Characterization of the Astrocyte Calcium Response to Norepinephrine in the Ventral Tegmental Area
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. AAV Delivery
2.3. Brain Slice Preparation
2.4. Ca2+ Imaging Experiments and Analysis
2.5. Immunohistochemistry and Cell Counting
2.6. Drugs
2.7. Data Analysis
3. Results
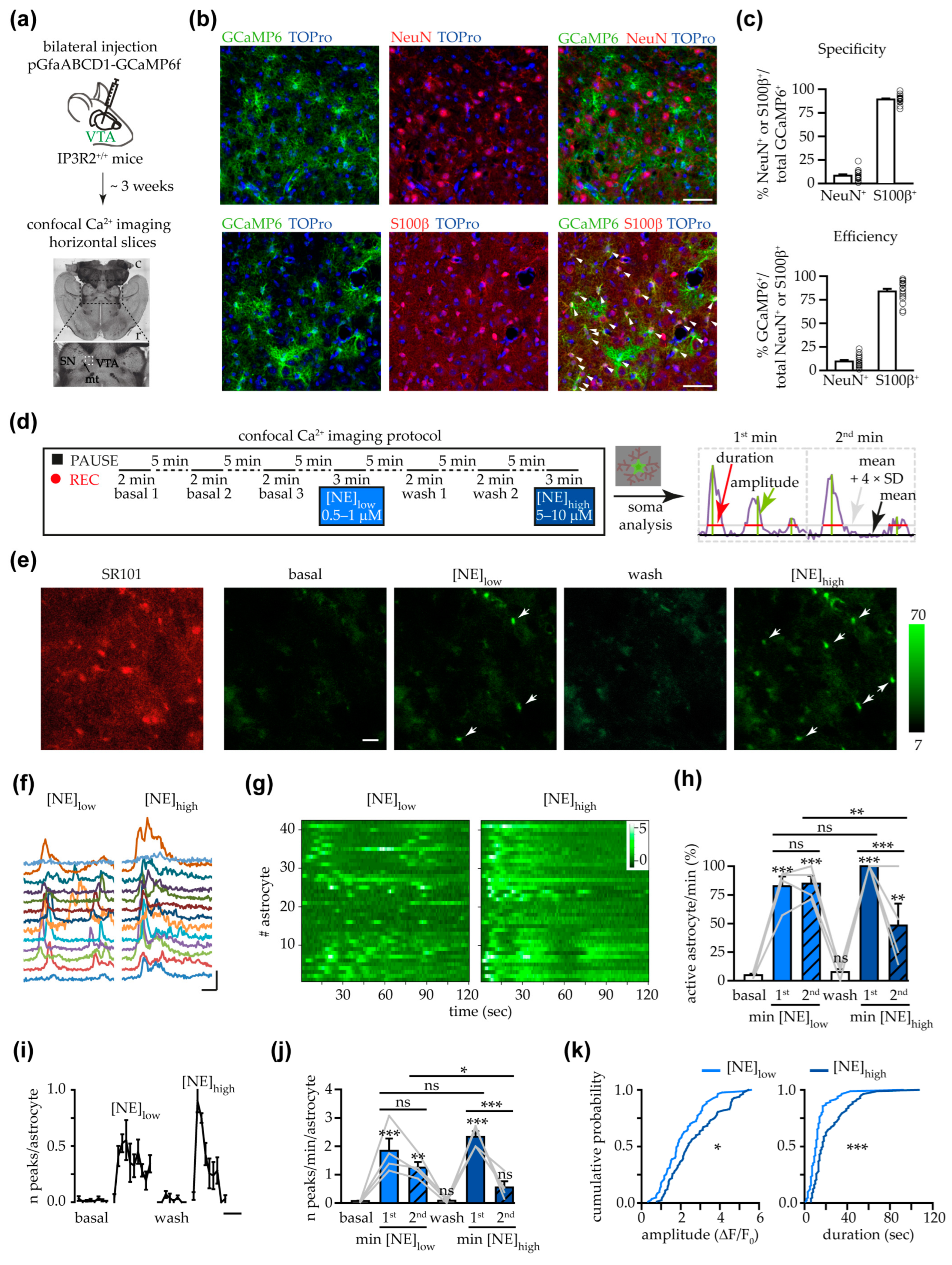
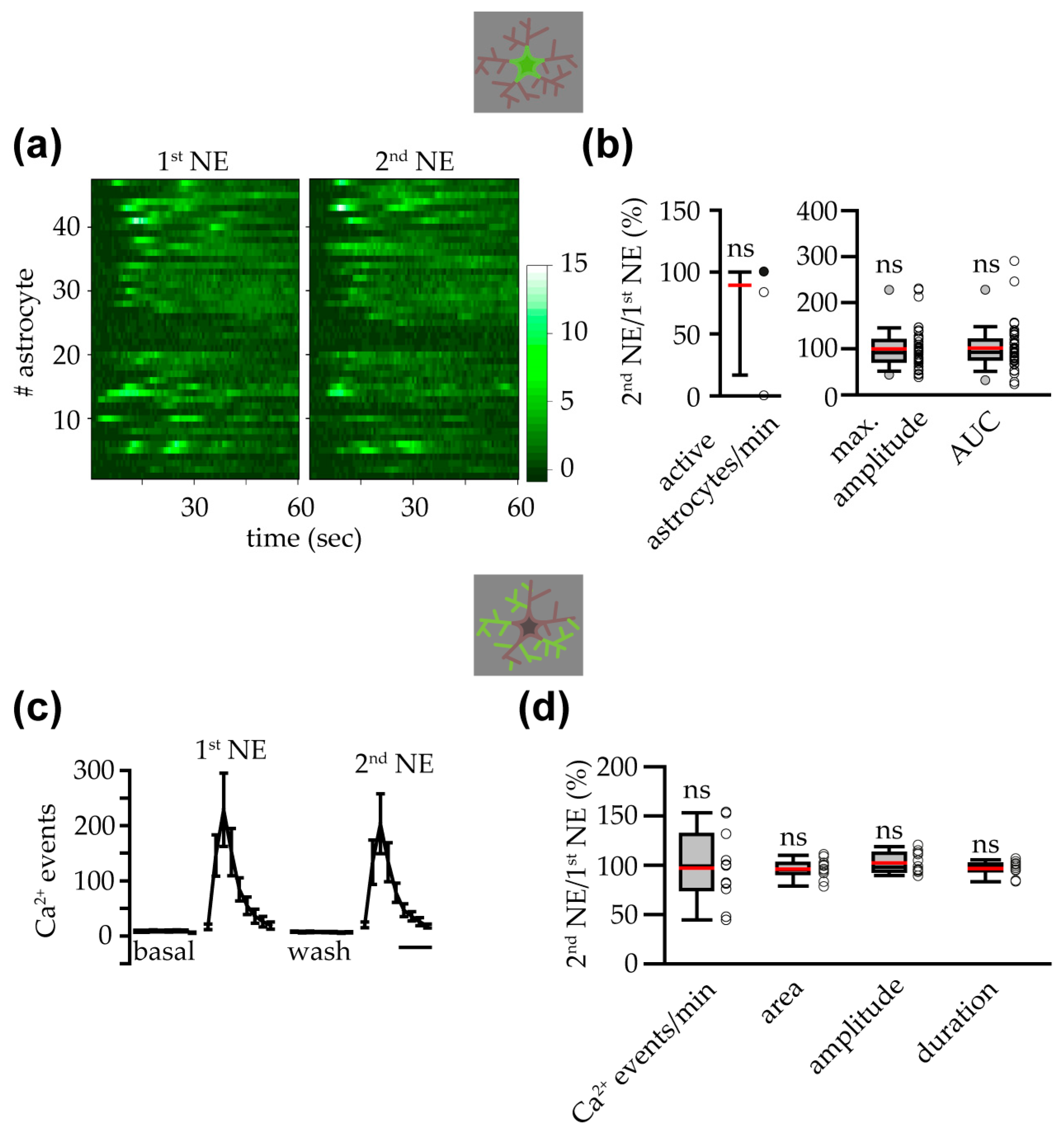
3.1. NE Triggered Somatic Ca2+ Increases in VTA Astrocytes
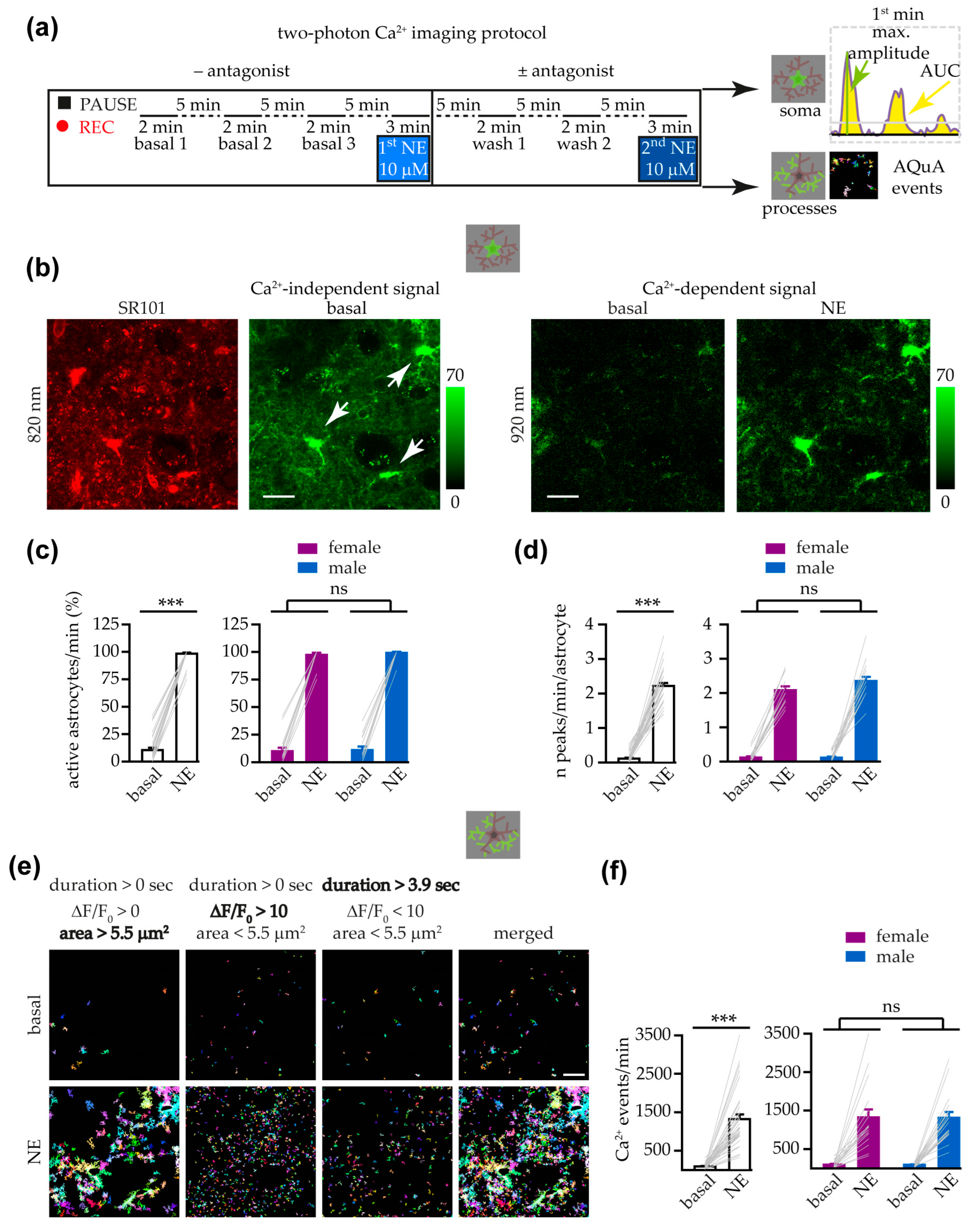
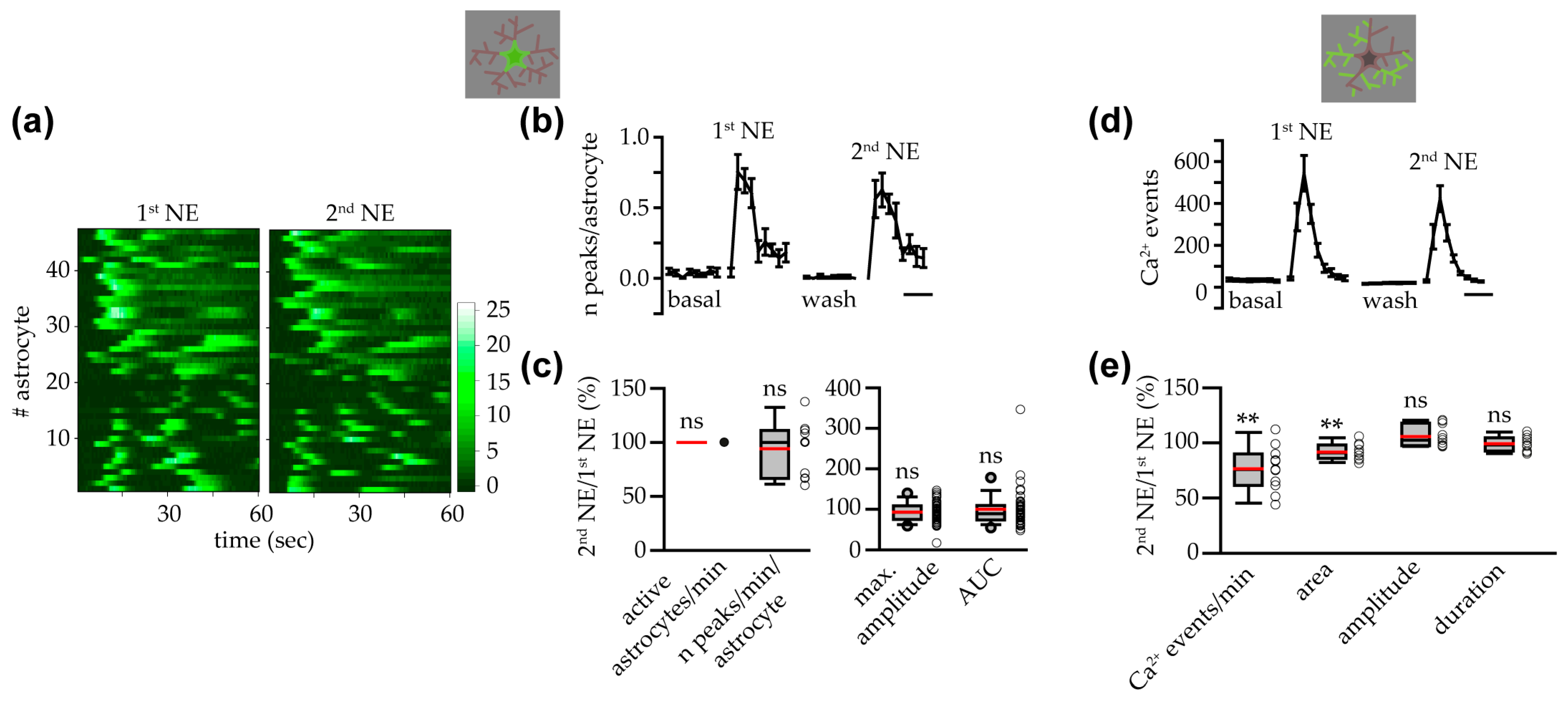
3.2. Astrocyte Response to NE at Soma and Processes Was Similar in Female and Male Mice
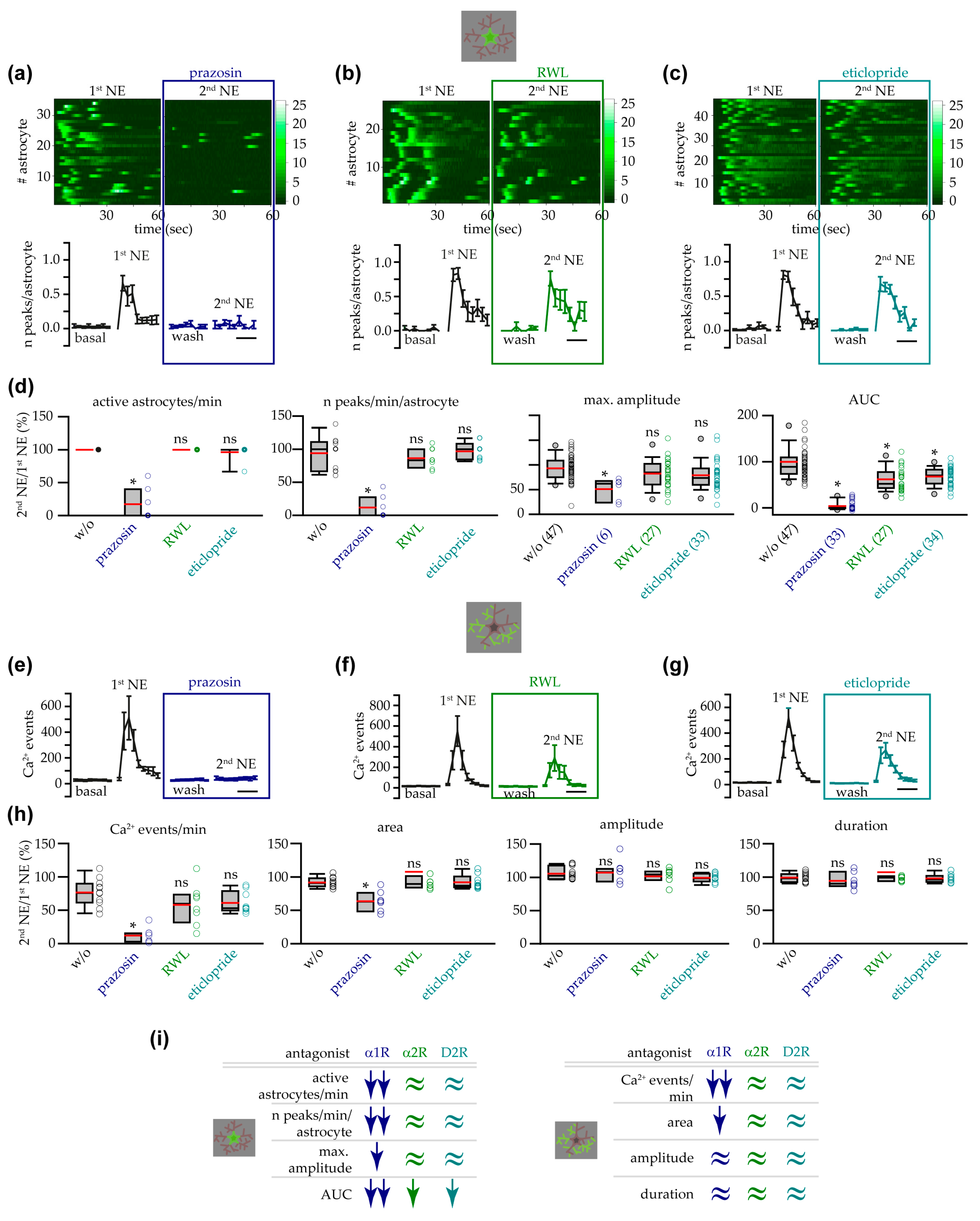
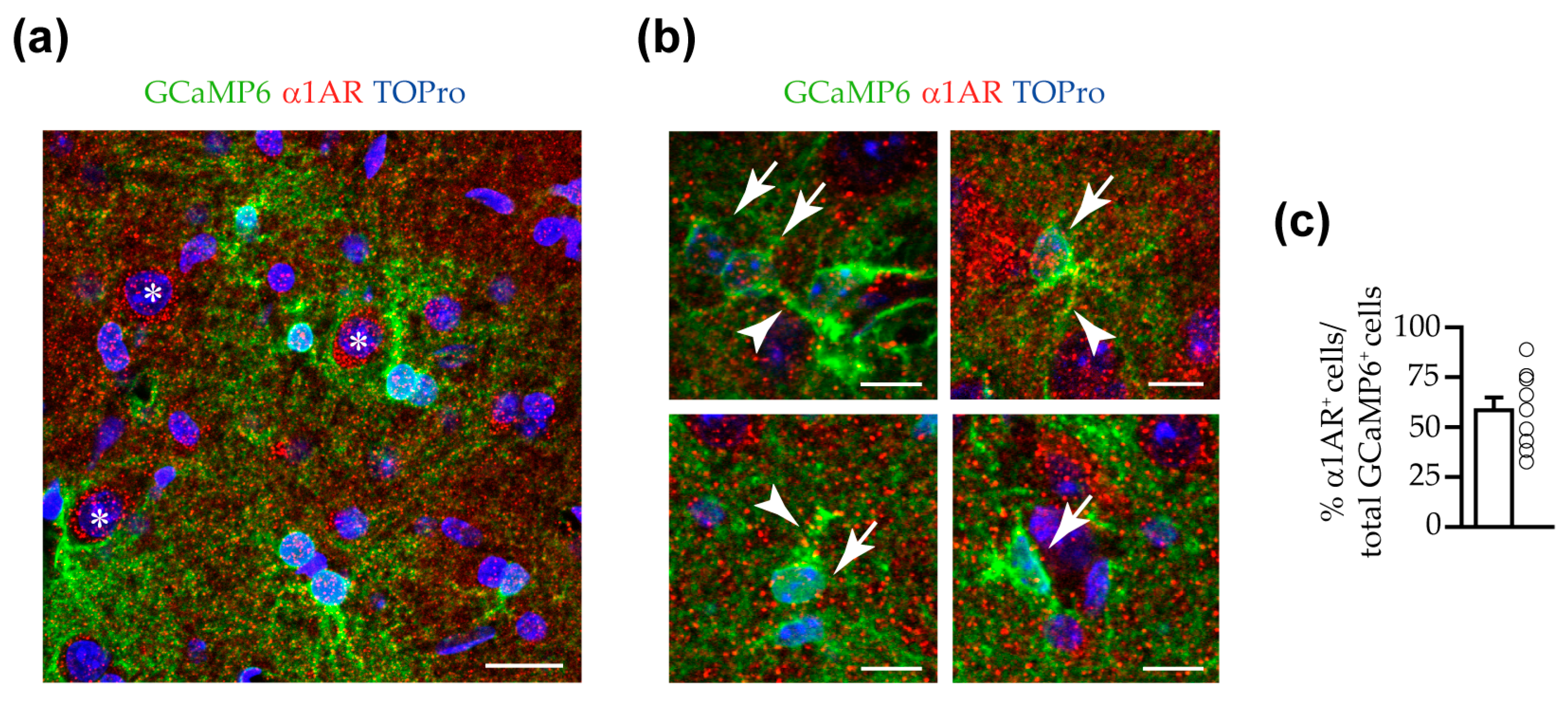
3.3. α1 ARs Mediated VTA Astrocyte Ca2+ Responses to NE
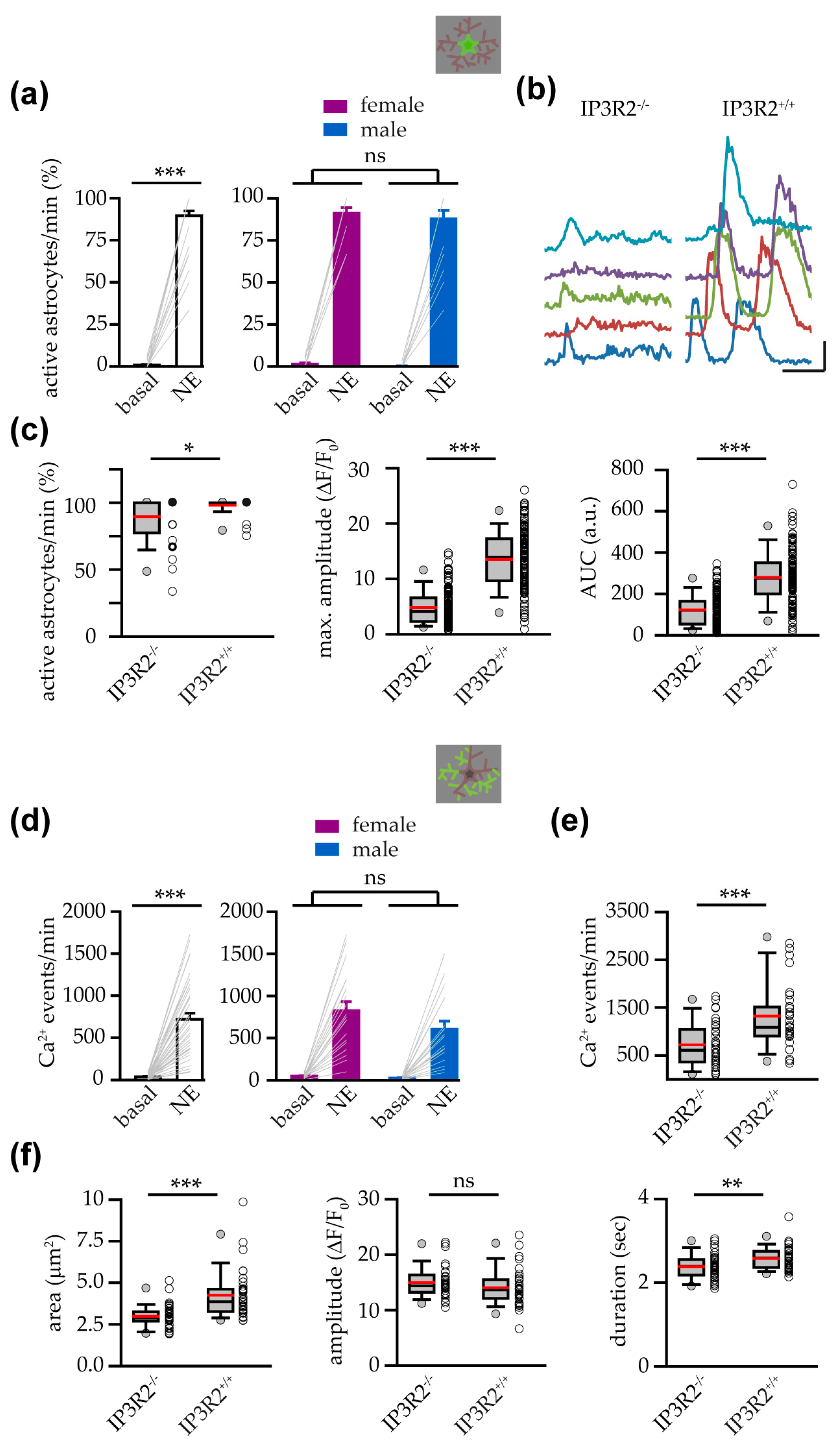
3.4. Astrocytes from IP3R2−/− Mice Showed a Reduced Response to NE
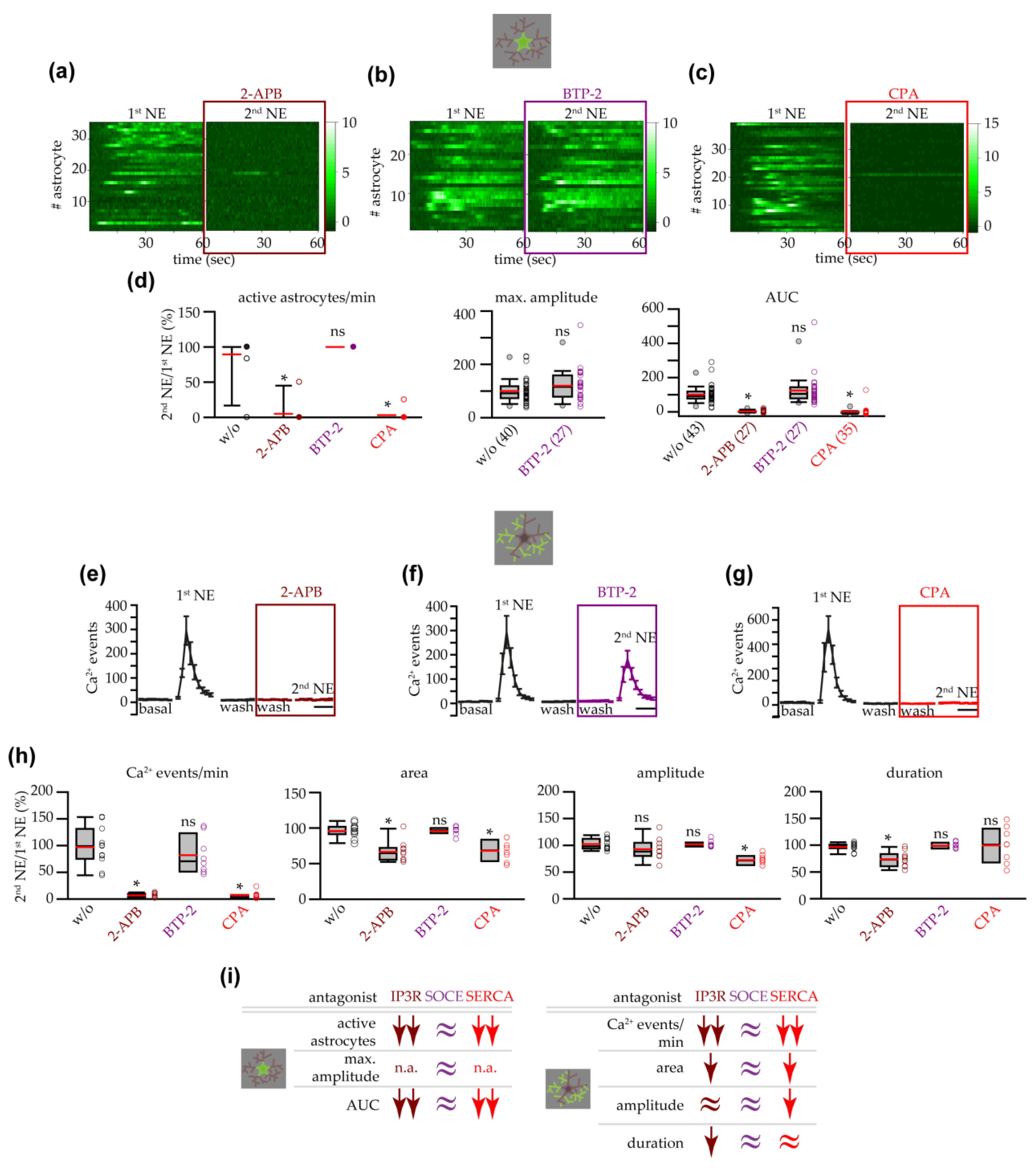
3.5. NE-Triggered Astrocyte Response in IP3R2−/− Mice Depended on ER Intracellular Ca2+ Stores and IP3Rs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nagai, J.; Yu, X.; Papouin, T.; Cheong, E.; Freeman, M.R.; Monk, K.R.; Hastings, M.H.; Haydon, P.G.; Rowitch, D.; Shaham, S.; et al. Behaviorally Consequential Astrocytic Regulation of Neural Circuits. Neuron 2021, 109, 576–596. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Nedergaard, M. Physiology of Astroglia. Physiol. Rev. 2018, 98, 239–389. [Google Scholar] [CrossRef] [PubMed]
- Dallérac, G.; Zapata, J.; Rouach, N. Versatile Control of Synaptic Circuits by Astrocytes: Where, When and How? Nat. Rev. Neurosci. 2018, 19, 729–743. [Google Scholar] [CrossRef]
- Ibáñez, I.; Bartolomé-Martín, D.; Piniella, D.; Giménez, C.; Zafra, F. Activity Dependent Internalization of the Glutamate Transporter GLT-1 Requires Calcium Entry through the NCX Sodium/Calcium Exchanger. Neurochem. Int. 2019, 123, 125–132. [Google Scholar] [CrossRef]
- Wang, F.; Smith, N.A.; Xu, Q.; Fujita, T.; Baba, A.; Matsuda, T.; Takano, T.; Bekar, L.; Nedergaard, M. Astrocytes Modulate Neural Network Activity by Ca2+-Dependent Uptake of Extracellular K+. Sci. Signal. 2012, 5, ra26. [Google Scholar] [CrossRef]
- Henriques, V.J.; Chiavegato, A.; Carmignoto, G.; Gómez-Gonzalo, M. Astrocytes Modulate Somatostatin Interneuron Signaling in the Visual Cortex. Cells 2022, 11, 1400. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar-Slamloo, Y.; Fazlali, Z. Dopamine and Noradrenaline in the Brain; Overlapping or Dissociate Functions? Front. Mol. Neurosci. 2020, 12, 334. [Google Scholar] [CrossRef] [PubMed]
- Kofuji, P.; Araque, A. G-Protein-Coupled Receptors in Astrocyte–Neuron Communication. Neuroscience 2021, 456, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Lia, A.; Henriques, V.J.; Zonta, M.; Chiavegato, A.; Carmignoto, G.; Gómez-Gonzalo, M.; Losi, G. Calcium Signals in Astrocyte Microdomains, a Decade of Great Advances. Front. Cell. Neurosci. 2021, 15, 673433. [Google Scholar] [CrossRef]
- Sherwood, M.W.; Arizono, M.; Panatier, A.; Mikoshiba, K.; Oliet, S.H.R. Astrocytic IP3Rs: Beyond IP3R2. Front. Cell. Neurosci. 2021, 15, 695817. [Google Scholar] [CrossRef]
- Navarrete, M.; Araque, A. Endocannabinoids Mediate Neuron-Astrocyte Communication. Neuron 2008, 57, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, L.; Losi, G.; Sessolo, M.; Marcon, I.; Carmignoto, G. The Inhibitory Neurotransmitter GABA Evokes Long-Lasting Ca2+ Oscillations in Cortical Astrocytes. Glia 2016, 64, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Durkee, C.A.; Covelo, A.; Lines, J.; Kofuji, P.; Aguilar, J.; Araque, A. Gi/o Protein-Coupled Receptors Inhibit Neurons but Activate Astrocytes and Stimulate Gliotransmission. Glia 2019, 67, 1076–1093. [Google Scholar] [CrossRef] [PubMed]
- Bari, B.A.; Chokshi, V.; Schmidt, K. Locus Coeruleus-Norepinephrine: Basic Functions and Insights into Parkinson’s Disease. Neural Regen. Res. 2020, 15, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.A.; Van Bockstaele, E.J. The Locus Coeruleus- Norepinephrine System in Stress and Arousal: Unraveling Historical, Current, and Future Perspectives. Front. Psychiatry 2021, 11, 601519. [Google Scholar] [CrossRef]
- Breton-Provencher, V.; Drummond, G.T.; Sur, M. Locus Coeruleus Norepinephrine in Learned Behavior: Anatomical Modularity and Spatiotemporal Integration in Targets. Front. Neural Circuits 2021, 15, 638007. [Google Scholar] [CrossRef]
- Özçete, Ö.D.; Banerjee, A.; Kaeser, P.S. Mechanisms of Neuromodulatory Volume Transmission. Mol. Psychiatry 2024, 29, 3680–3693. [Google Scholar] [CrossRef]
- Hirase, H.; Iwai, Y.; Takata, N.; Shinohara, Y.; Mishima, T. Volume Transmission Signalling via Astrocytes. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130604. [Google Scholar] [CrossRef]
- Pacholko, A.G.; Wotton, C.A.; Bekar, L.K. Astrocytes—The Ultimate Effectors of Long-Range Neuromodulatory Networks? Front. Cell. Neurosci. 2020, 14, 581075. [Google Scholar] [CrossRef]
- Gordon, G.R.J.; Baimoukhametova, D.V.; Hewitt, S.A.; Rajapaksha, W.K.J.; Fisher, T.E.; Bains, J.S. Norepinephrine Triggers Release of Glial ATP to Increase Postsynaptic Efficacy. Nat. Neurosci. 2005, 8, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; O’Donnell, J.; Thrane, A.S.; Zeppenfeld, D.; Kang, H.; Xie, L.; Wang, F.; Nedergaard, M. Alpha1-Adrenergic Receptors Mediate Coordinated Ca2+ Signaling of Cortical Astrocytes in Awake, Behaving Mice. Cell Calcium 2013, 54, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.; Prey, J.; Eschholz, L.; Rotermund, N.; Lohr, C. Norepinephrine-Induced Calcium Signaling and Store-Operated Calcium Entry in Olfactory Bulb Astrocytes. Front. Cell. Neurosci. 2021, 15, 639754. [Google Scholar] [CrossRef] [PubMed]
- Benton, K.C.; Wheeler, D.S.; Kurtoglu, B.; Ansari, M.B.Z.; Cibich, D.P.; Gonzalez, D.A.; Herbst, M.R.; Khursheed, S.; Knorr, R.C.; Lobner, D.; et al. Norepinephrine Activates Beta1-Adrenergic Receptors at the Inner Nuclear Membrane in Astrocytes. Glia 2022, 70, 1777–1794. [Google Scholar] [CrossRef]
- Wang, F.; Wang, W.; Gu, S.; Qi, D.; Smith, N.A.; Peng, W.; Dong, W.; Yuan, J.; Zhao, B.; Mao, Y.; et al. Distinct Astrocytic Modulatory Roles in Sensory Transmission during Sleep, Wakefulness, and Arousal States in Freely Moving Mice. Nat. Commun. 2023, 14, 2186. [Google Scholar] [CrossRef] [PubMed]
- Reitman, M.E.; Tse, V.; Mi, X.; Willoughby, D.D.; Peinado, A.; Aivazidis, A.; Myagmar, B.E.; Simpson, P.C.; Bayraktar, O.A.; Yu, G.; et al. Norepinephrine Links Astrocytic Activity to Regulation of Cortical State. Nat. Neurosci. 2023, 26, 579–593. [Google Scholar] [CrossRef]
- Del Franco, A.P.; Newman, E.A. Astrocyte Beta-Adrenergic Receptor Activity Regulates NMDA Receptor Signaling of Medial Prefrontal Cortex Pyramidal Neurons. J. Neurosci. 2024, 44, e0990232023. [Google Scholar] [CrossRef]
- Lefton, K.B.; Wu, Y.; Yen, A.; Okuda, T.; Zhang, Y.; Dai, Y.; Walsh, S.; Manno, R.; Dougherty, J.D.; Samineni, V.K.; et al. Norepinephrine Signals Through Astrocytes To Modulate Synapses. bioRxiv 2024. [Google Scholar] [CrossRef]
- Renden, R.B.; Institoris, A.; Sharma, K.; Tran, C.H.T. Modulatory Effects of Noradrenergic and Serotonergic Signaling Pathway on Neurovascular Coupling. Commun. Biol. 2024, 7, 287. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, X.; Dong, Y.; Liu, Y.; Wang, C.; Li, Y.; Ma, C.; Xu, G.; Wang, S.; Yang, C.; et al. Norepinephrine-Activated P38 MAPK Pathway Mediates Stress-Induced Cytotoxic Edema of Basolateral Amygdala Astrocytes. Brain Sci. 2024, 14, 161. [Google Scholar] [CrossRef]
- Wahis, J.; Akkaya, C.; Kirunda, A.M.; Mak, A.; Zeise, K.; Verhaert, J.; Gasparyan, H.; Hovhannisyan, S.; Holt, M.G. The Astrocyte Alfa1-Adrenoreceptor Is a Key Component of the Neuromodulatory System in Mouse Visual Cortex. Glia 2024, 72, 1955–1973. [Google Scholar] [CrossRef] [PubMed]
- Paukert, M.; Agarwal, A.; Cha, J.; Doze, V.A.; Kang, J.U.; Bergles, D.E. Norepinephrine Controls Astroglial Responsiveness to Local Circuit Activity. Neuron 2014, 82, 1263–1270. [Google Scholar] [CrossRef]
- Pankratov, Y.; Lalo, U. Role for Astroglial Alpha1-Adrenoreceptors in Gliotransmission and Control of Synaptic Plasticity in the Neocortex. Front. Cell. Neurosci. 2015, 9, 230. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Huang, B.S.; Venugopal, S.; Johnston, A.D.; Chai, H.; Zeng, H.; Golshani, P.; Khakh, B.S. Ca2+ Signaling in Astrocytes from Ip3r2-/- Mice in Brain Slices and during Startle Responses in Vivo. Nat. Neurosci. 2015, 18, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Gao, V.; Suzuki, A.; Magistretti, P.J.; Lengacher, S.; Pollonini, G.; Steinman, M.Q.; Alberini, C.M. Astrocytic Beta2- Adrenergic Receptors Mediate Hippocampal Long- Term Memory Consolidation. Proc. Natl. Acad. Sci. USA 2016, 113, 8526–8531. [Google Scholar] [CrossRef]
- Monai, H.; Ohkura, M.; Tanaka, M.; Oe, Y.; Konno, A.; Hirai, H.; Mikoshiba, K.; Itohara, S.; Nakai, J.; Iwai, Y.; et al. Calcium Imaging Reveals Glial Involvement in Transcranial Direct Current Stimulation-Induced Plasticity in Mouse Brain. Nat. Commun. 2016, 7, 11100. [Google Scholar] [CrossRef] [PubMed]
- Okubo, Y.; Kanemaru, K.; Suzuki, J.; Kobayashi, K.; Hirose, K.; Iino, M. Inositol 1,4,5-Trisphosphate Receptor Type 2-Independent Ca2+ Release from the Endoplasmic Reticulum in Astrocytes. Glia 2019, 67, 113–124. [Google Scholar] [CrossRef]
- Oe, Y.; Wang, X.; Patriarchi, T.; Konno, A.; Ozawa, K.; Yahagi, K.; Hirai, H.; Tian, L.; McHugh, T.J.; Hirase, H. Distinct Temporal Integration of Noradrenaline Signaling by Astrocytic Second Messengers during Vigilance. Nat. Commun. 2020, 11, 471. [Google Scholar] [CrossRef] [PubMed]
- Kohro, Y.; Matsuda, T.; Yoshihara, K.; Kohno, K.; Koga, K.; Katsuragi, R.; Oka, T.; Tashima, R.; Muneta, S.; Yamane, T.; et al. Spinal Astrocytes in Superficial Laminae Gate Brainstem Descending Control of Mechanosensory Hypersensitivity. Nat. Neurosci. 2020, 23, 1376–1387. [Google Scholar] [CrossRef] [PubMed]
- Sara, S.J. The Locus Coeruleus and Noradrenergic Modulation of Cognition. Nat. Rev. Neurosci. 2009, 10, 211–223. [Google Scholar] [CrossRef]
- Pignatelli, M.; Bonci, A. Role of Dopamine Neurons in Reward and Aversion: A Synaptic Plasticity Perspective. Neuron 2015, 86, 1145–1157. [Google Scholar] [CrossRef]
- Thiele, A.; Bellgrove, M.A. Neuromodulation of Attention. Neuron 2018, 97, 769–785. [Google Scholar] [CrossRef] [PubMed]
- Runegaard, A.H.; Fitzpatrick, C.M.; Woldbye, D.P.D.; Andreasen, J.T.; Sørensen, A.T.; Gether, U. Modulating Dopamine Signaling and Behavior with Chemogenetics: Concepts, Progress, and Challenges. Pharmacol. Rev. 2019, 71, 123–156. [Google Scholar] [CrossRef]
- Wise, R.A. Dopamine, Learning and Motivation. Nat. Rev. Neurosci. 2004, 5, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Wise, R.A.; Baler, R. The Dopamine Motive System: Implications for Drug and Food Addiction. Nat. Rev. Neurosci. 2017, 18, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Russo, S.J.; Nestler, E.J. The Brain Reward Circuitry in Mood Disorders. Nat. Rev. Neurosci. 2013, 14, 609–625. [Google Scholar] [CrossRef]
- Brancaccio, M.; Patton, A.P.; Chesham, J.E.; Maywood, E.S.; Hastings, M.H. Astrocytes Control Circadian Timekeeping in the Suprachiasmatic Nucleus via Glutamatergic Signaling. Neuron 2017, 93, 1420–1435.e5. [Google Scholar] [CrossRef]
- Brancaccio, M.; Edwards, M.D.; Patton, A.P.; Smyllie, N.J.; Chesham, J.E.; Maywood, E.S.; Hastings, M.H. Cell-Autonomous Clock of Astrocytes Drives Circadian Behavior in Mammals. Science 2019, 363, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.G.; Suyama, S.; Koch, M.; Jin, S.; Argente-Arizon, P.; Argente, J.; Liu, Z.W.; Zimmer, M.R.; Jeong, J.K.; Szigeti-Buck, K.; et al. Leptin Signaling in Astrocytes Regulates Hypothalamic Neuronal Circuits and Feeding. Nat. Neurosci. 2014, 17, 908–910. [Google Scholar] [CrossRef]
- Chen, N.; Sugihara, H.; Kim, J.; Fu, Z.; Barak, B.; Sur, M.; Feng, G.; Han, W. Direct Modulation of GFAP-Expressing Glia in the Arcuate Nucleus Bi-Directionally Regulates Feeding. eLife 2016, 5, e18716. [Google Scholar] [CrossRef]
- Varela, L.; Stutz, B.; Song, J.E.; Kim, J.G.; Liu, Z.W.; Gao, X.B.; Horvath, T.L. Hunger-Promoting AgRP Neurons Trigger an Astrocyte-Mediated Feed-Forward Autoactivation Loop in Mice. J. Clin. Investig. 2021, 131, 1–11. [Google Scholar] [CrossRef]
- Boender, A.J.; Bontempi, L.; Nava, L.; Pelloux, Y.; Tonini, R. Striatal Astrocytes Shape Behavioral Flexibility via Regulation of the Glutamate Transporter EAAT2. Biol. Psychiatry 2021, 89, 1045–1057. [Google Scholar] [CrossRef]
- Kang, S.; Hong, S.I.; Lee, J.; Peyton, L.; Baker, M.; Choi, S.; Kim, H.; Chang, S.Y.; Choi, D.S. Activation of Astrocytes in the Dorsomedial Striatum Facilitates Transition From Habitual to Goal-Directed Reward-Seeking Behavior. Biol. Psychiatry 2020, 88, 797–808. [Google Scholar] [CrossRef]
- Mederos, S.; Sánchez-Puelles, C.; Esparza, J.; Valero, M.; Ponomarenko, A.; Perea, G. GABAergic Signaling to Astrocytes in the Prefrontal Cortex Sustains Goal-Directed Behaviors. Nat. Neurosci. 2021, 24, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Martin-Fernandez, M.; Jamison, S.; Robin, L.M.; Zhao, Z.; Martin, E.D.; Aguilar, J.; Benneyworth, M.A.; Marsicano, G.; Araque, A. Synapse-Specific Astrocyte Gating of Amygdala-Related Behavior. Nat. Neurosci. 2017, 20, 1540–1548. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Förster, R.; He, W.; Liao, X.; Li, J.; Yang, C.; Qin, H.; Wang, M.; Ding, R.; Li, R.; et al. Fear Learning Induces Alpha7-Nicotinic Acetylcholine Receptor-Mediated Astrocytic Responsiveness That Is Required for Memory Persistence. Nat. Neurosci. 2021, 24, 1686–1698. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Kong, Y.; Wu, D.-Y.; Liu, J.-H.; Jie, W.; You, Q.-L.; Huang, L.; Hu, J.; Chu, H.-D.; Gao, F.; et al. Impaired Calcium Signaling in Astrocytes Modulates Autism Spectrum Disorder-like Behaviors in Mice. Nat. Commun. 2021, 12, 3321. [Google Scholar] [CrossRef]
- Noh, K.; Cho, W.H.; Lee, B.H.; Kim, D.W.; Kim, Y.S.; Park, K.; Hwang, M.; Barcelon, E.; Cho, Y.K.; Lee, C.J.; et al. Cortical Astrocytes Modulate Dominance Behavior in Male Mice by Regulating Synaptic Excitatory and Inhibitory Balance. Nat. Neurosci. 2023, 26, 1541–1554. [Google Scholar] [CrossRef]
- Cao, X.; Li, L.P.; Wang, Q.; Wu, Q.; Hu, H.H.; Zhang, M.; Fang, Y.Y.; Zhang, J.; Li, S.J.; Xiong, W.C.; et al. Astrocyte-Derived ATP Modulates Depressive-like Behaviors. Nat. Med. 2013, 19, 773–777. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yang, Y.; Ni, Z.; Dong, Y.; Cai, G.; Foncelle, A.; Ma, S.; Sang, K.; Tang, S.; Li, Y.; et al. Astroglial Kir4.1 in the Lateral Habenula Drives Neuronal Bursts in Depression. Nature 2018, 554, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Jun, M.; Xiaolong, Q.; Chaojuan, Y.; Ruiyuan, P.; Shukun, W.; Junbing, W.; Li, H.; Hong, C.; Jinbo, C.; Rong, W.; et al. Calhm2 Governs Astrocytic ATP Releasing in the Development of Depression-like Behaviors. Mol. Psychiatry 2018, 23, 883–891. [Google Scholar] [CrossRef]
- González-Arias, C.; Sánchez-Ruiz, A.; Esparza, J.; Sánchez-Puelles, C.; Arancibia, L.; Ramírez-Franco, J.; Gobbo, D.; Kirchhoff, F.; Perea, G. Dysfunctional Serotonergic Neuron-Astrocyte Signaling in Depressive-like States. Mol. Psychiatry 2023, 28, 3856–3873. [Google Scholar] [CrossRef] [PubMed]
- Gomez, J.A.; Perkins, J.M.; Beaudoin, G.M.; Cook, N.B.; Quraishi, S.A.; Szoeke, E.A.; Thangamani, K.; Tschumi, C.W.; Wanat, M.J.; Maroof, A.M.; et al. Ventral Tegmental Area Astrocytes Orchestrate Avoidance and Approach Behavior. Nat. Commun. 2019, 10, 1455. [Google Scholar] [CrossRef] [PubMed]
- Requie, L.M.; Gómez-Gonzalo, M.; Speggiorin, M.; Managò, F.; Melone, M.; Congiu, M.; Chiavegato, A.; Lia, A.; Zonta, M.; Losi, G.; et al. Astrocytes Mediate Long-Lasting Synaptic Regulation of Ventral Tegmental Area Dopamine Neurons. Nat. Neurosci. 2022, 25, 1639–1650. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, J.; Liu, Y.; Chen, K.H.; Baraban, J.M.; Qiu, Z. Ventral Tegmental Area Astrocytes Modulate Cocaine Reward by Tonically Releasing GABA. Neuron 2023, 111, 1104–1117. [Google Scholar] [CrossRef] [PubMed]
- Liprando, L.A.; Miner, L.H.; Blakely, R.D.; Lewis, D.A.; Sesack, S.R. Ultrastructural Interactions between Terminals Expressing the Norepinephrine Transporter and Dopamine Neurons in the Rat and Monkey Ventral Tegmental Area. Synapse 2004, 52, 233–244. [Google Scholar] [CrossRef]
- Rommelfanger, K.S.; Mitrano, D.A.; Smith, Y.; Weinshenker, D. Light and Electron Microscopic Localization of Alpha-1 Adrenergic Receptor Immunoreactivity in the Rat Striatum and Ventral Midbrain. Neuroscience 2009, 158, 1530–1540. [Google Scholar] [CrossRef]
- Bangasser, D.A.; Cuarenta, A. Sex Differences in Anxiety and Depression: Circuits and Mechanisms. Nat. Rev. Neurosci. 2021, 22, 674–684. [Google Scholar] [CrossRef]
- Li, X.; Zima, A.V.; Sheikh, F.; Blatter, L.A.; Chen, J. Endothelin-1-Induced Arrhythmogenic Ca2+ Signaling Is Abolished in Atrial Myocytes of Inositol-1,4,5-Trisphosphate(IP3)-Receptor Type 2-Deficient Mice. Circ. Res. 2005, 96, 1274–1281. [Google Scholar] [CrossRef] [PubMed]
- Haustein, M.D.; Kracun, S.; Lu, X.H.; Shih, T.; Jackson-Weaver, O.; Tong, X.; Xu, J.; Yang, X.W.; O’Dell, T.J.; Marvin, J.S.; et al. Conditions and Constraints for Astrocyte Calcium Signaling in the Hippocampal Mossy Fiber Pathway. Neuron 2014, 82, 413–429. [Google Scholar] [CrossRef] [PubMed]
- Dugué, G.P.; Dumoulin, A.; Triller, A.; Dieudonné, S. Target-Dependent Use of Coreleased Inhibitory Transmitters at Central Synapses. J. Neurosci. 2005, 25, 6490–6498. [Google Scholar] [CrossRef]
- Wang, Y.; DelRosso, N.V.; Vaidyanathan, T.V.; Cahill, M.K.; Reitman, M.E.; Pittolo, S.; Mi, X.; Yu, G.; Poskanzer, K.E. Accurate Quantification of Astrocyte and Neurotransmitter Fluorescence Dynamics for Single-Cell and Population-Level Physiology. Nat. Neurosci. 2019, 22, 1936–1944. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Wu, P.H.; Hughes, E.G.; Fukaya, M.; Tischfield, M.A.; Langseth, A.J.; Wirtz, D.; Bergles, D.E. Transient Opening of the Mitochondrial Permeability Transition Pore Induces Microdomain Calcium Transients in Astrocyte Processes. Neuron 2017, 93, 587–605.e7. [Google Scholar] [CrossRef]
- Grenhoff, J.; Nisell, M.; Ferré, S.; Aston-Jones, G.; Svensson, T.H. Noradrenergic Modulation of Midbrain Dopamine Cell Firing Elicited by Stimulation of the Locus Coeruleus in the Rat. J. Neural Transm. 1993, 93, 11–25. [Google Scholar] [CrossRef]
- Gómez-Gonzalo, M.; Navarrete, M.; Perea, G.; Covelo, A.; Martín-Fernández, M.; Shigemoto, R.; Luján, R.; Araque, A. Endocannabinoids Induce Lateral Long-Term Potentiation of Transmitter Release by Stimulation of Gliotransmission. Cereb. Cortex 2015, 25, 3699–3712. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Gonzalo, M.; Martin-Fernandez, M.; Martínez-Murillo, R.; Mederos, S.; Hernández-Vivanco, A.; Jamison, S.; Fernandez, A.P.; Serrano, J.; Calero, P.; Futch, H.S.; et al. Neuron–Astrocyte Signaling Is Preserved in the Aging Brain. Glia 2017, 65, 569–580. [Google Scholar] [CrossRef] [PubMed]
- De Young, G.W.; Keizer, J. A Single-Pool Inositol 1,4,5-Trisphosphate-Receptor-Based Model for Agonist-Stimulated Oscillations in Ca2+ Concentration. Proc. Natl. Acad. Sci. USA 1992, 89, 9895–9899. [Google Scholar] [CrossRef] [PubMed]
- Scemes, E.; Giaume, C. Astrocyte Calcium Waves: What They Are and What They Do. Glia 2006, 54, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Ikegaya, Y.; Koyama, R. The Astrocytic CAMP Pathway in Health and Disease. Int. J. Mol. Sci. 2019, 20, 779. [Google Scholar] [CrossRef] [PubMed]
- Papouin, T.; Dunphy, J.; Tolman, M.; Foley, J.C.; Haydon, P.G. Astrocytic Control of Synaptic Function. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160154. [Google Scholar] [CrossRef] [PubMed]
- Durkee, C.A.; Araque, A. Diversity and Specificity of Astrocyte–Neuron Communication. Neuroscience 2019, 396, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Velásquez-Martinez, M.C.; Vázquez-Torres, R.; Jiménez-Rivera, C.A. Activation of Alpha1-Adrenoceptors Enhances Glutamate Release onto Ventral Tegmental Area Dopamine Cells. Neuroscience 2012, 216, 18–30. [Google Scholar] [CrossRef]
- Velásquez-Martínez, M.C.; Vázquez-Torres, R.; Rojas, L.V.; Sanabria, P.; Jiménez-Rivera, C.A. Alpha-1 Adrenoreceptors Modulate GABA Release onto Ventral Tegmental Area Dopamine Neurons. Neuropharmacology 2015, 88, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Atzori, M.; Cuevas-Olguin, R.; Esquivel-Rendon, E.; Garcia-Oscos, F.; Salgado-Delgado, R.C.; Saderi, N.; Miranda-Morales, M.; Treviño, M.; Pineda, J.C.; Salgado, H. Locus Ceruleus Norepinephrine Release: A Central Regulator of CNS Spatio-Temporal Activation? Front. Synaptic Neurosci. 2016, 8, 25. [Google Scholar] [CrossRef]
- Wahis, J.; Holt, M.G. Astrocytes, Noradrenaline, Alpha1-Adrenoreceptors, and Neuromodulation: Evidence and Unanswered Questions. Front. Cell. Neurosci. 2021, 15, 645691. [Google Scholar] [CrossRef]
- McCall, J.G.; Al-Hasani, R.; Siuda, E.R.; Hong, D.Y.; Norris, A.J.; Ford, C.P.; Bruchas, M.R. CRH Engagement of the Locus Coeruleus Noradrenergic System Mediates Stress-Induced Anxiety. Neuron 2015, 87, 605–620. [Google Scholar] [CrossRef]
- Kanemaru, K.; Sekiya, H.; Xu, M.; Satoh, K.; Kitajima, N.; Yoshida, K.; Okubo, Y.; Sasaki, T.; Moritoh, S.; Hasuwa, H.; et al. In Vivo Visualization of Subtle, Transient, and Local Activity of Astrocytes Using an Ultrasensitive Ca2+ Indicator. Cell Rep. 2014, 8, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xie, Y.; Zhang, N.; Yu, Y.; Zhang, Q.; Ding, S. Disruption of IP3R2-Mediated Ca2+ Signaling Pathway in Astrocytes Ameliorates Neuronal Death and Brain Damage While Reducing Behavioral Deficits after Focal Ischemic Stroke. Cell Calcium 2015, 58, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Takano, T.; Wallace, J.T.; Baldwin, K.T.; Purkey, A.M.; Uezu, A.; Courtland, J.L.; Soderblom, E.J.; Shimogori, T.; Maness, P.F.; Eroglu, C.; et al. Chemico-Genetic Discovery of Astrocytic Control of Inhibition in Vivo. Nature 2020, 588, 296–302. [Google Scholar] [CrossRef]
- Shiratori-Hayashi, M.; Yamaguchi, C.; Eguchi, K.; Shiraishi, Y.; Kohno, K.; Mikoshiba, K.; Inoue, K.; Nishida, M.; Tsuda, M. Astrocytic STAT3 Activation and Chronic Itch Require IP3R1/TRPC-Dependent Ca2+ Signals in Mice. J. Allergy Clin. Immunol. 2021, 147, 1341–1353. [Google Scholar] [CrossRef]
- Rakers, C.; Petzold, G.C. Astrocytic Calcium Release Mediates Peri-Infarct Depolarizations in a Rodent Stroke Model. J. Clin. Investig. 2017, 127, 511–516. [Google Scholar] [CrossRef]
- Pinto-Duarte, A.; Roberts, A.J.; Ouyang, K.; Sejnowski, T.J. Impairments in Remote Memory Caused by the Lack of Type 2 IP3 Receptors. Glia 2019, 67, 1976–1989. [Google Scholar] [CrossRef] [PubMed]
- Butcher, J.B.; Sims, R.E.; Ngum, N.M.; Bazzari, A.H.; Jenkins, S.I.; King, M.; Hill, E.J.; Nagel, D.A.; Fox, K.; Parri, H.R.; et al. A Requirement for Astrocyte IP3R2 Signaling for Whisker Experience-Dependent Depression and Homeostatic Upregulation in the Mouse Barrel Cortex. Front. Cell. Neurosci. 2022, 16, 905285. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Speggiorin, M.; Chiavegato, A.; Zonta, M.; Gómez-Gonzalo, M. Characterization of the Astrocyte Calcium Response to Norepinephrine in the Ventral Tegmental Area. Cells 2025, 14, 24. https://doi.org/10.3390/cells14010024
Speggiorin M, Chiavegato A, Zonta M, Gómez-Gonzalo M. Characterization of the Astrocyte Calcium Response to Norepinephrine in the Ventral Tegmental Area. Cells. 2025; 14(1):24. https://doi.org/10.3390/cells14010024
Chicago/Turabian StyleSpeggiorin, Michele, Angela Chiavegato, Micaela Zonta, and Marta Gómez-Gonzalo. 2025. "Characterization of the Astrocyte Calcium Response to Norepinephrine in the Ventral Tegmental Area" Cells 14, no. 1: 24. https://doi.org/10.3390/cells14010024
APA StyleSpeggiorin, M., Chiavegato, A., Zonta, M., & Gómez-Gonzalo, M. (2025). Characterization of the Astrocyte Calcium Response to Norepinephrine in the Ventral Tegmental Area. Cells, 14(1), 24. https://doi.org/10.3390/cells14010024






