Cellular and Noncellular Approaches for Repairing the Damaged Blood–CNS–Barrier in Amyotrophic Lateral Sclerosis
Abstract
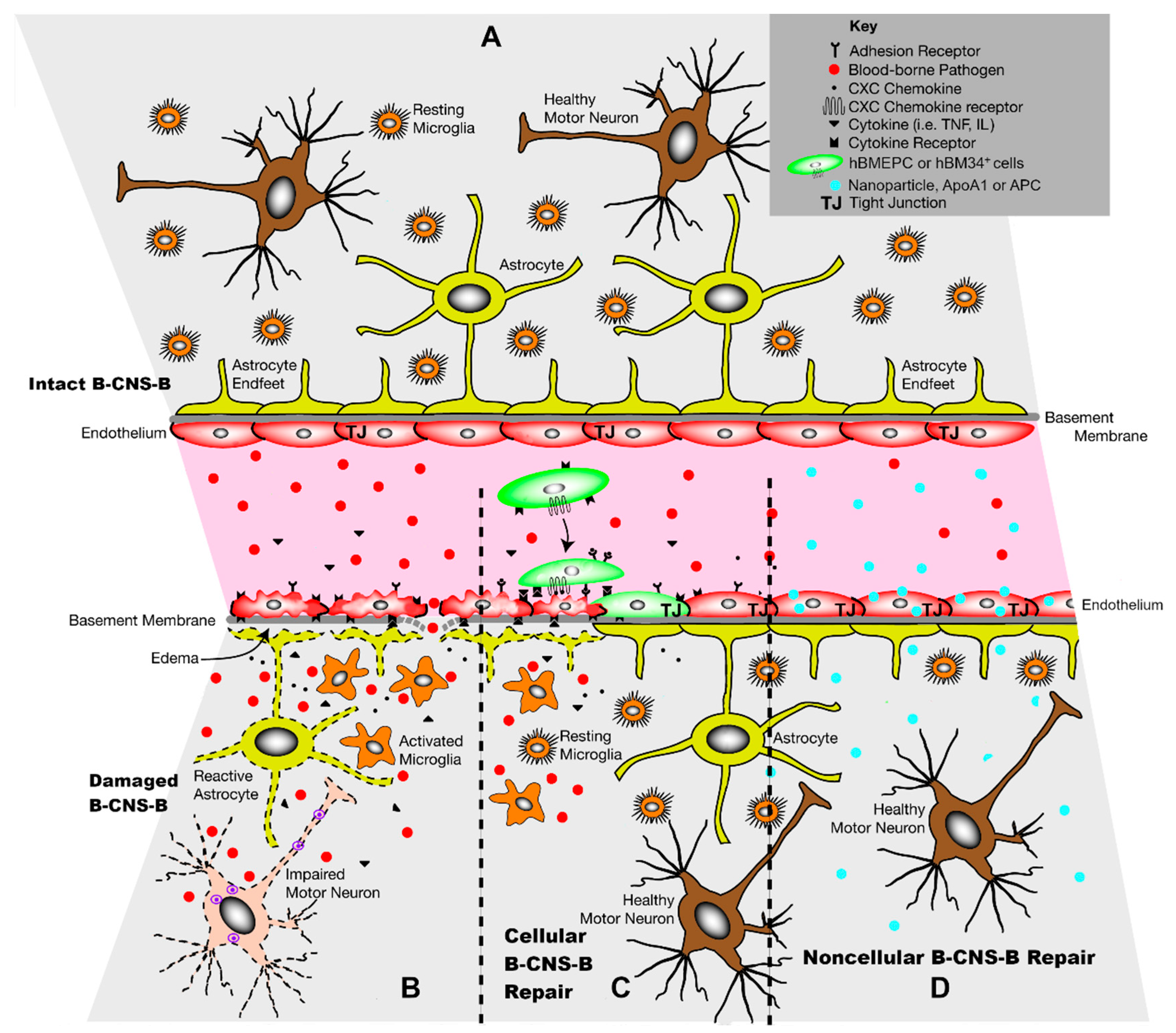
1. Introduction
2. Cellular Approach to B-CNS-B Repair
2.1. The Effects of Bone Marrow-Derived Stem Cells
2.2. The Effects of Mesenchymal Stem Cells
3. Noncellular Approach to B-CNS-B Repair
3.1. The Effects of Extracellular Vesicles Derived from Stem Cells
3.2. The Effects of Bioengineered EVs
3.3. Addressing B-CNS-B Repair in ALS with ApoA1
3.4. Addressing B-CNS-B Repair in ALS with Activated Protein C
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Rothstein, J.D. Current Hypotheses for the Underlying Biology of Amyotrophic Lateral Sclerosis. Ann. Neurol. 2009, 65 (Suppl. S1), S3–S9. [Google Scholar] [CrossRef]
- Strong, M.J.; Kesavapany, S.; Pant, H.C. The Pathobiology of Amyotrophic Lateral Sclerosis: A Proteinopathy? J. Neuropathol. Exp. Neurol. 2005, 64, 649–664. [Google Scholar] [CrossRef]
- Wijesekera, L.C.; Leigh, P.N. Amyotrophic Lateral Sclerosis. Orphanet J. Rare Dis. 2009, 4, 3. [Google Scholar] [CrossRef]
- Martin, L.J.; Price, A.C.; Kaiser, A.; Shaikh, A.Y.; Liu, Z. Mechanisms for Neuronal Degeneration in Amyotrophic Lateral Sclerosis and in Models of Motor Neuron Death (Review). Int. J. Mol. Med. 2000, 5, 3–13. [Google Scholar] [CrossRef]
- Garbuzova-Davis, S.; Haller, E.; Saporta, S.; Kolomey, I.; Nicosia, S.V.; Sanberg, P.R. Ultrastructure of Blood-Brain Barrier and Blood-Spinal Cord Barrier in SOD1 Mice Modeling ALS. Brain Res. 2007, 1157, 126–137. [Google Scholar] [CrossRef]
- Garbuzova-Davis, S.; Saporta, S.; Haller, E.; Kolomey, I.; Bennett, S.P.; Potter, H.; Sanberg, P.R. Evidence of Compromised Blood-Spinal Cord Barrier in Early and Late Symptomatic SOD1 Mice Modeling ALS. PLoS ONE 2007, 2, e1205. [Google Scholar] [CrossRef]
- Miyazaki, K.; Ohta, Y.; Nagai, M.; Morimoto, N.; Kurata, T.; Takehisa, Y.; Ikeda, Y.; Matsuura, T.; Abe, K. Disruption of Neurovascular Unit Prior to Motor Neuron Degeneration in Amyotrophic Lateral Sclerosis. J. Neurosci. Res. 2011, 89, 718–728. [Google Scholar] [CrossRef]
- Nicaise, C.; Mitrecic, D.; Demetter, P.; De Decker, R.; Authelet, M.; Boom, A.; Pochet, R. Impaired Blood-Brain and Blood-Spinal Cord Barriers in Mutant SOD1-Linked ALS Rat. Brain Res. 2009, 1301, 152–162. [Google Scholar] [CrossRef]
- Nicaise, C.; Soyfoo, M.S.; Delporte, C.; Pochet, R. Aquaporin-4 as a Potential Marker of BBB Disruption in ALS Models. Amyotroph. Lateral Scler. 2010, 11, 253–254. [Google Scholar] [CrossRef]
- Zhong, Z.; Deane, R.; Ali, Z.; Parisi, M.; Shapovalov, Y.; O’Banion, M.K.; Stojanovic, K.; Sagare, A.; Boillee, S.; Cleveland, D.W.; et al. ALS-Causing SOD1 Mutants Generate Vascular Changes Prior to Motor Neuron Degeneration. Nat. Neurosci. 2008, 11, 420–422. [Google Scholar] [CrossRef]
- Garbuzova-Davis, S.; Hernandez-Ontiveros, D.G.; Rodrigues, M.C.O.; Haller, E.; Frisina-Deyo, A.; Mirtyl, S.; Sallot, S.; Saporta, S.; Borlongan, C.V.; Sanberg, P.R. Impaired Blood-Brain/Spinal Cord Barrier in ALS Patients. Brain Res. 2012, 1469, 114–128. [Google Scholar] [CrossRef]
- Henkel, J.S.; Beers, D.R.; Wen, S.; Bowser, R.; Appel, S.H. Decreased MRNA Expression of Tight Junction Proteins in Lumbar Spinal Cords of Patients with ALS. Neurology 2009, 72, 1614–1616. [Google Scholar] [CrossRef]
- Winkler, E.A.; Sengillo, J.D.; Sullivan, J.S.; Henkel, J.S.; Appel, S.H.; Zlokovic, B.V. Blood-Spinal Cord Barrier Breakdown and Pericyte Reductions in Amyotrophic Lateral Sclerosis. Acta Neuropathol. 2013, 125, 111–120. [Google Scholar] [CrossRef]
- Yamadera, M.; Fujimura, H.; Inoue, K.; Toyooka, K.; Mori, C.; Hirano, H.; Sakoda, S. Microvascular Disturbance with Decreased Pericyte Coverage Is Prominent in the Ventral Horn of Patients with Amyotrophic Lateral Sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2015, 16, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Garbuzova-Davis, S.; Rodrigues, M.C.O.; Hernandez-Ontiveros, D.G.; Louis, M.K.; Willing, A.E.; Borlongan, C.V.; Sanberg, P.R. Amyotrophic Lateral Sclerosis: A Neurovascular Disease. Brain Res. 2011, 1398, 113–125. [Google Scholar] [CrossRef]
- Steinruecke, M.; Lonergan, R.M.; Selvaraj, B.T.; Chandran, S.; Diaz-Castro, B.; Stavrou, M. Blood-CNS Barrier Dysfunction in Amyotrophic Lateral Sclerosis: Proposed Mechanisms and Clinical Implications. J. Cereb. Blood Flow Metab. 2023, 43, 642–654. [Google Scholar] [CrossRef]
- Mirian, A.; Moszczynski, A.; Soleimani, S.; Aubert, I.; Zinman, L.; Abrahao, A. Breached Barriers: A Scoping Review of Blood-Central Nervous System Barrier Pathology in Amyotrophic Lateral Sclerosis. Front. Cell. Neurosci. 2022, 16, 851563. [Google Scholar] [CrossRef]
- Garbuzova-Davis, S.; Sanberg, P.R. Feasibility of Cell Therapy for Amyotrophic Lateral Sclerosis. Exp. Neurol. 2009, 216, 3–6. [Google Scholar] [CrossRef]
- Mitrecić, D.; Gajović, S.; Pochet, R. Toward the Treatments with Neural Stem Cells: Experiences from Amyotrophic Lateral Sclerosis. Anat. Rec. 2009, 292, 1962–1967. [Google Scholar] [CrossRef]
- Lunn, J.S.; Sakowski, S.A.; Feldman, E.L. Concise Review: Stem Cell Therapies for Amyotrophic Lateral Sclerosis: Recent Advances and Prospects for the Future. Stem Cells 2014, 32, 1099–1109. [Google Scholar] [CrossRef]
- Faravelli, I.; Riboldi, G.; Nizzardo, M.; Simone, C.; Zanetta, C.; Bresolin, N.; Comi, G.P.; Corti, S. Stem Cell Transplantation for Amyotrophic Lateral Sclerosis: Therapeutic Potential and Perspectives on Clinical Translation. Cell. Mol. Life Sci. 2014, 71, 3257–3268. [Google Scholar] [CrossRef] [PubMed]
- Boulis, N.M.; Federici, T.; Glass, J.D.; Lunn, J.S.; Sakowski, S.A.; Feldman, E.L. Translational Stem Cell Therapy for Amyotrophic Lateral Sclerosis. Nat. Rev. Neurol. 2011, 8, 172–176. [Google Scholar] [CrossRef]
- Mao, Z.; Zhang, S.; Chen, H. Stem Cell Therapy for Amyotrophic Lateral Sclerosis. Cell Regen. 2015, 4, 11. [Google Scholar] [CrossRef]
- Lin, T.-J.; Cheng, G.-C.; Wu, L.-Y.; Lai, W.-Y.; Ling, T.-Y.; Kuo, Y.-C.; Huang, Y.-H. Potential of Cellular Therapy for ALS: Current Strategies and Future Prospects. Front. Cell. Dev. Biol. 2022, 10, 851613. [Google Scholar] [CrossRef]
- Suzuki, M.; Svendsen, C.N. Combining Growth Factor and Stem Cell Therapy for Amyotrophic Lateral Sclerosis. Trends Neurosci. 2008, 31, 192–198. [Google Scholar] [CrossRef]
- Lunn, J.S.; Hefferan, M.P.; Marsala, M.; Feldman, E.L. Stem Cells: Comprehensive Treatments for Amyotrophic Lateral Sclerosis in Conjunction with Growth Factor Delivery. Growth Factors 2009, 27, 133–140. [Google Scholar] [CrossRef]
- Riley, J.; Sweeney, W.; Boulis, N. Shifting the Balance: Cell-Based Therapeutics as Modifiers of the Amyotrophic Lateral Sclerosis-Specific Neuronal Microenvironment. Neurosurg. Focus 2008, 24, E10. [Google Scholar] [CrossRef]
- Hur, Y.H.; Cerione, R.A.; Antonyak, M.A. Extracellular Vesicles and Their Roles in Stem Cell Biology. Stem Cells 2020, 38, 469–476. [Google Scholar] [CrossRef]
- Meamar, R.; Nasr-Esfahani, M.H.; Mousavi, S.A.; Basiri, K. Stem Cell Therapy in Amyotrophic Lateral Sclerosis. J. Clin. Neurosci. 2013, 20, 1659–1663. [Google Scholar] [CrossRef]
- Yoder, M.C. Defining Human Endothelial Progenitor Cells. J. Thromb. Haemost. 2009, 7 (Suppl. S1), 49–52. [Google Scholar] [CrossRef] [PubMed]
- Yoder, M.C. Endothelial Progenitor Cell: A Blood Cell by Many Other Names May Serve Similar Functions. J. Mol. Med. 2013, 91, 285–295. [Google Scholar] [CrossRef]
- Piatkowski, A.; Grieb, G.; Simons, D.; Bernhagen, J.; van der Hulst, R.R. Endothelial Progenitor Cells—Potential New Avenues to Improve Neoangiogenesis and Reendothelialization. Int. Rev. Cell Mol. Biol. 2013, 306, 43–81. [Google Scholar] [CrossRef]
- Richardson, M.R.; Yoder, M.C. Endothelial Progenitor Cells: Quo Vadis? J. Mol. Cell. Cardiol. 2011, 50, 266–272. [Google Scholar] [CrossRef]
- Bakhshandeh, B.; Kamaleddin, M.A.; Aalishah, K. A Comprehensive Review on Exosomes and Microvesicles as Epigenetic Factors. Curr. Stem Cell Res. Ther. 2017, 12, 31–36. [Google Scholar] [CrossRef]
- Gho, Y.S.; Lee, C. Emergent Properties of Extracellular Vesicles: A Holistic Approach to Decode the Complexity of Intercellular Communication Networks. Mol. Biosyst. 2017, 13, 1291–1296. [Google Scholar] [CrossRef] [PubMed]
- Iraci, N.; Leonardi, T.; Gessler, F.; Vega, B.; Pluchino, S. Focus on Extracellular Vesicles: Physiological Role and Signalling Properties of Extracellular Membrane Vesicles. Int. J. Mol. Sci. 2016, 17, 171. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R.F. Routes and Mechanisms of Extracellular Vesicle Uptake. J. Extracell. Vesicles 2014, 3, 24641. [Google Scholar] [CrossRef] [PubMed]
- Tetta, C.; Ghigo, E.; Silengo, L.; Deregibus, M.C.; Camussi, G. Extracellular Vesicles as an Emerging Mechanism of Cell-to-Cell Communication. Endocrine 2013, 44, 11–19. [Google Scholar] [CrossRef]
- Yoon, Y.J.; Kim, O.Y.; Gho, Y.S. Extracellular Vesicles as Emerging Intercellular Communicasomes. BMB Rep. 2014, 47, 531–539. [Google Scholar] [CrossRef]
- Garbuzova-Davis, S.; Willing, A.E.; Ehrhart, J.; Wang, L.; Sanberg, P.R.; Borlongan, C.V. Cell-Free Extracellular Vesicles Derived from Human Bone Marrow Endothelial Progenitor Cells as Potential Therapeutics for Microvascular Endothelium Restoration in ALS. Neuromol. Med. 2020, 22, 503–516. [Google Scholar] [CrossRef]
- Jang, S.C.; Kim, O.Y.; Yoon, C.M.; Choi, D.-S.; Roh, T.-Y.; Park, J.; Nilsson, J.; Lötvall, J.; Kim, Y.-K.; Gho, Y.S. Bioinspired Exosome-Mimetic Nanovesicles for Targeted Delivery of Chemotherapeutics to Malignant Tumors. ACS Nano 2013, 7, 7698–7710. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Kumar, H.; Jo, M.-J.; Kim, J.; Yoon, J.-K.; Lee, J.-R.; Kang, M.; Choo, Y.W.; Song, S.Y.; Kwon, S.P.; et al. Therapeutic Efficacy-Potentiated and Diseased Organ-Targeting Nanovesicles Derived from Mesenchymal Stem Cells for Spinal Cord Injury Treatment. Nano Lett. 2018, 18, 4965–4975. [Google Scholar] [CrossRef]
- Jin, Q.; Wu, P.; Zhou, X.; Qian, H.; Xu, W. Extracellular Vesicles: Novel Roles in Neurological Disorders. Stem Cells Int. 2021, 2021, 6640836. [Google Scholar] [CrossRef]
- Ingram, D.A.; Caplice, N.M.; Yoder, M.C. Unresolved Questions, Changing Definitions, and Novel Paradigms for Defining Endothelial Progenitor Cells. Blood 2005, 106, 1525–1531. [Google Scholar] [CrossRef]
- Srour, E.F.; Brandt, J.E.; Briddell, R.A.; Leemhuis, T.; van Besien, K.; Hoffman, R. Human CD34+ HLA-DR- Bone Marrow Cells Contain Progenitor Cells Capable of Self-Renewal, Multilineage Differentiation, and Long-Term in Vitro Hematopoiesis. Blood Cells 1991, 17, 287–295. [Google Scholar]
- Timmermans, F.; Van Hauwermeiren, F.; De Smedt, M.; Raedt, R.; Plasschaert, F.; De Buyzere, M.L.; Gillebert, T.C.; Plum, J.; Vandekerckhove, B. Endothelial Outgrowth Cells Are Not Derived from CD133+ Cells or CD45+ Hematopoietic Precursors. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1572–1579. [Google Scholar] [CrossRef]
- Garbuzova-Davis, S.; Kurien, C.; Thomson, A.; Falco, D.; Ahmad, S.; Staffetti, J.; Steiner, G.; Abraham, S.; James, G.; Mahendrasah, A.; et al. Endothelial and Astrocytic Support by Human Bone Marrow Stem Cell Grafts into Symptomatic ALS Mice towards Blood-Spinal Cord Barrier Repair. Sci. Rep. 2017, 7, 884. [Google Scholar] [CrossRef]
- Garbuzova-Davis, S.; Haller, E.; Navarro, S.; Besong, T.E.; Boccio, K.J.; Hailu, S.; Khatib, M.; Sanberg, P.R.; Appel, S.H.; Borlongan, C.V. Transplantation of Human Bone Marrow Stem Cells into Symptomatic ALS Mice Enhances Structural and Functional Blood-Spinal Cord Barrier Repair. Exp. Neurol. 2018, 310, 33–47. [Google Scholar] [CrossRef]
- Eve, D.J.; Steiner, G.; Mahendrasah, A.; Sanberg, P.R.; Kurien, C.; Thomson, A.; Borlongan, C.V.; Garbuzova-Davis, S. Reduction of Microhemorrhages in the Spinal Cord of Symptomatic ALS Mice after Intravenous Human Bone Marrow Stem Cell Transplantation Accompanies Repair of the Blood-Spinal Cord Barrier. Oncotarget 2018, 9, 10621–10634. [Google Scholar] [CrossRef][Green Version]
- Garbuzova-Davis, S.; Ehrhart, J.; Mustafa, H.; Llauget, A.; Boccio, K.J.; Sanberg, P.R.; Appel, S.H.; Borlongan, C.V. Phenotypic Characteristics of Human Bone Marrow-Derived Endothelial Progenitor Cells in vitro Support Cell Effectiveness for Repair of the Blood-Spinal Cord Barrier in ALS. Brain Res. 2019, 1724, 146428. [Google Scholar] [CrossRef] [PubMed]
- Garbuzova-Davis, S.; Kurien, C.; Haller, E.; Eve, D.J.; Navarro, S.; Steiner, G.; Mahendrasah, A.; Hailu, S.; Khatib, M.; Boccio, K.J.; et al. Human Bone Marrow Endothelial Progenitor Cell Transplantation into Symptomatic ALS Mice Delays Disease Progression and Increases Motor Neuron Survival by Repairing Blood-Spinal Cord Barrier. Sci. Rep. 2019, 9, 5280. [Google Scholar] [CrossRef]
- Garbuzova-Davis, S.; Boccio, K.J.; Ehrhart, J.; Sanberg, P.R.; Appel, S.H.; Borlongan, C.V. Detection of Endothelial Cell-Associated Human DNA Reveals Transplanted Human Bone Marrow Stem Cell Engraftment into CNS Capillaries of ALS Mice. Brain Res. Bull. 2021, 170, 22–28. [Google Scholar] [CrossRef]
- Garbuzova-Davis, S.; Boccio, K.J.; Llauget, A.; Shell, R.; Hailu, S.; Mustafa, H.; Ehrhart, J.; Sanberg, P.R.; Appel, S.H.; Borlongan, C.V. Beneficial Effects of Transplanted Human Bone Marrow Endothelial Progenitors on Functional and Cellular Components of Blood-Spinal Cord Barrier in ALS Mice. eNeuro 2021, 8, ENEURO.0314-21.2021. [Google Scholar] [CrossRef]
- Garbuzova-Davis, S.; Borlongan, C.V. Transplanted Human Bone Marrow Endothelial Progenitor Cells Prolong Functional Benefits and Extend Survival of ALS Mice Likely via Blood-Spinal Cord Barrier Repair. Stem Cell Rev. Rep. 2023, 19, 2284–2291. [Google Scholar] [CrossRef] [PubMed]
- Giordano, A.; Galderisi, U.; Marino, I.R. From the Laboratory Bench to the Patient’s Bedside: An Update on Clinical Trials with Mesenchymal Stem Cells. J. Cell Physiol. 2007, 211, 27–35. [Google Scholar] [CrossRef] [PubMed]
- de Girolamo, L.; Lucarelli, E.; Alessandri, G.; Avanzini, M.A.; Bernardo, M.E.; Biagi, E.; Brini, A.T.; D’Amico, G.; Fagioli, F.; Ferrero, I.; et al. Mesenchymal Stem/Stromal Cells: A New “‘cells as Drugs’” Paradigm. Efficacy and Critical Aspects in Cell Therapy. Curr. Pharm. Des. 2013, 19, 2459–2473. [Google Scholar] [CrossRef]
- Laroni, A.; Novi, G.; Kerlero de Rosbo, N.; Uccelli, A. Towards Clinical Application of Mesenchymal Stem Cells for Treatment of Neurological Diseases of the Central Nervous System. J. Neuroimmune Pharmacol. 2013, 8, 1062–1076. [Google Scholar] [CrossRef]
- Marconi, S.; Bonaconsa, M.; Scambi, I.; Squintani, G.M.; Rui, W.; Turano, E.; Ungaro, D.; D’Agostino, S.; Barbieri, F.; Angiari, S.; et al. Systemic Treatment with Adipose-Derived Mesenchymal Stem Cells Ameliorates Clinical and Pathological Features in the Amyotrophic Lateral Sclerosis Murine Model. Neuroscience 2013, 248, 333–343. [Google Scholar] [CrossRef]
- Magota, H.; Sasaki, M.; Kataoka-Sasaki, Y.; Oka, S.; Ukai, R.; Kiyose, R.; Onodera, R.; Kocsis, J.D.; Honmou, O. Intravenous Infusion of Mesenchymal Stem Cells Delays Disease Progression in the SOD1G93A Transgenic Amyotrophic Lateral Sclerosis Rat Model. Brain Res. 2021, 1757, 147296. [Google Scholar] [CrossRef]
- Vercelli, A.; Mereuta, O.M.; Garbossa, D.; Muraca, G.; Mareschi, K.; Rustichelli, D.; Ferrero, I.; Mazzini, L.; Madon, E.; Fagioli, F. Human Mesenchymal Stem Cell Transplantation Extends Survival, Improves Motor Performance and Decreases Neuroinflammation in Mouse Model of Amyotrophic Lateral Sclerosis. Neurobiol. Dis. 2008, 31, 395–405. [Google Scholar] [CrossRef]
- Zhao, C.-P.; Zhang, C.; Zhou, S.-N.; Xie, Y.-M.; Wang, Y.-H.; Huang, H.; Shang, Y.-C.; Li, W.-Y.; Zhou, C.; Yu, M.-J.; et al. Human Mesenchymal Stromal Cells Ameliorate the Phenotype of SOD1-G93A ALS Mice. Cytotherapy 2007, 9, 414–426. [Google Scholar] [CrossRef]
- Uccelli, A.; Milanese, M.; Principato, M.C.; Morando, S.; Bonifacino, T.; Vergani, L.; Giunti, D.; Voci, A.; Carminati, E.; Giribaldi, F.; et al. Intravenous Mesenchymal Stem Cells Improve Survival and Motor Function in Experimental Amyotrophic Lateral Sclerosis. Mol. Med. 2012, 18, 794–804. [Google Scholar] [CrossRef]
- Mazzini, L.; Mareschi, K.; Ferrero, I.; Vassallo, E.; Oliveri, G.; Boccaletti, R.; Testa, L.; Livigni, S.; Fagioli, F. Autologous Mesenchymal Stem Cells: Clinical Applications in Amyotrophic Lateral Sclerosis. Neurol. Res. 2006, 28, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Minguell, J.J.; Allers, C.; Lasala, G.P. Mesenchymal Stem Cells and the Treatment of Conditions and Diseases: The Less Glittering Side of a Conspicuous Stem Cell for Basic Research. Stem Cells Dev. 2013, 22, 193–203. [Google Scholar] [CrossRef]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Herrmann, I.K.; Wood, M.J.A.; Fuhrmann, G. Extracellular Vesicles as a Next-Generation Drug Delivery Platform. Nat. Nanotechnol. 2021, 16, 748–759. [Google Scholar] [CrossRef]
- Cheng, L.; Hill, A.F. Therapeutically Harnessing Extracellular Vesicles. Nat. Rev. Drug Discov. 2022, 21, 379–399. [Google Scholar] [CrossRef]
- Todorova, D.; Simoncini, S.; Lacroix, R.; Sabatier, F.; Dignat-George, F. Extracellular Vesicles in Angiogenesis. Circ. Res. 2017, 120, 1658–1673. [Google Scholar] [CrossRef]
- Suárez-Meade, P.; Carvajal, H.G.; Yasuhara, T.; Tajiri, N.; Date, I.; Borlongan, C.V. Regenerative Medicine for Central Nervous System Disorders: Role of Therapeutic Molecules in Stem Cell Therapy. Brain Circ. 2015, 1, 125. [Google Scholar] [CrossRef]
- Deregibus, M.C.; Cantaluppi, V.; Calogero, R.; Lo Iacono, M.; Tetta, C.; Biancone, L.; Bruno, S.; Bussolati, B.; Camussi, G. Endothelial Progenitor Cell Derived Microvesicles Activate an Angiogenic Program in Endothelial Cells by a Horizontal Transfer of MRNA. Blood 2007, 110, 2440–2448. [Google Scholar] [CrossRef]
- Mathiyalagan, P.; Liang, Y.; Kim, D.; Misener, S.; Thorne, T.; Kamide, C.E.; Klyachko, E.; Losordo, D.W.; Hajjar, R.J.; Sahoo, S. Angiogenic Mechanisms of Human CD34+ Stem Cell Exosomes in the Repair of Ischemic Hindlimb. Circ. Res. 2017, 120, 1466–1476. [Google Scholar] [CrossRef]
- Stone, M.L.; Zhao, Y.; Robert Smith, J.; Weiss, M.L.; Kron, I.L.; Laubach, V.E.; Sharma, A.K. Mesenchymal Stromal Cell-Derived Extracellular Vesicles Attenuate Lung Ischemia-Reperfusion Injury and Enhance Reconditioning of Donor Lungs after Circulatory Death. Respir. Res. 2017, 18, 212. [Google Scholar] [CrossRef]
- Ryu, Y.; Hwang, J.S.; Bo Noh, K.; Park, S.H.; Seo, J.H.; Shin, Y.J. Adipose Mesenchymal Stem Cell-Derived Exosomes Promote the Regeneration of Corneal Endothelium Through Ameliorating Senescence. Investig. Ophthalmol. Vis. Sci. 2023, 64, 29. [Google Scholar] [CrossRef]
- Gao, W.; Li, F.; Liu, L.; Xu, X.; Zhang, B.; Wu, Y.; Yin, D.; Zhou, S.; Sun, D.; Huang, Y.; et al. Endothelial Colony-Forming Cell-Derived Exosomes Restore Blood-Brain Barrier Continuity in Mice Subjected to Traumatic Brain Injury. Exp. Neurol. 2018, 307, 99–108. [Google Scholar] [CrossRef]
- Qiu, L.; Cai, Y.; Geng, Y.; Yao, X.; Wang, L.; Cao, H.; Zhang, X.; Wu, Q.; Kong, D.; Ding, D.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles Attenuate TPA-Induced Blood-Brain Barrier Disruption in Murine Ischemic Stroke Models. Acta Biomater. 2022, 154, 424–442. [Google Scholar] [CrossRef]
- Lu, Y.; Zhou, Y.; Zhang, R.; Wen, L.; Wu, K.; Li, Y.; Yao, Y.; Duan, R.; Jia, Y. Bone Mesenchymal Stem Cell-Derived Extracellular Vesicles Promote Recovery Following Spinal Cord Injury via Improvement of the Integrity of the Blood-Spinal Cord Barrier. Front. Neurosci. 2019, 13, 209. [Google Scholar] [CrossRef]
- Xue, C.; Ma, X.; Guan, X.; Feng, H.; Zheng, M.; Yang, X. Small Extracellular Vesicles Derived from Umbilical Cord Mesenchymal Stem Cells Repair Blood-Spinal Cord Barrier Disruption after Spinal Cord Injury through down-Regulation of Endothelin-1 in Rats. PeerJ 2023, 11, e16311. [Google Scholar] [CrossRef] [PubMed]
- Bonafede, R.; Turano, E.; Scambi, I.; Busato, A.; Bontempi, P.; Virla, F.; Schiaffino, L.; Marzola, P.; Bonetti, B.; Mariotti, R. ASC-Exosomes Ameliorate the Disease Progression in SOD1(G93A) Murine Model Underlining Their Potential Therapeutic Use in Human ALS. Int. J. Mol. Sci. 2020, 21, 3651. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Wang, Y.; Zhao, D.; Sun, C.; Zhou, S.; Xu, D.; Zhao, J. Exosomes Derived from CXCR4-Overexpressing BMSC Promoted Activation of Microvascular Endothelial Cells in Cerebral Ischemia/Reperfusion Injury. Neural Plast. 2020, 2020, 8814239. [Google Scholar] [CrossRef]
- Döring, Y.; Noels, H.; van der Vorst, E.P.C.; Neideck, C.; Egea, V.; Drechsler, M.; Mandl, M.; Pawig, L.; Jansen, Y.; Schröder, K.; et al. Vascular CXCR4 Limits Atherosclerosis by Maintaining Arterial Integrity: Evidence From Mouse and Human Studies. Circulation 2017, 136, 388–403. [Google Scholar] [CrossRef]
- Liang, Z.; Brooks, J.; Willard, M.; Liang, K.; Yoon, Y.; Kang, S.; Shim, H. CXCR4/CXCL12 Axis Promotes VEGF-Mediated Tumor Angiogenesis through Akt Signaling Pathway. Biochem. Biophys. Res. Commun. 2007, 359, 716–722. [Google Scholar] [CrossRef]
- Zheng, X.-B.; He, X.-W.; Zhang, L.-J.; Qin, H.-B.; Lin, X.-T.; Liu, X.-H.; Zhou, C.; Liu, H.-S.; Hu, T.; Cheng, H.-C.; et al. Bone Marrow-Derived CXCR4-Overexpressing MSCs Display Increased Homing to Intestine and Ameliorate Colitis-Associated Tumorigenesis in Mice. Gastroenterol. Rep. (Oxf.) 2019, 7, 127–138. [Google Scholar] [CrossRef]
- Rincon-Benavides, M.A.; Mendonca, N.C.; Cuellar-Gaviria, T.Z.; Salazar-Puerta, A.I.; Ortega-Pineda, L.; Blackstone, B.N.; Deng, B.; McComb, D.W.; Gallego-Perez, D.; Powell, H.M.; et al. Engineered Vasculogenic Extracellular Vesicles Drive Nonviral Direct Conversions of Human Dermal Fibroblasts into Induced Endothelial Cells and Improve Wound Closure. Adv. Ther. 2023, 6, 2200197. [Google Scholar] [CrossRef]
- Xie, Y.; Sun, Y.; Liu, Y.; Zhao, J.; Liu, Q.; Xu, J.; Qin, Y.; He, R.; Yuan, F.; Wu, T.; et al. Targeted Delivery of RGD-CD146+CD271+ Human Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes Promotes Blood-Spinal Cord Barrier Repair after Spinal Cord Injury. ACS Nano 2023, 17, 18008–18024. [Google Scholar] [CrossRef]
- González De Aguilar, J.-L. Lipid Biomarkers for Amyotrophic Lateral Sclerosis. Front. Neurol. 2019, 10, 284. [Google Scholar] [CrossRef]
- Chaves-Filho, A.B.; Pinto, I.F.D.; Dantas, L.S.; Xavier, A.M.; Inague, A.; Faria, R.L.; Medeiros, M.H.G.; Glezer, I.; Yoshinaga, M.Y.; Miyamoto, S. Alterations in Lipid Metabolism of Spinal Cord Linked to Amyotrophic Lateral Sclerosis. Sci. Rep. 2019, 9, 11642. [Google Scholar] [CrossRef] [PubMed]
- Frank, P.G.; Marcel, Y.L. Apolipoprotein A-I: Structure-Function Relationships. J. Lipid Res. 2000, 41, 853–872. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.G.; Talbot, K.; Turner, M.R. Higher Blood High Density Lipoprotein and Apolipoprotein A1 Levels Are Associated with Reduced Risk of Developing Amyotrophic Lateral Sclerosis. J. Neurol. Neurosurg. Psychiatry 2022, 93, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Garbuzova-Davis, S.; Willing, A.E.; Borlongan, C.V. Apolipoprotein A1 Enhances Endothelial Cell Survival in an In Vitro Model of ALS. eNeuro 2022, 9, 4. [Google Scholar] [CrossRef]
- Esmon, C.T. Inflammation and the Activated Protein C Anticoagulant Pathway. Semin. Thromb. Hemost. 2006, 32 (Suppl. S1), 49–60. [Google Scholar] [CrossRef]
- Esmon, C.T.; Glass, J.D. The APCs of Neuroprotection. J. Clin. Investig. 2009, 119, 3205–3207. [Google Scholar] [CrossRef]
- Mosnier, L.O.; Zlokovic, B.V.; Griffin, J.H. The Cytoprotective Protein C Pathway. Blood 2007, 109, 3161–3172. [Google Scholar] [CrossRef] [PubMed]
- Rezaie, A.R. Regulation of the Protein C Anticoagulant and Antiinflammatory Pathways. Curr. Med. Chem. 2010, 17, 2059–2069. [Google Scholar] [CrossRef] [PubMed]
- Danese, S.; Vetrano, S.; Zhang, L.; Poplis, V.A.; Castellino, F.J. The Protein C Pathway in Tissue Inflammation and Injury: Pathogenic Role and Therapeutic Implications. Blood 2010, 115, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Zlokovic, B.V. Neurovascular Pathways to Neurodegeneration in Alzheimer’s Disease and Other Disorders. Nat. Rev. Neurosci. 2011, 12, 723–738. [Google Scholar] [CrossRef] [PubMed]
- Deane, R.; LaRue, B.; Sagare, A.P.; Castellino, F.J.; Zhong, Z.; Zlokovic, B.V. Endothelial Protein C Receptor-Assisted Transport of Activated Protein C across the Mouse Blood-Brain Barrier. J. Cereb. Blood Flow. Metab. 2009, 29, 25–33. [Google Scholar] [CrossRef]
- Zhong, Z.; Ilieva, H.; Hallagan, L.; Bell, R.; Singh, I.; Paquette, N.; Thiyagarajan, M.; Deane, R.; Fernandez, J.A.; Lane, S.; et al. Activated Protein C Therapy Slows ALS-like Disease in Mice by Transcriptionally Inhibiting SOD1 in Motor Neurons and Microglia Cells. J. Clin. Investig. 2009, 119, 3437–3449. [Google Scholar] [CrossRef]
| Treatment Options | Advantages | Limitations | References |
|---|---|---|---|
| Cellular Approaches to Repairing Damaged B-CNS-B | |||
| hBM34+ cells | Dose–response iv study revealed that the highest cell dose improved motor function, enhanced mn survival, reduced gliosis, decreased EB permeability, and maintained astrocyte end-feet processes in G93A SOD1 mice. Transplanted cells engrafted within spinal cord capillaries. | Undifferentiated transplanted cells expressing CD45 antigen were detected within capillary lumen and at a distance from blood vessels in spinal cord. | Garbuzova-Davis et al. (2017) [47] |
| High cell dose showed ultrastructural morphology improvement. | Severely damaged capillaries were still detected in spinal cord via ultrastructural analysis. | Garbuzova-Davis et al. (2018) [48] | |
| Significant reduction of microhemorrhages noted in the gray and white matter spinal cords of mice with mid or high cell dose treatment. | Some microhemorrhages were present in the spinal cords of control mice. | Eve et al. (2018) [49] | |
| hBMEPCs | Cell iv transplantation enhanced behavioral disease outcomes and mn survival, restored capillary ultrastructure, reduced EB permeability, and re-established perivascular astrocyte end-feet in G93A SOD1 mice. Transplanted cells engrafted into capillaries of gray/white matter spinal cord and brain motor cortex/brainstem. | Ultrastructural capillary analysis was not performed, and vascular permeability was not analyzed in the brains of treated ALS mice. Post-transplant effects on TJ protein expressions were not identified. | Garbuzova-Davis et al. (2019b) [51] |
| Greater levels of human DNA were detected in mouse ECs isolated from brain and spinal cord tissues of ALS mice treated with hBMEPCs vs. hBM34+ at the same cell dose. | ECs were isolated from a combination of brain and spinal cord tissues. Determining human DNA in ECs isolated separately from the brain and spinal cords is needed to determine post-transplant cell distribution. | Garbuzova-Davis et al. (2021a) [52] | |
| Isolated EC viability was higher in ALS mice receiving hBMEPCs vs. hBM34+ cells. | |||
| Significantly upregulated TJ protein expressions, improved capillary pericyte coverage, amended basement membrane laminin, and enhanced endothelial cytoskeletal F-actin were detected in spinal cord capillaries from ALS mice treated with hBMEPCs vs. hBM34+ cells at the same cell dose. | TJ proteins in segmented regions of the brain and spinal cord were not analyzed. Additional basement membrane components were not evaluated. | Garbuzova-Davis et al. (2021b) [53] | |
| Behavioral outcomes were ameliorated near end-stage disease and significantly increased lifespan was detected in G93A SOD1 mice receiving hBMEPCs vs. hBM34+ cells at the same cell dose. | The modest increase in lifespan needs to be addressed for improving the treatment’s long-term effectiveness. | Garbuzova-Davis & Borlongan (2023) [54] | |
| MSCs | MSC iv administration improved behavioral motor function, reduced mn loss, decreased EB leakage, enhanced pericyte capillary coverage, and increased neurturin expression in lumbar spinal cords of treated G93A SOD1 rats. | MSCs engrafted outside of CNS endothelium. Specific cell type(s) differentiated from MSCs, contributing to BSCB repair, were not determined. | Magota et al. (2021) [59] |
| Noncellular Approaches to Repairing Damaged B-CNS-B | |||
| SC-derived nanovesicles | Colony-forming EPC-derived exosomes administered iv into mice with TBI significantly reduced EB leakage and brain edema. | A modest decrease in EB extravasation was detected in treated mice. | Gao et al. (2018) [74] |
| MSC-derived EVs administered iv into SCI rats improved locomotor function, reduced neuronal cell death, decreased EB leakage, and improved pericyte capillary coverage. | Direct EV interactions with damaged ECs were not determined. | Lu et al. (2019) [76] | |
| Repeated intranasal or iv administration of adipose MSC-derived exosomes improved motor function, increased mn survival, and decreased gliosis in G93A SOD1 mice. | Treatment was ineffective in delaying progression in late-stage disease. The effects of exosomes on the CNS endothelium were undetermined. | Bonafede et al. (2020) [78] | |
| hBMEPC-derived EVs dose-dependently increased mBEC survival in an ALS-like environment in vitro. Uptake of EVs into mBECs in pathological condition was established. | EVs at a dose of 5 µg/mL increased mBEC death in pathologic conditions. EV effects on endothelium repair in ALS were undetermined in vivo. | Garbuzova-Davis et al. (2020) [40] | |
| Human umbilical cord MSC-derived EVs delivered iv in a mouse model of ischemic stroke and tPA-induced injury model reduced hemorrhages, EB extravasation, and decreased neurological deficits. | The direct effects of EVs on ECs in the BBB were not determined. | Qiu et al. (2022) [75] | |
| MSC-EVs derived from human umbilical cord administered iv into SCI rats decreased BSCB permeability and increased TJ expressions in the spinal cord. | Only female rats were used. | Xue et al. (2023) [77] | |
| Bioengineered nanovesicles | NV-IONPs from hMSCs incorporating IONPs significantly reached the injured SC after iv administration and magnetic guidance to damaged site, resulting in reduction of cell apoptosis and neuroinflammation, and behavioral improvement in an SCI mouse model. In vitro, NV-IONPs enhanced human umbilical vein’s EC proliferation and migration. | The trafficking of NV-IONPs to the damaged CNS endothelial barrier has not been studied in vivo. BSCB integrity with EB permeability was not determined. | Kim et al. (2018) [42] |
| BM-MSC-derived exosomes transfected with CXCR4 increased angiogenesis and proliferation of ECs in vitro. In a rat ischemic model, injection of exosomes into lv improved behavioral recovery and reduced infarct volume in the brain. | CXCR4-overexpressing exosomes did not increase brain microvasculature EC migration more than control exosomes. | Li et al. (2020) [79] | |
| EVs derived from human dermal fibroblasts transfected with angiogenic factors promoted angiogenesis and enhanced wound healing in nude mice. These EVs also induced somatic cells towards EC differentiation. | CNS barrier integrity was not determined in vivo. EVs’ effects on EC status were not shown. | Rincon-Benavides et al. (2023) [83] | |
| Intranasal administration of RGD expressing-CD146+CD271+ human umbilical cord MSC-exosomes into mice with SCI reduced EB leakage, increased TJ protein expressions, and improved neurological recovery. | The exosome effects on ECs within brain capillaries were undetermined. | Xie et al. (2023) [84] | |
| Apolipoprotein A1 (ApoA1) | ApoA1 dose-dependently reduced mBEC death in an ALS-like environment in vitro. ApoA1 integrated into mBECs. | ApoA1 effects on endothelium status in ALS were undetermined in vivo. | Garbuzova-Davis et al. (2022) [89] |
| Activated protein C (APC) | IP injection of APC into symptomatic G93A SOD1 mice slowed disease progression and extended survival. APC treatment reduced serum protein leakage, restored TJ protein expression, and delayed microglia activation. | Whether APC’s protective effects on damaged ECs in ALS are primary or secondary results from treatment should be elucidated. | Zhong et al. (2009) [97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manora, L.; Borlongan, C.V.; Garbuzova-Davis, S. Cellular and Noncellular Approaches for Repairing the Damaged Blood–CNS–Barrier in Amyotrophic Lateral Sclerosis. Cells 2024, 13, 435. https://doi.org/10.3390/cells13050435
Manora L, Borlongan CV, Garbuzova-Davis S. Cellular and Noncellular Approaches for Repairing the Damaged Blood–CNS–Barrier in Amyotrophic Lateral Sclerosis. Cells. 2024; 13(5):435. https://doi.org/10.3390/cells13050435
Chicago/Turabian StyleManora, Larai, Cesario V. Borlongan, and Svitlana Garbuzova-Davis. 2024. "Cellular and Noncellular Approaches for Repairing the Damaged Blood–CNS–Barrier in Amyotrophic Lateral Sclerosis" Cells 13, no. 5: 435. https://doi.org/10.3390/cells13050435
APA StyleManora, L., Borlongan, C. V., & Garbuzova-Davis, S. (2024). Cellular and Noncellular Approaches for Repairing the Damaged Blood–CNS–Barrier in Amyotrophic Lateral Sclerosis. Cells, 13(5), 435. https://doi.org/10.3390/cells13050435








