis
3. Results
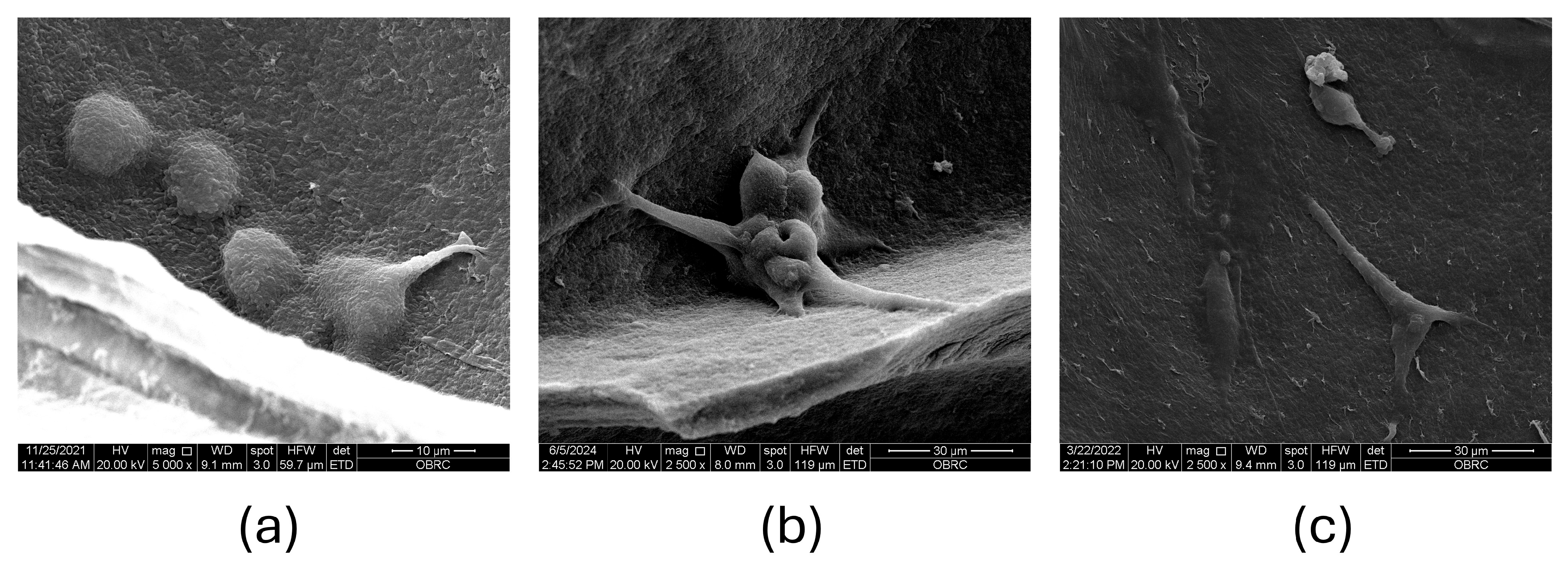
3.1. SEM Analysis of CS–BC Composite Scaffold Morphology
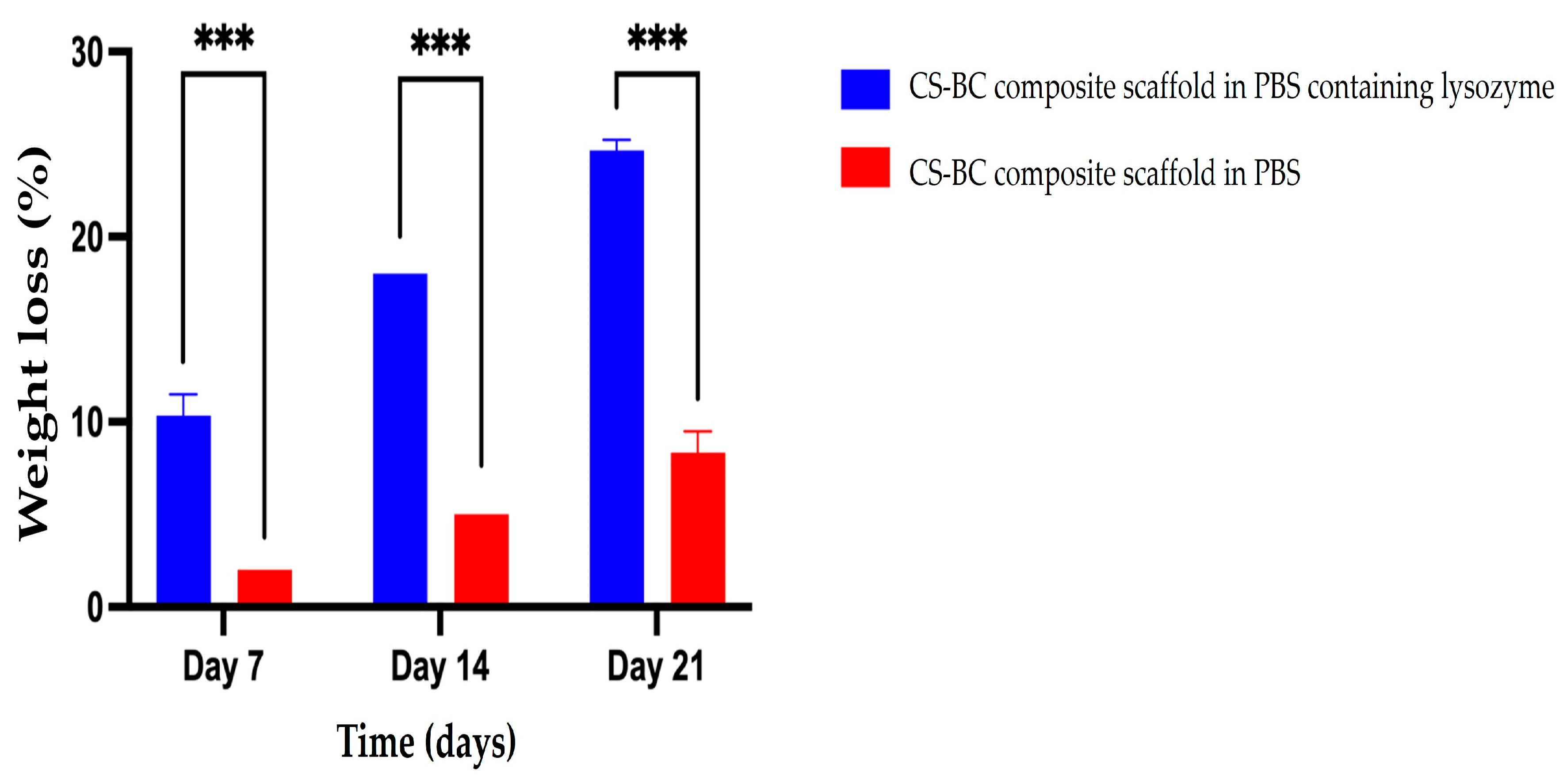
3.2. The Biodegradation of CS–BC Scaffold
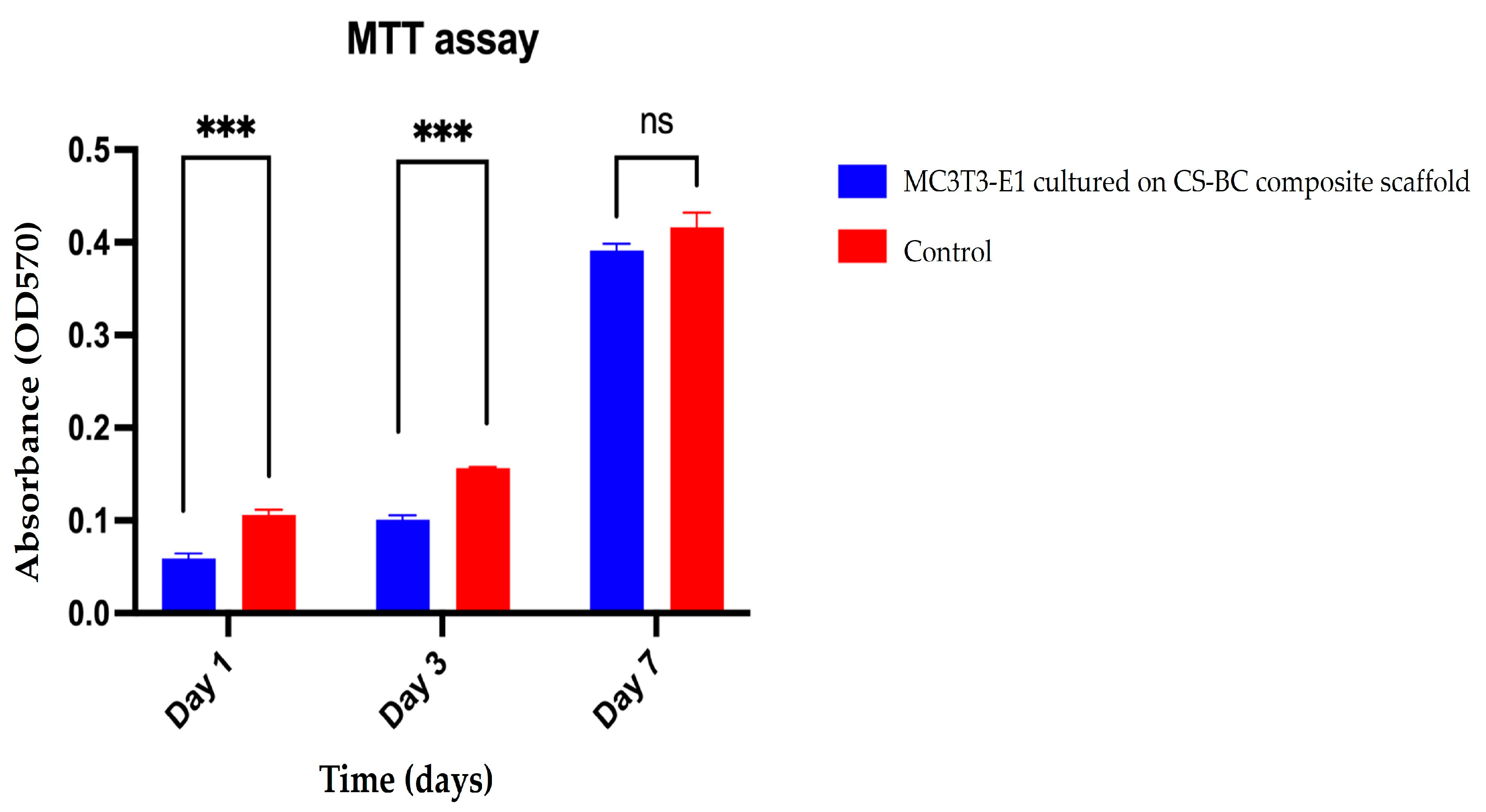
3.3. MC3T3-E1 Cell Attachment and Metabolic Activity on the CS–BC Composite Scaffold
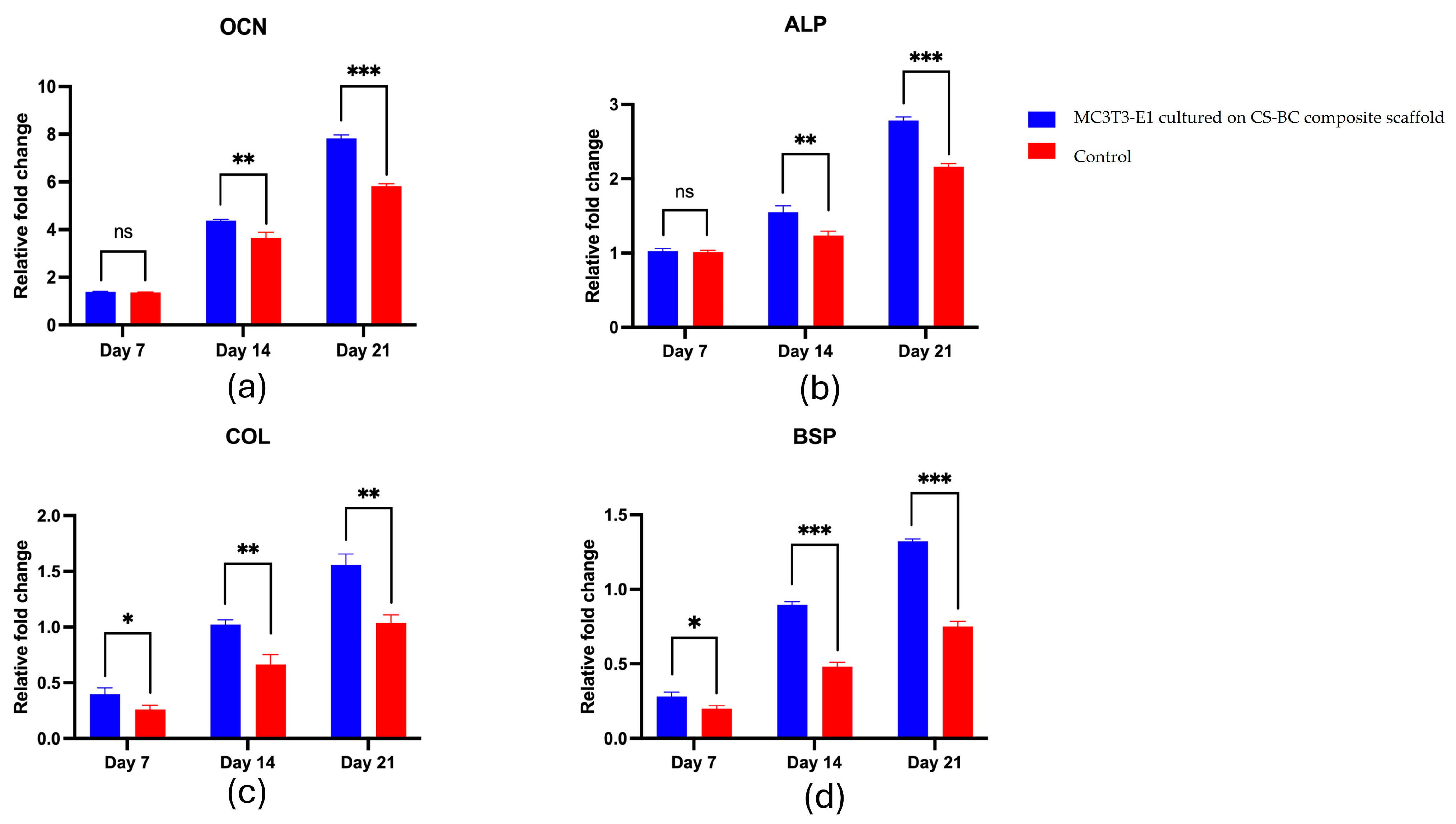
3.4. MC3C3-E1 Cell Differentiation on the CS–BC Composite Scaffold
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wubneh, A.; Tsekoura, E.K.; Ayranci, C.; Uludağ, H. Current state of fabrication technologies and materials for bone tissue engineering. Acta Biomater. 2018, 80, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.T.; Munguia-Lopez, J.G.; Cho, Y.W.; Ma, X.; Song, V.; Zhu, Z.; Tran, S.D. Polymeric scaffolds for dental, oral, and craniofacial regenerative medicine. Molecules 2021, 26, 7043. [Google Scholar] [CrossRef] [PubMed]
- Chocholata, P.; Kulda, V.; Babuska, V. Fabrication of scaffolds for bone-tissue regeneration. Materials 2019, 12, 568. [Google Scholar] [CrossRef]
- Ali, A.; Hasan, A.; Negi, Y.S. Effect of Carbon-based fillers on xylan/chitosan/nano-HAp composite matrix for bone tissue engineering application. Int. J. Biol. Macromol. 2022, 197, 1–11. [Google Scholar] [CrossRef]
- Kavya, K.C.; Jayakumar, R.; Nair, S.; Chennazhi, K.P. Fabrication and characterization of chitosan/gelatin/nSiO2 composite scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2013, 59, 255–263. [Google Scholar] [CrossRef]
- Lee, J.S.; Baek, S.D.; Venkatesan, J.; Bhatnagar, I.; Chang, H.K.; Kim, H.T.; Kim, S.K. In vivo study of chitosan-natural nano hydroxyapatite scaffolds for bone tissue regeneration. Int. J. Biol. Macromol. 2014, 67, 360–366. [Google Scholar] [CrossRef]
- Januariyasa, I.K.; Ana, I.D.; Yusuf, Y. Nanofibrous poly (vinyl alcohol)/chitosan contained carbonated hydroxyapatite nanoparticles scaffold for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110347. [Google Scholar] [CrossRef]
- Iqbal, H.; Ali, M.; Zeeshan, R.; Mutahir, Z.; Iqbal, F.; Nawaz, M.A.H.; Shahzadi, L.; Chaudhry, A.A.; Yar, M.; Luan, S.; et al. Chitosan/hydroxyapatite (HA)/hydroxypropylmethyl cellulose (HPMC) spongy scaffolds-synthesis and evaluation as potential alveolar bone substitutes. Colloids Surf. B Biointerfaces 2017, 160, 553–563. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Zhang, Z. Porous chitosan/nano-hydroxyapatite composite scaffolds incorporating simvastatin-loaded PLGA microspheres for bone repair. Cells Tissues Organs 2018, 205, 20–31. [Google Scholar] [CrossRef]
- Venkatesan, J.; Pallela, R.; Bhatnagar, I.; Kim, S.K. Chitosan-amylopectin/hydroxyapatite and chitosan-chondroitin sulphate/hydroxyapatite composite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2012, 51, 1033–1042. [Google Scholar] [CrossRef]
- Kanimozhi, K.; Khaleel-Basha, S.; Sugantha-Kumari, V. Processing and characterization of chitosan/PVA and methylcellulose porous scaffolds for tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, T.R.; Yang, X.; Zhang, J.; Cao, X. In situ and ex situ modifications of bacterial cellulose for applications in tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 82, 372–383. [Google Scholar] [CrossRef]
- Wahid, F.; Hu, X.H.; Chu, L.Q.; Jia, S.R.; Xie, Y.Y.; Zhong, C. Development of bacterial cellulose/chitosan based semi-interpenetrating hydrogels with improved mechanical and antibacterial properties. Int. J. Biol. Macromol. 2019, 122, 380–387. [Google Scholar] [CrossRef]
- de Oliveira Barud, H.G.; da Silva, R.R.; da Silva Barud, H.; Tercjak, A.; Gutierrez, J.; Lustri, W.R.; de Oliveira, O.B.J.; Ribeiro, S.J.L. A multipurpose natural and renewable polymer in medical applications: Bacterial cellulose. Carbohydr. Polym. 2016, 153, 406–420. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.C.; Lien, C.C.; Yeh, H.J.; Yu, C.M.; Hsu, S.H. Bacterial cellulose and bacterial cellulose-chitosan membranes for wound dressing applications. Carbohydr. Polym. 2013, 94, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Urbina, L.; Guaresti, O.; Requires, J.; Gabilondo, N.; Eceiza, A.; Corcuera, M.A.; Retegi, A. Design of reusable novel membranes based on bacterial cellulose and chitosan for the filtration of copper in wastewaters. Carbohydr. Polym. 2018, 193, 362–372. [Google Scholar] [CrossRef]
- Arikibe, J.E.; Lata, R.; Rohindra, D. Bacterial cellulose/chitosan hydrogels synthesized in situ for biomedical application. J. Appl. Biosci. 2021, 162, 16675–16693. [Google Scholar] [CrossRef]
- Piasecka-Zelga, J.; Zelga, P.; Szulc, J.; Wietecha, J.; Ciechańska, D. An in vivo biocompatibility study of surgical meshes made from bacterial cellulose modified with chitosan. Int. J. Biol. Macromol. 2018, 116, 1119–1127. [Google Scholar] [CrossRef]
- Yin, N.; Du, R.; Zhao, F.; Han, Y.; Zhou, Z. Characterization of antibacterial bacterial cellulose composite membranes modified with chitosan or chitooligosaccharide. Carbohydr. Polym. 2020, 229, 115520. [Google Scholar] [CrossRef]
- Petrova, V.A.; Gofman, I.V.; Dubashynskaya, N.V.; Golovkin, A.S.; Mishanin, A.I.; Ivan’kova, E.M.; Romanov, D.P.; Khripunov, A.K.; Vlasova, E.N.; Migunova, A.V.; et al. Chitosan composites with bacterial cellulose nanofibers doped with nanosized cerium oxide: Characterization and cytocompatibility evaluation. Int. J. Mol. Sci. 2023, 24, 5415. [Google Scholar] [CrossRef]
- Dubey, S.; Mishra, R.; Roy, P.; Singh, R.P. 3-D macro/microporous-nanofibrous bacterial cellulose scaffolds seeded with BMP-2 preconditioned mesenchymal stem cells exhibit remarkable potential for bone tissue engineering. Int. J. Biol. Macromol. 2021, 167, 934–946. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2017, 3, 278–314. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Lee, K.; Wang, X.; Yoshitomi, T.; Kawazoe, N.; Yang, Y.; Chen, G. Interconnected collagen porous scaffolds prepared with sacrificial PLGA sponge templates for cartilage tissue engineering. J. Mater. Chem. B 2021, 9, 8491–8500. [Google Scholar] [CrossRef] [PubMed]
- Sola, A.; Bertacchini, J.; D’Avella, D.; Anselmi, L.; Maraldi, T.; Marmiroli, S.; Messori, M. Development of solvent-casting particulate leaching (SCPL) polymer scaffolds as improved three-dimensional supports to mimic the bone marrow niche. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 96, 153–165. [Google Scholar] [CrossRef]
- Yodsanga, S.; Poeaim, S. Effect of NaOH/urea solution as a solvent and salt crystals as a porogen on the fabrication of porous composite scaffold of bacterial cellulose-chitosan for tissue engineering. Int. J. Agric. Technol. 2024, 20, 877–892. [Google Scholar]
- Shakir, M.; Zia, I.; Rehman, A.; Ullah, R. Fabrication and characterization of nanoengineered biocompatible n-HA/chitosan-tamarind seed polysaccharide: Bio-inspired nanocomposites for bone tissue engineering. Int. J. Biol. Macromol. 2018, 111, 903–916. [Google Scholar] [CrossRef]
- Wang, X.; Tang, S.; Chai, S.; Wang, P.; Qin, J.; Pei, W.; Bian, H.; Jiang, Q.; Huang, C. Preparing printable bacterial cellulose based gelatin gel to promote in vivo bone regeneration. Carbohydr. Polym. 2021, 270, 118342. [Google Scholar] [CrossRef]
- Rao, X.; Huang, X.; Zhou, Z.; Lin, X. An improvement of the 2^(−ΔΔCT) method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinform. Biomath. 2013, 3, 71–85. [Google Scholar]
- Motiee, E.S.; Karbasi, S.; Bidram, E.; Sheikholeslam, M. Investigation of physical, mechanical and biological properties of polyhydroxybutyrate-chitosan/graphene oxide nanocomposite scaffolds for bone tissue engineering applications. Int. J. Biol. Macromol. 2023, 247, 125593. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Jummaat, F.; Yahya, E.B.; Olaiya, N.G.; Adnan, A.; Abdat, M.; Nasir, N.A.M.; Halim, A.S.; Kumar, U.S.U.; Bairwan, R.D.; et al. A Review on micro- to nanocellulose biopolymer scaffold forming for tissue engineering applications. Polymers 2020, 12, 2043. [Google Scholar] [CrossRef]
- Le, T.D.H.; Liaudanskaya, V.; Bonani, W.; Migliaresi, C.; Motta, A. Enhancing bioactive properties of silk fibroin with diatom particles for bone tissue engineering applications. J. Tissue Eng. Regen. Med. 2018, 12, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Ul-Islam, M.; Ullah, M.W.; Ikram, M.; Subhan, F.; Kim, Y.; Jang, J.H.; Yoon, S.; Park, J.K. Engineered regenerated bacterial cellulose scaffolds for application in in vitro tissue regeneration. RSC Adv. 2015, 5, 84565–84573. [Google Scholar] [CrossRef]
- Khan, S.; Ul-Islam, M.; Ikram, M.; Ullah, M.W.; Israr, M.; Subhan, F.; Kim, Y.; Jang, J.H.; Yoon, S.; Park, J.K. Three-Dimensionally microporous and highly biocompatible bacterial cellulose–gelatin composite scaffolds for tissue engineering applications. RSC Adv. 2016, 6, 110840–110849. [Google Scholar] [CrossRef]
- Tian, Y.; Wu, Q.; Li, F.; Zhou, Y.; Huang, D.; Xie, R.; Wang, X.; Zheng, Z.; Li, G. A flexible and biocompatible bombyx mori silk fibroin/wool keratin composite scaffold with interconnective porous structure. Colloids Surf. B Biointerfaces 2021, 208, 112080. [Google Scholar] [CrossRef]
- Parvizifard, M.; Karbasi, S. Physical, mechanical and biological performance of PHB-chitosan/MWCNTs nanocomposite coating deposited on bioglass-based scaffold: Potential application in bone tissue engineering. Int. J. Biol. Macromol. 2020, 152, 645–662. [Google Scholar] [CrossRef]
- Zaborowska, M.; Bodin, A.; Bäckdahl, H.; Popp, J.; Goldstein, A.; Gatenholm, P. Microporous bacterial cellulose as a potential scaffold for bone regeneration. Acta Biomater. 2010, 7, 2540–2547. [Google Scholar] [CrossRef]
- Zhang, W.; Xia, W. Dissolution and stability of chitosan in a sodium hydroxide/urea aqueous solution. J. Appl. Polym. Sci. 2014, 131, 39819. [Google Scholar] [CrossRef]
- Pandey, M.; Abeer, M.M.; Mohd Amin, M.C.I. Dissolution study of bacterial cellulose (nata de coco) from local food industry: Solubility behavior & structural changes. Int. J. Pharm. Pharm. Sci. 2014, 6, 89–93. [Google Scholar]
- Savitskaya, I.S.; Kistaubayeva, A.S.; Digel, I.E.; Shokatayeva, D.H. Physicochemical and antibacterial properties of composite films based on bacterial cellulose and chitosan for wound dressing materials. Eurasian Chem. Technol. J. 2017, 19, 255–264. [Google Scholar] [CrossRef]
- Jia, Y.; Wei, Z.; Fu, W.; Huo, M.; Li, F.; Zhong, C.; Jia, S.; Zhou, Y. Biocompatibility evaluation on a bio-hydrogel composed of bacterial cellulose and chitosan. J. Biomater. Tissue Eng. 2014, 4, 118–125. [Google Scholar] [CrossRef]
- Liu, K.; Wang, Y.; Liu, W.; Zheng, C.; Xu, T.; Du, H.; Yuan, Z.; Si, C. Bacterial cellulose/chitosan composite materials for biomedical applications. Chem. Eng. J. 2024, 494, 153014. [Google Scholar] [CrossRef]
- Chang, C.; Chena, S.; Zhang, L. Novel hydrogels prepared via direct dissolution of chitin at low temperature: Structure and biocompatibility. J. Mater. Chem. 2011, 21, 3865–3871. [Google Scholar] [CrossRef]
- Huang, L.; Bi, S.; Pang, J.; Sun, M.; Feng, C.; Chen, X. Preparation and characterization of chitosan from crab shell (Portunus trituberculatus) by NaOH/urea solution freeze-thaw pretreatment procedure. Int. J. Biol. Macromol. 2020, 147, 931–936. [Google Scholar] [CrossRef]
- Xiong, B.; Zhao, P.; Hu, K.; Zhang, L.; Cheng, G. Dissolution of cellulose in aqueous NaOH/urea solution: Role of urea. Cellulose 2014, 21, 1183–1192. [Google Scholar] [CrossRef]
- Chen, X.; Chen, J.; You, T.; Wang, K.; Xu, F. Effects of polymorphs on dissolution of cellulose in NaOH/urea aqueous solution. Carbohydr. Polym. 2015, 125, 85–91. [Google Scholar] [CrossRef]
- Pasaribu, K.M.; Ilyas, S.; Tamrin, T.; Radecka, I.; Swingler, S.; Gupta, A.; Stamboulis, A.G.; Gea, S. Bioactive bacterial cellulose wound dressings for burns with collagen in-situ and chitosan ex-situ impregnation. Int. J. Biol. Macromol. 2023, 230, 123118. [Google Scholar] [CrossRef]
- Li, G.; Nandgaonkar, A.G.; Habibi, Y.; Krause, W.E.; Wei, Q.; Lucia, L.A. An environmentally benign approach to achieving vectorial alignment and high microporosity in bacterial cellulose/chitosan scaffolds. RSC Adv. 2017, 7, 13678–13688. [Google Scholar] [CrossRef]
- Jindal, A.; Mondal, T.; Bhattacharya, J. An in vitro evaluation of zinc silicate fortified chitosan scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2020, 164, 4252–4262. [Google Scholar] [CrossRef]
- Lowe, B.; Venkatesan, J.; Anil, S.; Shim, M.S.; Kim, S.K. Preparation and characterization of chitosan-natural nano hydroxyapatite-fucoidan nanocomposites for bone tissue engineering. Int. J. Biol. Macromol. 2016, 93, 1479–1487. [Google Scholar] [CrossRef]
- Torgbo, S.; Sukyai, P. Fabrication of microporous bacterial cellulose embedded with magnetite and hydroxyapatite nanocomposite scaffold for bone tissue engineering. Mater. Chem. Phys. 2019, 237, 121868. [Google Scholar] [CrossRef]
- Sharma, C.; Dinda, A.K.; Potdar, P.D.; Chou, C.F.; Mishra, N.C. Fabrication and characterization of novel nano-biocomposite scaffold of chitosan-gelatin-alginate-hydroxyapatite for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 64, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Atak, B.H.; Buyuk, B.; Huysal, M.; Isik, S.; Senel, M.; Metzger, W.; Cetin, G. Preparation and characterization of amine functional nano-hydroxyapatite/chitosan bionanocomposite for bone tissue engineering applications. Carbohydr. Polym. 2017, 164, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhao, Y.; Zha, X.; Zhang, Z.; Zhang, L.; Wu, Y.; Ren, R.; Zhao, Z.; Yang, W.; Zhao, L. A janus, robust, biodegradable bacterial cellulose/Ti3C2Tx MXene bilayer membranes for guided bone regeneration. Biomater. Adv. 2024, 161, 213892. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Hu, X.; Cao, J.; Zhang, Y.; Xiao, J.; Peng, L.; Chen, D.; Xiong, C.; Zhang, L. Chitosan-coated hydroxyapatite and drug-loaded polytrimethylene carbonate/polylactic acid scaffold for enhancing bone regeneration. Carbohydr. Polym. 2021, 253, 117198. [Google Scholar] [CrossRef]
- Jianqing, L.; Wang, Q.; Gu, Y.; Zhu, Y.; Chen, L.; Chen, Y. Production of composite scaffold containing silk fibroin, chitosan, and gelatin for 3D cell culture and bone tissue regeneration. Med. Sci. Monit. 2017, 23, 5311–5320. [Google Scholar] [CrossRef]
- Min, L.; Jia, W.; Zhang, X.; Weng, H.; Gu, G.; Chen, Z. Hyaluronic acid oligosaccharides modified mineralized collagen and chitosan with enhanced osteoinductive properties for bone tissue engineering. Carbohydr. Polym. 2021, 260, 117780. [Google Scholar] [CrossRef]
- Kim, H.L.; Jung, G.Y.; Yoon, J.H.; Han, J.S.; Park, Y.J.; Kim, D.G.; Zhang, M.; Kim, D.J. Preparation and characterization of nano-sized hydroxyapatite/alginate/chitosan composite scaffolds for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 54, 20–25. [Google Scholar] [CrossRef]
- Wang, X.; Yang, X.; Xiao, X.; Li, X.; Chen, C.; Sun, D. Biomimetic design of platelet-rich plasma controlled release bacterial cellulose/hydroxyapatite composite hydrogel for bone tissue engineering. Int. J. Biol. Macromol. 2024, 269, 132124. [Google Scholar] [CrossRef]
- Oryan, A.; Sahvieh, S. Effectiveness of chitosan scaffold in skin, bone and cartilage healing. Int. J. Biol. Macromol. 2017, 104, 1003–1011. [Google Scholar] [CrossRef]
- Chen, C.; Ding, W.; Zhang, H.; Zhang, L.; Huang, Y.; Fan, M.; Yang, J.; Sun, D. Bacterial cellulose-based biomaterials: From fabrication to application. Carbohydr. Polym. 2022, 278, 118995. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, H.; Wu, Z.; Wu, N.; Wang, Z.; Chen, X.; Wei, Y.; Zhang, P. Modulation of osteogenesis in MC3T3-E1 cells by different frequency electrical stimulation. PLoS ONE 2016, 11, e0154924. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, D.; Qin, Y.; Xu, M.; Zhou, L.; Xu, W.; Liu, X.; Ye, L.; Yue, S.; Zheng, Q.; et al. Astragalin promotes osteoblastic differentiation in MC3T3-E1 cells and bone formation in vivo. Front. Endocrinol. 2019, 10, 228. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.G.; Cho, I.H. Characteristics and osteogenic effect of zirconia porous scaffold coated with β-TCP/HA. J. Adv. Prosthodont. 2014, 6, 285–294. [Google Scholar] [CrossRef][Green Version]
- Lei, X.; Gao, J.; Xing, F.; Zhang, Y.; Ma, Y.; Zhang, G. Comparative evaluation of the physicochemical properties of nano-hydroxyapatite/collagen and natural bone ceramic/collagen scaffolds and their osteogenesis-promoting effect on MC3T3-E1 cells. Regen. Biomater. 2019, 6, 361–371. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, M.; Chen, X.; Pu, X.; Liao, X.; Huang, Z.; Yin, G. A novel akermanite/poly (lactic-co-glycolic acid) porous composite scaffold fabricated via a solvent casting-particulate leaching method improved by solvent self-proliferating process. Regen. Biomater. 2017, 4, 233–242. [Google Scholar] [CrossRef]
- Mondal, D.; Lin, S.; Rizkalla, A.S.; Mequanint, K. Porous and biodegradable polycaprolactone-borophosphosilicate hybrid scaffolds for osteoblast infiltration and stem cell differentiation. J. Mech. Behav. Biomed. Mater. 2019, 92, 162–171. [Google Scholar] [CrossRef]
- Jeong, M.J.; Lim, D.S.; Kim, S.O.; Park, C.; Choi, Y.H.; Jeong, S.J. Effect of rosmarinic acid on differentiation and mineralization of MC3T3-E1 osteoblastic cells on titanium surface. Anim. Cells Syst. 2021, 25, 46–55. [Google Scholar] [CrossRef]
- Arriero Mdel, M.; Ramis, J.M.; Perelló, J.; Monjo, M. Differential response of MC3T3-E1 and human mesenchymal stem cells to inositol hexakisphosphate. Cell Physiol. Biochem. 2012, 30, 974–986. [Google Scholar] [CrossRef]
- Xing, F.; Chi, Z.; Yang, R.; Xu, D.; Cui, J.; Huang, Y.; Zhou, C.; Liu, C. Chitin-hydroxyapatite-collagen composite scaffolds for bone regeneration. Int. J. Biol. Macromol. 2021, 184, 170–180. [Google Scholar] [CrossRef]
- Chen, D.; Liu, P.; Li, M.; Zhang, C.; Gao, Y.; Guo, Y. Nacre-mimetic hydroxyapatite/chitosan/gelatin layered scaffolds modifying substance P for subchondral bone regeneration. Carbohydr. Polym. 2022, 291, 119575. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.C.; Li, X.Y.; Zhang, L.L.; Jiang, F. A 3D porous microsphere with multistage structure and component based on bacterial cellulose and collagen for bone tissue engineering. Carbohydr. Polym. 2020, 236, 116043. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, J.; Yang, F.; Shao, Y.; Zhang, X.; Dai, K. Modification and evaluation of micro-nano structured porous bacterial cellulose scaffold for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 75, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, C.; Gao, G.; Yin, X.; Pu, X.; Shi, B.; Liu, Y.; Huang, Z.; Wang, J.; Li, J.; et al. MBG/PGA-PCL composite scaffolds provide highly tunable degradation and osteogenic features. Bioact. Mater. 2022, 15, 53–67. [Google Scholar] [CrossRef]
- Ran, J.; Jiang, P.; Liu, S.; Sun, G.; Yan, P.; Shen, X.; Tong, H. Constructing multi-component organic/inorganic composite bacterial cellulose-gelatin/hydroxyapatite double-network scaffold platform for stem cell-mediated bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 130–140. [Google Scholar] [CrossRef]
- Shahbazarab, Z.; Teimouri, A.; Chermahini, A.N.; Azadi, M. Fabrication and characterization of nanobiocomposite scaffold of zein/chitosan/nanohydroxyapatite prepared by freeze-drying method for bone tissue engineering. Int. J. Biol. Macromol. 2018, 108, 1017–1027. [Google Scholar] [CrossRef]
- Kim, Y.; Zharkinbekov, Z.; Raziyeva, K.; Tabyldiyeva, L.; Berikova, K.; Zhumagul, D.; Temirkhanova, K.; Saparov, A. Chitosan-based biomaterials for tissue regeneration. Pharmaceutics 2023, 15, 807. [Google Scholar] [CrossRef]
- Sukpaita, T.; Chirachanchai, S.; Pimkhaokham, A.; Ampornaramveth, R.S. Chitosan-Based scaffold for mineralized tissues regeneration. Mar. Drugs 2021, 19, 551. [Google Scholar] [CrossRef]
- Raut, M.P.; Asare, E.; Syed Mohamed, S.M.D.; Amadi, E.N.; Roy, I. Bacterial cellulose-based blends and composites: Versatile biomaterials for tissue engineering applications. Int. J. Mol. Sci. 2023, 24, 986. [Google Scholar] [CrossRef]
- Yan, H.; Chen, X.; Feng, M.; Shi, Z.; Zhang, D.; Lin, Q. Layer-by-layer assembly of 3D alginate-chitosan-gelatin composite scaffold incorporating bacterial cellulose nanocrystals for bone tissue engineering. Mater. Lett. 2017, 209, 492–496. [Google Scholar] [CrossRef]
- Strnad, S.; Zemljič, L.F. Cellulose-chitosan functional biocomposites. Polymers 2023, 15, 425. [Google Scholar] [CrossRef]
- Zhu, Q.; Chen, X.; Liu, Z.; Li, Z.; Li, D.; Yan, H.; Lin, Q. Development of alginate-chitosan composite scaffold incorporation of bacterial cellulose for bone tissue engineering. Int. J. Polym. Mater. Polym. Biomater. 2021, 72, 296–307. [Google Scholar] [CrossRef]
- Sukpaita, T.; Chirachanchai, S.; Chanamuangkon, T.; Nampuksa, K.; Monmaturapoj, N.; Sumrejkanchanakij, P.; Pimkhaokham, A.; Ampornaramveth, R.S. Novel epigenetic modulation chitosan-based scaffold as a promising bone regenerative Material. Cells 2022, 11, 3217. [Google Scholar] [CrossRef] [PubMed]
- Lekhavadhani, S.; Shanmugavadivu, A.; Selvamurugan, N. Role and architectural significance of porous chitosan-based scaffolds in bone tissue engineering. Int. J. Biol. Macromol. 2023, 251, 126238. [Google Scholar] [CrossRef] [PubMed]
- Busuioc, C.; Isopencu, G.; Banciu, A.; Banciu, D.D.; Oprea, O.; Mocanu, A.; Deleanu, I.; Zăuleţ, M.; Popescu, L.; Tănăsuică, R.; et al. Bacterial cellulose hybrid composites with calcium phosphate for bone tissue regeneration. Int. J. Mol. Sci. 2022, 23, 16180. [Google Scholar] [CrossRef]
- Black, C.R.M.; Goriainov, V.; Gibbs, D.; Kanczler, J.; Tare, R.S.; Oreffo, R.O.C. Bone tissue engineering. Curr. Mol. Biol. Rep. 2015, 1, 132–140. [Google Scholar] [CrossRef]
- Li, Y.; Chu, C.; Chen, C.; Sun, B.; Wu, J.; Wang, S.; Ding, W.; Sun, D. Quaternized chitosan/oxidized bacterial cellulose cryogels with shape recovery for noncompressible hemorrhage and wound healing. Carbohydr. Polym. 2024, 327, 121679. [Google Scholar] [CrossRef]
- Shuai, C.; Yu, L.; Feng, P.; Gao, C.; Peng, S. Interfacial reinforcement in bioceramic/biopolymer composite bone scaffold: The role of coupling agent. Colloids Surf. B Biointerfaces 2020, 193, 111083. [Google Scholar] [CrossRef]
- Kingkaew, J.; Kirdponpattara, S.; Sanchavanakit, N.; Pavasant, P.; Phisalaphong, M. Effect of molecular weight of chitosan on antimicrobial properties and tissue compatibility of chitosan-impregnated bacterial cellulose films. Biotechnol. Bioprocess E 2014, 19, 534–544. [Google Scholar] [CrossRef]
- Kim, J.; Cai, Z.; Lee, H.S.; Choi, G.S.; Lee, D.H.; Jo, C. Preparation and characterization of a bacterial cellulose/chitosan composite for potential biomedical application. J. Polym. Res. 2011, 18, 739–744. [Google Scholar] [CrossRef]
- Zuo, Y.; Li, Q.; Xiong, Q.; Li, J.; Tang, C.; Zhang, Y.; Wang, D. Naringin release from a nano-hydroxyapatite/collagen scaffold promotes osteogenesis and bone tissue reconstruction. Polymers 2022, 14, 3260. [Google Scholar] [CrossRef]
- Li, Z.; Chen, X.; Bao, C.; Liu, C.; Liu, C.; Li, D.; Yan, H.; Lin, Q. Fabrication and evaluation of alginate/bacterial cellulose nanocrystals-chitosan-gelatin composite scaffolds. Molecules 2021, 26, 5003. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Han, S.S. Efficacy of bacterial nanocellulose in hard tissue regeneration: A review. Materials 2021, 14, 4777. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, H.; Guo, S.; Qi, J.; Zhang, R.; Liu, X.; Sun, L.; Zong, M.; Cheng, H.; Wu, X.; et al. Applications of bacterial cellulose-based composite materials in hard tissue regenerative medicine. Tissue Eng. Regen. Med. 2023, 20, 1017–1039. [Google Scholar] [CrossRef] [PubMed]
- Horue, M.; Silva, J.M.; Berti, I.R.; Brandão, L.R.; Barud, H.D.S.; Castro, G.R. Bacterial cellulose-based materials as dressings for wound healing. Pharmaceutics 2023, 15, 424. [Google Scholar] [CrossRef]
- Torgbo, S.; Sukyai, P. Bacterial cellulose-based scaffold materials for bone tissue engineering. Appl. Mater. Today 2018, 11, 34–49. [Google Scholar] [CrossRef]
- Shah, N.; Ul-Islam, M.; Khattak, W.A.; Park, J.K. Overview of bacterial cellulose composites: A multipurpose advanced material. Carbohydr. Polym. 2013, 98, 1585–1598. [Google Scholar] [CrossRef]


| Gene Names | Forward/Reverse Primer Sequences |
|---|---|
| OCN | 5′-TGACCTCACAGATCCCAAGCC-3′/5′-ATACCGTAGATGCGTTTGTAGGC-3′ |
| ALP | 5′-CCTTGCCTGTATCTGGAATCCT-3′/5′-GTGCAGTCTGTGTCTTGCCTG-3′ |
| COL-1 | 5′-GGGTCTAGACATGTTCAGCTTTGTG-3′/5′-ACCCTTAGGCCATTGTGTATGC-3′ |
| BSP | 5′-CCTCCTCTGAAACGGTTTCCA-3′/5′-TCTGCATCTCCAGCCTCCTTG-3′ |
| GAPDH | 5′-AGGTCGGTGTGAACGGATTTG-3′/5′-GGGGTCGTTGATGGCAACA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yodsanga, S.; Poeaim, S.; Chantarangsu, S.; Swasdison, S. Investigation of Biodegradation and Biocompatibility of Chitosan–Bacterial Cellulose Composite Scaffold for Bone Tissue Engineering Applications. Cells 2025, 14, 723. https://doi.org/10.3390/cells14100723
Yodsanga S, Poeaim S, Chantarangsu S, Swasdison S. Investigation of Biodegradation and Biocompatibility of Chitosan–Bacterial Cellulose Composite Scaffold for Bone Tissue Engineering Applications. Cells. 2025; 14(10):723. https://doi.org/10.3390/cells14100723
Chicago/Turabian StyleYodsanga, Somchai, Supattra Poeaim, Soranun Chantarangsu, and Somporn Swasdison. 2025. "Investigation of Biodegradation and Biocompatibility of Chitosan–Bacterial Cellulose Composite Scaffold for Bone Tissue Engineering Applications" Cells 14, no. 10: 723. https://doi.org/10.3390/cells14100723
APA StyleYodsanga, S., Poeaim, S., Chantarangsu, S., & Swasdison, S. (2025). Investigation of Biodegradation and Biocompatibility of Chitosan–Bacterial Cellulose Composite Scaffold for Bone Tissue Engineering Applications. Cells, 14(10), 723. https://doi.org/10.3390/cells14100723






