Effects of Adipose-Derived Mesenchymal Stem Cell-Secretome on Pyroptosis of Laparoscopic Hepatic Ischemia Reperfusion Injury in a Porcine Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Establishment of HIRI and Hepatectomy Model
2.3. Culture of ADSCs
2.4. Preparation of ADSC-Secretome
2.5. ELISA
2.6. Histological Examination
2.7. Western Blotting
2.8. qRT-PCR
2.9. Immunohistochemical Staining
2.10. Statistical Analysis
3. Results
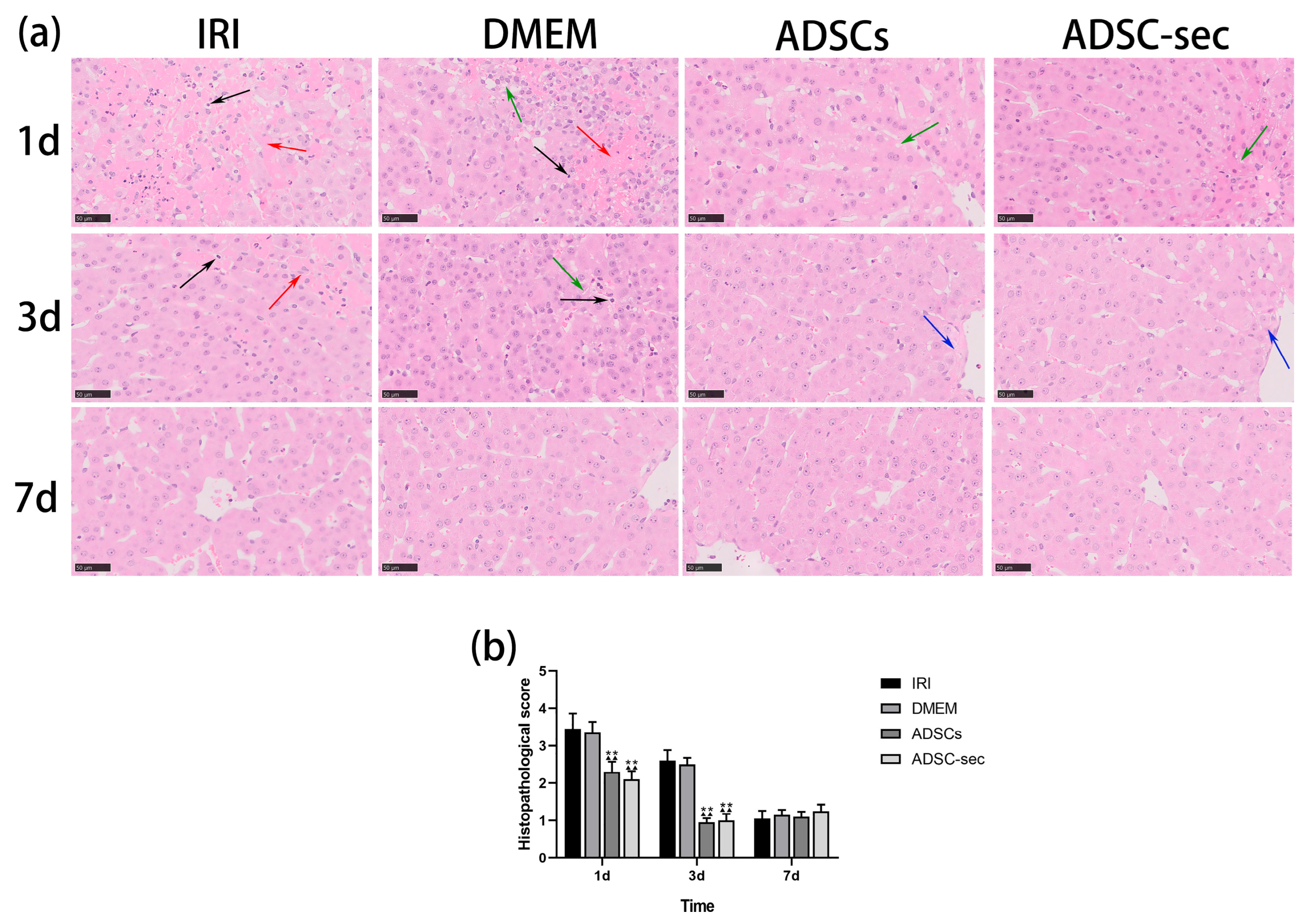
3.1. ADSC-Secretome Alleviated HIRI in a Minipig Model
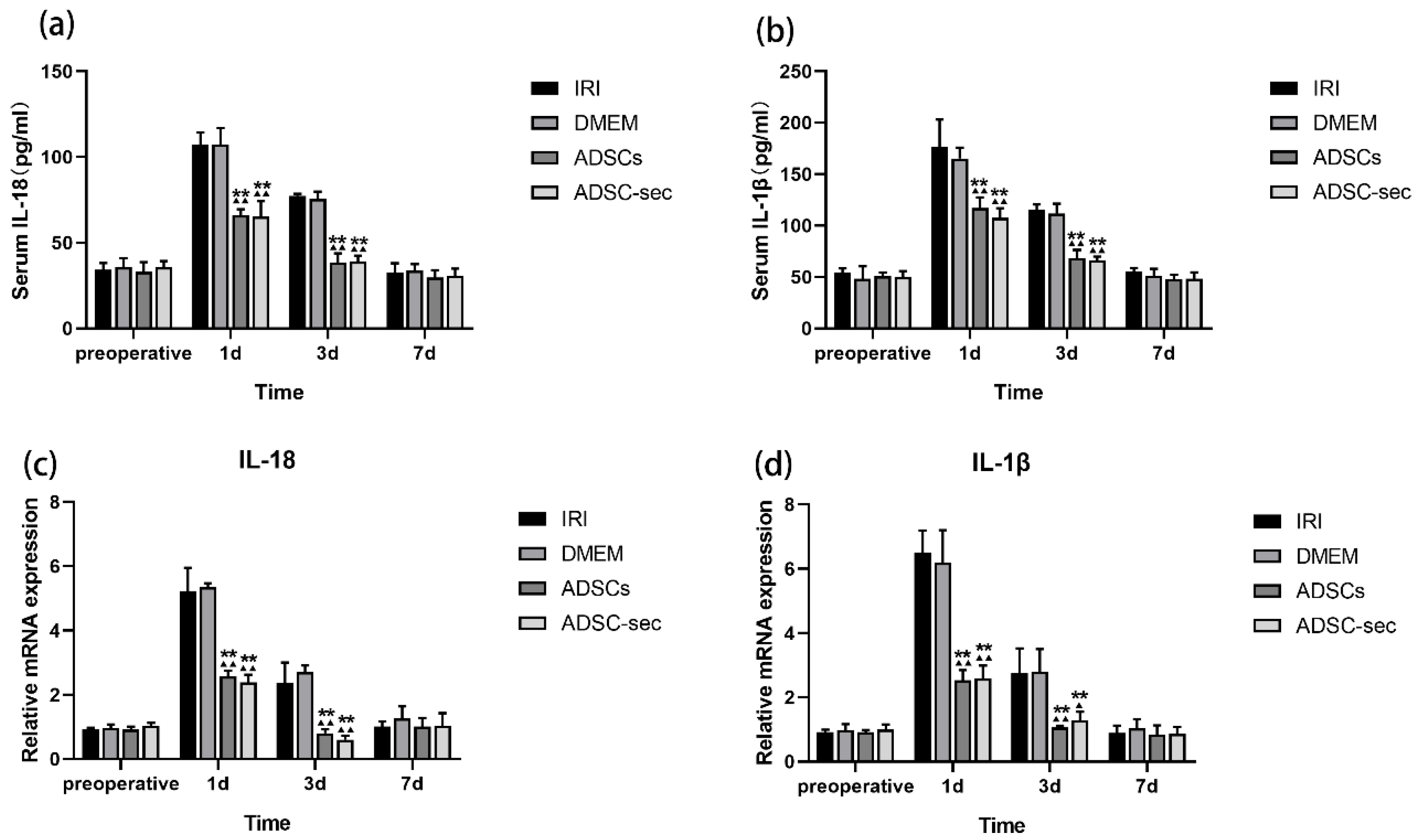
3.2. ADSC-Secretome Attenuated HIRI-Induced Inflammation
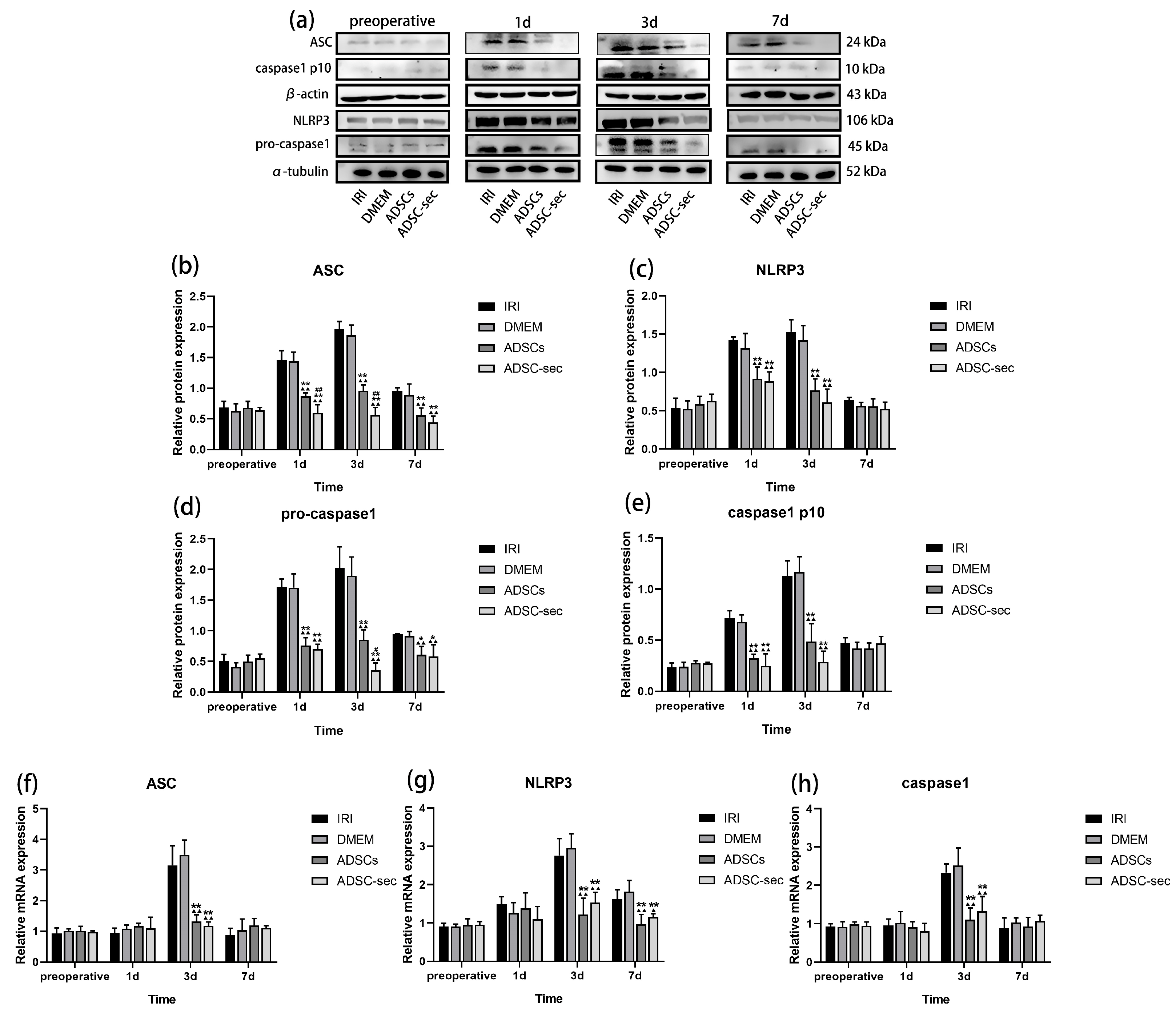
3.3. ADSC-Secretome Inhibited HIRI-Induced NLRP3 Inflammasome Activation
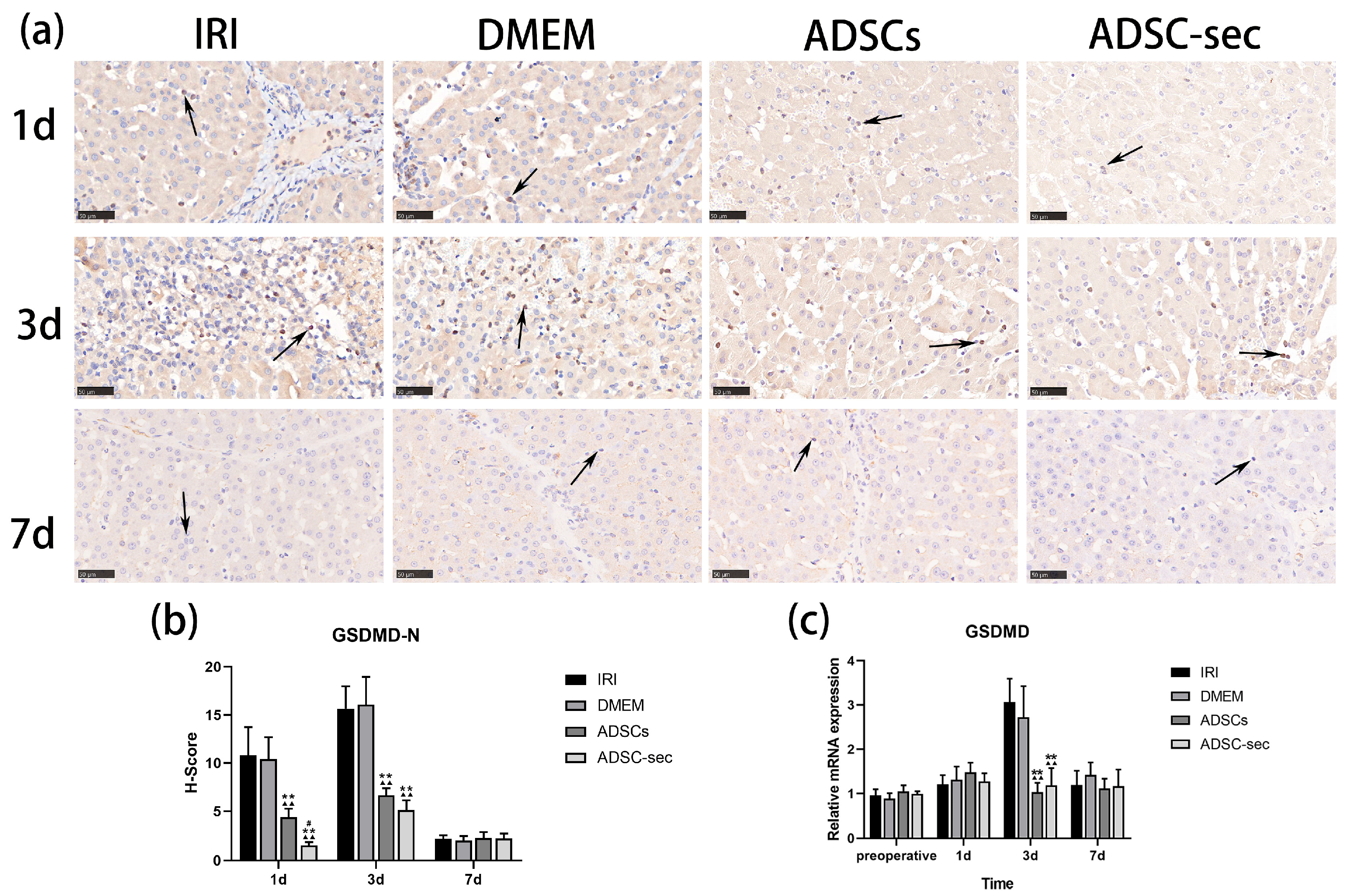
3.4. ADSC-Secretome Reduced GSDMD Expression in the Liver Tissues After IRI
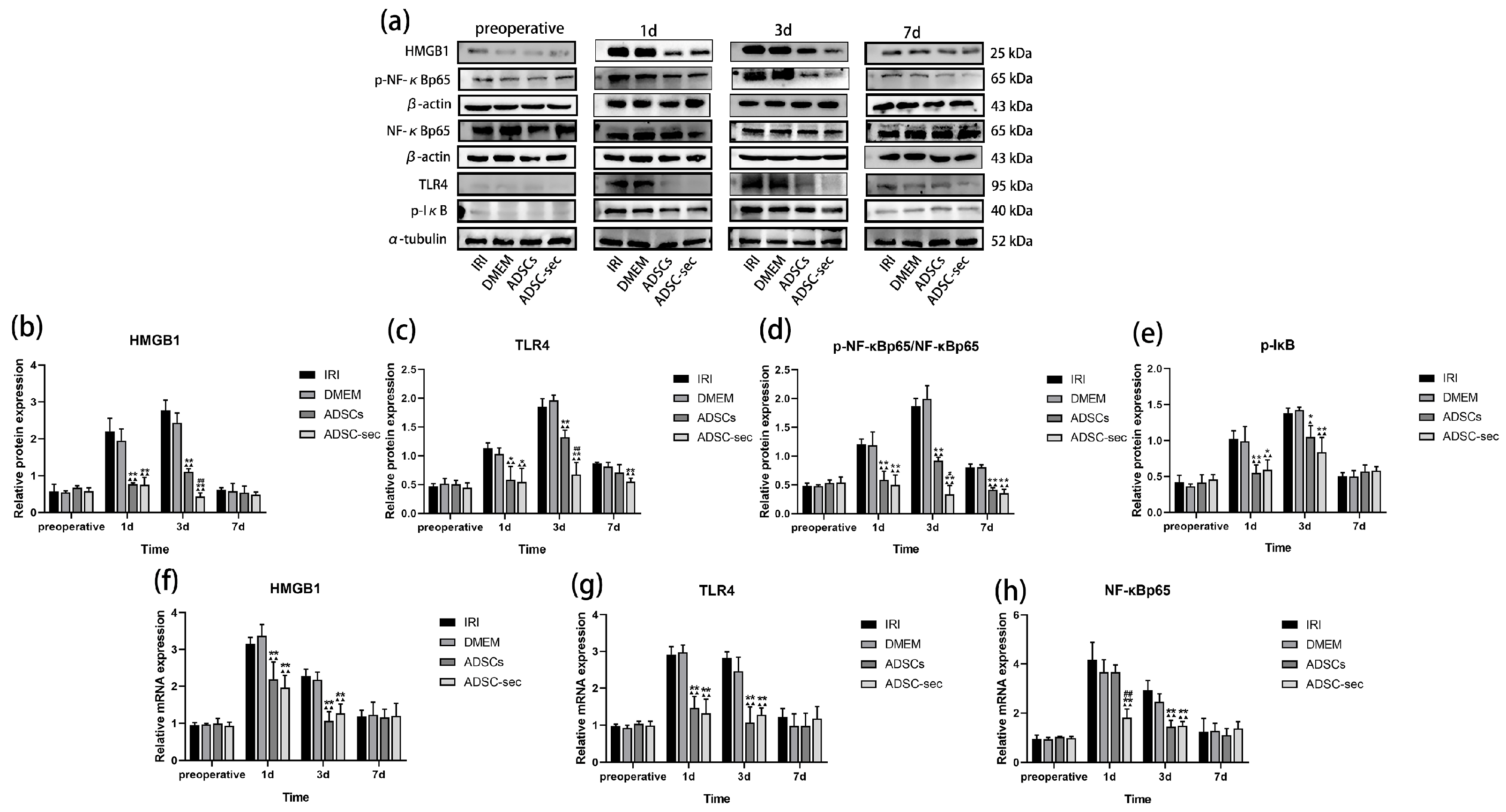
3.5. ADSC-Secretome Inhibited the HMGB1/TLR4/NF-κB Pathway During HIRI
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MSCs | Mesenchymal stem cells |
| ADSC-secretome | Adipose mesenchymal stem cell-secretome |
| HIRI | Hepatic ischemia–reperfusion injury |
| PH | Partial hepatectomy |
References
- Serracino-Inglott, F.; Habib, N.A.; Mathie, R.T. Hepatic ischemia-reperfusion injury. Am. J. Surg. 2001, 181, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am. J. Physiol.-Gastrointest. Liver Physiol. 2003, 284, G15–G26. [Google Scholar] [CrossRef]
- Lentsch, A.B.; Kato, A.; Yoshidome, H.; Mcmasters, K.M.; Edwards, M.J. Inflammatory mechanisms and therapeutic strategies for warm hepatic ischemia/reperfusion in-jury. Hepatology 2000, 32, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Fondevila, C.; Busuttil, R.W.; Kupiec-Weglinski, J.W. Hepatic ischemia/reperfusion injury—A fresh look. Exp. Mol. Pathol. 2003, 74, 86–93. [Google Scholar] [CrossRef]
- Yang, Z.; Deng, Y.; Su, D.; Tian, J.; Gao, Y.; He, Z. TLR4 as receptor for HMGB1-mediated acute lung injury after liver ischemia/reperfusion injury. Lab. Investig. 2013, 93, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Yan, H. Targeting AGEs-RAGE pathway inhibits inflammation and presents neuroprotective effect against hepatic ischemia-reperfusion induced hippocampus damage. Clin. Res. Hepatol. Gastroenterol. 2022, 46, 101792. [Google Scholar] [CrossRef]
- Deng, S.; Zhang, Y.; Xin, Y.; Hu, X. Vagus nerve stimulation attenuates acute kidney injury induced by hepatic ischemia/reperfusion injury in rats. Sci. Rep. 2022, 12, 21662. [Google Scholar] [CrossRef] [PubMed]
- Bergsbaken, T.; Fink, S.L.; Cookson, B. Pyroptosis: Host cell death and inflammation. Nat. Rev. Microbiol. 2009, 7, 99–109. [Google Scholar] [CrossRef]
- Li, J.; Li, R.; Lv, G.; Liu, H. The mechanisms and strategies to protect from hepatic ischemia-reperfusion injury. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 2036–2047. [Google Scholar]
- van Golen, R.F.; van Gulik, T.M.; Heger, M. The sterile immune response during hepatic ischemia/reperfusion. Cytokine Growth Factor Rev. 2012, 23, 69–84. [Google Scholar] [CrossRef]
- Dai, Q.; Jiang, W.; Liu, H.; Qing, X.; Wang, G.; Huang, F.; Yang, Z.; Wang, C.; Gu, E.; Zhao, H.; et al. Kupffer cell-targeting strategy for the protection of hepatic ischemia/reperfusion injury. Nanotechnology 2021, 32, 265101. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Cao, Q.; Guo, Y.; He, J.; Xu, D.; Lin, A. GSDMD knockdown attenuates phagocytic activity of microglia and exacerbates seizure susceptibility in TLE mice. J. Neuroinflammation 2023, 20, 193. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chen, H.; Evankovich, J.; Yan, W.; Rosborough, B.R.; Nace, G.W.; Ding, Q.; Loughran, P.; Beer-Stolz, D.; Billiar, T.R.; et al. Histones activate the NLRP3 inflammasome in Kupffer cells during sterile inflammatory liver injury. J. Immunol. 2013, 191, 2665–2679. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, J.; Xu, M.; Li, M.; Wang, B.; Qu, X.; Yu, C.; Huang, H.; Xia, Q.; Wu, H.; et al. Blocking GSDMD processing in innate immune cells but not in hepatocytes protects hepatic ischemia–reperfusion injury. Cell Death Dis. 2020, 11, 244. [Google Scholar] [CrossRef]
- Zheng, Y.; Xu, X.; Chi, F.; Cong, N. Pyroptosis: A Newly Discovered Therapeutic Target for Ischemia-Reperfusion Injury. Biomolecules 2022, 12, 1625. [Google Scholar] [CrossRef]
- Du, Y.; Zhong, F.; Cheng, H.; Li, T.; Chen, Y.; Tan, P.; Huang, M.; Liang, T.; Liu, Y.; Xia, X.; et al. The Dietary Supplement-Oryzanol Attenuates Hepatic Ischemia Reperfusion Injury via Inhibiting Endoplasmic Reticulum Stress and HMGB1/NLRP3 Inflammasome. Oxidative Med. Cell. Longev. 2021, 2021, 4628050. [Google Scholar] [CrossRef]
- Zhao, G.; Fu, C.; Wang, L.; Zhu, L.; Yan, Y.; Xiang, Y.; Zheng, F.; Gong, F.; Chen, S.; Chen, G. Down-regulation of nuclear HMGB1 reduces ischemia-induced HMGB1 translocation and release and protects against liver ischemia-reperfusion injury. Sci. Rep. 2017, 7, 46272. [Google Scholar] [CrossRef]
- Nace, G.W.; Huang, H.; Klune, J.R.; Eid, R.E.; Rosborough, B.R.; Korff, S.; Li, S.; Shapiro, R.A.; Stolz, D.B.; Sodhi, C.P.; et al. Cellular-specific role of toll-like receptor 4 in hepatic ischemia-reperfusion injury in mice. Hepatology 2013, 58, 374–387. [Google Scholar] [CrossRef]
- Tsung, A.; Sahai, R.; Tanaka, H.; Nakao, A.; Fink, M.P.; Lotze, M.T.; Yang, H.; Li, J.; Tracey, K.J.; Geller, D.A.; et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J. Exp. Med. 2005, 201, 1135–1143. [Google Scholar] [CrossRef]
- Mitchell, J.; Carmody, R. NF-κB and the Transcriptional Control of Inflammation. Int. Rev. Cell Mol. Biol. 2018, 335, 41–84. [Google Scholar] [CrossRef]
- Jin, X.; Liu, M.Y.; Zhang, D.F.; Zhong, X.; Du, K.; Qian, P.; Yao, W.F.; Gao, H.; Wei, M.J. Baicalin mitigates cognitive impairment and protects neurons from microglia-mediated neuroinflammation via suppressing NLRP 3 inflammasomes and TLR 4/NF-κB signaling pathway. CNS Neurosci. Ther. 2019, 25, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Peng, Y.; Sun, H.; Wang, L.; Jiang, L.; Zou, S. Silencing lncRNA KCNQ1OT1 reduced hepatic ischemia reperfusion injury-induced pyroptosis by regulating miR-142a-3p/HMGB1 axis. Mol. Cell. Biochem. 2023, 478, 1293–1305. [Google Scholar] [CrossRef]
- Ding, H.-S.; Huang, Y.; Qu, J.F.; Wang, Y.J.; Huang, Z.Y.; Wang, F.Y.; Yi, W.J.; Liu, X.X. Panaxynol ameliorates cardiac ischemia/reperfusion injury by suppressing NLRP3-induced pyroptosis and apoptosis via HMGB1/TLR4/NF-κB axis. Int. Immunopharmacol. 2023, 121, 110222. [Google Scholar] [CrossRef]
- Ayala-Cuellar, A.P.; Kang, J.H.; Jeung, E.B.; Choi, K.C. Roles of mesenchymal stem cells in tissue regeneration and immunomodulation. Biomol. Ther. 2018, 27, 25–33. [Google Scholar] [CrossRef]
- Vizoso, F.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medi-cine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef] [PubMed]
- Volarevic, V.; Markovic, B.S.; Gazdic, M.; Volarevic, A.; Jovicic, N.; Arsenijevic, N.; Armstrong, L.; Djonov, V.; Lako, M.; Stojkovic, M. Ethical and safety issues of stem cell-based therapy. Int. J. Med Sci. 2018, 15, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Hassanshahi, A.; Hassanshahi, M.; Khabbazi, S.; Hosseini-Khah, Z.; Peymanfar, Y.; Ghalamkari, S.; Su, Y.W.; Xian, C.J. Adipose-derived stem cells for wound healing. J. Cell. Physiol. 2019, 234, 7903–7914. [Google Scholar] [CrossRef] [PubMed]
- Abdolmohammadi, K.; Mahmoudi, T.; Nojehdehi, S.; Tayebi, L.; Hashemi, S.M.; Noorbakhsh, F.; Abdollahi, A.; Soleimani, M.; Nikbin, B.; Nicknam, M.H. Effect of hypoxia preconditioned adipose-derived mesenchymal stem cell conditioned medium on cerulein-induced acute pancreatitis in mice. Adv. Pharm. Bull. 2020, 10, 297–306. [Google Scholar] [CrossRef]
- Heidari, M.; Pouya, S.; Baghaei, K.; Aghdaei, H.A.; Namaki, S.; Zali, M.R.; Hashemi, S.M. The immunomodulatory effects of adipose-derived mesenchymal stem cells and mesenchymal stem cells-conditioned medium in chronic colitis. J. Cell. Physiol. 2018, 233, 8754–8766. [Google Scholar] [CrossRef]
- Lo Sicco, C.; Reverberi, D.; Balbi, C.; Ulivi, V.; Principi, E.; Pascucci, L.; Becherini, P.; Bosco, M.C.; Varesio, L.; Franzin, C.; et al. Mesenchymal stem cell-derived extracellular vesicles as mediators of anti-inflammatory effects: Endorsement of macrophage polarization. Stem Cells Transl. Med. 2017, 6, 1018–1028. [Google Scholar] [CrossRef]
- Pires, A.O.; Mendes-Pinheiro, B.; Teixeira, F.G.; Anjo, S.I.; Ribeiro-Samy, S.; Gomes, E.D.; Serra, S.C.; Silva, N.A.; Manadas, B.; Sousa, N.; et al. Unveiling the differences of secretome of human bone marrow mesenchymal stem cells, adipose tissue-derived stem cells, and human umbilical cord perivascular cells: A proteomic analysis. Stem Cells Dev. 2016, 25, 1073–1083. [Google Scholar] [CrossRef]
- Rigotti, G.; Marchi, A.; Sbarbati, A. Adipose-derived mesenchymal stem cells: Past, present, and future. Aesthetic Plast. Surg. 2009, 33, 271–273. [Google Scholar] [CrossRef]
- Konno, M.; Hamabe, A.; Hasegawa, S.; Ogawa, H.; Fukusumi, T.; Nishikawa, S.; Ohta, K.; Kano, Y.; Ozaki, M.; Noguchi, Y.; et al. Adipose-derived mesenchymal stem cells and regenerative medicine. Dev. Growth Differ. 2013, 55, 309–318. [Google Scholar] [CrossRef]
- Ge, Y.; Zhang, Q.; Li, H.; Bai, G.; Jiao, Z.; Wang, H. Adipose-derived stem cells alleviate liver apoptosis induced by ischemia-reperfusion and laparoscopic hepatectomy in swine. Sci. Rep. 2018, 8, 16878. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Z.; Ma, Y.; Liu, X.; Ge, Y.; Zhang, Q.; Liu, B.; Wang, H. Adipose-Derived Stem Cell Transplantation Attenuates Inflammation and Promotes Liver Regeneration after Ischemia-Reperfusion and Hemihepatectomy in Swine. Stem Cells Int. 2019, 2019, 2489584. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Z.; Ma, Y.; Wang, Y.; Liu, T.; Zhang, Q.; Liu, X.; Pao, C.; Liu, B.; Wang, H. Protective effect of adipose-derived mesenchymal stem cell secretome against hepatocyte apoptosis induced by liver ischemia-reperfusion with partial hepatectomy injury. Stem Cells Int. 2021, 2021, 9969372. [Google Scholar] [CrossRef]
- Jiao, Z.; Ma, Y.; Zhang, Q.; Wang, Y.; Liu, T.; Liu, X.; Pao, C.; Liu, B.; Wang, H. The adipose-derived mesenchymal stem cell secretome promotes hepatic regeneration in miniature pigs after liver ischaemia-reperfusion combined with partial resection. Stem Cell Res. Ther. 2021, 12, 218. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, T.; Wang, Y.; Liu, H.; Zhang, J.; Wu, Y.; Lei, L.; Wang, H. Laparoscopic left hepatectomy in swine: A safe and feasible technique. J. Vet. Sci. 2014, 15, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Toledo-Pereyra, L.H.; Rodriguez, F.J.; Cejalvo, D. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine. Transplantation 1993, 55, 1265–1271. [Google Scholar] [CrossRef]
- Wang, Y.; Piao, C.; Liu, T.; Lu, X.; Ma, Y.; Zhang, J.; Liu, G.; Wang, H. Effects of the exosomes of adipose-derived mesenchymal stem cells on apoptosis and pyroptosis of injured liver in miniature pigs. Biomed. Pharmacother. 2023, 169, 115873. [Google Scholar] [CrossRef]
- Kolachala, V.L.; Lopez, C.; Shen, M.; Shayakhmetov, D.; Gupta, N.A. Ischemia reperfusion injury induces pyroptosis and mediates injury in steatotic liver thorough Caspase 1 activation. Apoptosis 2021, 26, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Castro, M.B.; Cornide-Petronio, M.E.; Gracia-Sancho, J.; Peralta, C. Inflammasome-mediated inflammation in liver ischemia-reperfusion injury. Cells 2019, 8, 1131. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef]
- Liu, H.; Man, K. New insights in mechanisms and therapeutics for short-and long-term impacts of hepatic ischemia reperfusion injury post liver transplantation. Int. J. Mol. Sci. 2021, 22, 8210. [Google Scholar] [CrossRef] [PubMed]
- Pretzsch, E.; Nieß, H.; Khaled, N.B.; Bösch, F.; Guba, M.; Werner, J.; Angele, M.; Chaudry, I.H. Molecular Mechanisms of Ischaemia-Reperfusion Injury and Regeneration in the Liver-Shock and Surgery-Associated Changes. Int. J. Mol. Sci. 2022, 23, 12942. [Google Scholar] [CrossRef] [PubMed]
- Rao, Z.; Zhu, Y.; Yang, P.; Chen, Z.; Xia, Y.; Qiao, C.; Liu, W.; Deng, H.; Li, J.; Ning, P.; et al. Pyroptosis in inflammatory diseases and cancer. Theranostics 2022, 12, 4310–4329. [Google Scholar] [CrossRef]
- Kim, H.; Kim, S.; Lee, S.M. Activation of NLRP3 and AIM2 inflammasomes in Kupffer cells in hepatic ischemia/reperfusion. FEBS J. 2015, 282, 259–270. [Google Scholar] [CrossRef]
- Lin, J.; Li, F.; Jiao, J.; Qian, Y.; Xu, M.; Wang, F.; Sun, X.; Zhou, T.; Wu, H.; Kong, X. Quercetin, a natural flavonoid, protects against hepatic ischemia–reperfusion injury via inhibiting Caspase-8/ASC dependent macrophage pyroptosis. J. Adv. Res. 2024, 70, 555–569. [Google Scholar] [CrossRef]
- Chen, K.; Obara, H.; Matsubara, Y.; Fukuda, K.; Yagi, H.; Ono-Uruga, Y.; Matsubara, K.; Kitagawa, Y. Adipose-Derived Mesenchymal Stromal/Stem Cell Line Prevents Hepatic Ischemia/Reperfusion Injury in Rats by Inhibiting Inflammasome Activation. Cell Transplant. 2022, 31, 9636897221089629. [Google Scholar] [CrossRef]
- Xue, R.; Qiu, J.; Wei, S.; Liu, M.; Wang, Q.; Wang, P.; Sha, B.; Wang, H.; Shi, Y.; Zhou, J.; et al. Lycopene alleviates hepatic ischemia reperfusion injury via the Nrf2/HO-1 pathway mediated NLRP3 inflammasome inhibition in Kupffer cells. Ann. Transl. Med. 2021, 9, 631. [Google Scholar] [CrossRef]
- Si, Z.; Wang, X.; Sun, C.; Kang, Y.; Xu, J.; Wang, X.; Hui, Y. Adipose-derived stem cells: Sources, potency, and implications for regenerative therapies. Biomed. Pharmacother. 2019, 114, 108765. [Google Scholar] [CrossRef] [PubMed]
- Miceli, V.; Bulati, M.; Gallo, A.; Lannolo, G.; Busa, R.; Conaldi, P.G.; Zito, G. Role of Mesenchymal Stem/Stromal Cells in Modulating Ischemia/Reperfusion Injury: Current State of the Art and Future Perspectives. Biomedicines 2023, 11, 689. [Google Scholar] [CrossRef] [PubMed]
- Coll, R.; Schroder, K.; Pelegrin, P. NLRP3 and pyroptosis blockers for treating inflammatory diseases. Trends Pharmacol. Sci. 2022, 43, 653–668. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Scott, M. Caspase-1 as a multifunctional inflammatory mediator: Noncytokine maturation roles. J. Leukoc. Biol. 2016, 100, 961–967. [Google Scholar] [CrossRef]
- Li, X.; Luo, S.; Chen, X.; Li, S.; Hao, L.; Yang, D. Adipose-derived stem cells attenuate acne-related inflammation via suppression of NLRP3 inflammasome. Stem Cell Res. Ther. 2022, 13, 334. [Google Scholar] [CrossRef]
- Li, H.; Sun, L.; Wang, Y. Inhibition of LPS induced NLRP3 inflammasome activation by stem cell conditioned culture media in human gingival epithelial cells. Mol. Med. Rep. 2023, 27, 106. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, C.; Shao, T.; Chen, J.; Chen, D. The Role of NLRP3 Inflammasome Activation Pathway of Hepatic Macrophages in Liver Ische-mia-Reperfusion Injury. Front. Immunol. 2022, 13, 905423. [Google Scholar] [CrossRef]
- Ding, J.; Wang, K.; Liu, W.; She, Y.; Sun, Q.; Shi, J.; Sun, H.; Wang, D.C.; Shao, F. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 2016, 535, 111–116. [Google Scholar] [CrossRef]
- Lv, X.; Chen, J.; He, J.; Hou, L.; Ren, Y.; Shen, X.; Wang, Y.; Ji, T.; Cai, X. Gasdermin D–mediated pyroptosis suppresses liver regeneration after 70% partial hepatectomy. Hepatol. Commun. 2022, 6, 2340–2353. [Google Scholar] [CrossRef]
- Ni, Y.A.; Chen, H.; Nie, H.; Zheng, B.; Gong, Q. HMGB1: An overview of its roles in the pathogenesis of liver disease. J. Leukoc. Biol. 2021, 110, 987–998. [Google Scholar] [CrossRef]
- Park, J.S.; Svetkauskaite, D.; He, Q.; Kim, J.Y.; Strassheim, D. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J. Biol. Chem. 2004, 279, 7370–7377. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Chen, K.; Gu, Y.; Li, Y.; Wang, L.; Zhu, X.; Deng, Q. HMGB1/TLR4 axis promotes pyroptosis after ICH by activating the NLRP3 inflammasome. J. Neuroimmunol. 2024, 393, 578401. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lei, Z.; Chai, H.; Kang, Q.; Qin, X. Salidroside alleviates hepatic ischemia–reperfusion injury during liver transplant in rat through regulating TLR-4/NF-κB/NLRP3 inflammatory pathway. Sci. Rep. 2022, 12, 13973. [Google Scholar] [CrossRef]
- El, A.; Sokar, S.S.; Shebl, A.M.; Mohamed, D.Z.; Abu-Risha, S.E. Octreotide and melatonin alleviate inflammasome-induced pyroptosis through inhibition of TLR4-NF-κB-NLRP3 pathway in hepatic ischemia/reperfusion injury. Toxicol. Appl. Pharmacol. 2021, 410, 115340. [Google Scholar] [CrossRef]
- Teixeira, F.G.; Salgado, A.J. Mesenchymal stem cells secretome: Current trends and future challenges. Neural Regen. Res. 2020, 15, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Trigo, C.M.; Rodrigues, J.S.; Camões, S.P.; Solá, S.; Miranda, J.P. Mesenchymal stem cell secretome for regenerative medicine: Where do we stand? J. Adv. Res. 2025, 70, 103–124. [Google Scholar] [CrossRef]
- Műzes, G.; Sipos, F. Mesenchymal stem cell-derived secretome: A potential therapeutic option for autoimmune and immune-mediated inflammatory diseases. Cells 2022, 11, 2300. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Cao, L.; Li, P.; Jiao, Z.; Liu, X.; Lu, X.; Liu, T.; Wang, H. Effects of Adipose-Derived Mesenchymal Stem Cell-Secretome on Pyroptosis of Laparoscopic Hepatic Ischemia Reperfusion Injury in a Porcine Model. Cells 2025, 14, 722. https://doi.org/10.3390/cells14100722
Ma Y, Cao L, Li P, Jiao Z, Liu X, Lu X, Liu T, Wang H. Effects of Adipose-Derived Mesenchymal Stem Cell-Secretome on Pyroptosis of Laparoscopic Hepatic Ischemia Reperfusion Injury in a Porcine Model. Cells. 2025; 14(10):722. https://doi.org/10.3390/cells14100722
Chicago/Turabian StyleMa, Yajun, Lei Cao, Pujun Li, Zhihui Jiao, Xiaoning Liu, Xiangyu Lu, Tao Liu, and Hongbin Wang. 2025. "Effects of Adipose-Derived Mesenchymal Stem Cell-Secretome on Pyroptosis of Laparoscopic Hepatic Ischemia Reperfusion Injury in a Porcine Model" Cells 14, no. 10: 722. https://doi.org/10.3390/cells14100722
APA StyleMa, Y., Cao, L., Li, P., Jiao, Z., Liu, X., Lu, X., Liu, T., & Wang, H. (2025). Effects of Adipose-Derived Mesenchymal Stem Cell-Secretome on Pyroptosis of Laparoscopic Hepatic Ischemia Reperfusion Injury in a Porcine Model. Cells, 14(10), 722. https://doi.org/10.3390/cells14100722





