Abstract
From the moment a cell is on the path to malignant transformation, its interaction with other cells from the microenvironment becomes altered. The flow of molecular information is at the heart of the cellular and systemic fate in tumors, and various processes participate in conveying key molecular information from or to certain cancer cells. For instance, the loss of tight junction molecules is part of the signal sent to cancer cells so that they are no longer bound to the primary tumors and are thus free to travel and metastasize. Upon the targeting of a single cell by a therapeutic drug, gap junctions are able to communicate death information to by-standing cells. The discovery of the importance of novel modes of cell–cell communication such as different types of extracellular vesicles or tunneling nanotubes is changing the way scientists look at these processes. However, are they all actively involved in different contexts at the same time or are they recruited to fulfill specific tasks? What does the multiplicity of modes mean for the overall progression of the disease? Here, we extend an open invitation to think about the overall significance of these questions, rather than engage in an elusive attempt at a systematic repertory of the mechanisms at play.
1. Introduction: Membrane-to-Membrane Communication and Cancer
It would be a tedious task to survey every single mode through which cells communicate with others, for intercellular interaction is key to the homeostatic life of multicellular communities. Nevertheless, one can still have a relatively global yet analytical overview of these modes in search of their similarities and peculiarities.
First, there is contactless communication that uses a limitless range of molecules such as growth factors and cytokines, that are free released by cells in their immediate microenvironment. Subsequently, these factors either act locally or are transported by various body fluids to remote areas. This mode of communication, probably the most classically studied, does not involve direct membrane-to-membrane interaction, and is not covered by this review, although it would be of utmost importance to know how contact-based communication differs from contactless communication and what is the functional significance of these differences.
Then, there is a growing repertoire of modes of communication that involve one form or another of membranous contacts between cells (Figure 1). After briefly introducing the distinctive features of each of these modes, we will discuss the significance and impact of the diversity of membranous intercellular communication, not through the lens of a systematic repertory, but rather by looking at functional aspects.
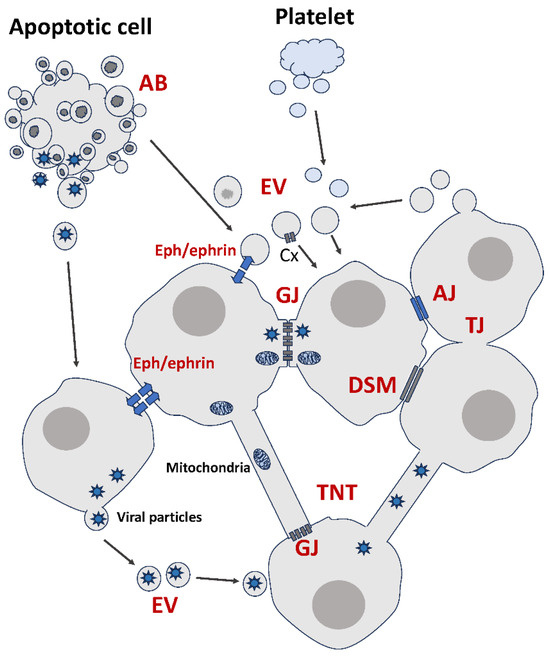
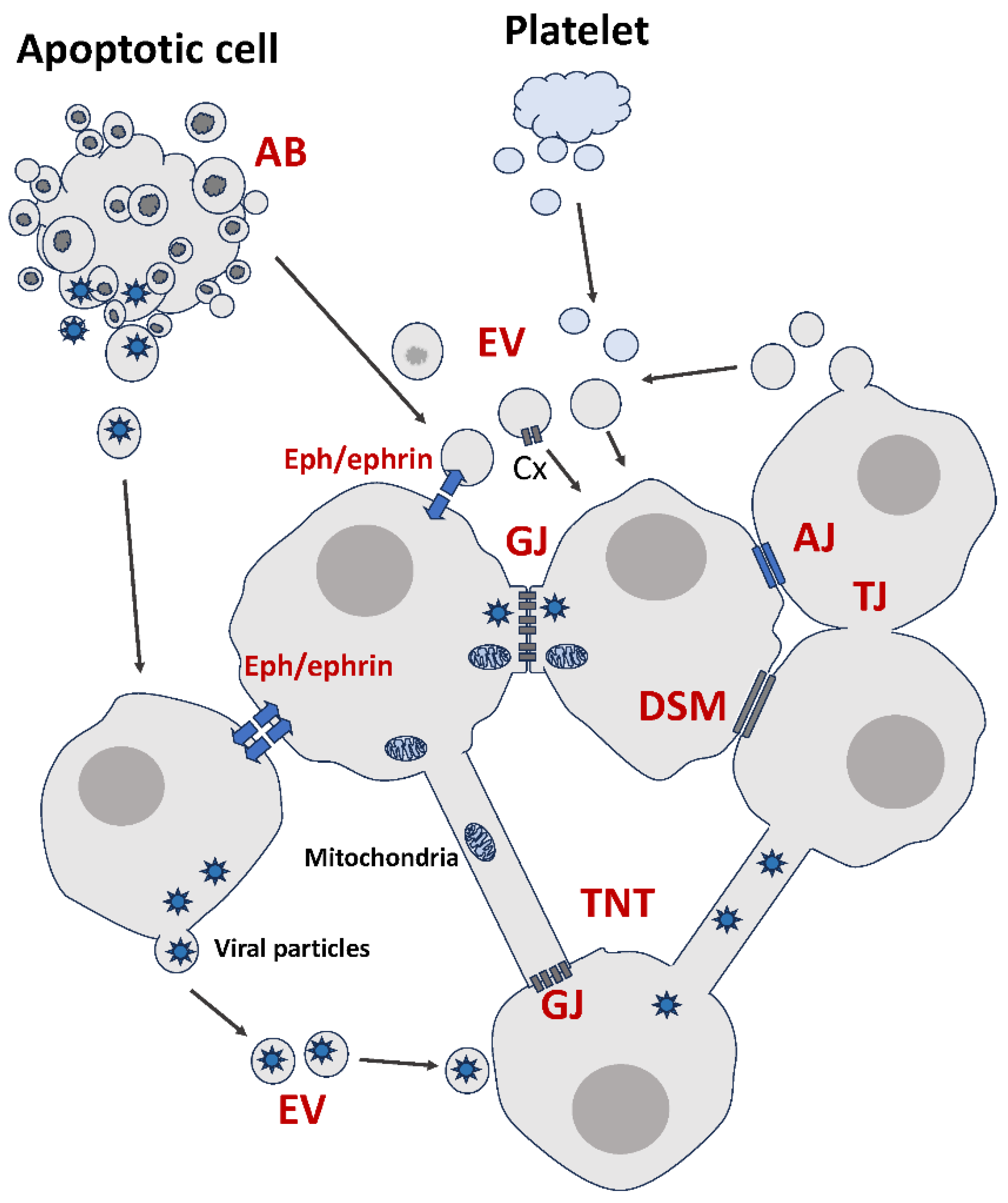
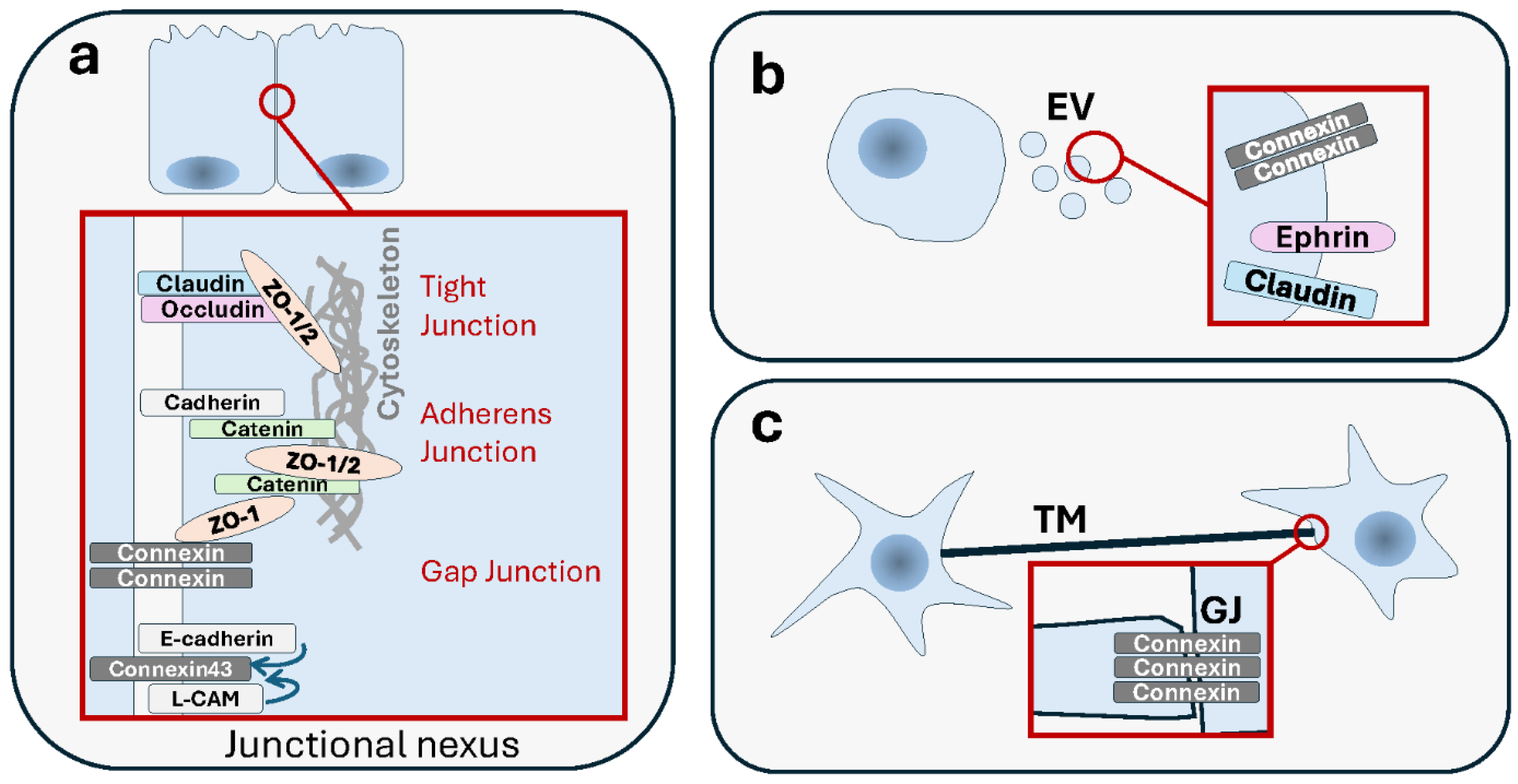
Figure 1.
Summary of membrane-based modes of intercellular communication. AJ: Adherens Junction. GJ: Gap Junction. TJ: Tight Junction. DSM: Desmosome. EV: Extracellular Vesicle. AB: Apoptotic Body. TNT: Tunneling Nanotube. Cx: Connexin. See text for details.
1.1. Junctions of All Kinds
Normal healthy cells are involved in complex organized multicellular tissues and are grouped into distinct tissular compartments endowed with specific functions. From that moment on, they become connected via different junctional structures. These specialized junctions, which allow intercellular communication and coordination, include gap junctions (GJs), tight junctions (TJs), adherens junctions (AJs) and desmosomes (DSMs). Each of these organizes into macromolecular complexes with structural and functional specificities. Based on their functions, cell junctions are classified into three groups. Communicating junctions, i.e., GJs, are involved in the exchange of molecules and electrical signals. Occluding junctions, i.e., TJs, prevent all molecular passage from cell to cell within epithelial tissues. Finally, structures responsible for cell–cell or cell–extracellular matrix (ECM) mechanical adherence are called anchoring junctions and include AJs, DSMs and focal adhesions (FAs).
1.1.1. Gap Junctions
There is abundant literature that associates the different junctions to cancer. The role of gap junctional intercellular communications (GJICs) in cancer development and progression, as well as its potential as a therapeutic target, has been known for decades [1,2,3]. GJs are made of channels that couple the cytoplasm of two neighboring cells, thus allowing the passive diffusion exchange of small molecules [4,5,6]. GJs are also involved in electrical coupling and the synchronization of cells [7]. The major component of these structures are transmembrane proteins of the connexin family [8].
Among junctions, the particularity of GJs is their ability to form a direct channel between the cytoplasm of adjoined cells, whereby hydrophilic molecules can diffuse directly [9]. Another feature of interest is that the main component of GJs, namely connexins, has the ability to form two different types of structures: GJs and connexin hemichannels. GJs are formed by two opposing hemichannels each of which are contributed by the two cells engaged in the junction. Hemichannels are made of hexamers of connexins also called connexons. GJs are clustered into plaques with central pores [10].
In addition to ensuring metabolic coupling between cells, a distinctive feature of GJs is the ability to provide electrical conductance and coupling. Interestingly, many decades ago this function was shown to be absent in liver cancer cells while present in normal hepatocytes [11,12]. Indeed, GJs and connexins are essential for the biology of various healthy tissues, e.g., cardiac, vascular or neural, ensuring the propagation of electrical and chemical signals and cell synchronization. However, the significance of this function in cancer has received very little attention [13].
Another important feature of GJs that is not available in many other modes is the existence of a rapid biochemical control mechanism of its gating potential, through connexin post-translational modifications such as by phosphorylation [14,15].
1.1.2. Tight Junctions
These structures are adhesive molecular complexes that are traditionally known for being important in preserving normal cellular integrity by establishing strong barriers. They are particularly essential for epithelial and endothelial tissues where they regulate cell polarity and paracellular permeability, respectively [16].
TJs are critical in cancer, particularly at the onset of metastasis. TJ loss affects tumor cell polarity, differentiation, adhesiveness, migration and invasiveness, all key steps in tumor metastasis [17,18,19,20].
Contrary to GJs, which function as movement facilitators, TJs are gatekeepers that tightly prevent intercellular molecular leakage and contribute to defining cellular structure and shape [16,21]. Understandably, the presence of TJs between cancer cells and neighboring cells, i.e., endothelial cells, impedes their efforts to undergo metastasis. TJ’s removal is thus a prerequisite for this process to occur [17,18,22,23,24,25]. However, TJs must not be regarded simply as idle stitches. While they bind to the cytoskeleton, they are also implicated in intracellular signaling [16], via components such as cytoplasmic adaptor proteins (e.g., zonula occludens (ZO)), or transmembrane linker proteins (e.g., occluding, claudins and junctional adhesion molecules (JAMs)) [19,21,26,27,28,29,30]. Claudins (CLDNs) for instance [31] have a signaling function that is bidirectional, sending signals to both cells involved in the interaction [19,32]. In this regard, they resemble junction-less proteins such as Eph/ephrins (see following sections).
1.1.3. Adherens Junctions and Desmosomes
Along with TJs, AJs and DSMs are other types of junctions with importance in epithelial tissues. Both make use of cadherins and related proteins as cell adhesion molecules that connect between cytoskeletal structures between cells rather than constituting a membrane-to-membrane seal as found in TJs. Therefore, they both provide mechanical support to cells. However, AJs and DSMs differ in that DSMs are much more structured in line with their higher specialization. DSMs represent an increase in functional sophistication and effectiveness. They provide strength and mechanical resistance to tissues [33]. There is evidence to indicate a role of DSMs and protein components in cancer [34,35]. Similar to TJs, there seems to be a more prominent impact of AJs’ and DSMs’ disaggregation or loss in enhancing cell motility and metastasis, although their actual role in tumor suppression vs. oncogenesis might be context-dependent [36,37]. On the other hand, like TJs, AJs and DSMs are not mere structural scaffolds; they also have signaling functions that are important in the regulation of multiple cancer-related processes such as proliferation, apoptosis, differentiation and migration [35,38].
The adhesive and intercellular communication modes differ in their role in tumors, whether that be temporally, spatially or functionally. Even when structurally close, they still show important specificities. For instance, in vivo genetic experiments indicate that DSMs’ loss precedes that of AJs in the course of tumorigenesis and early invasion. Loss of DSMs weakens cell–cell adhesion, increases cell survival, promotes recruitment of inflammatory cells and increases local invasion. Subsequent loss of AJs adds to the loss of cell–cell adhesion and increases the extent of cellular invasion and distant metastasis [39]. Nevertheless, a major advantage of junction-based communication modes is their ability to regenerate and persist, while extracellular vesicles for instance have not been shown to be able to do the same (although it is possible to view restricted action as an advantage under certain circumstances).
Finally, it must be noted that although the above-mentioned intercellular junctions are the major ones featured in textbooks, there are many lesser-known junctions. It is reasonable to speculate that these could possess specialized functions in specific cancers, tumorigenic stages or conditions [40].
1.2. Junction-Less Proteins
Although proteins are the main components of the intercellular junctions, there are other protein categories whose role in cell–cell communication does not involve the formation of junctions per se. Many of these belong to the guidance proteins, so-called because they were initially discovered in the nervous system, where they control normal development and functions such as axon guidance, synapse formation and plasticity. Typical examples which will be featured here are the Erythropoietin-producing human hepatocellular carcinoma (Eph) receptors and their ligands called ephrins. Ephs and ephrins have a plethora of functions, prominently in the formation of spatial boundaries during embryonic development, skeletal development and angiogenesis [41,42,43,44,45,46,47,48,49,50,51,52,53,54,55].
Among the cell–cell communication modes, a first distinctive property of Eph receptors is that they are receptor tyrosine kinases (RTKs); in fact, the largest family of all. Thanks to an exceptionally large body of literature demonstrating the role of RTKs in cancer and their potential use as therapeutic targets [56,57,58], it is easy to understand the double importance of Ephs and ephrins as both RTKs and communication systems. Furthermore, unlike other RTKs such as growth factor receptors, including epidermal growth factor receptor (EGFR) and platelet-derived growth factor receptor (PDGFR), which are activated by free-released soluble ligands, both Eph receptors and cognate ephrin ligands are carried by the cell membranes of interacting cells. This leads to another particularity for this cell–cell communication mode: it elicits bidirectional signaling. A cell is acted upon at the same time that it acts on its interacting partner.
Functionally, in addition to their role in major cell homeostatic processes such as proliferation, cell death, invasion, etc., Ephs and ephrins also have the particularity of regulating cell positioning and sorting [47,59]. For instance, Eph and ephrin signaling regulates the positioning of intestinal epithelial cells within the stem cell niche [60], thus ensuring a coordination between intercellular communication, migration and cell positioning [61]. As mentioned earlier, Ephs and ephrins are guidance molecules first, that help direct cells through their microenvironment, balancing cell sorting and segregation on one hand, repulsion or attraction on the other hand. These properties endow invasive and metastatic cells with the potential to skip through the multiple boundaries between the primary tumor, the normal microenvironment and the endothelial system [62,63,64,65]. They also promote intermingling with normal cells of the metastatic niches [66,67].
1.3. Extracellular Vesicles
The idea that two cells that are remote could still undergo long distance membrane-to-membrane communications was not common knowledge before the discovery of extracellular vesicles (EVs). This generic name actually refers to a diverse population of membranous vesicles released by a plethora of cell types into the extracellular milieu. Depending on their origin and mode of genesis, different forms can be distinguished, mainly exosomes (<100–150 nm), microvesicles (<500–1000 nm) and apoptotic bodies (ABs) (<2–5 µm) [68,69,70,71]. Regardless of type, the biogenesis of EVs always involves the formation of lipid membrane-bound vesicles derived from endosomes or plasma membranes. This could be due to exocytosis of intracellular multivesicular bodies for exosomes, shedding the cytoplasmic membrane to form microvesicles, or fragmentation of the cell into membranous blebs in the case of apoptosis [72,73,74,75,76]. Therefore, EVs are small particles limited by phospholipid membranes that encapsulate cellular content from their cells of origin, and are released into the extracellular environment [77]. In fact, EVs are released by many cell types both alive or dying, from all organisms, both normal and pathological [78,79].
While their functions in multiple tumorigenic processes is steadily unravelling [80], EVs can be regarded as bona fide intercellular communication devices that receive and send signals [81,82,83,84,85,86,87,88,89,90,91,92,93,94]. Study of their role in tumor progression by virtue of sharing oncogenic molecules has been prominent in the recent literature [95,96,97,98,99,100,101]. Indeed, tumors can share oncogenic moieties using EVs. This is shown to involve their role in cell–cell communication between cancer cells and other cellular types in their microenvironment, whether normal or pathogenic. Examples include interactions with stromal cells, but also long distance with cells in the metastatic niches. EVs can be pro-angiogenic and participate in multiple steps during metastasis by eliciting local tumor invasion and ECM remodeling and epithelial–mesenchymal transition (EMT) [102,103]. Perhaps the most spectacular aspect of EVs in tumor biology is their role as emissaries from primary tumors to the metastatic niche, where they contribute to making these microenvironments more hospitable for secondary tumor growth [104].
Interestingly, dying cells are also able to communicate with other cells using a type of EVs [105,106]. Classic descriptions of apoptosis involve a sequence of events that includes nuclear chromatin condensation, nuclear fragmentation and cytoplasmic membrane blebbing. The apoptotic process culminates in the disassembly of the cellular content into multiple membrane-enclosed vesicles, known as apoptotic bodies (ABs) and normally destined to removal by phagocytes [107,108,109,110,111,112,113]. While exosomes and microvesicles are formed by healthy cells, ABs result from a cell death process reaching its end stage [70,79,114]. This leads to questioning the significance and impact of this communication from the cell’s death bed. There appears to be another distinctive feature for ABs, namely that they are more enriched in miRNAs than other EVs [115], although the significance of this finding is not clear. ABs are involved in the communication of dying cells with neighboring cells, particularly within the immune system [73,116,117,118,119,120]. ABs affect various functions including polarization, cell proliferation and differentiation [121,122,123]. However, it is not clear whether or not the fact that apoptosis-derived EVs inherit their content from terminally dying parent cells, rather than living cells, has any impact on their role in cancer.
Activated platelets are small cellular fragments. Nonetheless, they too can release EVs that reflect their molecular content and possibly also their features. Admittedly, the role of these EVs in cancer is not understood, but their amounts seem to be higher in cancer [124,125,126,127,128]. Although it is still speculative, there have been suggestions that these free-travelling platelet-derived EVs might bypass the limitations imparted upon activated parent platelets trapped in thrombi or other aggregates. This second-line messaging system would have the benefit of extending the platelets’ reach to heterologous cells beyond the blood vessels and circulating metastatic tumor cells, into tumor microenvironments and possibly even in the metastatic niche [129,130,131].
The above-mentioned EVs are not the only ones on record. Very large EVs have been identified, including large oncosomes, which are a distinct type of vesicle released by cancer cells [132,133,134,135,136]; exophers that transport protein aggregates and organelles [137]; and migrasomes that are released during migracytosis, a cell migration-dependent mechanism of vesicular secretion [138].
Regardless of the type or size of EVs, they all mediate an intercellular communication that combines the ability to undergo long-range effects along with the use of a membrane-based interaction. This allows EV-releasing cells to package multiple materials, even multiprotein complexes that would not otherwise be allowed through junctions. Instead of being delivered as single free released agents, multiple molecules could be delivered as molecular cocktails, in a targeted manner that relies on specific receptors. This mode also shields this cargo material from degradation and dilution [139]. Finally, as we have previously mentioned, unless proven otherwise, EVs seem to work as single-use packages, delivered with a time limit, and are unable to self-sustain or regenerate.
1.4. Tubular Structures
In relatively recent history, new modes of cell–cell communication in the form of protrusive nanotubular structures have been reported. These structures are very heterogenous and diverse and sometimes exist in the form of complex networks. Tunneling nanotubes (TNTs), which are tunnels with a diameter of 50 to 800 nm [140,141], are involved in the transfer of multiple types of cargo, including proteins, non-coding RNAs, calcium ions, nucleic acids, and even entire organelles such as mitochondria, lysosomes and autophagosomes [142,143,144,145,146,147,148,149,150,151]. A distinctive characteristic of TNTs is that the communicating cells engage in targeted membrane-based interaction without having to be in close proximity. It has also been reported that TNT-like structures appear to facilitate the transfer of membrane vesicles and organelles more than they do small molecules [140,148].
Shortly after its initial characterization, TNT-like communication was identified between cancer cells, or connecting cancer cells to intra-tumoral stromal cells [152,153,154,155,156,157,158,159]. TNTs have been shown to contribute to tumor cells’ adaptation and survival in response to stress, whether metabolic or other [155,160]. The fact that TNTs are involved in the transfer of functional mitochondria has led authors to propose that TNTs might be involved in the metabolic rescue of damaged apoptotic cells [161]. In other respect, TNTs between macrophages and breast tumor cells have been found to stimulate tumor cell invasiveness [162].
Because they seem to possess distinctive features in gliomas, TNT-like protrusive tunnels involved in the intercellular transfer of material have been given a different name: tumor microtubes (TMs) [163]. TMs are thicker and much longer than TNTs and form an extended network in gliomas [163,164,165]. In fact, there are two different types of TMs: the non-connecting open-ended type, and the interconnecting type which is associated with GJs [140,166,167]. Like other TNT-like structures, TMs have been shown to have a role in cancer, specifically in glioma progression [165,168], where they are at the fore of the invasive front, and associated with resistance to cell death induced by radiotherapy [163,168]. While both EVs and TNTs have the advantage of serving as cargo for large complexes, TNTs differ from EVs in that the former seem more targeted and directed.
2. Range of Communication
A key difference between the above-described modes of intercellular communication is the range and directionality of their action.
TJs, AJs, DSMs and the junction-less proteins such as Ephs and ephrins need direct and immediate cell–cell contacts (Figure 2). TJ’s short range of action is consistent with a role in local gatekeeping functions and the complex architecture more enmeshed with signaling complexes [169,170,171]. In the context of cancer, this locality in a sense forfeits reliance on the presence of masses of cells such as those seen in pre-cancerous lesions, primary tumors and established metastases. The reliance of TJs and their component proteins on cellular proximity, along with their multiplicity of roles (e.g., differentiation, cell polarity, proliferation, migration, stemness and EMT), gives them a functional flexibility that would explain a key role in metastasis [17,32], a process that involves a multitude of steps and functional changes. Interestingly, TJ proteins have been shown to have an important role in clusters of circulating tumor cells (CTCs) [172], which are tumor cells that have detached from the primary tumor and underwent the process of extravasation, as part of the metastatic journey [173,174]. Similarly, the expression of components of DSMs and AJs such as plakoglobin are significantly enriched in breast cancer CTC clusters which have a much higher (23–50-fold) metastatic potential than single CTCs [175]. Furthermore, plakoglobin knockdown blocks intercellular interactions, reducing CTC clustering and metastatic potential [175]. Traditionally, tumor cells were believed to metastasize as single cells entering the blood stream. However, since this model has been questioned, with increasing evidence of clustered metastatic cells [176], cell–cell interactions have subsequently gained more importance in metastasis. Indeed, the significance of this function of TJs is high, as this process of cellular aggregation contributes to the survival of metastatic cells by evading anchorage-dependent apoptosis (a.k.a. anoikis) [177]. It should be noted that an increasing number of cell–cell interaction proteins, not all featured herein, are identified that have a role in CTC clustering and metastatic potential. For instance, the cell surface glycoprotein intercellular adhesion molecule 1 (ICAM1, CD54) also promotes CTC cluster formation associated with a strong pro-metastatic role [178].
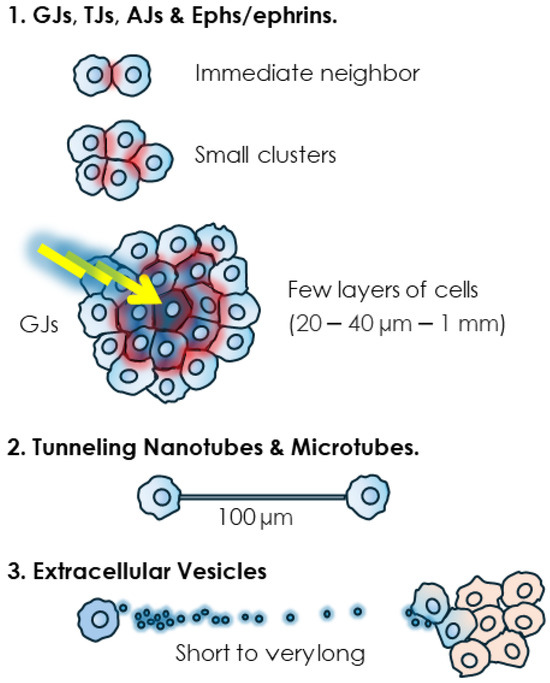
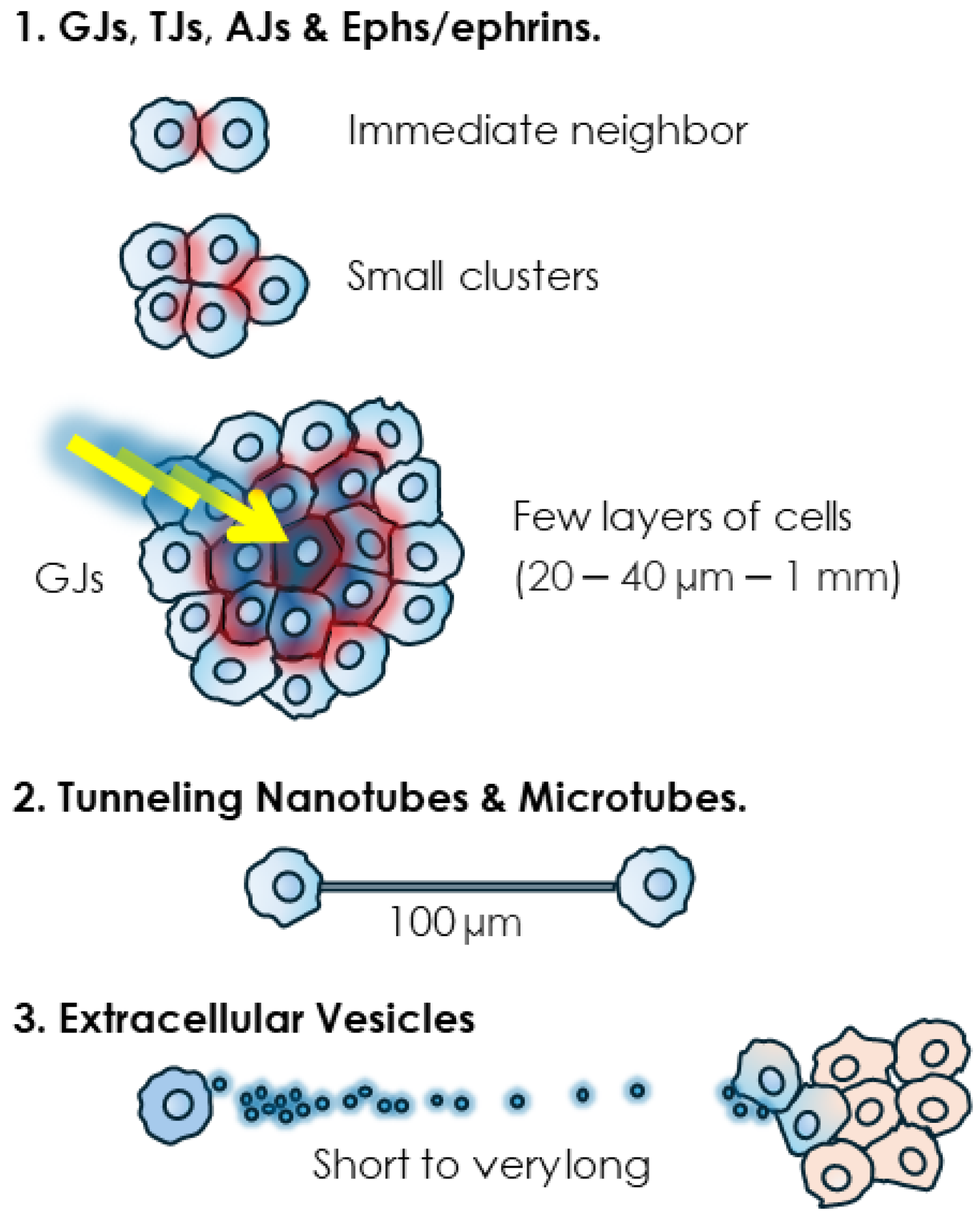
Figure 2.
Range of action of different modes of intercellular communication. While various short-range junctions such as TJs and AJs, and junction-less Eph/ephrin proteins involve tight contacts, GJs are able, thanks to the “bystander effect” (BE), to transfer cytotoxic signals to multiple cells within few surrounding layers. Tunneling nanotubes (TNTs) and extracellular vesicles (EVs) extend this range of action significantly.
Junctional structures couple the cytoplasm of neighboring cells and in the case of GJs, this coupling can reach multiple cells from a single emitting cell. This ability to use GJs to share a signal, generally a cytotoxic one, with by-standing neighboring cells, is known as the “bystander effect” (BE) [1]. This phenomenon can thus extend the range of communication of the GJs to multiple cells. The determination of the maximum range of the BE is complicated by multiple factors such as dosage and concentration of the cytotoxic agent, cellular models, culture setting, as well as the presence or not of functional GJs. Furthermore, it is arduous to distinguish between the GJ-mediated transfer of cytotoxic agents vs. the transmission of their effects relayed by secondary metabolites or signaling molecules such as peroxides or oxidative stress-mediated signals. Nevertheless, early studies have provided a general idea, particularly pertaining to radiation-induced BE. Low doses of radiation particles, directly delivered to only 1% of Chinese hamster ovary (CHO) cells, inflicted a genome-damaging effect to up to 30% of non-irradiated cells [179]. In another experiment, irradiation of only 10% of confluent cells with a single α particle per cell resulted in the transmission of mutagenic effects to 100% of the cell population, an effect that could be prevented by blocking GJ-mediated BE [180]. It was estimated that the BE generated by α particles can propagate up to 1 mm away from the cells that are directly irradiated [181]. In a more targeted estimate, using the effect on stress-responsive protein p21Waf1 as a biological marker, it was found that low-dose α particle exposure induced a BE within a mean propagation distance ranging from 20 to 40 µm around the target, corresponding to approximately 30 cells [182]. While these differences in estimates could be due to the cell type and experimental set up and could also vary when cytotoxic stimuli other than radiation are considered, the conclusion is that GJs contribute to short and middle range cell–cell communication mainly within a cellular mass. This conclusion supports the privileged role for GJs and connexins in early-stage cancer promotion and primary tumors [1,183,184,185,186,187,188,189,190,191]. Admittedly, this role is likely to vary depending on cancer stages and, while the ability of GJs to propagate cytotoxic effects is in line with their tumor suppressive role, these structures also have tumor promoter roles [192,193,194].
As for tubular structures, they can couple the cytoplasm of significantly distant cell partners. Indeed, lengths of 6 µm [140] to 100 µm [195] to a few hundred micrometers [153,163], even exceeding 500 μm in astrocytomas [163,164,165], have been determined. TNTs are longer in sub-confluent cultures. It was shown that their length decreases as mesothelioma cells proliferate and the space between them diminishes to reach full confluence in vitro, thus possibly making this mode of communication unnecessary [152,153]. The invasive front, where other cell–cell interactions are dismantled, is also conducive to the formation and lengthening of TNT and TNT-like protrusions, as observed for instance when protrusions from astrocytoma cells extend and infiltrate the normal brain at the invasive front [163]. In the clinical setting, there is in fact a positive correlation of tubular length and unfavorable prognosis in gliomas [163].
In other respect, as will be further discussed below, TNTs can be functionally co-opted by GJs to provide a long-range GJIC, and some TNTs have functional GJ channels at their ends. For example, for TNT-mediated electrical coupling, the presence of GJs is necessary [167]. In addition to extending the range of GJIC, the TNT–GJ connection provides fine-tuning, in terms of electrical signal selectivity and amplitude [167].
EVs are abundantly found in vivo because they are transported by body fluids to remote environments. They have been detected in almost all body fluids including blood, urine, saliva, cerebral spinal fluid, amniotic fluid, breast milk, etc. [196]. They can thus be considered as the farthest-reaching mode of membrane-based intercellular communication.
3. Directionality of Molecular Transfer
Communication via TJs [169] and Ephs/ephrins [197,198,199,200,201,202,203,204,205] is bidirectional. For the latter, signaling bidirectionality has an impact on heterotypic cell–cell communication between cancer and normal cells [64,65]. The Eph/ephrin family has a large number of members that can be found in different combinations and expression patterns, as well as variable spatio-temporal dynamics [206], which provides this mode of communication with a high level of flexibility and diversity that is unavailable to other modes.
Tubular coupling opens the possibility of a bidirectional and specific exchange of material. For instance, it has been shown that in coculture, macrophages extend TNTs towards fibroblasts that are deficient in the lysosomal membrane cystine transporter, cystinosin. This results in the transfer of cystinosin-containing lysosomes into the deficient cells, which reciprocally use the same route to transfer cystine-containing lysosomes to the macrophages [207]. Whether similar exchanges are possible between cancer cells or between cancer and non-cancer cells awaits further examination. In an instance, a bidirectional transfer of vesicles, proteins and mitochondria was shown to occur in mesothelioma cells via TNTs, between two malignant cells or between two normal mesothelial cells, but not between a malignant and a normal cell [152]. In glioblastoma (GBM) cells, intercellular calcium waves are propagated bidirectionally via tumor microtubes [163].
Additionally, there is some evidence in favor of bidirectional communication via EVs, particularly in the context of the tumor microenvironment (TME), which is known for its cellular diversity and complex repertoire of heterotypic cell–cell interactions. Within the TME, cancer cells use EVs to communicate with normal stromal cells such as cancer-associated fibroblasts (CAFs) and vice versa. This EV-based exchange of material results in the activation of CAFs and reciprocally enhances the proliferation of tumor cells and metastatic potential [208]. The immune system is responsible for an essential anti-tumorigenic function, known as immunosurveillance [209]. Part of this function is mediated by EVs released by immune cells to target tumorigenic cells [104], which, in turn, use EVs to impact the TME [210]. EVs released and exchanged by both tumor-associated neutrophils and tumor cells collaborate to act on cells within the TME. The ensuing bidirectional communication is key in deciding the final pro-inflammatory and immunosuppressive response of these cells [211]. Furthermore, bidirectional communication via EVs between cancer stem cells and the microenvironment seems to be widespread in many solid cancers including prostate, breast, lung and colon [212]. Nevertheless, in some cases, EV-mediated communication was shown to be unidirectional. For example, transfer of miRNA via exosomes between T cells and antigen-presenting cells (APCs) during immune synapsis was shown to be unidirectional [213]. Cancer stem cells (CSCs) and mesenchymal stem cells (MSCs) are important cellular entities of the TME, both of which are sources of EVs, acting in a multidirectional manner in concert with tumor cells [214]. Tumor cells in hematological malignancies secrete EVs that engage in both unidirectional and bidirectional interactions with other cells of their TME, i.e., CSCs, MSCs, in addition to hematopoietic stem cells (HSCs). These interactions contribute to evading immune antitumor functions and developing therapeutic resistance [215].
As a final note, while it is expected that communication directionality would be of no concern, due to the unidirectionality of flow in the vascular and lymphatic systems, it would be interesting to examine how the ability of EVs to perform a long-distance action impacts the strength and specificity of the signal.
4. Transfer of Viral Particles
Viruses use a multitude of ways to spread from one cell to another [216]. Cancer etiology that involves oncogenic viruses gains a completely new dimension with the discovery of a vesicular transfer of viral particles. Not only is EV biogenesis mechanistically close to that of viral particles [217], it is also known that upon viral infection, cells release specific EV populations with distinct molecular repertoires [218]. Many oncogenic molecules, including Latent membrane protein 1 (LMP1), the major viral oncogene expressed in tumors associated with Epstein–Barr virus (EBV), are present in EVs released by infected cancer cells [219,220,221,222]. They are responsible for activating various signaling pathways, e.g., Akt and ERK, in uninfected target cells [222,223]. EVs’ contribution to virus-associated cancers also involves making changes to the TME [223,224]. EVs are not the only vesicular vehicle used by oncoviruses. It is intriguing that apoptotic bodies derived from infected cells can contribute to virus dissemination [225,226,227], thus posing the question of whether, unlike other modes of communication, EVs can increase the reach of viral oncogenes [123] (Figure 1).
EV and AB-mediated modes of transfer of viral particles are different from the so-called Retrovirus-like Particles (RLPs). These are isolated from various tumor cells both in vitro and in vivo [228,229,230,231,232]. RLPs are similar to retroviral vesicles in the sense that they originate from the budding of the plasma membrane [233] and contain some of the retroviral proteins [234,235,236,237,238,239], but they lack infectivity [111]. Nevertheless, although the use of vesicular communication for viral transfer can appear reminiscent of the natural mechanism of viral particle formation and release, other modes of cell–cell communication are also known to contribute.
Intercellular transfer of the tumor virus human T-cell lymphotropic virus type 1 (HTLV-1) involves both TJs and tubular structures (a.k.a. cellular conduits), although the significance of the latter mode is not clear [240,241,242]. Similar data support TNT-mediated viral transfer, whether in influenza [243,244] or AIDS [245,246,247,248]. In spite of these and other data showing the role of TNTs in the long-distance spread of viral particles [249,250,251], it is not known whether the ability of TNTs and similar structures to transfer viral particles, proteins and genomes contributes to tumor formation and/or progression.
Last but not least, rather than using GJs as a mode of transfer, many oncogenic viruses including human papillomavirus (HPV), simian virus 40 (SV40) and avian sarcoma virus impair GJIC mainly by decreasing expression levels [252,253,254,255,256], altering trafficking and recycling to the membrane [257] of connexin-43 (Cx43). This effect is in accord with the fact that cancerous cells often lose their ability to communicate via GJIC [1].
5. Transfer of Organelles
The role of organelles, particularly mitochondria, in cancer progression and therapeutic response is well established [258,259,260,261,262]. In addition to their essential role in intracellular metabolism, intact or fragmented mitochondria have been found in the extracellular milieu [263,264]. Mitochondria sharing has multiple roles in a multicellular communication context. Healthy mitochondria, for instance, can fuse with and rescue damaged mitochondria in neighboring cells and rescue the cell itself from apoptosis [161,265]. In addition to existing as free intact organelles or fragments (e.g., proteins, lipids, mitochondrial DNA), mitochondria can also be shared between cancer and non-cancerous cells via different communication modes, including GJs and TNTs [140,266,267,268,269,270]. Mitochondria can also be transferred via EVs [271,272,273] (Figure 1).
Considering the metabolic and energetic functions of mitochondria, and the importance of metabolism in cancer [274,275], it is normal that mitochondrial transfer would contribute to tumorigenesis by changing the bioenergetic and metabolic state of receiving cells. For instance, it has been shown that mitochondria transfer from astrocytes to cancer cells is prevalent and involved in GBM [276]. This transfer is dependent on a network of intercellular communications between GBM cells and astrocytes [276]. Furthermore, this astrocyte-to-GBM cells organelle transfer drives an increase in mitochondrial respiration and upregulation of metabolic pathways, thus promoting cell proliferation and tumorigenicity [276]. This mechanism depends on a protein called growth-associated protein 43 (GAP43), a major component of tumor microtubes (MTs) [276], a TNT-like tubular structure so far only found in glioma tumors including GBMs.
It is noticeable that the main route of mitochondrial transfer seems to be TNTs and EVs. Conceptually, it is understandable why, unlike many other modes of communication, vesicular and tubular modes would be structurally more amenable to organelle transfer, and this could prove to be a major, functionally relevant distinction between modes of cell–cell communication. This is not to say that there is no link between other modes of communication and mitochondria or metabolic functions. Positioning and intracellular trafficking of organelles has an essential role in cellular polarity [277,278], a process that is governed by TJs. In addition, targeting mitochondria’s function in the oxidative equilibrium has an impact on TJs’ integrity and function [279,280]. However, a role for junction-based interactions other than GJs in mitochondrial transfer has yet to be demonstrated.
6. Physical Constraints and Mechanical Communication
The existence of flexible cell–cell communication entities and others constrained in space, raises an intriguing question. Would physical and mechanical tensions have a different impact on the functions of different cell–cell communication modes, and vice versa? Biological processes involved in virtually all developmental stages and all physiological processes involve a level of mechanical constraints, whether between cells/tissues, or with ECMs [281,282,283]. This is certainly true in the context of tumor progression [284,285,286,287,288]. Cancer progression is associated with defects in mechanotransduction, associated with the ECM, focal adhesions (FAs) and cytoskeletal tensions [289,290,291].
6.1. Holding Tight, Sensing External Forces
Multiple structures such as membrane-anchored receptors, FAs, AJs or DSMs, constitute major sensors and transmitters of extracellular mechanical forces inside the cell, establishing a link more specifically with the actin cytoskeleton [292,293]. This process is known as mechanotransduction, which subsequently translates this force into many biochemical signal transduction pathways and ultimately impacts biological functions [294]. Mechanotransduction is essential for the understanding of both normal and pathological development [292,295,296,297].
GJIC and connexins are involved in the mechano-regulation of the functions of tendon cells, the tenocytes, in response to mechanical forces imposed on the tendons [298,299,300]. Connexins and GJs also help osteocytes communicate beyond the mechanical constraints of the bone minerals and transmit mechanical stimulation signals into the bone [301,302,303,304,305]. The mechanical pressure of laminar shear stress, due to blood flow and the cardiac cycle, increases the expression of Cx43, which facilitates the endothelial-to-mesenchymal transition of endothelial cells, a process involved in cardiovascular diseases [306].
Surprisingly, despite this evidence found in normal tissues, the current literature provides only limited data about the role of GJs or connexins in mechanotransduction in cancer. Numerous studies have shown that endothelial connexins and GJs modulate angiogenesis, including in tumors, by regulating several aspects of cellular mechanics, including endothelial tube formation, cellular stiffness and shear stress [307,308,309,310,311]. In addition, earlier studies have shown that it was possible to mechanically induce the release of intracellular calcium in normal and cancerous cells, possibly by affecting GJIC [312,313,314]. Another way GJs could affect the mechanobiology of cancer cells is through the driving flow of ions and water, thus regulating cell volume and osmotic pressure in proliferating cancer cells [315]. Cx43 participates in cytoskeletal dynamics by binding with microtubules and actin [316,317,318,319,320]. When Cx43 expression is downregulated in colorectal cancer cells, cell stiffness is reduced and stemness is increased, ultimately resulting in increased drug resistance [321]. Increased stiffness, in connection with the cytoskeleton, is a feature of tumors that is under control from both cell–cell and cell–ECM interactions [290,322].
6.2. Responding to the Microenvironment
There is evidence that EVs have a role in transmitting the mechanical tensions that contribute to their role in cancer, particularly at multiple levels during the metastatic process. Stiffness of the ECM affects EV cargo of miRNAs, a mechanism involved in modulating prostate cancer cell metastasis via regulating cell motility and ECM remodeling [323]. Liver cancer cells produce EVs when subject to fluid shear stress generated by the tumor microenvironment (TME) (Figure 3). These EVs have been shown to promote activation of normal fibroblasts into cancer-associated fibroblasts (CAFs), due to enrichment and activation in the IGF2-PI3K signaling pathway [324]. Similarly, exposure to the TME mechanical forces promotes invasive and pro-tumorigenic phenotypes in triple negative, but not ER-positive, breast cancer cells, a role that involves the immunomodulatory profile of the released EVs [325]. In other respects, physical forces have been shown to affect EV production (Figure 3). For instance, while cancer cells interact with the ECM, the latter’s stiffening also promotes the production of EVs by cancer cells which, therefore, also use cell–cell communication to contribute to tumor progression [326] (Figure 3). EVs were also found to transfer CKAP4, a protein associated with tumor metastasis in bladder cancer, and is responsible for the maintenance of a central-to-peripheral gradient of stiffness on the cell membrane. This EV-mediated transfer of CKAP4 enhances the migratory and metastatic potential of the recipient cells [327]. In addition to metastasis, EVs can also regulate cellular mechanoresponses during angiogenesis; under mechanical stress, fibroblasts’ secretion of EVs is enhanced, thus promoting angiogenesis [328]. EVs also have an impact on the physical environment of the bone metastatic niche, by regulating the production of proteins important for bone matrix stiffness and mechanotransduction [329]. The role of EVs in mechanotransduction is indirect, thus seems different than that of junctional structures which are intimately and mechanistically involved. The contribution of quantitative vs. qualitative changes in particular need to be further discriminated, as some of these effects could “simply” reflect overall profile changes within the cells of origin, rather than the specific and genuine changes and effects of EVs.
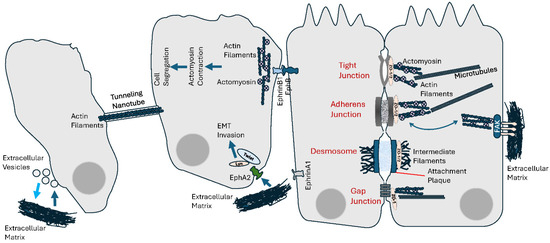
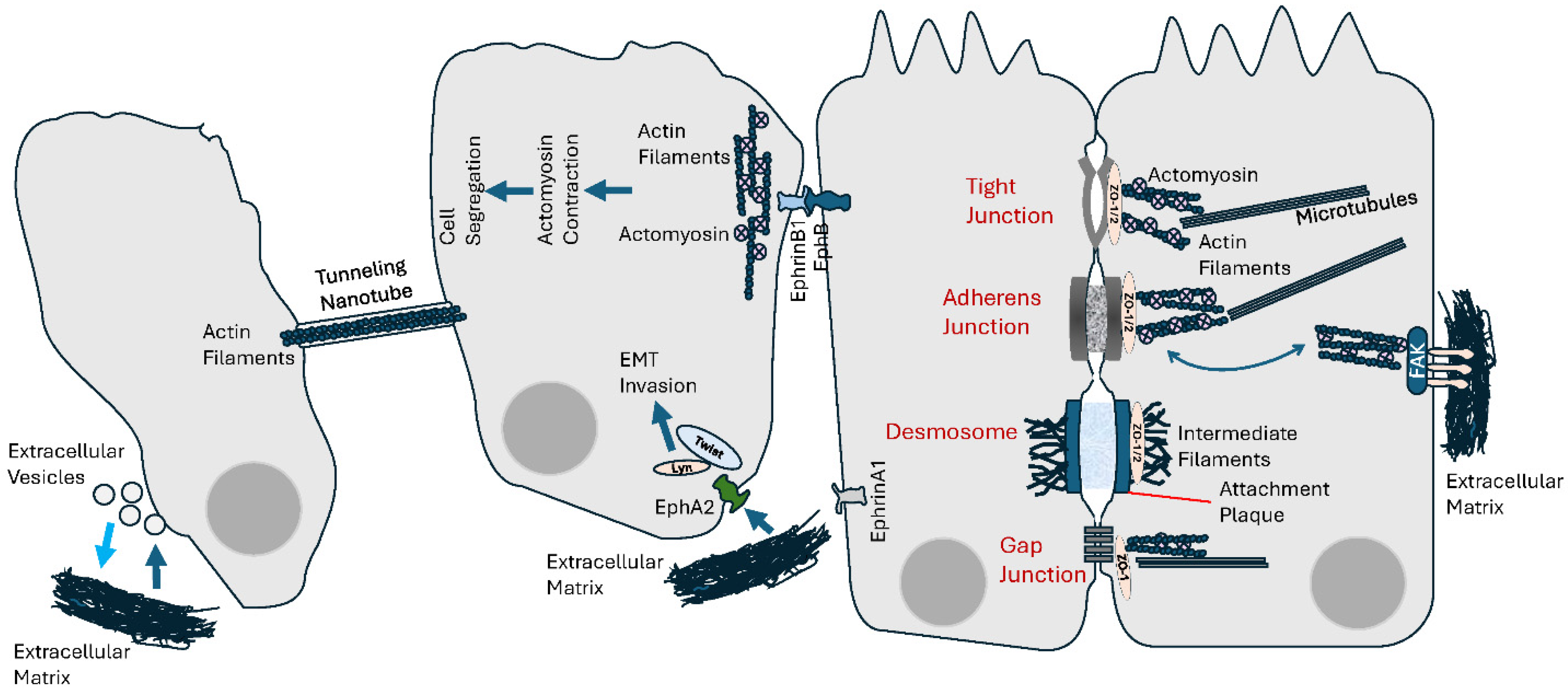
Figure 3.
Cell–cell and cell–extracellular matrix (ECM) mechanical communication. Intercellular communication not only generates mechanical tensions, by interacting with the cytoskeleton, it is also responsive to endogenous mechanical forces as well as physical features of the ECM. See text for details.
6.3. Spatial Positioning and Mechanical Communication
Together with other cell–cell interaction–regulatory proteins, Ephs/ephrins have critical functions during tissue development and organization, and morphogenesis [330]. The fact that Ephs/ephrins mediate cell–cell repulsion, cell and tissue segregation and boundary formation [331] is indicative of the extent of mechanical forces and tension at play [332]. Cell segregation driven by EphB/ephrinB1 signaling during tissue boundary formation is associated with actomyosin contractility, which generates a sustained mechanical cortical cytoskeleton tension [333,334] (Figure 3). In fact, the role of this signaling in cell segregation involves a decrease in the stability of heterotypic cell–cell contacts through increased cortical actomyosin contractility. In breast cancer cells, an EphA2/Lyn/Twist1 mechanosensitive signaling pathway has been identified in response to mechanical tension from the ECM (Figure 3). By inducing ligand-independent phosphorylation of the ephrin receptor EphA2, and subsequently recruiting Lyn/Twist1, ECM stiffness promotes EMT, cell invasion and metastasis [335]. The role of Ephs/ephrins involves an association with the mechanics of the plasma membrane as it connects its tension with the cytoskeletal machinery and signal transduction. Reciprocally, another particular feature of Ephs/ephrins is their bidirectionality, and it is very interesting to understand how mechanical forces could impact their spatiomechanical sensitivity. Spatial organization of Ephs/ephrins and sensitivity to mechanical influences are known to determine downstream signaling and cellular responses [336,337,338]. Spatial (re)organization of cell surface receptors and ligands under the influence of microenvironmental mechanical forces can determine the outcome of signal transduction [339,340,341,342]. The use of physical barriers to interfere with EphA2 receptor-ephrinA1 ligand binding, clustering and subsequent lateral transport results in changes to the cellular response of EphA2 to ephrinA1 in cancer cells [336] (Figure 3).
6.4. Collective Cell Migration
An example where mechanotransduction shows importance is the collective invasion of cancer cells into the surrounding non-cancerous tissue [343]. Migrating groups of cancer cells act as a cohesive and coordinated group, in which the link between and contraction of junctional cadherins and actomyosin cytoskeleton generates tension and physical forces [344]. Cancer-associated fibroblasts (CAFs), whose role in tumor invasion and metastasis is established, impose a physical force on cancer cells that enables their collective invasion [345]. Interestingly, many of the major communication structures involved, namely FAs, AJs and DSMs, use cadherins, whose role in mechanotransduction is well acknowledged [296,346,347]. There is also a relevant functional connection between FAs and AJs [347]. Mechanotransduction is sensitive to the nature of the cytoskeletal network’s anchorage sites. In other words, association with the actin cytoskeleton mediates the interplay between AJs and FAs in establishing a mechanical equilibrium between AJ-mediated cell–cell vs. FA-mediated cell–ECM interactions [296,347,348,349,350] (Figure 3). Reciprocally, the mechanical forces imposed on cells, particularly in the endothelium, affect junction formation [293,351,352,353].
The cellular group dynamic during migration can not only generate mechanical tension, it also affects other aspects as well. During collective cell migration (CCM), cells behave as either front leaders or rear followers. This structure is maintained by a polarized intercellular exchange of biochemical and mechanical information [354]. Due to their connection with the cytoskeleton, AJs and TJs are instrumental in establishing and maintaining this polarization during cell migration [355,356,357,358,359]. There is some evidence that the GJ connexin proteins might have some role in CCM, as their ability to transfer second messengers is crucial, at least in normal tissue such as during cardiac neural crest migration [360], wound closure [361] or feather elongation [362]. However, in cancer, evidence is not as abundant. GJIC and Cx43 have a role in facilitating collective migration in GBMs [363]. Another work showed that Cx43 hemichannels, but not GJIC, induce leader cell activity and collective migration of breast cancer cells [364]. Similarly, Ephs/ephrins seem to have a role in collective migration during thymus organogenesis [365] and directional collective cell migration of Schwann cells to help with peripheral nerve regeneration [366]. However, it is not clear if this cell–cell communication mode has any role in the CCM of cancer cells. The same could be said about EVs or TNTs, as the evidence is lacking in favor of their possible mechanistic involvement in or response to the collective dynamic of cellular migration.
7. Anti-Tumor Immunity
The establishment and function of host anti-tumor immunological defense mechanisms, including antigen (Ag) presentation, is part of the elements determining the fate of any developing malignant state. In spite of the long-standing interest in GJs, their contribution to these immune mechanisms has been largely understudied [367]. GJs allow antigenic viral peptides to be transferred from infected cells into by-standing noninfected neighbors, eliciting recognition by cytotoxic T lymphocyte (CTL) [367] (Figure 4). The advantage herein provided by GJIC to the immune defense mechanisms, as suggested by Neijssen et al., is that the elimination of bystanders mitigates risks of viral propagation and tumorigenesis [367,368]. In this regard, it would be understandable that the loss of GJs and connexins commonly observed in tumors [1,9,369] would be favorable to eluding anti-tumor immune defense mechanisms [370,371,372]. This hypothesis, nevertheless, awaits thorough exploration. In tumors, GJIC activity and connexin have been assigned both tumor suppression and tumor promotion functions [373]. Part of the reason behind this duality appears to be associated with their role in tumor immunity and interaction between tumor cells and stromal immune cells [374]. Evidence shows GJs as a way of communication, in particular with dendritic cells (DCs). For instance, GJs allow Ag transfer from melanoma to DCs, the latter subsequently being able to elicit a tumor-specific immune response in vivo [375,376]. GJICs are required for the activation of bone marrow-derived DCs [377]. In tumor cells, GJs are also involved in the transfer of antigenic peptides between tumor cells and the microenvironmental endothelial cells, leading to the latter’s recognition and elimination by cytotoxic T lymphocytes [378]. It has also been shown that GJs mediate the transfer of Ags produced by caspase cleavage, from apoptotic cells to be cross-presented by neighboring healthy and dendritic cells [379].
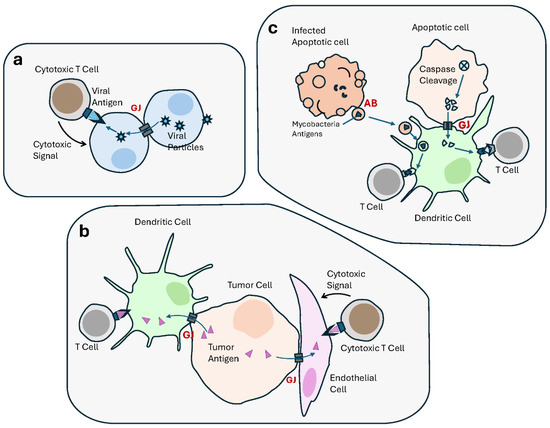
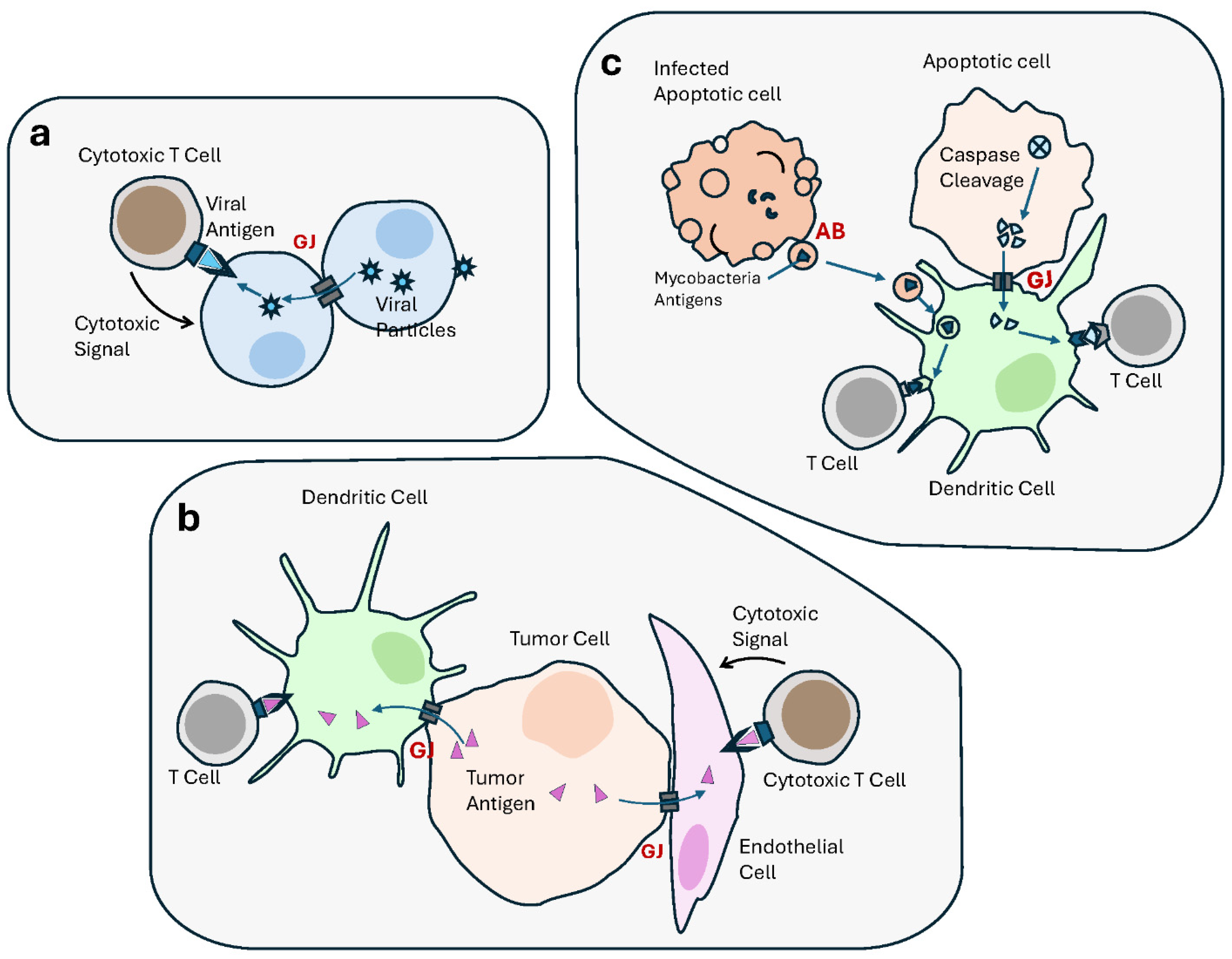
Figure 4.
Cell–cell communication contributes to the immune responses, including anti-tumor immunity. Anti-tumor immunological defense mechanisms, including antigen (Ag) presentation, involve multiple modes of intercellular communication. (a) Gap junctions (GJs) transfer antigenic viral peptides from infected cells into noninfected neighbors, resulting in the recognition and targeting by cytotoxic T lymphocyte (CTL). (b) GJs allow Ag transfer from cancer cells to dendritic cells (DCs), which is presented to T Cells, as part of an anti-tumor immune response. GJs also transfer antigenic peptides between tumor cells and endothelial cells, resulting in the latter’s recognition and elimination by cytotoxic T lymphocytes. (c) In apoptotic tumor cells, GJs transfer antigens generated by caspase cleavage, from apoptotic cells to DCs, to be presented to T Cells, thus activating an antitumor immune response. Similarly, apoptotic bodies derived from apoptotic mycobacteria-infected macrophages contain mycobacteria-derived antigens which are transferred to DCs and presented to T Cells.
There is a recent interest in the roles of EVs in immune processes, such as inflammation and antigen presentation [380,381], or in cancer progression and immunotherapy [382,383,384,385,386]. Data support a role for EVs in modulating innate immunity both positively and negatively [387]. EVs help connect tumor cells with the immune system, coagulation, parenchymal tissue remodeling [388,389,390,391] and transfer immunosuppressive molecules including cytokines such as IL-10 and TGF-β1 as well as death receptor ligands such as FasL or TRAIL [392,393]. These immune functions also concern apoptotic cells, which have attracted attention as contributors to the immune response, e.g., Ag processing and presentation, including through their release of ABs and EVs [394,395]. EVs derived from mycobacteria-infected macrophages that undergo apoptosis transfer mycobacterial Ags to dendritic cells that drive their phagocytosis [396,397] (Figure 4). While the GJ-mediated delivery of antigenic moieties necessitates direct contact, tumor-derived EVs are released in various body fluids, to remotely reach Ag-presenting cells (including DCs). The latter take in the EVs and present the Ags on MHC I molecules to T cells, thus activating an antitumor immune response [89,398,399,400]. Nevertheless, like GJs, EVs can also contribute to escaping anti-tumor immune defense mechanisms, for instance by “flooding” the natural killer (NK) cells’ receptor NKG2D with EV-transported ligands, which results in receptor downregulation and impairment of the cytotoxic function of the immune cells [401], or by transporting Fas ligands (FasL) to trigger Fas-dependent apoptosis [402,403,404]. Innate immunity is an instrumental barricade in the face of tumor progression.
Data support a role of TNTs in immunity [405,406,407]. It has been shown that B-cell precursor acute lymphoblastic leukemia cells use TNTs to signal primary mesenchymal stromal cells (MSCs) to release pro-survival cytokines within their bone marrow microenvironment [408]. In general, it is too early to extensively discuss the burgeoning data regarding the connection between TNTs and anti-tumor immune mechanisms.
Last but not least, Ephs and ephrins have immunological functions including in tumor immunity [409,410,411]. Historically, the discovery of their specific role in vascular development and angiogenesis was tightly associated with the nature and mechanisms of their function [412]. For instance, the interaction between the EphB4 receptor on tumor cell surface and the cognate ephrin-B2 ligand on endothelial cells stimulates the latter’s invasion, survival and proliferation, and ultimately angiogenesis and tumor growth [413]. In addition, there is a plethora of evidence involving this large family of RTKs in endothelial activation, monocyte activation, adhesion, transmigration [414,415,416] and T-cell development and activation [417,418,419,420], among other processes of importance in immune responses. Ephs and ephrins contribute to tumor immune evasion by directly interacting with cells within the TME. The Eph/ephrin interaction is involved in regulating both innate and adaptive immunity [421]. Indeed, it was shown in head and neck squamous cell carcinoma (HNSCC) that the interaction between the EphB4 receptor and ephrin–B2 ligand, on the membranes of tumor cells interacting with their immune neighbors, affects the numbers of intratumoral immunosuppressive regulatory T cells (Tregs) [421].
8. Diversity and Heterogeneity
Tumor heterogeneity is instrumental in cancer progression and therapeutic response [422]. The coexistence, within the same tumor, of cells endowed with differences in their profiles of gene expression and genetic mutations is behind the possibility that, even under the most tumor-hostile conditions, a few cells have the potential to survive, metastasize or resist therapy. In addition to intra-tumoral heterogeneity, inter-tumoral heterogeneity renders cancer prognostic and therapeutic efforts even more complicated [423]. How intercellular communication factors into tumor heterogeneity is still largely unknown. In an attempt to explain the origins of heterogeneity and plasticity in melanomas, a model was proposed in which direct intercellular communication of tumor cells with endothelial cells is established in a perivascular niche, which results in the identity switch of a subset of stem-like cells that initiate metastases, independently from stimulation of tumor growth [424]. One can expect that any mode of communication that shows a large level of diversity and flexibility could contribute to this phenomenon. In this regard, two modes could provide such attributes and thus prove of particular interest: EVs and Ephs/ephrins.
A specificity of EVs in comparison with other modes of intercellular communication is their own diversity and heterogeneity [425,426,427,428,429,430]. Not only do they carry a plethora of molecular types, but both their physical characteristics, contents and amounts produced by tumors and other cells are also very variable depending on a multitude of factors, including cells of origin and physiological state. A proteomic profiling of EVs has found that they can differentiate between breast cancer molecular subtypes to an extent that is better than the whole cellular proteome itself [431]. In other respect, since the TME is important for intra-tumoral heterogeneity [432,433,434], communication of cancer cells with various cellular components of the TME using EVs could indicate the latter’s contribution to the process.
GJs and Eph/ephrins have a commonality that allows them to show a large level of diversity. Indeed, both the GJ connexins [435,436] and Ephs/ephrins [437,438] enlist many family members. All tissues express more than one type of these proteins, thus allowing them to form a huge number of combinations of homomeric or heteromeric complexes and homotypic or heterotypic intercellular communications. The challenge therefore becomes to understand the biological significance and dynamic of establishment of such a plethora of combinations in various tissues, both in space and time. Indeed, different connexins can engage in different channels endowed with different gating properties and selective permeability, depending on the tissue and other factors [6,439,440,441,442]. These considerations have a critical bearing in cancer, for instance for the ability of tumor cells to establish GJICs with endothelial cells, to cross the endothelial barrier and metastasize [443,444,445,446,447,448]. Similarly, evidence has accumulated regarding the discriminatory role of Ephs/ephrins in metastasis [449,450,451]. The large number and combinatory nature of expression and interactions of Ephs/ephrins in various tissues and different tumor subtypes [438,452,453,454,455] is particularly favorable to a potential role in establishing and/or maintaining tumor heterogeneity. However, this can only be speculative at the moment, despite the publication of a finding of subtype-dependent frequency and exclusivity of mutations in Ephrins in gastric cancers [456]. Probably most importantly, it is our hypothesis that, thanks to their prominent role in the sorting, patterning and positioning of cells, Ephs and ephrins could regulate the group dynamic between distinct sub-clones that develop and co-exist within the same tumor. Indeed, subclonal diversification is key to heterogeneity [457,458,459,460,461] and the various clones within the tumor engage in interaction and competition [462,463,464].
The role of junction-based intercellular communications in tumor heterogeneity is less clear. Beyond the findings that junction-associated proteins such as connexins, cadherins or claudins are differentially expressed in different tumor subtypes [465,466,467,468], the importance of engaging in the junctional communications per se is not known. In GBM, brain cancers known for their extreme heterogeneity, exists a subpopulation of cells that express the GJ protein Cx43 and engage in GJIC with nontumorigenic astrocytes, an interaction that promotes GBM cells’ invasiveness [469,470]. A study found that interfering with this communication, by genetically eliminating Cx43 from astrocytes, counteracts the GBM invasive activity [471]. Similarly, genetic depletion of the TJ protein E-cadherin was shown to contribute to the gain by breast tumors of the ductal subtype (IDC) of molecular features of invasive lobular breast carcinoma (ILC) [472]. In both cases, the data indicate that GJs and TJs could have a role in specific cellular subsets, although whether this would ultimately contribute to tumor heterogeneity awaits further elucidation.
This being said, apart from clonal selection, it has been suggested that cancer stem cells (CSCs) could also contribute to tumor heterogeneity [473,474,475,476]. Therefore, intercellular communication could also have a role in tumor heterogeneity via a function in cellular stemness. In fact, there are many studies in favor of a role of GJs [477,478] and TJ proteins [479,480,481] in CSCs, and other studies are concerned with the impact of EVs secreted by CSCs on tumor growth, progression, immune surveillance, metastasis, drug resistance and disease relapse [482,483,484]. TNTs were found to transfer mitochondria between GBM stem cells [485]. There are also studies that show for instance that mesenchymal stem cells (MSC) use TNTs to transfer mitochondria to glioma stem cells (GSCs), a mechanism that enhances GSCs’ resistance to the GBM drug temozolomide (TMZ) [486]. Eph signaling was also reported to impact tumor cell dormancy and CSCs’ enrichment after chemotherapy [487]. However, intriguingly, there is little evidence, if any, formally establishing a causality link between any of these intercellular communication modes and development or maintenance of tumor heterogeneity via a function in cancer stemness.
Finally, the diversity of modes of intercellular communication and/or predominance of a specific mode or another might by itself contribute to tumor heterogeneity, if certain cancer cells or colonies are shown to have proclivity to communicate via specific mode(s) rather than others.
9. Integration of Multiple Communication Modes
An essential aspect of understanding the diversity of cell–cell interaction modes is the study of their collaboration or opposition in various contexts, as well as their enrollment in complex meshes of communication. In this regard, evidence is available to show that modal interplays involve structural and functional aspects (Figure 5).
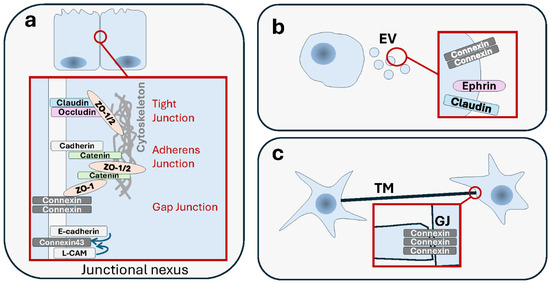
Figure 5.
Interplay between various modes of cell–cell communication. Intercellular communication can be mediated by multiple modes at the same time, in a collaborative or antagonistic manner, depending on the context. (a) Junctional communication modes tight junctions (TJs) and adherens junctions (AJs) are structurally and functionally related and form a junctional complex. This “junctional nexus” interacts also with gap junctions (GJs). (b) Junctional proteins such as connexins or claudins, as well as Eph/ephrin proteins are transported remotely by extracellular vesicles (EVs), whose functions they contribute to. (c) Functional GJs are present in the ends of some TNTs and contribute to electrical coupling.
9.1. Junctional Nexus
The oldest established interplays involved junctions, such as the structural and functional relatedness between desmosomes and AJs, that gave rise to distinct specialized functions [488], or between AJs and TJs [489,490,491]. Connexins interact with many AJ and TJ proteins [492]. An early study has shown that the neural cell adhesion molecule (NCAM)-mediated cell–cell adhesion promotes GJIC, and that blockade of the former interferes with the latter [493]. E-cadherin and the cell–cell adhesion molecule L-CAM exert a control over GJIC, possibly via post-translational modification of Cx43 [494,495]. Reciprocally, overexpression of connexin-26 (Cx26) enhances cancer cell motility in a GJIC-dependent manner, and it also reduces cell adhesiveness and loss of N-cadherins [496]. Association between Cx43 and N-cadherin was shown to be required for the formation of both GJs and AJs [497]. Another study extends the interplay even further by showing the existence of a “junctional nexus” involving connexins along with TJs and AJs, and that is remodeled during mammary gland development [498] (Figure 5). These examples of connections could reflect context-specific functional differences or adaptation processes. When two epithelial cells engage in a new interaction, the changes that occur in the organization of the actin cytoskeleton following AJ formation are a prerequisite for subsequent TJ formation [499]. Genetic loss of DSM-mediated adhesion was shown to disturb TJs’ formation and barrier function in the skin [500], a tissue where AJs and TJs have been shown to be structurally interconnected [501]. In cancer, the interplay between these junctions needs to be seen through the prism of adaptation of tumor cells to the stress induced by loss of adhesion, remodeling of the TME, changes in the identity of interacting neighbors, and engaging in movements. The task is not easy if one needs to obtain not only descriptive data but, more importantly, functional evidence as well. Still, the data for now support that the interplay observed in normal settings is also observed in tumors. Importantly, cell–cell junctions are very often responsive to the same pathological processes, such as disruption in cell polarity and architecture [502]. In the normal mammary epithelial cells, proteins that control cell polarity regulate the establishment of different types of cell junctions together (TJs, AJs and GJs) along the basoapical axis, an association that is important in preventing tumorigenesis [503]. This shared fate has a mechanistic basis that is perturbed in cancer cells. For instance, progastrin, a prohormone whose plasma levels are high in patients with colorectal carcinoma, causes a concomitant dissociation of both TJs and AJs, but via two distinct pathways [504]. IQGAP1, a pro-oncogenic scaffolding protein involved in TJ establishment by impacting claudin localization, also inhibits AJ formation by sequestering E-cadherin from cell–cell contacts [505].
9.2. Cooperation for Long-Distance Action
More recently, evidence started to appear of an interplay between junction-based intercellular communication and EVs. There are data in vitro and in vivo that at least a major component of GJs, i.e., Cx43, is transported by EVs in the form of hexameric channels. Far from being a mere passenger, this Cx43 impacts the function of the supporting EVs. However, more evidence is needed to envisage that EV-born GJ channels are able to elicit GJIC in a manner similar to their cellular counterparts. If proven true, this would raise the question of the added value of using GJs to transfer material that is destined to be delivered via EVs anyway. A possibility is that what matters here is the delivery of GJ structures themselves, making this a mode of long distance and heterologous transfer of readily made GJs destined for rapid use by recipient cells [506]. In addition to EVs, GJs have a connection with TNTs [144,167]. Functional GJs were found in the ends of the membrane projections of TNTs [507]. A study showed that TNT-dependent electrical coupling depends on the presence of GJs [167]. In fact, a type of TNTs which accumulates Cx43 is able to couple cells electrically via GJIC [508].
Uptake by brain microvascular endothelial cells of EVs produced by neutrophils results in the dysregulation of expression of genes associated with TJs, thus increasing the endothelial cells’ permeability, and decreasing their transendothelial electrical resistance (TEER) [509]. EV-mediated transfer of TJ proteins claudin5 was proposed to facilitate leukocyte transendothelial migration (TEM) across the strong TJ-based obstacles part of the blood–brain barrier (BBB), by allowing temporary leukocyte/endothelial contacts [510]. Similarly, long noncoding RNA lnc-MMP2-2 in EVs promotes non-small cell lung cancer (NSCLC) brain metastasis, by dismantling TJs, increasing vascular permeability and compromising the integrity of the BBB [511]. The microRNA miR-105 transported by EVs released by breast cancer metastatic cells was shown to regulate TJs in endothelial cells by downregulating the TJ protein ZO-1, thus increasing vascular permeability, and facilitating metastasis [512].
Another noticeable integrated communication that awaits further investigation occurs between EVs and TNTs [513]. For instance, in mesothelioma cells, EVs were able to accelerate the rate of TNT formation [514]. Furthermore, both TNTs and EVs were concomitantly released by GBM cells under cocaine treatment [515]. Although no mechanistic link was established between the two events, this is in support of the existence of a multimodal and coordinated communication between EVs and TNTs.
Breaking with the classical view that Eph/ephrin interaction necessitates direct proximity of cells, with the occasional possibility of shedding of soluble molecular forms, it was reported that both Ephs and ephrins could be transported by EVs [516]. Osteoclasts release EVs that express ephrin-A2, which allows them the interaction with EphA2-bearing osteoblasts [517]. While these data have been found in the context of neural development and synapse physiology, as well as bone homeostasis, respectively, it would be important to examine whether similar mechanisms occur in cancer. So far, the data are scarce. EphA2 was found to be enriched in a specific subpopulation of EVs from various cancer cells [430]. It was found that EphB2 expressed in EVs that are released by head and neck squamous cell carcinoma cells regulates angiogenesis by stimulating ephrin-B reverse signaling in endothelial cells [518]. As rightly pointed out by the authors of this work, the existence of such a mode of Eph/ephrin long-distance interaction has the advantage of allowing hypoxic cells at the center of the tumor, to release EVs which exit the tumor and stimulate angiogenesis [518]. Senescent cells were shown to secrete EphA2-expressing EVs which promote cancer cells’ proliferation by binding to ephrin-A1 and inducing reverse signaling [519]. Furthermore, not only are EVs released by drug-resistant cancer cells enriched with EphA2, but they also transmit the invasive and metastatic phenotype from drug-resistant to sensitive cancer cells [520]. As much as these findings extend the range of action of Ephs and ephrins, it would also eventually dissociate between forward and reverse arms of the Eph/ephrin-mediated bidirectional signaling.
9.3. Ephs/Ephrins and Junctions: Conflict and Cooperation
Ephs/ephrins and cadherins coordinate their actions to regulate cell segregation and tissue boundary establishment [55,521,522,523]. In fact, evidence is tilted in favor of an effect of Ephs/ephrins on cadherin-based adhesion, despite the fact that there is evidence to support the reciprocal regulation of Eph/ephrin expression and function by cadherins [524,525]. For example, the role of Ephs/ephrins in establishing embryonic boundaries was shown to be mediated by an effect on cadherin-mediated adhesion [526]. Similarly, in the intestinal epithelium, the interaction between EphB receptors and E-cadherins at the interface with ephrin-B1-expressing cells, results in metalloproteinase-mediated cleavage of the E-cadherins and weakening of cell–cell adhesion [527]. EphA2 was reported to regulate TJ formation in brain endothelial cells [528]. Ephrin-A1 stimulation disrupts TJs and AJs, suggesting a role in the regulation of pulmonary vascular permeability [529]. EphB2 and ephrin-B1 expression is upregulated during the process of skin wound repair, resulting in the downregulation of AJ and TJ proteins and weakening of the adhesion between epidermal cells [530]. EphA4 activation results in the loss of AJs more than TJs [531]. Ephrin-B1 binds AJ component Pick1, and overexpression of the former phenocopies the loss of the latter, both resulting in the dissociation of cancer epithelial cells via disruption of the AJs [532]. EphA2 associates with and modulates the localization and function of TJ protein claudin-4, hindering the latter’s cell–cell adhesive function and increasing paracellular permeability [533]. A collaboration between EphA4 and metalloprotease ADAM10 in the cleavage of E-cadherin regulates the integrity of AJs between adjacent pillar cells in the cochlear sensory epithelium [534]. Ephrin-B1 associates physically with TJ proteins claudin-1 or claudin-4, promoting ephrin-B1 phosphorylation, and affecting cell–cell adhesion in cancer cells [535]. A positive feedback loop has even been proposed, in which E-cadherin-based junctions and EphA/ephrinA signaling enhance each other [536]. A difference between Eph/ephrin-mediated and E-cadherin-based interactions could be the propensity of the former to regulate heterotypic contacts at the interface of segregated cell populations, while the latter is more associated with homophilic contacts [537]. In addition to the above-mentioned examples, more evidence exists linking Ephs/ephrins and either TJs or AJs [538,539,540]. We have previously published an extensive survey of the interplay between Ephs/ephrins and junction-based communications in cancer [437].
Furthermore, there is a link between Ephs/ephrins and GJs. For example, Ephs/Ephrins regulate GJIC at the boundary of hindbrain cell populations [541]. Ephrin-B2 enhances the assembly of Cx30-based GJ plaques between non-sensory Deiters’ cells [542]. Mutations in Ephrin-B1 lead to human craniofrontonasal syndrome (CFNS), an effect mediated by changing Cx43 distribution and inhibition of GJIC at ectopic Ephrin boundaries [543]. Tampering with either Ephrin-B1 or Cx32/Cx34-based GJIC results in similar effects on the adhesion and dissociation of embryonic cells [544,545]. At least some of these effects involve physical interactions. EphB1 and EphA1 increase GJIC via phosphorylation of the Cx32 C-terminal domain [546]. There is an interaction between EphB4 and Cx43 in cardiomyocytes and EphB activation results in GJIC inhibition [547]. Interestingly, the possibility for Eph/ephrins to undergo bidirectional and unidirectional signaling provides a basis for discrimination between their bidirectional function in restricting cell intermingling versus their unidirectional role in GJIC [541].
10. Concluding Remarks and Perspectives
Aiming to draw a global overview of membrane-to-membrane-based modes of intercellular communication would have been a colossal task. That was not our aim here. Focusing on a few aspects and illustrative examples, we pointed out distinctive features worth considering in a comparative view. Whenever possible, we discussed the importance of these distinctions in cancer progression. However, some aspects, despite being conceptually important, were left out either because of scarcity of data (such as target determination), immense volume of data (such as the nature of molecular cargo, or signal transduction coupling), prematurity of the task (such as implications for therapeutic targeting) or simply because they have been amply reviewed in previous publications.
What determinants drive and what machinery regulates contact establishment during intercellular communication is an area of research that is amply understudied, particularly for the more recently discovered modes of interaction such as EVs and TNTs. For instance, the presence of specific proteins and peptides such as receptors and adhesion molecules has been involved in cell-specific targeting by EVs [548,549,550]. Various elements such as the cell of origin, the target cells’ identity or the physiological state contribute to this determinism [551]. However, on a larger scale, the mechanistic and biological determinants of EV targeting are yet to be explored.
While it is essential to compare the molecular cargo profile between various modes of communication, possible predilection for the transfer of certain molecular types or functional categories, extent of transfer of complexes, structural vs. bioactive cargo, speed and extent of transfer, etc., recording cargo content would be a daunting task in view of the still rapidly ongoing developments in this area. Suffice it to say that virtually all categories of molecules have been shown to be transferred between cells using these modes, whether different types of nucleic acids, proteins, peptides, ions, etc. Technological profiling tools can only provide part of the answer to the large number of lingering questions. For instance, proteomic analysis of EVs collected from the NCI-60 human tumor cell line panel showed that they reflect the proteomes of their cells of origin [552], and showed an association between the abundance of a subgroup of proteins and specific cancer hallmark signatures [553]. More large-scale methods can evidently provide large data sets. A starting point towards a higher order analysis of the differences and their functional significance would be the establishment of centralized databases (e.g., the EVs’ database ExoCarta: http://www.exocarta.org). Nevertheless, as for all tools of global repertory, new concepts need to be developed to make sense of the collected data. Let us take the case of nucleic acids to illustrate some aspects of the complexity of knowledge that needs to be made sense of. We know that GJs are able to transmit DNA moieties. Small fragments of DNA such as short interfering RNAs (siRNAs) or microRNAs can move via GJs from a target cell to a neighboring cell, where they can inhibit gene expression and even induce resistance of cancer cells to chemotherapy [554,555,556,557]. However, it appears that GJs’ ability to transfer genetic material is somehow limited, at least concerning siRNAs, as the rate of transfer decreases with increasing length [554]. Nevertheless, small RNA transfer through GJs seems to be more efficient than through EVs [555,558]. In addition to proteins, EVs transfer a large variety of nucleic acids, including coding RNAs (mRNAs) and noncoding RNAs (long noncoding RNAs, microRNAs and circular RNAs) [559,560,561]. EV-transferred noncoding RNAs for instance have a role in cancer immune escape [562]. The particular example of ABs also carry nucleic acids, but they differ from EVs in their RNA cargo, with the former being more enriched in rRNA [563]. EVs are able to transfer DNA horizontally between cells [564,565,566,567]. EVs contain microRNAs which contribute to cancer progression by modulating intercellular communication [568]. Furthermore, not only has DNA been detected in ABs upon apoptotic fragmentation [569,570], but it is also used by cells involved in ABs’ phagocytosis [570,571]. EVs can also function as secondary carriers of genetic material, by transferring mitochondria loaded with DNA [572,573]. In fact, mitochondria can release EVs in their own right [574,575,576,577,578]. Interestingly, while higher levels of mitochondrial DNA in the plasma have been associated with cancer [579], it has been shown that a portion of it is carried by EVs [580], and that it can contribute to therapeutic resistance of breast cancer cells [581]. DNA fragments found in the bloodstream, a fraction of which is of mitochondrial origin, are known as circulating free DNA (cfDNAs), and there is some evidence that most of the cfDNAs is contained in EVs [132,582,583]. This is of significance since this blood-circulating genetic material is believed to have an oncogenic potential [584,585,586,587,588,589].
Admittedly, this review does not provide an answer to the intriguing question of the most functionally instrumental difference between modes of communication that rely on chemical release interaction versus those depending on direct membrane-to-membrane interaction. The task is rendered more challenging by the shared features between the two types of modes. Among membrane-based cell–cell communication modes, EVs might be the closest to released single molecules such as growth factors, in that both act at a long distance, including via the bloodstream, thus bypassing the imperative of close proximity which is limited by tissular rigidity and cell density. A major difference, though, is the EVs’ ability to transfer molecular complexes and cocktails rather than single molecules. In other respects, soluble and functional forms of Ephs/ephrins have been reported as a result of cleavage by matrix metalloproteases (e.g., [590,591,592,593,594,595]). However, a key particularity of Ephs and Ephrins is that, unlike communication via growth factor receptors (e.g., EGFR and PDGFR), which are activated by free soluble ligands, both the Eph receptors and the ephrin ligands are membrane-bound and both initiate signaling into their respective cells, whether it is the forward signaling that originates from the Eph receptor or the reverse signaling that originates from the ligand. This is a tremendous difference, as this signaling involves two partner cells that could be of similar or different types or origin, as well as either cancerous or normal. This bidirectionality is also observed in junction-based communication and EVs. It is also noteworthy that another distinctive feature of Ephs/ephrins is that they constitute the largest subgroup of RTKs. This has a double impact. First, biochemically they behave quite like other non-membranous communication receptors such as EGFR or PDGFR and phosphorylation is essential for their roles in cancer [596,597,598,599,600]. Secondly, they too attracted therapeutic targeting attempts based on kinase inhibitors, with more or less success [601,602,603,604].
To end, a chronological sequence of genetic and biochemical events that target specific components of each junction throughout tumor progression has yet to be established, in order to further understand the dynamic of the interplay between the different modes. For instance, the role of junctional complexes, including TJs, as adhesive structures that ensure intercellular association appears, understandably, to be the logical first barrier that tumor cells need to silence in order to metastasize [17,18,605]. However, TJs occasionally have an opposite function by promoting EMT and tumorigenesis [20]. In conclusion, an effort to obtain a global overview of these events is needed, that reaches beyond the critical biochemical and structural commonalities and interconnections, to elucidate a higher order functional significance.
Author Contributions
Conceptualization, M.K.; writing and editing, M.K., T.E. and A.S.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kandouz, M.; Batist, G. Gap junctions and connexins as therapeutic targets in cancer. Expert Opin. Ther. Targets 2010, 14, 681–692. [Google Scholar] [CrossRef]
- Zhou, M.; Zheng, M.; Zhou, X.; Tian, S.; Yang, X.; Ning, Y.; Li, Y.; Zhang, S. The roles of connexins and gap junctions in the progression of cancer. Cell Commun. Signal. 2023, 21, 8. [Google Scholar] [CrossRef] [PubMed]
- Aasen, T.; Mesnil, M.; Naus, C.C.; Lampe, P.D.; Laird, D.W. Gap junctions and cancer: Communicating for 50 years. Nat. Rev. Cancer 2016, 16, 775–788, Erratum in Nat. Rev. Cancer 2017, 17, 74. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.H.; Martin, P.E.M. Gap junctions: Structure and function (Review). Mol. Membr. Biol. 2002, 19, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Loewenstein, W.R. Junctional intercellular communication: The cell-to-cell membrane channel. Physiol. Rev. 1981, 61, 829–913. [Google Scholar] [CrossRef]
- Goldberg, G.S.; Valiunas, V.; Brink, P.R. Selective permeability of gap junction channels. Biochim. Biophys. Acta Biomembr. 2004, 1662, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Spray, D.C.; White, R.L.; Mazet, F.; Bennett, M.V. Regulation of gap junctional conductance. Am. J. Physiol.-Heart Circ. Physiol. 1985, 248, H753–H764. [Google Scholar] [CrossRef] [PubMed]
- Lampe, P.D.; Laird, D.W. Recent advances in connexin gap junction biology. Fac. Rev. 2022, 11, 14. [Google Scholar] [CrossRef]
- Wei, C.-J.; Xu, X.; Lo, C.W. Connexins and cell signaling in development and disease. Annu. Rev. Cell Dev. Biol. 2004, 20, 811–838. [Google Scholar] [CrossRef]
- Segretain, D.; Falk, M.M. Regulation of connexin biosynthesis, assembly, gap junction formation, and removal. Biochim. Biophys. Acta Biomembr. 2004, 1662, 3–21. [Google Scholar] [CrossRef]
- Loewenstein, W.R.; Kanno, Y. Intercellular communication and the control of tissue growth: Lack of communication between cancer cells. Nature 1966, 209, 1248–1249. [Google Scholar] [CrossRef]
- Borek, C.; Higashino, S.; Loewenstein, W.R. Intercellular communication and tissue growth: IV. Conductance of membrane junctions of normal and cancerous cells in culture. J. Membr. Biol. 1969, 1, 274–293. [Google Scholar] [CrossRef]
- Venkatesh, H.S.; Morishita, W.; Geraghty, A.C.; Silverbush, D.; Gillespie, S.M.; Arzt, M.; Tam, L.T.; Espenel, C.; Ponnuswami, A.; Ni, L.; et al. Electrical and synaptic integration of glioma into neural circuits. Nature 2019, 573, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Bukauskas, F.F.; Verselis, V.K. Gap junction channel gating. Biochim. Biophys. Acta Biomembr. 2004, 1662, 42–60. [Google Scholar] [CrossRef]
- Moreno, A.P.; Lau, A.F. Gap junction channel gating modulated through protein phosphorylation. Prog. Biophys. Mol. Biol. 2007, 94, 107–119. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zihni, C.; Mills, C.; Matter, K.; Balda, M.S. Tight junctions: From simple barriers to multifunctional molecular gates. Nat. Rev. Mol. Cell Biol. 2016, 17, 564–580. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.A. The role of tight junctions in cancer metastasis. Semin. Cell Dev. Biol. 2014, 36, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.A.; Jiang, W.G. Loss of tight junction barrier function and its role in cancer metastasis. Biochim. Biophys. Acta Biomembr. 2009, 1788, 872–891. [Google Scholar] [CrossRef]
- Nehme, Z.; Roehlen, N.; Dhawan, P.; Baumert, T.F. Tight Junction Protein Signaling and Cancer Biology. Cells 2023, 12, 243. [Google Scholar] [CrossRef]
- Kyuno, D.; Takasawa, A.; Kikuchi, S.; Takemasa, I.; Osanai, M.; Kojima, T. Role of tight junctions in the epithelial-to-mesenchymal transition of cancer cells. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183503. [Google Scholar] [CrossRef]
- Aijaz, S.; Balda, M.S.; Matter, K. Tight junctions: Molecular architecture and function. Int. Rev. Cytol. 2006, 248, 261–298. [Google Scholar] [CrossRef]
- Martin, T.A.; Mansel, R.E.; Jiang, W.G. Antagonistic effect of NK4 on HGF/SF induced changes in the transendothelial resistance (TER) and paracellular permeability of human vascular endothelial cells. J. Cell. Physiol. 2002, 192, 268–275. [Google Scholar] [CrossRef]
- Hoevel, T.; Macek, R.; Mundigl, O.; Swisshelm, K.; Kubbies, M. Expression and targeting of the tight junction protein CLDN1 in CLDN1-negative human breast tumor cells. J. Cell. Physiol. 2002, 191, 60–68. [Google Scholar] [CrossRef]
- Ren, J.; Hamada, J.; Takeichi, N.; Fujikawa, S.; Kobayashi, H. Ultrastructural differences in junctional intercellular communication between highly and weakly metastatic clones derived from rat mammary carcinoma. Cancer Res. 1990, 50, 358–362. [Google Scholar] [PubMed]
- Satoh, H.; Zhong, Y.; Isomura, H.; Saitoh, M.; Enomoto, K.; Sawada, N.; Mori, M. Localization of 7H6 tight junction-associated antigen along the cell border of vascular endothelial cells correlates with paracellular barrier function against ions, large molecules, and cancer cells. Exp. Cell Res. 1996, 222, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Cummins, P.M. Occludin: One protein, many forms. Mol. Cell. Biol. 2012, 32, 242–250. [Google Scholar] [CrossRef]
- Steed, E.; Balda, M.S.; Matter, K. Dynamics and functions of tight junctions. Trends Cell Biol. 2010, 20, 142–149. [Google Scholar] [CrossRef]
- Bhat, A.A.; Uppada, S.; Achkar, I.W.; Hashem, S.; Yadav, S.K.; Shanmugakonar, M.; Al-Naemi, H.A.; Haris, M.; Uddin, S. Tight Junction Proteins and Signaling Pathways in Cancer and Inflammation: A Functional Crosstalk. Front. Physiol. 2018, 9, 1942. [Google Scholar] [CrossRef]
- Garcia, M.A.; Nelson, W.J.; Chavez, N. Cell–Cell Junctions Organize Structural and Signaling Networks. Cold Spring Harb. Perspect. Biol. 2018, 10, a029181. [Google Scholar] [CrossRef]
- Singh, A.B.; Uppada, S.B.; Dhawan, P. Claudin proteins, outside-in signaling, and carcinogenesis. Pflügers Arch. Eur. J. Physiol. 2017, 469, 69–75. [Google Scholar] [CrossRef]
- Van Itallie, C.M.; Anderson, J.M. Claudin interactions in and out of the tight junction. Tissue Barriers 2013, 1, e25247. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-W.; Zhang, W.-H.; Danil, G.; Yang, K.; Hu, J.-K. The role and mechanism of claudins in cancer. Front. Oncol. 2022, 12, 1051497. [Google Scholar] [CrossRef]
- Hegazy, M.; Perl, A.L.; Svoboda, S.A.; Green, K.J. Desmosomal Cadherins in Health and Disease. Annu. Rev. Pathol. Mech. Dis. 2022, 17, 47–72. [Google Scholar] [CrossRef] [PubMed]
- Chidgey, M.; Dawson, C. Desmosomes: A role in cancer? Br. J. Cancer 2007, 96, 1783–1787. [Google Scholar] [CrossRef] [PubMed]
- Broussard, J.A.; Getsios, S.; Green, K.J. Desmosome regulation and signaling in disease. Cell Tissue Res. 2015, 360, 501–512. [Google Scholar] [CrossRef]
- Tselepis, C.; Chidgey, M.; North, A.; Garrod, D. Desmosomal adhesion inhibits invasive behavior. Proc. Natl. Acad. Sci. USA 1998, 95, 8064–8069. [Google Scholar] [CrossRef]
- Huber, O.; Petersen, I. 150th Anniversary Series: Desmosomes and the Hallmarks of Cancer. Cell Commun. Adhes. 2015, 22, 15–28. [Google Scholar] [CrossRef]
- Hatzfeld, M.; Wolf, A.; Keil, R. Plakophilins in desmosomal adhesion and signaling. Cell Commun. Adhes. 2014, 21, 25–42. [Google Scholar] [CrossRef]
- Dusek, R.L.; Attardi, L.D. Desmosomes: New perpetrators in tumour suppression. Nat. Rev. Cancer 2011, 11, 317–323. [Google Scholar] [CrossRef]
- Franke, W.W.; Rickelt, S.; Barth, M.; Pieperhoff, S. The junctions that don’t fit the scheme: Special symmetrical cell-cell junctions of their own kind. Cell Tissue Res. 2009, 338, 1–17. [Google Scholar] [CrossRef]
- Frisén, J.; Holmberg, J.; Barbacid, M. Ephrins and their Eph receptors: Multitalented directors of embryonic development. EMBO J. 1999, 18, 5159–5165. [Google Scholar] [CrossRef]
- Holder, N.; Klein, R. Eph receptors and ephrins: Effectors of morphogenesis. Development 1999, 126, 2033–2044. [Google Scholar] [CrossRef]
- O’Leary, D.D.; Wilkinson, D.G. Eph receptors and ephrins in neural development. Curr. Opin. Neurobiol. 1999, 9, 65–73. [Google Scholar] [CrossRef]
- Yancopoulos, G.D.; Klagsbrun, M.; Folkman, J. Vasculogenesis, angiogenesis, and growth factors: Ephrins enter the fray at the border. Cell 1998, 93, 661–664. [Google Scholar] [CrossRef]
- Flenniken, A.M.; Gale, N.W.; Yancopoulos, G.D.; Wilkinson, D.G. Distinct and overlapping expression patterns of ligands for Eph-related receptor tyrosine kinases during mouse embryogenesis. Dev. Biol. 1996, 179, 382–401. [Google Scholar] [CrossRef]
- Friedman, G.C.; O’Leary, D.D. Eph receptor tyrosine kinases and their ligands in neural development. Curr. Opin. Neurobiol. 1996, 6, 127–133. [Google Scholar] [CrossRef]
- Merlos-Suárez, A.; Batlle, E. Eph–ephrin signalling in adult tissues and cancer. Curr. Opin. Cell Biol. 2008, 20, 194–200. [Google Scholar] [CrossRef]
- Pasquale, E.B. Eph-ephrin bidirectional signaling in physiology and disease. Cell 2008, 133, 38–52. [Google Scholar] [CrossRef]
- Hruska, M.; Dalva, M.B. Ephrin regulation of synapse formation, function and plasticity. Mol. Cell. Neurosci. 2012, 50, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Compagni, A.; Logan, M.; Klein, R.; Adams, R.H. Control of skeletal patterning by ephrinB1-EphB interactions. Dev. Cell 2003, 5, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Kuijper, S.; Turner, C.J.; Adams, R.H. Regulation of angiogenesis by Eph–ephrin interactions. Trends Cardiovasc. Med. 2007, 17, 145–151. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Pasquale, E.B. Eph receptors in the adult brain. Curr. Opin. Neurobiol. 2004, 14, 288–296. [Google Scholar] [CrossRef]
- Klein, R. Eph/ephrin signalling during development. Development 2012, 139, 4105–4109. [Google Scholar] [CrossRef]
- Coulthard, M.G.; Duffy, S.; Down, M.; Evans, B.; Power, M.; Smith, F.; Stylianou, C.; Kleikamp, S.; Oates, A.; Lackmann, M.; et al. The role of the Eph-ephrin signalling system in the regulation of developmental patterning. Int. J. Dev. Biol. 2002, 46, 375–384. [Google Scholar] [PubMed]
- Cayuso, J.; Xu, Q.; Wilkinson, D.G. Mechanisms of boundary formation by Eph receptor and ephrin signaling. Dev. Biol. 2015, 401, 122–131. [Google Scholar] [CrossRef]
- Anderton, M.; van der Meulen, E.; Blumenthal, M.J.; Schäfer, G. The Role of the Eph Receptor Family in Tumorigenesis. Cancers 2021, 13, 206. [Google Scholar] [CrossRef] [PubMed]
- Janes, P.W.; Vail, M.E.; Ernst, M.; Scott, A.M. Eph Receptors in the Immunosuppressive Tumor Microenvironment. Cancer Res. 2021, 81, 801–805. [Google Scholar] [CrossRef]
- Zhou, X.; Tu, P.; Chen, X.; Guo, S.; Wang, J. Eph Receptors: Actors in Tumor Microenvironment. Crit. Rev. Oncog. 2017, 22, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Genander, M.; Frisén, J. Eph receptors tangled up in two: Independent control of cell positioning and proliferation. Cell Cycle 2010, 9, 1865–1866. [Google Scholar] [CrossRef]
- Holmberg, J.; Genander, M.; Halford, M.M.; Annerén, C.; Sondell, M.; Chumley, M.J.; Silvany, R.E.; Henkemeyer, M.; Frisén, J. EphB receptors coordinate migration and proliferation in the intestinal stem cell niche. Cell 2006, 125, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Solanas, G.; Batlle, E. Control of cell adhesion and compartmentalization in the intestinal epithelium. Exp. Cell Res. 2011, 317, 2695–2701. [Google Scholar] [CrossRef]
- Mayor, R.; Carmona-Fontaine, C. Keeping in touch with contact inhibition of locomotion. Trends Cell Biol. 2010, 20, 319–328. [Google Scholar] [CrossRef]
- Batson, J.; Astin, J.W.; Nobes, C.D. Regulation of contact inhibition of locomotion by Eph–ephrin signalling. J. Microsc. 2013, 251, 232–241. [Google Scholar] [CrossRef]
- Wang, B. Cancer cells exploit the Eph-ephrin system to promote invasion and metastasis: Tales of unwitting partners. Sci. Signal. 2011, 4, pe28. [Google Scholar] [CrossRef]
- Astin, J.W.; Batson, J.; Kadir, S.; Charlet, J.; Persad, R.A.; Gillatt, D.; Oxley, J.D.; Nobes, C.D. Competition amongst Eph receptors regulates contact inhibition of locomotion and invasiveness in prostate cancer cells. Nat. Cell Biol. 2010, 12, 1194–1204. [Google Scholar] [CrossRef]
- Batlle, E.; Henderson, J.T.; Beghtel, H.; van den Born, M.M.; Sancho, E.; Huls, G.; Meeldijk, J.; Robertson, J.; van de Wetering, M.; Pawson, T.; et al. β-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell 2002, 111, 251–263. [Google Scholar] [CrossRef]
- Cortina, C.; Palomo-Ponce, S.; Iglesias, M.; Fernández-Masip, J.L.; Vivancos, A.; Whissell, G.; Humà, M.; Peiró, N.; Gallego, L.; Jonkheer, S.; et al. EphB–ephrin-B interactions suppress colorectal cancer progression by compartmentalizing tumor cells. Nat. Genet. 2007, 39, 1376–1383. [Google Scholar] [CrossRef]
- Santavanond, J.P.; Rutter, S.F.; Atkin-Smith, G.K.; Poon, I.K.H. Apoptotic Bodies: Mechanism of Formation, Isolation and Functional Relevance. Subcell. Biochem. 2021, 97, 61–88. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- György, B.; Szabó, T.G.; Pásztói, M.; Pál, Z.; Misják, P.; Aradi, B.; László, V.; Pállinger, É.; Pap, E.; Kittel, Á.; et al. Membrane vesicles, current state-of-the-art: Emerging role of extracellular vesicles. Cell. Mol. Life Sci. 2011, 68, 2667–2688. [Google Scholar] [CrossRef] [PubMed]
- Tixeira, R.; Caruso, S.; Paone, S.; Baxter, A.A.; Atkin-Smith, G.K.; Hulett, M.D.; Poon, I.K.H. Defining the morphologic features and products of cell disassembly during apoptosis. Apoptosis 2017, 22, 475–477. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Gueydan, C.; Han, J. Plasma membrane changes during programmed cell deaths. Cell Res. 2018, 28, 9–21. [Google Scholar] [CrossRef]
- Schiller, M.; Bekeredjian-Ding, I.; Heyder, P.; Blank, N.; Ho, A.D.; Lorenz, H.-M. Autoantigens are translocated into small apoptotic bodies during early stages of apoptosis. Cell Death Differ. 2008, 15, 183–191. [Google Scholar] [CrossRef]
- Maas, S.L.N.; Breakefield, X.O.; Weaver, A.M. Extracellular Vesicles: Unique Intercellular Delivery Vehicles. Trends Cell Biol. 2017, 27, 172–188. [Google Scholar] [CrossRef]
- Théry, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef]
- Nieuwland, R.; Falcon-Perez, J.M.; Soekmadji, C.; Boilard, E.; Carter, D.; Buzas, E.I. Essentials of extracellular vesicles: Posters on basic and clinical aspects of extracellular vesicles. J. Extracell. Vesicles 2018, 7, 1548234. [Google Scholar] [CrossRef]
- Tricarico, C.; Clancy, J.; D’Souza-Schorey, C. Biology and biogenesis of shed microvesicles. Small GTPases 2017, 8, 220–232. [Google Scholar] [CrossRef]
- Abels, E.R.; Breakefield, X.O. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell. Mol. Neurobiol. 2016, 36, 301–312. [Google Scholar] [CrossRef]
- El Andaloussi, S.; Mäger, I.; Breakefield, X.O.; Wood, M.J.A. Extracellular vesicles: Biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 2013, 12, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Gézsi, A.; Kovács, Á.; Visnovitz, T.; Buzás, E.I. Systems biology approaches to investigating the roles of extracellular vesicles in human diseases. Exp. Mol. Med. 2019, 51, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Moita, C.; van Niel, G.; Kowal, J.; Vigneron, J.; Benaroch, P.; Manel, N.; Moita, L.F.; Théry, C.; Raposo, G. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J. Cell Sci. 2013, 126, 5553–5565. [Google Scholar] [CrossRef]
- Guescini, M.; Guidolin, D.; Vallorani, L.; Casadei, L.; Gioacchini, A.M.; Tibollo, P.; Battistelli, M.; Falcieri, E.; Battistin, L.; Agnati, L.F.; et al. C2C12 myoblasts release micro-vesicles containing mtDNA and proteins involved in signal transduction. Exp. Cell Res. 2010, 316, 1977–1984. [Google Scholar] [CrossRef]
- Loyer, X.; Vion, A.-C.; Tedgui, A.; Boulanger, C.M. Microvesicles as cell–cell messengers in cardiovascular diseases. Circ. Res. 2014, 114, 345–353. [Google Scholar] [CrossRef]
- Simons, M.; Raposo, G. Exosomes–vesicular carriers for intercellular communication. Curr. Opin. Cell Biol. 2009, 21, 575–581. [Google Scholar] [CrossRef]
- Ludwig, A.-K.; Giebel, B. Exosomes: Small vesicles participating in intercellular communication. Int. J. Biochem. Cell Biol. 2012, 44, 11–15. [Google Scholar] [CrossRef]
- Lopatina, T.; Gai, C.; Deregibus, M.C.; Kholia, S.; Camussi, G. Cross Talk between Cancer and Mesenchymal Stem Cells through Extracellular Vesicles Carrying Nucleic Acids. Front. Oncol. 2016, 6, 125. [Google Scholar] [CrossRef] [PubMed]
- Frühbeis, C.; Fröhlich, D.; Kuo, W.P.; Krämer-Albers, E.-M. Extracellular vesicles as mediators of neuron-glia communication. Front. Cell Neurosci. 2013, 7, 182. [Google Scholar] [CrossRef] [PubMed]
- Wendler, F.; Bota-Rabassedas, N.; Franch-Marro, X. Cancer becomes wasteful: Emerging roles of exosomes† in cell-fate determination. J. Extracell. Vesicles 2013, 2, 22390. [Google Scholar] [CrossRef] [PubMed]
- Wolfers, J.; Lozier, A.; Raposo, G.; Regnault, A.; Théry, C.; Masurier, C.; Flament, C.; Pouzieux, S.; Faure, F.; Tursz, T.; et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat. Med. 2001, 7, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Ostrowski, M.; Segura, E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009, 9, 581–593. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Taylor, D.D.; Gerçel-Taylor, C. Tumour-derived exosomes and their role in cancer-associated T-cell signalling defects. Br. J. Cancer 2005, 92, 305–311. [Google Scholar] [CrossRef]
- Théry, C.; Regnault, A.; Garin, J.; Wolfers, J.; Zitvogel, L.; Ricciardi-Castagnoli, P.; Raposo, G.; Amigorena, S. Molecular characterization of dendritic cell-derived exosomes: Selective accumulation of the heat shock protein hsc73. J. Cell Biol. 1999, 147, 599–610. [Google Scholar] [CrossRef]
- Wilson, C.M.; Naves, T.; Vincent, F.; Melloni, B.; Bonnaud, F.; Lalloué, F.; Jauberteau, M.-O. Sortilin mediates the release and transfer of exosomes in concert with two tyrosine kinase receptors. J. Cell Sci. 2014, 127, 3983–3997. [Google Scholar] [CrossRef]
- Verma, M.; Lam, T.K.; Hebert, E.; Divi, R.L. Extracellular vesicles: Potential applications in cancer diagnosis, prognosis, and epidemiology. BMC Clin. Pathol. 2015, 15, 6. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, J. Malignant messengers. Science 2016, 352, 164–166. [Google Scholar] [CrossRef]
- Al-Nedawi, K.; Meehan, B.; Rak, J. Microvesicles: Messengers and mediators of tumor progression. Cell Cycle 2009, 8, 2014–2018. [Google Scholar] [CrossRef]
- Kalluri, R. The biology and function of exosomes in cancer. J. Clin. Investig. 2016, 126, 1208–1215. [Google Scholar] [CrossRef]
- Zoller, M. Exosomes in Cancer Disease. Methods Mol. Biol. 2016, 1381, 111–149. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, T.L. Tumor-Derived Exosomes and Their Role in Cancer Progression. Adv. Clin. Chem. 2016, 74, 103–141. [Google Scholar] [CrossRef]
- Webber, J.; Yeung, V.; Clayton, A. Extracellular vesicles as modulators of the cancer microenvironment. Semin. Cell Dev. Biol. 2015, 40, 27–34. [Google Scholar] [CrossRef]
- Hood, J.L.; San, R.S.; Wickline, S.A. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res. 2011, 71, 3792–3801. [Google Scholar] [CrossRef]
- Rak, J. Microparticles in cancer. Semin. Thromb. Hemost. 2010, 36, 888–906. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.; Thakur, B.K.; Weiss, J.M.; Kim, H.S.; Peinado, H.; Lyden, D. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell 2016, 30, 836–848. [Google Scholar] [CrossRef]
- Zhang, M.; Lin, Y.; Chen, R.; Yu, H.; Li, Y.; Chen, M.; Dou, C.; Yin, P.; Zhang, L.; Tang, P. Ghost messages: Cell death signals spread. Cell Commun. Signal. 2023, 21, 6. [Google Scholar] [CrossRef]
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef]
- Cline, A.M.; Radic, M.Z. Apoptosis, subcellular particles, and autoimmunity. Clin. Immunol. 2004, 112, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Lane, J.D.; Allan, V.J.; Woodman, P.G. Active relocation of chromatin and endoplasmic reticulum into blebs in late apoptotic cells. J. Cell Sci. 2005, 118, 4059–4071. [Google Scholar] [CrossRef]
- Orlando, K.A.; Stone, N.L.; Pittman, R.N. Rho kinase regulates fragmentation and phagocytosis of apoptotic cells. Exp. Cell Res. 2006, 312, 5–15. [Google Scholar] [CrossRef]
- Sebbagh, M.; Hamelin, J.; Bertoglio, J.; Solary, E.; Breard, J. Direct cleavage of ROCK II by granzyme B induces target cell membrane blebbing in a caspase-independent manner. J. Exp. Med. 2005, 201, 465–471. [Google Scholar] [CrossRef]
- Akers, J.C.; Gonda, D.; Kim, R.; Carter, B.S.; Chen, C.C. Biogenesis of extracellular vesicles (EV): Exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J. Neuro-Oncol. 2013, 113, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Povea-Cabello, S.; Oropesa-Ávila, M.; De la Cruz-Ojeda, P.; Villanueva-Paz, M.; De la Mata, M.; Suárez-Rivero, J.M.; Álvarez-Córdoba, M.; Villalón-García, I.; Cotán, D.; Ybot-González, P.; et al. Dynamic Reorganization of the Cytoskeleton during Apoptosis: The Two Coffins Hypothesis. Int. J. Mol. Sci. 2017, 18, 2393. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Kalra, H.; Drummen, G.P.C.; Mathivanan, S. Focus on Extracellular Vesicles: Introducing the Next Small Big Thing. Int. J. Mol. Sci. 2016, 17, 170. [Google Scholar] [CrossRef]
- Than, U.T.T.; Guanzon, D.; Broadbent, J.A.; Leavesley, D.I.; Salomon, C.; Parker, T.J. Differential Expression of Keratinocyte-Derived Extracellular Vesicle Mirnas Discriminate Exosomes from Apoptotic Bodies and Microvesicles. Front. Endocrinol. 2018, 9, 535. [Google Scholar] [CrossRef] [PubMed]
- Cocca, B.A.; Cline, A.M.; Radic, M.Z. Blebs and apoptotic bodies are B cell autoantigens. J. Immunol. 2002, 169, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Segura, E.; Guerin, C.; Hogg, N.; Amigorena, S.; Thery, C. CD8+ dendritic cells use LFA-1 to capture MHC-peptide complexes from exosomes in vivo. J. Immunol. 2007, 179, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Duban, L.; Segura, E.; Véron, P.; Lantz, O.; Amigorena, S. Indirect activation of naïve CD4+ T cells by dendritic cell–derived exosomes. Nat. Immunol. 2002, 3, 1156–1162. [Google Scholar] [CrossRef]
- Martin, S.; Tesse, A.; Hugel, B.; Martinez, M.C.; Morel, O.; Freyssinet, J.-M.; Andriantsitohaina, R. Shed membrane particles from T lymphocytes impair endothelial function and regulate endothelial protein expression. Circulation 2004, 109, 1653–1659. [Google Scholar] [CrossRef]
- Murray, P.J. Macrophage Polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef]
- Ma, Q.; Liang, M.; Wu, Y.; Luo, F.; Ma, Z.; Dong, S.; Xu, J.; Dou, C. Osteoclast-derived apoptotic bodies couple bone resorption and formation in bone remodeling. Bone Res. 2021, 9, 5. [Google Scholar] [CrossRef]
- Brock, C.K.; Wallin, S.T.; Ruiz, O.E.; Samms, K.M.; Mandal, A.; Sumner, E.A.; Eisenhoffer, G.T. Stem cell proliferation is induced by apoptotic bodies from dying cells during epithelial tissue maintenance. Nat. Commun. 2019, 10, 1044. [Google Scholar] [CrossRef]
- Zhou, M.; Li, Y.-J.; Tang, Y.-C.; Hao, X.-Y.; Xu, W.-J.; Xiang, D.-X.; Wu, J.-Y. Apoptotic bodies for advanced drug delivery and therapy. J. Control. Release 2022, 351, 394–406. [Google Scholar] [CrossRef]
- Aatonen, M.T.; Öhman, T.; Nyman, T.A.; Laitinen, S.; Grönholm, M.; Siljander, P.R.-M. Isolation and characterization of platelet-derived extracellular vesicles. J. Extracell. Vesicles 2014, 3, 24692. [Google Scholar] [CrossRef]
- Goubran, H.; Sabry, W.; Kotb, R.; Seghatchian, J.; Burnouf, T. Platelet microparticles and cancer: An intimate cross-talk. Transfus. Apher. Sci. 2015, 53, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Song, K.S.; Park, Y.S.; Kang, Y.H.; Lee, Y.J.; Lee, K.R.; Kim, H.K.; Ryu, K.W.; Bae, J.M.; Kim, S. Elevated levels of circulating platelet microparticles, VEGF, IL-6 and RANTES in patients with gastric cancer: Possible role of a metastasis predictor. Eur. J. Cancer 2003, 39, 184–191. [Google Scholar] [CrossRef]
- Mezouar, S.; Mege, D.; Darbousset, R.; Farge, D.; Debourdeau, P.; Dignat-George, F.; Panicot-Dubois, L.; Dubois, C. Involvement of platelet-derived microparticles in tumor progression and thrombosis. Semin. Oncol. 2014, 41, 346–358. [Google Scholar] [CrossRef] [PubMed]
- Lazar, S.; Goldfinger, L.E. Platelet Microparticles and miRNA Transfer in Cancer Progression: Many Targets, Modes of Action, and Effects Across Cancer Stages. Front. Cardiovasc. Med. 2018, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Lazar, S.; Goldfinger, L.E. Platelets and extracellular vesicles and their cross talk with cancer. Blood 2021, 137, 3192–3200. [Google Scholar] [CrossRef] [PubMed]
- Aatonen, M.; Grönholm, M.; Siljander, P.R.-M. Platelet-derived microvesicles: Multitalented participants in intercellular communication. Semin. Thromb. Hemost. 2012, 38, 102–113. [Google Scholar] [CrossRef]
- Edelstein, L.C. The role of platelet microvesicles in intercellular communication. Platelets 2017, 28, 222–227. [Google Scholar] [CrossRef]
- Vagner, T.; Spinelli, C.; Minciacchi, V.R.; Balaj, L.; Zandian, M.; Conley, A.; Zijlstra, A.; Freeman, M.R.; Demichelis, F.; De, S.; et al. Large extracellular vesicles carry most of the tumour DNA circulating in prostate cancer patient plasma. J. Extracell. Vesicles 2018, 7, 1505403. [Google Scholar] [CrossRef] [PubMed]
- Di Vizio, D.; Morello, M.; Dudley, A.C.; Schow, P.W.; Adam, R.M.; Morley, S.; Mulholland, D.; Rotinen, M.; Hager, M.H.; Insabato, L.; et al. Large oncosomes in human prostate cancer tissues and in the circulation of mice with metastatic disease. Am. J. Pathol. 2012, 181, 1573–1584. [Google Scholar] [CrossRef]
- Minciacchi, V.R.; Freeman, M.R.; Di Vizio, D. Extracellular vesicles in cancer: Exosomes, microvesicles and the emerging role of large oncosomes. Semin. Cell Dev. Biol. 2015, 40, 41–51. [Google Scholar] [CrossRef]
- Minciacchi, V.R.; You, S.; Spinelli, C.; Morley, S.; Zandian, M.; Aspuria, P.-J.; Cavallini, L.; Ciardiello, C.; Sobreiro, M.R.; Morello, M.; et al. Large oncosomes contain distinct protein cargo and represent a separate functional class of tumor-derived extracellular vesicles. Oncotarget 2015, 6, 11327–11341. [Google Scholar] [CrossRef]
- Zijlstra, A.; Di Vizio, D. Size matters in nanoscale communication. Nat. Cell Biol. 2018, 20, 228–230. [Google Scholar] [CrossRef] [PubMed]
- Melentijevic, I.; Toth, M.L.; Arnold, M.L.; Guasp, R.J.; Harinath, G.; Nguyen, K.C.; Taub, D.; Parker, J.A.; Neri, C.; Gabel, C.V.; et al. C. elegans neurons jettison protein aggregates and mitochondria under neurotoxic stress. Nature 2017, 542, 367–371. [Google Scholar] [CrossRef]
- Ma, L.; Li, Y.; Peng, J.; Wu, D.; Zhao, X.; Cui, Y.; Chen, L.; Yan, X.; Du, Y.; Yu, L. Discovery of the migrasome, an organelle mediating release of cytoplasmic contents during cell migration. Cell Res. 2015, 25, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, W.; Freeman, M.L.; Lederman, M.M.; Vasilieva, E.; Romero, R.; Margolis, L. A System of Cytokines Encapsulated in ExtraCellular Vesicles. Sci. Rep. 2018, 8, 8973. [Google Scholar] [CrossRef]
- Rustom, A.; Saffrich, R.; Markovic, I.; Walther, P.; Gerdes, H.-H. Nanotubular highways for intercellular organelle transport. Science 2004, 303, 1007–1010. [Google Scholar] [CrossRef]
- Ariazi, J.; Benowitz, A.; De Biasi, V.; Den Boer, M.L.; Cherqui, S.; Cui, H.; Douillet, N.; Eugenin, E.A.; Favre, D.; Goodman, S.; et al. Tunneling Nanotubes and Gap Junctions–Their Role in Long-Range Intercellular Communication during Development, Health, and Disease Conditions. Front. Mol. Neurosci. 2017, 10, 333. [Google Scholar] [CrossRef]
- Kimura, S.; Hase, K.; Ohno, H. Tunneling nanotubes: Emerging view of their molecular components and formation mechanisms. Exp. Cell Res. 2012, 318, 1699–1706. [Google Scholar] [CrossRef]
- Marzo, L.; Gousset, K.; Zurzolo, C. Multifaceted roles of tunneling nanotubes in intercellular communication. Front. Physiol. 2012, 3, 72. [Google Scholar] [CrossRef]
- Wang, X.; Gerdes, H.-H. Long-distance electrical coupling via tunneling nanotubes. Biochim. Biophys. Acta Biomembr. 2012, 1818, 2082–2086. [Google Scholar] [CrossRef]
- Dagar, S.; Pathak, D.; Oza, H.V.; Mylavarapu, S.V.S. Tunneling nanotubes and related structures: Molecular mechanisms of formation and function. Biochem. J. 2021, 478, 3977–3998. [Google Scholar] [CrossRef]
- Cordero Cervantes, D.; Zurzolo, C. Peering into tunneling nanotubes—The path forward. EMBO J. 2021, 40, e105789. [Google Scholar] [CrossRef] [PubMed]
- Zurzolo, C. Tunneling nanotubes: Reshaping connectivity. Curr. Opin. Cell Biol. 2021, 71, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Abounit, S.; Zurzolo, C. Wiring through tunneling nanotubes—From electrical signals to organelle transfer. J. Cell Sci. 2012, 125, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.M.; Inaba, M.; Buszczak, M. Specialized Intercellular Communications via Cytonemes and Nanotubes. Annu. Rev. Cell Dev. Biol. 2018, 34, 59–84. [Google Scholar] [CrossRef] [PubMed]
- Korenkova, O.; Pepe, A.; Zurzolo, C. Fine intercellular connections in development: TNTs, cytonemes, or intercellular bridges? Cell Stress. 2020, 4, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Austefjord, M.W.; Gerdes, H.-H.; Wang, X. Tunneling nanotubes: Diversity in morphology and structure. Commun. Integr. Biol. 2014, 7, e27934. [Google Scholar] [CrossRef] [PubMed]
- Lou, E.; Fujisawa, S.; Morozov, A.; Barlas, A.; Romin, Y.; Dogan, Y.; Gholami, S.; Moreira, A.L.; Manova-Todorova, K.; Moore, M.A.S. Tunneling nanotubes provide a unique conduit for intercellular transfer of cellular contents in human malignant pleural mesothelioma. PLoS ONE 2012, 7, e33093. [Google Scholar] [CrossRef] [PubMed]
- Ady, J.W.; Desir, S.; Thayanithy, V.; Vogel, R.I.; Moreira, A.L.; Downey, R.J.; Fong, Y.; Manova-Todorova, K.; Moore, M.A.S.; Lou, E. Intercellular communication in malignant pleural mesothelioma: Properties of tunneling nanotubes. Front. Physiol. 2014, 5, 400. [Google Scholar] [CrossRef] [PubMed]
- Antanavičiūtė, I.; Rysevaitė, K.; Liutkevičius, V.; Marandykina, A.; Rimkutė, L.; Sveikatienė, R.; Uloza, V.; Skeberdis, V.A. Long-distance communication between laryngeal carcinoma cells. PLoS ONE 2014, 9, e99196. [Google Scholar] [CrossRef] [PubMed]
- Desir, S.; Dickson, E.L.; Vogel, R.I.; Thayanithy, V.; Wong, P.; Teoh, D.; Geller, M.A.; Steer, C.J.; Subramanian, S.; Lou, E. Tunneling nanotube formation is stimulated by hypoxia in ovarian cancer cells. Oncotarget 2016, 7, 43150–43161. [Google Scholar] [CrossRef] [PubMed]
- Burtey, A.; Wagner, M.; Hodneland, E.; Skaftnesmo, K.O.; Schoelermann, J.; Mondragon, I.R.; Espedal, H.; Golebiewska, A.; Niclou, S.P.; Bjerkvig, R.; et al. Intercellular transfer of transferrin receptor by a contact-, Rab8-dependent mechanism involving tunneling nanotubes. FASEB J. 2015, 29, 4695–4712. [Google Scholar] [CrossRef]
- Thayanithy, V.; Dickson, E.L.; Steer, C.; Subramanian, S.; Lou, E. Tumor-stromal cross talk: Direct cell-to-cell transfer of oncogenic microRNAs via tunneling nanotubes. Transl. Res. 2014, 164, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Webber, J.P.; Spary, L.K.; Sanders, A.J.; Chowdhury, R.; Jiang, W.G.; Steadman, R.; Wymant, J.; Jones, A.T.; Kynaston, H.; Mason, M.D.; et al. Differentiation of tumour-promoting stromal myofibroblasts by cancer exosomes. Oncogene 2015, 34, 290–302. [Google Scholar] [CrossRef]
- Pinto, G.; Brou, C.; Zurzolo, C. Tunneling Nanotubes: The Fuel of Tumor Progression? Trends Cancer 2020, 6, 874–888. [Google Scholar] [CrossRef]
- Kretschmer, A.; Zhang, F.; Somasekharan, S.P.; Tse, C.; Leachman, L.; Gleave, A.; Li, B.; Asmaro, I.; Huang, T.; Kotula, L.; et al. Stress-induced tunneling nanotubes support treatment adaptation in prostate cancer. Sci. Rep. 2019, 9, 7826. [Google Scholar] [CrossRef]
- Wang, X.; Gerdes, H.-H. Transfer of mitochondria via tunneling nanotubes rescues apoptotic PC12 cells. Cell Death Differ. 2015, 22, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Hanna, S.J.; McCoy-Simandle, K.; Leung, E.; Genna, A.; Condeelis, J.; Cox, D. Tunneling nanotubes, a novel mode of tumor cell-macrophage communication in tumor cell invasion. J. Cell Sci. 2019, 132, jcs223321. [Google Scholar] [CrossRef]
- Osswald, M.; Jung, E.; Sahm, F.; Solecki, G.; Venkataramani, V.; Blaes, J.; Weil, S.; Horstmann, H.; Wiestler, B.; Syed, M.; et al. Brain tumour cells interconnect to a functional and resistant network. Nature 2015, 528, 93–98. [Google Scholar] [CrossRef]
- Venkataramani, V.; Schneider, M.; Giordano, F.A.; Kuner, T.; Wick, W.; Herrlinger, U.; Winkler, F. Disconnecting multicellular networks in brain tumours. Nat. Rev. Cancer 2022, 22, 481–491. [Google Scholar] [CrossRef]
- Osswald, M.; Solecki, G.; Wick, W.; Winkler, F. A malignant cellular network in gliomas: Potential clinical implications. Neuro-Oncology 2016, 18, 479–485. [Google Scholar] [CrossRef]
- Jung, E.; Osswald, M.; Blaes, J.; Wiestler, B.; Sahm, F.; Schmenger, T.; Solecki, G.; Deumelandt, K.; Kurz, F.T.; Xie, R.; et al. Tweety-Homolog 1 Drives Brain Colonization of Gliomas. J. Neurosci. 2017, 37, 6837–6850. [Google Scholar] [CrossRef]
- Wang, X.; Veruki, M.L.; Bukoreshtliev, N.V.; Hartveit, E.; Gerdes, H.H. Animal cells connected by nanotubes can be electrically coupled through interposed gap-junction channels. Proc. Natl. Acad. Sci. USA 2010, 107, 17194–17199. [Google Scholar] [CrossRef]
- Weil, S.; Osswald, M.; Solecki, G.; Grosch, J.; Jung, E.; Lemke, D.; Ratliff, M.; Hänggi, D.; Wick, W.; Winkler, F. Tumor microtubes convey resistance to surgical lesions and chemotherapy in gliomas. Neuro-Oncology 2017, 19, 1316–1326. [Google Scholar] [CrossRef]
- González-Mariscal, L.; Tapia, R.; Chamorro, D. Crosstalk of tight junction components with signaling pathways. Biochim. Biophys. Acta Biomembr. 2008, 1778, 729–756. [Google Scholar] [CrossRef]
- Paris, L.; Tonutti, L.; Vannini, C.; Bazzoni, G. Structural organization of the tight junctions. Biochim. Biophys. Acta Biomembr. 2008, 1778, 646–659. [Google Scholar] [CrossRef]
- Miyoshi, J.; Takai, Y. Structural and functional associations of apical junctions with cytoskeleton. Biochim. Biophys. Acta Biomembr. 2008, 1778, 670–691. [Google Scholar] [CrossRef]
- Schuster, E.; Taftaf, R.; Reduzzi, C.; Albert, M.K.; Romero-Calvo, I.; Liu, H. Better together: Circulating tumor cell clustering in metastatic cancer. Trends Cancer 2021, 7, 1020–1032. [Google Scholar] [CrossRef]
- Massagué, J.; Obenauf, A.C. Metastatic colonization by circulating tumour cells. Nature 2016, 529, 298–306. [Google Scholar] [CrossRef]
- Pereira-Veiga, T.; Schneegans, S.; Pantel, K.; Wikman, H. Circulating tumor cell-blood cell crosstalk: Biology and clinical relevance. Cell Rep. 2022, 40, 111298. [Google Scholar] [CrossRef]
- Aceto, N.; Bardia, A.; Miyamoto, D.T.; Donaldson, M.C.; Wittner, B.S.; Spencer, J.A.; Yu, M.; Pely, A.; Engstrom, A.; Zhu, H.; et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 2014, 158, 1110–1122. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.J.; Padmanaban, V.; Silvestri, V.; Schipper, K.; Cohen, J.D.; Fairchild, A.N.; Gorin, M.A.; Verdone, J.E.; Pienta, K.J.; Bader, J.S.; et al. Polyclonal breast cancer metastases arise from collective dissemination of keratin 14-expressing tumor cell clusters. Proc. Natl. Acad. Sci. USA 2016, 113, E854–E863. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Barclay, M.; Hilkens, J.; Guo, X.; Barrow, H.; Rhodes, J.M.; Yu, L.G. Interaction between circulating galectin-3 and cancer-associated MUC1 enhances tumour cell homotypic aggregation and prevents anoikis. Mol. Cancer 2010, 9, 154. [Google Scholar] [CrossRef] [PubMed]
- Taftaf, R.; Liu, X.; Singh, S.; Jia, Y.; Dashzeveg, N.K.; Hoffmann, A.D.; El-Shennawy, L.; Ramos, E.K.; Adorno-Cruz, V.; Schuster, E.J.; et al. ICAM1 initiates CTC cluster formation and trans-endothelial migration in lung metastasis of breast cancer. Nat. Commun. 2021, 12, 4867. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, H.; Little, J.B. Induction of sister chromatid exchanges by extremely low doses of alpha-particles. Cancer Res. 1992, 52, 6394–6396. [Google Scholar] [PubMed]
- Zhou, H.; Suzuki, M.; Randers-Pehrson, G.; Vannais, D.; Chen, G.; Trosko, J.E.; Waldren, C.A.; Hei, T.K. Radiation risk to low fluences of α particles may be greater than we thought. Proc. Natl. Acad. Sci. USA 2001, 98, 14410–14415. [Google Scholar] [CrossRef]
- Belyakov, O.V.; Mitchell, S.A.; Parikh, D.; Randers-Pehrson, G.; Marino, S.A.; Amundson, S.A.; Geard, C.R.; Brenner, D.J. Biological effects in unirradiated human tissue induced by radiation damage up to 1 mm away. Proc. Natl. Acad. Sci. USA 2005, 102, 14203–14208. [Google Scholar] [CrossRef]
- Gaillard, S.; Pusset, D.; de Toledo, S.M.; Fromm, M.; Azzam, E.I. Propagation distance of the α-particle-induced bystander effect: The role of nuclear traversal and gap junction communication. Radiat. Res. 2009, 171, 513–520. [Google Scholar] [CrossRef]
- Murray, A.W.; Fitzgerald, D.J. Tumor promoters inhibit metabolic cooperation in cocultures of epidermal and 3T3 cells. Biochem. Biophys. Res. Commun. 1979, 91, 395–401. [Google Scholar] [CrossRef]
- Yotti, L.P.; Chang, C.C.; Trosko, J.E. Elimination of metabolic cooperation in Chinese hamster cells by a tumor promoter. Science 1979, 206, 1089–1091. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, H.; Katoh, F. Further evidence for the involvement of gap-junctional intercellular communication in induction and maintenance of transformed foci in BALB/c 3T3 cells. Cancer Res. 1988, 48, 3490–3495. [Google Scholar] [PubMed]
- Fitzgerald, D.J.; Mesnil, M.; Oyamada, M.; Tsuda, H.; Ito, N.; Yamasaki, H. Changes in gap junction protein (connexin 32) gene expression during rat liver carcinogenesis. J. Cell. Biochem. 1989, 41, 97–102. [Google Scholar] [CrossRef]
- Kamibayashi, Y.; Oyamada, Y.; Mori, M.; Oyamada, M. Aberrant expression of gap junction proteins (connexins) is associated with tumor progression during multistage mouse skin carcinogenesis in vivo. Carcinogenesis 1995, 16, 1287–1297. [Google Scholar] [CrossRef] [PubMed]
- Temme, A.; Buchmann, A.; Gabriel, H.D.; Nelles, E.; Schwarz, M.; Willecke, K. High incidence of spontaneous and chemically induced liver tumors in mice deficient for connexin32. Curr. Biol. 1997, 7, 713–716. [Google Scholar] [CrossRef]
- Avanzo, J.L.; Mesnil, M.; Hernandez-Blazquez, F.J.; Mackowiak, I.I.; Mori, C.M.C.; da Silva, T.C.; Oloris, S.C.S.; Gárate, A.P.; Massironi, S.M.G.; Yamasaki, H.; et al. Increased susceptibility to urethane-induced lung tumors in mice with decreased expression of connexin43. Carcinogenesis 2004, 25, 1973–1982. [Google Scholar] [CrossRef]
- Pointis, G.; Fiorini, C.; Gilleron, J.; Carette, D.; Segretain, D. Connexins as precocious markers and molecular targets for chemical and pharmacological agents in carcinogenesis. Curr. Med. Chem. 2007, 14, 2288–2303. [Google Scholar] [CrossRef]
- Hokaiwado, N.; Asamoto, M.; Ogawa, K.; Shirai, T. Transgenic disruption of gap junctional intercellular communication enhances early but not late stage hepatocarcinogenesis in the rat. Toxicol. Pathol. 2005, 33, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Naus, C.C.; Laird, D.W. Implications and challenges of connexin connections to cancer. Nat. Rev. Cancer 2010, 10, 435–441. [Google Scholar] [CrossRef]
- Omori, Y.; Zaidan Dagli, M.L.; Yamakage, K.; Yamasaki, H. Involvement of gap junctions in tumor suppression: Analysis of genetically-manipulated mice. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2001, 477, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Sinyuk, M.; Mulkearns-Hubert, E.E.; Reizes, O.; Lathia, J. Cancer Connectors: Connexins, Gap Junctions, and Communication. Front. Oncol. 2018, 8, 646. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Luo, W.; Zhang, Y.; Liu, F.; Liu, D.; Xu, L.; Qin, L.; Xiong, C.; Lu, Z.; Fang, X.; et al. Intercellular transportation of quantum dots mediated by membrane nanotubes. ACS Nano 2010, 4, 3015–3022. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Thery, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Davy, A.; Soriano, P. Ephrin signaling in vivo: Look both ways. Dev. Dyn. 2005, 232, 1–10. [Google Scholar] [CrossRef]
- Arvanitis, D.; Davy, A. Eph/ephrin signaling: Networks. Genes Dev. 2008, 22, 416–429. [Google Scholar] [CrossRef]
- Klein, R. Bidirectional modulation of synaptic functions by Eph/ephrin signaling. Nat. Neurosci. 2009, 12, 15–20. [Google Scholar] [CrossRef]
- Lisabeth, E.M.; Falivelli, G.; Pasquale, E.B. Eph receptor signaling and ephrins. Cold Spring Harb. Perspect. Biol. 2013, 5, a009159. [Google Scholar] [CrossRef]
- Egea, J.; Klein, R. Bidirectional Eph–ephrin signaling during axon guidance. Trends Cell Biol. 2007, 17, 230–238. [Google Scholar] [CrossRef]
- Aoto, J.; Chen, L. Bidirectional ephrin/Eph signaling in synaptic functions. Brain Res. 2007, 1184, 72–80. [Google Scholar] [CrossRef]
- Noren, N.K.; Pasquale, E.B. Eph receptor–ephrin bidirectional signals that target Ras and Rho proteins. Cell. Signal. 2004, 16, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Murai, K.K.; Pasquale, E.B. ‘Eph’ective signaling: Forward, reverse and crosstalk. J. Cell Sci. 2003, 116, 2823–2832. [Google Scholar] [CrossRef] [PubMed]
- Cowan, C.A.; Henkemeyer, M. Ephrins in reverse, park and drive. Trends Cell Biol. 2002, 12, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Pasquale, E.B. Eph receptor signalling casts a wide net on cell behaviour. Nat. Rev. Mol. Cell Biol. 2005, 6, 462–475. [Google Scholar] [CrossRef] [PubMed]
- Naphade, S.; Sharma, J.; Gaide Chevronnay, H.P.; Shook, M.A.; Yeagy, B.A.; Rocca, C.J.; Ur, S.N.; Lau, A.J.; Courtoy, P.J.; Cherqui, S. Brief reports: Lysosomal cross-correction by hematopoietic stem cell-derived macrophages via tunneling nanotubes. Stem Cells 2015, 33, 301–309. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Wang, L.; Li, L.; Zhu, G. Extracellular vesicle-orchestrated crosstalk between cancer-associated fibroblasts and tumors. Transl. Oncol. 2021, 14, 101231. [Google Scholar] [CrossRef]
- Dunn, G.P.; Bruce, A.T.; Ikeda, H.; Old, L.J.; Schreiber, R.D. Cancer immunoediting: From immunosurveillance to tumor escape. Nat. Immunol. 2002, 3, 991–998. [Google Scholar] [CrossRef]
- Pretti, M.A.M.; Bernardes, S.S.; da Cruz, J.G.V.; Boroni, M.; Possik, P.A. Extracellular vesicle-mediated crosstalk between melanoma and the immune system: Impact on tumor progression and therapy response. J. Leukoc. Biol. 2020, 108, 1101–1115. [Google Scholar] [CrossRef]
- Rubenich, D.S.; Omizzollo, N.; Szczepański, M.J.; Reichert, T.E.; Whiteside, T.L.; Ludwig, N.; Braganhol, E. Small extracellular vesicle-mediated bidirectional crosstalk between neutrophils and tumor cells. Cytokine Growth Factor Rev. 2021, 61, 16–26. [Google Scholar] [CrossRef]
- Ciardiello, C.; Leone, A.; Budillon, A. The Crosstalk between Cancer Stem Cells and Microenvironment Is Critical for Solid Tumor Progression: The Significant Contribution of Extracellular Vesicles. Stem Cells Int. 2018, 2018, 6392198. [Google Scholar] [CrossRef] [PubMed]
- Mittelbrunn, M.; Gutiérrez-Vázquez, C.; Villarroya-Beltri, C.; González, S.; Sánchez-Cabo, F.; González, M.A.; Bernad, A.; Sánchez-Madrid, F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat. Commun. 2011, 2, 282. [Google Scholar] [CrossRef] [PubMed]
- Lindoso, R.S.; Collino, F.; Vieyra, A. Extracellular vesicles as regulators of tumor fate: Crosstalk among cancer stem cells, tumor cells and mesenchymal stem cells. Stem Cell Investig. 2017, 4, 75. [Google Scholar] [CrossRef]
- Laurenzana, I.; Lamorte, D.; Trino, S.; De Luca, L.; Ambrosino, C.; Zoppoli, P.; Ruggieri, V.; Del Vecchio, L.; Musto, P.; Caivano, A.; et al. Extracellular Vesicles: A New Prospective in Crosstalk between Microenvironment and Stem Cells in Hematological Malignancies. Stem Cells Int. 2018, 2018, 9863194. [Google Scholar] [CrossRef]
- Cifuentes-Munoz, N.; El Najjar, F.; Dutch, R.E. Viral cell-to-cell spread: Conventional and non-conventional ways. Adv. Virus Res. 2020, 108, 85–125. [Google Scholar] [CrossRef]
- Meckes, D.G., Jr.; Raab-Traub, N. Microvesicles and viral infection. J. Virol. 2011, 85, 12844–12854. [Google Scholar] [CrossRef]
- Meckes, D.G., Jr.; Gunawardena, H.P.; Dekroon, R.M.; Heaton, P.R.; Edwards, R.H.; Ozgur, S.; Griffith, J.D.; Damania, B.; Raab-Traub, N. Modulation of B-cell exosome proteins by gamma herpesvirus infection. Proc. Natl. Acad. Sci. USA 2013, 110, E2925–E2933. [Google Scholar] [CrossRef]
- Flanagan, J.; Middeldorp, J.; Sculley, T. Localization of the Epstein–Barr virus protein LMP 1 to exosomes. J. Gen. Virol. 2003, 84, 1871–1879. [Google Scholar] [CrossRef] [PubMed]
- Dukers, D.F.; Meij, P.; Vervoort, M.B.; Vos, W.; Scheper, R.J.; Meijer, C.J.; Bloemena, E.; Middeldorp, J.M. Direct immunosuppressive effects of EBV-encoded latent membrane protein 1. J. Immunol. 2000, 165, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Keryer-Bibens, C.; Pioche-Durieu, C.; Villemant, C.; Souquère, S.; Nishi, N.; Hirashima, M.; Middeldorp, J.; Busson, P. Exosomes released by EBV-infected nasopharyngeal carcinoma cells convey the viral latent membrane protein 1 and the immunomodulatory protein galectin 9. BMC Cancer 2006, 6, 283. [Google Scholar] [CrossRef] [PubMed]
- Meckes, D.G., Jr.; Shair, K.H.Y.; Marquitz, A.R.; Kung, C.-P.; Edwards, R.H.; Raab-Traub, N. Human tumor virus utilizes exosomes for intercellular communication. Proc. Natl. Acad. Sci. USA 2010, 107, 20370–20375. [Google Scholar] [CrossRef] [PubMed]
- Meckes, D.G., Jr. Exosomal communication goes viral. J. Virol. 2015, 89, 5200–5203. [Google Scholar] [CrossRef] [PubMed]
- Guenat, D.; Hermetet, F.; Prétet, J.-L.; Mougin, C. Exosomes and Other Extracellular Vesicles in HPV Transmission and Carcinogenesis. Viruses 2017, 9, 211. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Goel, H.; Husain, M.; Lan, X.; Mikulak, J.; Malthotra, A.; Teichberg, S.; Schmidtmayerova, H.; Singhal, P.C. Tubular cell HIV-entry through apoptosed CD4 T cells: A novel pathway. Virology 2012, 434, 68–77. [Google Scholar] [CrossRef]
- Atkin-Smith, G.K.; Duan, M.; Zanker, D.J.; Loh, L.; Nguyen, T.H.O.; Koutsakos, M.; Nguyen, T.; Jiang, X.; Carrera, J.; Phan, T.K.; et al. Monocyte apoptotic bodies are vehicles for influenza A virus propagation. Commun. Biol. 2020, 3, 223. [Google Scholar] [CrossRef]
- Ganesan, M.; Poluektova, L.Y.; Enweluzo, C.; Kharbanda, K.K.; Osna, N.A. Hepatitis C Virus-Infected Apoptotic Hepatocytes Program Macrophages and Hepatic Stellate Cells for Liver Inflammation and Fibrosis Development: Role of Ethanol as a Second Hit. Biomolecules 2018, 8, 113. [Google Scholar] [CrossRef]
- Muster, T.; Waltenberger, A.; Grassauer, A.; Hirschl, S.; Caucig, P.; Romirer, I.; Födinger, D.; Seppele, H.; Schanab, O.; Magin-Lachmann, C.; et al. An endogenous retrovirus derived from human melanoma cells. Cancer Res. 2003, 63, 8735–8741. [Google Scholar]
- Büscher, K.; Hahn, S.; Hofmann, M.; Trefzer, U.; Özel, M.; Sterry, W.; Löwer, J.; Löwer, R.; Kurth, R.; Denner, J. Expression of the human endogenous retrovirus-K transmembrane envelope, Rec and Np9 proteins in melanomas and melanoma cell lines. Melanoma Res. 2006, 16, 223–234. [Google Scholar] [CrossRef]
- Seifarth, W.; Skladny, H.; Krieg-Schneider, F.; Reichert, A.; Hehlmann, R.; Leib-Mösch, C. Retrovirus-like particles released from the human breast cancer cell line T47-D display type B- and C-related endogenous retroviral sequences. J. Virol. 1995, 69, 6408–6416. [Google Scholar] [CrossRef]
- Lai, O.Y.; Chen, H.; Michaud, H.-A.; Hayashi, G.; Kuebler, P.J.; Hultman, G.K.; Ariza, M.-E.; Williams, M.V.; Batista, M.D.; Nixon, D.F.; et al. Protective effect of human endogenous retrovirus K dUTPase variants on psoriasis susceptibility. J. Investig. Dermatol. 2012, 132, 1833–1840. [Google Scholar] [CrossRef]
- Al-Sumidaie, A.M.; Leinster, S.J.; Hart, C.A.; Green, C.D.; McCarthy, K. Particles with properties of retroviruses in monocytes from patients with breast cancer. Lancet 1988, 1, 5–9. [Google Scholar] [CrossRef]
- Bieda, K.; Hoffmann, A.; Boller, K. Phenotypic heterogeneity of human endogenous retrovirus particles produced by teratocarcinoma cell lines. J. Gen. Virol. 2001, 82, 591–596. [Google Scholar] [CrossRef]
- Bronson, D.L.; Fraley, E.E.; Fogh, J.; Kalter, S.S. Induction of retrovirus particles in human testicular tumor (Tera-1) cell cultures: An electron microscopic study. J. Natl. Cancer Inst. 1979, 63, 337–339. [Google Scholar]
- Boller, K.; König, H.; Sauter, M.; Mueller-Lantzsch, N.; Löwer, R.; Löwer, J.; Kurth, R. Evidence that HERV-K is the endogenous retrovirus sequence that codes for the human teratocarcinoma-derived retrovirus HTDV. Virology 1993, 196, 349–353. [Google Scholar] [CrossRef]
- Mueller-Lantzsch, N.; Sauter, M.; Weiskircher, A.; Kramer, K.; Best, B.; Buck, M.; Gräasser, F. Human endogenous retroviral element K10 (HERV-K10) encodes a full-length gag homologous 73-kDa protein and a functional protease. AIDS Res. Hum. Retroviruses 1993, 9, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Dewannieux, M.; Blaise, S.; Heidmann, T. Identification of a functional envelope protein from the HERV-K family of human endogenous retroviruses. J. Virol. 2005, 79, 15573–15577. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, M.; Maire, C.L.; Nuppenau, N.; Habiballa, M.; Uhde, A.; Kolbe, K.; Schröder, T.; Lamszus, K.; Fehse, B.; Głów, D. Deep Characterization and Comparison of Different Retrovirus-like Particles Preloaded with CRISPR/Cas9 RNPs. Int. J. Mol. Sci. 2023, 24, 11399. [Google Scholar] [CrossRef] [PubMed]
- Prachar, J.; Hlubinová, K.; Vrbenská, A.; Matoska, J.; Simkovic, D. Retrovirus-like particles produced by human embryonal cells and cell lines derived from human malignancies. Neoplasma 1986, 33, 551–554. [Google Scholar] [PubMed]
- Van Prooyen, N.; Gold, H.; Andresen, V.; Schwartz, O.; Jones, K.; Ruscetti, F.; Lockett, S.; Gudla, P.; Venzon, D.; Franchini, G. Human T-cell leukemia virus type 1 p8 protein increases cellular conduits and virus transmission. Proc. Natl. Acad. Sci. USA 2010, 107, 20738–20743. [Google Scholar] [CrossRef]
- Malbec, M.; Roesch, F.; Schwartz, O. A new role for the HTLV-1 p8 protein: Increasing intercellular conduits and viral cell-to-cell transmission. Viruses 2011, 3, 254–259. [Google Scholar] [CrossRef]
- Gross, C.; Thoma-Kress, A.K. Molecular Mechanisms of HTLV-1 Cell-to-Cell Transmission. Viruses 2016, 8, 74. [Google Scholar] [CrossRef]
- Kumar, A.; Kim, J.H.; Ranjan, P.; Metcalfe, M.G.; Cao, W.; Mishina, M.; Gangappa, S.; Guo, Z.; Boyden, E.S.; Zaki, S.; et al. Influenza virus exploits tunneling nanotubes for cell-to-cell spread. Sci. Rep. 2017, 7, 40360. [Google Scholar] [CrossRef]
- Peralta, B.; Gil-Carton, D.; Castaño-Díez, D.; Bertin, A.; Boulogne, C.; Oksanen, H.M.; Bamford, D.H.; Abrescia, N.G.A. Mechanism of membranous tunnelling nanotube formation in viral genome delivery. PLoS Biol. 2013, 11, e1001667. [Google Scholar] [CrossRef]
- Eugenin, E.A.; Gaskill, P.J.; Berman, J.W. Tunneling nanotubes (TNT): A potential mechanism for intercellular HIV trafficking. Commun. Integr. Biol. 2009, 2, 243–244. [Google Scholar] [CrossRef]
- Eugenin, E.A.; Gaskill, P.J.; Berman, J.W. Tunneling nanotubes (TNT) are induced by HIV-infection of macrophages: A potential mechanism for intercellular HIV trafficking. Cell. Immunol. 2009, 254, 142–148. [Google Scholar] [CrossRef]
- Sowinski, S.; Jolly, C.; Berninghausen, O.; Purbhoo, M.A.; Chauveau, A.; Köhler, K.; Oddos, S.; Eissmann, P.; Brodsky, F.M.; Hopkins, C.; et al. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat. Cell Biol. 2008, 10, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Bhuyan, F.; Hiyoshi, M.; Noyori, O.; Nasser, H.; Miyazaki, M.; Saito, T.; Kondoh, Y.; Osada, H.; Kimura, S.; et al. Potential Role of the Formation of Tunneling Nanotubes in HIV-1 Spread in Macrophages. J. Immunol. 2016, 196, 1832–1841. [Google Scholar] [CrossRef]
- Jansens, R.J.J.; Tishchenko, A.; Favoreel, H.W. Bridging the Gap: Virus Long-Distance Spread via Tunneling Nanotubes. J. Virol. 2020, 94, e02120-19. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.L.; Manicassamy, B.; Lamb, R.A. Influenza A virus uses intercellular connections to spread to neighboring cells. J. Virol. 2015, 89, 1537–1549. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Katz, B.B.; Tomich, J.M.; Gallagher, T.; Fang, Y. Porcine Reproductive and Respiratory Syndrome Virus Utilizes Nanotubes for Intercellular Spread. J. Virol. 2016, 90, 5163–5175. [Google Scholar] [CrossRef]
- Oelze, I.; Kartenbeck, J.; Crusius, K.; Alonso, A. Human papillomavirus type 16 E5 protein affects cell-cell communication in an epithelial cell line. J. Virol. 1995, 69, 4489–4494. [Google Scholar] [CrossRef]
- Azarnia, R.; Loewenstein, W.R. Intercellular communication and the control of growth: XII. Alteration of junctional permeability by simian virus 40. Roles of the large and small T antigens. J. Membr. Biol. 1984, 82, 213–220. [Google Scholar] [CrossRef]
- Atkinson, M.M.; Menko, A.S.; Johnson, R.G.; Sheppard, J.R.; Sheridan, J.D. Rapid and reversible reduction of junctional permeability in cells infected with a temperature-sensitive mutant of avian sarcoma virus. J. Cell Biol. 1981, 91, 573–578. [Google Scholar] [CrossRef]
- Khan, Z.; Yaiw, K.-C.; Wilhelmi, V.; Lam, H.; Rahbar, A.; Stragliotto, G.; Söderberg-Nauclér, C. Human cytomegalovirus immediate early proteins promote degradation of connexin 43 and disrupt gap junction communication: Implications for a role in gliomagenesis. Carcinogenesis 2014, 35, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Gallego, C.; Jaracz-Ros, A.; Laganà, M.; Mercier-Nomé, F.; Domenichini, S.; Fumagalli, A.; Roingeard, P.; Herfs, M.; Pidoux, G.; Bachelerie, F.; et al. Reprogramming of connexin landscape fosters fast gap junction intercellular communication in human papillomavirus-infected epithelia. Front. Cell. Infect. Microbiol. 2023, 13, 1138232. [Google Scholar] [CrossRef]
- Sun, P.; Dong, L.; MacDonald, A.I.; Akbari, S.; Edward, M.; Hodgins, M.B.; Johnstone, S.R.; Graham, S.V. HPV16 E6 Controls the Gap Junction Protein Cx43 in Cervical Tumour Cells. Viruses 2015, 7, 5243–5256. [Google Scholar] [CrossRef] [PubMed]
- Zong, W.-X.; Rabinowitz, J.D.; White, E. Mitochondria and Cancer. Mol. Cell 2016, 61, 667–676. [Google Scholar] [CrossRef]
- Wallace, D.C. Mitochondria and cancer. Nat. Rev. Cancer 2012, 12, 685–698. [Google Scholar] [CrossRef]
- Klein, K.; He, K.; Younes, A.I.; Barsoumian, H.B.; Chen, D.; Ozgen, T.; Mosaffa, S.; Patel, R.R.; Gu, M.; Novaes, J.; et al. Role of Mitochondria in Cancer Immune Evasion and Potential Therapeutic Approaches. Front. Immunol. 2020, 11, 573326. [Google Scholar] [CrossRef] [PubMed]
- Vasan, K.; Werner, M.; Chandel, N.S. Mitochondrial Metabolism as a Target for Cancer Therapy. Cell Metab. 2020, 32, 341–352. [Google Scholar] [CrossRef]
- Altieri, D.C. Mitochondria in cancer: Clean windmills or stressed tinkerers? Trends Cell Biol. 2023, 33, 293–299. [Google Scholar] [CrossRef]
- Al Amir Dache, Z.; Thierry, A.R. Mitochondria-derived cell-to-cell communication. Cell Rep. 2023, 42, 112728. [Google Scholar] [CrossRef] [PubMed]
- Al Amir Dache, Z.; Otandault, A.; Tanos, R.; Pastor, B.; Meddeb, R.; Sanchez, C.; Arena, G.; Lasorsa, L.; Bennett, A.; Grange, T.; et al. Blood contains circulating cell-free respiratory competent mitochondria. FASEB J. 2020, 34, 3616–3630. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Cordes, N. ATM controls DNA repair and mitochondria transfer between neighboring cells. Cell Commun. Signal. 2019, 17, 144. [Google Scholar] [CrossRef] [PubMed]
- Zampieri, L.X.; Silva-Almeida, C.; Rondeau, J.D.; Sonveaux, P. Mitochondrial Transfer in Cancer: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 3245. [Google Scholar] [CrossRef] [PubMed]
- Torralba, D.; Baixauli, F.; Sánchez-Madrid, F. Mitochondria Know No Boundaries: Mechanisms and Functions of Intercellular Mitochondrial Transfer. Front. Cell Dev. Biol. 2016, 4, 107. [Google Scholar] [CrossRef] [PubMed]
- Norris, R.P. Transfer of mitochondria and endosomes between cells by gap junction internalization. Traffic 2021, 22, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Rogers, R.S.; Bhattacharya, J. When cells become organelle donors. Physiology 2013, 28, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Murray, L.M.A.; Krasnodembskaya, A.D. Concise Review: Intercellular Communication Via Organelle Transfer in the Biology and Therapeutic Applications of Stem Cells. Stem Cells 2019, 37, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, K.; Esposito, E.; Wang, X.; Terasaki, Y.; Liu, Y.; Xing, C.; Ji, X.; Lo, E.H. Transfer of mitochondria from astrocytes to neurons after stroke. Nature 2016, 535, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.U.; Minhas, P.S.; Liddelow, S.A.; Haileselassie, B.; Andreasson, K.I.; Dorn, G.W., 2nd; Mochly-Rosen, D. Fragmented mitochondria released from microglia trigger A1 astrocytic response and propagate inflammatory neurodegeneration. Nat. Neurosci. 2019, 22, 1635–1648. [Google Scholar] [CrossRef] [PubMed]
- Peruzzotti-Jametti, L.; Bernstock, J.D.; Willis, C.M.; Manferrari, G.; Rogall, R.; Fernandez-Vizarra, E.; Williamson, J.C.; Braga, A.; van den Bosch, A.; Leonardi, T.; et al. Neural stem cells traffic functional mitochondria via extracellular vesicles. PLoS Biol. 2021, 19, e3001166. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, N.N.; Zhu, J.; Thompson, C.B. The hallmarks of cancer metabolism: Still emerging. Cell Metab. 2022, 34, 355–377. [Google Scholar] [CrossRef] [PubMed]
- Elia, I.; Haigis, M.C. Metabolites and the tumour microenvironment: From cellular mechanisms to systemic metabolism. Nat. Metab. 2021, 3, 21–32. [Google Scholar] [CrossRef]
- Watson, D.C.; Bayik, D.; Storevik, S.; Moreino, S.S.; Sprowls, S.A.; Han, J.; Augustsson, M.T.; Lauko, A.; Sravya, P.; Røsland, G.V.; et al. GAP43-dependent mitochondria transfer from astrocytes enhances glioblastoma tumorigenicity. Nat. Cancer 2023, 4, 648–664. [Google Scholar] [CrossRef]
- Vaidžiulytė, K.; Coppey, M.; Schauer, K. Intracellular organization in cell polarity—Placing organelles into the polarity loop. J. Cell Sci. 2019, 132, jcs230995. [Google Scholar] [CrossRef]
- Madan, S.; Uttekar, B.; Chowdhary, S.; Rikhy, R. Mitochondria Lead the Way: Mitochondrial Dynamics and Function in Cellular Movements in Development and Disease. Front. Cell Dev. Biol. 2021, 9, 781933. [Google Scholar] [CrossRef]
- Fedorov, A.V.; Chelombitko, M.A.; Chernyavskij, D.A.; Galkin, I.I.; Pletjushkina, O.Y.; Vasilieva, T.V.; Zinovkin, R.A.; Chernyak, B.V. Mitochondria-Targeted Antioxidant SkQ1 Prevents the Development of Experimental Colitis in Mice and Impairment of the Barrier Function of the Intestinal Epithelium. Cells 2022, 11, 3441. [Google Scholar] [CrossRef]
- Gangwar, R.; Meena, A.S.; Shukla, P.K.; Nagaraja, A.S.; Dorniak, P.L.; Pallikuth, S.; Waters, C.M.; Sood, A.; Rao, R. Calcium-mediated oxidative stress: A common mechanism in tight junction disruption by different types of cellular stress. Biochem. J. 2017, 474, 731–749. [Google Scholar] [CrossRef]
- Goodwin, K.; Nelson, C.M. Mechanics of Development. Dev. Cell 2021, 56, 240–250. [Google Scholar] [CrossRef]
- Agarwal, P.; Zaidel-Bar, R. Mechanosensing in embryogenesis. Curr. Opin. Cell Biol. 2021, 68, 1–9. [Google Scholar] [CrossRef]
- Piccolo, S.; Sladitschek-Martens, H.L.; Cordenonsi, M. Mechanosignaling in vertebrate development. Dev. Biol. 2022, 488, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Butcher, D.T.; Alliston, T.; Weaver, V.M. A tense situation: Forcing tumour progression. Nat. Rev. Cancer 2009, 9, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Broders-Bondon, F.; Nguyen Ho-Bouldoires, T.H.; Fernandez-Sanchez, M.-E.; Farge, E. Mechanotransduction in tumor progression: The dark side of the force. J. Cell Biol. 2018, 217, 1571–1587. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.-H.; Jeon, T.J.; Shin, Y.K.; Lee, H.J. Role of extrinsic physical cues in cancer progression. BMB Rep. 2023, 56, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Schwager, S.C.; Taufalele, P.V.; Reinhart-King, C.A. Cell–Cell Mechanical Communication in Cancer. Cell. Mol. Bioeng. 2019, 12, 1–14. [Google Scholar] [CrossRef]
- Northcott, J.M.; Dean, I.S.; Mouw, J.K.; Weaver, V.M. Feeling Stress: The Mechanics of Cancer Progression and Aggression. Front. Cell Dev. Biol. 2018, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Paszek, M.J.; Zahir, N.; Johnson, K.R.; Lakins, J.N.; Rozenberg, G.I.; Gefen, A.; Reinhart-King, C.A.; Margulies, S.S.; Dembo, M.; Boettiger, D.; et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 2005, 8, 241–254. [Google Scholar] [CrossRef]
- Huang, S.; Ingber, D.E. Cell tension, matrix mechanics, and cancer development. Cancer Cell 2005, 8, 175–176. [Google Scholar] [CrossRef]
- Jaalouk, D.E.; Lammerding, J. Mechanotransduction gone awry. Nat. Rev. Mol. Cell Biol. 2009, 10, 63–73. [Google Scholar] [CrossRef]
- Paluch, E.K.; Nelson, C.M.; Biais, N.; Fabry, B.; Moeller, J.; Pruitt, B.L.; Wollnik, C.; Kudryasheva, G.; Rehfeldt, F.; Federle, W. Mechanotransduction: Use the force(s). BMC Biol. 2015, 13, 47. [Google Scholar] [CrossRef] [PubMed]
- Pannekoek, W.-J.; de Rooij, J.; Gloerich, M. Force transduction by cadherin adhesions in morphogenesis. F1000Research 2019, 8, 1044. [Google Scholar] [CrossRef]
- Bosch-Fortea, M.; Martín-Belmonte, F. Mechanosensitive adhesion complexes in epithelial architecture and cancer onset. Curr. Opin. Cell Biol. 2018, 50, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Iskratsch, T.; Wolfenson, H.; Sheetz, M.P. Appreciating force and shape—The rise of mechanotransduction in cell biology. Nat. Rev. Mol. Cell Biol. 2014, 15, 825–833. [Google Scholar] [CrossRef]
- Han, M.K.L.; de Rooij, J. Converging and Unique Mechanisms of Mechanotransduction at Adhesion Sites. Trends Cell Biol. 2016, 26, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Martino, F.; Perestrelo, A.R.; Vinarsky, V.; Pagliari, S.; Forte, G. Cellular Mechanotransduction: From Tension to Function. Front. Physiol. 2018, 9, 824. [Google Scholar] [CrossRef]
- Qi, J.; Chi, L.; Bynum, D.; Banes, A.J. Gap junctions in IL-1β-mediated cell survival response to strain. J. Appl. Physiol. 2011, 110, 1425–1431. [Google Scholar] [CrossRef]
- Maeda, E.; Ye, S.; Wang, W.; Bader, D.L.; Knight, M.M.; Lee, D.A. Gap junction permeability between tenocytes within tendon fascicles is suppressed by tensile loading. Biomech. Model. Mechanobiol. 2012, 11, 439–447. [Google Scholar] [CrossRef]
- Maeda, E.; Ohashi, T. Mechano-regulation of gap junction communications between tendon cells is dependent on the magnitude of tensile strain. Biochem. Biophys. Res. Commun. 2015, 465, 281–286. [Google Scholar] [CrossRef]
- Zhang, Y.; Paul, E.M.; Sathyendra, V.; Davison, A.; Sharkey, N.; Bronson, S.; Srinivasan, S.; Gross, T.S.; Donahue, H.J. Enhanced osteoclastic resorption and responsiveness to mechanical load in gap junction deficient bone. PLoS ONE 2011, 6, e23516. [Google Scholar] [CrossRef]
- Grimston, S.K.; Watkins, M.P.; Brodt, M.D.; Silva, M.J.; Civitelli, R. Enhanced periosteal and endocortical responses to axial tibial compression loading in conditional connexin43 deficient mice. PLoS ONE 2012, 7, e44222. [Google Scholar] [CrossRef]
- Bivi, N.; Pacheco-Costa, R.; Brun, L.R.; Murphy, T.R.; Farlow, N.R.; Robling, A.G.; Bellido, T.; Plotkin, L.I. Absence of Cx43 selectively from osteocytes enhances responsiveness to mechanical force in mice. J. Orthop. Res. 2013, 31, 1075–1081. [Google Scholar] [CrossRef]
- Lloyd, S.A.; Lewis, G.S.; Zhang, Y.; Paul, E.M.; Donahue, H.J. Connexin 43 deficiency attenuates loss of trabecular bone and prevents suppression of cortical bone formation during unloading. J. Bone Miner. Res. 2012, 27, 2359–2372. [Google Scholar] [CrossRef]
- Plotkin, L.I.; Speacht, T.L.; Donahue, H.J. Cx43 and mechanotransduction in bone. Curr. Osteoporos. Rep. 2015, 13, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Zhou, E.; Zhou, J.; Bi, C.; Zhang, Z. Cx43 Facilitates Mesenchymal Transition of Endothelial Cells Induced by Shear Stress. J. Vasc. Res. 2023, 60, 204–212. [Google Scholar] [CrossRef]
- Okamoto, T.; Kawamoto, E.; Takagi, Y.; Akita, N.; Hayashi, T.; Park, E.J.; Suzuki, K.; Shimaoka, M. Gap junction-mediated regulation of endothelial cellular stiffness. Sci. Rep. 2017, 7, 6134. [Google Scholar] [CrossRef]
- Okamoto, T.; Usuda, H.; Tanaka, T.; Wada, K.; Shimaoka, M. The Functional Implications of Endothelial Gap Junctions and Cellular Mechanics in Vascular Angiogenesis. Cancers 2019, 11, 237. [Google Scholar] [CrossRef] [PubMed]
- Stroka, K.M.; Aranda-Espinoza, H. Effects of Morphology vs. Cell–Cell Interactions on Endothelial Cell Stiffness. Cell. Mol. Bioeng. 2011, 4, 9–27. [Google Scholar] [CrossRef]
- Meens, M.J.; Pfenniger, A.; Kwak, B.R.; Delmar, M. Regulation of cardiovascular connexins by mechanical forces and junctions. Cardiovasc. Res. 2013, 99, 304–314. [Google Scholar] [CrossRef]
- Cowan, D.B.; Lye, S.J.; Langille, B.L. Regulation of vascular connexin43 gene expression by mechanical loads. Circ. Res. 1998, 82, 786–793. [Google Scholar] [CrossRef]
- Enomoto, K.; Furuya, K.; Yamagishi, S.; Maeno, T. Mechanically induced electrical and intracellular calcium responses in normal and cancerous mammary cells. Cell Calcium 1992, 13, 501–511. [Google Scholar] [CrossRef]
- Furuya, K.; Enomoto, K.; Yamagishi, S. Spontaneous calcium oscillations and mechanically and chemically induced calcium responses in mammary epithelial cells. Pflügers Arch. 1993, 422, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Gomez, P.; Vereecke, J.; Himpens, B. Intra- and intercellular Ca2+-transient propagation in normal and high glucose solutions in ROS cells during mechanical stimulation. Cell Calcium 2001, 29, 137–148. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, E.; Han, Y.L.; Guo, M.; Shenoy, V.B. Gap junctions amplify spatial variations in cell volume in proliferating tumor spheroids. Nat. Commun. 2020, 11, 6148. [Google Scholar] [CrossRef] [PubMed]
- Giepmans, B.N.G.; Verlaan, I.; Moolenaar, W.H. Connexin-43 interactions with ZO-1 and α- and β-tubulin. Cell Commun. Adhes. 2001, 8, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Giepmans, B.N.; Verlaan, I.; Hengeveld, T.; Janssen, H.; Calafat, J.; Falk, M.M.; Moolenaar, W.H. Gap junction protein connexin-43 interacts directly with microtubules. Curr. Biol. 2001, 11, 1364–1368. [Google Scholar] [CrossRef] [PubMed]
- Basheer, W.A.; Xiao, S.; Epifantseva, I.; Fu, Y.; Kleber, A.G.; Hong, T.; Shaw, R.M. GJA1-20k Arranges Actin to Guide Cx43 Delivery to Cardiac Intercalated Discs. Circ. Res. 2017, 121, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, C.; Ren, C.; Spagnol, G.; Cavin, G.; Cone, A.; Grintsevich, E.E.; Sosinsky, G.E.; Sorgen, P.L. Connexin43 Forms Supramolecular Complexes through Non-Overlapping Binding Sites for Drebrin, Tubulin, and ZO-1. PLoS ONE 2016, 11, e0157073. [Google Scholar] [CrossRef]
- Giepmans, B.N.; Moolenaar, W.H. The gap junction protein connexin43 interacts with the second PDZ domain of the zona occludens-1 protein. Curr. Biol. 1998, 8, 931–934. [Google Scholar] [CrossRef]
- Han, Y.; Wang, H.; Chen, H.; Tan, T.; Wang, Y.; Yang, H.; Ding, Y.; Wang, S. CX43 down-regulation promotes cell aggressiveness and 5-fluorouracil-resistance by attenuating cell stiffness in colorectal carcinoma. Cancer Biol. Ther. 2023, 24, 2221879. [Google Scholar] [CrossRef]
- Nagelkerke, A.; Bussink, J.; Rowan, A.E.; Span, P.N. The mechanical microenvironment in cancer: How physics affects tumours. Semin. Cancer Biol. 2015, 35, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jin, Q.; Yan, T.; Wo, Y.; Liu, H.; Wang, Y. Exosome-mediated transduction of mechanical force regulates prostate cancer migration via microRNA. Biochem. Biophys. Rep. 2022, 31, 101299. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Fang, F.; Zhang, C.; Li, T.; He, J.; Shen, Y.; Yu, H.; Liu, X. Fluid Shear Stress-Induced Exosomes from Liver Cancer Cells Promote Activation of Cancer-Associated Fibroblasts via IGF2-PI3K Axis. Front. Biosci. (Landmark Ed.) 2022, 27, 104. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Goliwas, K.F.; Severino, P.E.; Hough, K.P.; Van Vessem, D.; Wang, H.; Tousif, S.; Koomullil, R.P.; Frost, A.R.; Ponnazhagan, S.; et al. Mechanical strain induces phenotypic changes in breast cancer cells and promotes immunosuppression in the tumor microenvironment. Lab. Investig. 2020, 100, 1503–1516. [Google Scholar] [CrossRef]
- Wu, B.; Liu, D.-A.; Guan, L.; Myint, P.K.; Chin, L.; Dang, H.; Xu, Y.; Ren, J.; Li, T.; Yu, Z.; et al. Stiff matrix induces exosome secretion to promote tumour growth. Nat. Cell Biol. 2023, 25, 415–424. [Google Scholar] [CrossRef]
- Sun, X.; Xie, L.; Qiu, S.; Li, H.; Zhou, Y.; Zhang, H.; Zhang, Y.; Zhang, L.; Xie, T.; Chen, Y.; et al. Elucidation of CKAP4-remodeled cell mechanics in driving metastasis of bladder cancer through aptamer-based target discovery. Proc. Natl. Acad. Sci. USA 2022, 119, e2110500119. [Google Scholar] [CrossRef]
- Xie, F.; Wen, G.; Sun, W.; Jiang, K.; Chen, T.; Chen, S.; Wen, J. Mechanical stress promotes angiogenesis through fibroblast exosomes. Biochem. Biophys. Res. Commun. 2020, 533, 346–353. [Google Scholar] [CrossRef]
- Urciuoli, E.; Peruzzi, B. Mutual Modulation Between Extracellular Vesicles and Mechanoenvironment in Bone Tumors. Front. Cell Dev. Biol. 2021, 9, 789674. [Google Scholar] [CrossRef]
- Kindberg, A.; Hu, J.K.; Bush, J.O. Forced to communicate: Integration of mechanical and biochemical signaling in morphogenesis. Curr. Opin. Cell Biol. 2020, 66, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Batlle, E.; Wilkinson, D.G. Molecular mechanisms of cell segregation and boundary formation in development and tumorigenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008227. [Google Scholar] [CrossRef]
- Rodríguez-Franco, P.; Brugués, A.; Marín-Llauradó, A.; Conte, V.; Solanas, G.; Batlle, E.; Fredberg, J.J.; Roca-Cusachs, P.; Sunyer, R.; Trepat, X. Long-lived force patterns and deformation waves at repulsive epithelial boundaries. Nat. Mater. 2017, 16, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, A.K.; Kindberg, A.A.; Niethamer, T.K.; Larson, A.R.; Ho, H.-Y.H.; Greenberg, M.E.; Bush, J.O. Unidirectional Eph/ephrin signaling creates a cortical actomyosin differential to drive cell segregation. J. Cell Biol. 2016, 215, 217–229. [Google Scholar] [CrossRef]
- Cayuso, J.; Xu, Q.; Addison, M.; Wilkinson, D.G. Actomyosin regulation by Eph receptor signaling couples boundary cell formation to border sharpness. eLife 2019, 8, e49696. [Google Scholar] [CrossRef] [PubMed]
- Fattet, L.; Jung, H.-Y.; Matsumoto, M.W.; Aubol, B.E.; Kumar, A.; Adams, J.A.; Chen, A.C.; Sah, R.L.; Engler, A.J.; Pasquale, E.B.; et al. Matrix Rigidity Controls Epithelial-Mesenchymal Plasticity and Tumor Metastasis via a Mechanoresponsive EPHA2/LYN Complex. Dev. Cell 2020, 54, 302–316.e7. [Google Scholar] [CrossRef]
- Salaita, K.; Nair, P.M.; Petit, R.S.; Neve, R.M.; Das, D.; Gray, J.W.; Groves, J.T. Restriction of receptor movement alters cellular response: Physical force sensing by EphA2. Science 2010, 327, 1380–1385. [Google Scholar] [CrossRef]
- Walker, D. Mechanotransduction: Using the force. Nat. Rev. Mol. Cell Biol. 2010, 11, 311. [Google Scholar] [CrossRef] [PubMed]
- Paszek, M.; Weaver, V. Enforcing order on signaling. Science 2010, 327, 1335–1336. [Google Scholar] [CrossRef]
- Mossman, K.D.; Campi, G.; Groves, J.T.; Dustin, M.L. Altered TCR signaling from geometrically repatterned immunological synapses. Science 2005, 310, 1191–1193. [Google Scholar] [CrossRef]
- Zhang, T.; Hu, W.; Chen, W. Plasma Membrane Integrates Biophysical and Biochemical Regulation to Trigger Immune Receptor Functions. Front. Immunol. 2021, 12, 613185. [Google Scholar] [CrossRef]
- Hurtley, S. Spatial cell biology: Location, location, location. Science 2009, 326, 1205. [Google Scholar] [CrossRef]
- Scott, J.D.; Pawson, T. Cell signaling in space and time: Where proteins come together and when they’re apart. Science 2009, 326, 1220–1224. [Google Scholar] [CrossRef]
- Ladoux, B.; Mège, R.-M. Mechanobiology of collective cell behaviours. Nat. Rev. Mol. Cell Biol. 2017, 18, 743–757. [Google Scholar] [CrossRef]
- Khalil, A.A.; de Rooij, J. Cadherin mechanotransduction in leader-follower cell specification during collective migration. Exp. Cell Res. 2019, 376, 86–91. [Google Scholar] [CrossRef]
- Labernadie, A.; Kato, T.; Brugués, A.; Serra-Picamal, X.; Derzsi, S.; Arwert, E.; Weston, A.; González-Tarragó, V.; Elosegui-Artola, A.; Albertazzi, L.; et al. A mechanically active heterotypic E-cadherin/N-cadherin adhesion enables fibroblasts to drive cancer cell invasion. Nat. Cell Biol. 2017, 19, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Leckband, D.E.; de Rooij, J. Cadherin adhesion and mechanotransduction. Annu. Rev. Cell Dev. Biol. 2014, 30, 291–315. [Google Scholar] [CrossRef] [PubMed]
- Mui, K.L.; Chen, C.S.; Assoian, R.K. The mechanical regulation of integrin–cadherin crosstalk organizes cells, signaling and forces. J. Cell Sci. 2016, 129, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Gumbiner, B.M. Crosstalk between different adhesion molecules. Curr. Opin. Cell Biol. 2006, 18, 572–578. [Google Scholar] [CrossRef]
- Weber, G.F.; Bjerke, M.A.; DeSimone, D.W. Integrins and cadherins join forces to form adhesive networks. J. Cell Sci. 2011, 124, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Zuidema, A.; Wang, W.; Sonnenberg, A. Crosstalk between Cell Adhesion Complexes in Regulation of Mechanotransduction. Bioessays 2020, 42, e2000119. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Tan, J.L.; Cohen, D.M.; Yang, M.T.; Sniadecki, N.J.; Ruiz, S.A.; Nelson, C.M.; Chen, C.S. Mechanical tugging force regulates the size of cell–cell junctions. Proc. Natl. Acad. Sci. USA 2010, 107, 9944–9949. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, J.; de Rooij, J. Mechanical control of the endothelial barrier. Cell Tissue Res. 2014, 355, 545–555. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, E.; Sneh, T.; Moeendarbary, E.; Javanmardi, Y.; Efimova, N.; Yang, C.; Marino-Bravante, G.E.; Chen, X.; Escribano, J.; Spill, F.; et al. Feedback between mechanosensitive signaling and active forces governs endothelial junction integrity. Nat. Commun. 2022, 13, 7089. [Google Scholar] [CrossRef] [PubMed]
- Capuana, L.; Boström, A.; Etienne-Manneville, S. Multicellular scale front-to-rear polarity in collective migration. Curr. Opin. Cell Biol. 2020, 62, 114–122. [Google Scholar] [CrossRef]
- Venhuizen, J.-H.; Zegers, M.M. Making Heads or Tails of It: Cell–Cell Adhesion in Cellular and Supracellular Polarity in Collective Migration. Cold Spring Harb. Perspect. Biol. 2017, 9, a027854. [Google Scholar] [CrossRef]
- Bazellières, E.; Conte, V.; Elosegui-Artola, A.; Serra-Picamal, X.; Bintanel-Morcillo, M.; Roca-Cusachs, P.; Muñoz, J.J.; Sales-Pardo, M.; Guimerà, R.; Trepat, X. Control of cell–cell forces and collective cell dynamics by the intercellular adhesome. Nat. Cell Biol. 2015, 17, 409–420. [Google Scholar] [CrossRef]
- Mayor, R.; Etienne-Manneville, S. The front and rear of collective cell migration. Nat. Rev. Mol. Cell Biol. 2016, 17, 97–109. [Google Scholar] [CrossRef]
- Friedl, P.; Mayor, R. Tuning Collective Cell Migration by Cell–Cell Junction Regulation. Cold Spring Harb. Perspect. Biol. 2017, 9, a029199. [Google Scholar] [CrossRef]
- Collins, C.; Nelson, W.J. Running with neighbors: Coordinating cell migration and cell–cell adhesion. Curr. Opin. Cell Biol. 2015, 36, 62–70. [Google Scholar] [CrossRef]
- Huang, G.Y.; Cooper, E.S.; Waldo, K.; Kirby, M.L.; Gilula, N.B.; Lo, C.W. Gap junction–mediated cell–cell communication modulates mouse neural crest migration. J. Cell Biol. 1998, 143, 1725–1734. [Google Scholar] [CrossRef]
- Lorraine, C.; Wright, C.S.; Martin, P.E. Connexin43 plays diverse roles in co-ordinating cell migration and wound closure events. Biochem. Soc. Trans. 2015, 43, 482–488. [Google Scholar] [CrossRef]
- Li, A.; Cho, J.-H.; Reid, B.; Tseng, C.-C.; He, L.; Tan, P.; Yeh, C.-Y.; Wu, P.; Li, Y.; Widelitz, R.B.; et al. Calcium oscillations coordinate feather mesenchymal cell movement by SHH dependent modulation of gap junction networks. Nat. Commun. 2018, 9, 5377. [Google Scholar] [CrossRef] [PubMed]
- Aftab, Q.; Sin, W.-C.; Naus, C.C. Reduction in gap junction intercellular communication promotes glioma migration. Oncotarget 2015, 6, 11447–11464. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.A.; Ilina, O.; Vasaturo, A.; Venhuizen, J.-H.; Vullings, M.; Venhuizen, V.; Bilos, A.; Figdor, C.G.; Span, P.N.; Friedl, P. Collective invasion induced by an autocrine purinergic loop through connexin-43 hemichannels. J. Cell Biol. 2020, 219, e201911120. [Google Scholar] [CrossRef] [PubMed]
- Foster, K.E.; Gordon, J.; Cardenas, K.; Veiga-Fernandes, H.; Makinen, T.; Grigorieva, E.; Wilkinson, D.G.; Blackburn, C.C.; Richie, E.; Manley, N.R.; et al. EphB–ephrin-B2 interactions are required for thymus migration during organogenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 13414–13419. [Google Scholar] [CrossRef] [PubMed]
- Parrinello, S.; Napoli, I.; Ribeiro, S.; Wingfield Digby, P.; Fedorova, M.; Parkinson, D.B.; Doddrell, R.D.; Nakayama, M.; Adams, R.H.; Lloyd, A.C. EphB signaling directs peripheral nerve regeneration through Sox2-dependent Schwann cell sorting. Cell 2010, 143, 145–155. [Google Scholar] [CrossRef]
- Neijssen, J.; Herberts, C.; Drijfhout, J.W.; Reits, E.; Janssen, L.; Neefjes, J. Cross-presentation by intercellular peptide transfer through gap junctions. Nature 2005, 434, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Herlyn, M. Information sharing and collateral damage. Trends Mol. Med. 2005, 11, 350–352. [Google Scholar] [CrossRef]
- Leithe, E.; Sirnes, S.; Omori, Y.; Rivedal, E. Downregulation of gap junctions in cancer cells. Crit. Rev. Oncog. 2006, 12, 225–256. [Google Scholar] [CrossRef]
- Tittarelli, A.; Mendoza-Naranjo, A.; Farías, M.; Guerrero, I.; Ihara, F.; Wennerberg, E.; Riquelme, S.; Gleisner, A.; Kalergis, A.; Lundqvist, A.; et al. Gap junction intercellular communications regulate NK cell activation and modulate NK cytotoxic capacity. J. Immunol. 2014, 192, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, F.; Navarrete, M.; Álvarez, J.; Guerrero, I.; Gleisner, M.A.; Tittarelli, A.; Salazar-Onfray, F. Cx43-Gap Junctions Accumulate at the Cytotoxic Immunological Synapse Enabling Cytotoxic T Lymphocyte Melanoma Cell Killing. Int. J. Mol. Sci. 2019, 20, 4509. [Google Scholar] [CrossRef]
- Benlalam, H.; Carré, T.; Jalil, A.; Noman, Z.; Caillou, B.; Vielh, P.; Tittarelli, A.; Robert, C.; Chouaib, S. Regulation of gap junctions in melanoma and their impact on Melan-A/MART-1-specific CD8+ T lymphocyte emergence. J. Mol. Med. 2013, 91, 1207–1220. [Google Scholar] [CrossRef] [PubMed]
- Mulkearns-Hubert, E.E.; Reizes, O.; Lathia, J.D. Connexins in Cancer: Jekyll or Hyde? Biomolecules 2020, 10, 1654. [Google Scholar] [CrossRef]
- Gleisner, M.A.; Navarrete, M.; Hofmann, F.; Salazar-Onfray, F.; Tittarelli, A. Mind the Gaps in Tumor Immunity: Impact of Connexin-Mediated Intercellular Connections. Front. Immunol. 2017, 8, 1067. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Naranjo, A.; Saez, P.J.; Johansson, C.C.; Ramirez, M.; Mandakovic, D.; Pereda, C.; Lopez, M.N.; Kiessling, R.; Saez, J.C.; Salazar-Onfray, F. Functional gap junctions facilitate melanoma antigen transfer and cross-presentation between human dendritic cells. J. Immunol. 2007, 178, 6949–6957. [Google Scholar] [CrossRef] [PubMed]
- Escobar, A.; López, M.; Serrano, A.; Ramirez, M.; Pérez, C.; Aguirre, A.; González, R.; Alfaro, J.; Larrondo, M.; Fodor, M.; et al. Dendritic cell immunizations alone or combined with low doses of interleukin-2 induce specific immune responses in melanoma patients. Clin. Exp. Immunol. 2005, 142, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Matsue, H.; Yao, J.; Matsue, K.; Nagasaka, A.; Sugiyama, H.; Aoki, R.; Kitamura, M.; Shimada, S. Gap junction-mediated intercellular communication between dendritic cells (DCs) is required for effective activation of DCs. J. Immunol. 2006, 176, 181–190. [Google Scholar] [CrossRef]
- Benlalam, H.; Jalil, A.; Hasmim, M.; Pang, B.; Tamouza, R.; Mitterrand, M.; Godet, Y.; Lamerant, N.; Robert, C.; Avril, M.-F.; et al. Gap junction communication between autologous endothelial and tumor cells induce cross-recognition and elimination by specific CTL. J. Immunol. 2009, 182, 2654–2664. [Google Scholar] [CrossRef]
- Pang, B.; Neijssen, J.; Qiao, X.; Janssen, L.; Janssen, H.; Lippuner, C.; Neefjes, J. Direct antigen presentation and gap junction mediated cross-presentation during apoptosis. J. Immunol. 2009, 183, 1083–1090. [Google Scholar] [CrossRef]
- Buzas, E.I. The roles of extracellular vesicles in the immune system. Nat. Rev. Immunol. 2023, 23, 236–250. [Google Scholar] [CrossRef]
- Robbins, P.D.; Morelli, A.E. Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 2014, 14, 195–208. [Google Scholar] [CrossRef]
- Yang, P.; Peng, Y.; Feng, Y.; Xu, Z.; Feng, P.; Cao, J.; Chen, Y.; Chen, X.; Cao, X.; Yang, Y.; et al. Immune Cell-Derived Extracellular Vesicles—New Strategies in Cancer Immunotherapy. Front. Immunol. 2021, 12, 771551. [Google Scholar] [CrossRef]
- Xu, Z.; Zeng, S.; Gong, Z.; Yan, Y. Exosome-based immunotherapy: A promising approach for cancer treatment. Mol. Cancer 2020, 19, 160. [Google Scholar] [CrossRef] [PubMed]
- Marar, C.; Starich, B.; Wirtz, D. Extracellular vesicles in immunomodulation and tumor progression. Nat. Immunol. 2021, 22, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Jiang, S. Immune Cell-Derived Exosomes in the Cancer-Immunity Cycle. Trends Cancer 2020, 6, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Lucotti, S.; Kenific, C.M.; Zhang, H.; Lyden, D. Extracellular vesicles and particles impact the systemic landscape of cancer. EMBO J. 2022, 41, e109288. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun, J.; Dastgheyb, R.M.; Li, Z. Tumor-derived extracellular vesicles modulate innate immune responses to affect tumor progression. Front. Immunol. 2022, 13, 1045624. [Google Scholar] [CrossRef] [PubMed]
- Peinado, H.; Lavotshkin, S.; Lyden, D. The secreted factors responsible for pre-metastatic niche formation: Old sayings and new thoughts. Semin. Cancer Biol. 2011, 21, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Ciardiello, C.; Cavallini, L.; Spinelli, C.; Yang, J.; Reis-Sobreiro, M.; de Candia, P.; Minciacchi, V.R.; Di Vizio, D. Focus on Extracellular Vesicles: New Frontiers of Cell-to-Cell Communication in Cancer. Int. J. Mol. Sci. 2016, 17, 175. [Google Scholar] [CrossRef]
- van der Pol, E.; Böing, A.N.; Harrison, P.; Sturk, A.; Nieuwland, R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol. Rev. 2012, 64, 676–705. [Google Scholar] [CrossRef]
- Ratajczak, J.; Wysoczynski, M.; Hayek, F.; Janowska-Wieczorek, A.; Ratajczak, M.Z. Membrane-derived microvesicles: Important and underappreciated mediators of cell-to-cell communication. Leukemia 2006, 20, 1487–1495. [Google Scholar] [CrossRef]
- Zhang, H.-G.; Grizzle, W.E. Exosomes and cancer: A newly described pathway of immune suppression. Clin. Cancer Res. 2011, 17, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, T.L. Exosomes and tumor-mediated immune suppression. J. Clin. Investig. 2016, 126, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.L. Death-defying immunity: Do apoptotic cells influence antigen processing and presentation? Nat. Rev. Immunol. 2004, 4, 223–231. [Google Scholar] [CrossRef]
- Rovere, P.; Vallinoto, C.; Bondanza, A.; Crosti, M.C.; Rescigno, M.; Ricciardi-Castagnoli, P.; Rugarli, C.; Manfredi, A.A. Bystander apoptosis triggers dendritic cell maturation and antigen-presenting function. J. Immunol. 1998, 161, 4467–4471. [Google Scholar] [CrossRef]
- Winau, F.; Kaufmann, S.H.E.; Schaible, U.E. Apoptosis paves the detour path for CD8 T cell activation against intracellular bacteria. Cell Microbiol. 2004, 6, 599–607. [Google Scholar] [CrossRef]
- Winau, F.; Weber, S.; Sad, S.; de Diego, J.; Hoops, S.L.; Breiden, B.; Sandhoff, K.; Brinkmann, V.; Kaufmann, S.H.; Schaible, U.E. Apoptotic vesicles crossprime CD8 T cells and protect against tuberculosis. Immunity 2006, 24, 105–117. [Google Scholar] [CrossRef]
- Bu, N.; Wu, H.; Sun, B.; Zhang, G.; Zhan, S.; Zhang, R.; Zhou, L. Exosome-loaded dendritic cells elicit tumor-specific CD8+ cytotoxic T cells in patients with glioma. J. Neuro-Oncol. 2011, 104, 659–667. [Google Scholar] [CrossRef]
- Bu, N.; Wu, H.; Zhang, G.; Zhan, S.; Zhang, R.; Sun, H.; Du, Y.; Yao, L.; Wang, H. Exosomes from Dendritic Cells Loaded with Chaperone-Rich Cell Lysates Elicit a Potent T Cell Immune Response Against Intracranial Glioma in Mice. J. Mol. Neurosci. 2015, 56, 631–643. [Google Scholar] [CrossRef]
- Andre, F.; Schartz, N.E.; Movassagh, M.; Flament, C.; Pautier, P.; Morice, P.; Pomel, C.; Lhomme, C.; Escudier, B.; Le Chevalier, T.; et al. Malignant effusions and immunogenic tumour-derived exosomes. Lancet 2002, 360, 295–305. [Google Scholar] [CrossRef]
- Mincheva-Nilsson, L.; Baranov, V. Cancer exosomes and NKG2D receptor–ligand interactions: Impairing NKG2D-mediated cytotoxicity and anti-tumour immune surveillance. Semin. Cancer Biol. 2014, 28, 24–30. [Google Scholar] [CrossRef]
- Andreola, G.; Rivoltini, L.; Castelli, C.; Huber, V.; Perego, P.; Deho, P.; Squarcina, P.; Accornero, P.; Lozupone, F.; Lugini, L.; et al. Induction of lymphocyte apoptosis by tumor cell secretion of FasL-bearing microvesicles. J. Exp. Med. 2002, 195, 1303–1316. [Google Scholar] [CrossRef] [PubMed]
- Huber, V.; Fais, S.; Iero, M.; Lugini, L.; Canese, P.; Squarcina, P.; Zaccheddu, A.; Colone, M.; Arancia, G.; Gentile, M.; et al. Human colorectal cancer cells induce T-cell death through release of proapoptotic microvesicles: Role in immune escape. Gastroenterology 2005, 128, 1796–1804. [Google Scholar] [CrossRef]
- Kim, J.W.; Wieckowski, E.; Taylor, D.D.; Reichert, T.E.; Watkins, S.; Whiteside, T.L. Fas ligand-positive membranous vesicles isolated from sera of patients with oral cancer induce apoptosis of activated T lymphocytes. Clin. Cancer Res. 2005, 11, 1010–1020. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Shi, Y.; You, J. Immune Cell Connection by Tunneling Nanotubes: The Impact of Intercellular Cross-Talk on the Immune Response and Its Therapeutic Applications. Mol. Pharm. 2021, 18, 772–786. [Google Scholar] [CrossRef] [PubMed]
- Önfelt, B.; Davis, D.M. Can membrane nanotubes facilitate communication between immune cells? Biochem. Soc. Trans. 2004, 32, 676–678. [Google Scholar] [CrossRef] [PubMed]
- Onfelt, B.; Nedvetzki, S.; Yanagi, K.; Davis, D.M. Cutting edge: Membrane nanotubes connect immune cells. J. Immunol. 2004, 173, 1511–1513. [Google Scholar] [CrossRef]
- Polak, R.; de Rooij, B.; Pieters, R.; den Boer, M.L. B-cell precursor acute lymphoblastic leukemia cells use tunneling nanotubes to orchestrate their microenvironment. Blood 2015, 126, 2404–2414. [Google Scholar] [CrossRef]
- Shiuan, E.; Chen, J. Eph Receptor Tyrosine Kinases in Tumor Immunity. Cancer Res. 2016, 76, 6452–6457. [Google Scholar] [CrossRef]
- Darling, T.K.; Lamb, T.J. Emerging Roles for Eph Receptors and Ephrin Ligands in Immunity. Front. Immunol. 2019, 10, 1473. [Google Scholar] [CrossRef]
- Funk, S.D.; Orr, A.W. Ephs and ephrins resurface in inflammation, immunity, and atherosclerosis. Pharmacol. Res. 2013, 67, 42–52. [Google Scholar] [CrossRef]
- Cheng, N.; Brantley, D.M.; Chen, J. The ephrins and Eph receptors in angiogenesis. Cytokine Growth Factor Rev. 2002, 13, 75–85. [Google Scholar] [CrossRef]
- Noren, N.K.; Lu, M.; Freeman, A.L.; Koolpe, M.; Pasquale, E.B. Interplay between EphB4 on tumor cells and vascular ephrin-B2 regulates tumor growth. Proc. Natl. Acad. Sci. USA 2004, 101, 5583–5588. [Google Scholar] [CrossRef]
- Pfaff, D.; Heroult, M.; Riedel, M.; Reiss, Y.; Kirmse, R.; Ludwig, T.; Korff, T.; Hecker, M.; Augustin, H.G. Involvement of endothelial ephrin-B2 in adhesion and transmigration of EphB-receptor-expressing monocytes. J. Cell Sci. 2008, 121, 3842–3850. [Google Scholar] [CrossRef]
- Braun, J.; Hoffmann, S.C.; Feldner, A.; Ludwig, T.; Henning, R.; Hecker, M.; Korff, T. Endothelial cell ephrinB2-dependent activation of monocytes in arteriosclerosis. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 297–305. [Google Scholar] [CrossRef]
- Funk, S.D.; Yurdagul, A., Jr.; Albert, P.; Traylor, J.G., Jr.; Jin, L.; Chen, J.; Orr, A.W. EphA2 activation promotes the endothelial cell inflammatory response: A potential role in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 686–695. [Google Scholar] [CrossRef]
- Kawano, H.; Katayama, Y.; Minagawa, K.; Shimoyama, M.; Henkemeyer, M.; Matsui, T. A novel feedback mechanism by Ephrin-B1/B2 in T-cell activation involves a concentration-dependent switch from costimulation to inhibition. Eur. J. Immunol. 2012, 42, 1562–1572. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Luo, H.; Wu, J. Effect of reduced EPHB4 expression in thymic epithelial cells on thymocyte development and peripheral T cell function. Mol. Immunol. 2014, 58, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Luo, H.; Wu, Y.; Wu, J. Ephrin B2 induces T cell costimulation. J. Immunol. 2003, 171, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Wohlfahrt, J.G.; Karagiannidis, C.; Kunzmann, S.; Epstein, M.M.; Kempf, W.; Blaser, K.; Schmidt-Weber, C.B. Ephrin-A1 suppresses Th2 cell activation and provides a regulatory link to lung epithelial cells. J. Immunol. 2004, 172, 843–850. [Google Scholar] [CrossRef]
- Bhatia, S.; Oweida, A.; Lennon, S.; Darragh, L.B.; Milner, D.; Phan, A.V.; Mueller, A.C.; Van Court, B.; Raben, D.; Serkova, N.J.; et al. Inhibition of EphB4–Ephrin-B2 Signaling Reprograms the Tumor Immune Microenvironment in Head and Neck Cancers. Cancer Res. 2019, 79, 2722–2735. [Google Scholar] [CrossRef]
- Meacham, C.E.; Morrison, S.J. Tumour heterogeneity and cancer cell plasticity. Nature 2013, 501, 328–337. [Google Scholar] [CrossRef]
- Sutherland, K.D.; Visvader, J.E. Cellular Mechanisms Underlying Intertumoral Heterogeneity. Trends Cancer 2015, 1, 15–23. [Google Scholar] [CrossRef]
- Karras, P.; Bordeu, I.; Pozniak, J.; Nowosad, A.; Pazzi, C.; Van Raemdonck, N.; Landeloos, E.; Van Herck, Y.; Pedri, D.; Bervoets, G.; et al. A cellular hierarchy in melanoma uncouples growth and metastasis. Nature 2022, 610, 190–198. [Google Scholar] [CrossRef]
- Bordanaba-Florit, G.; Royo, F.; Kruglik, S.G.; Falcón-Pérez, J.M. Using single-vesicle technologies to unravel the heterogeneity of extracellular vesicles. Nat. Protoc. 2021, 16, 3163–3185. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Lin, H.-Y.; Chou, C.-Y.; Huang, C.-H.; Small, J.; Sadik, N.; Ayinon, C.M.; Lansbury, E.; Cruz, L.; Yekula, A.; et al. Navigating the Landscape of Tumor Extracellular Vesicle Heterogeneity. Int. J. Mol. Sci. 2019, 20, 1349. [Google Scholar] [CrossRef] [PubMed]
- Vagner, T.; Chin, A.; Mariscal, J.; Bannykh, S.; Engman, D.M.; Di Vizio, D. Protein Composition Reflects Extracellular Vesicle Heterogeneity. Proteomics 2019, 19, e1800167. [Google Scholar] [CrossRef] [PubMed]
- Willms, E.; Cabañas, C.; Mäger, I.; Wood, M.J.A.; Vader, P. Extracellular Vesicle Heterogeneity: Subpopulations, Isolation Techniques, and Diverse Functions in Cancer Progression. Front. Immunol. 2018, 9, 738. [Google Scholar] [CrossRef]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Théry, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef]
- Willms, E.; Johansson, H.J.; Mäger, I.; Lee, Y.; Blomberg, K.E.M.; Sadik, M.; Alaarg, A.; Smith, C.I.E.; Lehtiö, J.; El Andaloussi, S.; et al. Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci. Rep. 2016, 6, 22519. [Google Scholar] [CrossRef]
- Rontogianni, S.; Synadaki, E.; Li, B.; Liefaard, M.C.; Lips, E.H.; Wesseling, J.; Wu, W.; Altelaar, M. Proteomic profiling of extracellular vesicles allows for human breast cancer subtyping. Commun. Biol. 2019, 2, 325. [Google Scholar] [CrossRef] [PubMed]
- Junttila, M.R.; de Sauvage, F.J. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature 2013, 501, 346–354. [Google Scholar] [CrossRef]
- DeCordova, S.; Shastri, A.; Tsolaki, A.G.; Yasmin, H.; Klein, L.; Singh, S.K.; Kishore, U. Molecular Heterogeneity and Immunosuppressive Microenvironment in Glioblastoma. Front. Immunol. 2020, 11, 1402. [Google Scholar] [CrossRef] [PubMed]
- Hass, R.; von der Ohe, J.; Ungefroren, H. Impact of the Tumor Microenvironment on Tumor Heterogeneity and Consequences for Cancer Cell Plasticity and Stemness. Cancers 2020, 12, 3716. [Google Scholar] [CrossRef]
- Lucaciu, S.A.; Leighton, S.E.; Hauser, A.; Yee, R.; Laird, D.W. Diversity in connexin biology. J. Biol. Chem. 2023, 299, 105263. [Google Scholar] [CrossRef]
- Willecke, K.; Eiberger, J.; Degen, J.; Eckardt, D.; Romualdi, A.; Güldenagel, M.; Deutsch, U.; Söhl, G. Structural and functional diversity of connexin genes in the mouse and human genome. Biol. Chem. 2002, 383, 725–737. [Google Scholar] [CrossRef]
- Amessou, M.; Kandouz, M. Role of the Family of Ephs and Ephrins in Cell-Cell Communication in Cancer. In Intercellular Communication in Cancer; Kandouz, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 255–286. [Google Scholar]
- Lackmann, M.; Boyd, A.W. Eph, a protein family coming of age: More confusion, insight, or complexity? Sci. Signal. 2008, 1, re2. [Google Scholar] [CrossRef]
- Sosinsky, G. Mixing of connexins in gap junction membrane channels. Proc. Natl. Acad. Sci. USA 1995, 92, 9210–9214. [Google Scholar] [CrossRef]
- Bevans, C.G.; Kordel, M.; Rhee, S.K.; Harris, A.L. Isoform composition of connexin channels determines selectivity among second messengers and uncharged molecules. J. Biol. Chem. 1998, 273, 2808–2816. [Google Scholar] [CrossRef]
- Plum, A.; Hallas, G.; Magin, T.; Dombrowski, F.; Hagendorff, A.; Schumacher, B.; Wolpert, C.; Kim, J.; Lamers, W.H.; Evert, M.; et al. Unique and shared functions of different connexins in mice. Curr. Biol. 2000, 10, 1083–1091. [Google Scholar] [CrossRef]
- Weber, P.A.; Chang, H.-C.; Spaeth, K.E.; Nitsche, J.M.; Nicholson, B.J. The permeability of gap junction channels to probes of different size is dependent on connexin composition and permeant-pore affinities. Biophys. J. 2004, 87, 958–973. [Google Scholar] [CrossRef]
- Kapoor, P.; Saunders, M.M.; Li, Z.; Zhou, Z.; Sheaffer, N.; Kunze, E.L.; Samant, R.S.; Welch, D.R.; Donahue, H.J. Breast cancer metastatic potential: Correlation with increased heterotypic gap junctional intercellular communication between breast cancer cells and osteoblastic cells. Int. J. Cancer 2004, 111, 693–697. [Google Scholar] [CrossRef]
- El-Sabban, M.E.; Pauli, B.U. Cytoplasmic dye transfer between metastatic tumor cells and vascular endothelium. J. Cell Biol. 1991, 115, 1375–1382. [Google Scholar] [CrossRef]
- el-Sabban, M.E.; Pauli, B.U. Adhesion-mediated gap junctional communication between lung-metastatatic cancer cells and endothelium. Invasion Metastasis 1994, 14, 164–176. [Google Scholar]
- Ito, A.; Katoh, F.; Kataoka, T.R.; Okada, M.; Tsubota, N.; Asada, H.; Yoshikawa, K.; Maeda, S.; Kitamura, Y.; Yamasaki, H.; et al. A role for heterologous gap junctions between melanoma and endothelial cells in metastasis. J. Clin. Investig. 2000, 105, 1189–1197. [Google Scholar] [CrossRef]
- Pollmann, M.-A.; Shao, Q.; Laird, D.W.; Sandig, M. Connexin 43 mediated gap junctional communication enhances breast tumor cell diapedesis in culture. Breast Cancer Res. 2005, 7, R522–R534. [Google Scholar] [CrossRef]
- Saunders, M.M.; Seraj, M.J.; Li, Z.; Zhou, Z.; Winter, C.R.; Welch, D.R.; Donahue, H.J. Breast cancer metastatic potential correlates with a breakdown in homospecific and heterospecific gap junctional intercellular communication. Cancer Res. 2001, 61, 1765–1767. [Google Scholar]
- Kandouz, M. The Eph/Ephrin family in cancer metastasis: Communication at the service of invasion. Cancer Metastasis Rev. 2012, 31, 353–373. [Google Scholar] [CrossRef] [PubMed]
- Héroult, M.; Schaffner, F.; Augustin, H.G. Eph receptor and ephrin ligand-mediated interactions during angiogenesis and tumor progression. Exp. Cell Res. 2006, 312, 642–650. [Google Scholar] [CrossRef]
- Pasquale, E.B. Eph receptors and ephrins in cancer progression. Nat. Rev. Cancer 2023, 24, 5–27. [Google Scholar] [CrossRef] [PubMed]
- Rohani, N.; Parmeggiani, A.; Winklbauer, R.; Fagotto, F. Variable combinations of specific ephrin ligand/Eph receptor pairs control embryonic tissue separation. PLoS Biol. 2014, 12, e1001955. [Google Scholar] [CrossRef] [PubMed]
- Nikas, I.; Ryu, H.S.; Theocharis, S. Viewing the Eph receptors with a focus on breast cancer heterogeneity. Cancer Lett. 2018, 434, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, A.S.; Hailat, Z.; Bandyopadhyay, S.; Neill, D.; Kandouz, M. The Value of EphB2 Receptor and Cognate Ephrin Ligands in Prognostic and Predictive Assessments of Human Breast Cancer. Int. J. Mol. Sci. 2021, 22, 8098. [Google Scholar] [CrossRef]
- Huang, S.; Dong, C.; Zhang, J.; Fu, S.; Lv, Y.; Wu, J. A comprehensive prognostic and immunological analysis of ephrin family genes in hepatocellular carcinoma. Front. Mol. Biosci. 2022, 9, 943384. [Google Scholar] [CrossRef]
- Wong, S.S.; Kim, K.-M.; Ting, J.C.; Yu, K.; Fu, J.; Liu, S.; Cristescu, R.; Nebozhyn, M.; Gong, L.; Yue, Y.G.; et al. Genomic landscape and genetic heterogeneity in gastric adenocarcinoma revealed by whole-genome sequencing. Nat. Commun. 2014, 5, 5477. [Google Scholar] [CrossRef]
- Yates, L.R.; Gerstung, M.; Knappskog, S.; Desmedt, C.; Gundem, G.; Van Loo, P.; Aas, T.; Alexandrov, L.B.; Larsimont, D.; Davies, H.; et al. Subclonal diversification of primary breast cancer revealed by multiregion sequencing. Nat. Med. 2015, 21, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.P.; Roth, A.; Goya, R.; Oloumi, A.; Ha, G.; Zhao, Y.; Turashvili, G.; Ding, J.; Tse, K.; Haffari, G.; et al. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature 2012, 486, 395–399. [Google Scholar] [CrossRef]
- Green, M.R.; Gentles, A.J.; Nair, R.V.; Irish, J.M.; Kihira, S.; Liu, C.L.; Kela, I.; Hopmans, E.S.; Myklebust, J.H.; Ji, H.; et al. Hierarchy in somatic mutations arising during genomic evolution and progression of follicular lymphoma. Blood 2013, 121, 1604–1611. [Google Scholar] [CrossRef]
- Cooper, C.S.; Eeles, R.; Wedge, D.C.; Van Loo, P.; Gundem, G.; Alexandrov, L.B.; Kremeyer, B.; Butler, A.; Lynch, A.G.; Camacho, N.; et al. Analysis of the genetic phylogeny of multifocal prostate cancer identifies multiple independent clonal expansions in neoplastic and morphologically normal prostate tissue. Nat. Genet. 2015, 47, 367–372. [Google Scholar] [CrossRef]
- Jacoby, M.A.; Duncavage, E.J.; Walter, M.J. Implications of Tumor Clonal Heterogeneity in the Era of Next-Generation Sequencing. Trends Cancer 2015, 1, 231–241. [Google Scholar] [CrossRef]
- Parker, T.M.; Gupta, K.; Palma, A.M.; Yekelchyk, M.; Fisher, P.B.; Grossman, S.R.; Won, K.J.; Madan, E.; Moreno, E.; Gogna, R. Cell competition in intratumoral and tumor microenvironment interactions. EMBO J. 2021, 40, e107271. [Google Scholar] [CrossRef]
- Madan, E.; Peixoto, M.L.; Dimitrion, P.; Eubank, T.D.; Yekelchyk, M.; Talukdar, S.; Fisher, P.B.; Mi, Q.-S.; Moreno, E.; Gogna, R. Cell Competition Boosts Clonal Evolution and Hypoxic Selection in Cancer. Trends Cell Biol. 2020, 30, 967–978. [Google Scholar] [CrossRef]
- Zhou, H.; Neelakantan, D.; Ford, H.L. Clonal cooperativity in heterogenous cancers. Semin. Cell Dev. Biol. 2017, 64, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Lee, H.E.; Li, H.; Shipitsin, M.; Gelman, R.; Polyak, K. Heterogeneity for stem cell–related markers according to tumor subtype and histologic stage in breast cancer. Clin. Cancer Res. 2010, 16, 876–887. [Google Scholar] [CrossRef]
- Matsuda, Y.; Semba, S.; Ueda, J.; Fuku, T.; Hasuo, T.; Chiba, H.; Sawada, N.; Kuroda, Y.; Yokozaki, H. Gastric and intestinal claudin expression at the invasive front of gastric carcinoma. Cancer Sci. 2007, 98, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Kuo, W.-L.; Lee, L.-Y.; Wu, C.-M.; Wang, C.-C.; Yu, J.-S.; Liang, Y.; Lo, C.-H.; Huang, K.-H.; Hwang, T.-L. Differential expression of claudin-4 between intestinal and diffuse-type gastric cancer. Oncol. Rep. 2006, 16, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Prat, A.; Parker, J.S.; Karginova, O.; Fan, C.; Livasy, C.; Herschkowitz, J.I.; He, X.; Perou, C.M. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 2010, 12, R68. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Couldwell, W.T.; Simard, M.F.; Song, H.; Lin, J.H.; Nedergaard, M. Direct gap junction communication between malignant glioma cells and astrocytes. Cancer Res. 1999, 59, 1994–2003. [Google Scholar]
- Sin, W.C.; Aftab, Q.; Bechberger, J.F.; Leung, J.H.; Chen, H.; Naus, C.C. Astrocytes promote glioma invasion via the gap junction protein connexin43. Oncogene 2016, 35, 1504–1516. [Google Scholar] [CrossRef]
- McCutcheon, S.; Spray, D.C. Glioblastoma–Astrocyte Connexin 43 Gap Junctions Promote Tumor Invasion. Mol. Cancer Res. 2022, 20, 319–331. [Google Scholar] [CrossRef]
- Chen, F.; Ding, K.; Priedigkeit, N.; Elangovan, A.; Levine, K.M.; Carleton, N.; Savariau, L.; Atkinson, J.M.; Oesterreich, S.; Lee, A.V. Single-Cell Transcriptomic Heterogeneity in Invasive Ductal and Lobular Breast Cancer Cells. Cancer Res. 2021, 81, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Shackleton, M.; Quintana, E.; Fearon, E.R.; Morrison, S.J. Heterogeneity in cancer: Cancer stem cells versus clonal evolution. Cell 2009, 138, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Prasetyanti, P.R.; Medema, J.P. Intra-tumor heterogeneity from a cancer stem cell perspective. Mol. Cancer 2017, 16, 41. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, E.M.F.; Vermeulen, L.; Fessler, E.; Medema, J.P. Cancer heterogeneity—A multifaceted view. EMBO Rep. 2013, 14, 686–695. [Google Scholar] [CrossRef]
- Yabo, Y.A.; Niclou, S.P.; Golebiewska, A. Cancer cell heterogeneity and plasticity: A paradigm shift in glioblastoma. Neuro-Oncology 2022, 24, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, A.; Hainz, N.; Tschernig, T.; Meier, C. Facets of Communication: Gap Junction Ultrastructure and Function in Cancer Stem Cells and Tumor Cells. Cancers 2019, 11, 288. [Google Scholar] [CrossRef] [PubMed]
- Sinha, G.; Ferrer, A.I.; Moore, C.A.; Naaldijk, Y.; Rameshwar, P. Gap Junctions and Breast Cancer Dormancy. Trends Cancer 2020, 6, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.J. Emerging roles of claudins in human cancer. Int. J. Mol. Sci. 2013, 14, 18148–18180. [Google Scholar] [CrossRef]
- Gowrikumar, S.; Singh, A.B.; Dhawan, P. Role of Claudin Proteins in Regulating Cancer Stem Cells and Chemoresistance-Potential Implication in Disease Prognosis and Therapy. Int. J. Mol. Sci. 2019, 21, 53. [Google Scholar] [CrossRef]
- Farahani, E.; Patra, H.K.; Jangamreddy, J.R.; Rashedi, I.; Kawalec, M.; Rao Pariti, R.K.; Batakis, P.; Wiechec, E. Cell adhesion molecules and their relation to (cancer) cell stemness. Carcinogenesis 2014, 35, 747–759. [Google Scholar] [CrossRef]
- Scioli, M.G.; Terriaca, S.; Fiorelli, E.; Storti, G.; Fabbri, G.; Cervelli, V.; Orlandi, A. Extracellular Vesicles and Cancer Stem Cells in Tumor Progression: New Therapeutic Perspectives. Int. J. Mol. Sci. 2021, 22, 10572. [Google Scholar] [CrossRef]
- Adnani, L.; Spinelli, C.; Tawil, N.; Rak, J. Role of extracellular vesicles in cancer-specific interactions between tumour cells and the vasculature. Semin. Cancer Biol. 2022, 87, 196–213. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Shi, X.; Jiang, M.; Liu, H. Cross-talk between cancer stem cells and immune cells: Potential therapeutic targets in the tumor immune microenvironment. Mol. Cancer 2023, 22, 38. [Google Scholar] [CrossRef] [PubMed]
- Pinto, G.; Saenz-de-Santa-Maria, I.; Chastagner, P.; Perthame, E.; Delmas, C.; Toulas, C.; Moyal-Jonathan-Cohen, E.; Brou, C.; Zurzolo, C. Patient-derived glioblastoma stem cells transfer mitochondria through tunneling nanotubes in tumor organoids. Biochem. J. 2021, 478, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Nakhle, J.; Khattar, K.; Özkan, T.; Boughlita, A.; Abba Moussa, D.; Darlix, A.; Lorcy, F.; Rigau, V.; Bauchet, L.; Gerbal-Chaloin, S.; et al. Mitochondria Transfer from Mesenchymal Stem Cells Confers Chemoresistance to Glioblastoma Stem Cells through Metabolic Rewiring. Cancer Res. Commun. 2023, 3, 1041–1056. [Google Scholar] [CrossRef]
- Wang, L.; Peng, Q.; Xie, Y.; Yin, N.; Xu, J.; Chen, A.; Yi, J.; Shi, W.; Tang, J.; Xiang, J. Cell-cell contact-driven EphB1 cis- and trans- signalings regulate cancer stem cells enrichment after chemotherapy. Cell Death Dis. 2022, 13, 980. [Google Scholar] [CrossRef] [PubMed]
- Green, K.J.; Jaiganesh, A.; Broussard, J.A. Desmosomes: Essential contributors to an integrated intercellular junction network. F1000Research 2019, 8, 2150. [Google Scholar] [CrossRef] [PubMed]
- Campbell, H.K.; Maiers, J.L.; DeMali, K.A. Interplay between tight junctions & adherens junctions. Exp. Cell Res. 2017, 358, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Hartsock, A.; Nelson, W.J. Adherens and tight junctions: Structure, function and connections to the actin cytoskeleton. Biochim. Biophys. Acta Biomembr. 2008, 1778, 660–669. [Google Scholar] [CrossRef]
- Rusu, A.D.; Georgiou, M. The multifarious regulation of the apical junctional complex. Open Biol. 2020, 10, 190278. [Google Scholar] [CrossRef]
- Toyofuku, T.; Yabuki, M.; Otsu, K.; Kuzuya, T.; Hori, M.; Tada, M. Direct association of the gap junction protein connexin-43 with ZO-1 in cardiac myocytes. J. Biol. Chem. 1998, 273, 12725–12731. [Google Scholar] [CrossRef] [PubMed]
- Keane, R.W.; Mehta, P.P.; Rose, B.; Honig, L.S.; Loewenstein, W.R.; Rutishauser, U. Neural differentiation, NCAM-mediated adhesion, and gap junctional communication in neuroectoderm. A study in vitro. J. Cell Biol. 1988, 106, 1307–1319. [Google Scholar] [CrossRef] [PubMed]
- Jongen, W.M.; Fitzgerald, D.J.; Asamoto, M.; Piccoli, C.; Slaga, T.J.; Gros, D.; Takeichi, M.; Yamasaki, H. Regulation of connexin 43-mediated gap junctional intercellular communication by Ca2+ in mouse epidermal cells is controlled by E-cadherin. J. Cell Biol. 1991, 114, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Musil, L.S.; Cunningham, B.A.; Edelman, G.M.; Goodenough, D.A. Differential phosphorylation of the gap junction protein connexin43 in junctional communication-competent and -deficient cell lines. J. Cell Biol. 1990, 111, 2077–2088. [Google Scholar] [CrossRef] [PubMed]
- Polusani, S.R.; Kalmykov, E.A.; Chandrasekhar, A.; Zucker, S.N.; Nicholson, B.J. Cell coupling mediated by connexin 26 selectively contributes to reduced adhesivity and increased migration. J. Cell Sci. 2016, 129, 4399–4410. [Google Scholar] [CrossRef]
- Wei, C.-J.; Francis, R.; Xu, X.; Lo, C.W. Connexin43 associated with an N-cadherin-containing multiprotein complex is required for gap junction formation in NIH3T3 cells. J. Biol. Chem. 2005, 280, 19925–19936. [Google Scholar] [CrossRef]
- Dianati, E.; Poiraud, J.; Weber-Ouellette, A.; Plante, I. Connexins, E-cadherin, Claudin-7 and β-catenin transiently form junctional nexuses during the post-natal mammary gland development. Dev. Biol. 2016, 416, 52–68. [Google Scholar] [CrossRef]
- Shigetomi, K.; Ikenouchi, J. Cell Adhesion Structures in Epithelial Cells Are Formed in Dynamic and Cooperative Ways. Bioessays 2019, 41, e1800227. [Google Scholar] [CrossRef]
- Rietscher, K.; Wolf, A.; Hause, G.; Rother, A.; Keil, R.; Magin, T.M.; Glass, M.; Niessen, C.M.; Hatzfeld, M. Growth Retardation, Loss of Desmosomal Adhesion, and Impaired Tight Junction Function Identify a Unique Role of Plakophilin 1 In Vivo. J. Investig. Dermatol. 2016, 136, 1471–1478. [Google Scholar] [CrossRef]
- Schlüter, H.; Wepf, R.; Moll, I.; Franke, W.W. Sealing the live part of the skin: The integrated meshwork of desmosomes, tight junctions and curvilinear ridge structures in the cells of the uppermost granular layer of the human epidermis. Eur. J. Cell Biol. 2004, 83, 655–665. [Google Scholar] [CrossRef]
- Feigin, M.E.; Muthuswamy, S.K. Polarity proteins regulate mammalian cell–cell junctions and cancer pathogenesis. Curr. Opin. Cell Biol. 2009, 21, 694–700. [Google Scholar] [CrossRef]
- Bazzoun, D.; Lelièvre, S.; Talhouk, R. Polarity proteins as regulators of cell junction complexes: Implications for breast cancer. Pharmacol. Ther. 2013, 138, 418–427. [Google Scholar] [CrossRef]
- Hollande, F.; Lee, D.J.; Choquet, A.; Roche, S.; Baldwin, G.S. Adherens junctions and tight junctions are regulated via different pathways by progastrin in epithelial cells. J. Cell Sci. 2003, 116, 1187–1197. [Google Scholar] [CrossRef]
- Tanos, B.E.; Yeaman, C.; Rodriguez-Boulan, E. An emerging role for IQGAP1 in tight junction control. Small GTPases 2018, 9, 375–383. [Google Scholar] [CrossRef]
- Soares, A.R.; Martins-Marques, T.; Ribeiro-Rodrigues, T.; Ferreira, J.V.; Catarino, S.; Pinho, M.J.; Zuzarte, M.; Isabel Anjo, S.; Manadas, B.; Sluijter, J.P.G.; et al. Gap junctional protein Cx43 is involved in the communication between extracellular vesicles and mammalian cells. Sci. Rep. 2015, 5, 13243. [Google Scholar] [CrossRef]
- Okafo, G.; Prevedel, L.; Eugenin, E. Tunneling nanotubes (TNT) mediate long-range gap junctional communication: Implications for HIV cell to cell spread. Sci. Rep. 2017, 7, 16660. [Google Scholar] [CrossRef]
- Sherer, N.M. Long-distance relationships: Do membrane nanotubes regulate cell–cell communication and disease progression? Mol. Biol. Cell 2013, 24, 1095–1098. [Google Scholar] [CrossRef] [PubMed]
- Ajikumar, A.; Long, M.B.; Heath, P.R.; Wharton, S.B.; Ince, P.G.; Ridger, V.C.; Simpson, J.E. Neutrophil-Derived Microvesicle Induced Dysfunction of Brain Microvascular Endothelial Cells In Vitro. Int. J. Mol. Sci. 2019, 20, 5227. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.; Baena, V.; Ge, S.; Jiang, X.; Jellison, E.R.; Kiprono, T.; Agalliu, D.; Pachter, J.S. Appearance of claudin-5+ leukocytes in the central nervous system during neuroinflammation: A novel role for endothelial-derived extracellular vesicles. J. Neuroinflamm. 2016, 13, 292. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Deng, S.; Li, L.; Liu, T.; Zhang, T.; Li, J.; Yu, Y.; Xu, Y. TGF-β1-mediated exosomal lnc-MMP2-2 increases blood–brain barrier permeability via the miRNA-1207-5p/EPB41L5 axis to promote non-small cell lung cancer brain metastasis. Cell Death Dis. 2021, 12, 721. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Fong, M.Y.; Min, Y.; Somlo, G.; Liu, L.; Palomares, M.R.; Yu, Y.; Chow, A.; O’Connor, S.T.F.; Chin, A.R.; et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell 2014, 25, 501–515. [Google Scholar] [CrossRef]
- Nawaz, M.; Fatima, F. Extracellular Vesicles, Tunneling Nanotubes, and Cellular Interplay: Synergies and Missing Links. Front. Mol. Biosci. 2017, 4, 50. [Google Scholar] [CrossRef] [PubMed]
- Thayanithy, V.; Babatunde, V.; Dickson, E.L.; Wong, P.; Oh, S.; Ke, X.; Barlas, A.; Fujisawa, S.; Romin, Y.; Moreira, A.L.; et al. Tumor exosomes induce tunneling nanotubes in lipid raft-enriched regions of human mesothelioma cells. Exp. Cell Res. 2014, 323, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Carone, C.; Genedani, S.; Leo, G.; Filaferro, M.; Fuxe, K.; Agnati, L.F. In vitro effects of cocaine on tunneling nanotube formation and extracellular vesicle release in glioblastoma cell cultures. J. Mol. Neurosci. 2015, 55, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Körner, R.; Gaitanos, L.; Klein, R. Exosomes mediate cell contact–independent ephrin-Eph signaling during axon guidance. J. Cell Biol. 2016, 214, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zhao, C.; Li, Y.; Wang, L.; Nie, G.; Peng, J.; Wang, A.; Zhang, P.; Tian, W.; Li, Q.; et al. Osteoclast-derived microRNA-containing exosomes selectively inhibit osteoblast activity. Cell Discov. 2016, 2, 16015. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Vasaikar, S.; Eskaros, A.; Kim, Y.; Lewis, J.S.; Zhang, B.; Zijlstra, A.; Weaver, A.M. EPHB2 carried on small extracellular vesicles induces tumor angiogenesis via activation of ephrin reverse signaling. JCI Insight 2019, 4, e132447. [Google Scholar] [CrossRef]
- Takasugi, M.; Okada, R.; Takahashi, A.; Virya Chen, D.; Watanabe, S.; Hara, E. Small extracellular vesicles secreted from senescent cells promote cancer cell proliferation through EphA2. Nat. Commun. 2017, 8, 15729. [Google Scholar] [CrossRef]
- Gao, Z.; Han, X.; Zhu, Y.; Zhang, H.; Tian, R.; Wang, Z.; Cui, Y.; Wang, Z.; Niu, R.; Zhang, F. Drug-resistant cancer cell-derived exosomal EphA2 promotes breast cancer metastasis via the EphA2-Ephrin A1 reverse signaling. Cell Death Dis. 2021, 12, 414. [Google Scholar] [CrossRef]
- Wilkinson, D.G. Interplay of Eph-Ephrin Signalling and Cadherin Function in Cell Segregation and Boundary Formation. Front. Cell Dev. Biol. 2021, 9, 784039. [Google Scholar] [CrossRef]
- Foty, R.A.; Steinberg, M.S. Cadherin-mediated cell-cell adhesion and tissue segregation in relation to malignancy. Int. J. Dev. Biol. 2004, 48, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Beamish, I.V.; Hinck, L.; Kennedy, T.E. Making Connections: Guidance Cues and Receptors at Nonneural Cell-Cell Junctions. Cold Spring Harb. Perspect. Biol. 2018, 10, a029165. [Google Scholar] [CrossRef]
- Orsulic, S.; Kemler, R. Expression of Eph receptors and ephrins is differentially regulated by E-cadherin. J. Cell Sci. 2000, 113 Pt 10, 1793–1802. [Google Scholar] [CrossRef] [PubMed]
- Zantek, N.D.; Azimi, M.; Fedor-Chaiken, M.; Wang, B.; Brackenbury, R.; Kinch, M.S. E-cadherin regulates the function of the EphA2 receptor tyrosine kinase. Cell Growth Differ. 1999, 10, 629–638. [Google Scholar] [PubMed]
- Fagotto, F.; Rohani, N.; Touret, A.-S.; Li, R. A molecular base for cell sorting at embryonic boundaries: Contact inhibition of cadherin adhesion by ephrin/Eph-dependent contractility. Dev. Cell 2013, 27, 72–87. [Google Scholar] [CrossRef]
- Solanas, G.; Cortina, C.; Sevillano, M.; Batlle, E. Cleavage of E-cadherin by ADAM10 mediates epithelial cell sorting downstream of EphB signalling. Nat. Cell Biol. 2011, 13, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Zhao, W.-D.; Liu, D.-X.; Liang, Y.; Fang, W.-G.; Li, B.; Chen, Y.-H. Inactivation of EphA2 promotes tight junction formation and impairs angiogenesis in brain endothelial cells. Microvasc. Res. 2011, 82, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Larson, J.; Schomberg, S.; Schroeder, W.; Carpenter, T.C. Endothelial EphA receptor stimulation increases lung vascular permeability. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 295, L431–L439. [Google Scholar] [CrossRef]
- Nunan, R.; Campbell, J.; Mori, R.; Pitulescu, M.E.; Jiang, W.G.; Harding, K.G.; Adams, R.H.; Nobes, C.D.; Martin, P. Ephrin-Bs Drive Junctional Downregulation and Actin Stress Fiber Disassembly to Enable Wound Re-epithelialization. Cell Rep. 2015, 13, 1380–1395. [Google Scholar] [CrossRef]
- Winning, R.S.; Wyman, T.L.; Walker, G.K. EphA4 activity causes cell shape change and a loss of cell polarity in Xenopus laevis embryos. Differentiation 2001, 68, 126–132. [Google Scholar] [CrossRef]
- Son, J.; Park, M.S.; Park, I.; Lee, H.-K.; Lee, S.-H.; Kang, B.; Min, B.-H.; Ryoo, J.; Lee, S.; Bae, J.-S.; et al. Pick1 modulates ephrinB1-induced junctional disassembly through an association with ephrinB1. Biochem. Biophys. Res. Commun. 2014, 450, 659–665. [Google Scholar] [CrossRef]
- Tanaka, M.; Kamata, R.; Sakai, R. EphA2 phosphorylates the cytoplasmic tail of Claudin-4 and mediates paracellular permeability. J. Biol. Chem. 2005, 280, 42375–42382. [Google Scholar] [CrossRef]
- Defourny, J.; Peuckert, C.; Kullander, K.; Malgrange, B. EphA4-ADAM10 Interplay Patterns the Cochlear Sensory Epithelium through Local Disruption of Adherens Junctions. iScience 2019, 11, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Kamata, R.; Sakai, R. Phosphorylation of ephrin-B1 via the interaction with claudin following cell–cell contact formation. EMBO J. 2005, 24, 3700–3711. [Google Scholar] [CrossRef]
- Miura, K.; Nam, J.-M.; Kojima, C.; Mochizuki, N.; Sabe, H. EphA2 engages Git1 to suppress Arf6 activity modulating epithelial cell–cell contacts. Mol. Biol. Cell 2009, 20, 1949–1959. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.B.; Khuong, A.; Wu, Z.; Xu, Q.; Morley, R.; Gregory, L.; Poliakov, A.; Taylor, W.R.; Wilkinson, D.G. Cell segregation and border sharpening by Eph receptor–ephrin-mediated heterotypic repulsion. J. R. Soc. Interface 2017, 14, 20170338. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.A.; Son, A.I.; Komlos, D.; Sun, Y.; Kleiman, N.J.; Zhou, R. Loss of ephrin-A5 function disrupts lens fiber cell packing and leads to cataract. Proc. Natl. Acad. Sci. USA 2008, 105, 16620–16625. [Google Scholar] [CrossRef] [PubMed]
- Hahn, A.C.; Emmons, S.W. The roles of an ephrin and a semaphorin in patterning cell–cell contacts in C. elegans sensory organ development. Dev. Biol. 2003, 256, 379–388. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Harrington, R.J.; Gutch, M.J.; Hengartner, M.O.; Tonks, N.K.; Chisholm, A.D. The C. elegans LAR-like receptor tyrosine phosphatase PTP-3 and the VAB-1 Eph receptor tyrosine kinase have partly redundant functions in morphogenesis. Development 2002, 129, 2141–2153. [Google Scholar] [CrossRef]
- Mellitzer, G.; Xu, Q.; Wilkinson, D.G. Eph receptors and ephrins restrict cell intermingling and communication. Nature 1999, 400, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Defourny, J.; Audouard, C.; Davy, A.; Thiry, M. Efnb2 haploinsufficiency induces early gap junction plaque disassembly and endocytosis in the cochlea. Brain Res. Bull. 2021, 174, 153–160. [Google Scholar] [CrossRef]
- Davy, A.; Bush, J.O.; Soriano, P. Inhibition of gap junction communication at ectopic Eph/ephrin boundaries underlies craniofrontonasal syndrome. PLoS Biol. 2006, 4, e315. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.L.; Chong, L.D.; Kim, J.; Xu, R.-H.; Kung, H.-F.; Daar, I.O. Loss of cell adhesion in Xenopus laevis embryos mediated by the cytoplasmic domain of XLerk, an erythropoietin-producing hepatocellular ligand. Proc. Natl. Acad. Sci. USA 1998, 95, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.L.; Yu, K.; Bruzzone, R.; Gimlich, R.L.; Goodenough, D.A. Expression of a dominant negative inhibitor of intercellular communication in the early Xenopus embryo causes delamination and extrusion of cells. Development 1995, 121, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Trease, A.J.; Li, H.; Spagnol, G.; Zheng, L.; Stauch, K.L.; Sorgen, P.L. Regulation of Connexin32 by ephrin receptors and T-cell protein-tyrosine phosphatase. J. Biol. Chem. 2019, 294, 341–350. [Google Scholar] [CrossRef]
- Ishii, M.; Mueller, I.; Nakajima, T.; Pasquale, E.B.; Ogawa, K. EphB signaling inhibits gap junctional intercellular communication and synchronized contraction in cultured cardiomyocytes. Basic Res. Cardiol. 2011, 106, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- Mallegol, J.; Van Niel, G.; Lebreton, C.; Lepelletier, Y.; Candalh, C.; Dugave, C.; Heath, J.K.; Raposo, G.; Cerf–Bensussan, N.; Heyman, M. T84-intestinal epithelial exosomes bear MHC class II/peptide complexes potentiating antigen presentation by dendritic cells. Gastroenterology 2007, 132, 1866–1876. [Google Scholar] [CrossRef]
- Denzer, K.; van Eijk, M.; Kleijmeer, M.J.; Jakobson, E.; de Groot, C.; Geuze, H.J. Follicular dendritic cells carry MHC class II-expressing microvesicles at their surface. J. Immunol. 2000, 165, 1259–1265. [Google Scholar] [CrossRef]
- Rana, S.; Yue, S.; Stadel, D.; Zöller, M. Toward tailored exosomes: The exosomal tetraspanin web contributes to target cell selection. Int. J. Biochem. Cell Biol. 2012, 44, 1574–1584. [Google Scholar] [CrossRef]
- Buzás, E.I.; Tóth, E.; Sódar, B.W.; Szabó-Taylor, K. Molecular interactions at the surface of extracellular vesicles. Semin. Immunopathol. 2018, 40, 453–464. [Google Scholar] [CrossRef]
- Hurwitz, S.N.; Rider, M.A.; Bundy, J.L.; Liu, X.; Singh, R.K.; Meckes, D.G., Jr. Proteomic profiling of NCI-60 extracellular vesicles uncovers common protein cargo and cancer type-specific biomarkers. Oncotarget 2016, 7, 86999–87015. [Google Scholar] [CrossRef]
- Carvalho, A.S.; Baeta, H.; Silva, B.C.; Moraes, M.C.S.; Bodo, C.; Beck, H.C.; Rodriguez, M.S.; Saraswat, M.; Pandey, A.; Matthiesen, R. Extra-cellular vesicles carry proteome of cancer hallmarks. Front. Biosci. (Landmark Ed.) 2020, 25, 398–436. [Google Scholar] [CrossRef] [PubMed]
- Valiunas, V.; Polosina, Y.Y.; Miller, H.; Potapova, I.A.; Valiuniene, L.; Doronin, S.; Mathias, R.T.; Robinson, R.B.; Rosen, M.R.; Cohen, I.S.; et al. Connexin-specific cell-to-cell transfer of short interfering RNA by gap junctions. J. Physiol. 2005, 568, 459–468. [Google Scholar] [CrossRef]
- Zong, L.; Zhu, Y.; Liang, R.; Zhao, H.-B. Gap junction mediated miRNA intercellular transfer and gene regulation: A novel mechanism for intercellular genetic communication. Sci. Rep. 2016, 6, 19884. [Google Scholar] [CrossRef] [PubMed]
- Lemcke, H.; Steinhoff, G.; David, R. Gap junctional shuttling of miRNA—A novel pathway of intercellular gene regulation and its prospects in clinical application. Cell. Signal. 2015, 27, 2506–2514. [Google Scholar] [CrossRef] [PubMed]
- Menachem, A.; Makovski, V.; Bodner, O.; Pasmanik-Chor, M.; Stein, R.; Shomron, N.; Kloog, Y. Intercellular transfer of small RNAs from astrocytes to lung tumor cells induces resistance to chemotherapy. Oncotarget 2016, 7, 12489–12504. [Google Scholar] [CrossRef] [PubMed]
- Brink, P.R.; Valiunas, V.; Gordon, C.; Rosen, M.R.; Cohen, I.S. Can gap junctions deliver? Biochim. Biophys. Acta Biomembr. 2012, 1818, 2076–2081. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Abdelmohsen, K.; Mustapic, M.; Kapogiannis, D.; Gorospe, M. RNA in extracellular vesicles. Wiley Interdiscip. Rev. RNA 2017, 8, e1413. [Google Scholar] [CrossRef]
- Turchinovich, A.; Drapkina, O.; Tonevitsky, A. Transcriptome of Extracellular Vesicles: State-of-the-Art. Front. Immunol. 2019, 10, 202. [Google Scholar] [CrossRef]
- O’Brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606. [Google Scholar] [CrossRef]
- Huang, M.; Peng, X.; Yang, L.; Yang, S.; Li, X.; Tang, S.; Li, B.; Jin, H.; Wu, B.; Liu, J.; et al. Non-coding RNA derived from extracellular vesicles in cancer immune escape: Biological functions and potential clinical applications. Cancer Lett. 2021, 501, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Battistelli, M.; Falcieri, E. Apoptotic Bodies: Particular Extracellular Vesicles Involved in Intercellular Communication. Biology 2020, 9, 21. [Google Scholar] [CrossRef]
- Elzanowska, J.; Semira, C.; Costa-Silva, B. DNA in extracellular vesicles: Biological and clinical aspects. Mol. Oncol. 2021, 15, 1701–1714. [Google Scholar] [CrossRef]
- Valcz, G.; Újvári, B.; Buzás, E.I.; Krenács, T.; Spisák, S.; Kittel, Á.; Tulassay, Z.; Igaz, P.; Takács, I.; Molnár, B. Small extracellular vesicle DNA-mediated horizontal gene transfer as a driving force for tumor evolution: Facts and riddles. Front. Oncol. 2022, 12, 945376. [Google Scholar] [CrossRef]
- Emamalipour, M.; Seidi, K.; Zununi Vahed, S.; Jahanban-Esfahlan, A.; Jaymand, M.; Majdi, H.; Amoozgar, Z.; Chitkushev, L.T.; Javaheri, T.; Jahanban-Esfahlan, R.; et al. Horizontal Gene Transfer: From Evolutionary Flexibility to Disease Progression. Front. Cell Dev. Biol. 2020, 8, 229. [Google Scholar] [CrossRef]
- Cai, J.; Wu, G.; Jose, P.A.; Zeng, C. Functional transferred DNA within extracellular vesicles. Exp. Cell Res. 2016, 349, 179–183. [Google Scholar] [CrossRef]
- Mills, J.; Capece, M.; Cocucci, E.; Tessari, A.; Palmieri, D. Cancer-Derived Extracellular Vesicle-Associated MicroRNAs in Intercellular Communication: One Cell’s Trash Is Another Cell’s Treasure. Int. J. Mol. Sci. 2019, 20, 6109. [Google Scholar] [CrossRef]
- Halicka, H.D.; Bedner, E.; Darzynkiewicz, Z. Segregation of RNA and separate packaging of DNA and RNA in apoptotic bodies during apoptosis. Exp. Cell Res. 2000, 260, 248–256. [Google Scholar] [CrossRef]
- Holmgren, L.; Szeles, A.; Rajnavolgyi, E.; Folkman, J.; Klein, G.; Ernberg, I.; Falk, K.I. Horizontal transfer of DNA by the uptake of apoptotic bodies. Blood 1999, 93, 3956–3963. [Google Scholar] [CrossRef]
- Bergsmedh, A.; Szeles, A.; Henriksson, M.; Bratt, A.; Folkman, M.J.; Spetz, A.-L.; Holmgren, L. Horizontal transfer of oncogenes by uptake of apoptotic bodies. Proc. Natl. Acad. Sci. USA 2001, 98, 6407–6411. [Google Scholar] [CrossRef]
- Phinney, D.G.; Di Giuseppe, M.; Njah, J.; Sala, E.; Shiva, S.; St Croix, C.M.; Stolz, D.B.; Watkins, S.C.; Di, Y.P.; Leikauf, G.D.; et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat. Commun. 2015, 6, 8472. [Google Scholar] [CrossRef]
- Liu, D.; Dong, Z.; Wang, J.; Tao, Y.; Sun, X.; Yao, X. The existence and function of mitochondrial component in extracellular vesicles. Mitochondrion 2020, 54, 122–127. [Google Scholar] [CrossRef] [PubMed]
- D’Acunzo, P.; Kim, Y.; Ungania, J.M.; Pérez-González, R.; Goulbourne, C.N.; Levy, E. Isolation of mitochondria-derived mitovesicles and subpopulations of microvesicles and exosomes from brain tissues. Nat. Protoc. 2022, 17, 2517–2549. [Google Scholar] [CrossRef]
- D’Acunzo, P.; Pérez-González, R.; Kim, Y.; Hargash, T.; Miller, C.; Alldred, M.J.; Erdjument-Bromage, H.; Penikalapati, S.C.; Pawlik, M.; Saito, M.; et al. Mitovesicles are a novel population of extracellular vesicles of mitochondrial origin altered in Down syndrome. Sci. Adv. 2021, 7, eabe5085. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.C.; Crescitelli, R.; Cvjetkovic, A.; Belgrano, V.; Olofsson Bagge, R.; Sundfeldt, K.; Ochiya, T.; Kalluri, R.; Lötvall, J. Mitochondrial protein enriched extracellular vesicles discovered in human melanoma tissues can be detected in patient plasma. J. Extracell. Vesicles 2019, 8, 1635420. [Google Scholar] [CrossRef] [PubMed]
- Puhm, F.; Afonyushkin, T.; Resch, U.; Obermayer, G.; Rohde, M.; Penz, T.; Schuster, M.; Wagner, G.; Rendeiro, A.F.; Melki, I.; et al. Mitochondria Are a Subset of Extracellular Vesicles Released by Activated Monocytes and Induce Type I IFN and TNF Responses in Endothelial Cells. Circ. Res. 2019, 125, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Guescini, M.; Genedani, S.; Stocchi, V.; Agnati, L.F. Astrocytes and Glioblastoma cells release exosomes carrying mtDNA. J. Neural Transm. 2010, 117, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, H.; Hoon, D.S.B.; Pantel, K. Cell-free nucleic acids as biomarkers in cancer patients. Nat. Rev. Cancer 2011, 11, 426–437. [Google Scholar] [CrossRef]
- Lazo, S.; Noren Hooten, N.; Green, J.; Eitan, E.; Mode, N.A.; Liu, Q.R.; Zonderman, A.B.; Ezike, N.; Mattson, M.P.; Ghosh, P.; et al. Mitochondrial DNA in extracellular vesicles declines with age. Aging Cell 2021, 20, e13283. [Google Scholar] [CrossRef]
- Sansone, P.; Savini, C.; Kurelac, I.; Chang, Q.; Amato, L.B.; Strillacci, A.; Stepanova, A.; Iommarini, L.; Mastroleo, C.; Daly, L.; et al. Packaging and transfer of mitochondrial DNA via exosomes regulate escape from dormancy in hormonal therapy-resistant breast cancer. Proc. Natl. Acad. Sci. USA 2017, 114, E9066–E9075. [Google Scholar] [CrossRef]
- Fernando, M.R.; Jiang, C.; Krzyzanowski, G.D.; Ryan, W.L. New evidence that a large proportion of human blood plasma cell-free DNA is localized in exosomes. PLoS ONE 2017, 12, e0183915. [Google Scholar] [CrossRef]
- Torralba, D.; Baixauli, F.; Villarroya-Beltri, C.; Fernández-Delgado, I.; Latorre-Pellicer, A.; Acín-Pérez, R.; Martín-Cófreces, N.B.; Jaso-Tamame, Á.L.; Iborra, S.; Jorge, I.; et al. Priming of dendritic cells by DNA-containing extracellular vesicles from activated T cells through antigen-driven contacts. Nat. Commun. 2018, 9, 2658. [Google Scholar] [CrossRef] [PubMed]
- Trejo-Becerril, C.; Pérez-Cárdenas, E.; Taja-Chayeb, L.; Anker, P.; Herrera-Goepfert, R.; Medina-Velázquez, L.A.; Hidalgo-Miranda, A.; Pérez-Montiel, D.; Chávez-Blanco, A.; Cruz-Velázquez, J.; et al. Cancer progression mediated by horizontal gene transfer in an in vivo model. PLoS ONE 2012, 7, e52754. [Google Scholar] [CrossRef] [PubMed]
- García-Olmo, D.; García-Olmo, D.C.; Ontañón, J.; Martinez, E.; Vallejo, M. Tumor DNA circulating in the plasma might play a role in metastasis: The hypothesis of the genometastasis. Histol. Histopathol. 1999, 14, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Mittra, I.; Khare, N.K.; Raghuram, G.V.; Chaubal, R.; Khambatti, F.; Gupta, D.; Gaikwad, A.; Prasannan, P.; Singh, A.; Iyer, A.; et al. Circulating nucleic acids damage DNA of healthy cells by integrating into their genomes. J. Biosci. 2015, 40, 91–111. [Google Scholar] [CrossRef]
- Mittra, I.; Samant, U.; Sharma, S.; Raghuram, G.V.; Saha, T.; Tidke, P.; Pancholi, N.; Gupta, D.; Prasannan, P.; Gaikwad, A.; et al. Cell-free chromatin from dying cancer cells integrate into genomes of bystander healthy cells to induce DNA damage and inflammation. Cell Death Discov. 2017, 3, 17015. [Google Scholar] [CrossRef]
- Fűri, I.; Kalmár, A.; Wichmann, B.; Spisák, S.; Schöller, A.; Barták, B.; Tulassay, Z.; Molnár, B. Cell Free DNA of Tumor Origin Induces a ‘Metastatic’ Expression Profile in HT-29 Cancer Cell Line. PLoS ONE 2015, 10, e0131699. [Google Scholar] [CrossRef]
- Cai, J.; Han, Y.; Ren, H.; Chen, C.; He, D.; Zhou, L.; Eisner, G.M.; Asico, L.D.; Jose, P.A.; Zeng, C. Extracellular vesicle-mediated transfer of donor genomic DNA to recipient cells is a novel mechanism for genetic influence between cells. J. Mol. Cell Biol. 2013, 5, 227–238. [Google Scholar] [CrossRef]
- Hattori, M.; Osterfield, M.; Flanagan, J.G. Regulated cleavage of a contact-mediated axon repellent. Science 2000, 289, 1360–1365. [Google Scholar] [CrossRef]
- Janes, P.W.; Saha, N.; Barton, W.A.; Kolev, M.V.; Wimmer-Kleikamp, S.H.; Nievergall, E.; Blobel, C.P.; Himanen, J.-P.; Lackmann, M.; Nikolov, D.B. Adam meets Eph: An ADAM substrate recognition module acts as a molecular switch for ephrin cleavage in trans. Cell 2005, 123, 291–304. [Google Scholar] [CrossRef]
- Alford, S.C.; Bazowski, J.; Lorimer, H.; Elowe, S.; Howard, P.L. Tissue transglutaminase clusters soluble A-type ephrins into functionally active high molecular weight oligomers. Exp. Cell Res. 2007, 313, 4170–4179. [Google Scholar] [CrossRef]
- Beauchamp, A.; Lively, M.O.; Mintz, A.; Gibo, D.; Wykosky, J.; Debinski, W. EphrinA1 is released in three forms from cancer cells by matrix metalloproteases. Mol. Cell. Biol. 2012, 32, 3253–3264. [Google Scholar] [CrossRef]
- Georgakopoulos, A.; Litterst, C.; Ghersi, E.; Baki, L.; Xu, C.; Serban, G.; Robakis, N.K. Metalloproteinase/Presenilin1 processing of ephrinB regulates EphB-induced Src phosphorylation and signaling. EMBO J. 2006, 25, 1242–1252. [Google Scholar] [CrossRef] [PubMed]
- Litterst, C.; Georgakopoulos, A.; Shioi, J.; Ghersi, E.; Wisniewski, T.; Wang, R.; Ludwig, A.; Robakis, N.K. Ligand binding and calcium influx induce distinct ectodomain/γ-secretase-processing pathways of EphB2 receptor. J. Biol. Chem. 2007, 282, 16155–16163. [Google Scholar] [CrossRef] [PubMed]
- Nakada, M.; Niska, J.A.; Miyamori, H.; McDonough, W.S.; Wu, J.; Sato, H.; Berens, M.E. The phosphorylation of EphB2 receptor regulates migration and invasion of human glioma cells. Cancer Res. 2004, 64, 3179–3185. [Google Scholar] [CrossRef]
- Nakada, M.; Anderson, E.M.; Demuth, T.; Nakada, S.; Reavie, L.B.; Drake, K.L.; Hoelzinger, D.B.; Berens, M.E. The phosphorylation of ephrin-B2 ligand promotes glioma cell migration and invasion. Int. J. Cancer 2010, 126, 1155–1165. [Google Scholar] [CrossRef]
- Tanaka, M.; Kamata, R.; Takigahira, M.; Yanagihara, K.; Sakai, R. Phosphorylation of ephrin-B1 regulates dissemination of gastric scirrhous carcinoma. Am. J. Pathol. 2007, 171, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Huynh-Do, U.; Vindis, C.; Liu, H.; Cerretti, D.P.; McGrew, J.T.; Enriquez, M.; Chen, J.; Daniel, T.O. Ephrin-B1 transduces signals to activate integrin-mediated migration, attachment and angiogenesis. J. Cell Sci. 2002, 115, 3073–3081. [Google Scholar] [CrossRef]
- Hu, T.; Shi, G.; Larose, L.; Rivera, G.M.; Mayer, B.J.; Zhou, R. Regulation of process retraction and cell migration by EphA3 is mediated by the adaptor protein Nck1. Biochemistry 2009, 48, 6369–6378. [Google Scholar] [CrossRef]
- Herington, A.C.; Mertens-Walker, I.; Lisle, J.E.; Maharaj, M.; Stephenson, S.-A. Inhibiting Eph kinase activity may not be “Eph”ective for cancer treatment. Growth Factors 2014, 32, 207–213. [Google Scholar] [CrossRef]
- Noberini, R.; Lamberto, I.; Pasquale, E.B. Targeting Eph receptors with peptides and small molecules: Progress and challenges. Semin. Cell Dev. Biol. 2012, 23, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Lamminmaki, U.; Nikolov, D.; Himanen, J. Eph Receptors as Drug Targets: Single-Chain Antibodies and Beyond. Curr. Drug Targets 2015, 16, 1021–1030. [Google Scholar] [CrossRef]
- Giorgio, C.; Zanotti, I.; Lodola, A.; Tognolini, M. Ephrin or not? Six tough questions on Eph targeting. Expert. Opin. Ther. Targets 2020, 24, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Savagner, P. Epithelial-mesenchymal transitions: From cell plasticity to concept elasticity. Curr. Top. Dev. Biol. 2015, 112, 273–300. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).