Firing Alterations of Neurons in Alzheimer’s Disease: Are They Merely a Consequence of Pathogenesis or a Pivotal Component of Disease Progression?
Abstract
1. Introduction
2. Neuronal Excitability in AD
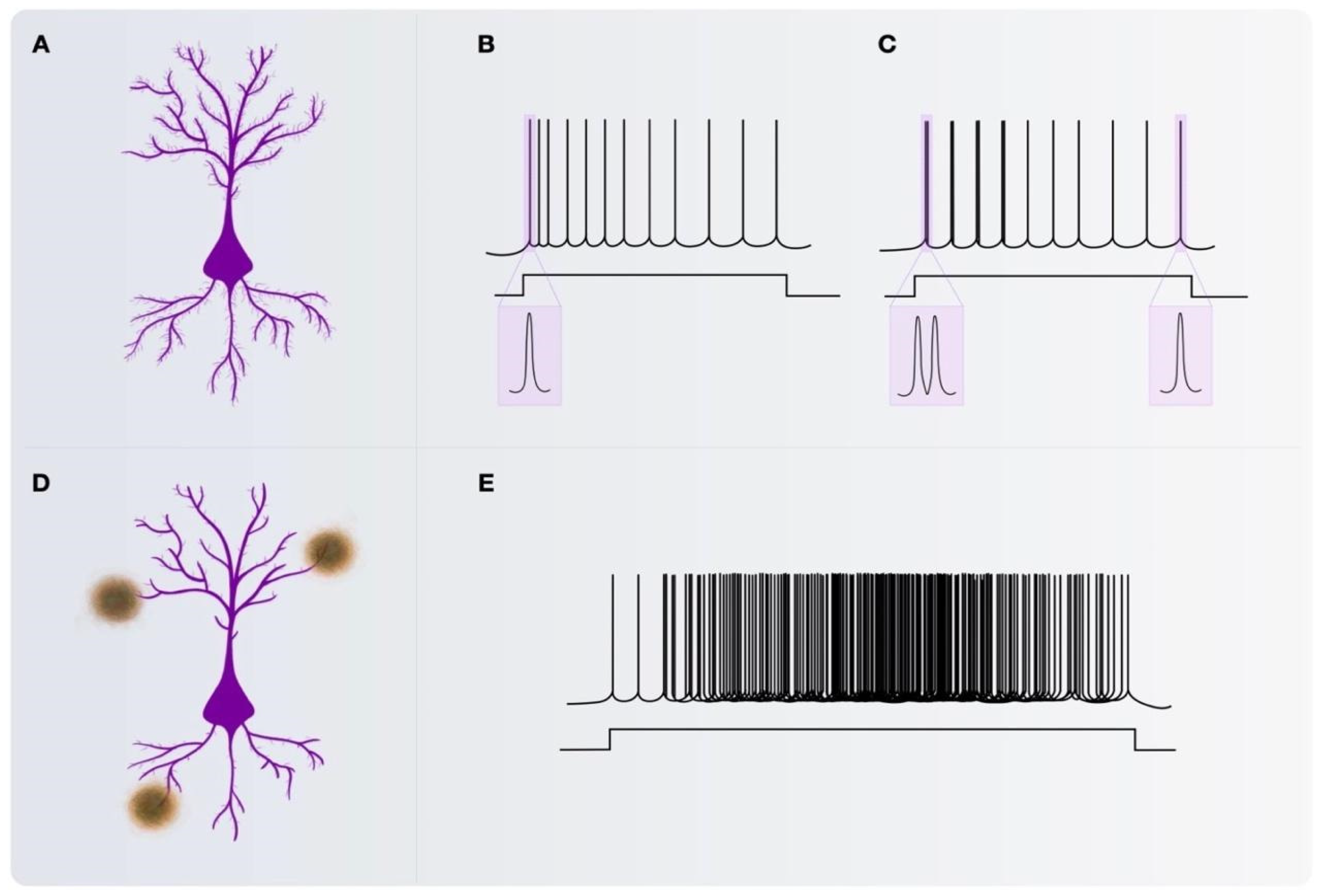
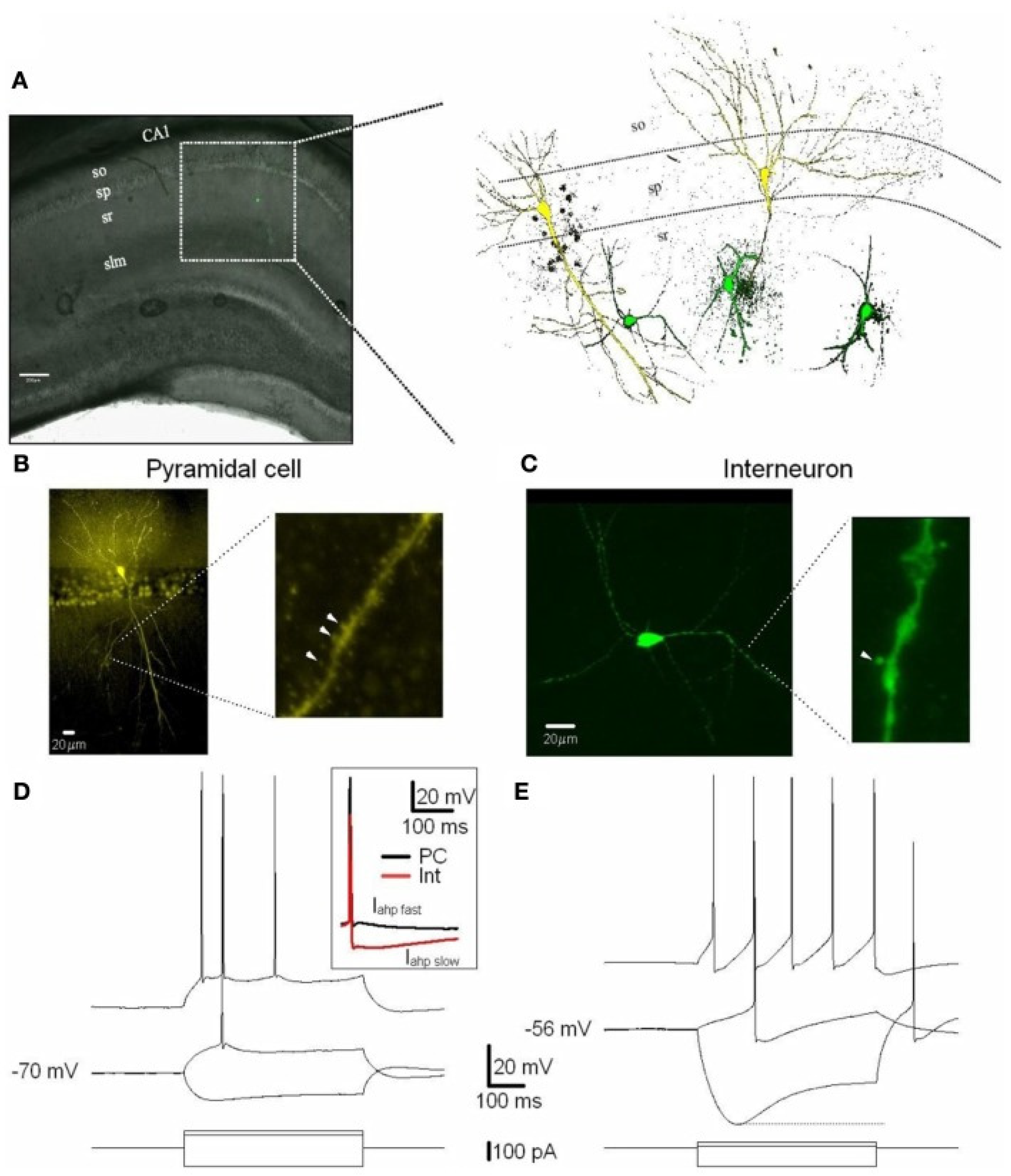
2.1. Pyramidal Neurons
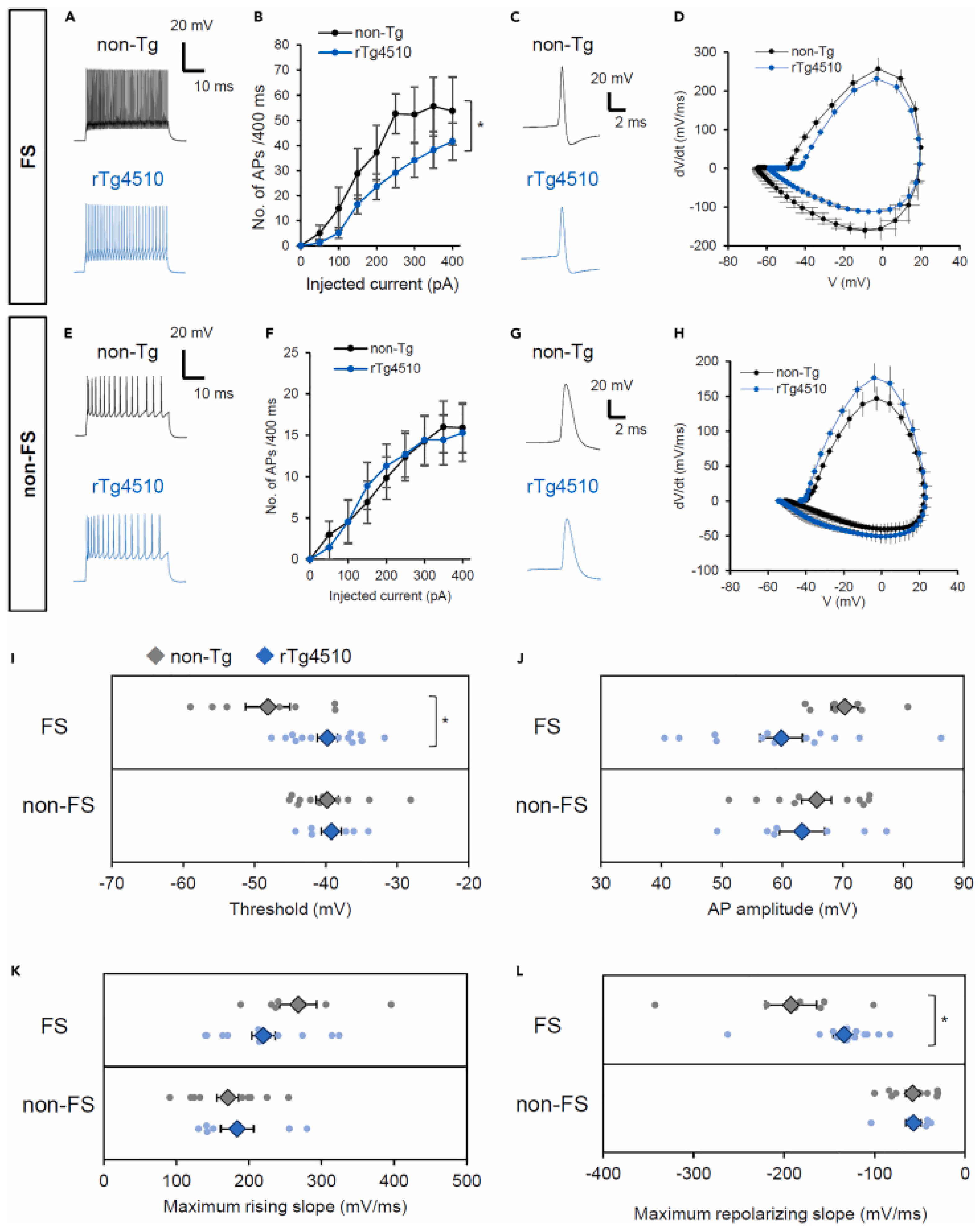
2.2. Interneurons
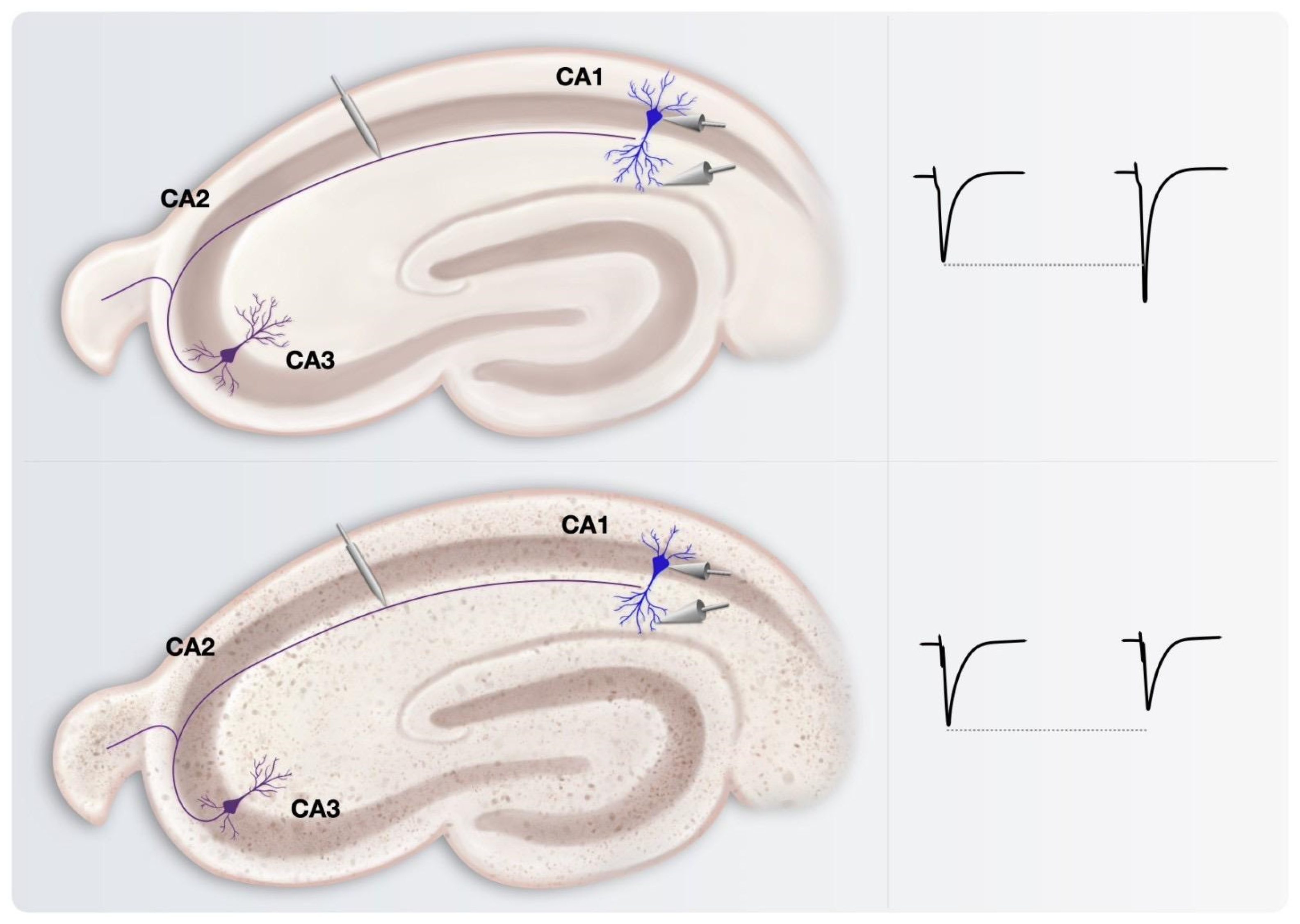
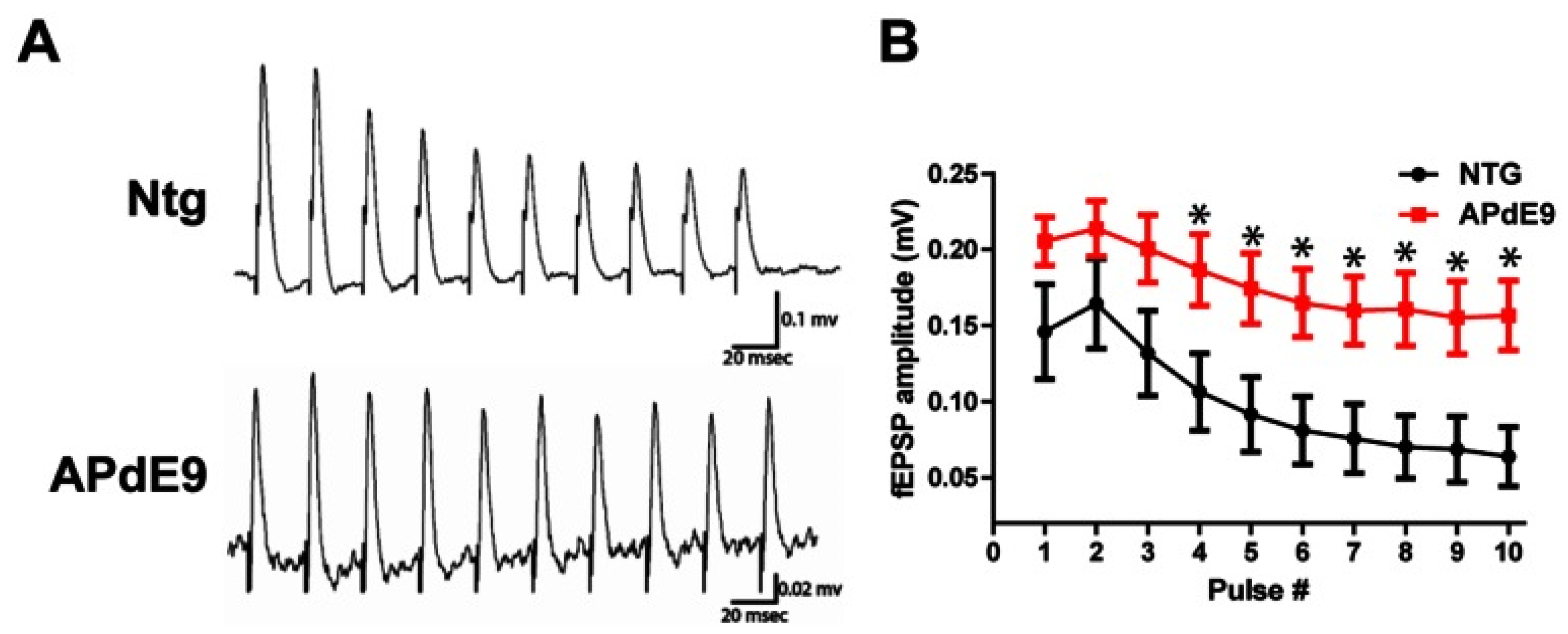
3. Insights on Neuronal Networks Firing Alterations in AD from Studies on Long-Term Potentiation (LTP)
4. Neurophysiological Impact of Morphologic and Synaptic Alterations on Neurons and Neuronal Circuits
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pei, Y.A.; Davies, J.; Zhang, M.; Zhang, H.-T. The Role of Synaptic Dysfunction in Alzheimer’s Disease. J. Alzheimer’s Dis. 2020, 76, 49–62. [Google Scholar] [CrossRef]
- Babiloni, C.; Blinowska, K.; Bonanni, L.; Cichocki, A.; De Haan, W.; Del Percio, C.; Dubois, B.; Escudero, J.; Fernández, A.; Frisoni, G.; et al. What electrophysiology tells us about Alzheimer’s disease: A window into the synchronization and connectivity of brain neurons. Neurobiol. Aging 2020, 85, 58–73. [Google Scholar] [CrossRef]
- Monllor, P.; Cervera-Ferri, A.; Lloret, M.-A.; Esteve, D.; Lopez, B.; Leon, J.-L.; Lloret, A. Electroencephalography as a Non-Invasive Biomarker of Alzheimer’s Disease: A Forgotten Candidate to Substitute CSF Molecules? Int. J. Mol. Sci. 2021, 22, 10889. [Google Scholar] [CrossRef]
- Nuñez, A.; Buño, W. The Theta Rhythm of the Hippocampus: From Neuronal and Circuit Mechanisms to Behavior. Front. Cell. Neurosci. 2021, 15, 649262. [Google Scholar] [CrossRef]
- Wu, L.; Cao, T.; Li, S.; Yuan, Y.; Zhang, W.; Huang, L.; Cai, C.; Fan, L.; Li, L.; Wang, J.; et al. Long-term gamma transcranial alternating current stimulation improves the memory function of mice with Alzheimer’s disease. Front. Aging Neurosci. 2022, 14, 980636. [Google Scholar] [CrossRef]
- Martorell, A.J.; Paulson, A.L.; Suk, H.-J.; Abdurrob, F.; Drummond, G.T.; Guan, W.; Young, J.Z.; Kim, D.N.-W.; Kritskiy, O.; Barker, S.J.; et al. Multi-sensory Gamma Stimulation Ameliorates Alzheimer’s-Associated Pathology and Improves Cognition. Cell 2019, 177, 256–271.e22. [Google Scholar] [CrossRef]
- Iaccarino, H.F.; Singer, A.C.; Martorell, A.J.; Rudenko, A.; Gao, F.; Gillingham, T.Z.; Mathys, H.; Seo, J.; Kritskiy, O.; Abdurrob, F.; et al. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature 2016, 540, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Staff, N.P.; Jung, H.Y.; Thiagarajan, T.; Yao, M.; Spruston, N. Resting and active properties of pyramidal neurons in subiculum and CA1 of rat hippocampus. J. Neurophysiol. 2000, 84, 2398–2408. [Google Scholar] [CrossRef] [PubMed]
- McGinley, M.J.; Westbrook, G.L. Membrane and synaptic properties of pyramidal neurons in the anterior olfactory nucleus. J. Neurophysiol. 2011, 105, 1444–1453. [Google Scholar] [CrossRef] [PubMed]
- Michalikova, M.; Remme, M.W.H.; Schmitz, D.; Schreiber, S.; Kempter, R. Spikelets in pyramidal neurons: Generating mechanisms, distinguishing properties, and functional implications. Rev. Neurosci. 2019, 31, 101–119. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, K.A. Differential developmental refinement of the intrinsic electrophysiological properties of CA1 pyramidal neurons from the rat dorsal and ventral hippocampus. Hippocampus 2020, 30, 233–249. [Google Scholar] [CrossRef]
- Graves, A.R.; Moore, S.J.; Bloss, E.B.; Mensh, B.D.; Kath, W.L.; Spruston, N. Hippocampal pyramidal neurons comprise two distinct cell types that are countermodulated by metabotropic receptors. Neuron 2012, 76, 776–789. [Google Scholar] [CrossRef]
- Menschik, E.D.; Finkel, L.H. Neuromodulatory control of hippocampal function: Towards a model of Alzheimer’s disease. Artif. Intell. Med. 1998, 13, 99–121. [Google Scholar] [CrossRef] [PubMed]
- Palop, J.J.; Chin, J.; Roberson, E.D.; Wang, J.; Thwin, M.T.; Bien-Ly, N.; Yoo, J.; Ho, K.O.; Yu, G.-Q.; Kreitzer, A.; et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron 2007, 55, 697–711. [Google Scholar] [CrossRef]
- Palop, J.J.; Mucke, L. Epilepsy and cognitive impairments in Alzheimer disease. Arch. Neurol. 2009, 66, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Busche, M.A.; Konnerth, A. Impairments of neural circuit function in Alzheimer’s disease. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150429. [Google Scholar] [CrossRef] [PubMed]
- Tamagnini, F.; Walsh, D.A.; Brown, J.T.; Bondulich, M.K.; Hanger, D.P.; Randall, A.D. Hippocampal neurophysiology is modified by a disease-associated C-terminal fragment of tau protein. Neurobiol. Aging 2017, 60, 44–56. [Google Scholar] [CrossRef]
- Royeck, M.; Horstmann, M.-T.; Remy, S.; Reitze, M.; Yaari, Y.; Beck, H. Role of axonal NaV1.6 sodium channels in action potential initiation of CA1 pyramidal neurons. J. Neurophysiol. 2008, 100, 2361–2380. [Google Scholar] [CrossRef]
- Stafstrom, C.E. Persistent sodium current and its role in epilepsy. Epilepsy Curr. 2007, 7, 15–22. [Google Scholar] [CrossRef]
- Brand, S.; Seeger, T.; Alzheimer, C. Enhancement of persistent Na+ current by sea anemone toxin (ATX II) exerts dual action on hippocampal excitability. Eur. J. Neurosci. 2000, 12, 2387–2396. [Google Scholar] [CrossRef]
- Shemer, I.; Holmgren, C.; Min, R.; Fülöp, L.; Zilberter, M.; Sousa, K.M.; Farkas, T.; Härtig, W.; Penke, B.; Burnashev, N.; et al. Non-fibrillar beta-amyloid abates spike-timing-dependent synaptic potentiation at excitatory synapses in layer 2/3 of the neocortex by targeting postsynaptic AMPA receptors. Eur. J. Neurosci. 2006, 23, 2035–2047. [Google Scholar] [CrossRef]
- Brown, J.T.; Chin, J.; Leiser, S.C.; Pangalos, M.N.; Randall, A.D. Altered intrinsic neuronal excitability and reduced Na+ currents in a mouse model of Alzheimer’s disease. Neurobiol. Aging 2011, 32, 2109.e1–2109.e14. [Google Scholar] [CrossRef]
- Busche, M.A.; Chen, X.; Henning, H.A.; Reichwald, J.; Staufenbiel, M.; Sakmann, B.; Konnerth, A. Critical role of soluble amyloid-β for early hippocampal hyperactivity in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2012, 109, 8740–8745. [Google Scholar] [CrossRef] [PubMed]
- Kerrigan, T.L.; Brown, J.T.; Randall, A.D. Characterization of altered intrinsic excitability in hippocampal CA1 pyramidal cells of the Aβ-overproducing PDAPP mouse. Neuropharmacology 2014, 79, 515–524. [Google Scholar] [CrossRef]
- Hall, A.M.; Throesch, B.T.; Buckingham, S.C.; Markwardt, S.J.; Peng, Y.; Wang, Q.; Hoffman, D.A.; Roberson, E.D. Tau-dependent Kv4.2 depletion and dendritic hyperexcitability in a mouse model of Alzheimer’s disease. J. Neurosci. 2015, 35, 6221–6230. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Wang, H.; Zhang, H.; Gao, N.; Zhang, T.; Yang, Z. Resveratrol Attenuates Aβ-Induced Early Hippocampal Neuron Excitability Impairment via Recovery of Function of Potassium Channels. Neurotox. Res. 2017, 32, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, D.A.; Magee, J.C.; Colbert, C.M.; Johnston, D. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature 1997, 387, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Coyle, D.; Wong-Lin, K.; Maguire, L. Beta-amyloid induced changes in A-type K+ current can alter hippocampo-septal network dynamics. J. Comput. Neurosci. 2012, 32, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Tazerart, S.; Blanchard, M.G.; Miranda-Rottmann, S.; Mitchell, D.E.; Navea Pina, B.; Thomas, C.I.; Kamasawa, N.; Araya, R. Selective activation of BK channels in small-headed dendritic spines suppresses excitatory postsynaptic potentials. J. Physiol. 2022, 600, 2165–2187. [Google Scholar] [CrossRef]
- Martina, M.; Comas, T.; Mealing, G.A.R. Selective Pharmacological Modulation of Pyramidal Neurons and Interneurons in the CA1 Region of the Rat Hippocampus. Front. Pharmacol. 2013, 4, 24. [Google Scholar] [CrossRef]
- Freund, T.F.; Buzsáki, G. Interneurons of the hippocampus. Hippocampus 1996, 6, 347–470. [Google Scholar] [CrossRef]
- Li, Y.; Sun, H.; Chen, Z.; Xu, H.; Bu, G.; Zheng, H. Implications of GABAergic Neurotransmission in Alzheimer’s Disease. Front. Aging Neurosci. 2016, 8, 31. [Google Scholar] [CrossRef]
- Jiménez-Balado, J.; Eich, T.S. GABAergic dysfunction, neural network hyperactivity and memory impairments in human aging and Alzheimer’s disease. Semin. Cell Dev. Biol. 2021, 116, 146–159. [Google Scholar] [CrossRef]
- O’Brien, R.J.; Wong, P.C. Amyloid precursor protein processing and Alzheimer’s disease. Annu. Rev. Neurosci. 2011, 34, 185–204. [Google Scholar] [CrossRef]
- Wang, J.; Fang, Y.; Wang, X.; Yang, H.; Yu, X.; Wang, H. Enhanced Gamma Activity and Cross-Frequency Interaction of Resting-State Electroencephalographic Oscillations in Patients with Alzheimer’s Disease. Front. Aging Neurosci. 2017, 9, 243. [Google Scholar] [CrossRef]
- Amatniek, J.C.; Hauser, W.A.; DelCastillo-Castaneda, C.; Jacobs, D.M.; Marder, K.; Bell, K.; Albert, M.; Brandt, J.; Stern, Y. Incidence and predictors of seizures in patients with Alzheimer’s disease. Epilepsia 2006, 47, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Hijazi, S.; Smit, A.B.; van Kesteren, R.E. Fast-spiking parvalbumin-positive interneurons in brain physiology and Alzheimer’s disease. Mol. Psychiatry 2023. Online ahead of print. [Google Scholar] [CrossRef]
- Rudy, B.; Fishell, G.; Lee, S.; Hjerling-Leffler, J. Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev. Neurobiol. 2011, 71, 45–61. [Google Scholar] [CrossRef]
- Runyan, C.A.; Schummers, J.; Van Wart, A.; Kuhlman, S.J.; Wilson, N.R.; Huang, Z.J.; Sur, M. Response features of parvalbumin-expressing interneurons suggest precise roles for subtypes of inhibition in visual cortex. Neuron 2010, 67, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Schiff, M.L.; Reyes, A.D. Characterization of thalamocortical responses of regular-spiking and fast-spiking neurons of the mouse auditory cortex in vitro and in silico. J. Neurophysiol. 2012, 107, 1476–1488. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Hu, H.; Huang, Z.J.; Agmon, A. Robust but delayed thalamocortical activation of dendritic-targeting inhibitory interneurons. Proc. Natl. Acad. Sci. USA 2008, 105, 2187–2192. [Google Scholar] [CrossRef] [PubMed]
- Canter, R.G.; Penney, J.; Tsai, L.-H. The road to restoring neural circuits for the treatment of Alzheimer’s disease. Nature 2016, 539, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Vargova, G.; Vogels, T.; Kostecka, Z.; Hromadka, T. Inhibitory interneurons in Alzheimer’s disease. Bratisl. Lek. Listy 2018, 119, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Gan, J.; Jonas, P. Interneurons. Fast-spiking, parvalbumin+ GABAergic interneurons: From cellular design to microcircuit function. Science 2014, 345, 1255263. [Google Scholar] [CrossRef]
- Verret, L.; Mann, E.O.; Hang, G.B.; Barth, A.M.I.; Cobos, I.; Ho, K.; Devidze, N.; Masliah, E.; Kreitzer, A.C.; Mody, I.; et al. Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell 2012, 149, 708–721. [Google Scholar] [CrossRef]
- Cattaud, V.; Bezzina, C.; Rey, C.C.; Lejards, C.; Dahan, L.; Verret, L. Early disruption of parvalbumin expression and perineuronal nets in the hippocampus of the Tg2576 mouse model of Alzheimer’s disease can be rescued by enriched environment. Neurobiol. Aging 2018, 72, 147–158. [Google Scholar] [CrossRef]
- Petrache, A.L.; Rajulawalla, A.; Shi, A.; Wetzel, A.; Saito, T.; Saido, T.C.; Harvey, K.; Ali, A.B. Aberrant Excitatory-Inhibitory Synaptic Mechanisms in Entorhinal Cortex Microcircuits During the Pathogenesis of Alzheimer’s Disease. Cereb. Cortex 2019, 29, 1834–1850. [Google Scholar] [CrossRef]
- Sanchez-Mejias, E.; Nuñez-Diaz, C.; Sanchez-Varo, R.; Gomez-Arboledas, A.; Garcia-Leon, J.A.; Fernandez-Valenzuela, J.J.; Mejias-Ortega, M.; Trujillo-Estrada, L.; Baglietto-Vargas, D.; Moreno-Gonzalez, I.; et al. Distinct disease-sensitive GABAergic neurons in the perirhinal cortex of Alzheimer’s mice and patients. Brain Pathol. 2020, 30, 345–363. [Google Scholar] [CrossRef]
- Hijazi, S.; Heistek, T.S.; Scheltens, P.; Neumann, U.; Shimshek, D.R.; Mansvelder, H.D.; Smit, A.B.; van Kesteren, R.E. Early restoration of parvalbumin interneuron activity prevents memory loss and network hyperexcitability in a mouse model of Alzheimer’s disease. Mol. Psychiatry 2020, 25, 3380–3398. [Google Scholar] [CrossRef]
- Chung, H.; Park, K.; Jang, H.J.; Kohl, M.M.; Kwag, J. Dissociation of somatostatin and parvalbumin interneurons circuit dysfunctions underlying hippocampal theta and gamma oscillations impaired by amyloid β oligomers in vivo. Brain Struct. Funct. 2020, 225, 935–954. [Google Scholar] [CrossRef]
- Hijazi, S.; Heistek, T.S.; van der Loo, R.; Mansvelder, H.D.; Smit, A.B.; van Kesteren, R.E. Hyperexcitable Parvalbumin Interneurons Render Hippocampal Circuitry Vulnerable to Amyloid Beta. iScience 2020, 23, 101271. [Google Scholar] [CrossRef] [PubMed]
- Giesers, N.K.; Wirths, O. Loss of Hippocampal Calretinin and Parvalbumin Interneurons in the 5XFAD Mouse Model of Alzheimer’s Disease. ASN Neuro 2020, 12, 1759091420925356. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Losa, M.; Tracy, T.E.; Ma, K.; Verret, L.; Clemente-Perez, A.; Khan, A.S.; Cobos, I.; Ho, K.; Gan, L.; Mucke, L.; et al. Nav1.1-Overexpressing Interneuron Transplants Restore Brain Rhythms and Cognition in a Mouse Model of Alzheimer’s Disease. Neuron 2018, 98, 75–89.e5. [Google Scholar] [CrossRef]
- Kudo, T.; Takuwa, H.; Takahashi, M.; Urushihata, T.; Shimojo, M.; Sampei, K.; Yamanaka, M.; Tomita, Y.; Sahara, N.; Suhara, T.; et al. Selective dysfunction of fast-spiking inhibitory interneurons and disruption of perineuronal nets in a tauopathy mouse model. iScience 2023, 26, 106342. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.-Q.; Yao, W.; Yan, J.-Z.; Jin, C.; Yin, J.-J.; Yuan, J.; Yu, S.; Cheng, Z. Amyloid β causes excitation/inhibition imbalance through dopamine receptor 1-dependent disruption of fast-spiking GABAergic input in anterior cingulate cortex. Sci. Rep. 2018, 8, 302. [Google Scholar] [CrossRef] [PubMed]
- Shi, A.; Petrache, A.L.; Shi, J.; Ali, A.B. Preserved Calretinin Interneurons in an App Model of Alzheimer’s Disease Disrupt Hippocampal Inhibition via Upregulated P2Y1 Purinoreceptors. Cereb. Cortex 2020, 30, 1272–1290. [Google Scholar] [CrossRef]
- Li, H.; Zhao, J.; Lai, L.; Xia, Y.; Wan, C.; Wei, S.; Liang, J.; Chen, Y.; Xu, N. Loss of SST and PV positive interneurons in the ventral hippocampus results in anxiety-like behavior in 5xFAD mice. Neurobiol. Aging 2022, 117, 165–178. [Google Scholar] [CrossRef]
- Saganich, M.J.; Schroeder, B.E.; Galvan, V.; Bredesen, D.E.; Koo, E.H.; Heinemann, S.F. Deficits in synaptic transmission and learning in amyloid precursor protein (APP) transgenic mice require C-terminal cleavage of APP. J. Neurosci. 2006, 26, 13428–13436. [Google Scholar] [CrossRef]
- Trinchese, F.; Liu, S.; Battaglia, F.; Walter, S.; Mathews, P.M.; Arancio, O. Progressive age-related development of Alzheimer-like pathology in APP/PS1 mice. Ann. Neurol. 2004, 55, 801–814. [Google Scholar] [CrossRef]
- Harris, J.A.; Devidze, N.; Halabisky, B.; Lo, I.; Thwin, M.T.; Yu, G.-Q.; Bredesen, D.E.; Masliah, E.; Mucke, L. Many neuronal and behavioral impairments in transgenic mouse models of Alzheimer’s disease are independent of caspase cleavage of the amyloid precursor protein. J. Neurosci. 2010, 30, 372–381. [Google Scholar] [CrossRef]
- Knobloch, M.; Farinelli, M.; Konietzko, U.; Nitsch, R.M.; Mansuy, I.M. Abeta oligomer-mediated long-term potentiation impairment involves protein phosphatase 1-dependent mechanisms. J. Neurosci. 2007, 27, 7648–7653. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gong, B.; Vitolo, O.V.; Trinchese, F.; Liu, S.; Shelanski, M.; Arancio, O. Persistent improvement in synaptic and cognitive functions in an Alzheimer mouse model after rolipram treatment. J. Clin. Investig. 2004, 114, 1624–1634. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, C.; Marie, H. Hippocampal synaptic plasticity in Alzheimer’s disease: What have we learned so far from transgenic models? Rev. Neurosci. 2011, 22, 373–402. [Google Scholar] [CrossRef]
- Fitzjohn, S.M.; Kuenzi, F.; Morton, R.A.; Rosahl, T.W.; Lewis, H.; Smith, D.; Seabrook, G.R.; Collingridge, G.L. A study of long-term potentiation in transgenic mice over-expressing mutant forms of both amyloid precursor protein and presenilin-1. Mol. Brain 2010, 3, 21. [Google Scholar] [CrossRef]
- Stranahan, A.M.; Mattson, M.P. Selective vulnerability of neurons in layer II of the entorhinal cortex during aging and Alzheimer’s disease. Neural Plast. 2010, 2010, 108190. [Google Scholar] [CrossRef] [PubMed]
- Danysz, W.; Parsons, C.G. Alzheimer’s disease, β-amyloid, glutamate, NMDA receptors and memantine--searching for the connections. Br. J. Pharmacol. 2012, 167, 324–352. [Google Scholar] [CrossRef]
- Kim, J.H.; Roberts, D.S.; Hu, Y.; Lau, G.C.; Brooks-Kayal, A.R.; Farb, D.H.; Russek, S.J. Brain-derived neurotrophic factor uses CREB and Egr3 to regulate NMDA receptor levels in cortical neurons. J. Neurochem. 2012, 120, 210–219. [Google Scholar] [CrossRef]
- Nomura, I.; Kato, N.; Kita, T.; Takechi, H. Mechanism of impairment of long-term potentiation by amyloid beta is independent of NMDA receptors or voltage-dependent calcium channels in hippocampal CA1 pyramidal neurons. Neurosci. Lett. 2005, 391, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Cuestas Torres, D.M.; Cardenas, F.P. Synaptic plasticity in Alzheimer’s disease and healthy aging. Rev. Neurosci. 2020, 31, 245–268. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.H.; Savage, M.J.; Flood, D.G.; Thomas, J.M.; Levy, R.B.; Mahadomrongkul, V.; Shirao, T.; Aoki, C.; Huerta, P.T. AMPA receptor downscaling at the onset of Alzheimer’s disease pathology in double knockin mice. Proc. Natl. Acad. Sci. USA 2006, 103, 3410–3415. [Google Scholar] [CrossRef]
- Shipton, O.A.; Leitz, J.R.; Dworzak, J.; Acton, C.E.J.; Tunbridge, E.M.; Denk, F.; Dawson, H.N.; Vitek, M.P.; Wade-Martins, R.; Paulsen, O.; et al. Tau protein is required for amyloid {beta}-induced impairment of hippocampal long-term potentiation. J. Neurosci. 2011, 31, 1688–1692. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Dani, J.A. Nicotinic acetylcholine receptors at glutamate synapses facilitate long-term depression or potentiation. J. Neurosci. 2005, 25, 6084–6091. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Li, Z.; Li, H.; Arancio, O.; Zhang, W. Notoginsenoside R1 increases neuronal excitability and ameliorates synaptic and memory dysfunction following amyloid elevation. Sci. Rep. 2014, 4, 6352. [Google Scholar] [CrossRef] [PubMed]
- Larson, J.; Lynch, G.; Games, D.; Seubert, P. Alterations in synaptic transmission and long-term potentiation in hippocampal slices from young and aged PDAPP mice. Brain Res. 1999, 840, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Eslami, M.; Sadeghi, B.; Goshadrou, F. Chronic ghrelin administration restores hippocampal long-term potentiation and ameliorates memory impairment in rat model of Alzheimer’s disease. Hippocampus 2018, 28, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ma, X.; Wei, J.; Shakir, N.; Zhang, J.K.; Zhang, L.; Nehme, A.; Cui, Y.; Ferguson, D.; Bai, F.; et al. Early impairment of cortical circuit plasticity and connectivity in the 5XFAD Alzheimer’s disease mouse model. Transl. Psychiatry 2022, 12, 371. [Google Scholar] [CrossRef]
- Kapay, N.A.; Isaev, N.K.; Stelmashook, E.V.; Popova, O.V.; Zorov, D.B.; Skrebitsky, V.G.; Skulachev, V.P. In vivo injected mitochondria-targeted plastoquinone antioxidant SkQR1 prevents β-amyloid-induced decay of long-term potentiation in rat hippocampal slices. Biochemistry 2011, 76, 1367–1370. [Google Scholar] [CrossRef]
- Shu, B.; Zhang, X.; Du, G.; Fu, Q.; Huang, L. MicroRNA-107 prevents amyloid-β-induced neurotoxicity and memory impairment in mice. Int. J. Mol. Med. 2018, 41, 1665–1672. [Google Scholar] [CrossRef]
- Roder, S.; Danober, L.; Pozza, M.F.; Lingenhoehl, K.; Wiederhold, K.-H.; Olpe, H.-R. Electrophysiological studies on the hippocampus and prefrontal cortex assessing the effects of amyloidosis in amyloid precursor protein 23 transgenic mice. Neuroscience 2003, 120, 705–720. [Google Scholar] [CrossRef]
- Xu, L.; Zhou, Y.; Hu, L.; Jiang, H.; Dong, Y.; Shen, H.; Lou, Z.; Yang, S.; Ji, Y.; Ruan, L.; et al. Deficits in N-Methyl-D-Aspartate Receptor Function and Synaptic Plasticity in Hippocampal CA1 in APP/PS1 Mouse Model of Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 772980. [Google Scholar] [CrossRef]
- Zeng, H.; Sanes, J.R. Neuronal cell-type classification: Challenges, opportunities and the path forward. Nat. Rev. Neurosci. 2017, 18, 530–546. [Google Scholar] [CrossRef]
- Mateos-Aparicio, P.; Rodríguez-Moreno, A. The Impact of Studying Brain Plasticity. Front. Cell. Neurosci. 2019, 13, 66. [Google Scholar] [CrossRef]
- Fuchs, E.; Flügge, G. Adult neuroplasticity: More than 40 years of research. Neural Plast. 2014, 2014, 541870. [Google Scholar] [CrossRef]
- Moolman, D.L.; Vitolo, O.V.; Vonsattel, J.-P.G.; Shelanski, M.L. Dendrite and dendritic spine alterations in Alzheimer models. J. Neurocytol. 2004, 33, 377–387. [Google Scholar] [CrossRef]
- Šišková, Z.; Justus, D.; Kaneko, H.; Friedrichs, D.; Henneberg, N.; Beutel, T.; Pitsch, J.; Schoch, S.; Becker, A.; von der Kammer, H.; et al. Dendritic structural degeneration is functionally linked to cellular hyperexcitability in a mouse model of Alzheimer’s disease. Neuron 2014, 84, 1023–1033. [Google Scholar] [CrossRef]
- Alvarez, V.A.; Sabatini, B.L. Anatomical and physiological plasticity of dendritic spines. Annu. Rev. Neurosci. 2007, 30, 79–97. [Google Scholar] [CrossRef] [PubMed]
- Chidambaram, S.B.; Rathipriya, A.G.; Bolla, S.R.; Bhat, A.; Ray, B.; Mahalakshmi, A.M.; Manivasagam, T.; Thenmozhi, A.J.; Essa, M.M.; Guillemin, G.J.; et al. Dendritic spines: Revisiting the physiological role. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 92, 161–193. [Google Scholar] [CrossRef] [PubMed]
- Kasai, H.; Hayama, T.; Ishikawa, M.; Watanabe, S.; Yagishita, S.; Noguchi, J. Learning rules and persistence of dendritic spines. Eur. J. Neurosci. 2010, 32, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Penzes, P.; Cahill, M.E.; Jones, K.A.; VanLeeuwen, J.-E.; Woolfrey, K.M. Dendritic spine pathology in neuropsychiatric disorders. Nat. Neurosci. 2011, 14, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Maiti, P.; Piacentini, R.; Ripoli, C.; Grassi, C.; Bitan, G. Surprising toxicity and assembly behaviour of amyloid β-protein oxidized to sulfone. Biochem. J. 2011, 433, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Attar, A.; Ripoli, C.; Riccardi, E.; Maiti, P.; Li Puma, D.D.; Liu, T.; Hayes, J.; Jones, M.R.; Lichti-Kaiser, K.; Yang, F.; et al. Protection of primary neurons and mouse brain from Alzheimer’s pathology by molecular tweezers. Brain 2012, 135, 3735–3748. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, J.S.; Wu, C.-C.; Redwine, J.M.; Comery, T.A.; Arias, R.; Bowlby, M.; Martone, R.; Morrison, J.H.; Pangalos, M.N.; Reinhart, P.H.; et al. Early-onset behavioral and synaptic deficits in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2006, 103, 5161–5166. [Google Scholar] [CrossRef] [PubMed]
- Lanz, T.A.; Carter, D.B.; Merchant, K.M. Dendritic spine loss in the hippocampus of young PDAPP and Tg2576 mice and its prevention by the ApoE2 genotype. Neurobiol. Dis. 2003, 13, 246–253. [Google Scholar] [CrossRef]
- Garcia-Alloza, M.; Dodwell, S.A.; Meyer-Luehmann, M.; Hyman, B.T.; Bacskai, B.J. Plaque-derived oxidative stress mediates distorted neurite trajectories in the Alzheimer mouse model. J. Neuropathol. Exp. Neurol. 2006, 65, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Spires-Jones, T.L.; Meyer-Luehmann, M.; Osetek, J.D.; Jones, P.B.; Stern, E.A.; Bacskai, B.J.; Hyman, B.T. Impaired spine stability underlies plaque-related spine loss in an Alzheimer’s disease mouse model. Am. J. Pathol. 2007, 171, 1304–1311. [Google Scholar] [CrossRef]
- Tsai, J.; Grutzendler, J.; Duff, K.; Gan, W.-B. Fibrillar amyloid deposition leads to local synaptic abnormalities and breakage of neuronal branches. Nat. Neurosci. 2004, 7, 1181–1183. [Google Scholar] [CrossRef]
- Yu, W.; Lu, B. Synapses and dendritic spines as pathogenic targets in Alzheimer’s disease. Neural Plast. 2012, 2012, 247150. [Google Scholar] [CrossRef]
- Kim, E.; Sheng, M. PDZ domain proteins of synapses. Nat. Rev. Neurosci. 2004, 5, 771–781. [Google Scholar] [CrossRef]
- Shankar, G.M.; Bloodgood, B.L.; Townsend, M.; Walsh, D.M.; Selkoe, D.J.; Sabatini, B.L. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J. Neurosci. 2007, 27, 2866–2875. [Google Scholar] [CrossRef]
- Rolland, M.; Powell, R.; Jacquier-Sarlin, M.; Boisseau, S.; Reynaud-Dulaurier, R.; Martinez-Hernandez, J.; André, L.; Borel, E.; Buisson, A.; Lanté, F. Effect of Aβ Oligomers on Neuronal APP Triggers a Vicious Cycle Leading to the Propagation of Synaptic Plasticity Alterations to Healthy Neurons. J. Neurosci. 2020, 40, 5161–5176. [Google Scholar] [CrossRef]
- Müller-Thomsen, L.; Borgmann, D.; Morcinek, K.; Schröder, S.; Dengler, B.; Moser, N.; Neumaier, F.; Schneider, T.; Schröder, H.; Huggenberger, S. Consequences of hyperphosphorylated tau on the morphology and excitability of hippocampal neurons in aged tau transgenic mice. Neurobiol. Aging 2020, 93, 109–123. [Google Scholar] [CrossRef]
- Auffret, A.; Gautheron, V.; Repici, M.; Kraftsik, R.; Mount, H.T.J.; Mariani, J.; Rovira, C. Age-dependent impairment of spine morphology and synaptic plasticity in hippocampal CA1 neurons of a presenilin 1 transgenic mouse model of Alzheimer’s disease. J. Neurosci. 2009, 29, 10144–10152. [Google Scholar] [CrossRef]
- Androuin, A.; Potier, B.; Nägerl, U.V.; Cattaert, D.; Danglot, L.; Thierry, M.; Youssef, I.; Triller, A.; Duyckaerts, C.; El Hachimi, K.H.; et al. Evidence for altered dendritic spine compartmentalization in Alzheimer’s disease and functional effects in a mouse model. Acta Neuropathol. 2018, 135, 839–854. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.-Z.; He, Y.-C.; Ma, X.K.; Li, S.-T.; Chen, D.-J.; Gao, M.; Qiu, S.-F.; Yin, J.-X.; Shi, J.; Wu, J. Hippocampal synaptic and neural network deficits in young mice carrying the human APOE4 gene. CNS Neurosci. Ther. 2017, 23, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Ammassari-Teule, M. Early-Occurring Dendritic Spines Alterations in Mouse Models of Alzheimer’s Disease Inform on Primary Causes of Neurodegeneration. Front. Synaptic Neurosci. 2020, 12, 566615. [Google Scholar] [CrossRef] [PubMed]
- Rocher, A.B.; Crimins, J.L.; Amatrudo, J.M.; Kinson, M.S.; Todd-Brown, M.A.; Lewis, J.; Luebke, J.I. Structural and functional changes in tau mutant mice neurons are not linked to the presence of NFTs. Exp. Neurol. 2010, 223, 385–393. [Google Scholar] [CrossRef]
- Hsieh, H.; Boehm, J.; Sato, C.; Iwatsubo, T.; Tomita, T.; Sisodia, S.; Malinow, R. AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron 2006, 52, 831–843. [Google Scholar] [CrossRef]
- Kim, J.; Wei, D.-S.; Hoffman, D.A. Kv4 potassium channel subunits control action potential repolarization and frequency-dependent broadening in rat hippocampal CA1 pyramidal neurones. J. Physiol. 2005, 569, 41–57. [Google Scholar] [CrossRef]
- Lugo, J.N.; Brewster, A.L.; Spencer, C.M.; Anderson, A.E. Kv4.2 knockout mice have hippocampal-dependent learning and memory deficits. Learn. Mem. 2012, 19, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.J.; Krimer, L.S.; Goldman-Rakic, P.S. Presynaptic regulation of recurrent excitation by D1 receptors in prefrontal circuits. Proc. Natl. Acad. Sci. USA 2001, 98, 295–300. [Google Scholar] [CrossRef]
- Gao, W.-J.; Wang, Y.; Goldman-Rakic, P.S. Dopamine Modulation of Perisomatic and Peridendritic Inhibition in Prefrontal Cortex. J. Neurosci. 2003, 23, 1622–1630. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Khan, G.M.; Nichols, R.A. Dopamine release in prefrontal cortex in response to β-amyloid activation of α7∗ nicotinic receptors. Brain Res. 2007, 1182, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Parnetti, L.; D’Amelio, M.; Tozzi, A.; Tantucci, M.; Romigi, A.; Siliquini, S.; Cavallucci, V.; Di Filippo, M.; Mazzocchetti, P.; et al. Epilepsy, amyloid-β, and D1 dopamine receptors: A possible pathogenetic link? Neurobiol. Aging 2016, 48, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S. High prevalence of parkinsonism in patients with MCI or mild Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 1615–1622. [Google Scholar] [CrossRef] [PubMed]
- D’Amelio, M.; Puglisi-Allegra, S.; Mercuri, N. The role of dopaminergic midbrain in Alzheimer’s disease: Translating basic science into clinical practice. Pharmacol. Res. 2018, 130, 414–419. [Google Scholar] [CrossRef] [PubMed]
- D’Amelio, M.; Serra, L.; Bozzali, M. Ventral Tegmental Area in Prodromal Alzheimer’s Disease: Bridging the Gap between Mice and Humans. J. Alzheimer’s Dis. 2018, 63, 181–183. [Google Scholar] [CrossRef]
- Ceyzériat, K.; Gloria, Y.; Tsartsalis, S.; Fossey, C.; Cailly, T.; Fabis, F.; Millet, P.; Tournier, B.B. Alterations in dopamine system and in its connectivity with serotonin in a rat model of Alzheimer’s disease. Brain Commun. 2021, 3, fcab029. [Google Scholar] [CrossRef]
- Hazra, A.; Gu, F.; Aulakh, A.; Berridge, C.; Eriksen, J.L.; Ziburkus, J. Inhibitory neuron and hippocampal circuit dysfunction in an aged mouse model of Alzheimer’s disease. PLoS ONE 2013, 8, e64318. [Google Scholar] [CrossRef]
- Chen, Q.S.; Kagan, B.L.; Hirakura, Y.; Xie, C.W. Impairment of hippocampal long-term potentiation by Alzheimer amyloid beta-peptides. J. Neurosci. Res. 2000, 60, 65–72. [Google Scholar] [CrossRef]
- Townsend, M.; Shankar, G.M.; Mehta, T.; Walsh, D.M.; Selkoe, D.J. Effects of secreted oligomers of amyloid beta-protein on hippocampal synaptic plasticity: A potent role for trimers. J. Physiol. 2006, 572, 477–492. [Google Scholar] [CrossRef] [PubMed]
- Targa Dias Anastacio, H.; Matosin, N.; Ooi, L. Neuronal hyperexcitability in Alzheimer’s disease: What are the drivers behind this aberrant phenotype? Transl. Psychiatry 2022, 12, 257. [Google Scholar] [CrossRef] [PubMed]
- Scheff, S.W.; Price, D.A.; Schmitt, F.A.; DeKosky, S.T.; Mufson, E.J. Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology 2007, 68, 1501–1508. [Google Scholar] [CrossRef]
- Šimko, P.; Kent, J.A.; Rektorova, I. Is non-invasive brain stimulation effective for cognitive enhancement in Alzheimer’s disease? An updated meta-analysis. Clin. Neurophysiol. 2022, 144, 23–40. [Google Scholar] [CrossRef]
- Gu, L.; Xu, H.; Qian, F. Effects of Non-Invasive Brain Stimulation on Alzheimer’s Disease. J. Prev. Alzheimer’s Dis. 2022, 9, 410–424. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Mao, C.; Hu, X.; Zhang, S.; Yang, Z.; Hu, Z.; Sun, H.; Fan, Y.; Dong, Y.; Yang, J.; et al. New Insights Into the Pathogenesis of Alzheimer’s Disease. Front. Neurol. 2019, 10, 1312. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tzavellas, N.P.; Tsamis, K.I.; Katsenos, A.P.; Davri, A.S.; Simos, Y.V.; Nikas, I.P.; Bellos, S.; Lekkas, P.; Kanellos, F.S.; Konitsiotis, S.; et al. Firing Alterations of Neurons in Alzheimer’s Disease: Are They Merely a Consequence of Pathogenesis or a Pivotal Component of Disease Progression? Cells 2024, 13, 434. https://doi.org/10.3390/cells13050434
Tzavellas NP, Tsamis KI, Katsenos AP, Davri AS, Simos YV, Nikas IP, Bellos S, Lekkas P, Kanellos FS, Konitsiotis S, et al. Firing Alterations of Neurons in Alzheimer’s Disease: Are They Merely a Consequence of Pathogenesis or a Pivotal Component of Disease Progression? Cells. 2024; 13(5):434. https://doi.org/10.3390/cells13050434
Chicago/Turabian StyleTzavellas, Nikolaos P., Konstantinos I. Tsamis, Andreas P. Katsenos, Athena S. Davri, Yannis V. Simos, Ilias P. Nikas, Stefanos Bellos, Panagiotis Lekkas, Foivos S. Kanellos, Spyridon Konitsiotis, and et al. 2024. "Firing Alterations of Neurons in Alzheimer’s Disease: Are They Merely a Consequence of Pathogenesis or a Pivotal Component of Disease Progression?" Cells 13, no. 5: 434. https://doi.org/10.3390/cells13050434
APA StyleTzavellas, N. P., Tsamis, K. I., Katsenos, A. P., Davri, A. S., Simos, Y. V., Nikas, I. P., Bellos, S., Lekkas, P., Kanellos, F. S., Konitsiotis, S., Labrakakis, C., Vezyraki, P., & Peschos, D. (2024). Firing Alterations of Neurons in Alzheimer’s Disease: Are They Merely a Consequence of Pathogenesis or a Pivotal Component of Disease Progression? Cells, 13(5), 434. https://doi.org/10.3390/cells13050434







