Effects of Repeated Cisplatin and Monosodium Glutamate on Visceral Sensitivity in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
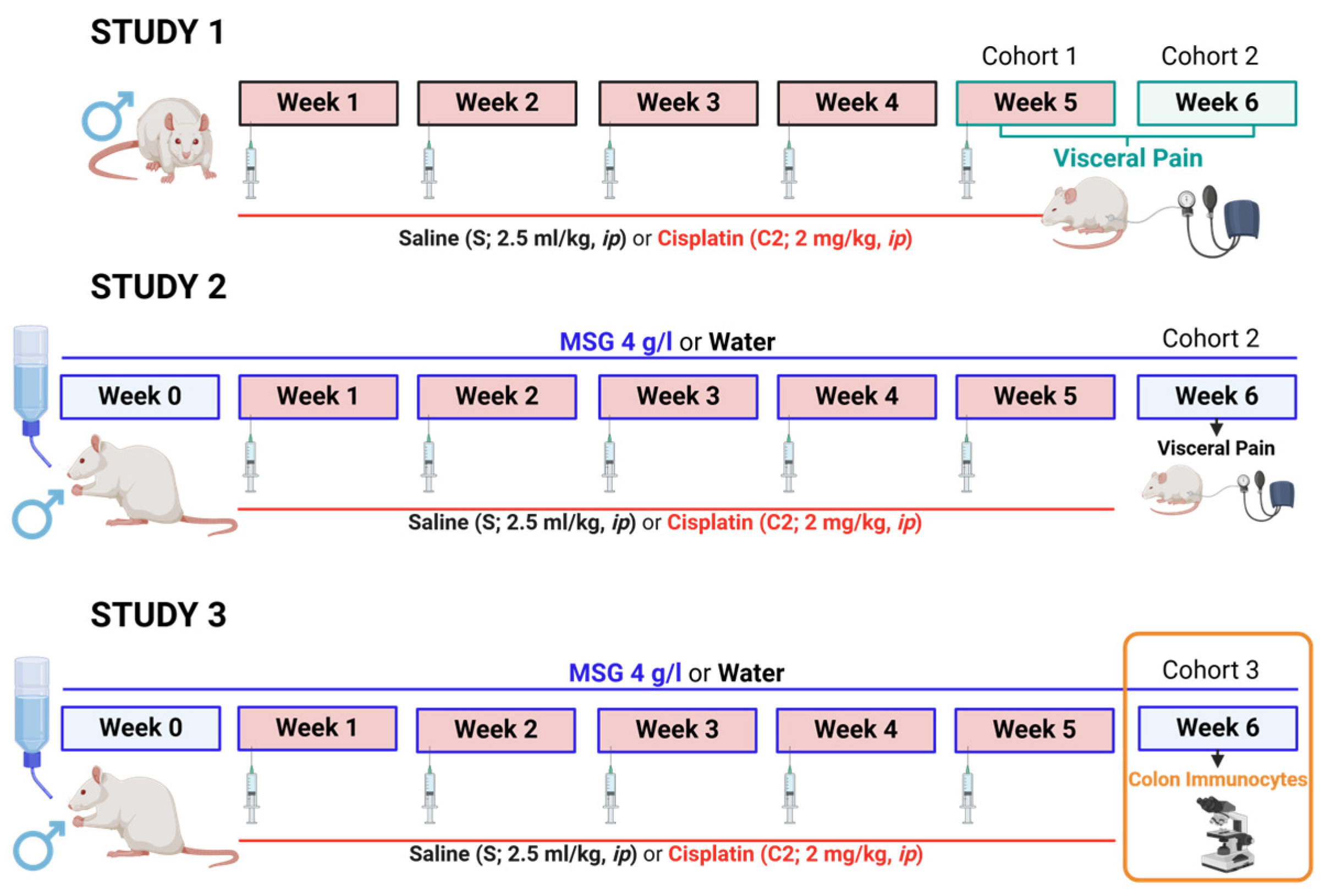
2.2. Experimental Protocol
2.2.1. Study 1
2.2.2. Study 2
2.2.3. Study 3
2.3. Assessment of Colorectal Sensitivity
2.4. Evaluation of Colonic Immunocytes
2.5. Compounds and Drugs
2.6. Statistical Analysis
3. Results
3.1. Effects of Cisplatin on Colorectal Sensitivity (Study 1)
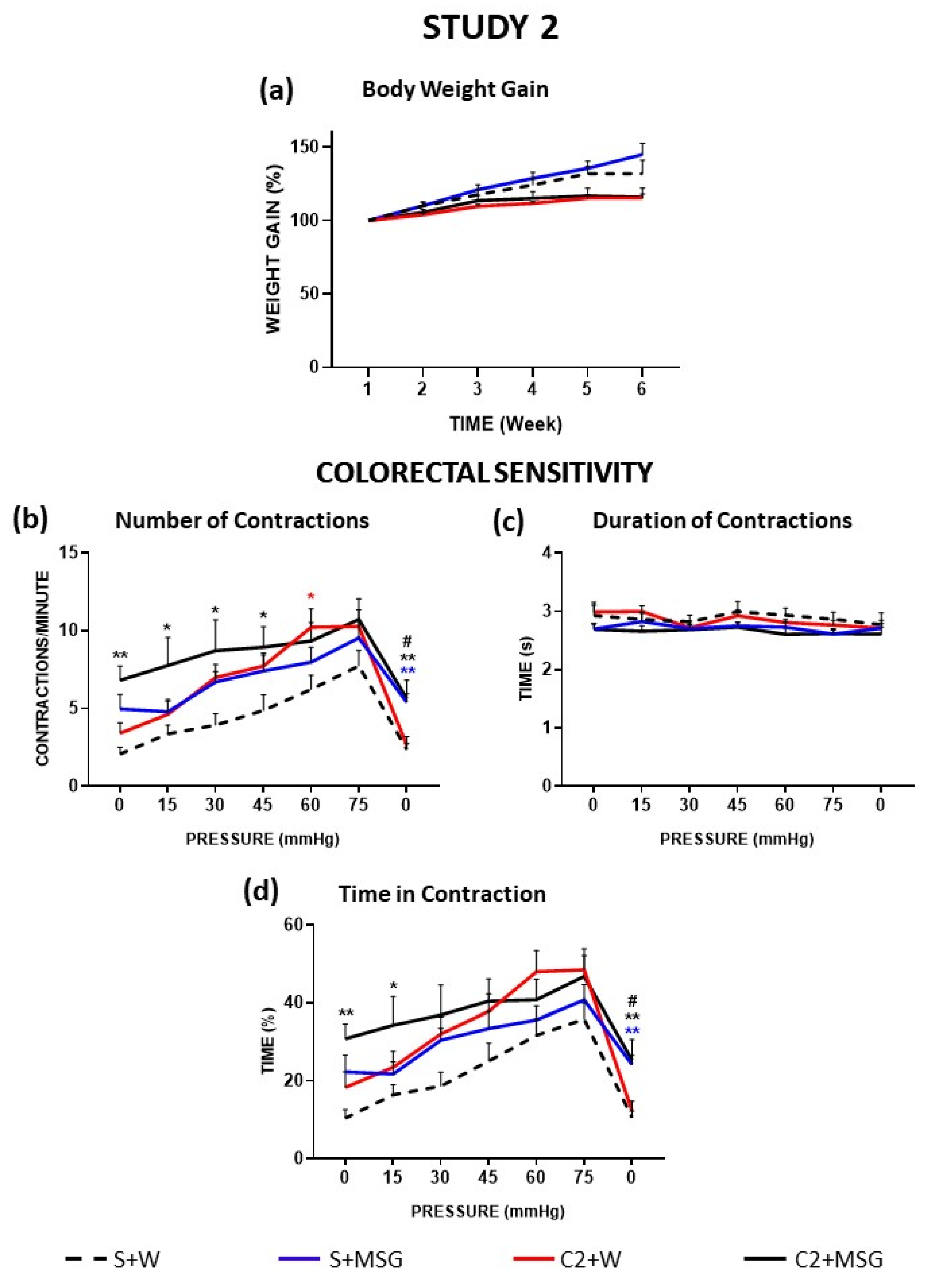
3.2. Effects of Monosodium Glutamate on Colorectal Sensitivity (Study 2)
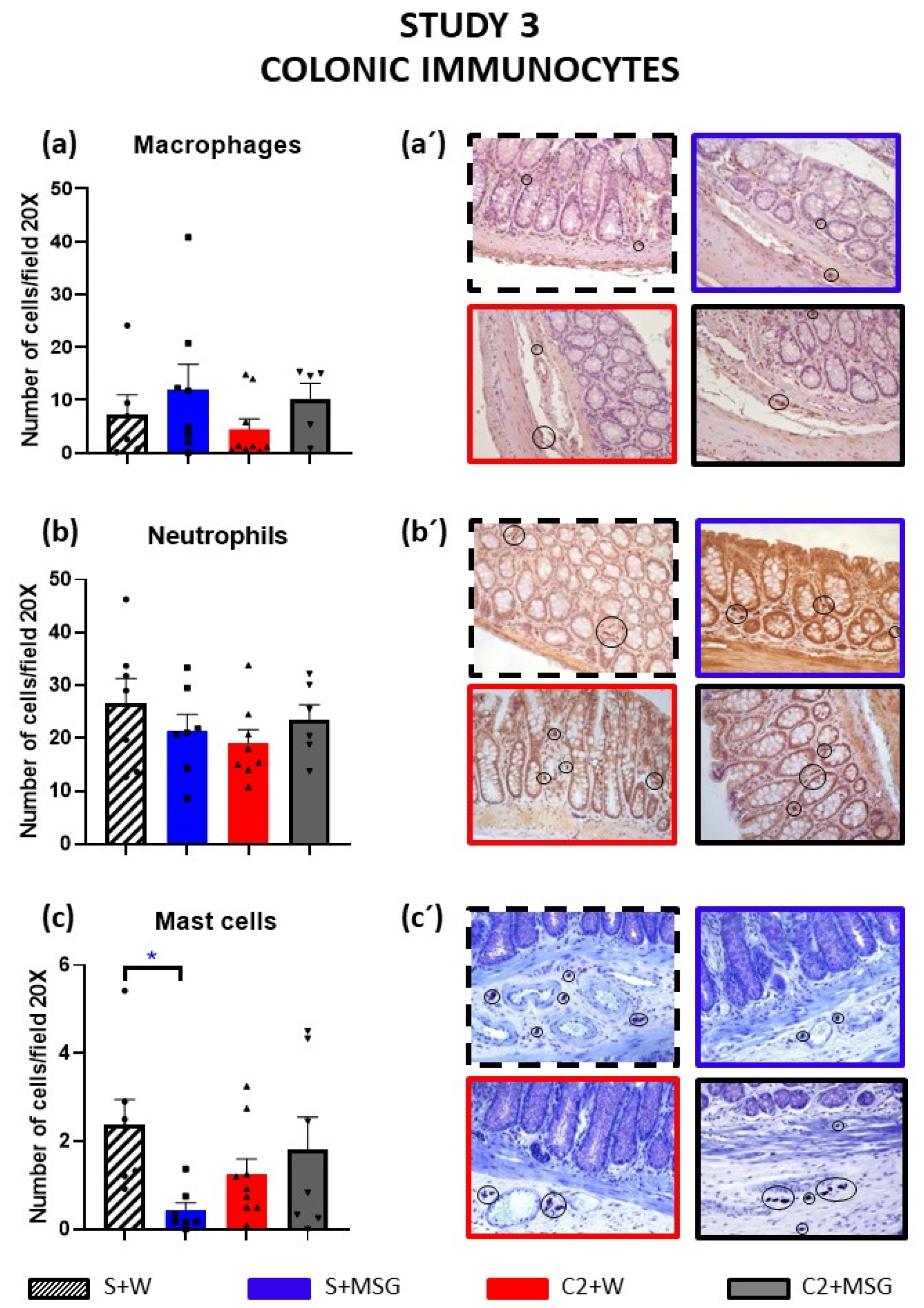
3.3. Effects of Cisplatin and Monosodium Glutamate on Colonic Immunocytes (Study 3)
4. Discussion
4.1. Effects of Cisplatin on Body Weight Gain and Visceral Pain
4.2. Effects of MSG on Cisplatin-Induced Alterations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Cutsem, E.; Arends, J. The causes and consequences of cancer-associated malnutrition. Eur. J. Oncol. Nurs. 2005, 9 (Suppl. S2), S51–S63. [Google Scholar] [CrossRef] [PubMed]
- Cinausero, M.; Aprile, G.; Ermacora, P.; Basile, D.; Vitale, M.G.; Fanotto, V.; Parisi, G.; Calvetti, L.; Sonis, S.T. New Frontiers in the Pathobiology and Treatment of Cancer Regimen-Related Mucosal Injury. Front. Pharmacol. 2017, 8, 354. [Google Scholar] [CrossRef] [PubMed]
- Kerckhove, N.; Collin, A.; Condé, S.; Chaleteix, C.; Pezet, D.; Balayssac, D. Long-Term Effects, Pathophysiological Mechanisms, and Risk Factors of Chemotherapy-Induced Peripheral Neuropathies: A Comprehensive Literature Review. Front. Pharmacol. 2017, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, B.L. Delayed Chemotherapy-Induced Nausea and Vomiting: Pathogenesis, Incidence, and Current Management. Front. Pharmacol. 2017, 8, 19. [Google Scholar] [CrossRef]
- Was, H.; Borkowska, A.; Bagues, A.; Tu, L.; Liu, J.Y.H.; Lu, Z.; Rudd, J.A.; Nurgali, K.; Abalo, R. Mechanisms of Chemotherapy-Induced Neurotoxicity. Front. Pharmacol. 2022, 13, 750507. [Google Scholar] [CrossRef]
- Navari, R.M. Prevention of emesis from multiple-day and high-dose chemotherapy regimens. J. Natl. Compr. Cancer Netw. 2007, 5, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Bagues, A.; López-Tofiño, Y.; Llorente-Berzal, Á.; Abalo, R. Cannabinoid drugs against chemotherapy-induced adverse effects: Focus on nausea/vomiting, peripheral neuropathy and chemofog in animal models. Behav. Pharmacol. 2022, 33, 105–129. [Google Scholar] [CrossRef] [PubMed]
- Yoshiya, T.; Mimae, T.; Ito, M.; Sasada, S.; Tsutani, Y.; Satoh, K.; Masuda, T.; Miyata, Y.; Hattori, N.; Okada, M. Prospective, randomized, cross-over pilot study of the effects of Rikkunshito, a Japanese traditional herbal medicine, on anorexia and plasma-acylated ghrelin levels in lung cancer patients undergoing cisplatin-based chemotherapy. Investig. New Drugs 2020, 38, 485–492. [Google Scholar] [CrossRef]
- Hattori, T.; Yakabi, K.; Takeda, H. Cisplatin-induced anorexia and ghrelin. Vitam. Horm. 2013, 92, 301–317. [Google Scholar] [CrossRef]
- Scarborough, B.M.; Smith, C.B. Optimal pain management for patients with cancer in the modern era. CA Cancer J. Clin. 2018, 68, 182–196. [Google Scholar] [CrossRef]
- Authier, N.; Gillet, J.P.; Fialip, J.; Eschalier, A.; Coudore, F. An animal model of nociceptive peripheral neuropathy following repeated cisplatin injections. Exp. Neurol. 2003, 182, 12–20. [Google Scholar] [CrossRef]
- Vera, G.; Cabezos, P.A.; Martín, M.I.; Abalo, R. Characterization of cannabinoid-induced relief of neuropathic pain in a rat model of cisplatin-induced neuropathy. Pharmacol. Biochem. Behav. 2013, 105, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Viana-Cardoso, K.V.; da Silva, M.T.; Júnior, R.C.; Peixoto Junior, A.A.; Pinho, L.G.; Santos, A.A.; Ribeiro, R.A.; Rola, F.H.; Gondim Fde, A. Repeated cisplatin treatments inhibit gastrointestinal motility and induces baroreflex changes and mechanical hyperalgesia in rats. Cancer Investig. 2011, 29, 494–500. [Google Scholar] [CrossRef]
- Feng, B.; Guo, T. Visceral pain from colon and rectum: The mechanotransduction and biomechanics. J. Neural Transm. 2020, 127, 415–429. [Google Scholar] [CrossRef]
- Bielefeldt, K.; Christianson, J.A.; Davis, B.M. Basic and clinical aspects of visceral sensation: Transmission in the CNS. Neurogastroenterol. Motil. 2005, 17, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Grundy, L.; Erickson, A.; Brierley, S.M. Visceral Pain. Annu. Rev. Physiol. 2019, 81, 261–284. [Google Scholar] [CrossRef] [PubMed]
- Vera, G.; Castillo, M.; Cabezos, P.A.; Chiarlone, A.; Martín, M.I.; Gori, A.; Pasquinelli, G.; Barbara, G.; Stanghellini, V.; Corinaldesi, R.; et al. Enteric neuropathy evoked by repeated cisplatin in the rat. Neurogastroenterol. Motil. 2011, 23, 370–378, e162–e163. [Google Scholar] [CrossRef] [PubMed]
- Uranga, J.A.; García-Martínez, J.M.; García-Jiménez, C.; Vera, G.; Martín-Fontelles, M.I.; Abalo, R. Alterations in the small intestinal wall and motor function after repeated cisplatin in rat. Neurogastroenterol. Motil. 2017, 29, e13047. [Google Scholar] [CrossRef]
- Barbara, G.; Stanghellini, V.; De Giorgio, R.; Corinaldesi, R. Functional gastrointestinal disorders and mast cells: Implications for therapy. Neurogastroenterol. Motil. 2006, 18, 6–17. [Google Scholar] [CrossRef]
- Domoto, R.; Sekiguchi, F.; Tsubota, M.; Kawabata, A. Macrophage as a Peripheral Pain Regulator. Cells 2021, 10, 1881. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, T.; Shiotani, A. The Cutting Edge Research of Functional Gastrointestinal Disorders in Japan: Review on JGA Core Symposium 2018–2020. Digestion 2021, 102, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Rossato, M.F.; Rigo, F.K.; Oliveira, S.M.; Guerra, G.P.; Silva, C.R.; Cunha, T.M.; Gomez, M.V.; Ferreira, J.; Trevisan, G. Participation of transient receptor potential vanilloid 1 in paclitaxel-induced acute visceral and peripheral nociception in rodents. Eur. J. Pharmacol. 2018, 828, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Martín-Ruíz, M.; Uranga, J.A.; Mosinska, P.; Fichna, J.; Nurgali, K.; Martín-Fontelles, M.I.; Abalo, R. Alterations of colonic sensitivity and gastric dysmotility after acute cisplatin and granisetron. Neurogastroenterol. Motil. 2019, 31, e13499. [Google Scholar] [CrossRef] [PubMed]
- Jinap, S.; Hajeb, P. Glutamate. Its applications in food and contribution to health. Appetite 2010, 55, 1–10. [Google Scholar] [CrossRef]
- Behrens, M.; Meyerhof, W.; Hellfritsch, C.; Hofmann, T. Sweet and umami taste: Natural products, their chemosensory targets, and beyond. Angew. Chem. Int. Ed. Engl. 2011, 50, 2220–2242. [Google Scholar] [CrossRef]
- Bellisle, F. Glutamate and the UMAMI taste: Sensory, metabolic, nutritional and behavioural considerations. A review of the literature published in the last 10 years. Neurosci. Biobehav. Rev. 1999, 23, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Tapiero, H.; Mathé, G.; Couvreur, P.; Tew, K.D. II. Glutamine and glutamate. Biomed. Pharmacother. 2002, 56, 446–457. [Google Scholar] [CrossRef]
- Newsholme, P.; Procopio, J.; Lima, M.M.; Pithon-Curi, T.C.; Curi, R. Glutamine and glutamate–their central role in cell metabolism and function. Cell Biochem. Funct. 2003, 21, 1–9. [Google Scholar] [CrossRef]
- Bak, L.K.; Schousboe, A.; Waagepetersen, H.S. The glutamate/GABA-glutamine cycle: Aspects of transport, neurotransmitter homeostasis and ammonia transfer. J. Neurochem. 2006, 98, 641–653. [Google Scholar] [CrossRef]
- Shono, H.; Tsutsumi, R.; Beppu, K.; Matsushima, R.; Watanabe, S.; Fujimoto, C.; Kanamura, R.; Ohnishi, H.; Kondo, E.; Azuma, T.; et al. Dietary Supplementation with Monosodium Glutamate Suppresses Chemotherapy-Induced Downregulation of the T1R3 Taste Receptor Subunit in Head and Neck Cancer Patients. Nutrients 2021, 13, 2921. [Google Scholar] [CrossRef] [PubMed]
- Kendig, D.M.; Hurst, N.R.; Bradley, Z.L.; Mahavadi, S.; Kuemmerle, J.F.; Lyall, V.; DeSimone, J.; Murthy, K.S.; Grider, J.R. Activation of the umami taste receptor (T1R1/T1R3) initiates the peristaltic reflex and pellet propulsion in the distal colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 307, G1100–G1107. [Google Scholar] [CrossRef]
- Crowe, M.S.; Wang, H.; Blakeney, B.A.; Mahavadi, S.; Singh, K.; Murthy, K.S.; Grider, J.R. Expression and function of umami receptors T1R1/T1R3 in gastric smooth muscle. Neurogastroenterol. Motil. 2020, 32, e13737. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, H.; Shimizu, T.; Yogo, H.; Nishimiya, Y.; Hori, S.; Kosugi, T.; Nakayama, S. Gastric emptying and duodenal motility upon intake of a liquid meal with monosodium glutamate in healthy subjects. Physiol. Rep. 2014, 2, e00187. [Google Scholar] [CrossRef] [PubMed]
- Boyle, F.M.; Wheeler, H.R.; Shenfield, G.M. Amelioration of experimental cisplatin and paclitaxel neuropathy with glutamate. J. Neurooncol. 1999, 41, 107–116. [Google Scholar] [CrossRef]
- Bhadri, N.; Sanji, T.; Madakasira Guggilla, H.; Razdan, R. Amelioration of behavioural, biochemical, and neurophysiological deficits by combination of monosodium glutamate with resveratrol/alpha-lipoic acid/coenzyme Q10 in rat model of cisplatin-induced peripheral neuropathy. Sci. World J. 2013, 2013, 565813. [Google Scholar] [CrossRef]
- López-Tofiño, Y.; Vera, G.; López-Gómez, L.; Girón, R.; Nurgali, K.; Uranga, J.A.; Abalo, R. Effects of the food additive monosodium glutamate on cisplatin-induced gastrointestinal dysmotility and peripheral neuropathy in the rat. Neurogastroenterol. Motil. 2021, 33, e14020. [Google Scholar] [CrossRef] [PubMed]
- López-Miranda, V.; Soto-Montenegro, M.L.; Uranga-Ocio, J.A.; Vera, G.; Herradón, E.; González, C.; Blas, C.; Martínez-Villaluenga, M.; López-Pérez, A.E.; Desco, M.; et al. Effects of chronic dietary exposure to monosodium glutamate on feeding behavior, adiposity, gastrointestinal motility, and cardiovascular function in healthy adult rats. Neurogastroenterol. Motil. 2015, 27, 1559–1570. [Google Scholar] [CrossRef]
- Jacenik, D.; Bagüés, A.; López-Gómez, L.; López-Tofiño, Y.; Iriondo-DeHond, A.; Serra, C.; Banovcanová, L.; Gálvez-Robleño, C.; Fichna, J.; Del Castillo, M.D.; et al. Changes in Fatty Acid Dietary Profile Affect the Brain-Gut Axis Functions of Healthy Young Adult Rats in a Sex-Dependent Manner. Nutrients 2021, 13, 1864. [Google Scholar] [CrossRef]
- Cervero, F. Visceral hyperalgesia revisited. Lancet 2000, 356, 1127–1128. [Google Scholar] [CrossRef] [PubMed]
- Ta, L.E.; Espeset, L.; Podratz, J.; Windebank, A.J. Neurotoxicity of oxaliplatin and cisplatin for dorsal root ganglion neurons correlates with platinum-DNA binding. Neurotoxicology 2006, 27, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Santos, N.A.G.D.; Ferreira, R.S.; Santos, A.C.D. Overview of cisplatin-induced neurotoxicity and ototoxicity, and the protective agents. Food Chem. Toxicol. 2020, 136, 111079. [Google Scholar] [CrossRef]
- Alotaibi, M.; Al-Aqil, F.; Alqahtani, F.; Alanazi, M.; Nadeem, A.; Ahmad, S.F.; Lapresa, R.; Alharbi, M.; Alshammari, A.; Alotaibi, M.; et al. Alleviation of cisplatin-induced neuropathic pain, neuronal apoptosis, and systemic inflammation in mice by rapamycin. Front. Aging Neurosci. 2022, 14, 891593. [Google Scholar] [CrossRef]
- Boyer, J.; Saint-Paul, M.C.; Dadone, B.; Patouraux, S.; Vivinus, M.H.; Ouvrier, D.; Michiels, J.F.; Piche, T.; Tulic, M.K. Inflammatory cell distribution in colon mucosa as a new tool for diagnosis of irritable bowel syndrome: A promising pilot study. Neurogastroenterol. Motil. 2018, 30, e13223. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, T.; Oshima, T.; Huang, X.; Tomita, T.; Fukui, H.; Miwa, H. Microinflammation in the intestinal mucosa and symptoms of irritable bowel syndrome. J. Gastroenterol. 2022, 57, 62–69. [Google Scholar] [CrossRef]
- Lauritano, D.; Mastrangelo, F.; D’Ovidio, C.; Ronconi, G.; Caraffa, A.; Gallenga, C.E.; Frydas, I.; Kritas, S.K.; Trimarchi, M.; Carinci, F.; et al. Activation of Mast Cells by Neuropeptides: The Role of Pro-Inflammatory and Anti-Inflammatory Cytokines. Int. J. Mol. Sci. 2023, 24, 4811. [Google Scholar] [CrossRef]
- Riaz, B.; Sohn, S. Neutrophils in Inflammatory Diseases: Unraveling the Impact of Their Derived Molecules and Heterogeneity. Cells 2023, 12, 2621. [Google Scholar] [CrossRef]
- Castro, J.; Harrington, A.M.; Chegini, F.; Matusica, D.; Spencer, N.J.; Brierley, S.M.; Haberberger, R.V.; Barry, C.M. Clodronate Treatment Prevents Vaginal Hypersensitivity in a Mouse Model of Vestibulodynia. Front. Cell Infect. Microbiol. 2022, 11, 784972. [Google Scholar] [CrossRef]
- Yantiss, R.K. Eosinophils in the GI tract: How many is too many and what do they mean? Mod. Pathol. 2015, 28 (Suppl. S1), S7–S21. [Google Scholar] [CrossRef] [PubMed]
- Jordan, K.; Kasper, C.; Schmoll, H.J. Chemotherapy-induced nausea and vomiting: Current and new standards in the antiemetic prophylaxis and treatment. Eur. J. Cancer 2005, 41, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Andrews, P.L.; Horn, C.C. Signals for nausea and emesis: Implications for models of upper gastrointestinal diseases. Auton. Neurosci. 2006, 125, 100–115. [Google Scholar] [CrossRef]
- Andrews, P.L.; Sanger, G.J. Nausea and the quest for the perfect anti-emetic. Eur. J. Pharmacol. 2014, 722, 108–121. [Google Scholar] [CrossRef] [PubMed]
- De Jonghe, B.C.; Horn, C.C. Chemotherapy-Induced Pica and Anorexia Are Reduced by Common Hepatic Branch Va-gotomy in the Rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R756–R765. [Google Scholar] [CrossRef] [PubMed]
- Sanger, G.J.; Andrews, P.L.R. A History of Drug Discovery for Treatment of Nausea and Vomiting and the Implications for Future Research. Front. Pharmacol. 2018, 9, 913. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Dong, X.; Liu, Z.L.; Mo, J.Z.; Fang, J.Y.; Xiao, S.D.; Li, Y.; Chen, S.L. Luminal serotonin time-dependently modulates vagal afferent driven antinociception in response to colorectal distention in rats. Neurogastroenterol. Motil. 2011, 23, 62-e6. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.C.; Yan, X.J.; Chen, X.; Wang, E.M.; Liu, Q.; Zhang, L.Y.; Chen, J.; Fang, J.Y.; Chen, S.L. Vagal anandamide signaling via cannabinoid receptor 1 contributes to luminal 5-HT modulation of visceral nociception in rats. Pain 2014, 155, 1591–1604. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.Y.; Yang, Y.T.; She, M.P.; Tu, C.H.; Lee, T.C.; Wu, M.S.; Sun, C.H.; Hsin, L.W.; Yu, L.C. 5-HT7 receptor-dependent intestinal neurite outgrowth contributes to visceral hypersensitivity in irritable bowel syndrome. Lab. Investig. 2022, 102, 1023–1037. [Google Scholar] [CrossRef]
- Ben Rehouma, M.; Kfoury, T.; Hamdi, L.; Bouchouareb, M.; Soued, M.; Benhamou, D.; Mazoit, J.X. Acute Visceral Pain in Rats: Vagal Nerve Block Compared to Bupivacaine Administered Intramuscularly. Anesth. Analg. 2021, 133, 1311–1320. [Google Scholar] [CrossRef]
- Gottfried-Blackmore, A.; Habtezion, A.; Nguyen, L. Noninvasive vagal nerve stimulation for gastroenterology pain disorders. Pain. Manag. 2021, 11, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Soffer, E. Electroceuticals for Neurogastroenterology and Motility Disorders. Curr. Gastroenterol. Rep. 2023, 25, 91–97. [Google Scholar] [CrossRef]
- Ossipov, M.H.; Morimura, K.; Porreca, F. Descending pain modulation and chronification of pain. Curr. Opin. Support. Palliat. Care 2014, 8, 143–151. [Google Scholar] [CrossRef]
- López-Tofiño, Y.; de Sosa, F.; Vera, G.; López-Gómez, L.; Herradón, E.; López-Miranda, V.; Nurgali, K.; Uranga, J.A.; Abalo, R. Effects of vincristine and monosodium glutamate on gastrointestinal motility and visceral sensitivity. Neurogastroenterol. Motil. 2024, 36, e14704. [Google Scholar] [CrossRef]
- Willard, S.S.; Koochekpour, S. Glutamate, glutamate receptors, and downstream signaling pathways. Int. J. Biol. Sci. 2013, 9, 948–959. [Google Scholar] [CrossRef] [PubMed]
- Pereira, V.; Goudet, C. Emerging Trends in Pain Modulation by Metabotropic Glutamate Receptors. Front. Mol. Neurosci. 2019, 11, 464. [Google Scholar] [CrossRef]
- Frisby, C.L.; Mattsson, J.P.; Jensen, J.M.; Lehmann, A.; Dent, J.; Blackshaw, L.A. Inhibition of transient lower esophageal sphincter relaxation and gastroesophageal reflux by metabotropic glutamate receptor ligands. Gastroenterology 2005, 129, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.; Lehmann, A.; Uvebrant, A.; Carlsson, A.; Jerndal, G.; Nilsson, K.; Frisby, C.; Blackshaw, L.A.; Mattsson, J.P. Transient lower esophageal sphincter relaxations in dogs are inhibited by a metabotropic glutamate receptor 5 antagonist. Eur. J. Pharmacol. 2005, 519, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Slattery, J.A.; Page, A.J.; Dorian, C.L.; Brierley, S.M.; Blackshaw, L.A. Potentiation of mouse vagal afferent mechanosensitivity by ionotropic and metabotropic glutamate receptors. J. Physiol. 2006, 577 Pt 1, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.Z.; Ren, J.; Liu, S.; Gao, C.; Xia, Y.; Wood, J.D. Functional group I metabotropic glutamate receptors in submucous plexus of guinea-pig ileum. Br. J. Pharmacol. 1999, 128, 1631–1635. [Google Scholar] [CrossRef]
- Ferrigno, A.; Berardo, C.; Di Pasqua, L.G.; Siciliano, V.; Richelmi, P.; Vairetti, M. Localization and role of metabotropic glutamate receptors subtype 5 in the gastrointestinal tract. World J. Gastroenterol. 2017, 23, 4500–4507. [Google Scholar] [CrossRef] [PubMed]
- Nasser, Y.; Keenan, C.M.; Ma, A.C.; McCafferty, D.M.; Sharkey, K.A. Expression of a functional metabotropic glutamate receptor 5 on enteric glia is altered in states of inflammation. Glia 2007, 55, 859–872. [Google Scholar] [CrossRef] [PubMed]
- Lindström, E.; Brusberg, M.; Hughes, P.A.; Martin, C.M.; Brierley, S.M.; Phillis, B.D.; Martinsson, R.; Abrahamsson, C.; Larsson, H.; Martinez, V.; et al. Involvement of metabotropic glutamate 5 receptor in visceral pain. Pain 2008, 137, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Zanfirescu, A.; Ungurianu, A.; Tsatsakis, A.M.; Nițulescu, G.M.; Kouretas, D.; Veskoukis, A.; Tsoukalas, D.; Engin, A.B.; Aschner, M.; Margină, D. A review of the alleged health hazards of monosodium glutamate. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1111–1134. [Google Scholar] [CrossRef] [PubMed]
- Peláez, B.; Blázquez, J.L.; Pastor, F.E.; Sánchez, A.; Amat, P. Lectin histochemistry and ultrastructure of microglial response to monosodium glutamate-mediated neurotoxicity in the arcuate nucleus. Histol. Histopathol. 1999, 14, 165–174. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Tofiño, Y.; López-Gómez, L.; Martín-Ruíz, M.; Uranga, J.A.; Nurgali, K.; Vera, G.; Abalo, R. Effects of Repeated Cisplatin and Monosodium Glutamate on Visceral Sensitivity in Rats. Cells 2025, 14, 26. https://doi.org/10.3390/cells14010026
López-Tofiño Y, López-Gómez L, Martín-Ruíz M, Uranga JA, Nurgali K, Vera G, Abalo R. Effects of Repeated Cisplatin and Monosodium Glutamate on Visceral Sensitivity in Rats. Cells. 2025; 14(1):26. https://doi.org/10.3390/cells14010026
Chicago/Turabian StyleLópez-Tofiño, Yolanda, Laura López-Gómez, Marta Martín-Ruíz, Jose Antonio Uranga, Kulmira Nurgali, Gema Vera, and Raquel Abalo. 2025. "Effects of Repeated Cisplatin and Monosodium Glutamate on Visceral Sensitivity in Rats" Cells 14, no. 1: 26. https://doi.org/10.3390/cells14010026
APA StyleLópez-Tofiño, Y., López-Gómez, L., Martín-Ruíz, M., Uranga, J. A., Nurgali, K., Vera, G., & Abalo, R. (2025). Effects of Repeated Cisplatin and Monosodium Glutamate on Visceral Sensitivity in Rats. Cells, 14(1), 26. https://doi.org/10.3390/cells14010026








