Lamin A/C and PI(4,5)P2—A Novel Complex in the Cell Nucleus
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Transfections
2.2. Constructs and Expression of Recombinant Proteins
2.3. Antibodies and Proteins
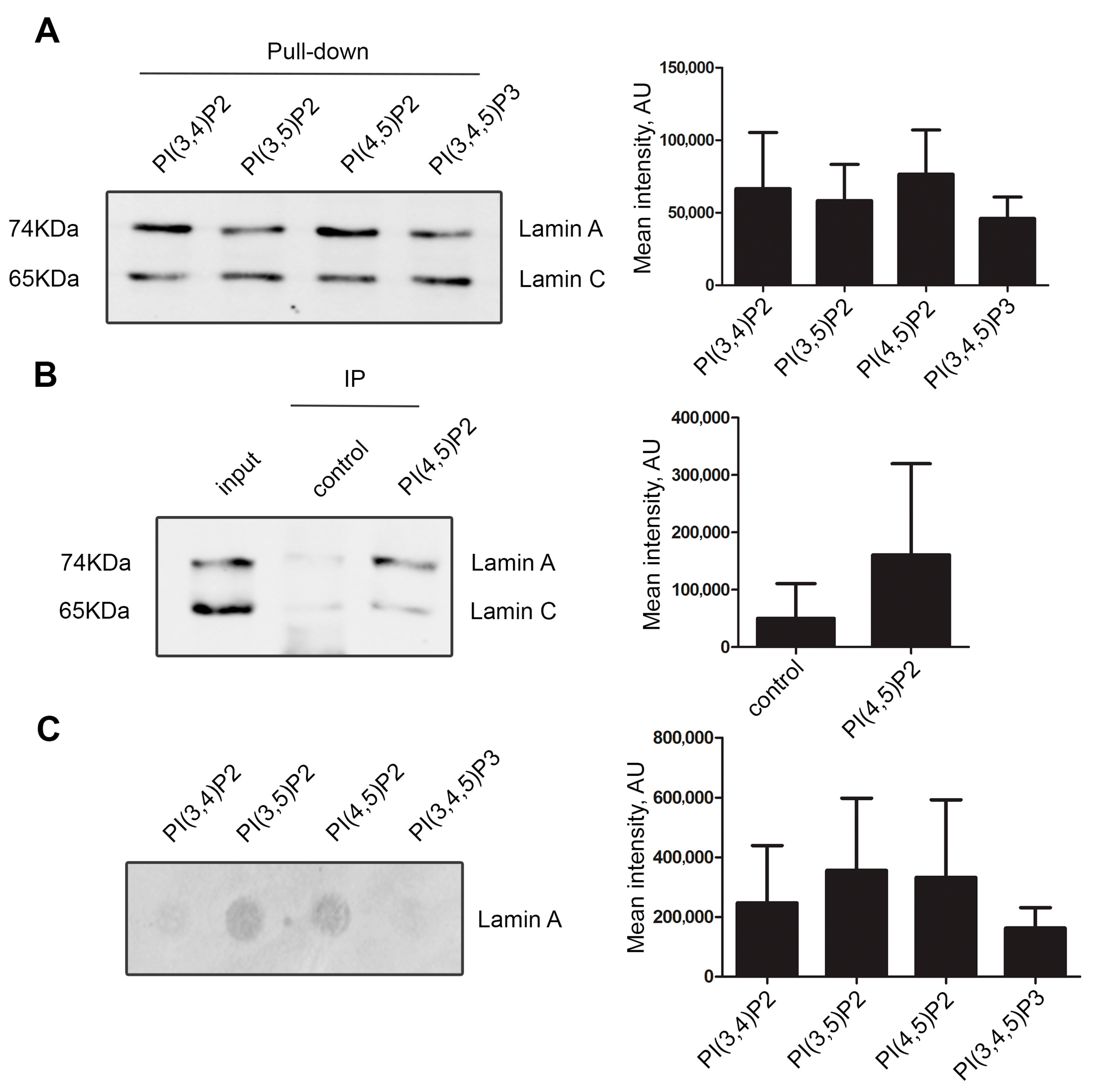
2.4. Pull down and Co-Immunoprecipitation
2.5. Western Blot
2.6. Protein-lipid Overlay Assay
2.7. Cellular Fractionation
2.8. Phosphatase Treatment
2.9. Indirect Immunofluorescence, Confocal Microscopy and dSTORM
2.10. Two-Dimensional Electrophoresis (2D-E)
2.11. Data Analysis
3. Results
3.1. Lamin A/C, but Not Progerin, Is in Complex with Nuclear PI(4,5)P2
3.2. A Portion of Intranuclear Lamin A/C Interacts with NM1 in a PI(4,5)P2-Dependent Manner
3.3. Lamin A/C Phosphorylation Is Important for the PI(4,5)P2-Dependent Binding to NM1
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gruenbaum, Y.; Foisner, R. Lamins: Nuclear intermediate filament proteins with fundamental functions in nuclear mechanics and genome regulation. Annu. Rev. Biochem. 2015, 84, 131–164. [Google Scholar] [CrossRef] [PubMed]
- Prokocimer, M.; Davidovich, M.; Nissim-Rafinia, M.; Wiesel-Motiuk, N.; Bar, D.Z.; Barkan, R.; Meshorer, E.; Gruenbaum, Y. Nuclear lamins: Key regulators of nuclear structure and activities. J. Cell. Mol. Med. 2009, 13, 1059–1085. [Google Scholar] [CrossRef] [PubMed]
- Dechat, T.; Pfleghaar, K.; Sengupta, K.; Shimi, T.; Shumaker, D.K.; Solimando, L.; Goldman, R.D. Nuclear lamins: Major factors in the structural organization and function of the nucleus and chromatin. Genes. Dev. 2008, 22, 832–853. [Google Scholar] [CrossRef] [PubMed]
- Shimi, T.; Pfleghaar, K.; Kojima, S.; Pack, C.G.; Solovei, I.; Goldman, A.E.; Adam, S.A.; Shumaker, D.K.; Kinjo, M.; Cremer, T.; et al. The A- and B-type nuclear lamin networks: Microdomains involved in chromatin organization and transcription. Genes. Dev. 2008, 22, 3409–3421. [Google Scholar] [CrossRef] [PubMed]
- Kreienkamp, R.; Graziano, S.; Coll-Bonfill, N.; Bedia-Diaz, G.; Cybulla, E.; Vindigni, A.; Dorsett, D.; Kubben, N.; Batista, L.F.Z.; Gonzalo, S. A Cell-Intrinsic Interferon-like Response Links Replication Stress to Cellular Aging Caused by Progerin. Cell Rep. 2018, 22, 2006–2015. [Google Scholar] [CrossRef] [PubMed]
- Oca, R.M.d.; Shoemaker, C.J.; Gucek, M.; Cole, R.N.; Wilson, K.L. Barrier-to-Autointegration Factor Proteome Reveals Chromatin-Regulatory Partners. PLoS ONE 2009, 4, e7050. [Google Scholar]
- Dittmer, T.; Misteli, T. The lamin protein family. Genome Biol. 2011, 12, 222. [Google Scholar] [CrossRef]
- Gruenbaum, Y.; Medalia, O. Lamins: The structure and protein complexes. Curr. Opin. Cell Biol. 2015, 32, 7–12. [Google Scholar] [CrossRef]
- Maraldi, N.M.; Capanni, C.; Cenni, V.; Fini, M.; Lattanzi, G. Laminopathies and lamin-associated signaling pathways. J. Cell. Biochem. 2011, 112, 979–992. [Google Scholar] [CrossRef]
- Cattin, M.E.; Ferry, A.; Vignaud, A.; Mougenot, N.; Jacquet, A.; Wahbi, K.; Bertrand, A.T.; Bonne, G. Mutation in lamin A/C sensitizes the myocardium to exercise-induced mechanical stress but has no effect on skeletal muscles in mouse. Neuromuscul. Disord. 2016, 26, 490–499. [Google Scholar] [CrossRef]
- Bertrand, A.T.; Brull, A.; Azibani, F.; Benarroch, L.; Chikhaoui, K.; Stewart, C.L.; Medalia, O.; Ben Yaou, R.; Bonne, G. Lamin A/C Assembly Defects in LMNA-Congenital Muscular Dystrophy Is Responsible for the Increased Severity of the Disease Compared with Emery-Dreifuss Muscular Dystrophy. Cells 2020, 9, 844. [Google Scholar] [CrossRef]
- Worman, H.J. Nuclear lamins and laminopathies. J. Pathol. 2012, 226, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Prokocimer, M.; Barkan, R.; Gruenbaum, Y. Hutchinson-Gilford progeria syndrome through the lens of transcription. Aging Cell 2013, 12, 533–543. [Google Scholar] [CrossRef]
- Benarroch, L.; Cohen, E.; Atalaia, A.; Ben Yaou, R.; Bonne, G.; Bertrand, A.T. Preclinical Advances of Therapies for Laminopathies. J. Clin. Med. 2021, 10, 4834. [Google Scholar] [CrossRef] [PubMed]
- Turgay, Y.; Eibauer, M.; Goldman, A.E.; Shimi, T.; Khayat, M.; Ben-Harush, K.; Dubrovsky-Gaupp, A.; Sapra, K.T.; Goldman, R.D.; Medalia, O. The molecular architecture of lamins in somatic cells. Nature 2017, 543, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Hozák, P.; Sasseville, A.M.-J.; Raymond, Y.; Cook, P.R. Lamin proteins form an internal nucleoskeleton as well as a peripheral lamina in human cells. J. Cell Sci. 1995, 108, 635–644. [Google Scholar] [CrossRef]
- Moir, R.D.; Yoon, M.; Khuon, S.; Goldman, R.D. Nuclear Lamins A and B1: Different Pathways of Assembly during Nuclear Envelope Formation in Living Cells. J. Cell Biol. 2000, 151, 1155–1168. [Google Scholar] [CrossRef]
- Naetar, N.; Korbei, B.; Kozlov, S.; Kerenyi, M.A.; Dorner, D.; Kral, R.; Gotic, I.; Fuchs, P.; Cohen, T.V.; Bittner, R.; et al. Loss of nucleoplasmic LAP2alpha-lamin A complexes causes erythroid and epidermal progenitor hyperproliferation. Nat. Cell Biol. 2008, 10, 1341–1348. [Google Scholar] [CrossRef]
- Kolb, T.; Maass, K.; Hergt, M.; Aebi, U.; Herrmann, H. Lamin A and lamin C form homodimers and coexist in higher complex forms both in the nucleoplasmic fraction and in the lamina of cultured human cells. Nucleus 2011, 2, 425–433. [Google Scholar] [CrossRef]
- Eriksson, J.E.; Dechat, T.; Grin, B.; Helfand, B.; Mendez, M.; Pallari, H.M.; Goldman, R.D. Introducing intermediate filaments: From discovery to disease. J. Clin. Investig. 2009, 119, 1763–1771. [Google Scholar] [CrossRef]
- Liu, S.Y.; Ikegami, K. Nuclear lamin phosphorylation: An emerging role in gene regulation and pathogenesis of laminopathies. Nucleus 2020, 11, 299–314. [Google Scholar] [CrossRef]
- Buxboim, A.; Swift, J.; Irianto, J.; Spinler, K.R.; Dingal, P.C.; Athirasala, A.; Kao, Y.R.; Cho, S.; Harada, T.; Shin, J.W.; et al. Matrix elasticity regulates lamin-A,C phosphorylation and turnover with feedback to actomyosin. Curr. Biol. 2014, 24, 1909–1917. [Google Scholar] [CrossRef]
- Naetar, N.; Ferraioli, S.; Foisner, R. Lamins in the nuclear interior—Life outside the lamina. J. Cell Sci. 2017, 130, 2087–2096. [Google Scholar] [CrossRef]
- Kochin, V.; Shimi, T.; Torvaldson, E.; Adam, S.A.; Goldman, A.; Pack, C.G.; Melo-Cardenas, J.; Imanishi, S.Y.; Goldman, R.D.; Eriksson, J.E. Interphase phosphorylation of lamin A. J. Cell Sci. 2014, 127 Pt. 12, 2683–2696. [Google Scholar] [CrossRef]
- Ikegami, K.; Secchia, S.; Almakki, O.; Lieb, J.D.; Moskowitz, I.P. Phosphorylated Lamin A/C in the Nuclear Interior Binds Active Enhancers Associated with Abnormal Transcription in Progeria. Dev. Cell 2020, 52, 699–713. [Google Scholar] [CrossRef]
- Towbin, B.D.; Meister, P.; Pike, B.L.; Gasser, S.M. Repetitive Transgenes in C. elegans Accumulate Heterochromatic Marks and Are Sequestered at the Nuclear Envelope in a Copy Numberand Lamin-Dependent Manner. In Cold Spring Harbor Symposia on Quantitative Biology; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY. USA, 2010; Volume 75, pp. 555–565. [Google Scholar]
- Gesson, K.; Rescheneder, P.; Skoruppa, M.P.; von Haeseler, A.; Dechat, T.; Foisner, R. A-type lamins bind both hetero- and euchromatin, the latter being regulated by lamina-associated polypeptide 2 alpha. Genome Res. 2016, 26, 462–473. [Google Scholar] [CrossRef]
- Capanni, C.; Del Coco, R.; Mattioli, E.; Camozzi, D.; Columbaro, M.; Schena, E.; Merlini, L.; Squarzoni, S.; Maraldi, N.M.; Lattanzi, G. Emerin-prelamin A interplay in human fibroblasts. Biol. Cell 2009, 101, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Holaska, J.M.; Wilson, K.L. An emerin “proteome”: Purification of distinct emerin-containing complexes from HeLa cells suggests molecular basis for diverse roles including gene regulation, mRNA splicing, signaling, mechanosensing, and nuclear architecture. Biochemistry 2007, 46, 8897–8908. [Google Scholar] [CrossRef] [PubMed]
- Ranade, D.; Pradhan, R.; Jayakrishnan, M.; Hegde, S.; Sengupta, K. Lamin A/C and Emerin depletion impacts chromatin organization and dynamics in the interphase nucleus. BMC Mol. Cell Biol. 2019, 20, 11. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, R.; Nallappa, M.J.; Sengupta, K. Lamin A/C modulates spatial organization and function of the Hsp70 gene locus via nuclear myosin I. J. Cell Sci. 2020, 133, jcs236265. [Google Scholar] [CrossRef] [PubMed]
- Fomproix, N.; Percipalle, P. An actin-myosin complex on actively transcribing genes. Exp. Cell Res. 2004, 294, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Kysela, K.; Philimonenko, A.A.; Philimonenko, V.V.; Janacek, J.; Kahle, M.; Hozak, P. Nuclear distribution of actin and myosin I depends on transcriptional activity of the cell. Histochem. Cell Biol. 2005, 124, 347–358. [Google Scholar] [CrossRef]
- Percipalle, P.; Visa, N. Molecular functions of nuclear actin in transcription. J. Cell Biol. 2006, 172, 967–971. [Google Scholar] [CrossRef] [PubMed]
- Sarshad, A.; Sadeghifar, F.; Louvet, E.; Mori, R.; Bohm, S.; Al-Muzzaini, B.; Vintermist, A.; Fomproix, N.; Ostlund, A.K.; Percipalle, P. Nuclear myosin 1c facilitates the chromatin modifications required to activate rRNA gene transcription and cell cycle progression. PLoS Genet. 2013, 9, e1003397. [Google Scholar] [CrossRef]
- Sarshad, A.A.; Percipalle, P. New insight into role of myosin motors for activation of RNA polymerases. Int. Rev. Cell Mol. Biol. 2014, 311, 183–230. [Google Scholar] [CrossRef]
- Ye, J.; Zhao, J.; Hoffmann-Rohrer, U.; Grummt, I. Nuclear myosin I acts in concert with polymeric actin to drive RNA polymerase I transcription. Genes. Dev. 2008, 22, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Hokanson, D.E.; Ostap, E.M. Myo1c binds tightly and specifically to phosphatidylinositol 4,5-bisphosphate and inositol 1,4,5-trisphosphate. Proc. Natl. Acad. Sci. USA 2005, 103, 3118–3123. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.L.; Janmey, P.A. Phosphoinositide regulation of the actin cytoskeleton. Annu. Rev. Physiol. 2003, 65, 761–789. [Google Scholar] [CrossRef]
- Skare, P.; Karlsson, R. Evidence for two interaction regions for phosphatidylinositol(4,5)-bisphosphate on mammalian pro¢lin I. FEBS Lett. 2002, 522, 119–124. [Google Scholar] [CrossRef]
- Rando, O.J.; Zhao, K.; Janmey, P.; Crabtree, G.R. Phosphatidylinositol-dependent actin filament binding by the SWI/SNF-like BAF chromatin remodeling complex. Proc. Natl. Acad. Sci. USA 2002, 99, 2824–2829. [Google Scholar] [CrossRef]
- York, J.D. Regulation of nuclear processes by inositol polyphosphates. Biochim. Biophys. Acta 2006, 1761, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.E.; Sommer, L.; Arntzen, M.O.; Strahm, Y.; Morrice, N.A.; Divecha, N.; D’Santos, C.S. Identification of nuclear phosphatidylinositol 4,5-bisphosphate-interacting proteins by neomycin extraction. Mol. Cell. Proteom. 2011, 10, S1–S15. [Google Scholar] [CrossRef]
- Philimonenko, V.V.; Zhao, J.; Iben, S.; Dingova, H.; Kysela, K.; Kahle, M.; Zentgraf, H.; Hofmann, W.A.; de Lanerolle, P.; Hozak, P.; et al. Nuclear actin and myosin I are required for RNA polymerase I transcription. Nat. Cell Biol. 2004, 6, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Sobol, M.; Yildirim, S.; Philimonenko, V.V.; Marasek, P.; Castano, E.; Hozak, P. UBF complexes with phosphatidylinositol 4,5-bisphosphate in nucleolar organizer regions regardless of ongoing RNA polymerase I activity. Nucleus 2013, 4, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, W.A.; Johnson, T.; Klapczynski, M.; Fan, J.L.; de Lanerolle, P. From transcription to transport: Emerging roles for nuclear myosin I. Biochem. Cell Biol. 2006, 84, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Venit, T.; Dzijak, R.; Kalendova, A.; Kahle, M.; Rohozkova, J.; Schmidt, V.; Rulicke, T.; Rathkolb, B.; Hans, W.; Bohla, A.; et al. Mouse nuclear myosin I knock-out shows interchangeability and redundancy of myosin isoforms in the cell nucleus. PLoS ONE 2013, 8, e61406. [Google Scholar] [CrossRef] [PubMed]
- Sobol, M.; Krausova, A.; Yildirim, S.; Kalasova, I.; Faberova, V.; Vrkoslav, V.; Philimonenko, V.; Marasek, P.; Pastorek, L.; Capek, M.; et al. Nuclear phosphatidylinositol 4,5-bisphosphate islets contribute to efficient RNA polymerase II-dependent transcription. J. Cell Sci. 2018, 131, jcs211094. [Google Scholar] [CrossRef]
- Jungmichel, S.; Sylvestersen, K.B.; Choudhary, C.; Nguyen, S.; Mann, M.; Nielsen, M.L. Specificity and commonality of the phosphoinositide-binding proteome analyzed by quantitative mass spectrometry. Cell Rep. 2014, 6, 578–591. [Google Scholar] [CrossRef]
- Stierle, V.; Couprie, J.; Ostlund, C.; Krimm, I.; Zinn-Justin, S.; Hossenlopp, P.; Worman, H.J.; Courvalin, J.-C.; Duband-Goulet, I. The Carboxyl-Terminal Region Common to Lamins A and C Contains a DNA Binding Domain. Biochemistry 2003, 42, 4819–4828. [Google Scholar] [CrossRef]
- Goldberg, M.; Harel, A.; Gruenbaum, Y. The Nuclear Lamina: Molecular Organization and Interaction with Chromatin. Crit. Rev. Eukaryot. Gene Expr. 1999, 9, 285–293. [Google Scholar] [CrossRef]
- Mattout, A.; Goldberg, M.; Tzur, Y.; Margalit, A.; Gruenbaum, Y. Specific and conserved sequences in D. melanogaster and C. elegans lamins and histone H2A mediate the attachment of lamins to chromosomes. J. Cell Sci. 2007, 120 Pt 1, 77–85. [Google Scholar] [CrossRef]
- Hodge, K.; ten Have, S. Cell Fractionation—Cytoplasmic, Nucleoplasmic and Nucleoli Fractionation. 2012. Available online: https://www.lamondlab.com/newwebsite/Protocols%20for%20Website/Cellular%20Fractionation%20Protocol.pdf (accessed on 5 October 2023).
- Hoboth, P.; Šebesta, O.; Sztacho, M.; Castano, E.; Hozák, P. Dual-color dSTORM imaging and ThunderSTORM image reconstruction and analysis to study the spatial organization of the nuclear phosphatidylinositol phosphates. MethodsX 2021, 8, 101372. [Google Scholar] [CrossRef]
- Hoboth, P.; Šebesta, O.; Hozák, P. How Single-Molecule Localization Microscopy Expanded Our Mechanistic Understanding of RNA Polymerase II Transcription. Int. J. Mol. Sci. 2021, 22, 6694. [Google Scholar] [CrossRef]
- Mou, F.; Forest, T.; Baines, J.D. US3 of herpes simplex virus type 1 encodes a promiscuous protein kinase that phosphorylates and alters localization of lamin A/C in infected cells. J. Virol. 2007, 81, 6459–6470. [Google Scholar] [CrossRef]
- Al-Saaidi, R.; Bross, P. Do lamin A and lamin C have unique roles? Chromosoma 2015, 124, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Zhou, Z. Genetics of aging, progeria and lamin disorders. Curr. Opin. Genet. Dev. 2014, 26, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Rana, R.A.; Cataldi, A.; Di Pietro, R.; Mazzotti, G.; Centurione, L.; Robuffo, I.; Vitale, M.; Miscia, S. Evidence for an early and transient involvement of nuclear inositol lipids in subcellular signalling events related to DNA repair processes. Cell. Signal. 1994, 6, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Hariharan, A.; Bastianello, G.; Toyama, Y.; Shivashankar, G.V.; Foiani, M.; Sheetz, M.P. DNA damage causes rapid accumulation of phosphoinositides for ATR signaling. Nat. Commun. 2017, 8, 2118. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Sheetz, M.P. When PIP(2) Meets p53: Nuclear Phosphoinositide Signaling in the DNA Damage Response. Front. Cell Dev. Biol. 2022, 10, 903994. [Google Scholar] [CrossRef]
- Liu, B.; Wang, J.; Chan, K.M.; Tjia, W.M.; Deng, W.; Guan, X.; Huang, J.D.; Li, K.M.; Chau, P.Y.; Chen, D.J.; et al. Genomic instability in laminopathy-based premature aging. Nat. Med. 2005, 11, 780–785. [Google Scholar] [CrossRef]
- Liu, Y.; Rusinol, A.; Sinensky, M.; Wang, Y.; Zou, Y. DNA Damage Responses in Progeroid Syndromes Arising from Defective Maturation of Prelamin A. J. Cell Sci. 2006, 119, 4644–4649. [Google Scholar] [CrossRef]
- Sztacho, M.; Sobol, M.; Balaban, C.; Lopes, S.E.E.; Hozák, P. Nuclear phosphoinositides and phase separation: Important players in nuclear compartmentalization. Adv. Biol. Regul. 2019, 71, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.N.; Zastrow, M.S.; Wilson, K.L. Direct actin binding to A- and B-type lamin tails and actin filament bundling by the lamin A tail. Nucleus 2010, 1, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, A.; Kristensen, D.B.; Takeda, Y.; Miyamoto, Y.; Okada, K.; Inamatsu, M.; Yoshizato, K. Mapping of phosphorylated proteins on two-dimensional polyacrylamide gels using protein phosphatase. Proteomics 2002, 2, 1267–1276. [Google Scholar] [CrossRef]
- Ardito, F.; Giuliani, M.; Perrone, D.; Troiano, G.; Lo Muzio, L. The crucial role of protein phosphorylation in cell signaling and its use as targeted therapy (Review). Int. J. Mol. Med. 2017, 40, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Nishi, H.; Hashimoto, K.; Panchenko, A.R. Phosphorylation in protein-protein binding: Effect on stability and function. Structure 2011, 19, 1807–1815. [Google Scholar] [CrossRef]
- Torvaldson, E.; Kochin, V.; Eriksson, J.E. Phosphorylation of lamins determine their structural properties and signaling functions. Nucleus 2015, 6, 166–171. [Google Scholar] [CrossRef]
- Machowska, M.; Piekarowicz, K.; Rzepecki, R. Regulation of lamin properties and functions: Does phosphorylation do it all? Open Biol. 2015, 5, 150094. [Google Scholar] [CrossRef]
- Shimizu, T.; Cao, C.X.; Shao, R.G.; Pommier, Y. Lamin B phosphorylation by protein kinase calpha and proteolysis during apoptosis in human leukemia HL60 cells. J. Biol. Chem. 1998, 273, 8669–8674. [Google Scholar] [CrossRef]
- Karoutas, A.; Akhtar, A. Functional mechanisms and abnormalities of the nuclear lamina. Nat. Cell Biol. 2021, 23, 116–126. [Google Scholar] [CrossRef]
- Ovsiannikova, N.L.; Lavrushkina, S.V.; Ivanova, A.V.; Mazina, L.M.; Zhironkina, O.A.; Kireev, I.I. Lamin A as a Determinant of Mechanical Properties of the Cell Nucleus in Health and Disease. Biochem. (Mosc.) 2021, 86, 1288–1300. [Google Scholar] [CrossRef] [PubMed]
- Tenga, R.; Medalia, O. Structure and unique mechanical aspects of nuclear lamin filaments. Curr. Opin. Struct. Biol. 2020, 64, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Boubriak, I.I.; Malhas, A.N.; Drozdz, M.M.; Pytowski, L.; Vaux, D.J. Stress-induced release of Oct-1 from the nuclear envelope is mediated by JNK phosphorylation of lamin B1. PLoS ONE 2017, 12, e0177990. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, S.; Castano, E.; Sobol, M.; Philimonenko, V.V.; Dzijak, R.; Venit, T.; Hozak, P. Involvement of phosphatidylinositol 4,5-bisphosphate in RNA polymerase I transcription. J. Cell Sci. 2013, 126, 2730–2739. [Google Scholar] [CrossRef] [PubMed]
- Ulicna, L.; Kalendova, A.; Kalasova, I.; Vacik, T.; Hozak, P. PIP2 epigenetically represses rRNA genes transcription interacting with PHF8. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Brangwynne, C.P. Liquid phase condensation in cell physiology and disease. Science 2017, 357, eaaf4382. [Google Scholar] [CrossRef] [PubMed]
- Owen, I.; Shewmaker, F. The Role of Post-Translational Modifications in the Phase Transitions of Intrinsically Disordered Proteins. Int. J. Mol. Sci. 2019, 20, 5501. [Google Scholar] [CrossRef]
- Dumelie, J.G.; Chen, Q.; Miller, D.; Attarwala, N.; Gross, S.S.; Jaffrey, S.R. Biomolecular condensates create phospholipid-enriched microenvironments. Nat. Chem. Biol. 2023. [Google Scholar] [CrossRef]
- Nevzorov, I.; Sidorenko, E.; Wang, W.; Zhao, H.; Vartiainen, M.K. Myosin-1C uses a novel phosphoinositide-dependent pathway for nuclear localization. EMBO Rep. 2018, 19, 290–304. [Google Scholar] [CrossRef]
- Venit, T.; Kalendova, A.; Petr, M.; Dzijak, R.; Pastorek, L.; Rohozkova, J.; Malohlava, J.; Hozak, P. Nuclear myosin I regulates cell membrane tension. Sci. Rep. 2016, 6, 30864. [Google Scholar] [CrossRef]
- Osborne, S.L.; Thomas, C.L.; Gschmeissner, S.; Schiavo, G. Nuclear PtdIns(4,5)P2 assembles in a mitotically regulated particle involved in pre-mRNA splicing. J. Cell Sci. 2001, 114, 2501–2511. [Google Scholar] [CrossRef]
- YU, H.; FUKAMI, K.; WATANABE, Y.; OZAKI, C.; TAKENAWA, T. Phosphatidylinositol 4,5-bisphosphate reverses the inhibition of RNA transcription caused by histone H1. Eur. J. Biochem. 1998, 251, 281–287. [Google Scholar] [CrossRef]
- Ulicna, L.; Rohozkova, J.; Hozak, P. Multiple Aspects of PIP2 Involvement in C. elegans Gametogenesis. Int. J. Mol. 2018, 18, 2679. [Google Scholar] [CrossRef]
- Venit, T.; Semesta, K.; Farrukh, S.; Endara-Coll, M.; Havalda, R.; Hozak, P.; Percipalle, P. Nuclear myosin 1 activates p21 gene transcription in response to DNA damage through a chromatin-based mechanism. Commun. Biol. 2020, 3, 115. [Google Scholar] [CrossRef] [PubMed]
- Vidak, S.; Foisner, R. Molecular insights into the premature aging disease progeria. Histochem. Cell Biol. 2016, 145, 401–417. [Google Scholar] [CrossRef] [PubMed]
- Cenni, V.; Capanni, C.; Mattioli, E.; Schena, E.; Squarzoni, S.; Bacalini, M.G.; Garagnani, P.; Salvioli, S.; Franceschi, C.; Lattanzi, G. Lamin A involvement in ageing processes. Ageing Res. Rev. 2020, 62, 101073. [Google Scholar] [CrossRef] [PubMed]
- Olive, M.; Harten, I.; Mitchell, R.; Beers, J.; Djabali, K.; Cao, K.; Erdos, M.R.; Blair, C.; Funke, B.; Smoot, L.; et al. Cardiovascular Pathology in Hutchinson-Gilford Progeria: Correlation with the Vascular Pathology of Aging. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2301–2309. [Google Scholar] [CrossRef] [PubMed]
- Kurz, E.U.; Lees-Miller, S.P. DNA damage-induced activation of ATM and ATM-dependent signaling pathways. DNA Repair 2004, 3, 889–900. [Google Scholar] [CrossRef] [PubMed]
- Moiseeva, O.; Lopes-Paciencia, S.; Huot, G.; Lessard, F.; Ferbeyre, G. Permanent farnesylation of lamin A mutants linked to progeria impairs its phosphorylation at serine 22 during interphase. Aging 2016, 8, 366–381. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escudeiro-Lopes, S.; Filimonenko, V.V.; Jarolimová, L.; Hozák, P. Lamin A/C and PI(4,5)P2—A Novel Complex in the Cell Nucleus. Cells 2024, 13, 399. https://doi.org/10.3390/cells13050399
Escudeiro-Lopes S, Filimonenko VV, Jarolimová L, Hozák P. Lamin A/C and PI(4,5)P2—A Novel Complex in the Cell Nucleus. Cells. 2024; 13(5):399. https://doi.org/10.3390/cells13050399
Chicago/Turabian StyleEscudeiro-Lopes, Sara, Vlada V. Filimonenko, Lenka Jarolimová, and Pavel Hozák. 2024. "Lamin A/C and PI(4,5)P2—A Novel Complex in the Cell Nucleus" Cells 13, no. 5: 399. https://doi.org/10.3390/cells13050399
APA StyleEscudeiro-Lopes, S., Filimonenko, V. V., Jarolimová, L., & Hozák, P. (2024). Lamin A/C and PI(4,5)P2—A Novel Complex in the Cell Nucleus. Cells, 13(5), 399. https://doi.org/10.3390/cells13050399







