Abstract
Research suggests the potential of using cannabinoid-derived compounds to function as anticancer agents against melanoma cells. Our recent study highlighted the remarkable in vitro anticancer effects of PHEC-66, an extract from Cannabis sativa, on the MM418-C1, MM329, and MM96L melanoma cell lines. However, the complete molecular mechanism behind this action remains to be elucidated. This study aims to unravel how PHEC-66 brings about its antiproliferative impact on these cell lines, utilising diverse techniques such as real-time polymerase chain reaction (qPCR), assays to assess the inhibition of CB1 and CB2 receptors, measurement of reactive oxygen species (ROS), apoptosis assays, and fluorescence-activated cell sorting (FACS) for apoptosis and cell cycle analysis. The outcomes obtained from this study suggest that PHEC-66 triggers apoptosis in these melanoma cell lines by increasing the expression of pro-apoptotic markers (BAX mRNA) while concurrently reducing the expression of anti-apoptotic markers (Bcl-2 mRNA). Additionally, PHEC-66 induces DNA fragmentation, halting cell progression at the G1 cell cycle checkpoint and substantially elevating intracellular ROS levels. These findings imply that PHEC-66 might have potential as an adjuvant therapy in the treatment of malignant melanoma. However, it is essential to conduct further preclinical investigations to delve deeper into its potential and efficacy.
1. Introduction
Melanomas are cancerous cells arising from epidermal melanocytes [1]. While melanoma accounts for ~6% of skin cancers, it is the cause of >80% of related skin cancer deaths [2]. This cancer is highly prone to metastasising and can generate tumours through local and distant invasions [3] and has been shown to be highly resistant to traditional treatments [4]. Melanoma development is influenced by various factors, including chronic inflammatory conditions like psoriasis and eczema, genetic factors, and prolonged exposure to environmental hazards such as ultraviolet (UV) radiation [5].
The current pharmacological treatment options for metastatic melanoma are limited, resulting in a low median survival time of ~8–9 months, with <15% of patients surviving beyond three years [6]. Approximately 50% of melanomas have BRAF mutations, with the majority being BRAFV600E, which causes intrinsic activation of the BRAF-MEK-ERK-MAPK signalling pathway, also known as the Mitogen-activated protein kinase (MAPK) pathway [7,8], leading to enhanced melanoma cell proliferation and survival [7]. Inhibition of the MAPK signalling pathway through agents such as dabrafenib, vemurafenib, binimetinib, encorafernib, trametinib, selumetinib, and cobimetinib, which bind to signal cascade kinases BRAF and MEK, has become a crucial treatment strategy for patients with BRAF-mutant melanoma [9,10,11,12].
Immunotherapy has revolutionised cancer treatment in recent years. Tumour necrosis factor-alpha (TNFα), interleukin-2 (IL-2), and interferon-gamma (IFNγ) are shown to be critical in fighting melanoma cells [13,14]. Moreover, immune checkpoint inhibitors (ICIs) (e.g., pembrolizumab, nivolumab, atezolizumab, ipilimumab, and relatlimab) have demonstrated effective antitumour activity in melanoma. ICIs, including PD-1 and its ligand PD-L1, along with CTLA-4, are evolving treatment options for several types of cancer [15,16,17,18,19]; however, a limited number of patients have been shown to benefit from such therapy [20,21]. Combined therapy between BRAK/MEK and immunotherapy or “checkpoints” effectors has enhanced melanoma patient survival [22]. However, sequential administration instead of concurrent treatment is recommended to avoid treatment-limiting toxicities [22]. Considering the above-mentioned issues, an additional treatment strategy is imperative to mitigate the adverse effects of the current pharmacotherapies and complement the single-drug treatment approach.
Evidence suggests that certain plant-derived natural compounds, including polyphenols, flavonoids, and terpenes, may possess anticancer properties helpful in treating melanoma [23]. For instance, some compounds present in cannabis may exert antitumour effects and potentially impede the growth and spread of melanoma cells [24]. The endogenous cannabinoid system (ECS) is a ubiquitous neuromodulator system that modulates a broad range of physiological functions in the human body [25]. This system comprises cannabinoid receptors and their endogenous ligands, as well as the enzymes that synthesise and degrade them [25]. The primary mediators of the ECS system are the cannabinoid receptors CB1 and CB2, which are distributed widely in the central nervous and periphery system [26]. These G protein-coupled receptors are known to influence various intracellular signalling pathways that regulate different molecular processes, such as gene transcription, cell motility, angiogenesis, and apoptosis [26,27]. Cannabinoids can also ligand via the G protein-coupled receptor GPR55 and the peroxisome proliferator-activated receptors (PPARs) [28]. Moreover, recent research has revealed that cannabinoids can influence specific transient receptor potential (TRP) channels [29]. There are three subfamilies of TRP channels: TRP vanilloid (TRPV), TRP ankyrin (TRPA), and TRP melastatin (TRPM) [29]. Notably, six TRP channels have been identified as mediators of cannabinoid activity: TRPV1, TRPV2, TRPV3, TRPV4, TRPA1, and TRPM8 [29].
Previous studies have demonstrated that the activation of CB1 and CB2 by external plant-derived compounds results in the inhibition of early tumour growth in various malignancies, including non-small cell lung carcinoma [30], prostate cancer [31], and melanoma [32], where these receptors function as mediators that stimulate diverse molecular signalling pathways [26]. Recently, we demonstrated that the Cannabis sativa (C. sativa)-derived extract PHEC-66 inhibited the growth and motility of different melanoma cells [33]. In this study, we have explored the mechanisms underlying the effects of PHEC-66 on melanoma cells, which may reveal potential targets for drug development and shed light on the impact of cannabinoids on these cells.
2. Materials and Methods
2.1. Materials
RPMI-1640 media, heat-inactivated foetal bovine serum (FBS), streptomycin, penicillin, PowerUp SYBR Green Master Mix, Annexin V-FITC (11-8005-74), propidium iodide (PI) staining in annexin binding buffer (00-6990-42), Thiazoyl blue tetrazolium bromide (MTT), and 5-(and-6)-carboxy-2′,7′-dichlorodihydrofluorescein (DCF-DA) were obtained from Thermo Fisher Scientific (Melbourne, Australia). AM251 (A6226), AM630 (SML0327), and TRIzol reagent were purchased from Sigma (Sydney, Australia). The Propidium Iodide flow cytometry kit (Abcam139418) was purchased from Abcam (Melbourne, Australia). The SensiFASTTM cDNA Synthesis Kit was obtained from Meridian Bioscience (Cincinnati, OH, USA), and the PCR primers were purchased from Bioneer (Melbourne, Australia).
Cell Culture
Human melanoma MM418-C1 (primary (1°) melanoma possessing the oncogenic BRAFV600E mutation), MM329 (Primary (1°) melanoma possessing wild type BRAF (BRAFWT)), and MM96L (metastatic or secondary (2°) melanoma possessing the oncogenic BRAFV600E mutation) cells, and human immortalised keratinocytes (HaCaT), were used in this study. The MM418-C1, MM329, MM96L, and HaCaT cells were kindly supplied by Prof Nicholas Hayward and Peter Parsons of the Queensland Institute of Medical Research, Brisbane, Australia.
2.2. Methods
Cells were cultured in RPMI-1640 tissue culture medium supplemented with 10% (v/v) FBS and 1% (v/v) penicillin and streptomycin. The cells were incubated at 37 °C in a humidified 5% CO2 incubator and passaged every 3–4 days until they reached 80–90% confluency.
2.2.1. Quantitative Polymerase Chain Reaction (qPCR)
In 6-well plates, cells were seeded at a density of 5 × 105 cells/well in RPMI media supplemented with 10% (v/v) FBS and 1% (v/v) penicillin and streptomycin. The cells were then incubated in a humidified 5% CO2 incubator at 37 °C until they reached ~75% confluency. The cells were then treated with RPMI media containing PHEC-66-IC50 (half-maximal inhibitory concentration of PHEC-66) for 24 h. Total RNA was extracted using TRIzol™ Reagent according to the manufacturer’s instructions. The RNA was quantified at 260 nm using a spectrophotometer (Nanodrop ND1000), and the purity was assessed by measuring the absorbance ratios at 260/280 nm and 260/230 nm. SensiFASTTM cDNA Synthesis Kit was used to synthesise cDNA from 1 µg of total RNA. Each transcript quantification was performed in triplicate. Then, 1 µL of cDNA was mixed with 3.6 µL of PowerUp SYBR Green Master Mix, 0.4 µL of each forward and reverse primer, and 2.9 µL of DEPC H2O. The QuantStudioTM 7 Real-Time PCR (Thermofisher, Melbourne, Australia) was used to perform qPCR with the following thermal cycling conditions: an initial activation at 95 °C for 2 min, 39 cycles of 95 °C for 5 s, 60 °C for 10 s, 72 °C for 15 s, and a melting curve from 65 °C to 95 °C. The 2–ΔΔCq method was used for relative quantitative analysis of the expression of the genes of interest normalised to the housekeeping gene glucuronidase (GUSB). The expression was calculated as n-fold induction of the gene of interest in treated cells relative to that of untreated control cells, which was expressed as unity (1).
2.2.2. CB1/CB2 Receptors Antagonism Assay
The cytotoxicity of antagonists combined with PHEC-66 on MM418-C1, MM329, and MM96L cells was evaluated using the MTT assay. Cells were seeded into 96-well plates at a density of 3000–10,000 cells per well depending on their doubling times and allowed to adhere for 24 h at 37 °C and 5% CO2. After 24 h incubation, this medium was replaced with tissue culture medium containing CB1 and CB2 antagonists combined with PHEC-66 dissolved in (0.05% v/v) DMSO. DMSO was used as the solvent control. After 48 h incubation, the medium containing the test compounds was aspirated, and 100 µL of culture media containing 5 mg/mL MTT was added to each well. The cells were incubated for 3 h in the dark at 37 °C, after which this solution was removed, and 100 µL DMSO was added to each well to solubilise the crystallised formazan product. The plates were read on a microplate reader at 570 nm and a reference wavelength of 630 nm. The growth percentage of inhibition was calculated as 100 − [(Mean OD of the treated cell × 100)/Mean OD of vehicle-treated cells (DMSO)]. The IC50 values were calculated using Prism Software. All experiments were performed three times independently.
2.2.3. Reactive Oxygen Species (ROS)
The cells were plated in a 6-well plate with a density of 4 × 105 cells per well and allowed to adhere overnight. The cells were then treated with PHEC-66 at a concentration of 50% and 100% of their respective IC50 values for 24 h. After treatment, the cells were collected and suspended in 1 mL of phosphate-buffered saline (PBS) containing 10 μM DCF-DA at room temperature (RT) and placed in the dark for 15 min. Next, the cells were washed with PBS to remove excess dye before being examined using a BD LSRFortessa Cell Analyzer.
2.2.4. Cell Cycle Arrest Assay
The cells were plated in a 6-well plate at a density of 4 × 105 cells per well and allowed to adhere overnight. The cells were treated with PHEC-66 at a concentration of 50%, 100%, and 200% of their respective IC50 values for 24 h. The cells were trypsined, rinsed with ice-cold PBS, and then fixed with 70% ice-cold ethanol. Next, the cells were washed with PBS and stained with propidium iodide (PI) staining buffer. Finally, 10,000 cells from each sample were examined for propidium iodide fluorescence using the BD LSRFortessaTM Cell Analyzer Flow Cytometer.
2.2.5. Annexin V-FITC/PI Staining Assay
To determine the apoptotic events induced by PHEC-66, annexin V and PI staining were used, following the manufacturer’s protocol for the Dead Cell Apoptosis Kit with annexin V-FITC and PI and analysed using flow cytometry. The cells were plated in a 6-well plate at a density of 4 × 105 cells per well and allowed to adhere overnight. The cells were treated with PHEC-66 at a concentration of 50%, 100%, and 200% of their respective IC50 values for 48 h. The cells were harvested, washed with PBS, and resuspended in 100 μL annexin V binding buffer as per the manufacturer’s instructions. Next, Annexin V (5 μL) and PI (1 μL of 100 μg/mL) were added to the suspended cells and the cells incubated for 15 min at RT in the dark. Next, the cells were washed with PBS to remove excess dye before being examined. Subsequently, a total of 10,000 events were acquired to assess green fluorescence using a BD LSRFortessa Cell Analyzer. Cells were considered to be apoptotic if they were Annexin V+/PI− (early apoptotic) or Annexin V+/PI+ (late apoptotic/necrotic). Live cells were defined as Annexin V−/PI−, and cell debris was Annexin V−/PI+ as necrotic.
2.3. Statistical Analysis
Statistical analyses were conducted using GraphPad Prism version 8 (GraphPad Software Inc., San Diego, CA, USA). Bar plots and graphs were generated using GraphPad software. The data are presented as mean ± standard deviation (SD) for three independent experiments. For comparisons involving three or more groups, either one-way or two-way ANOVA was performed, followed by Tukey’s multiple comparison test. In cases where there were only two groups, the Mann–Whitney U test was used. A two-sided p-value of less than 0.05 was considered statistically significant, denoted as * (p < 0.05), ** (p < 0.01), *** (p < 0.001), or **** (p < 0.0001).
3. Results
We have previously reported that PHEC-66 reduced the viability of human melanoma cell lines (MM418-C1, MM329, and MM96L) in a concentration-dependent manner [33]. PHEC-66 decreased, to a lesser extent, the viability of non-transformed epidermal cells [33]. In the melanoma cells, the IC50 for PHEC-66 was approximately half of that seen in the untransformed HaCaT cells, as seen in Table 1. Inspired by the remarkable melanoma cancer cell growth inhibition properties of PHEC66, in this study, we have investigated the mechanism of cell growth inhibition.

Table 1.
Comparison of the IC50 value for epidermal cells treated with PHEC-66. Data represent the mean ± standard deviation of three independent experiments performed in triplicate [33].
3.1. Quantitative Polymerase Chain Reaction (qPCR)
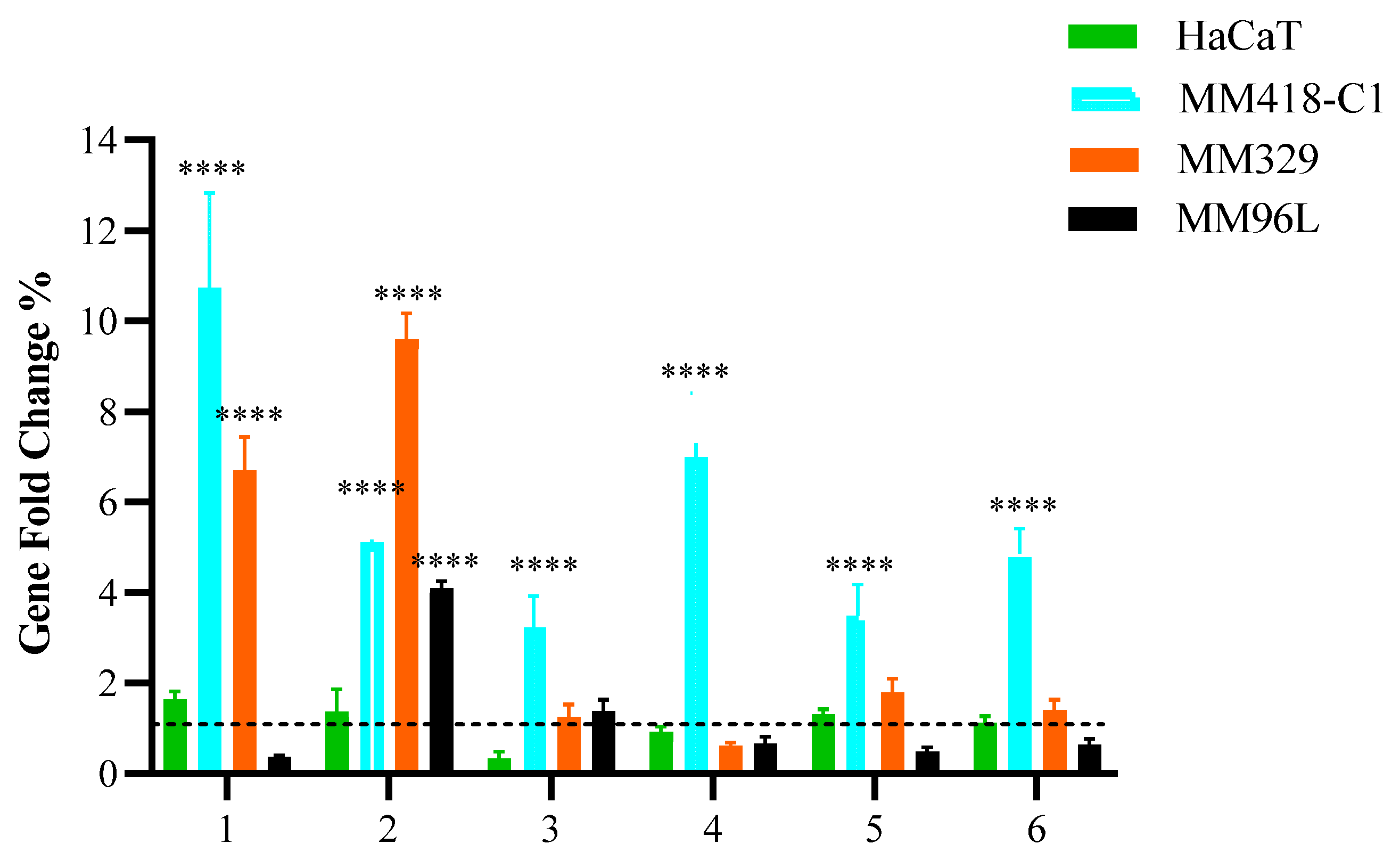
To comprehend the effect of PHEC-66, we examined its effect on the expression of membrane cannabinoid receptors, including CB1, CB2, TRPV1, TRPV2, TRPM8, and GPR55, as seen in Figure 1.
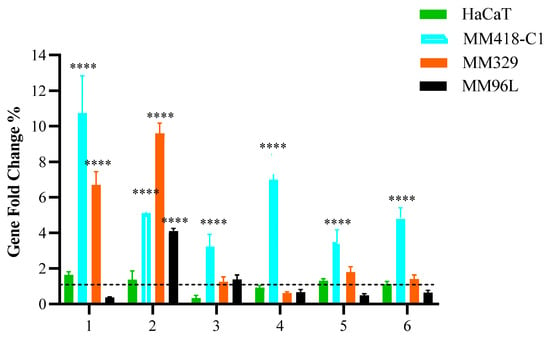
Figure 1.
Effect of PHEC-66 on the expression of cell membrane receptors. Melanoma (MM418-C1, MM329, and MM96L) and keratinocyte-derived cells (HaCaT) were treated with PHEC-66 at its IC50 concentration for 24 h. Asterisks indicate statistically significant differences between PHEC-66-IC50-treated melanoma cell lines and the corresponding non-treated cells. All data represent the mean ± SEM of three independent experiments (**** p < 0.0001). The dotted line represents untreated cells which ranked number one across all cells on the Y-axis scale.
Following PHEC-66-IC50 treatment of MM418-C1 cells, there was a considerable increase in the expression of some of the receptor genes compared to the untreated cells. When the MM418-C1 cells were treated with PHEC-66-IC50, there was a significantly higher expression of CB1, CB2, TRPV1, TRPV2, TRPM8, and GPR55 genes, while in the MM329 cells, only CB1 and CB2 significantly increased, and there were insignificant changes in the expression of TRPV1, TRPV2, TRPM8, and GPR55. Additionally, PHEC-66 at its IC50 concentration significantly induced the expression of CB2 genes in MM96L and decreased the expression of CB1, TRPV2, TRPM8, and GPR55 genes but insignificantly. HaCaT cells did not exhibit significant changes in the expression of these genes after treatment with PHEC-66-IC50. These findings suggest that CB1 and CB2 play a significant role in the growth-inhibitory mechanism of PHEC-66 in melanoma cell lines. Consequently, the impact of CB1 and CB2 was further investigated by employing antagonists to block their activity and examine the effects this inhibition had on the viability of melanoma cells.
3.2. CB1 & CB2 Antagonist
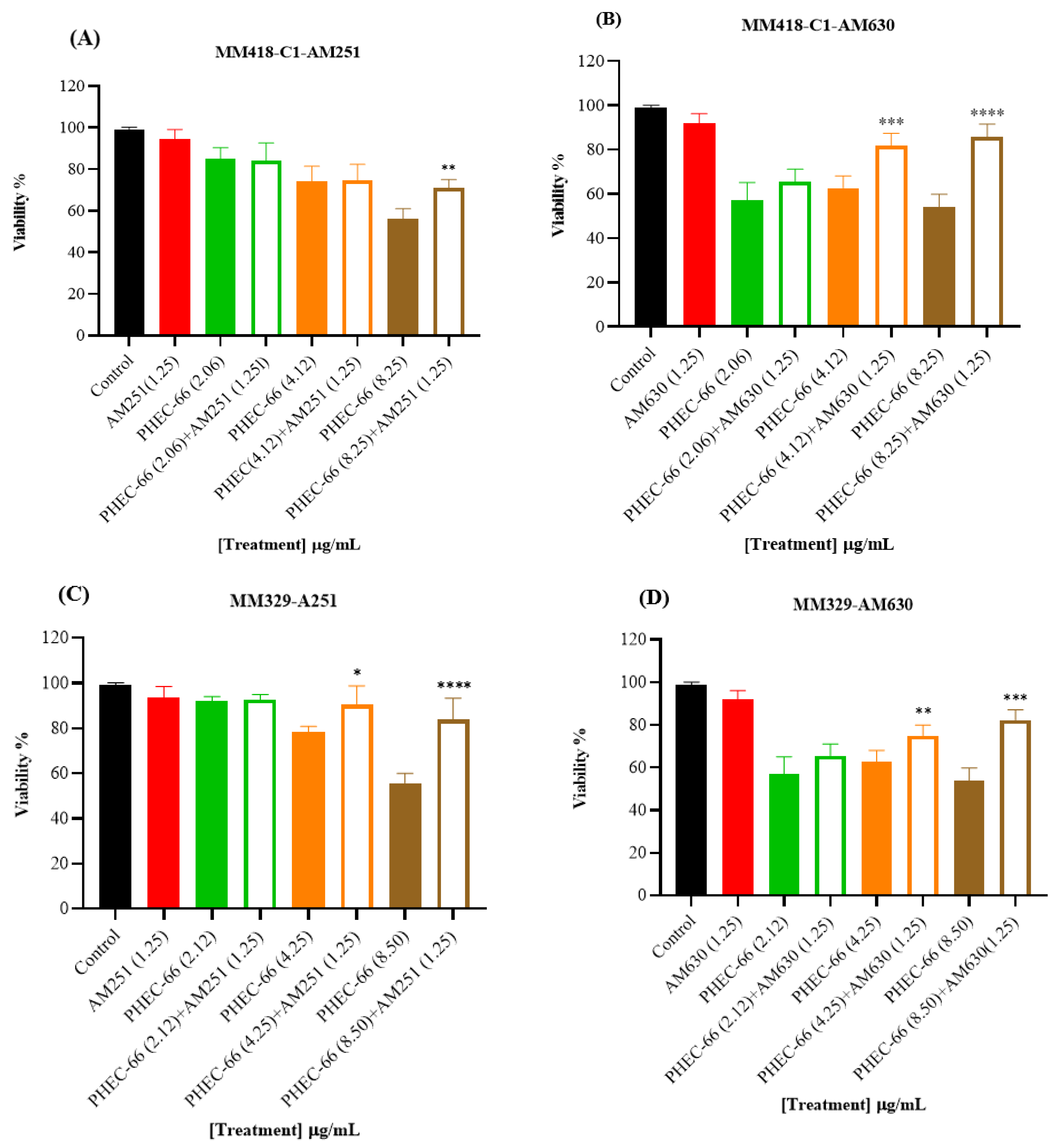
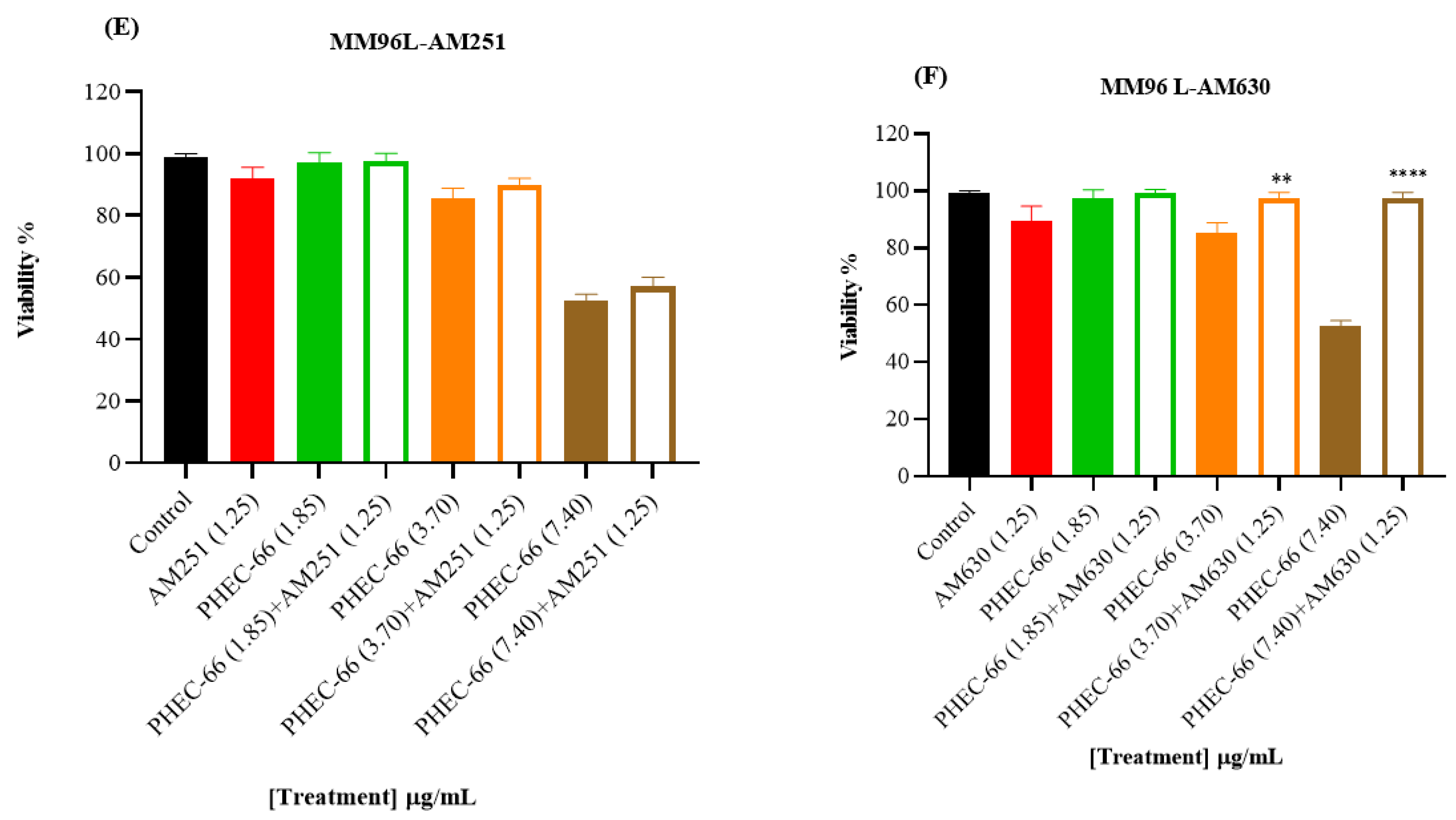
In order to confirm the qPCR results, the melanoma cells were exposed to CB1 (AM261) and CB2 (AM630) antagonists to block these receptors. This approach aimed to confirm whether blocking either the CB1 or CB2 receptor would influence the impact of PHEC-66 on cell viability. The results reveal a significant increase in cell viability for the MM418-C1, MM329, and MM96L cell lines when treated with CB2 antagonist (AM630) plus PHEC-66 compared with untreated cells. Similarly, administering CB1 antagonist (AM251) plus PHEC-66 to these melanoma cell lines significantly improved treated MM418-C1 and MM329 cell viability compared with the untreated cells (Figure 2).
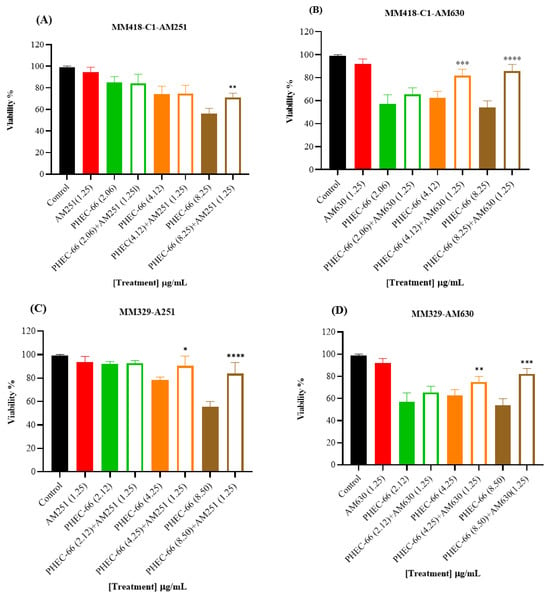
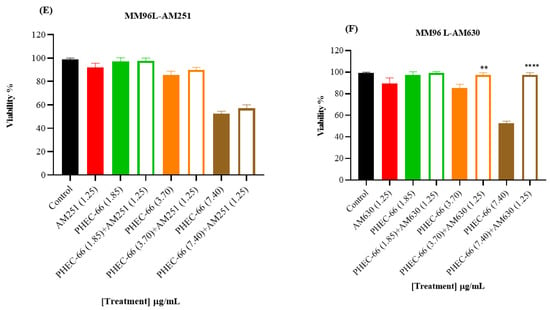
Figure 2.
The effect of PHEC-66 extract on the viability of melanoma cells treated with CB1 and CB2 antagonists. MM418-C1 cells treated with PHEC-66 in the presence or absence of (A) CB1 antagonist (1.25 µg/mL AM251), (B) CB2 antagonist (1.25 µg/mL AM630). MM329 cells treated with PHEC-66 in the presence or absence of (C) CB1 antagonist (1.25 µg/mL AM251), (D) CB2 antagonist (1.25 µg/mL AM630). MM96L cells treated with PHEC-66 in the presence or absence of (E) CB1 antagonist (1.25 µg/mL AM251), (F) CB2 antagonist (1.25 µg/mL AM630). The data are presented as means ± standard deviations for each group (n = 3). Significant differences between groups are indicated by * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
When the melanoma cells were exposed to PHEC-66 alone, their viability was approximately 55%. The addition of PHEC-66 to AM251-treated cells increased cell viability to 71% for MM418-C1 cells and 83% for MM329 cells (Figure 2A,C). However, for MM96L cells, using the CB1 antagonist to block PHEC-66 did not change the viability of these cells compared to untreated cells (Figure 2E).
3.3. Reactive Oxygen Species (ROS)
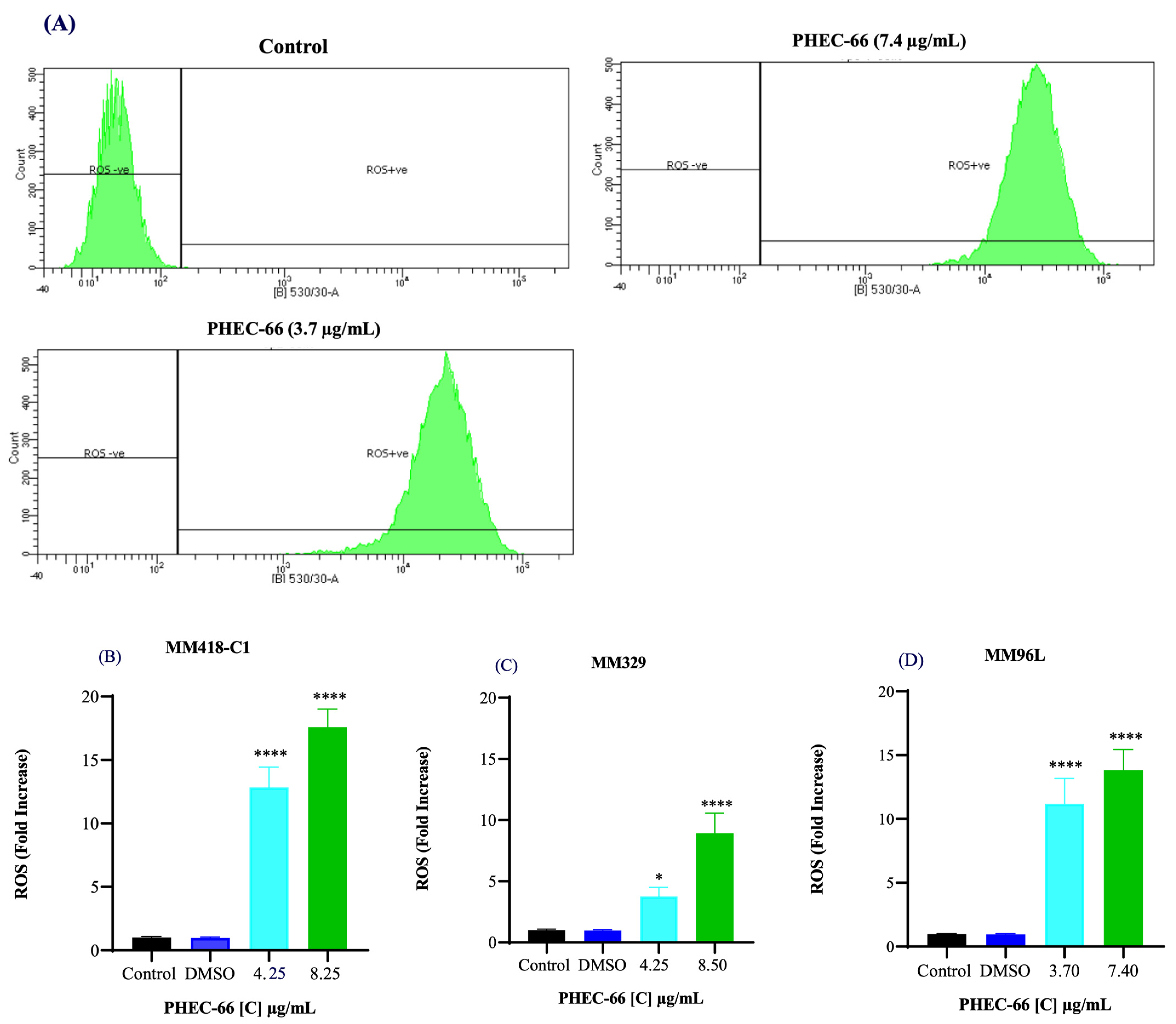
We investigated whether PHEC-66 increased ROS levels in these cells. We exposed the melanoma cells to two different PHEC-66 concentrations (50% and 100% of their corresponding IC50 values) for 24 h. PHEC-66 at 50% of its IC50 concentration caused a 12-, 3-, and 11-fold increase in ROS levels in MM418-C1, MM329, and MM96L cells, respectively, compared to the corresponding untreated controls. The ROS levels increased by factors of 17, 8, and 13 when PHEC-66 was introduced to these cells at its IC50 concentration, and this augmentation was not attributable to the solvent carrier, DMSO (Figure 3).
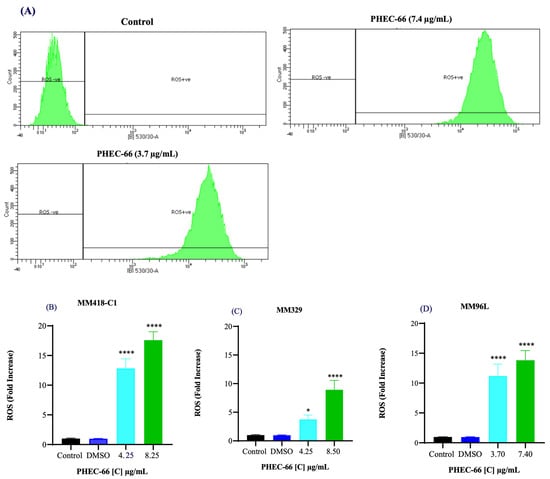
Figure 3.
Effect of PHEC-66 treatment on cellular ROS level in melanoma cells. (A) ROS levels for MM96L were measured using BD LSRFortessa, (B) MM418-C1, (C) MM329, and (D) MM96L were treated for 24 h with PHEC-66 h at both 50% and 100% of its IC50 concentration. Data are expressed as the means ± standard deviations of triplicate experiments (n = 3). Significant differences between PHEC-66-treated cells and vehicle-control cells are marked with * p < 0.05, **** p < 0.0001.
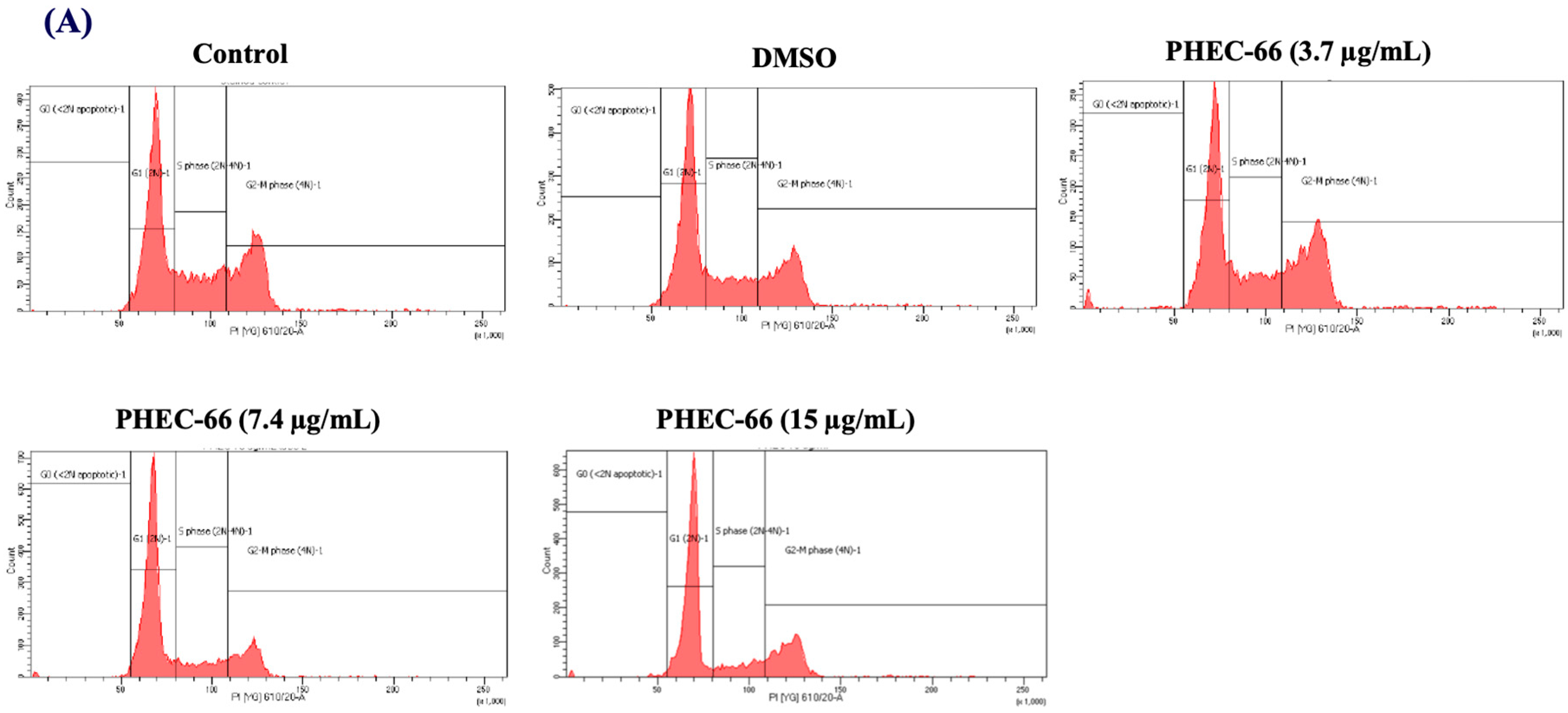
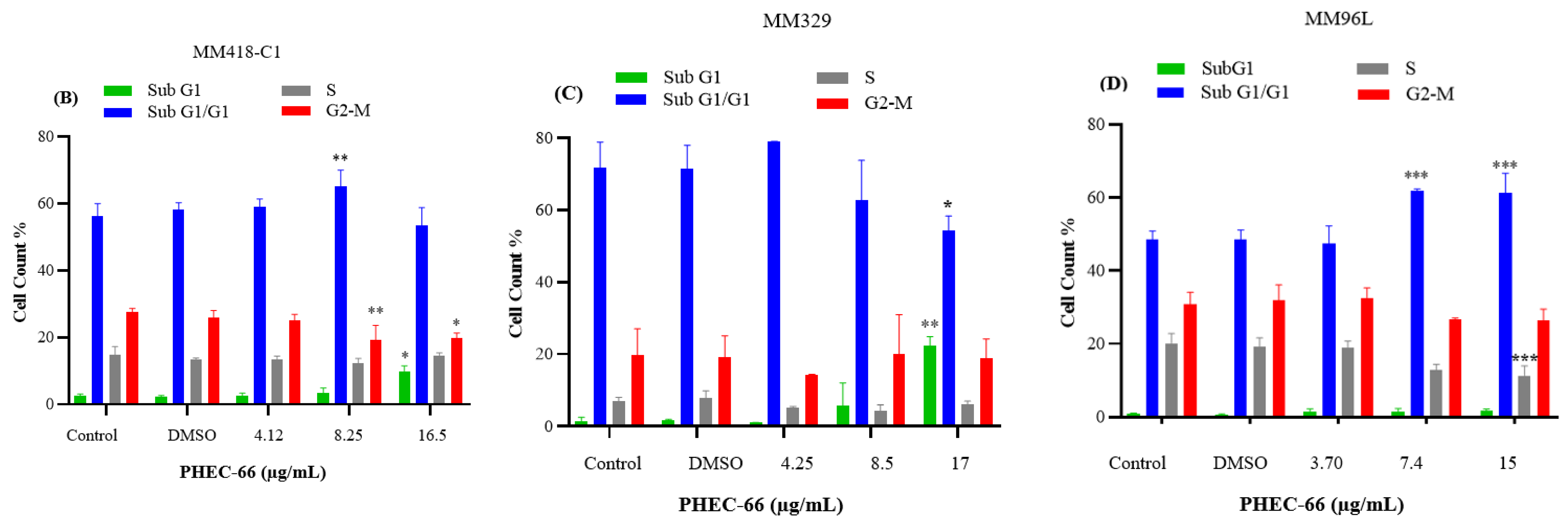
3.4. Effect of PHEC-66 on Cell Cycle Dynamics
In order to further comprehend the mechanism by which PHEC-66 inhibits the growth of cancer cells, a cell cycle analysis of its effect on melanoma cells was investigated. PHEC-66 (16.5 µg/mL) treatment for 24 h increased the percentage of MM418-C1 cells in subG1, indicating damaged and fragmented DNA, which was not observed in the untreated controls (Figure 4A). A similar observation was made for MM329 cells, when PHEC-66 (17 µg/mL) increased the percentage of cells in subG1 (20%) compared to the untreated controls (1.5%). This increase in the number of cells in the sub-G1phase of MM329 cells corresponded to a reduction in the number of cells in the subG1/G1 phase (Figure 4B). Of interest was that in MM96L cells, the PHEC-66 (15 µg/mL) treatment increased the percentage of cells in the subG1/G1 phase by ~13%, which corresponded to the reduction (~10%) in cells in the S phase (Figure 4C).
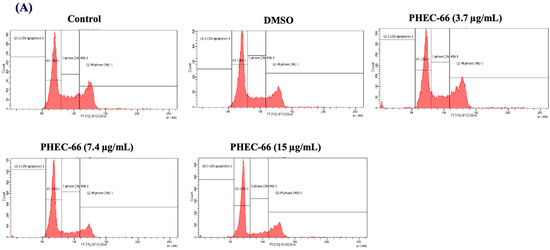
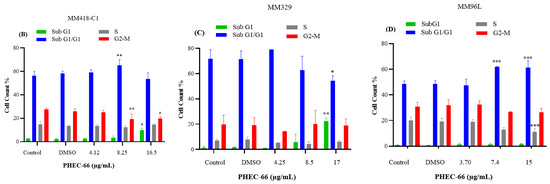
Figure 4.
The effect of PHEC-66 treatment on melanoma cell cycle kinetics was assessed for three different cell lines. These cells were exposed to PHEC-66 at concentrations equivalent to 50%, 100%, and 200% of their respective IC50 values, and the treatment duration was 24 h. (A) Cell cycle distribution for MM96L was analysed for MM96L using BD LSRFortessa, (B) MM418C1, (C) MM329, and (D) MM96L. The data presented represent the mean ± standard deviations for each group (n = 3). Significant differences between groups are indicated * p < 0.05, ** p < 0.01, *** p < 0.001.
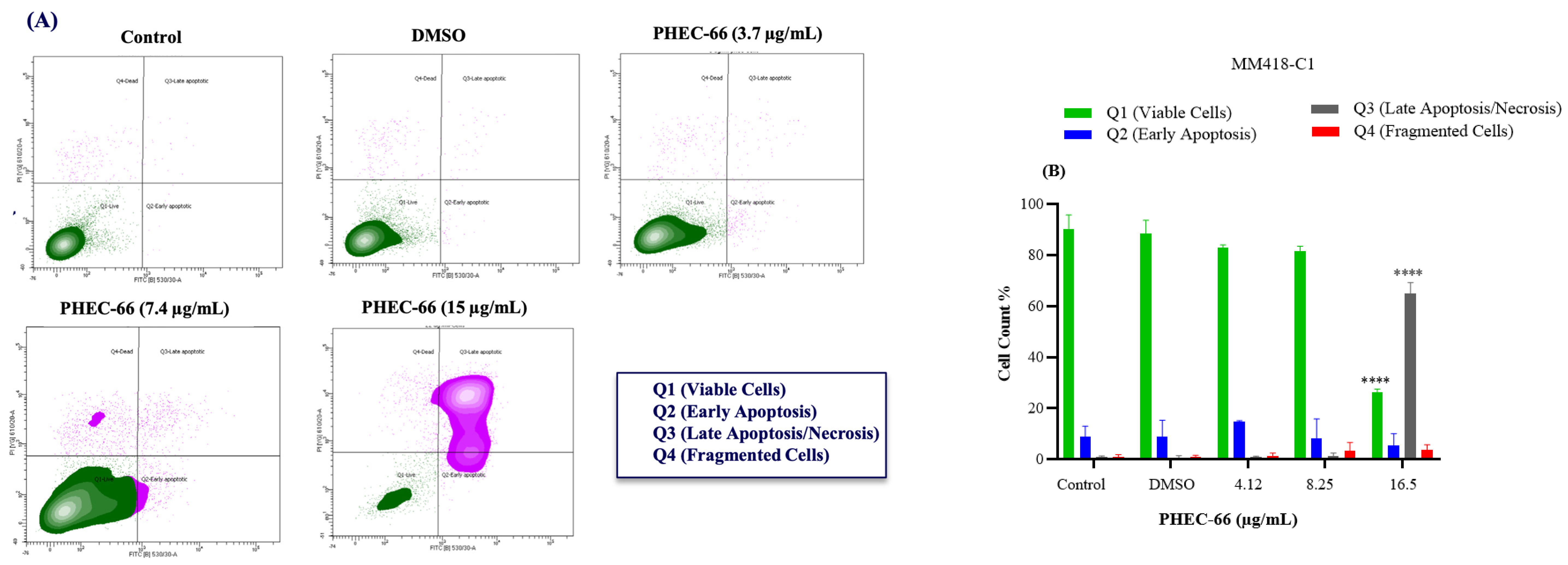
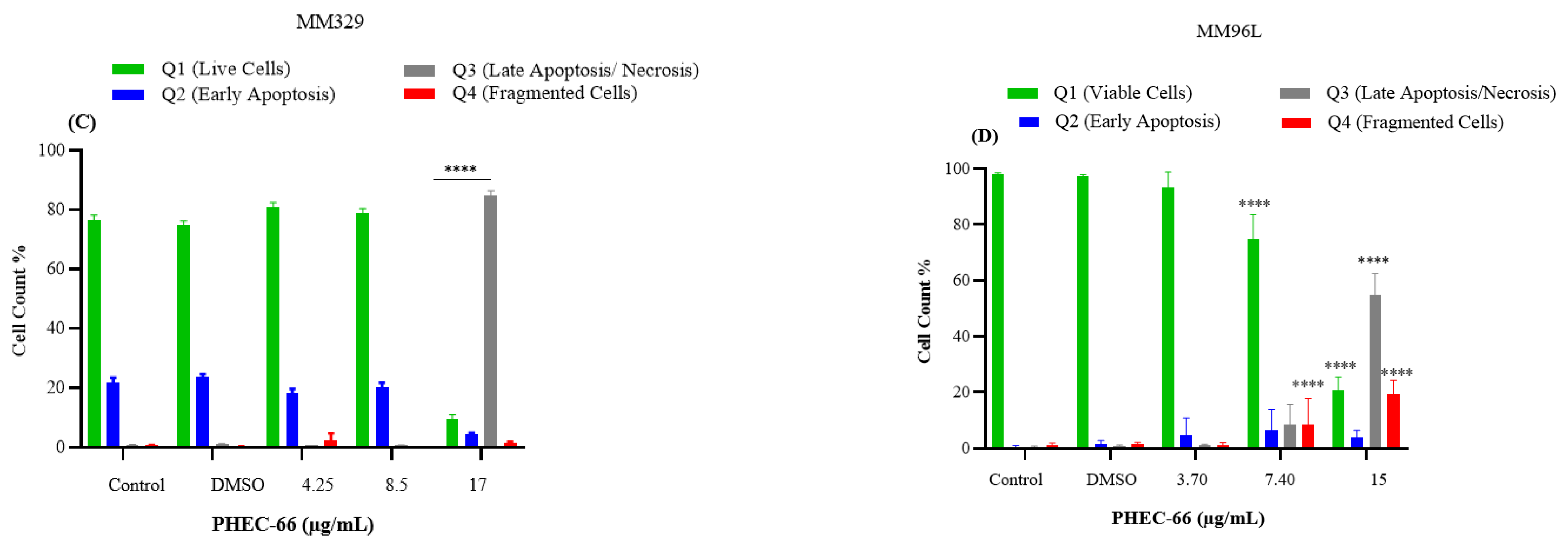
3.5. Apoptosis Assay
After subjecting MM418-C1 cells to PHEC-66 treatment at a concentration of 17 µg/mL for 48 h, the population of viable cells decreased by 64%, due to their progress to a late apoptosis/necrosis state (Figure 5A). In MM329 cells, using 17 µg/mL of PHEC-66 treatment increases the number of cells experiencing late apoptosis (from 1% to 84%) (Figure 5B). Concerning the third cell line, MM96L, Figure 5C displays an increase in late apoptosis by ~64% following the application of 15 µg/mL of PHEC-66.
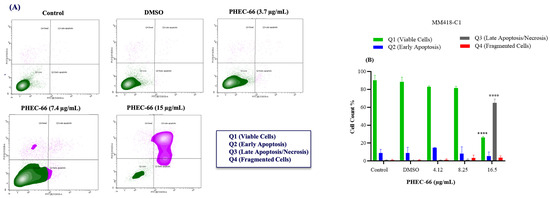
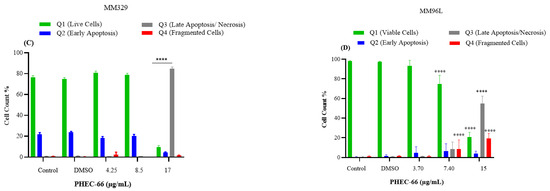
Figure 5.
The effect of PHEC-66 treatment on melanoma was assessed for three different cell lines. These cells were exposed to PHEC-66 at concentrations equivalent to 50%, 100%, and 200% of their respective IC50 values, and the treatment duration was 48 h. (A) Apoptosis for MM96L was analysed using BD LSRFortessa, (B) MM418-C1, (C) MM329, and (D) MM96L. Data are expressed as means ± standard deviations of each group (n = 3). Significant differences between groups are marked with **** p < 0.0001.
3.6. BAX and Bcl-2 Gene Expression
In order to gain a more comprehensive understanding of the underlying mechanism of PHEC-66 in triggering apoptosis, we assessed its effect on the expression of Bcl-2, an anti-apoptotic protein, and BAX, a pro-apoptotic protein. The administration of PHEC-66 led to a reduction in the expression of the anti-apoptotic Bcl-2 gene while increasing the expression of the pro-apoptotic BAX gene when compared to untreated cells. This reaffirms the previous findings that PHEC-66 induces apoptosis in melanoma cell lines.
PHEC-66 reduced the release of Bcl-2 in MM418-C1 and MM329, leading to augmented expression of BAX in these cell lines; nevertheless, no significant changes in the expression of BAX and Bcl-2 were observed in MM96L. In MM418-C1 cells treated with PHEC-66-IC50, the expression of Bcl-2 gene decreased significantly, while BAX gene expression notably increased five-fold compared to untreated cells. In MM329 cells, there was approximately a 1.9-fold increase in BAX gene expression, as indicated in Table 2.

Table 2.
Evaluation of the gene expression level of BAX and Bcl-2 in melanoma cells (MM418, MM329, MM96L) and HaCaT cells following treatment with IC50 of PHEC-66 for 24 h in comparison to untreated cells.
Interestingly, when we applied PHEC-66-IC50 to non-cancerous keratinocyte cells, there was a 2.27-fold increase in the expression of Bcl-2 and no reduction in BAX expression. This suggests that PHEC-66 is less likely to induce apoptosis (cell death) in non-cancerous cells than in melanoma cells, supporting our earlier findings regarding cell death.
4. Discussion
We have previously shown that PHEC-66 exerts a cytotoxic effect on several melanoma cell lines [33]. PHEC-66 prevented the formation of cell colonies, impeded cell migration, induced cellular immobility, and induced morphological changes indicative of apoptosis [33].
Using the quantitative polymerase chain reaction (qPCR), we observed PHEC-66 at its IC50 concentration induced differential changes in the expression of receptor genes of the melanoma cell lines. These results suggest that the signalling pathways triggered by PHEC-66 may involve CB1 and CB2 receptors. Moreover, it is evident that the response to PHEC-66 treatment differs among various cell lines, with MM418-C1 cells being the most responsive in terms of gene expression changes as indicated in Table 3. Drozd et al. observed the anticancer effect of cannabinoids by activating CB1 and CB2 receptors in tissue-cultured Lewis lung adenocarcinoma cells [34]. Another preclinical study, both in vivo and in vitro, also demonstrated that cannabinoids exert their anticancer effects primarily by inducing apoptosis through CB1, CB2, and TRPV1 receptors [35]. Furthermore, cannabinoids have been shown to decrease the expression of the epidermal growth factor receptor (EGFR) as well as plasminogen activator inhibitor-1 (PAI-1) while increasing the expression of the tissue inhibitors of matrix metalloproteinases-1 (TIMP-1) in non-small cell lung cancer (NSCLC) A549, H358, and H460 cell lines, and human-derived NSCLC cells via CB1, CB2, and TRPV1 [36]. Therefore, in a broad sense, cannabis extract, such as PHEC-66, which is primarily composed of CBD (60%), hinders cell proliferation [36], diminishes cell migration, and suppresses the invasive potential of melanoma cells [37], thereby inhibiting angiogenesis and impeding the development of metastases [36].

Table 3.
Effect of PHEC-66 on various mechanisms of melanoma cell lines. PHEC-66 either increased (↑), had no effect (=) or reduced (↓) gene expression and ROS levels, while the CB antagonists either reduced (+) or had no effect (−) on PHEC-66’s cytotoxic effect.
Nonetheless, fully establishing the effect of PHEC-66 on CB1/CB2 receptors in melanoma cells necessitates further investigations at both the protein level and functional aspects. These additional studies should explore whether the increased receptor expression results in enhanced receptor functionality and evaluate the potential therapeutic implications of modulating these receptors in melanoma. This comprehensive analysis would provide a deeper understanding of the mechanisms underlying PHEC-66′s impact on melanoma and its potential for future therapeutic development. Consequently, the impact of CB1 and CB2 was further investigated by employing antagonists to block their activity and examine the effects this inhibition had on the viability of melanoma cells as seen in Table 3.
The interaction between PHEC-66 and its receptors was examined in the presence of specific antagonists, namely, AM251 and AM630, which selectively block CB1 and CB2 receptors, respectively. The use of these specific CB1 and CB2 receptor blockers confirmed that the reduction in growth caused by PHEC-66 was primarily associated with CB2 receptors in all examined melanoma cells.
These findings suggest that the CB1 receptor may be involved to a minor extent in PHEC-66 activity on MM96L cells, while CB2 antagonist significantly blocked PHEC-66 cytotoxicity in all tested cell lines. These results aligned with a previous study that demonstrated the cytotoxic impact of cannabis sativa extract on bladder urothelial carcinoma cell lines, including T24 and HBT-9 cell lines. Notably, the cytotoxic effect was diminished when CB1 and CB2 antagonists were introduced [38].
Cannabinoids have been shown to induce ER stress, resulting in increased ROS levels, which can trigger apoptosis in non-melanoma cells [39,40,41]. The administration of PHEC-66 at half of its IC50 dose resulted in a significant increase in ROS levels within melanoma cells. Moreover, a more substantial elevation in ROS levels was observed when PHEC-66 was applied at its full IC50 dose.
ROS is known to be important for CBD-induced cell death in glioma and leukaemia cells [42,43]. Reduced mitochondrial function leads to higher production of ROS through a process known as electron leakage in the electron transport chain of mitochondria [44]. Moreover, recent studies suggest elevated ROS levels are associated with initiating apoptosis and autophagy [37].
Multiple studies have consistently demonstrated the involvement of endocannabinoids and cannabinoids in exerting antiproliferative effects through metabolic pathways, particularly those involving ROS [37,41,42,43,44]. Park et al. observed a substantial elevation in ROS levels when treating head and neck squamous cell carcinoma (HNSCC) lines with the endocannabinoid anandamide (AEA) [45]. Utilising such cell lines, increased ROS production was observed after AEA treatment. Moreover, antioxidants such as hydrogen peroxide (H2O2) partially reversed the AEA-dependent inhibition of cell proliferation by inducing oxidative stress and damaging cellular components such as DNA, proteins, and lipids [45]. This oxidative damage triggers cell cycle arrest and programmed cell death (apoptosis), ultimately preventing cells from dividing and proliferating [46]. AEA treatment increased intracellular ROS levels in mice cholangiocytes [47]. When these cells were treated with 10 µM AEA for 2 h, ROS levels rose 30-fold [47]. These findings imply that cannabinoids, such as AEA, can trigger ROS generation and accumulation in different cell types. Consequently, this process can induce alterations in cellular signalling pathways, potentially prompting apoptosis cell death. However, further investigation is required to understand the precise mechanism by which PHEC-66 exerts its action on these melanoma cells.
Apoptotic cell death is generally associated with alterations in the cell cycle program [48,49]. When MM418-C1 cells were exposed to 16.5 µg/mL PHEC-66, there was an increase in the sub- G1 phase, which typically indicates cells with fragmented or degraded DNA, a characteristic feature of apoptosis/necrosis [49]. PHEC-66 treatment of MM329 resulted in an elevated sub- G1 phase (indicating apoptotic/necrotic cells) and a corresponding decrease in cells in the sub- G1/G1 phase [48]. In the MM96L cells, there was a notable increase in cells in the sub- G1/G1 phase and a significant reduction in the S phase after PHEC-66 administration as seen in Table 3. DNA synthesis occurs in the S phase, so this decrease suggests that the G1/S cell cycle checkpoint has been blocked [50]. In light of this, the impact of PHEC-66 treatment on three different cell lines highlights its impact on their cell cycle phases. It suggests that PHEC-66 treatment may induce apoptosis and affect cell distribution across various cell cycle phases in these cell lines. These findings are essential for understanding the mechanism of action of PHEC-66 on these cells, and further studies are needed on PHEC-66 to understand why it affected these cells differently.
In order to gain a more comprehensive understanding of the underlying mechanism of PHEC-66 in triggering apoptosis, we assessed its effect on the expression of Bcl-2, an anti-apoptotic protein, and BAX, a pro-apoptotic protein.
BAX and Bcl-2 are both members of the Bcl-2 family, BAX is a central regulator in the intrinsic apoptosis pathway [51]. When stimulated by apoptotic signals, it becomes activated and assembles into oligomers on the mitochondrial outer membrane (MOM), promoting its permeabilization and releasing cytochrome c during mitochondrial-mediated intrinsic apoptosis [52,53], whereas Bcl-2 acts as an inhibitor of apoptosis by forming a heterodimer with BAX, ensuring the inhibition of BAX activity, leading to cell survival sustained by regulating the Ca2+ concentration [54]. The balance between Bcl-2 and BAX proteins is critical in determining whether cytochrome c is released from the mitochondria and whether apoptosis is initiated [55]. High levels of Bcl-2 tend to inhibit cytochrome c release and promote cell survival, while increased BAX levels favour cytochrome c release and apoptosis. Hence, the ratio of Bcl-2 to BAX can be a critical regulatory factor in determining a cell’s fate regarding apoptosis. The administration of PHEC-66 led to a reduction in the expression of the anti-apoptotic Bcl-2 gene while increasing the expression of the pro-apoptotic BAX gene when compared to untreated cells. This reaffirms the previous findings that PHEC-66 induces apoptosis in melanoma cell lines. The contrasting cellular dynamics observed in HaCaT cells, when compared to melanoma cell lines, offer valuable context for interpreting the impact of PHEC-66. Specifically, the observed rise in Bcl-2 expression suggests a pro-survival effect, as it appears to coincide with a decrease in BAX expression, resulting in a favourable ratio that suppresses pro-apoptotic signals. This dual effect implies a cellular response that leans towards enhanced cell viability in the HaCaT cell line. Such observations underscore the importance of recognising cell type-specific responses to PHEC-66 and suggest that PHEC-66 may have a relatively reduced inhibitory effect on HaCaT cells, in contrast to its potential impact on melanoma cell lines. Further investigations are warranted to elucidate the underlying molecular mechanisms governing these effects and to ascertain the broader implications for the application of PHEC-66 in the context of cell-specific therapeutic strategies.
5. Conclusions
In conclusion, we have shown that PHEC-66 impedes the growth of MM418-C1, MM329, and MM96L melanoma cells. This inhibitory effect arises from interactions with CB1 and CB2 receptors. PHEC-66’s impact extends to the modulation of cell cycle progression, particularly evident in the sub G1 and sub G1/G1 phases. Furthermore, PHEC-66 influences metabolic pathways by inducing the accumulation of ROS within these cells, thereby tilting the balance toward pro-apoptotic signalling pathways while diminishing anti-apoptotic ones. All these actions together start the process of apoptosis and slow down the growth of melanoma cells. Further studies are required for a comprehensive understanding of its potential use in advanced-stage melanoma treatment, preferably involving more sophisticated models and assessing its viability within combination therapies.
Author Contributions
Conceptualisation, A.B., N.M., R.Z. and T.J.P.; methodology, A.B.; formal analysis, A.B., N.N., T.J.P. and S.T.; investigation, A.B., N.M. and S.T.; writing—original draft preparation, A.B., N.N. and T.J.P.; writing—review and editing, T.J.P., S.T., R.Z. and N.M.; supervision, S.T., N.M. and T.J.P.; funding acquisition, N.M. and R.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partly funded by MGC Pharmaceuticals Ltd., Australia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data for this manuscript is confidential. However, it can be provided to researcher if the request is reasonable.
Conflicts of Interest
Author Ava Bachari and Roby Zomer was employed by the MGC Pharmaceuticals Limited. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Liu, J.; Fukunaga-Kalabis, M.; Li, L.; Herlyn, M. Developmental pathways activated in melanocytes and melanoma. Arch. Biochem. Biophys. 2014, 563, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Barsouk, A. Epidemiology of Melanoma. Med. Sci. 2021, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Zbytek, B.; Carlson, J.A.; Granese, J.; Ross, J.; Mihm, M.C., Jr.; Slominski, A. Current concepts of metastasis in melanoma. Expert Rev. Dermatol. 2008, 3, 569–585. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Vecchio, D.; Avci, P.; Yin, R.; Garcia-Diaz, M.; Hamblin, M.R. Melanoma resistance to photodynamic therapy: New insights. Biol. Chem. 2013, 394, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Volkovova, K.; Bilanicova, D.; Bartonova, A.; Letašiová, S.; Dusinska, M. Associations between environmental factors and incidence of cutaneous melanoma. Review. Environ. Health 2012, 11 (Suppl. 1), S12. [Google Scholar] [CrossRef]
- Nguyen, D.; Holien, J.; Dekiwadia, C.; Thrimawithana, T.; Piva, T.; Huynh, T. Momordica cochinchinensis (Gấc) Seed Extracts Induce Apoptosis and Necrosis in Melanoma Cells. Pharmaceuticals 2023, 16, 100. [Google Scholar] [CrossRef]
- Sanchez, J.N.; Wang, T.; Cohen, M.S. BRAF and MEK Inhibitors: Use and Resistance in BRAF-Mutated Cancers. Drugs 2018, 78, 549–566. [Google Scholar] [CrossRef]
- Chan, X.Y.; Singh, A.; Osman, N.; Piva, T.J. Role Played by Signalling Pathways in Overcoming BRAF Inhibitor Resistance in Melanoma. Int. J. Mol. Sci. 2017, 18, 1527. [Google Scholar] [CrossRef]
- Eroglu, Z.; Ribas, A. Combination therapy with BRAF and MEK inhibitors for melanoma: Latest evidence and place in therapy. Ther. Adv. Med. Oncol. 2016, 8, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Schulz, A.; Raetz, J.; Karitzky, P.C.; Dinter, L.; Tietze, J.K.; Kolbe, I.; Käubler, T.; Renner, B.; Beissert, S.; Meier, F.; et al. Head-to-Head Comparison of BRAF/MEK Inhibitor Combinations Proposes Superiority of Encorafenib Plus Trametinib in Melanoma. Cancers 2022, 14, 4930. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.; Wayman, M. Encorafenib and Binimetinib Combination Therapy in Metastatic Melanoma. J. Adv. Pract. Oncol. 2022, 13, 450–455. [Google Scholar] [CrossRef]
- Carvajal, R.D.; Piperno-Neumann, S.; Kapiteijn, E.; Chapman, P.B.; Frank, S.; Joshua, A.M.; Piulats, J.M.; Wolter, P.; Cocquyt, V.; Chmielowski, B.; et al. Selumetinib in Combination with Dacarbazine in Patients with Metastatic Uveal Melanoma: A Phase III, Multicenter, Randomized Trial (SUMIT). J. Clin. Oncol. 2018, 36, 1232–1239. [Google Scholar] [CrossRef]
- Mercogliano, M.F.; Bruni, S.; Mauro, F.; Elizalde, P.V.; Schillaci, R. Harnessing Tumor Necrosis Factor Alpha to Achieve Effective Cancer Immunotherapy. Cancers 2021, 13, 564. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Zhou, C.; Ren, S. Role of IL-2 in cancer immunotherapy. OncoImmunology 2016, 5, e1163462. [Google Scholar] [CrossRef]
- Faghfuri, E.; Faramarzi, M.A.; Nikfar, S.; Abdollahi, M. Nivolumab and pembrolizumab as immune-modulating monoclonal antibodies targeting the PD-1 receptor to treat melanoma. Expert Rev. Anticancer Ther. 2015, 15, 981–993. [Google Scholar] [CrossRef]
- de Azevedo, S.J.; de Melo, A.C.; Roberts, L.; Caro, I.; Xue, C.; Wainstein, A. First-line atezolizumab monotherapy in patients with advanced BRAF(V600) wild-type melanoma. Pigment Cell Melanoma Res. 2021, 34, 973–977. [Google Scholar] [CrossRef] [PubMed]
- Rausch, M.P.; Hastings, K.T. Immune Checkpoint Inhibitors in the Treatment of Melanoma: From Basic Science to Clinical Application. In Cutaneous Melanoma: Etiology and Therapy; Ward, W.H., Farma, J.M., Eds.; Codon Publications: Brisbane, Australia, 2017. [Google Scholar]
- Moniuszko, M.; Radziwon, P.; Tucker, S.C.; Honn, K.V. Inhibitors of immune checkpoints-PD-1, PD-L1, CTLA-4-new opportunities for cancer patients and a new challenge for internists and general practitioners. Cancer Metastasis Rev. 2021, 40, 949–982. [Google Scholar]
- Carlino, M.S.; Larkin, J.; Long, G.V. Immune checkpoint inhibitors in melanoma. Lancet 2021, 398, 1002–1014. [Google Scholar] [CrossRef] [PubMed]
- Bachari, A.; Nassar, N.; Schanknecht, E.; Telukutla, S.; Piva, T.J.; Mantri, N. Rationalizing a prospective coupling effect of cannabinoids with the current pharmacotherapy for melanoma treatment. Wiley Interdiscip. Rev. Syst. Biol. Med. 2024, 16, e1633. [Google Scholar] [CrossRef]
- Saad, M.B.; Hong, L.; Aminu, M.; I Vokes, N.; Chen, P.; Salehjahromi, M.; Qin, K.; Sujit, S.J.; Lu, X.; Young, E.; et al. Predicting benefit from immune checkpoint inhibitors in patients with non-small-cell lung cancer by CT-based ensemble deep learning: A retrospective study. Lancet Digit. Health 2023, 5, e404–e420. [Google Scholar] [CrossRef]
- Darvin, P.; Toor, S.M.; Sasidharan Nair, V.; Elkord, E. Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef]
- Smalley, K.S.; Eroglu, Z.; Sondak, V.K. Combination Therapies for Melanoma: A New Standard of Care? Am. J. Clin. Dermatol. 2016, 17, 99–105. [Google Scholar] [CrossRef]
- Chinembiri, T.N.; Du Plessis, L.H.; Gerber, M.; Hamman, J.H.; Du Plessis, J. Review of natural compounds for potential skin cancer treatment. Molecules 2014, 19, 11679–11721. [Google Scholar] [CrossRef]
- Bachari, A.; Piva, T.J.; Salami, S.A.; Jamshidi, N.; Mantri, N. Roles of Cannabinoids in Melanoma: Evidence from In Vivo Studies. Int. J. Mol. Sci. 2020, 21, 6040. [Google Scholar] [CrossRef]
- Lu, H.-C.; Mackie, K. An Introduction to the Endogenous Cannabinoid System. Biol. Psychiatry 2016, 79, 516–525. [Google Scholar] [CrossRef]
- Zou, S.; Kumar, U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar] [CrossRef] [PubMed]
- Hermanson, D.J.; Marnett, L.J. Cannabinoids, endocannabinoids, and cancer. Cancer Metastasis Rev. 2011, 30, 599–612. [Google Scholar] [CrossRef]
- Ramer, R.; Schwarz, R.; Hinz, B. Modulation of the Endocannabinoid System as a Potential Anticancer Strategy. Front. Pharmacol. 2019, 10, 430. [Google Scholar] [CrossRef]
- Muller, C.; Morales, P.; Reggio, P.H. Cannabinoid Ligands Targeting TRP Channels. Front. Mol. Neurosci. 2018, 11, 487. [Google Scholar] [CrossRef]
- Preet, A.; Qamri, Z.; Nasser, M.W.; Prasad, A.; Shilo, K.; Zou, X.; Groopman, J.E.; Ganju, R.K. Cannabinoid receptors, CB1 and CB2, as novel targets for inhibition of non-small cell lung cancer growth and metastasis. Cancer Prev. Res. 2011, 4, 65–75. [Google Scholar] [CrossRef]
- Sarfaraz, S.; Afaq, F.; Adhami, V.M.; Mukhtar, H. Cannabinoid receptor as a novel target for the treatment of prostate cancer. Cancer Res. 2005, 65, 1635–1641. [Google Scholar] [CrossRef] [PubMed]
- Bachari, A.; Nassar, N.; Telukutla, S.; Zomer, R.; Dekiwadia, C.; Piva, T.J.; Mantri, N. In Vitro Antiproliferative Effect of Cannabis Extract PHEC-66 on Melanoma Cell Lines. Cells 2023, 12, 2450. [Google Scholar] [CrossRef]
- Dariš, B.; Tancer Verboten, M.; Knez, Z.; Ferk, P. Cannabinoids in cancer treatment: Therapeutic potential and legislation. Bosn. J. Basic Med. Sci. 2019, 19, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Heider, C.G.; Itenberg, S.A.; Rao, J.; Ma, H.; Wu, X. Mechanisms of Cannabidiol (CBD) in Cancer Treatment: A Review. Biology 2022, 11, 817. [Google Scholar] [CrossRef]
- Ramer, R.; Rohde, A.; Merkord, J.; Rohde, H.; Hinz, B. Decrease of plasminogen activator inhibitor-1 may contribute to the anti-invasive action of cannabidiol on human lung cancer cells. Pharm. Res. 2010, 27, 2162–2174. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gibson, S.B. Is mitochondrial generation of reactive oxygen species a trigger for autophagy? Autophagy 2008, 4, 246–248. [Google Scholar] [CrossRef]
- Anis, O.; Vinayaka, A.C.; Shalev, N.; Namdar, D.; Nadarajan, S.; Anil, S.M.; Cohen, O.; Belausov, E.; Ramon, J.; Gati, E.M.; et al. Cannabis-Derived Compounds Cannabichromene and Δ9-Tetrahydrocannabinol Interact and Exhibit Cytotoxic Activity against Urothelial Cell Carcinoma Correlated with Inhibition of Cell Migration and Cytoskeleton Organization. Molecules 2021, 26, 465. [Google Scholar] [CrossRef]
- Soliman, E.; Van Dross, R. Anandamide-induced endoplasmic reticulum stress and apoptosis are mediated by oxidative stress in non-melanoma skin cancer: Receptor-independent endocannabinoid signaling. Mol. Carcinog. 2016, 55, 1807–1821. [Google Scholar] [CrossRef]
- Pagano, C.; Savarese, B.; Coppola, L.; Navarra, G.; Avilia, G.; Laezza, C.; Bifulco, M. Cannabinoids in the Modulation of Oxidative Signaling. Int. J. Mol. Sci. 2023, 24, 2513. [Google Scholar] [CrossRef]
- Donadelli, M.; Dando, I.; Zaniboni, T.; Costanzo, C.; Pozza, E.D.; Scupoli, M.T.; Scarpa, A.; Zappavigna, S.; Marra, M.; Abbruzzese, A.; et al. Gemcitabine/cannabinoid combination triggers autophagy in pancreatic cancer cells through a ROS-mediated mechanism. Cell Death Dis. 2011, 2, e152. [Google Scholar] [CrossRef]
- Massi, P.; Vaccani, A.; Bianchessi, S.; Costa, B.; Macchi, P.; Parolaro, D. The non-psychoactive cannabidiol triggers caspase activation and oxidative stress in human glioma cells. Cell. Mol. Life Sci. 2006, 63, 2057–2066. [Google Scholar] [CrossRef] [PubMed]
- McKallip, R.J.; Jia, W.; Schlomer, J.; Warren, J.W.; Nagarkatti, P.S.; Nagarkatti, M. Cannabidiol-induced apoptosis in human leukemia cells: A novel role of cannabidiol in the regulation of p22phox and Nox4 expression. Mol. Pharmacol. 2006, 70, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.-Z.; Jiang, S.; Zhang, L.; Yu, Z.-B. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef]
- Park, S.-W.; Hah, J.H.; Oh, S.-M.; Jeong, W.-J.; Sung, M.-W. 5-lipoxygenase mediates docosahexaenoyl ethanolamide and N-arachidonoyl-L-alanine-induced reactive oxygen species production and inhibition of proliferation of head and neck squamous cell carcinoma cells. BMC Cancer 2016, 16, 458. [Google Scholar] [CrossRef]
- Vilema-Enríquez, G.; Arroyo, A.; Grijalva, M.; Amador-Zafra, R.I.; Camacho, J. Molecular and Cellular Effects of Hydrogen Peroxide on Human Lung Cancer Cells: Potential Therapeutic Implications. Oxidative Med. Cell. Longev. 2016, 2016, 1908164. [Google Scholar] [CrossRef]
- DeMorrow, S.; Francis, H.; Gaudio, E.; Ueno, Y.; Venter, J.; Onori, P.; Franchitto, A.; Vaculin, B.; Vaculin, S.; Alpini, G. Anandamide inhibits cholangiocyte hyperplastic proliferation via activation of thioredoxin 1/redox factor 1 and AP-1 activation. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G506–G519. [Google Scholar] [CrossRef] [PubMed]
- Murad, H.; Hawat, M.; Ekhtiar, A.; AlJapawe, A.; Abbas, A.; Darwish, H.; Sbenati, O.; Ghannam, A. Induction of G1-phase cell cycle arrest and apoptosis pathway in MDA-MB-231 human breast cancer cells by sulfated polysaccharide extracted from Laurencia papillosa. Cancer Cell Int. 2016, 16, 39. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Lu, B.; Gan, J.; Wang, S.; Jiang, X.; Li, H. Apoptosis detection: A purpose-dependent approach selection. Cell Cycle 2021, 20, 1033–1040. [Google Scholar] [CrossRef]
- Limas, J.C.; Cook, J.G. Preparation for DNA replication: The key to a successful S phase. FEBS Lett. 2019, 593, 2853–2867. [Google Scholar] [CrossRef]
- McKenna, S.; García-Gutiérrez, L.; Matallanas, D.; Fey, D. BAX and SMAC regulate bistable properties of the apoptotic caspase system. Sci. Rep. 2021, 11, 3272. [Google Scholar] [CrossRef]
- Peña-Blanco, A.; García-Sáez, A.J. Bax, Bak and beyond-mitochondrial performance in apoptosis. FEBS J. 2018, 285, 416–431. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, Y. Role of Bcl-2 family proteins in apoptosis: Apoptosomes or mitochondria? Genes Cells 1998, 3, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Park, H.A.; Broman, K.; Jonas, E.A. Oxidative stress battles neuronal Bcl-xL in a fight to the death. Neural Regen. Res. 2021, 16, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Wei, Z.; Yang, W.; Huang, J.; Yang, Y.; Wang, J. The role of BCL-2 family proteins in regulating apoptosis and cancer therapy. Front. Oncol. 2022, 12, 985363. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).