Reversible Modulation of Motile Cilia by a Benzo[e][1,2,4]triazinone: A Potential Non-Hormonal Approach to Male Contraception
Abstract
1. Introduction
2. Materials and Methods
2.1. General Methods and Materials
2.2. Stock Solutions
2.3. Xenopus laevis Embryo Manipulation and Microinjections
2.4. Chemical Deciliation of Embryos
2.5. Immunofluorescence
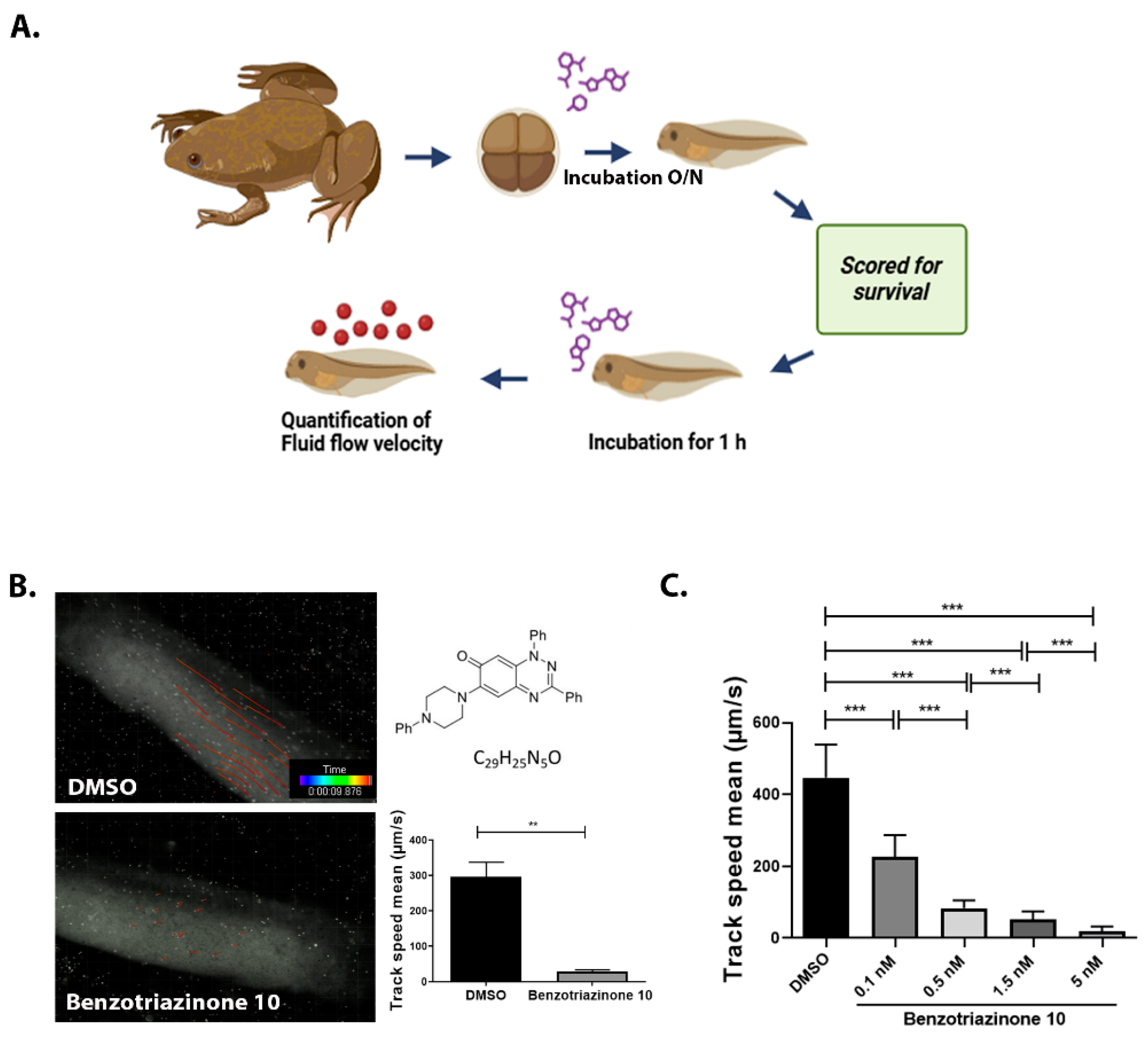
2.6. Assessment of Cilia Generated Fluid Flow
2.7. Quantifications and Statistics
3. Results
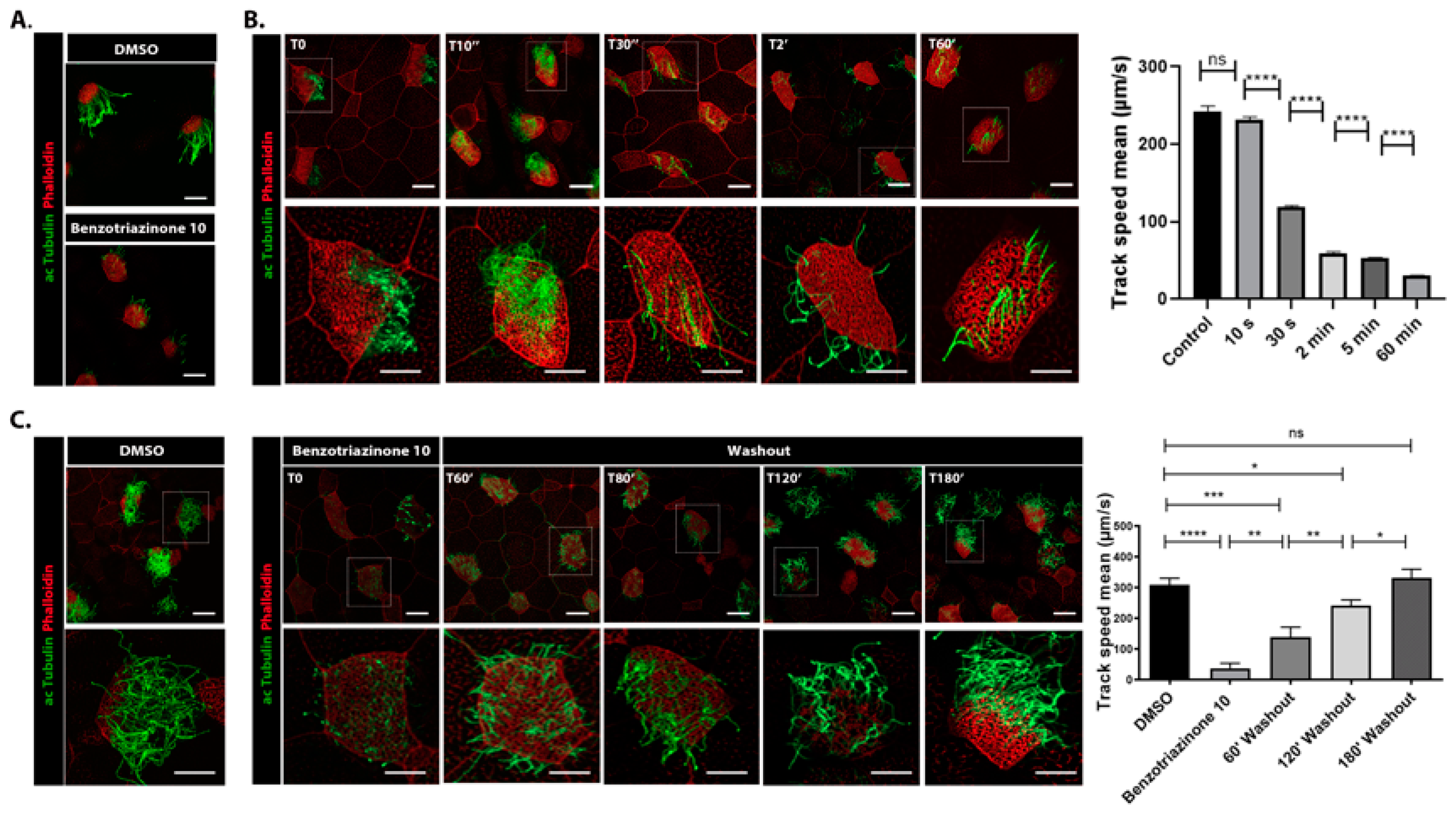
3.1. 1,3-Diphenyl-6-(4-phenylpiperazin-1-yl)benzo[e][1,2,4]triazin-7(1H)-one (10) Robustly and Reversibly Inhibits Fluid Flow Generation
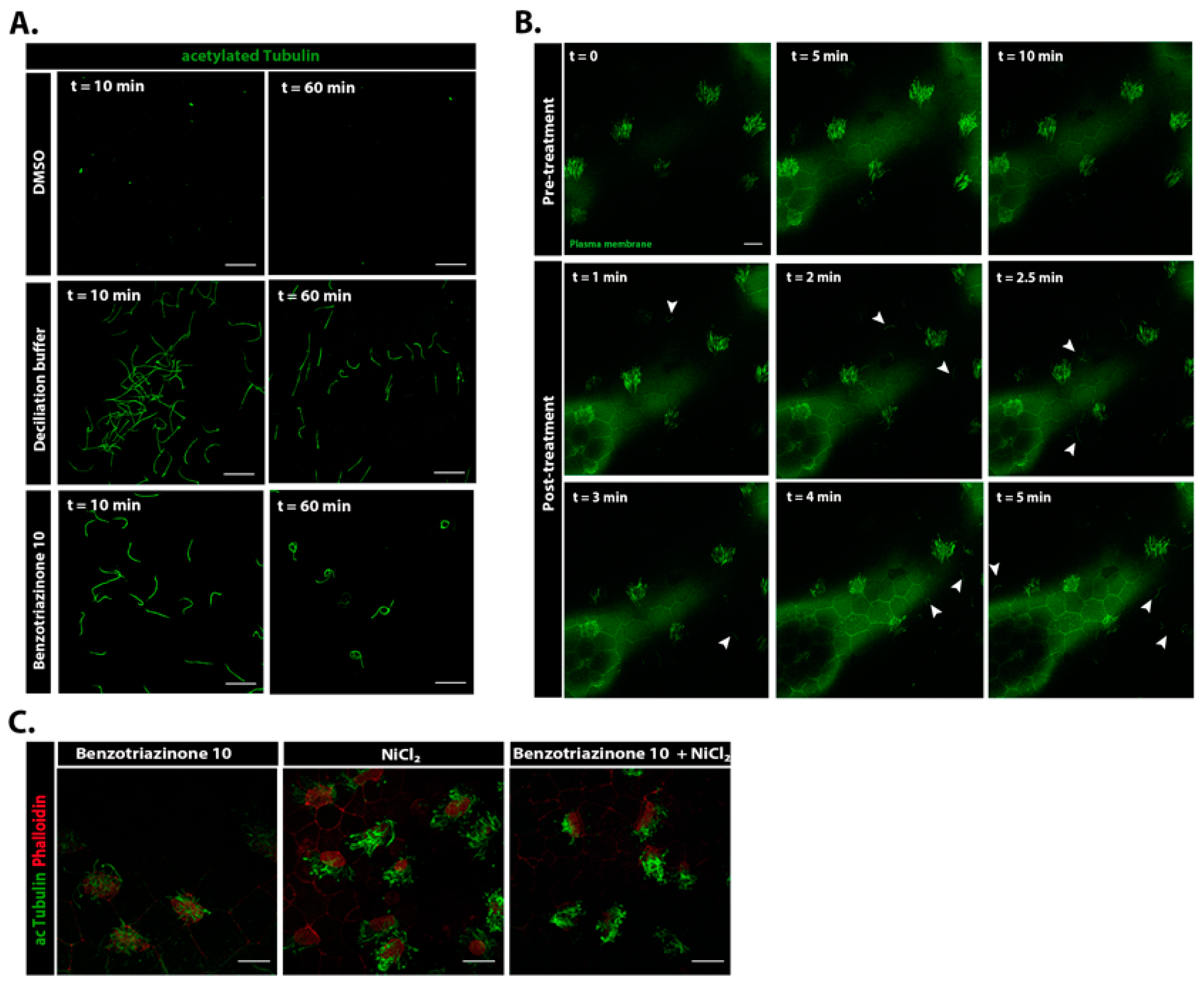
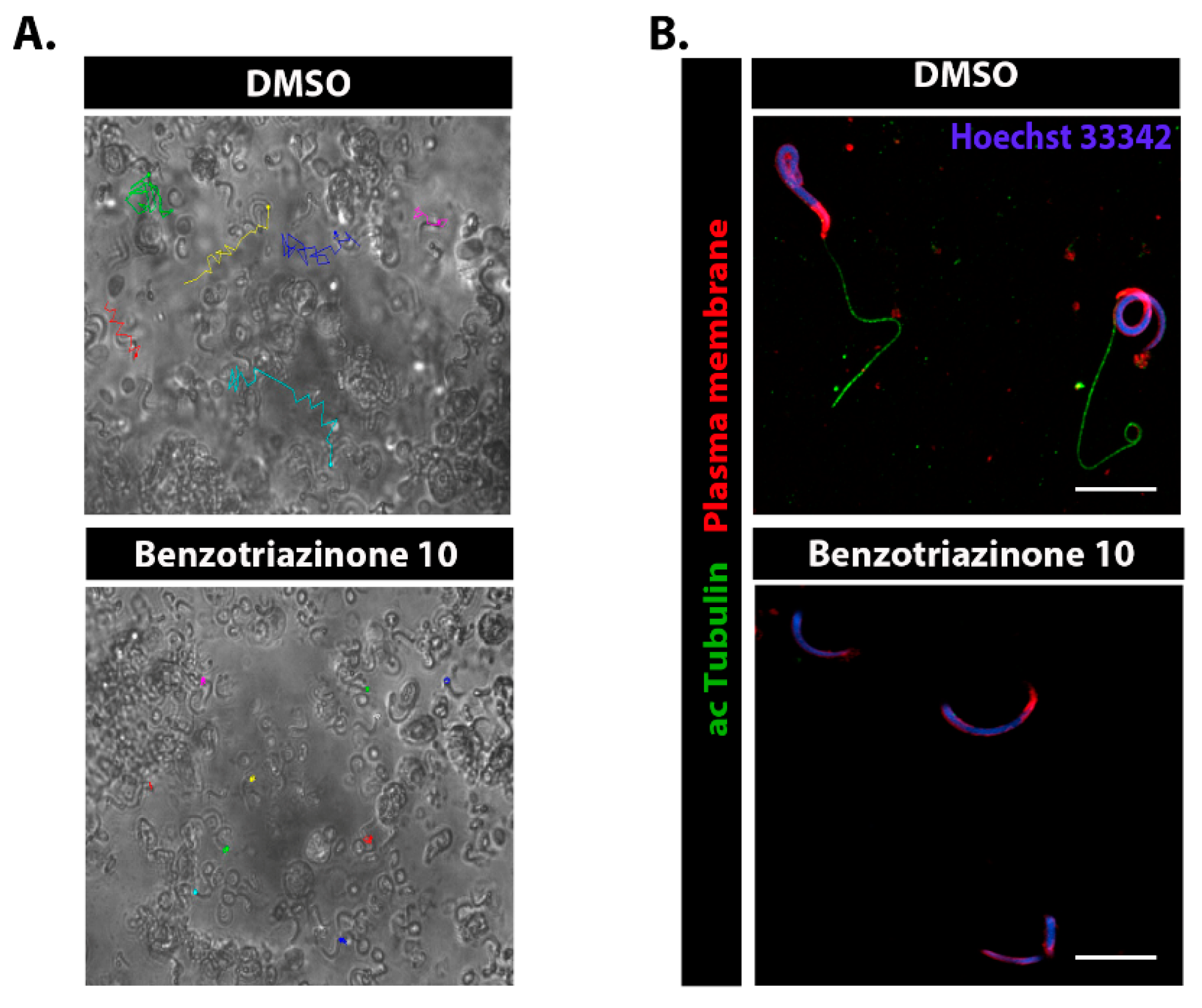
3.2. 1,3-Diphenyl-6-(4-phenylpiperazin-1-yl)benzo[e][1,2,4]triazin-7(1H)-one (10) Promotes Shear Stress-Driven Deciliation
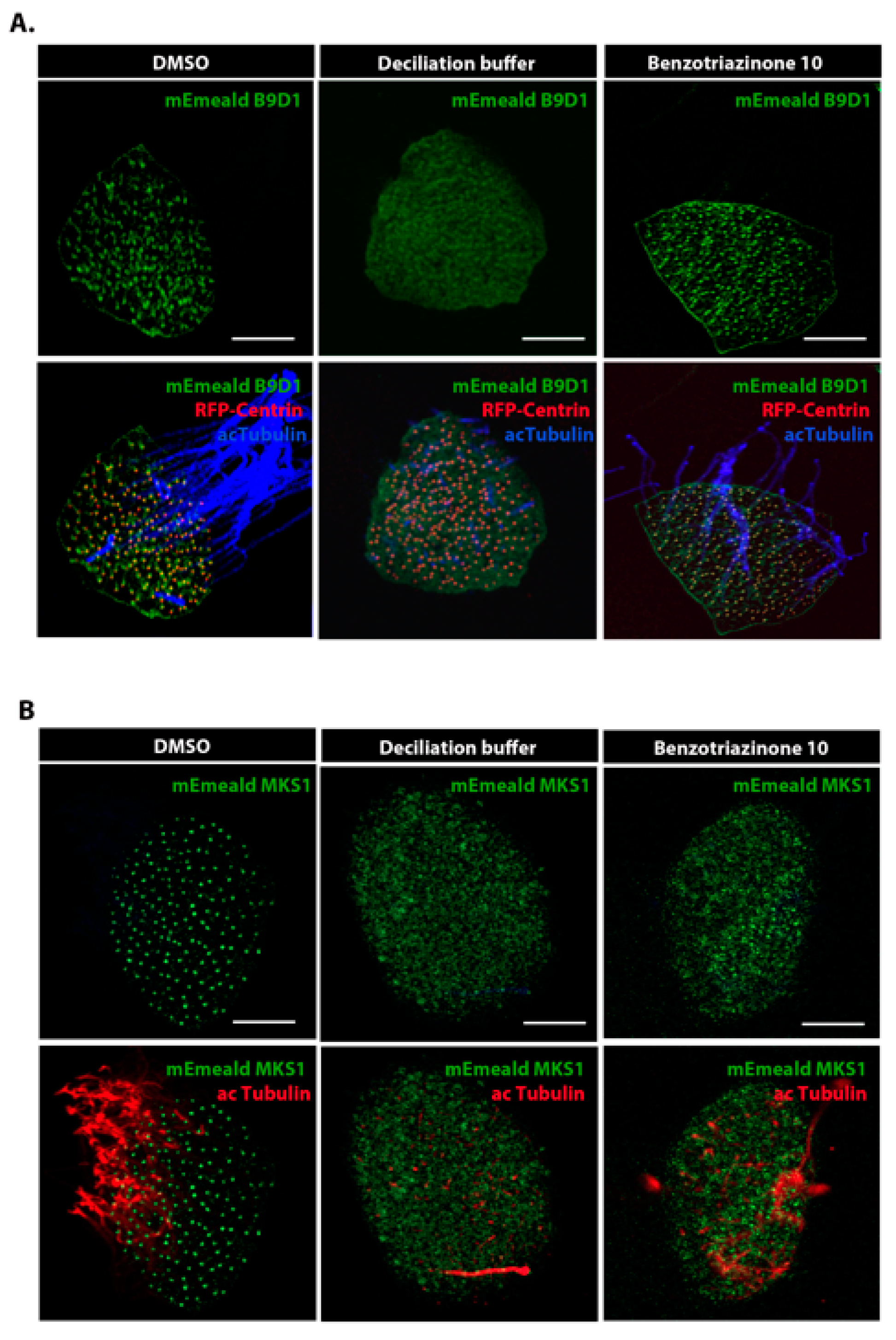
3.3. 1,3-Diphenyl-6-(4-phenylpiperazin-1-yl)benzo[e][1,2,4]triazin-7(1H)-one (10) Induces Cilia Shedding at the Distal Region of the Transition Zone
3.4. Structural Requirements for 1,3-Diphenyl-6-(4-phenylpiperazin-1-yl)benzo[e][1,2,4]triazin-7(1H)-one (10) Activity
3.5. 6-(4-Phenylpiperazin-1-yl)-Substituted Benzotriazinone 10 Inhibits Sperm Motility by Inducing Flagellar Loss
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quarmby, L.M. Cellular Deflagellation. Int. Rev. Cytol. 2004, 233, 47–91. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, J.L.; Witman, G.B. Intraflagellar Transport. Nat. Rev. Mol. Cell Biol. 2002, 3, 813–825. [Google Scholar] [CrossRef]
- Pugacheva, E.N.; Jablonski, S.A.; Hartman, T.R.; Henske, E.P.; Golemis, E.A. HEF1-Dependent Aurora A Activation Induces Disassembly of the Primary Cilium. Cell 2007, 129, 1351–1363. [Google Scholar] [CrossRef] [PubMed]
- Blum, J.J. Existence of a Breaking Point in Cilia and Flagella. J. Theor. Biol. 1971, 33, 257–263. [Google Scholar] [CrossRef]
- Tucker, R.W.; Scher, C.D.; Stiles, C.D. Centriole Deciliation Associated with the Early Response of 3T3 Cells to Growth Factors but Not to SV40. Cell 1979, 18, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Tucker, R.W.; Pardee, A.B.; Fujiwara, K. Centriole Ciliation Is Related to Quiescence and DNA Synthesis in 3T3 Cells. Cell 1979, 17, 527–535. [Google Scholar] [CrossRef]
- Huisgen, R.; Wulff, J. 1.3-Dipolare Cycloadditionen, LII. Umsetzungen von 1.3-Dipolen Mit Iminophosphoranen. Chem. Ber. 1969, 102, 1848–1858. [Google Scholar] [CrossRef]
- Zissimou, G.A.; Kourtellaris, A.; Manoli, M.; Koutentis, P.A. Redox Active Quinoidal 1,2,4-Benzotriazines. J. Org. Chem. 2018, 83, 9391–9402. [Google Scholar] [CrossRef]
- Koutentis, P.A.; Krassos, H.; Lo Re, D. 1,3-Diphenylbenzo[e][1,2,4]triazin-7(1H)-one: Selected Chemistry at the C-6, C-7 and C-8 Positions. Org. Biomol. Chem. 2011, 9, 5228–5237. [Google Scholar] [CrossRef]
- Ioannou, T.A.; Koutentis, P.A.; Krassos, H.; Loizou, G.; Lo Re, D. Some Cyclization Reactions of 1,3-Diphenylbenzo[e][1,2,4]triazin-7(1H)-one: Preparation and Computational Analysis of Non Symmetrical Zwitterionic Biscyanines. Org. Biomol. Chem. 2012, 10, 1339–1348. [Google Scholar] [CrossRef]
- Sweeney, M.; Coyle, R.; Kavanagh, P.; Berezin, A.A.; Lo Re, D.; Zissimou, G.A.; Koutentis, P.A.; Carty, M.P.; Aldabbagh, F. Discovery of Anti-Cancer Activity for Benzo[1,2,4]triazin-7-ones: Very Strong Correlation to Pleurotin and Thioredoxin Reductase Inhibition. Bioorg. Med. Chem. 2016, 24, 3565–3570. [Google Scholar] [CrossRef]
- Catto, M.; Berezin, A.A.; Lo Re, D.; Loizou, G.; Demetriades, M.; De Stradis, A.; Campagna, F.; Koutentis, P.A.; Carotti, A. Design, Synthesis and Biological Evaluation of Benzo[e][1,2,4]triazin-7(1H)-one and [1,2,4]-Triazino[5,6,1-jk]carbazol-6-one Derivatives as Dual Inhibitors of Beta-Amyloid Aggregation and Acetyl/Butyryl Cholinesterase. Eur. J. Med. Chem. 2012, 58, 84–97. [Google Scholar] [CrossRef]
- Koutentis, P.A.; Lo Re, D. Catalytic Oxidation of N-Phenylamidrazones to 1,3-Diphenyl-1,4-dihydro-1,2,4-benzotriazin-4-yls:An Improved Synthesis of Blatter’s Radical. Synthesis 2010, 2010, 2075–2079. [Google Scholar] [CrossRef]
- Koutentis, P.A.; Loizou, G.; Lo Re, D. Synthesis of Triazafluoranthenones via Silver(I)-Mediated Nonoxidative and Oxidative Intramolecular Palladium-Catalyzed Cyclizations. J. Org. Chem. 2011, 76, 5793–5802. [Google Scholar] [CrossRef]
- Dong, Y.; Chen, Y.; Zhang, Z.Y.; Qian, J.H.; Peng, Z.Z.; Chang, B.; Shi, Z.C.; Li, Z.H.; He, B. A One-Pot Access to 2-(N-Substituted Amino)-Quinones or 3-Indolyl-Quinones from Naphthol/Hydroquinone. Tetrahedron 2023, 135, 133337. [Google Scholar] [CrossRef]
- Chatzifrangkeskou, M.; Kouis, P.; Skourides, P.A. JNK Regulates Ciliogenesis through the Interflagellar Transport Complex and Actin Networks. J. Cell Biol. 2023, 222, e202303052. [Google Scholar] [CrossRef]
- Werner, M.E.; Mitchell, B.J. Using Xenopus Skin to Study Cilia Development and Function. Methods Enzymol. 2013, 525, 191–217. [Google Scholar] [CrossRef] [PubMed]
- Antoniades, I.; Koulle, A.; Chatzifrangkeskou, M.; Konstantinou, T.; Skourides, P. Development of a Xenopus-Based Assay for High-Throughput Evaluation of Mucociliary Flow. bioRxiv 2022. [Google Scholar] [CrossRef]
- Tian, X.; Liu, Z.; Niu, B.; Zhang, J.; Tan, T.K.; Lee, S.R.; Zhao, Y.; Harris, D.C.H.; Zheng, G. E-Cadherin/β-Catenin Complex and the Epithelial Barrier. J. Biomed. Biotechnol. 2011, 2011, 567305. [Google Scholar] [CrossRef]
- Valenta, T.; Hausmann, G.; Basler, K. The Many Faces and Functions of β-Catenin. EMBO J. 2012, 31, 2714–2736. [Google Scholar] [CrossRef]
- Romanelli, M.N.; Manetti, D.; Braconi, L.; Dei, S.; Gabellini, A.; Teodori, E. The Piperazine Scaffold for Novel Drug Discovery Efforts: The Evidence to Date. Expert Opin. Drug Discov. 2022, 17, 969–984. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.G.; Subramanianbalachandar, V.; Magaj, M.M.; Redemann, S.; Kulkarni, S.S. Mechanisms of Cilia Regeneration in Xenopus Multiciliated Epithelium in Vivo. bioRxiv Prepr. Serv. Biol. 2025, 26, 2192–2220. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhao, M.; Li, R.H.W.; Lin, Z.; Chung, J.P.W.; Li, T.C.; Lee, T.L.; Chan, D.Y.L. Effects of Nonoxynol-9 (N-9) on Sperm Functions: Systematic Review and Meta-Analysis. Reprod. Fertil. 2022, 3, R19–R33. [Google Scholar] [CrossRef] [PubMed]
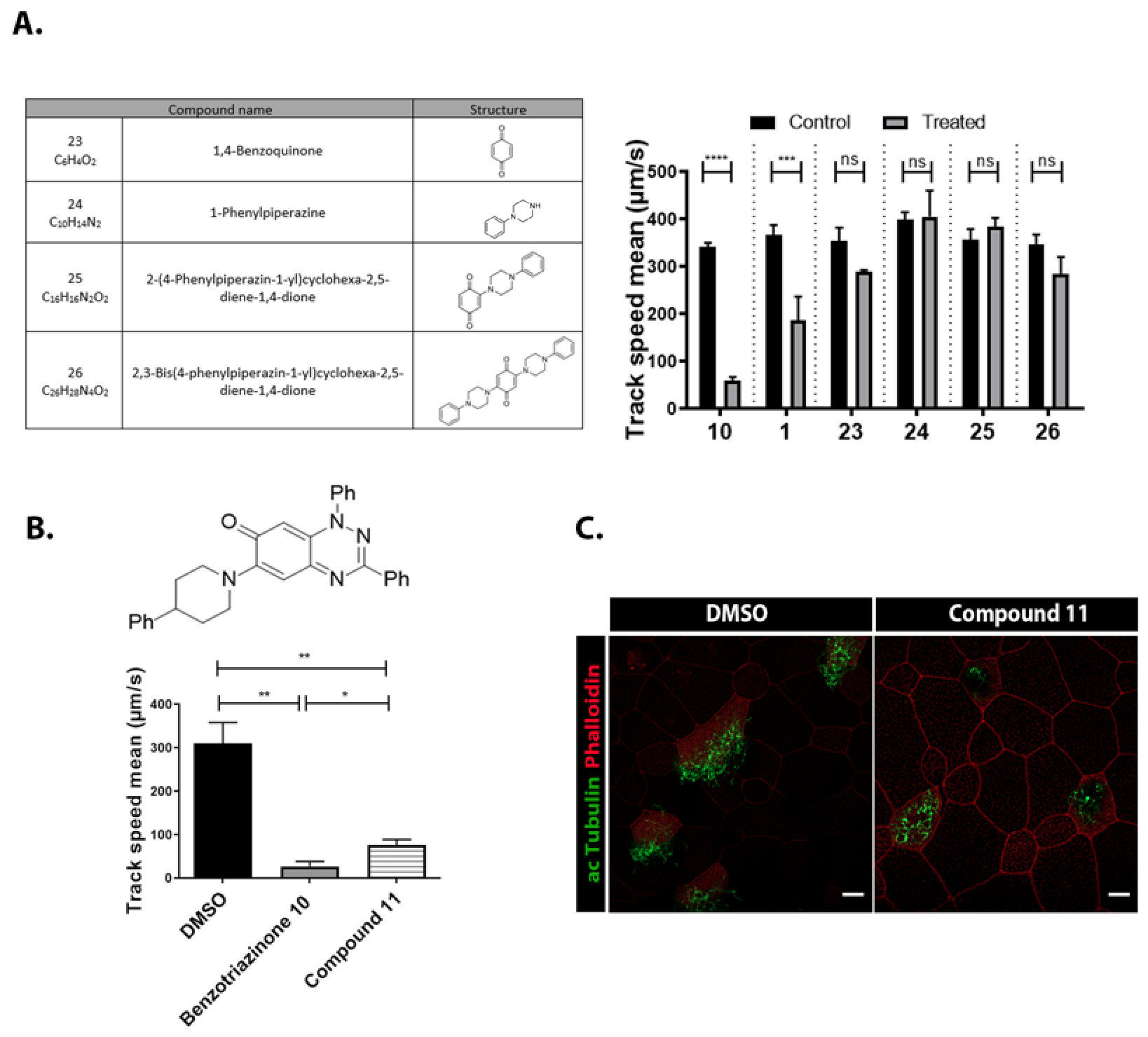
| Compound Number & Formula | Structure | Effect on Ciliary Function |
|---|---|---|
| 1 C19H13N3O | 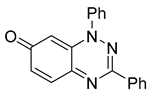 |  |
| 2 C14H8F3N3O | 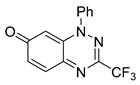 |  |
| 3 C19H14N4O |  |  |
| 4 C21H18N4O | 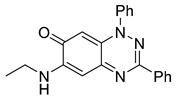 | 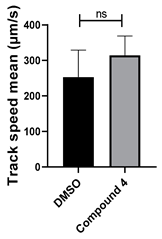 |
| 5 C25H24N4O |  | 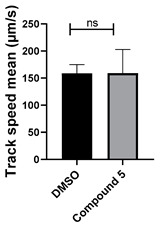 |
| 6 C25H24N4O | 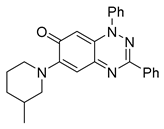 | 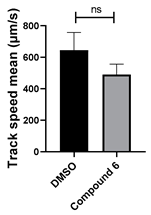 |
| 7 C25H24N4O | 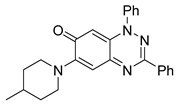 |  |
| 8 C23H20N4O2 |  | 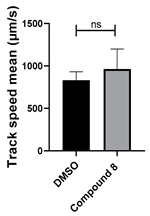 |
| 9 C24H23N5O |  | 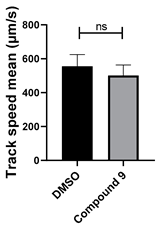 |
| 10 C29H25N5O | 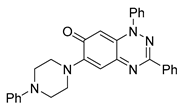 | 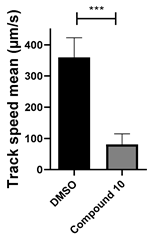 |
| 11 C30H26N4O |  |  |
| 12 C25H19N5O |  | 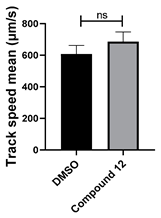 |
| 13 C25H27N5O |  | 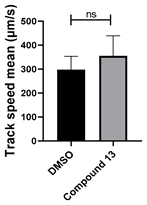 |
| 14 C20H15N3O |  |  |
| 15 C25H17N3OS | 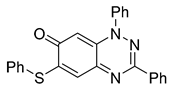 |  |
| 16 C23H15N3OS | 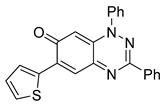 | 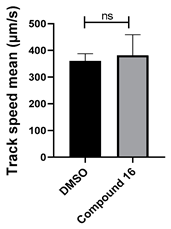 |
| 17 C23H18N4O |  |  |
| 18 C25H15N3OS |  |  |
| 19 C25H17N3O |  | 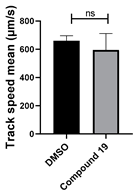 |
| 20 C26H19N3O2 |  | 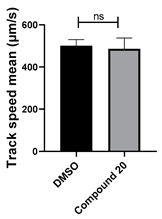 |
| 21 C25H16ClN3O |  | 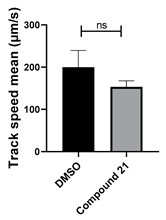 |
| 22 C23H15N3O2 |  | 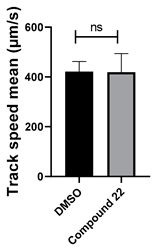 |
| Antibody | Catalogue Number | Dilution |
|---|---|---|
| Acetylated alpha tubulin | Santa Cruz Biotechnology (Dallas, TX, USA), sc-23950 | 1:500 |
| β-Tubulin (E7-s) | DSHB Hybridoma Products (Iowa City, IA, USA), AB2315513 | 1:500 |
| Beta-catenin | Sino Biological (Chesterbrook, PA, USA), 11279-R021 | 1:250 |
| Centrin 1 | Proteintech (Rosemont, IL, USA), 12794-1-AP | 1:500 |
| CellMask plasma membrane stain | Invitrogen, C10046 | 1:1000 |
| Phalloidin Alexa Fluor Plus 647 | Thermo Fischer Scientific | 1:500 |
| Secondary donkey anti-rabbit Alexa 488 | Invitrogen | 1:500 |
| Secondary donkey anti-mouse Alexa 488 | Invitrogen | 1:500 |
| Secondary donkey anti-mouse Alexa 647 | Invitrogen | 1:500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatzifrangkeskou, M.; Perdiou, A.; Kreouzou, R.; Zissimou, G.A.; Flesariu, D.F.; Koutentis, P.A.; Skourides, P.A. Reversible Modulation of Motile Cilia by a Benzo[e][1,2,4]triazinone: A Potential Non-Hormonal Approach to Male Contraception. Cells 2025, 14, 688. https://doi.org/10.3390/cells14100688
Chatzifrangkeskou M, Perdiou A, Kreouzou R, Zissimou GA, Flesariu DF, Koutentis PA, Skourides PA. Reversible Modulation of Motile Cilia by a Benzo[e][1,2,4]triazinone: A Potential Non-Hormonal Approach to Male Contraception. Cells. 2025; 14(10):688. https://doi.org/10.3390/cells14100688
Chicago/Turabian StyleChatzifrangkeskou, Maria, Alexandra Perdiou, Revekka Kreouzou, Georgia A. Zissimou, Dragos F. Flesariu, Panayiotis A. Koutentis, and Paris A. Skourides. 2025. "Reversible Modulation of Motile Cilia by a Benzo[e][1,2,4]triazinone: A Potential Non-Hormonal Approach to Male Contraception" Cells 14, no. 10: 688. https://doi.org/10.3390/cells14100688
APA StyleChatzifrangkeskou, M., Perdiou, A., Kreouzou, R., Zissimou, G. A., Flesariu, D. F., Koutentis, P. A., & Skourides, P. A. (2025). Reversible Modulation of Motile Cilia by a Benzo[e][1,2,4]triazinone: A Potential Non-Hormonal Approach to Male Contraception. Cells, 14(10), 688. https://doi.org/10.3390/cells14100688






